Abstract
The efficacy and safety of anticoagulant therapy in patients with acute ischemic stroke (AIS) and atrial fibrillation (AF) remain uncertain. This study enrolled 431 AIS and AF patients from Nanjing Drum Tower Hospital between January 2019 and December 2021 and followed for 365 days to determine the associations between anticoagulants and clinical outcomes by assessing modified Rankin Scale (mRS) score, recurrent ischemic stroke/systemic embolism (IS/SE), all-cause mortality, intracranial hemorrhage (ICH) and major bleeding. Final analysis included 400 eligible patients and divided them into antiplatelet group (n = 191) and anticoagulant group (n = 209). Anticoagulant therapy was associated with excellent (mRS 0–1; adjusted odds ratio (aOR), 2.63; 95% confidence interval (CI), 1.61–4.30) and favorable functional outcomes (mRS 0–2; aOR, 2.82; 95% CI, 1.69–4.70) and lower risk of all-cause mortality (adjusted hazard ratio (aHR), 0.35; 95% CI, 0.21–0.57), ICH (aHR, 0.45; 95% CI, 0.23–0.87) and major bleeding (aHR, 0.51; 95% CI, 0.28–0.94), without increasing the risk of recurrent IS/SE (aHR, 0.75; 95% CI, 0.45–1.24). In conclusion, anticoagulant therapy may be a more effective and safer option than antiplatelet therapy for AIS patients with AF.
Keywords: anticoagulant therapy, antiplatelet therapy, acute ischemic stroke, atrial fibrillation
Introduction
Acute ischemic stroke (AIS) remains the most common type of stroke, which affects more than 11 million people worldwide each year. Moreover, AIS is a leading cause of disability and death worldwide.1,2 Atrial fibrillation (AF) is linked to a five-fold increase in the risk of AIS and accounts for 15% to 30% of all AIS cases.3,4 Given the high mortality and morbidity, appropriate anticoagulant treatment, which is required as part of intervention for secondary stroke prevention strategy, should be widely researched in AIS patients with AF.
Currently, oral anticoagulants (OACs) are recommended for patients with AF in order to prevent stroke. 5 The initiation of anticoagulant therapy after AIS is complicated by the fact that patients with AF following AIS have a significant risk of hemorrhagic transformation. 6 The American Heart Association/American Stroke Association and the European Stroke Organization guidelines have already outlined this treatment problem in detail.7,8 However, there is a significant discrepancy between the clinical use of anticoagulant medication in AIS patients with AF and the recommendations. Patients typically undergo antiplatelet medication alone rather than anticoagulant therapy due to worries about the risk of bleeding. Many patients also did not obtain long-term anticoagulant medication because of a history of bleeding, frailty, cognitive impairment, or poor adherence. 9 According to earlier studies, only 11% to 19% of AF patients in China received anticoagulants following the development of AIS.10,11 Studies on the prevention of stroke in people with AF currently tend to compare various anticoagulant types or antiplatelet plus anticoagulant therapy versus anticoagulant therapy alone, as there are still insufficient studies comparing anticoagulant therapy alone with antiplatelet therapy.12–14 Therefore, the purpose of this study was to compare anticoagulant therapy with antiplatelet therapy in real-world patients with AIS and AF.
Methods
Study Design and Data Sources
From January 2019 to December 2021, 431 patients with AIS and AF were retrospectively enrolled in this study at Nanjing Drum Tower Hospital. The inclusion criteria were as follows: (1) diagnosed with AIS and confirmed by computerized tomography (CT) or magnetic resonance imaging (MRI) of the head in accordance with the Chinese guidelines for diagnosis and treatment of AIS; (2) patients with a history of AF or who have been diagnosed with AF at admission based on 24-h dynamic electrocardiography (ECG); (3) age of 18 years or older. Patients were excluded if they met any of the following criteria: (1) patients admitted with intracranial hemorrhage (ICH) or upper gastrointestinal hemorrhage; (2) patients without antithrombotic medications after onset; (3) clinical data missing; (4) lost to follow-up.
According to the principle of intention-to-treat (ITT) analysis, patients were divided into two groups based on the type of antithrombotic medication they received after onset during hospitalization: (1) the antiplatelet group; (2) the anticoagulant group. This study was carried out in accordance with the Declaration of Helsinki and approved by the Human Ethical Committee and Medical Research Council of Nanjing Drum Tower Hospital (Ethics Number: 2023–026-02), and written informed consents were obtained from all the patients or their guardians.
Baseline Assessment
Only the clinical information relating to the first hospitalization was recorded for patients who had several hospitalization experiences. Demographic characteristics, medical and medication histories, and in-hospital treatment were all documented at the time of enrolment. Co-infection was used to describe the infection that developed while the patient was hospitalized. Physical examinations were carried out by senior clinicians. The National Institutes of Health Stroke Scale (NIHSS) score was used to assess the severity of stroke at baseline. The CHA2DS2VASc score and the HAS-BLED score were used to evaluate the risk of ischemic events and the risk of bleeding, respectively. Laboratory parameters such as platelet count, estimated glomerular filtration rate (eGFR), and coagulation function for the enrolled patients were measured.
Treatment
Patients in the antiplatelet group were given either single antiplatelet therapy (SAPT; aspirin 100 mg qd or clopidogrel 75 mg qd) or dual antiplatelet therapy (DAPT; aspirin 100 mg qd plus clopidogrel 75 mg qd). The anticoagulant group included patients who were treated with OACs, such as warfarin (doses adjusted to control the international normalized ratio (INR) between 2 and 3), as well as novel oral anticoagulants (NOACs), such as rivaroxaban (10 mg qd, 15 mg qd, or 20 mg qd) and dabigatran (110 mg bid). The antithrombotic regimens after onset were developed for each patient based on the American Heart Association/American Stroke Association recommendations and the clinician's experience. 7 Other medical interventions followed the recommendations for secondary prevention of stroke.
Outcome Measurement
The safety outcomes were ICH and major bleeding. ICH was defined as hemorrhagic infarction or parenchymal hemorrhage after antithrombotic therapy. Major bleeding was defined as clinically overt bleeding that resulted in a drop in hemoglobin ≥20 g/L, transfusion of ≥2 units of packed red blood cells or whole blood, or involved a critical site (intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal hemorrhage), or had a fatal outcome.
