Abstract
Background
The combination of different anesthesia techniques or adjuvant drugs can relieve the stress response to surgery, reduce adverse reactions and improve the clinical outcome. We investigated the effects of subcostal anterior quadratus lumborum block (SQLB) with and without dexmedetomidine (DEX) on postoperative rehabilitation for laparoscopic renal surgery (LRS).
Methods
We included 90 patients in this single-center study. All were scheduled for elective laparoscopic radical or partial nephrectomy under general anesthesia (GA). We randomly and evenly assigned them to three groups: Group GA (GA alone), Group QG (SQLB with 30 mL of 0.25% ropivacaine and GA), and Group DQG (SQLB with 30 mL of 0.25% ropivacaine plus 1 μg/kg DEX and GA). The primary outcomes were serum creatinine (Cr) and blood urea nitrogen (BUN) levels; the secondary outcomes included the average numeric rating scale (NRS) scores at rest and during activity within 48 h postoperatively; perioperative opioid consumption; the time to first ambulation, exhaust, and fluid intake, and postoperative adverse reactions.
Results
The serum Cr and BUN levels in Group DQG decreased significantly compared with Group GA (P < 0.05). The average NRS scores in Group DQG were significantly lower than other two groups (P < 0.05). Furthermore, the indexes reduced significantly in Group QG compared with Group GA (P < 0.05). Groups DQG and QG had lower consumption of opioid compared with Group GA (P < 0.05). The recovery indicators in Groups DQG and QG were higher quality than Group GA (P < 0.05). The incidences of adverse reactions in Group DQG was significantly lower than the other groups (P < 0.05).
Conclusion
SQLB with and without DEX could attenuate postoperative pain, reduce opioids requirement and side effects, as well as facilitate postoperative early rehabilitation. More interesting, SQLB with DEX could confer kidney protection.
Clinical Trial Registration Number
The Chinese Clinical Trial Registry (ChiCTR2200061554).
Keywords: dexmedetomidine, subcostal anterior quadratus lumborum block, renal function, laparoscopic renal surgery
Introduction
Kidney carcinoma is a common malignant tumor of the urinary system, and the incidence of renal carcinoma has increased in recent years, accounting for 2.2% of all cancers.1,2 Laparoscopic renal surgery (LRS) is recognized as the optimal approach for the surgical management of renal carcinoma. Its advantages include minimal invasiveness, less post-operative pain, faster recovery, and a shorter hospital length of stay.3,4 Moreover, laparoscopic partial nephrectomy provides better renal functional preservation without surgical complications.5
Currently, general anesthesia (GA) alone is the most frequently-used approach to the surgery. Nevertheless, recent studies have shown that various types of anesthesia methods could affect postoperative rehabilitation. In particular, some anesthesia procedure or drugs might influence renal function.6–12 Thus, an urgent clinical problem is to protect perioperative renal function and to promote early postoperative recovery. Quadratus lumborum block (QLB) is an abdominal truncal block that provides analgesia for abdominal surgery including laparoscopic gastrectomy, cesarean section, laparoscopic colorectal surgery, laparoscopic hepatectomy, and LRS. This relatively novel technique was first described by Blanco in 2007.13–17 QLB can lower pain scores, provide a greater opioid-sparing effect and a shorter postoperative hospital stay, and improve postoperative cognitive function.18–20 As a new approach to QLB, subcostal anterior quadratus lumborum block (SQLB) has been used effectively for analgesia in abdominal surgery by blocking more dermatomes, including the appropriate thoracic dermatome level between T6-7 and L1-2.21,22 DEX, a highly selective specific α2-adrenergic agonist with centrally mediated sympatholytic, sedative and analgesic properties, has been used as a new adjuvant during regional anaesthesia.23 To make things more interesting, El Sherif et al24 discovered that systemic absorption of DEX administered in transversus abdominis plane block (TAPB) was common, which might cause direct central effects on the locus coeruleus via systemic absorption, thus affecting the analgesic and hemodynamic effects of TAPB. Several clinical trials have revealed that DEX in peripheral nerve blocks prolonged the duration of local anesthetics and provided better analgesia. Moreover, DEX reduced postoperative opioids requirements, increase patient satisfaction, and enhance postoperative recovery of gastrointestinal function.25–29 In addition, DEX appears to have postoperative renal protective effects.30 Nevertheless, so far, the exact protective mechanism of DEX in the kidney has still not been elucidated, which is thought to act mainly by inhibiting sympathetic activity, attenuating inflammation and reducing ischemic–reperfusion injury.24,28,30
Despite the growing interest in DEX as an adjunct to regional block, to our knowledge there are no data available on the use of DEX in SQLB for LRS. Hence, we investigated the effects of SQLB with and without DEX on rehabilitation in patients undergoing LRS. We hypothesized that the SQLB with DEX may improve the analgesic effect, reduce opioid requirements, alleviate the renal function impairment and facilitate postoperative rehabilitation for LRS.
Materials and Methods
Study Design and Setting
This single-center, prospective, double-blind, randomized, controlled clinical trial was approved by the Ethics Committee of the Second Hospital of Shandong University (No: KYLL-2022LW-024). The study was registered at the Chinese Clinical Trial Center (ChiCTR2200061554) and also adhered to the Declaration of Helsinki. Before enrollment, all patients who participated in the study provided written informed consent.
