Abstract
Rapid detection of Vibrio vulnificus can be enhanced by optimizing the components of enrichment broth. PNC (5% peptone, 1% NaCl, and 0.08% cellobiose [pH 8.0]) enhanced the growth of V. vulnificus compared to alkaline peptone broth. PNCC (PNC with 1.0 to 4.1 U of colistin methanesulfonate per ml) increased the growth of low levels of V. vulnificus while suppressing non-target bacteria.
Vibrio vulnificus causes invasive and life-threatening disease in persons with weakened host defenses (7, 10, 13). Preventing infection depends in part on early detection. Currently, environmental and clinical data indicate that a level of ≥1,000 V. vulnificus organisms per g of oyster meat is associated with human infections (6). This concentration is below or near the minimum detection limits of most diagnostic assays, necessitating the use of enrichment broths.
Virtually all bacterial cultivations can be complicated by replication of non-target bacteria that compete for limited nutrients, produce bacteriocins, and/or effect pH (8, 11, 17). Another factor affecting method sensitivity is differentiation of target organisms among various colonies on agar. For example, TCBS (thiosulfate-citrate-bile salt-sucrose) agar is an excellent medium for selective isolation of vibrios; however, because marine samples normally contain multiple Vibrio spp., especially dominant vibrios such as Vibrio parahaemolyticus and Vibrio alginolyticus, the target vibrio is often difficult to locate among colonies (3, 15–17).
Currently, diagnostic assays require sample enrichment to elevate low numbers of bacteria to minimum detection limits (1, 3–5, 12). For example, the lower limit of detection for PCR in oysters is 10,000 CFU/ml-g (1). Immunoassays usually require 105 cells/ml, with false-negative reactions observed in low dilutions of samples, possibly resulting from overgrowth by non-target organisms and/or inhibitory substances in sample homogenates (15).
The purpose of this study was to define components of enrichment broth that enhance and select for growth of V. vulnificus. First, the effects of alkaline peptone broth (APB) components on V. vulnificus growth were evaluated. From these results, a basal broth was prepared and used to evaluate additional medium supplements (i.e., selected antibiotics and carbohydrates).
Bacterial strains.
V. vulnificus environmental strains 4600, 4832, and 4965 (provided by D. A. Cook, U.S. Food and Drug Administration [FDA]), V. parahaemolyticus HC5C-1C (provided by C. Kaysner, U.S. FDA), and V. alginolyticus ATCC 17749 were studied. All strains were authenticated by established techniques (3, 15). One day prior to experimentation, frozen cultures were plated on tryptic soy agar (TSA) (Difco Laboratories, Detroit, Mich.) containing 1.0% NaCl. Results showed that the three V. vulnificus strains had indistinguishable growth patterns under all test conditions; unless otherwise specified, only V. vulnificus 4832 data are shown.
V. vulnificus strains were incubated on TSA–1% NaCl at 35°C for 16 to 24 h, and then one isolated colony was transferred to 10 ml of APB and incubated until logarithmic growth phase. Twenty microliters was transferred to 8 ml of experimental broth, and bacterial growth was monitored by the absorbance at 420 nm. All experiments consisted of static cultures and were conducted in triplicate.
Effects of peptone.
Deionized water containing 1% NaCl was supplemented with 0.5 to 5.0% peptone at pH 8.4.
Effects of NaCl.
Deionized water containing peptone was supplemented with 0 to 5.0% NaCl.
Effects of pH.
Two experiments were conducted to determine the effects of pH. First, media containing 5.0% peptone and 1.0% NaCl were made in 0.1 M citrate-phosphate, potassium phosphate, or Tris-HCl to produce respective pH values of 5.0 and 5.5; 6.0, 6.5, 7.0, 7.5, and 8.0; and 8.5 and 9.0. Second, media containing 5% peptone and 1% NaCl were made in 0.01, 0.03, 0.05, and 0.1 M Tris buffer to produce pH values of 7.5, 8.0, and 8.5 for each buffer concentration.
Effects of temperature.
Media containing 5.0% peptone–1.0% NaCl (pH 8.0) were incubated at 25, 30, 35, 40, and 45°C to determine the effects of temperature.