The efficacy outcomes were modified Rankin Scale (mRS) score, recurrent ischemic stroke/systemic embolism (IS/SE), and all-cause mortality. Excellent functional outcome was defined as mRS score of 0 to 1, whereas favorable functional outcome was defined as mRS score of 0 to 2. SE was defined as abrupt vascular insufficiency associated with clinical or radiological evidence of arterial occlusion in the absence of other likely mechanisms (eg, trauma, atherosclerosis or instrumentation); in the presence of atherosclerotic peripheral vascular disease, the diagnosis of the lower-extremity embolism required angiographic evidence of abrupt arterial occlusion.
Follow-up was defined as the 365th day from the date of onset via phone call or in a face-to-face interview. For composite clinical outcome, the event time was defined as the time to first occurrence among components. When all attempts to contact patients failed, these patients were recorded as those lost in follow-up.
Statistical Analysis
Normally distributed continuous variables were expressed as mean ± standard deviation (SD) and compared by the t-test. Non-normally distributed continuous variables were expressed as median (interquartile range (IQR)) and analyzed by the Mann-Whitney U test. Categorical variables were presented as frequency (percentage (%)) and compared using the chi-squared test or Fisher exact test. Univariate and multivariate logistic regression were carried out to determine the association between anticoagulant therapy and functional outcomes. Univariate and multivariate Cox proportional hazard regression analysis models were performed to examine the relationship between anticoagulant therapy and the efficacy and safety outcomes. Candidate variables with P < 0.1 in the univariate analysis were included in the multivariate analysis. The cumulative incidence of the outcomes was estimated by the Kaplan-Meier and analyzed by the log-rank test (anticoagulants vs antiplatelets). To test the robustness of the findings, subgroup analysis in age, co-infection, baseline NIHSS score, thrombolytic therapy, thrombectomy therapy and types of AF was further performed. We dichotomized ages above and below 80 years based on the definition of elderly. The threshold of baseline NIHSS score was set at 15, according to the neurological severity of stroke. The statistical significance of the interaction between anticoagulant therapy and subgroups was defined as Pinteraction <0.1. Two-tailed P < 0.05 was considered statistically significant. All analyses were performed with STATA 17 (Stata Corp LP, College Station, Texas).
Results
Baseline Characteristics
Figure 1 shows the flowchart of this study. Of the 431 patients diagnosed with AIS complicated with AF, 3 patients with ICH and 1 patient with upper gastrointestinal bleeding at admission, 13 patients without antithrombotic drugs after onset, 1 patient's clinical data missing and 13 patients lost to follow-up were excluded. A total of 400 eligible patients entered into the final analysis, of which 191 patients in the antiplatelet group and 209 patients in the anticoagulant group.
Figure 1.
Flowchart of the study. AIS, acute ischemic stroke; AF, atrial fibrillation; ICH, intracranial hemorrhage.
The baseline characteristics of the patients according to the different treatment regimens are shown in Table 1. There were 206 (51.5%) men, with a median age of 79 years. Overall, 91 (22.8%) patients were treated with thrombolytic therapy, and 95 (23.8%) patients received thrombectomy therapy after onset. There were 150 (37.5%) patients had a history of stroke/transient ischemic attack and 327 (81.8%) patients with chronic AF. Co-infection occurred in 40.5% of patients. The median CHA2DS2-VASc score, HAS-BLED score, and NIHSS score at baseline were 4.0, 2.0, and 7.0, respectively. Prior thromboembolism and prior use of antithrombotic drugs were identified in 14 (3.5%) and 154 (38.5%) patients, respectively.
Table 1.
Baseline Characteristics of AIS Patients with AF Using Anticoagulants Versus Antiplatelets.
| Variables | All (n = 400) | Anticoagulants (n = 209) | Antiplatelets (n = 191) | P value |
|---|---|---|---|---|
| Male, n (%) | 206 (51.5) | 115 (55.0) | 91 (47.6) | 0.140 |
| Age, years, median (IQR) | 79.0 (71.0–84.0) | 77.0 (69.0–83.0) | 81.5 (73.0–86.8) | <0.001 |
| Co-infection, n (%) | 162 (40.5) | 68 (32.5) | 94 (49.2) | 0.001 |
| Thrombolytic therapy, n (%) | 91 (22.8) | 53 (25.4) | 38 (19.9) | 0.193 |
| Thrombectomy therapy, n (%) | 95 (23.8) | 46 (22.0) | 49 (25.7) | 0.392 |
| Types of AF, n (%) | ||||
| Chronic AF | 327 (81.8) | 182 (87.1) | 145 (75.9) | 0.004 |
| Newly diagnosed AF | 73 (18.2) | 27 (12.9) | 46 (24.1) | |
| Baseline score, median (IQR) | ||||
| CHA2DS2-VASc score | 4.0 (3.0–6.0) | 5.0 (3.0–6.0) | 4.5 (3.3–6.0) | 0.161 |
| HAS-BLED score | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.718 |
| NIHSS score | 7.0 (2.0–14.0) | 5.0 (2.0–11.0) | 9.5 (3.0–16.0) | <0.001 |
| Medical history, n (%) | ||||
| Smoking | 63 (15.8) | 33 (15.8) | 30 (15.7) | 0.982 |
| Drinking | 50 (12.5) | 32 (15.3) | 18 (9.4) | 0.075 |
| Hypertension | 291 (72.8) | 149 (71.3) | 142 (74.3) | 0.493 |
| Diabetes mellitus | 107 (26.8) | 56 (26.8) | 51 (26.7) | 0.983 |
| Heart failure | 47 (11.8) | 22 (10.5) | 25 (13.1) | 0.427 |
| Stroke/TIA | 150 (37.5) | 78 (37.3) | 72 (37.7) | 0.938 |
| Thromboembolism | 14 (3.5) | 11 (5.3) | 3 (1.6) | 0.045 |
| Bleeding | 17 (4.3) | 5 (2.4) | 12 (6.3) | 0.054 |
| Malignancy | 26 (6.5) | 13 (6.2) | 13 (6.8) | 0.812 |
| Medication history, n (%) | ||||
| Antithrombotic drugs | 154 (38.5) | 100 (47.8) | 54 (28.3) | <0.001 |
| Antihypertensive drugs | 268 (67.0) | 143 (68.4) | 125 (65.4) | 0.527 |
| Diuretics | 21 (5.3) | 12 (5.7) | 9 (4.7) | 0.645 |
| Laboratory parameters | ||||
| PLT, × 109/L, median (IQR) | 171.0 (138.0–215.5) | 166.0 (140.0–213.0) | 176.5 (144.8–218.8) | 0.039 |
| eGFR, ml/min/1.73 m2, mean ± SD | 89.3 ± 26.3 | 87.7 ± 26.4 | 91.0 ± 26.1 | 0.207 |
| PT, s, median (IQR) | 12.1 (11.4–12.8) | 12.1 (11.5–13.1) | 12.1 (11.3–12.8) | 0.204 |
| APTT, s, median (IQR) | 26.4 (25.0–28.3) | 26.8 (25.0–28.6) | 26.4 (24.9–28.0) | 0.273 |
| TT, s, median (IQR) | 18.1 (17.3–19.0) | 18.4 (17.6–19.3) | 18.0 (17.2–18.8) | 0.560 |
| FIB, g/L, median (IQR) | 2.9 (2.5–3.5) | 3.0 (2.4–3.6) | 2.9 (2.5–3.5) | 0.257 |
| D-dimer, mg/L, median (IQR) | 1.0 (0.5–2.5) | 0.9 (0.5–1.8) | 1.2 (0.5–2.3) | 0.562 |
AIS, acute ischemic stroke; AF, atrial fibrillation; APTT, activated partial thromboplastin time; eGFR, estimated glomerular filtration rate; FIB, fibrinogen; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; PLT, platelet count; PT, prothrombin time; SD, standard deviation; TIA, transient ischemic attack; TT, thrombin time.