Subjects
A total of 90 patients scheduled to undergo elective LRS under GA at the Second Hospital of Shandong University between July 2022 and July 2023 were enrolled in this study. Inclusion criteria: Aged 18 to 75 years; American Society of Anesthesiologists (ASA) grade I~II; Body mass index (BMI) of 18.5–28 kg/m2; Signed the informed consent form for 48-h patient-controlled intravenous analgesia (PCIA), and were explained in detail the numerical rating scale (NRS) scoring rules (scores from 0 to 10: 0 = no pain, 10 = most severe pain). Exclusion criteria: Preoperative cognitive dysfunction; Severe cardiopulmonary dysfunction; Serious liver and kidney dysfunction; Distant organ metastasis or other malignant tumors; Pregnancy; Solitary kidney; Recent use of antidepressants, sedatives, analgesics or nephrotoxic drugs within 6 months; Allergy or contraindication to medications used for the standardized anesthesia protocol; A history of chronic opioid addiction; A history of kidney surgery; A recent history of radiotherapy, chemotherapy, or related immunotherapy within 6 months; Contradictions or complications related to QLB; Conversion to open renal surgery; Intraoperative infusion of blood products or colloids; Failed block; Failed to complete data collection; Refusal to participate in the study.
Randomization and Blinding
All participants were randomly and evenly assigned to three groups in a blinded fashion (with a sealed opaque envelope) by the administrator who did not take part in the treatment. The groups included: Group GA: the patients received GA alone; Group QG: the patients received bilateral SQLB with 30 mL of 0.25% ropivacaine and GA; Group DQG: the patients received bilateral SQLB with 30 mL of 0.25% ropivacaine plus 1 μg/kg DEX and GA.
The participants, chief anesthesiologists, ward staff, and the outcome assessor were unaware of the group assignments. Only the independent anesthesia nurse was responsible for the group allocation and prepared the experimental drugs, as well as the regional anesthesiologist who performed the blocks knew the group allocation, but they did not involve in the other parts of the study.
Study Interventions and Anesthesia Procedure
All patients were regularly monitored by electrocardiography, invasive blood pressure, pulse oxygen saturation, end-tidal carbon dioxide concentration, body temperature, and bispectral index (BIS) values. Preoperative SQLB was performed by an experienced regional anesthesiologist who had completed at least 100 QLBs. Before the bilateral block procedure, sufentanil 2.5 to 5μg IV was administered to raise the pain threshold.
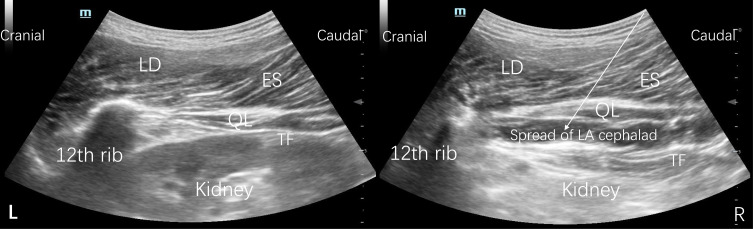
This study employed a previously described approach for SQLB22 (Figure 1). In brief, the patients were positioned in lateral decubitus with the spine flexed. According to current aseptic practice standards, a curvilinear probe (6–2 MHz, 1202, BK Medical ApS) was placed posteriorly below the 12 th rib in a parasagittal oblique plane tilted medially at the L1-L2 level. When the probe was moved further medially, the quadratus lumborum (QL) muscle, the latissimus dorsi (LD) muscle, the kidney, the transversalis fascia (TF), the erector spinae (ES) muscle, and the psoas major (PM) muscle could be visualized. A 21 G Facet Nanoline needle was advanced in plane in the caudal-to-cranial direction under ultrasound guidance until the needle tip was positioned anterior to the QL muscle and between the investing layer of the QL muscle and the TF. After negative aspiration, 1 to 2 mL of 0.9% saline was injected first to ensure correct positioning of the needle tip, 30 mL of the solution per side (with the content based on the group) was injected in the interfascial plane, and the local anesthetic (LA) was observed spreading cephalad to the 12 th rib.31–33
Figure 1.
Sonography of SQLB before local anesthetic injection (L) and after local anesthetic injection (R).
Note: The white arrow indicates the needle trajectory.
Abbreviations: SQLB, subcostal anterior quadratus lumborum block; QL, quadratus lumborum; ES, erector spinae; LA, local anesthetic; LD, latissimus dorsi; TF transversalis fascia.