Effects of selective carbohydrates.
Media containing 0.1, 1.0, or 5.0% peptone and 1.0% NaCl (pH 8.0) were supplemented with salicin (0 to 0.5%), lactose (0 to 2.5%), or cellobiose (0 to 5.0%). A simplex-centroid experimental design was used to determine if combinations of carbohydrates had synergistic effects (2). Concentrations of each carbohydrate that supported optimum growth were chosen from single carbohydrate studies. Seven combinations of three carbohydrates (0.1% lactose, 0.1% salicin, 0.08% cellobiose) were studied.
Effects of antibiotics.
The susceptibilities of V. vulnificus, V. alginolyticus, and V. parahaemolyticus to polymyxin B (PB) and colistin methanesulfonate (CM) were determined. The three species were separately prepared at 101 to 105 CFU/ml, and 1 ml was added to 10 ml of broth containing 5% peptone–1% NaCl (pH 8.0) supplemented with PB at 0.25, 0.31, 0.50, or 0.62 U/ml or CM at 1.0, 2.1, 4.1, or 8.2 U/ml. Triplicate tubes were used for each bacterial strain at each inoculum dose and for each antibiotic concentration. Following incubation at 35°C for 4 and 8 h, broth absorbance was recorded.
Statistics.
All statistical tests were conducted with the General Linear Model procedures of the Statistical Analysis System (SAS Institute, Inc., Cary, N.C.). In laboratory studies of the effects of peptone, NaCl, pH, and temperature, mean comparisons were determined by Duncan’s multiple-range test. Data for carbohydrate studies were determined by Dunnett’s test (2).
PNC medium (5.0% peptone, 1.0% NaCl, 0.08% cellobiose [35°C, pH 8.0]) increased V. vulnificus growth compared to APB. PNCC medium (PNC with 1.0 to 4.1 U of CM/ml) inhibited growth of V. parahaemolyticus and V. alginolyticus while promoting growth of V. vulnificus strains.
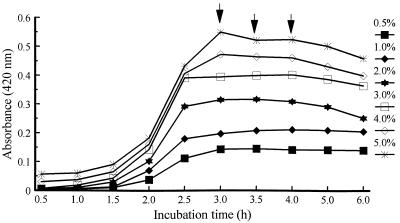
Normal growth patterns were observed at peptone concentrations of 2.0 to 5.0% for all three strains (Fig. 1). Five percent peptone produced the highest turbidity at 3, 3.5, and 4 h for all three strains tested (P < 0.05).
FIG. 1.
Effects of peptone concentration on growth of V. vulnificus 4832 in broth containing 1% NaCl, pH 8.4. , statistical analysis performed at time point.
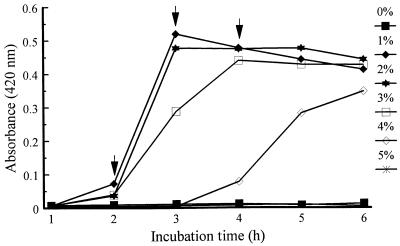
One and 2.0% NaCl supported high growth for all strains in 1.0, 3.0, 4.0 (not shown), and 5.0% peptone (Fig. 2), with 5.0% peptone and 1.0% NaCl being optimal.
FIG. 2.
Effects of NaCl concentration on growth of V. vulnificus 4832 in broth containing 5% peptone, pH 8.4. , statistical analysis performed at time point.
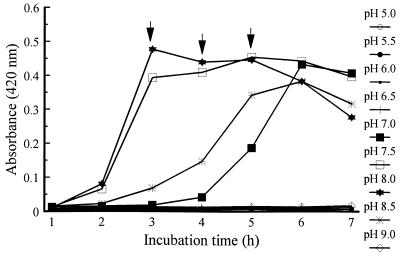
Results showed that pHs of 7.5 and 8.0 produced fast and abundant growth (Fig. 3). In 0.01, 0.03, and 0.05 M Tris, the growth rate was higher at pHs of 8.0 and 8.5 but lower at pH 7.5. In 0.1 M Tris, a lower growth rate occurred at pH 8.5.