Patients in the antiplatelet group were older and had higher baseline NIHSS scores than those in the anticoagulant group, and the differences were statistically significant (all P < 0.001). Patients in the anticoagulant group had a lower percentage of co-infection (32.5% vs 49.2%, P = 0.001) and lower median platelet counts (166.0 vs 176.5, P = 0.039) than those in the antiplatelet group. Patients taking anticoagulants were more likely to have a history of thromboembolism (P = 0.045) and chronic AF (P = 0.004). Prior to this hospitalization, more patients took antithrombotic drugs in the anticoagulant group than in the antiplatelet group (47.8% vs 28.3%, P < 0.001).
Antithrombotic Strategies
As shown in Supplementary Table 1, of the 191 (47.8%) patients in the antiplatelet group, 160 (83.8%) patients were treated with SAPT, and 31 (16.2%) patients were treated with DAPT. Among the patients treated with SAPT, 115 (71.9%) patients were treated with aspirin and 45 (28.1%) patients with clopidogrel. The anticoagulant group of 209 (52.2%) patients consisted of 177 (84.7%) patients on NOACs and 32 (15.3%) patients on warfarin. Among patients on NOACs, 142 (80.2%) patients were on rivaroxaban, including 59 (41.5%) patients on 10 mg qd, 68 (47.9%) patients on 15 mg qd, and 15 (10.6%) patients on 20 mg qd, and 35 (19.8%) patients on dabigatran 110 mg bid.
Functional Outcomes
The differences in efficacy and safety outcomes between the anticoagulant and antiplatelet groups were summarized in Figure 2. The percentage of patients with an mRS score of 0–1 at 365 days was 65.1% (n = 136) in the anticoagulant group and 34.0% (n = 65) in the antiplatelet group. Furthermore, the percentage of patients with an mRS score of 0–2 was 69.4% (n = 145) in the anticoagulant group and 37.2% (n = 71) in the antiplatelet group at 365 days. During the follow-up, more patients in the anticoagulant group achieved excellent and favorable functional outcomes than those in the antiplatelet group (all P < 0.001) (Figure 2A&2B). The distribution of mRS scores in patients was shown in Figure 3. These findings suggested that patients who received anticoagulant therapy might achieve significantly better functional outcomes at the end of this study.
Figure 2.
Proportions of efficacy and safety outcomes in the anticoagulant group and antiplatelet group during follow-up. (A) Excellent functional outcome; (B) Favorable functional outcome; (C) Recurrent IS/SE; (D) All-cause mortality; (E) ICH; (F) Major bleeding. mRS, modified Rankin Scale; IS, ischemic stroke; SE, systemic embolism; ICH, intracranial hemorrhage.
Figure 3.
Distribution of mRS Score by treatment groups at 365 days. mRS, modified Rankin Scale.
The multivariate logistic regression analysis revealed that anticoagulant therapy was associated with excellent functional outcome (adjusted odds ratio (aOR), 2.63; 95% CI, 1.61–4.30; P < 0.001) and favorable functional outcome (aOR, 2.82; 95% CI, 1.69–4.70; P < 0.001) after adjusting for statistically significant baseline variables (Table 2; Supplementary Table 2).
Table 2.
Univariate and Multivariate Logistic and Cox Regression Analysis for the Associations Between Anticoagulant Therapy and the Efficacy and Safety Outcomes.
| Functional outcomes | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | aOR (95% CI) | P value | |
| Excellent functional outcome | 3.61 (2.39–5.46) | <0.001 | 2.63 (1.61–4.30) | <0.001 |
| Favorable functional outcome | 3.83 (2.53–5.80) | <0.001 | 2.82 (1.69–4.70) | <0.001 |
OR, odds ratio; CI, confidence interval; aOR, adjusted odds ratio; HR, hazard ratio; aHR, adjusted hazard ratio; IS, ischemic stroke; SE, systemic embolism; ICH, intracranial hemorrhage.