After SQLB had been completed, all patients received standardized GA induction with an intravenous slow bolus injection of propofol 2–5 mg/kg + rocuronium 0.6 mg/kg + sufentanil 3 ug/kg by tracheal intubation. They were maintained with inhaled sevoflurane 2–3% to keep the BIS value between 40 and 60. Additional sufentanil and rocuronium were given as necessary. A reduction or elevation in the mean arterial by more than 20% of the baseline value was treated with intravenous 50 μg norepinephrine or 12.5 mg urapidil, respectively. Bradycardia, denoted by a heart rate of < 50 beats/min, was treated with intravenous atropine 0.25–0.5 mg. Tachycardia was treated with intravenous esmolol 20 mg. The above treatments were repeated, if needed. PCIA was established with sufentanil 2 μg/kg and ondansetron 8 mg diluted to a final volume of 100 mL in normal saline. The pump was programmed to deliver 0.5 mL boluses with a lockout interval of 15 min, an initial load of 5 mL, and a background infusion of 2 mL/h. Additionally, if the postoperative NRS score was > 4 and there was no relief after two boluses of PCIA, then intravenous sufentanil 5 µg was injected as a rescue and repeated, if needed. In addition, a full-time anesthesia nurse who was blinded to the anesthesia method was arranged to follow up and evaluate the pain NRS scores at 0, 12, 24 and 48 h after surgery.
Outcomes
The primary outcomes were serum creatinine (Cr) and blood urea nitrogen (BUN) levels at 24 h after surgery. The secondary outcomes were: the average NRS scores at rest and during activity within 48 h after surgery; the perioperative opioid consumption (sufentanil) preoperatively, intraoperatively and 48 h postoperatively; the time to first ambulation, the time to first exhaust, the time to first fluid intake, and the postoperative adverse reactions including somnolence, postoperative nausea and vomiting (PONV), itching, respiratory depression, urinary retention, hypertension, hypotension, bradycardia, and postoperative cognitive dysfunction (POCD).
Sample Size Calculation
The sample size was estimated using PASS 11.0 (NCSS-PASS 11, USA). According to the results of a preparatory experiment, the serum Cr level, representing a major endpoint after surgery, was 115.0 ± 30.4 in Group GA, 86.0 ± 9.2 in Group QG, and 82.0 ± 15.1 in Group DQG. The serum BUN level, representing another major endpoint after surgery, was 7.8 ± 1.6 in Group GA, 6.1 ± 2.5 in Group QG, and 5.2 ± 0.8 in Group DQG. The sample size was estimated separately based on the serum Cr and BUN levels using a one-way analysis of variance (ANOVA) power analysis with a significance level of 5% and power (β) of 0.10. The size of each group was estimated to be 24 cases based on the serum Cr and 20 cases based on the serum BUN. The larger of the two sample sizes (24) was chosen. Considering a 20% dropout rate, a sample of size was N1=N2=24/0.8=30 cases per group, for a total of 90 patients would be sufficient in this trial.
Statistical Analysis
All statistical analyses were performed using SPSS Statistics 23.0 (IBM Corp, Armonk, NY, USA). The Shapiro–Wilk test was used to test the normality of the data distribution. The Levene-type test was used to access the homogeneity of variances of the data. Quantitative variables were presented as the mean ± standard deviation (SD) and were compared using one-way ANOVA. Qualitative variables were presented as the number (proportion) and were analyzed with the chi-square test or Fisher’s exact test among groups. All comparisons were two sided and P < 0.05 indicated a statistically significant difference. In addition, post hoc pairwise comparisons of the randomized groups were performed; P < 0.05 after Bonferroni correction was considered a statistically significant difference.
Results
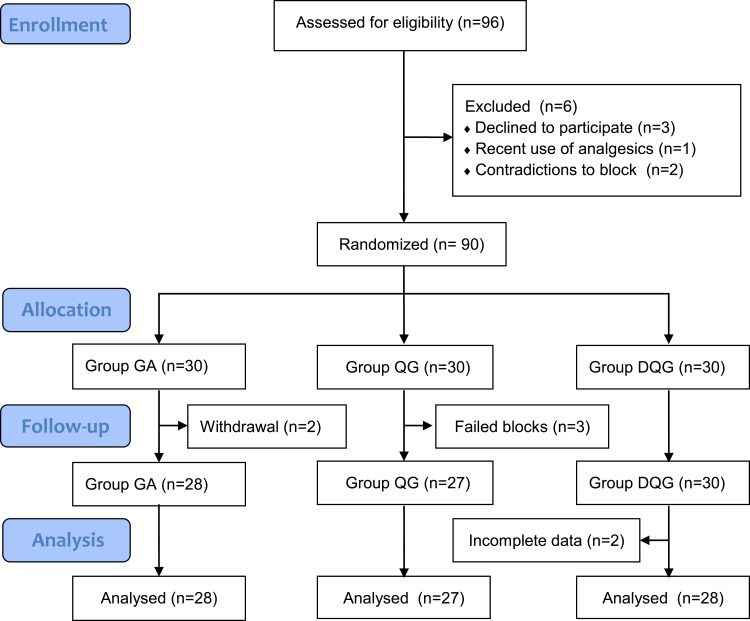
Eventually, we enrolled a total of 90 patients undergoing LRS and allocated them randomly to the three groups (n=30/group). Two patients in Group GA withdrew because of severe postoperative vomiting. Three patients in Group QG withdrew because poor ultrasound imaging guidance resulted in failed block. We excluded two patients in Group DQG due to incomplete data collection. Therefore, we used data from 83 patients in the final analysis (Figure 2). There was no occurrence of local anesthetic toxicity during the entire period. The patient characteristics at baseline were well balanced among the three groups (P > 0.05, Table 1).