FIG. 3.
Effects of medium pH on growth of V. vulnificus 4832 in broth containing 5% peptone and 1% NaCl. , statistical analysis performed at time point.
The effects of incubation temperature were measured in 5.0% peptone–1.0% NaCl (pH 8.0) at a temperature range of 25 to 45°C. Maximum growth occurred at 35°C for all strains (not shown).
Lactose, salicin, and cellobiose are commonly used to differentiate V. vulnificus from other Vibrio spp. (9). Five percent peptone had a protective effect against the inhibitory properties of lactose observed at lower peptone concentrations (Fig. 4). Growth was enhanced in 0.1% peptone containing 0.5 or 1.0% lactose (Fig. 4C). Salicin showed effects similar to those of lactose (not shown).
FIG. 4.
Effects of lactose supplementation on growth of V. vulnificus 4832 in broth containing various peptone and lactose concentrations: 5% peptone with 0 to 2.5% lactose (A), 1% peptone with 0 to 5% lactose (B), and 0.1% peptone with 0 to 3% lactose (C). , statistical analysis performed at time interval.
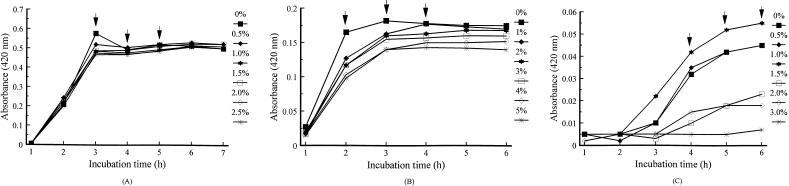
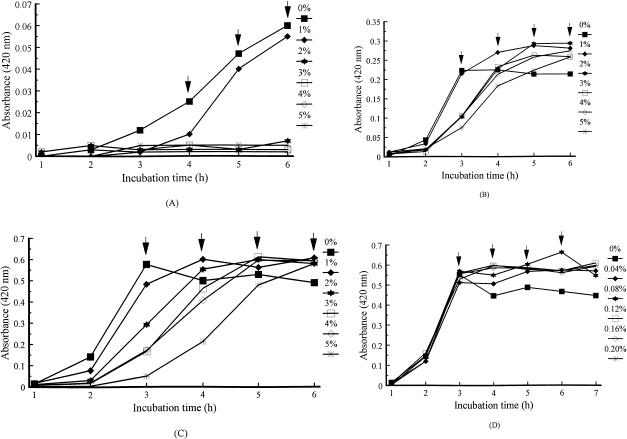
In contrast to lactose and salicin, cellobiose markedly inhibited growth in 0.1% peptone at concentrations of 1.0% or greater (Fig. 5A). However, in 1.0% peptone, 1.0% cellobiose enhanced growth after 3 h (Fig. 5B). Higher concentrations of cellobiose increased growth after 4 h in comparison to the control (Fig. 5B). In 5.0% peptone, 2.0% or higher cellobiose markedly delayed growth (Fig. 5C). In contrast, greater growth occurred at 0.04 to 0.20% cellobiose, particularly at 0.08% (Fig. 5D). No synergy among carbohydrates was observed (not shown).
FIG. 5.
Effects of cellobiose supplementation on growth of V. vulnificus 4832 in broth containing various peptone and cellobiose concentrations: 0.1% peptone with 0 to 5% cellobiose (A), 1% peptone with 0 to 5% cellobiose (B), 5% peptone with 0 to 5% cellobiose (C), and 5% peptone with 0 to 0.2% cellobiose (D). , statistical analysis performed at time interval.
Approximately 3% of V. vulnificus strains are susceptible to polymyxin antibiotics, in contrast to other Vibrio species (9). At inocula of 104 CFU/ml of broth (each), V. vulnificus, V. alginolyticus, and V. parahaemolyticus were inhibited at all test concentrations of CM (1.0 to 8.2 U/ml) and PB (0.2 to 0.6 U/ml) during 4 h of incubation (data not shown). At 8 h, 8.2 U of CM/ml inhibited all three Vibrio spp. at inoculum levels of 1 to 10,000 CFU/ml (Table 1); an initial inoculum of 100 V. vulnificus organisms per ml of broth was required for 8 h of growth in 1.0 to 4.1 U of CM/ml, while 102 V. parahaemolyticus organisms per ml and more than 104 V. alginolyticus organisms per ml were necessary under the same conditions.