Recurrent IS/SE and All-Cause Mortality
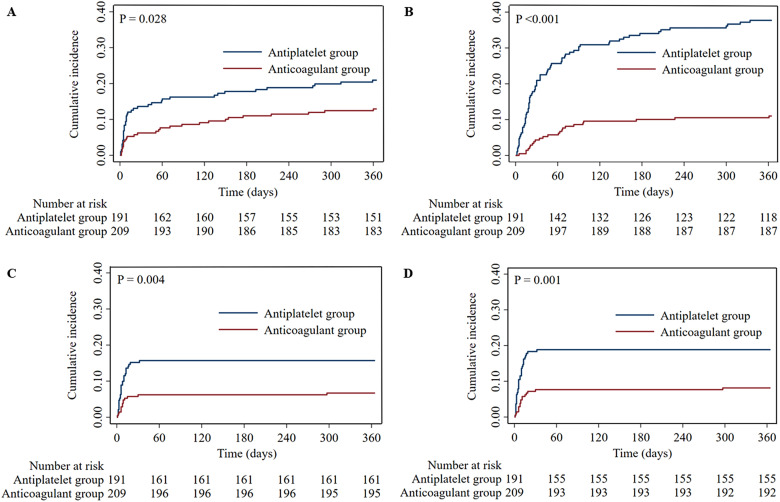
During the follow-up, recurrent IS/SE occurred in 67 (16.8%) patients, and all-cause mortality occurred in 95 (23.8%) patients. In the anticoagulant group, the proportions of patients with recurrent IS/SE (12.9% vs 20.9%, P = 0.032) and all-cause mortality (11.0% vs 37.7%, P < 0.001) were significantly lower than in the antiplatelet group (Figure 2C&2D). The Cox regression analysis comparing other outcomes was presented in Table 2. The relevant adjusting variables were presented in Supplementary Table 3 in detail. After multivariable adjustment, treatment with anticoagulants was associated with a decreased risk of all-cause mortality (adjusted hazard ratio (aHR), 0.35; 95% CI, 0.21–0.57; P < 0.001). However, no significant difference in recurrent IS/SE was observed between the patients with anticoagulant therapy and with antiplatelet therapy (aHR, 0.75; 95% CI, 0.45–1.24; P = 0.264). Moreover, Kaplan-Meier curves showed that patients receiving anticoagulant therapy had a significantly lower cumulative incidence rate of recurrent IS/SE (P = 0.028) and all-cause mortality (P < 0.001) than those receiving antiplatelet therapy (Figure 4A&4B).
Figure 4.
Kaplan-Meier curves estimate the efficacy and safety outcomes between patients with anticoagulant therapy and those with antiplatelet therapy during the follow-up. (A) Recurrent IS/SE; (B) All-cause mortality; (C) ICH; (D) Major bleeding. IS, ischemic stroke; SE, systemic embolism; ICH, intracranial hemorrhage.
Safety Outcomes
In terms of safety outcomes, ICH occurred in 44 (11.0%) patients, and major bleeding occurred in 53 (13.3%) patients. In the anticoagulant group, the proportions of patients with ICH (6.7% vs 15.7%, P = 0.004) and major bleeding (8.1% vs 18.8%, P = 0.002) were significantly lower than in the antiplatelet group (Figure 2E&2F). Furthermore, in multivariate Cox regression analysis, the decreased risks of ICH (aHR, 0.45; 95% CI, 0.23–0.87; P = 0.017) and major bleeding (aHR, 0.51; 95% CI, 0.28–0.94; P = 0.031) were significantly associated with anticoagulant therapy (Table 2). Additionally, Kaplan–Meier curves revealed that the cumulative incidence rate of ICH (P = 0.004) and major bleeding (P = 0.001) were significantly lower in patients receiving anticoagulant therapy compared to those receiving antiplatelet therapy (Figure 4C&4D).
Subgroup Analysis Age (<80 Years, ≥ 80 Years)
No significant interactions were found between the treatment (anticoagulant therapy vs antiplatelet therapy) and age for recurrent IS/SE, ICH and major bleeding (all Pinteraction >0.1) except for all-cause mortality (Pinteraction = 0.001) (Figure 5). Anticoagulant therapy can significantly reduce the risk of all-cause mortality regardless of whether patients were aged <80 years (HR, 0.17; 95% CI, 0.06–0.45) or ≥80 years (HR, 0.31; 95% CI, 0.14–0.64). In patients aged <80 years, anticoagulant therapy was associated with a significant lower incidence of recurrent IS/SE (HR, 0.17; 95% CI, 0.05–0.54).
Figure 5.
Subgroup analysis for the risk of efficacy and safety outcomes in patients with anticoagulant therapy and antiplatelet therapy. (A) Efficacy outcomes; (B) Safety outcomes. AF, atrial fibrillation; HR, hazard ratio; CI, confidence interval; IS, ischemic stroke; SE, systemic embolism; NIHSS, National Institutes of Health Stroke Scale; ICH, intracranial hemorrhage.
Co-Infection
The significant treatment interactions in the co-infection subgroup were observed for all-cause mortality (Pinteraction = 0.079), ICH (Pinteraction = 0.078) and major bleeding (Pinteraction = 0.033), except for recurrent IS/SE (Pinteraction >0.1) (Figure 5). In patients with co-infection, anticoagulant therapy was associated with a significantly reduced risk of all-cause mortality (HR, 0.18; 95%CI, 0.09–0.36) and ICH (HR, 0.22; 95% CI, 0.06–0.78).
Baseline NIHSS Score (<15, ≥ 15)
There were no significant interactions between the treatment and baseline NIHSS score for recurrent IS/SE and major bleeding (all Pinteraction >0.1) (Figure 5). Anticoagulant therapy can significantly reduce the risk of all-cause mortality (Pinteraction <0.001) whether in patients with a baseline NIHSS score <15 (HR, 0.31; 95% CI, 0.15–0.64) or with a baseline NIHSS score ≥15 (HR, 0.06; 95% CI, 0.01–0.26). In patients with a higher baseline NIHSS score (≥15), anticoagulant therapy showed a significant trend towards a lower risk of ICH (HR, 0.04; 95% CI, 0.01–0.64, Pinteraction = 0.067).
Thrombolytic Therapy
There were no significant interactions between treatment and thrombolytic therapy for recurrent IS/SE, ICH and major bleeding (all Pinteraction >0.1), while there was a significant interaction for all-cause mortality (Pinteraction <0.001) (Figure 5). In patients who received thrombolytic therapy, anticoagulant therapy was significantly associated with a reduced risk of recurrent IS/SE (HR, 0.08; 95% CI, 0.01–0.51). In patients without thrombolytic therapy, anticoagulant therapy can significantly reduce the risk of all-cause mortality (HR, 0.24; 95% CI, 0.13–0.46).