Figure 2.
Flow diagram of the study.
Notes: Group GA, GA alone; Group QG, SQLB with 30 mL of 0.25% ropivacaine and GA; Group DQG, SQLB with 30 mL of 0.25% ropivacaine plus 1 μg/kg DEX and GA.
Abbreviations: GA, general anesthesia; SQLB, subcostal anterior quadratus lumborum block.
Table 1.
Comparison of Demographic Data and Characteristics in the Groups
| Characteristics | Group GA (n=28) | Group QG (n=27) | Group DQG (n=28) | P value |
|---|---|---|---|---|
| Age, (year) | 56.6±13.9 | 60.9±12.9 | 59.6±6.7 | 0.362* |
| Sex, (n, M/F) | 16/12 | 18/9 | 16/12 | 0.789# |
| Weight (kg) | 67.6±9.6 | 66.3±8.6 | 63.9±9.0 | 0.304* |
| BMI (kg/m2) | 23.5±2.3 | 23.1±1.8 | 22.4±2.4 | 0.194* |
| ASA grade, (n, I/II) | 22/6 | 20/7 | 25/3 | 0.338# |
| Comorbidities (n, H/C/D/A) | 5/3/2/3 | 6/4/2/2 | 7/6/4/2 | 0.530# |
| Preoperative hemoglobin (mmol/L) | 135.1±15.7 | 133.7±17.4 | 130.7±15.9 | 0.588* |
| Preoperative Cr (umol/L) | 72.1±26.7 | 68.5±26.1 | 69.9±19.9 | 0.857* |
| Preoperative BUN (mmol/L) | 5.5±1.9 | 5.0±2.0 | 4.6±1.5 | 0.161* |
| Lesion side (n, left/right) | 10/18 | 8/19 | 15/13 | 0.167# |
| Type of surgery (n, radical/partial) | 23/5 | 18/9 | 16/12 | 0.126# |
| Sevoflurane (mL) | 38.9±8.3 | 39.8±20.6 | 35.7±6.9 | 0.486* |
| Propofol (mg) | 1026.8±119.0 | 975.6±222.8 | 991.1±152.8 | 0.518* |
| Norepinephrine (n (%)) | 3 (14.3) | 2 (7.4) | 1 (3.6) | 0.587# |
| Anesthesia duration (min) | 206.6±19.9 | 201.1±42.3 | 204.5 ± 15.7 | 0.803* |
| Operation duration (min) | 175.6±20.9 | 176.9±25.9 | 174.3 ±19.6 | 0.908* |
| Renal artery occlusion time (min) | 28.7±8.1 | 33.4±8.8 | 30.0±7.8 | 0.095* |
| Intraoperative blood loss (mL) | 49.6±22.2 | 74.8±88.3 | 55.0±20.8 | 0.191* |
| Intraoperative crystal fluid (mL) | 1707.1±312.9 | 1816.7±312.3 | 1867.9±344.1 | 0.172* |
| Urine volume (mL) | 357.1±100.7 | 416.7±191.2 | 382.1±93.5 | 0.267* |
Notes: The data are given as mean ± SD or number. *Quantitative variables were compared by one-way ANOVA; #Qualitative variables were compared by Chi-square (χ2).
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; H, Hypertension; C, Chronic heart disease; D, Diabetes mellitus; A, Abnormal kidney function; Cr, creatinine; BUN, blood urea nitrogen; SD, standard deviation.
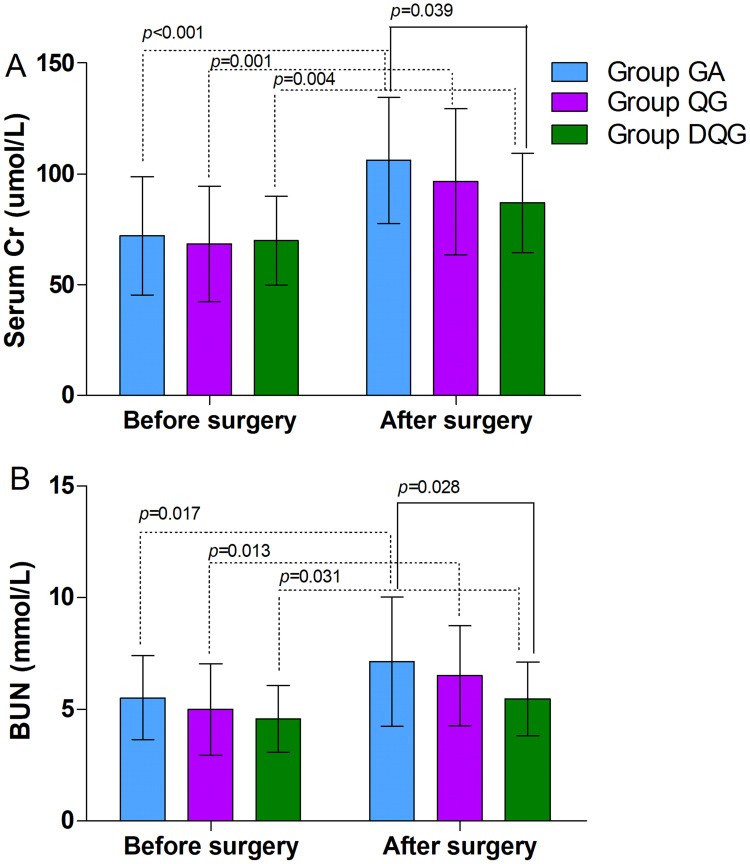
Regarding the primary outcomes, the preoperative serum Cr and BUN levels were similar between the groups (P > 0.05, Figure 3), but they were higher after surgery than before surgery in each group (P < 0.05, Figure 3). The serum Cr and BUN levels were significantly lower in Group DQG compared with Group GA (P < 0.05, Figure 3). However, there were no differences in the serum Cr and BUN levels between Groups QG and GA, as well as between Groups QG and DQG (P > 0.05, Figure 3).
