Abstract
Excised Zea mays L. embryos were cultured on Linsmaier and Skoog medium. Coleoptiles were sampled at regular intervals and the length, fresh weight, cell wall weight, and cell wall neutral sugar composition were determined. A specific β-d-glucanase from Bacillus subtilis was used to determine the content of a (1 → 3),(1 → 4)-β-d-glucan.
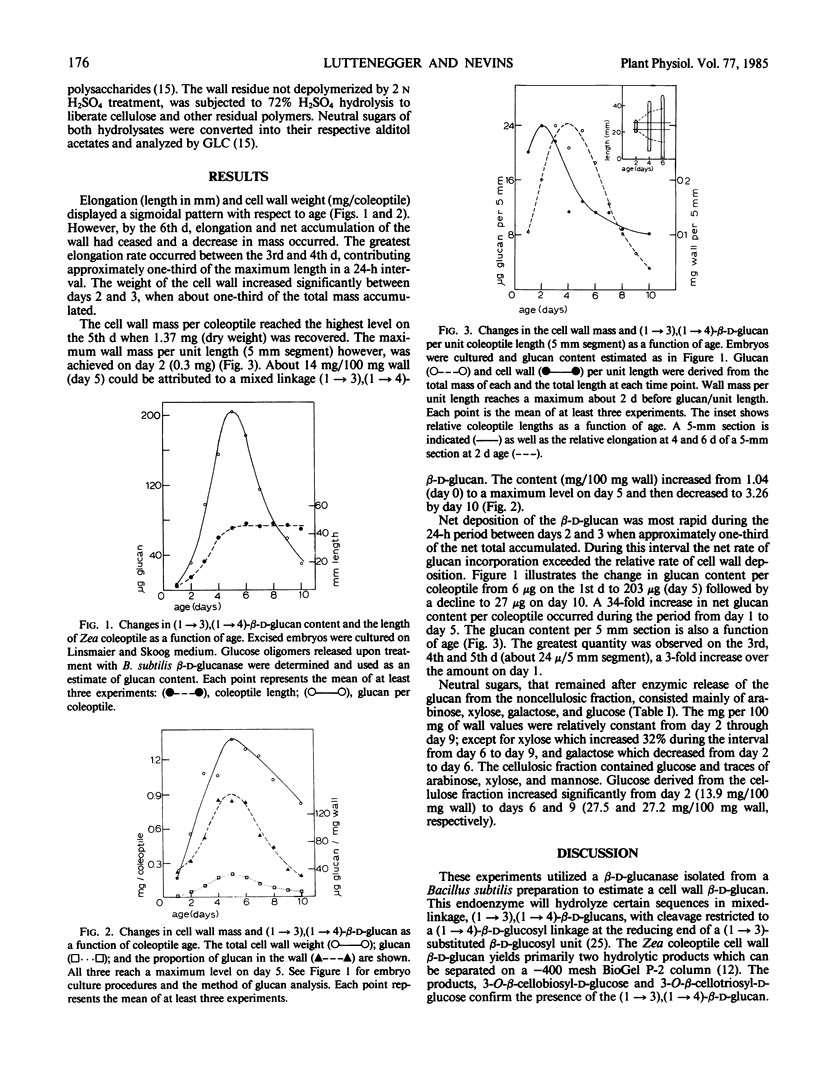
Coleoptiles elongated through the 5th day following imbibition with the most rapid elongation occurring between days 3 and 4. The greatest net rate of incorporation of cell wall per coleoptile occurred between the 2nd and 3rd days when deposition of approximately one-third of the maximum net glucan level was observed. By day 5, the amount of glucan present had increased 34-fold from the 6 micrograms per coleoptile on day 1 and accounted for about 14% of the cell wall (w/w). Thereafter, the glucan content declined until only 3.3% (w/w) remained by day 10. In this 10-day interval, xylose increased 32% and cellulose content doubled, while proportions of other neutral sugars changed less dramatically.
These results are consistent with a possible role for the β-d-glucan in elongation of the Zea coleoptile. Moreover, changes in the quantity of this wall component clearly reflect the dynamic nature of plant cell wall polysaccharides. An evaluation of glucan dynamics in vivo suggests that in vitro autolysis studies employing Zea coleoptile walls may overestimate the actual rate of glucan turnover in the intact tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASPINALL G. O. Structural chemistry of the hemicelluloses. Adv Carbohydr Chem. 1959;14:429–468. doi: 10.1016/s0096-5332(08)60228-3. [DOI] [PubMed] [Google Scholar]
- Carpita N. C., Brown R. A., Weller K. M. Uptake and Metabolic Fate of Glucose, Arabinose, and Xylose by Zea mays Coleoptiles in Relation to Cell Wall Synthesis. Plant Physiol. 1982 May;69(5):1173–1180. doi: 10.1104/pp.69.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Factors Influencing beta-Glucan Synthesis by Particulate Enzymes from Suspension-Cultured Lolium multiflorum Endosperm Cells. Plant Physiol. 1982 Mar;69(3):632–636. doi: 10.1104/pp.69.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. Preparation and Properties of a beta-d-Glucanase for the Specific Hydrolysis of beta-d-Glucans. Plant Physiol. 1977 Aug;60(2):300–304. doi: 10.1104/pp.60.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nevins D. J. Enzymic Dissociation of Zea Shoot Cell Wall Polysaccharides : II. Dissociation of (1 --> 3),(1 --> 4)-beta-d-Glucan by Purified (1 --> 3),(1 --> 4)-beta-d-Glucan 4-Glucanohydrolase from Bacillus subtilis. Plant Physiol. 1984 Jul;75(3):745–752. doi: 10.1104/pp.75.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nevins D. J. Enzymic dissociation of zea shoot cell wall polysaccharides : I. Preliminary characterization of the water-insoluble fraction of zea shoot cell walls. Plant Physiol. 1984 Jul;75(3):740–744. doi: 10.1104/pp.75.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M., Ordin L. A cell wall polysaccharide-hydrolyzing enzyme system in Avena sativa L. coleoptiles. Biochim Biophys Acta. 1967 Jun 13;141(1):126–134. doi: 10.1016/0304-4165(67)90251-6. [DOI] [PubMed] [Google Scholar]
- Kivilaan A., Bandurski R. S., Schulze A. A partial characterization of an autolytically solubilized cell wall glucan. Plant Physiol. 1971 Oct;48(4):389–393. doi: 10.1104/pp.48.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W. H., Nevins D. J. Turgor-dependent Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1973 Sep;52(3):248–251. doi: 10.1104/pp.52.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REESE E. T., PERLIN A. S. Enzymic preparation of 3-O-beta-cellobiosyl D-glucose. Biochem Biophys Res Commun. 1963 Jul 26;12:194–197. doi: 10.1016/0006-291x(63)90188-8. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Sugar composition of oat-coleoptile cell walls. Biochem J. 1963 Oct;89(1):144–150. doi: 10.1042/bj0890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHABANOVA I. A. Osobennosti sostava i uglovodnofosfornogo obmena myshts krys pri eksperimental'noi myshechnoi distrofii. Biokhimiia. 1953 Jul-Aug;18(4):385–392. [PubMed] [Google Scholar]