Thrombectomy Therapy
There were no significant interactions between treatments and thrombectomy therapy in regards to recurrent IS/SE, ICH, and major bleeding (all Pinteraction >0.1) (Figure 5). Anticoagulant therapy can significantly reduce the risk of all-cause mortality regardless of whether or not the patients received thrombectomy therapy (Pinteraction <0.001).
Types of AF
There were no significant interactions between treatments and types of AF in related to all four clinical outcomes (all Pinteraction >0.1) (Figure 5). In patients with chronic AF, anticoagulant therapy was associated with a significantly reduced risk of all-cause mortality (HR, 0.27; 95%CI, 0.15–0.50).
Efficacy and Safety of NOACs Versus Warfarin in AIS Patients with AF
We also compared the efficacy and safety of NOACs to warfarin. Baseline characteristics between the NOACs group and the warfarin group were compared in Supplementary Table 4. More patients in the warfarin group occurred excellent and favorable functional outcomes, all-cause mortality, ICH and major bleeding during follow-up. On the contrary, the NOACs group had a higher rate of recurrent IS/SE. However, there were no statistically significant differences in the occurrence of all the efficacy and safety outcomes between the two groups (Supplementary Table 5). Multivariate logistic regression analysis and Cox regression analysis indicated that NOACs were not associated with the risk of the efficacy and safety outcomes, as detailed in Supplementary Table 6–7.
Discussion
In this study, a real-world exploratory investigation was conducted to evaluate the efficacy and safety of anticoagulant therapy in AIS patients with AF. Anticoagulant therapy had several advantages over antiplatelet therapy, as follows: (1) significantly improves functional outcomes, as well as lowers the rate and risk of all-cause mortality without increasing the risk of recurrent IS/SE at 365 days; (2) prominently reduces the rate and risk of ICH and major bleeding. This implies that anticoagulant therapy can be safely applied in patients with AIS and AF. To the best of our knowledge, this is the first real-world population-based trial that directly compared the efficacy and safety of anticoagulant therapy alone against antiplatelet therapy alone in patients with AIS and AF.
AIS patients with AF typically have a high rate of all-cause mortality and recurrent embolic events. This association arises from the propensity of AF to cause blood pooling in the atria, subsequently fostering the formation of thrombus. 15 Ray et al discovered that 3 807 patients experienced ischemic events (IS/SE), and 20 336 patients died during follow-up in a cohort analysis of 581 451 AF patients. 16 As a result, rational antithrombotic treatments are urgently required to reduce the impact of embolic events and death on patients’ lives and quality of life. In previous studies, OACs have exhibited superior efficacy than antiplatelets in stroke prevention among AF patients.17–19 Currently, both the European Heart Rhythm Association and the Asia Pacific Heart Rhythm Society clinical guidelines recommend OACs as the preferred choice for preventing cardioembolic stroke in AF patients.5,20 However, due to concerns regarding the potential bleeding risks associated with OACs have led to a reluctance among AF patients in clinical practice to receive appropriate anticoagulant therapy after AIS. According to recent clinical data, approximately 21.7% of Chinese patients with AF-related IS received no antithrombotic therapy after discharge, and the proportions of patients receiving warfarin (29.9%) and NOACs (2.1%) are significantly lower than in Europe and the United States. 21 Only 52.2% of AF patients were treated with anticoagulants following AIS in our analysis, which was close to the proportion recently reported (42.7%) in patients with AIS and AF in China. 22
Several studies have reported the benefit of anticoagulant therapy on functional outcomes. Anticoagulants were associated with a superior functional outcome at 3 months compared to no anticoagulants in a trial of 395 patients with AF-related AIS. 23 A systematic review and meta-analysis of 8 studies involving 3 552 patients with AF-related stroke discovered that initiation of OACs at stroke onset can lead to a favorable functional outcome at 1 year. 24 Similarly, our study found that anticoagulant therapy enhanced not only the proportion of patients with an excellent functional outcome, but also the proportion of patients with a favorable functional outcome.
OACs have also played an important role in improving survival in patients with AF. In high-risk patients with AF, Li et al found that OACs were associated with decreased risk of all-cause mortality when compared to antiplatelets. 25 However, evidence for prognostic benefit of anticoagulant therapy in patients with AIS and AF is still insufficient. A cohort analysis of 1121 IS patients (17.8% with AF) indicated that anticoagulant therapy was associated with a significantly reduced risk of all-cause mortality. 26 This finding is highly consistent with the results of our study. In this study, we demonstrated that anticoagulant therapy was associated with a lower rate and risk of all-cause mortality compared with antiplatelet therapy in patients with AIS and AF. In addition, the subgroup analysis demonstrated evidence of a reduced risk of all-cause mortality with anticoagulant therapy in the subgroups of age, co-infection, baseline NIHSS score, thrombolytic therapy, and thrombectomy therapy. In AIS patients, innate immune cells are known to contribute to ischemic damage, activating systemic immunity and thus increasing the risk of all-cause mortality from fatal infections. 27 Although previous studies have indicated that thrombolytic therapy can improve cardiac embolism recanalization and therefore reduce the risk of all-cause mortality in AIS patients. 28 The subgroup analysis of this study indicated that anticoagulant therapy can significantly reduce the risk of all-cause mortality, even in patients with co-infection or those who did not receive thrombolytic therapy, regardless of age, baseline NIHSS score, or whether received thrombectomy therapy. Furthermore, the subgroup analysis brought to light the observation that the effect of anticoagulant therapy in decreasing the risk of all-cause mortality was particularly pronounced in patients with chronic AF. Given that the majority of stroke patients tend to not receive OACs until AF is diagnosed, it is noteworthy that they may share some similar characteristics with those with chronic AF. 29 Therefore, patients with chronic AF may derive notable benefits from a continuous regimen of OACs, leading to a reduced risk of all-cause mortality.