Figure 3.
The serum Cr and BUN levels in the groups.
Notes: The preoperative serum Cr (A) and BUN (B) levels were similar between the groups (P > 0.05), but the postoperative levels were significantly higher in each group (P < 0.05). The postoperative serum Cr (A) and BUN (B) levels in Group DQG were significantly lower than Group GA (P < 0.05). However, the postoperative levels did not differ significantly between Groups QG and GA, as well as between Groups QG and DQG (P > 0.05). The data were given as mean ± SD. A P-value < 0.05 was considered statistically significant. Data were compared by one-way ANOVA among groups and post hoc pairwise comparison of the groups. Group GA, GA alone; Group QG, SQLB with 30 mL of 0.25% ropivacaine and GA; Group DQG, SQLB with 30 mL of 0.25% ropivacaine plus 1 μg/kg DEX and GA.
Abbreviations: GA, general anesthesia; SQLB, subcostal anterior quadratus lumborum block; BUN, blood urea nitrogen; Cr, creatinine; DEX, dexmedetomidine.
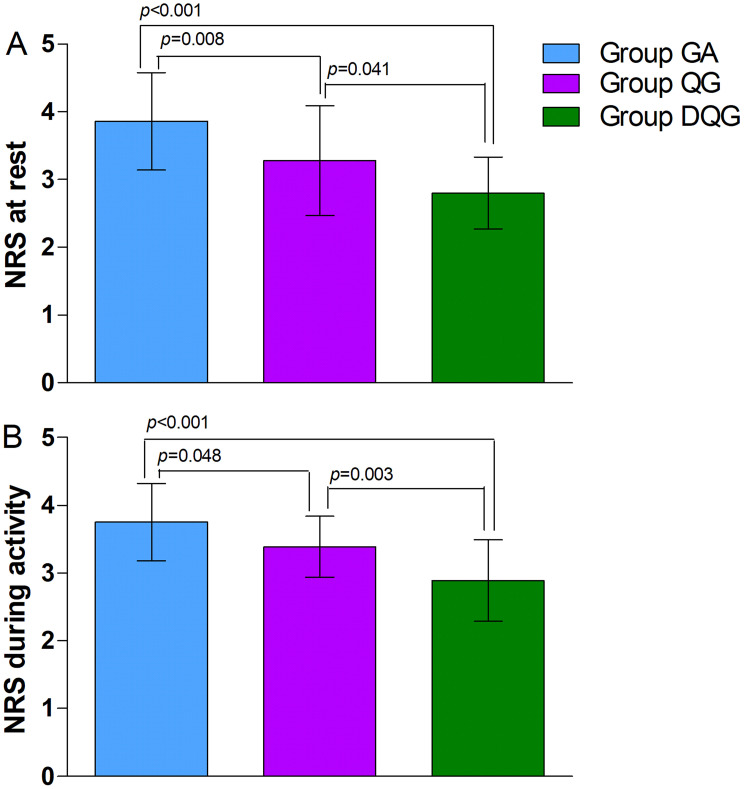
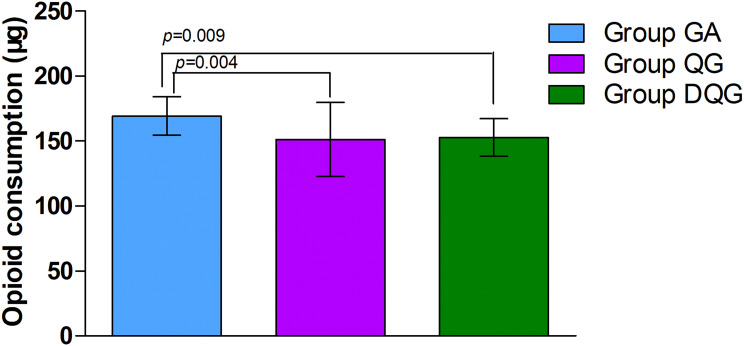
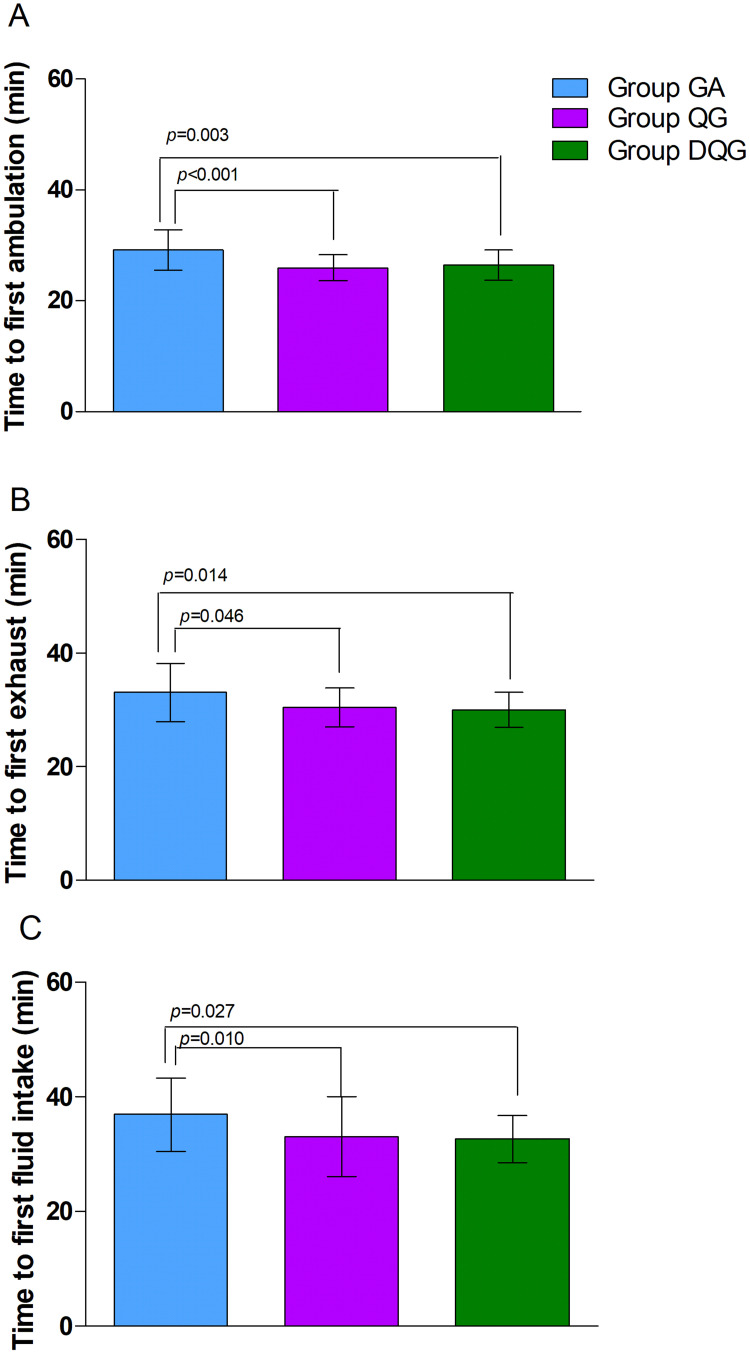
Regarding the secondary outcomes, the average NRS scores at rest and during activity were significantly lower in Group DQG compared with the other two groups (P < 0.05, Figure 4). Furthermore, these scores were significantly reduced in Group QG compared with Group GA (P < 0.05, Figure 4). Groups DQG and QG had lower perioperative cumulative opioid consumption (sufentanil) compared with Group GA (P < 0.05, Figure 5); However, the opioid