TABLE 1.
Inhibitory effects of CM on V. vulnificus 4832, V. parahaemolyticus, and V. alginolyticus at various inoculum sizes
| No. of cells inoculated/ml | Effect of CM
(U/ml)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.2
|
4.1
|
2.1
|
1.0
|
|||||||||
| Vv | Va | Vp | Vv | Va | Vp | Vv | Va | Vp | Vv | Va | Vp | |
| 100 | − | − | − | − | − | − | − | − | − | − | − | − |
| 101 | − | − | − | − | − | − | − | − | − | + | − | − |
| 102 | − | − | − | + | − | − | + | − | + | + | − | + |
| 103 | − | − | − | + | − | + | + | − | + | + | − | + |
| 104 | − | − | − | + | − | + | + | − | + | + | − | + |
Bacterial cultures were added to broth containing 5% peptone–1% NaCl (pH 8.0) and CM. Cultures were incubated at 35°C for 8 h. Vv, V. vulnificus; Va, V. alginolyticus; Vp, V. parahaemolyticus; −, no growth; +, growth (turbidity).
PB at 0.2 to 0.6 U/ml inhibited V. alginolyticus at all inoculum levels during 8 h of incubation (Table 2); V. parahaemolyticus was able to grow in PB up to 0.6 U/ml. An inoculum of 103 V. vulnificus CFU/ml was needed for growth in 0.6 U CM/ml. Overall, CM was more inhibitory than PB for V. alginolyticus and V. parahaemolyticus and less inhibitory than PB for V. vulnificus. Therefore, PNC with 1.0 to 4.1 U of CM/ml (PNCC medium) is proposed for field evaluations.
TABLE 2.
Inhibitory effects of PB on V. vulnificus 4832, V. parahaemolyticus, and V. alginolyticus at various inoculum sizes
| No. of cells inoculated/ml | Effect of PB
(U/ml)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.6
|
0.5
|
0.3
|
0.2
|
|||||||||
| Vv | Va | Vp | Vv | Va | Vp | Vv | Va | Vp | Vv | Va | Vp | |
| 100 | − | − | − | − | − | − | − | − | − | − | − | − |
| 101 | − | − | − | − | − | − | − | − | − | + | − | + |
| 102 | − | − | − | − | − | − | + | − | + | + | − | + |
| 103 | + | − | + | + | − | + | + | − | + | + | − | + |
| 104 | + | − | + | + | − | + | + | − | + | + | − | + |
Bacterial cultures were added to broth containing 5% peptone–1% NaCl (pH 8.0) and PB. Cultures were incubated at 35°C for 8 h. Vv, V. vulnificus; Va, V. alginolyticus; Vp, V. parahaemolyticus; −, no growth; +, growth (turbidity).
Although originally developed for Vibrio cholerae, APB has been routinely used as an enrichment broth for detection and enumeration of V. vulnificus and other Vibrio spp. in shellfish meats and environmental samples (3, 15). Sloan et al. (14) compared five selective enrichment broths for recovery of V. vulnificus in oysters and reported that APB produced the highest most probable number. The present study is the first to optimize the components of APB and supplements for enhanced recovery of V. vulnificus.
Acknowledgments
These studies were supported, in part, by U.S. Department of Commerce National Oceanographic and Atmospheric Administration grant NA27FD0117-01.
Footnotes
Florida Agricultural Experiment Station Journal Series no. R-06330.