Antiplatelets and anticoagulants can prevent thrombosis by blocking platelet activation pathways and inhibiting the function of clotting factors, respectively. 30 Compared with placebo, antiplatelets can significantly reduce the risk of recurrent stroke in patients with mild AIS, 31 and OACs can approximately reduce the overall risk of stroke in AF patients by 64%. 32 In this study, the anticoagulant group had a lower rate of recurrent IS/SE than the antiplatelet group. However, when controlling for characteristics like age, thrombectomy therapy, and chronic AF, the effect of anticoagulant therapy on reducing the risk of recurrent IS/SE was not statistically significant. Additionally, our subgroup analysis did not reveal any significant interactions between the treatments and recurrent IS/SE in the various subgroups. Patients after thrombectomy therapy may develop ongoing embolization as result of vessel wall injury, which has been significantly associated with the recurrence of IS/SE. 33 Furthermore, patients require absolute bed immobilization after thrombectomy therapy, resulting in slow and stagnant blood flow in the lower limb veins, which might contribute to embolic events. Meanwhile, the risk of stroke and SE in AF patients increases with age. 34 It has been reported that patients newly diagnosed with AF have a higher risk of recurrent IS/SE than those with chronic AF within 1 year.35,36 Given these considerations, disparities in the distribution of patients undergoing thrombectomy therapy, with chronic AF, and variations in age between the groups in our study may have implications for the efficacy of anticoagulant therapy in reducing the risk of recurrent IS/SE. Importantly, our study found an independent association between anticoagulant therapy and a lower risk of recurrent IS/SE in AIS patients with AF at 365 days, particularly among patients under the age of 80 years or who received thrombolytic therapy. It has been reported that OACs are frequently underused in elderly AF patients, as increasing age is sometimes regarded as a contraindication to anticoagulants, particularly in patients over 75 years.37,38 Besides, patients with age ≥80 years were more likely to discontinue OACs. 39 Thrombolytic therapy is recognized as an effective way to induce tissue reperfusion. Therefore, young AF patients and those undergoing thrombolytic therapy are more likely to benefit from anticoagulant therapy in reducing the risk of recurrent IS/SE.
Although OACs serve an important role in the secondary prevention of stroke, the risk of bleeding is a concern for clinicians. The ACTIVE W trial found that the incidence of major bleeding was comparable between anticoagulant therapy and antiplatelet therapy in 6 706 AF patients with at least one risk factor for stroke. 40 A network meta-analysis of published data from 24 studies encompassing 203 394 AF patients showed that NOACs significantly lowered the risk of ICH more than antiplatelets or placebo. 41 In this study, anticoagulant therapy exhibited a significant capacity to reduce the risk of major bleeding, and this effect was also observed to interact with antiplatelet therapy in the co-infection subgroup. Moreover, our study also found an independent association between anticoagulant therapy and a lower risk of ICH in AIS patients with AF at 365 days, especially in patients with co-infection or baseline NIHSS score ≥15. It has been reported that systemic inflammatory responses triggered by infections and elevated baseline NIHSS scores are associated with a high risk of ICH.42,43 As a result, anticoagulant therapy is particularly promising in reducing the incidence of ICH in patients, especially those who face a high risk of developing ICH.
The comparative efficacy and safety of NOACs and warfarin treatment among patients with AF have been the focus of clinical trials. A meta-analysis based on four clinical trials reported that NOACs may reduce the incidence of stroke/SE, all-cause mortality, ICH, and major bleeding in Asians with AF when compared with warfarin. 44 The multicenter prospective cohort study (da Vinci study) found that NOACs had a better efficacy profile than warfarin in terms of all-cause mortality and ischemic events in AIS patients with AF, although the rate of hemorrhagic events was comparable. 45 However, no substantial advantages of NOACs over warfarin were discovered in terms of efficacy and safety outcomes in this study. Although warfarin is highly efficient in preventing thromboembolism, INR has major influence on its effectiveness. Monitoring and strict control of INR in patients on warfarin can lower the risk of bleeding and thrombosis and patients with a higher therapeutic INR range (TTR) tend to have better outcomes. 46 Moreover, the risk of an outcome event varies with NOACs doses. For example, the risk of major bleeding was lower with the J-ROCKET AF dose (rivaroxaban 15/10 mg/d) than with the ROCKET AF dose (rivaroxaban 20/15 mg/d). 47 Therefore, the findings of this study regarding the efficacy and safety of NOACs against warfarin may be skewed by the lack of monitoring of TTR and dose harmonization for NOACs.
There were several limitations in our study. First, as a single-center retrospective study, the multivariate regression analysis may be susceptible to some selection bias. Therefore, some efforts should be made to construct large-scale studies in order to conduct more effective and in-depth researches. Moreover, patients were classified according to treatment options in the study rather than the random allocation. Although relevant variables were controlled, there was still a possibility of residual confounding. Second, our study did not look into how different anticoagulant dosages and antiplatelet regimens affecting outcomes. Third, the initiation timing of anticoagulant therapy is substantially associated with efficacy and safety outcomes. 48 Therefore, further studies are needed to investigate the efficacy and safety of early and delayed anticoagulant therapy compared with antiplatelet therapy.
Conclusion
In this study, we found that anticoagulant therapy was associated with a lower risk of ICH and major bleeding, as well as a better functional prognosis and lower all-cause mortality, when compared to antiplatelet therapy at 365 days. Our study contributes to a better knowledge of anticoagulant therapy in AIS patients with AF.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296231213070 for Efficacy and Safety of Anticoagulant Therapy Versus Antiplatelet Therapy in Acute Ischemic Stroke Patients with Atrial Fibrillation by Xiaodi Yan, Baoyan Wang, Peng Xia, Chen Lan, Qian Wang, Weihong Ge and Yujie Zhou, Chenxiao Jiang in Clinical and Applied Thrombosis/Hemostasis
Supplemental material, sj-xlsx-2-cat-10.1177_10760296231213070 for Efficacy and Safety of Anticoagulant Therapy Versus Antiplatelet Therapy in Acute Ischemic Stroke Patients with Atrial Fibrillation by Xiaodi Yan, Baoyan Wang, Peng Xia, Chen Lan, Qian Wang, Weihong Ge and Yujie Zhou, Chenxiao Jiang in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The authors thank all the patients and the participants.
Abbreviations
- AIS
Acute Ischemic Stroke
- AF
Atrial Fibrillation
- mRS
modified Rankin Scale
- IS
Ischemic Stroke
- SE
Systemic Embolism
- ICH
Intracranial Hemorrhage
- OACs
Oral Anticoagulants
- CT
Computerized Tomography
- MRI
Magnetic Resonance Imaging
- ECG
Electrocardiography
- NIHSS
National Institutes of Health Stroke Scale
- eGFR
estimated Glomerular Filtration Rate
- SAPT
Single Antiplatelet Therapy
- DAPT
Dual Antiplatelet Therapy
- NOACs
Novel Oral Anticoagulants
- INR
international normalized ratio
- TTR
therapeutic range.