consumption did not differ between Groups DQG and QG (P > 0.05, Figure 5). Postoperative early recovery indicators including the time to first ambulation, the time to first exhaust, and the time to first fluid intake were higher quality in Groups DQG and QG compared with Group GA (p < 0.05, Figure 6). However, the above-mentioned parameters were not significantly different between Groups DQG and QG (P > 0.05, Figure 6). The total incidence of postoperative adverse reactions was significantly lower in Group DQG compared with the other groups. Moreover, it was also decreased dramatically in Group QG in contrast to Group GA (P < 0.05, Table 2).
Figure 4.
The average NRS scores at rest and during activity within 48 h after surgery in the groups.
Notes: The average NRS scores at rest (A) and during activity (B) were significantly lower in Group DQG compared with the other two groups (P < 0.05). Furthermore, these scores were significantly reduced in Group QG compared with Group GA (P < 0.05). The data were given as mean ± SD. A P-value < 0.05 was considered statistically significant. Data were compared by one-way ANOVA among groups and post hoc pairwise comparison of the groups. Group GA, GA alone; Group QG, SQLB with 30 mL of 0.25% ropivacaine and GA; Group DQG, SQLB with 30 mL of 0.25% ropivacaine plus 1 μg/kg DEX and GA.
Abbreviations: GA, general anesthesia; SQLB, subcostal anterior quadratus lumborum block; NRS, numerical rating scale; DEX, dexmedetomidine.
Figure 5.
Cumulative perioperative opioid consumption in the groups.