REFERENCES
- 1.Brauns L A, Hudson M C, Oliver J D. Use of the polymerase chain reaction in detection of culturable and nonculturable Vibrio vulnificuscells. Appl Environ Microbiol. 1991;57:2651–2655. doi: 10.1128/aem.57.9.2651-2655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornell J A. Experiments with mixtures: designs, models, and the analysis of mixture data. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 22–65. [Google Scholar]
- 3.Elliot E L, Kaysner C A, Tamplin M L. U.S. Food and Drug Administration, Bacteriological analytical manual. 7th ed. Arlington, Va: AOAC International; 1992. V. cholerae, V. parahaemolyticus, V. vulnificus, and other Vibriospp; pp. 111–140. [Google Scholar]
- 4.Hill W E, Datta A R, Feng P, Lampel K A, Payne W L. U.S. Food and Drug Administration, Bacteriological analytical manual. 7th ed. Arlington, Va: AOAC International; 1992. Identification of foodborne bacterial pathogens by gene probes; pp. 383–417. [Google Scholar]
- 5.Hill W E, Keasler S P, Trucksess M W, Feng P, Kaysner C A, Lampel K A. Polymerase chain reaction identification of Vibrio vulnificusin artificially contaminated oysters. Appl Environ Microbiol. 1991;57:707–711. doi: 10.1128/aem.57.3.707-711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson J K, Murphree R L, Tamplin M L. Evidence that mortality from Vibrio vulnificusinfection results from single strains among heterogeneous populations in shellfish. J Clin Microbiol. 1997;35:2098–2101. doi: 10.1128/jcm.35.8.2098-2101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janda J M, Powers C, Bryant R, Abbott S. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibriospp. Clin Microbiol Rev. 1988;1:245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaysner C A, Tamplin M L, Wekell M M, Stott R F, Colburn K G. Survival of Vibrio vulnificus in shellfish and shucked oysters (Crassostrea gigas and Crassostrea virginica) and effects of isolation medium on recovery. Appl Environ Microbiol. 1989;55:3072–3079. doi: 10.1128/aem.55.12.3072-3079.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly M T, Hickman-Brenner F W, Farmer J J., III . Vibrio. In: Lennette E H, Balows A, Hausler W J, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 282–301. [Google Scholar]
- 10.Kelly M T, McCormick W F. Acute bacterial myositis caused by Vibrio vulnificus. JAMA. 1981;264:72–73. [PubMed] [Google Scholar]
- 11.Lotz M J, Tamplin M L, Rodrick G E. Thiosulfate-citrate-bile salts-sucrose agar and its selectivity for clinical and marine Vibrioorganisms. Ann Clin Lab Sci. 1983;13:45–48. [PubMed] [Google Scholar]
- 12.Morris J G, Wright A C, Roberts D M, Wood P K, Simpson L M, Oliver J D. Identification of environmental Vibrio vulnificusisolates with a DNA probe for the cytotoxin-hemolysin gene. Appl Environ Microbiol. 1987;53:193–195. doi: 10.1128/aem.53.1.193-195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rippey S R. Shellfish borne disease outbreaks, July 1992 compilation. FDA Report. North Kingston, R.I: Northeast Seafood Laboratory; 1992. [Google Scholar]
- 14.Sloan E M, Hagen C J, Lancette G A, Peeler J T, Sofos J N. Comparison of five selective enrichment broths and two selective agars for recovery of Vibrio vulnificusfrom oysters. J Food Prot. 1992;55:356–359. doi: 10.4315/0362-028X-55.5.356. [DOI] [PubMed] [Google Scholar]
- 15.Tamplin M L, Martin A L, Ruple A D, Cook D W, Kaspar C W. Enzyme immunoassay for identification of Vibrio vulnificusin seawater, sediment, and oysters. Appl Environ Microbiol. 1991;57:1235–1240. doi: 10.1128/aem.57.4.1235-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamplin M L, Rodrick G E, Blake N J, Bundy D A P, Alexander L. Public health aspects of halophilic Vibrioin Jamaica. West Indian Med J. 1983;32:147–151. [PubMed] [Google Scholar]
- 17.Tamplin M L, Rodrick G E, Blake N J, Cuba T. Isolation and characterization of Vibrio vulnificusfrom two Florida estuaries. Appl Environ Microbiol. 1982;44:1466–1470. doi: 10.1128/aem.44.6.1466-1470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]