Author Contributions: Xiaodi Yan: Conceptualization, Methodology, Data curation, Writing—Original Draft; Baoyan Wang: Investigation, Validation; Peng Xia: Data curation; Chen Lan: Writing—Review & Editing; Qian Wang: Writing—Review & Editing; Weihong Ge: Funding acquisition; Yujie Zhou: Supervision; Chenxiao Jiang: Writing—Review & Editing, Formal analysis, Funding acquisition. All authors reviewed the manuscript and approved the final version.
Data Availability Statement: Data will be made available on request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: Ethical approval to report this case was obtained from the Ethics Committee of Nanjing Drum Tower Hospital (Ethics Number: 2023–026-02).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University [grant number 2023-LCYJ-PY-24]; the Jiangsu Research Hospital Association for Precision Medication [grant number JY202120]; the Jiangsu Pharmaceutical Association for Jinpeiying Project [grant number J2021001]; and the Nanjing Medical Center for Clinical Pharmacy [grant number 15]. The funders had no involvement in the preparation or writing up of this research.
Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
ORCID iDs: Xiaodi Yan https://orcid.org/0000-0002-2807-9684
Chenxiao Jiang https://orcid.org/0000-0002-7545-5515
Baoyan Wang MSc https://orcid.org/0000-0003-2661-2382
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Boot E, Ekker MS, Putaala J, et al. Ischaemic stroke in young adults: A global perspective. J Neurol Neurosurg Psychiatry. 2020;91(4):411-417. [DOI] [PubMed] [Google Scholar]
- 2.Yi X, Lin J, Han Z, et al. Preceding antithrombotic treatment is associated with acute ischemic stroke severity and functional outcome at 90 days among patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2019;28(7):2003-2010. [DOI] [PubMed] [Google Scholar]
- 3.Jung YH, Choi HY, Lee KY, et al. Stroke severity in patients on non-vitamin K antagonist oral anticoagulants with a standard or insufficient dose. Thromb Haemost. 2018;118(12):2145-2151. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125-e151. [DOI] [PubMed] [Google Scholar]
- 5.Steffel J, Collins R, Antz M, et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.England TJ, Bath PM, Sare GM, et al. Asymptomatic hemorrhagic transformation of infarction and its relationship with functional outcome and stroke subtype: Assessment from the Tinzaparin in Acute Ischaemic Stroke Trial. Stroke. 2010;41(12):2834-2839. [DOI] [PubMed] [Google Scholar]
- 7.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364-e467. [DOI] [PubMed] [Google Scholar]
- 8.Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur Stroke J. 2019;4(3):198-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidoux C, Meseguer E, Ong E, et al. Twelve-month outcome in patients with stroke and atrial fibrillation not suitable to oral anticoagulant strategy: The WATCH-AF registry. Open Heart. 2019;6(2):e001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Li Z, Zhao X, et al. Use of Warfarin at discharge among acute ischemic stroke patients with nonvalvular atrial fibrillation in China. Stroke. 2016;47(2):464-470. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Yang Z, Wang C, et al. Significant underuse of warfarin in patients with nonvalvular atrial fibrillation: Results from the China national stroke registry. J Stroke Cerebrovasc Dis. 2014;23(5):1157-1163. [DOI] [PubMed] [Google Scholar]
- 12.Park CW, Nam HS, Heo JH, et al. Non-vitamin K oral anticoagulants as first-line regimen for acute ischemic stroke with non-valvular atrial fibrillation. J Stroke Cerebrovasc Dis. 2020;29(9):105025. [DOI] [PubMed] [Google Scholar]
- 13.Kim JT, Lee JS, Kim BJ, et al. Effectiveness of adding antiplatelets to oral anticoagulants in patients with acute ischemic stroke with atrial fibrillation and concomitant large artery steno-occlusion. Transl Stroke Res. 2020;11(6):1322-1331. [DOI] [PubMed] [Google Scholar]
- 14.Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry. 2022;93(6):588-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH, Roh SY. The mechanism of and preventive therapy for stroke in patients with atrial fibrillation. J Stroke. 2016;18(2):129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray WA, Chung CP, Stein CM, et al. Association of rivaroxaban vs apixaban with major ischemic or hemorrhagic events in patients with atrial fibrillation. JAMA. 2021;326(23):2395-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806-817. [DOI] [PubMed] [Google Scholar]
- 18.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial . Lancet. 2007;370(9586):493-503. [DOI] [PubMed] [Google Scholar]
- 19.López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: Systematic review, network meta-analysis, and cost effectiveness analysis. Br Med J. 2017;359:j5058. 10.1136/bmj.j5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao TF, Joung B, Takahashi Y, et al. 2021 Focused update of the 2017 consensus guidelines of the Asia Pacific Heart Rhythm Society (APHRS) on stroke prevention in atrial fibrillation. J Arrhythm. 2021;37(6):1389-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Wang Y, Guo W, et al. Temporal trends of atrial fibrillation and/or rheumatic heart disease-related ischemic stroke, and anticoagulant use in Chinese population: An 8-year study. Int J Cardiol. 2021;322:258-264. 10.1016/j.ijcard.2020.08.046 [DOI] [PubMed] [Google Scholar]
- 22.Gong X, Chen H, Wang J, et al. Undertreatment of anticoagulant therapy in hospitalized acute ischemic stroke patients with atrial fibrillation. Front Cardiovasc Med. 2022;9:841020. 10.3389/fcvm.2022.841020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matos-Ribeiro J, Castro-Chaves P, Oliveira-Ferreira M, et al. Early anticoagulation in atrial fibrillation-related acute ischaemic stroke: Efficacy and safety profile. J Neurol. 2022;269(4):2099-2112. [DOI] [PubMed] [Google Scholar]