Notes: Groups DQG and QG had lower perioperative cumulative opioid consumption (sufentanil) compared with Group GA (P < 0.05); however, the opioid consumption did not differ between Groups DQG and QG (P > 0.05). The data were given as mean ± SD. A P-value < 0.05 was considered statistically significant. Data were compared by one-way ANOVA among groups and post hoc pairwise comparison of the groups. Group GA, GA alone; Group QG, SQLB with 30 mL of 0.25% ropivacaine and GA; Group DQG, SQLB with 30 mL of 0.25% ropivacaine plus 1 μg/kg DEX and GA.
Abbreviations: GA, general anesthesia; SQLB, subcostal anterior quadratus lumborum block; DEX, dexmedetomidine.
Figure 6.
Early postoperative recovery in the groups.
Notes: The time to first ambulation (A), the time to first exhaust (B), and the time to first fluid intake (C) in Groups DQG and QG were higher quality compared with Group GA (P < 0.05). However, the above-mentioned parameters were not significantly different between Groups DQG and QG (P > 0.05). The data were given as mean ± SD. A P-value < 0.05 was considered statistically significant. Data were compared by one-way ANOVA among groups and post hoc pairwise comparison of the groups. Group GA, GA alone; Group QG, SQLB with 30 mL of 0.25% ropivacaine and GA; Group DQG, SQLB with 30 mL of 0.25% ropivacaine plus 1 μg/kg DEX and GA.
Abbreviations: GA, general anesthesia; SQLB, subcostal anterior quadratus lumborum block; DEX, dexmedetomidine.
Table 2.
Comparison of Side Effects After Operation
| Indicators | Group GA (n=28) | Group QG (n=27) | Group DQG (n=28) | P value |
|---|---|---|---|---|
| Somnolence (n) | 2 | 0 | 1 | |
| PONV (n) | 3 | 1 | 1 | |
| Itching (n) | 1 | 1 | 0 | |
| Respiratory depression (n) | 1 | 0 | 0 | |
| Urinary retention (n) | 1 | 0 | 0 | |
| Hypertension (n) | 1 | 0 | 0 | |
| Hypotension (n) | 0 | 1 | 0 | |
| Bradycardia (n) | 1 | 0 | 0 | |
| POCD (n) | 1 | 1 | 0 | |
| Total incidence (%) | 39.3 | 14.8 | 7.1 | <0.001 |
Notes: The data are given as number or percentage. Qualitative variables were compared by Chi-square (χ2).
Abbreviations: PONV, postoperative nausea and vomiting, POCD, postoperative cognitive dysfunction.
Discussion
The most important finding of our study was that SQLB with DEX could alleviate kidney impairment after LRS. In addition, SQLB with and without DEX could attenuate postoperative pain, reduce opioids requirement and side effects, as well as facilitate postoperative early rehabilitation.
Based on the literature, acute kidney injury (AKI) affected 6–8% of patients undergoing surgery and was associated with increased mortality and risk of chronic kidney disease.34–36 According to the current relevant studies, the choice of anesthesia technique or anesthetics can affect perioperative renal function and the incidence of AKI after surgery.6,36–38 Wang et al9 demonstrated that continuous epidural block in addition to GA in elderly patients undergoing laparoscopic colorectal resection surgery provided better protection of perioperative liver and kidney function than GA alone. Kwon et al10 revealed that spinal anesthesia had advantages in renal function change compared with GA at 3 months after surgery in patients submitted to retrograde intrarenal surgery.
DEX is a highly selective drug α2-adrenergic receptor agonist with characteristics including sedation, analgesia, anti-anxiety, inhibition of sympathetic activity, mild respiratory inhibition, and stable hemodynamics.23–25 The mechanism of DEX as an adjuvant to local anesthetics enhances their effect is a multifactorial theory still being controversial, which works mainly through peripheral level, spinal cord level and supraspinal level.25–29 Furthermore, there is a lack of research about the mechanism of DEX on renal.
A recent study suggested that DEX could modulate inflammation by enhancing parasympathetic tone while reducing sympathetic tone, protect against ischemia-reperfusion injury, and patients receiving DEX had better early postoperative renal function in renal transplant recipients.39 A 2021 meta-analysis confirmed that DEX exerted kidney-protective effects after surgery, with neutrophil gelatinase-associated lipocalin (NGAL) levels reduced and creatinine clearance significantly increased in patients treated with it.28 Besides, DEX exerted renoprotective effects and was thought to act mainly by reducing norepinephrine release, improving hemodynamic stability, and maintaining myocardial oxygen supply balance, thereby significantly reducing the incidence of AKI, especially in patients with normal or mildly impaired preoperative renal function.40
There have been a few other studies on DEX as an adjuvant in SQLB for LRS. We found that serum Cr and BUN levels were lower in Group DQG than Group GA (P < 0.05). These results were basically similar to the previous studies. The key potential mechanisms of the protective effect might be as follows: First, DEX might inhibit sympathetic activity, stabilize hemodynamics, attenuate inflammation, and reduce ischemic–reperfusion injury to a certain extent, which played a role in renal protection.24,28,30 Second, SQLB had a more cranial intrathoracic distribution, consistently rising to the T7-T8 level, leading to better analgesic effects, suppressing stress reactions more effectively, partly blocking sympathetic nerve excitation, reducing the degree of renal vascular contraction, improving renal perfusion, and was conducive to the recovery of renal function to some extent.