- 24.Hannon N, Arsava EM, Audebert HJ, et al. Antithrombotic treatment at onset of stroke with atrial fibrillation, functional outcome, and fatality: A systematic review and meta-analysis. Int J Stroke. 2015;10(6):808-814. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Pathadka S, Man KKC, et al. Comparative outcomes between direct oral anticoagulants, warfarin, and antiplatelet monotherapy among Chinese patients with atrial fibrillation: A population-based cohort study. Drug Saf. 2020;43(10):1023-1033. [DOI] [PubMed] [Google Scholar]
- 26.Goulart AC, Olmos RD, Santos IS, et al. The impact of atrial fibrillation and long-term oral anticoagulant use on all-cause and cardiovascular mortality: A 12-year evaluation of the prospective Brazilian Study of Stroke Mortality and Morbidity. Int J Stroke. 2022;17(1):48-58. [DOI] [PubMed] [Google Scholar]
- 27.Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130(6):2777-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matute MC, Masjuan J, Egido JA, et al. Safety and outcomes following thrombolytic treatment in stroke patients who had received prior treatment with anticoagulants. Cerebrovasc Dis. 2012;33(3):231-239. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Macha K, Haupenthal D, et al. Acute care and secondary prevention of stroke with newly detected versus known atrial fibrillation. Eur J Neurol. 2022;29(7):1963-1971. [DOI] [PubMed] [Google Scholar]
- 30.Kapil N, Datta YH, Alakbarova N, et al. Antiplatelet and anticoagulant therapies for prevention of ischemic stroke. Clin Appl Thromb Hemost. 2017;23(4):301-318. [DOI] [PubMed] [Google Scholar]
- 31.Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: Time-course analysis of randomised trials. Lancet. 2016;388(10042):365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Essa H, Hill AM, Lip GYH. Atrial fibrillation and stroke. Card Electrophysiol Clin. 2021;13(1):243-255. [DOI] [PubMed] [Google Scholar]
- 33.Romoli M, Vandelli L, Bigliardi G, et al. Fibrinogen depletion coagulopathy predicts Major bleeding after thrombolysis for ischemic stroke: A multicenter study. Stroke. 2022;53(12):3671-3678. [DOI] [PubMed] [Google Scholar]
- 34.Kim BJ, Han MK, Park TH, et al. Low-Versus standard-dose alteplase for ischemic strokes within 4.5 hours: A comparative effectiveness and safety study. Stroke. 2015;46(9):2541-2548. [DOI] [PubMed] [Google Scholar]
- 35.Cerasuolo JO, Cipriano LE, Sposato LA. The complexity of atrial fibrillation newly diagnosed after ischemic stroke and transient ischemic attack: Advances and uncertainties. Curr Opin Neurol. 2017;30(1):28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sposato LA, Cerasuolo JO, Cipriano LE, et al. Atrial fibrillation detected after stroke is related to a low risk of ischemic stroke recurrence. Neurology. 2018;90(11):e924-e931. [DOI] [PubMed] [Google Scholar]
- 37.Best JG, Bell R, Haque M, et al. Atrial fibrillation and stroke: A practical guide. Pract Neurol. 2019;19(3):208-224. [DOI] [PubMed] [Google Scholar]
- 38.Hylek EM, D'antonio J, Evans-Molina C, et al. Translating the results of randomized trials into clinical practice: The challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37(4):1075-1080. [DOI] [PubMed] [Google Scholar]
- 39.Potpara TS, Lip GY. Oral anticoagulant therapy in atrial fibrillation patients at high stroke and bleeding risk. Prog Cardiovasc Dis. 2015;58(2):177-194. [DOI] [PubMed] [Google Scholar]
- 40.Su Y, Cheng X, Dong Q. Dual antiplatelet therapy of clopidogrel and aspirin in secondary prevention of ischemic stroke: Evidence and indications. CNS Neurosci Ther. 2015;21(11):870-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blann AD, Skjøth F, Rasmussen LH, et al. Edoxaban versus placebo, aspirin, or aspirin plus clopidogrel for stroke prevention in atrial fibrillation. An indirect comparison analysis. Thromb Haemost. 2015;114(2):403-409. [DOI] [PubMed] [Google Scholar]
- 42.Wang RH, Wen WX, Jiang ZP, et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. 2023;14:1115031. 10.3389/fimmu.2023.1115031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Åsberg S, Farahmand B, Hasvold P, et al. Non-cardioembolic TIA and ischemic stroke: Implications of severity. Acta Neurol Scand. 2018;138(4):369-376. [DOI] [PubMed] [Google Scholar]
- 44.Wang KL, Lip GY, Lin SJ, et al. Non-Vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: Meta-analysis. Stroke. 2015;46(9):2555-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saji N, Kimura K, Tateishi Y, et al. Safety and efficacy of non-vitamin K oral anticoagulant treatment compared with warfarin in patients with non-valvular atrial fibrillation who develop acute ischemic stroke or transient ischemic attack: A multicenter prospective cohort study (daVinci study). J Thromb Thrombolysis. 2016;42(4):453-462. [DOI] [PubMed] [Google Scholar]
- 46.Ababneh M, Nasser SA, Rababa'h A, et al. Warfarin adherence and anticoagulation control in atrial fibrillation patients: A systematic review. Eur Rev Med Pharmacol Sci. 2021;25(24):7926-7933. [DOI] [PubMed] [Google Scholar]
- 47.Chan YH, Lee HF, Wang CL, et al. Comparisons of rivaroxaban following different dosage criteria (ROCKET AF or J-ROCKET AF trials) in Asian Patients With Atrial Fibrillation. J Am Heart Assoc. 2019;8(21):e013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seiffge DJ, Werring DJ, Paciaroni M, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019;18(1):117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296231213070 for Efficacy and Safety of Anticoagulant Therapy Versus Antiplatelet Therapy in Acute Ischemic Stroke Patients with Atrial Fibrillation by Xiaodi Yan, Baoyan Wang, Peng Xia, Chen Lan, Qian Wang, Weihong Ge and Yujie Zhou, Chenxiao Jiang in Clinical and Applied Thrombosis/Hemostasis
Supplemental material, sj-xlsx-2-cat-10.1177_10760296231213070 for Efficacy and Safety of Anticoagulant Therapy Versus Antiplatelet Therapy in Acute Ischemic Stroke Patients with Atrial Fibrillation by Xiaodi Yan, Baoyan Wang, Peng Xia, Chen Lan, Qian Wang, Weihong Ge and Yujie Zhou, Chenxiao Jiang in Clinical and Applied Thrombosis/Hemostasis