As a commonly performed minimally invasive procedure, laparoscopy can reduce the pain level, but the patients still suffer moderate to severe postoperative pain at the incision site and in internal organs, leading to physical stringent state, and thus influencing prognosis.13,17,19 As a consequence, achieving a more ideal prognosis for patients requires adequate pain relief, which should block both visceral and somatic nerve fibers.41 QLB can provide both somatic and visceral sensory blockade, making it an ideal regional anesthetic technique for abdominal surgery.18–20 According to the literature, DEX is a powerful adjuvant to regional anesthesia and provides more satisfactory analgesic effects, which was absorbed both locally and throughout systemically.24,26–28
We found a significantly lower average NRS scores within 48 h after surgery in Group DQG compared with the other two groups (P < 0.05). Moreover, the NRS score was significantly lower in Group QG compared with Group GA (P < 0.05). Similarly, previous studies with the addition of DEX to regional blockade have shown a longer duration of analgesia with lower pain scores, which agreed with our results.25,26 Furthermore, our findings were consistent with the results of a randomized trial from Nie et al18 who demonstrated that SQLB provided lower visual analogue (VAS) score in comparison with TAPB within 48 h after laparoscopic radical gastrectomy. Our results was also similar to a study by Elsharkawy et al22 indicating that SQLB provided the appropriate thoracic dermatome level needed for analgesia following open urological surgical procedures. What is more, Alansary et al42 reported that a prolonged duration of initial analgesia in the QLB plus DEX group compared to the QLB group in patients undergoing open kidney surgery. El Sherif et al24 revealed that from 2 to 8 h postoperatively, VAS scores at rest and during movement were significantly lower in the TAPB-DEX group compared with the TAPB group, results that are consistent with our study.
We clearly found that Groups DQG and QG had lower cumulative perioperative opioid consumption (sufentanil) compared with Group GA (P < 0.05). This finding indicated that SQLB with and without DEX could significantly reduce the perioperative opioid requirement, finding consistent with the studies by Nie et al18 and Yang et al26 who showed that QLB significantly decreased perioperative opioid consumption. In addition, randomized controlled trials have shown that DEX combined with QLB or QLB alone appeared to reduce the opioid requirement.21,25,43
Although we implemented laparoscopic surgery, which is less traumatic than open surgery, other factors are important to consider in early recovery, such as the time to first ambulation, the time to first exhaust, and the time to first fluid intake, etc.13,20 We found that these indicators were significantly higher quality in Groups DQG and QG compared with Group GA (P < 0.05). The results are similar to that of Zhu et al21 study revealed that time to recovery of intestinal function and the mobilization time after surgery were significantly earlier in SQLB group than the control group for laparoscopic nephrectomy in a randomized controlled trial. Similarly, in a triple-blind, randomized, controlled study for laparoscopic partial nephrectomy, by Zhu et al44 showed the time to the patient’s first excretion, ambulation, and drainage tube removal were shorter in the QLB group compared with the control group. Likewise, other studies have reported that compared with GA, QLB for patients undergoing abdominal surgery shortened the first time of getting up, flatus and taking semi-liquid diet, findings consistent with our study.13,45 Interestingly, Wu et al25 found that ultrasound-guided deep serratus anterior plane block (dSAPB) combined with ropivacaine plus DEX might improve the QoR-15 in patients undergoing modified radical mastectomy. Nevertheless, there was no statistical difference between Groups DQG and QG. In any case, SQLB with DEX might be a useful intervention to promote early recovery following LRS. In other words, it is consistent with the concept of enhanced recovery after surgery to restore preoperative organ function and to expedite recovery with a faster return of bowel function, fewer complications, and a shorter hospital length of stay.
We considered several adverse reactions, including somnolence, PONV, itching, respiratory depression, urinary retention, hypertension, hypotension, bradycardia, and POCD, that might result from surgical factors, residual anesthetics, and analgesics. In a randomized trial, Zha et al46 showed that thoracic paravertebral block (TPVB) with DEX significantly reduced the incidence of PONV compared with the TPVB combined with GA. A study by Zhu et al21 that the incidence of PONV was significantly lower in the QLB group than in the Control group in patients undergoing laparoscopic nephrectomy. In our trial, the total incidence of postoperative adverse reactions in Group DQG was significantly lower than the other groups, which was decreased dramatically in Group QG in contrast to Group GA. These findings were consistent with the previous investigations. The possible reasons include the multimodal analgesia from DEX or QLB, the relieved lower pain, the lower opioid requirement, and the rapid restoration of gastrointestinal function. Although the most common side effects of DEX are bradycardia and hypotension, we did not observe these in our patients. This finding indicates we used an appropriate dosage of DEX.
Our study has some limitations. First, the participants were from a single center and lacked external validity. Second, we failed to consider that some drugs that could impair renal function, such as non-steroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, some antibiotics, and injectable contrast agents, all of which probably had an impact on the results. Additionally, we did not record the size of tumor resection. Third, although the patients did not experience severe hypotension and there was no difference in the use of vasoactive drugs, the lack of statistical analysis of perioperative blood pressure between the three groups might have affected the results. Forth, the follow-up periods were relatively short: 24 h for renal function and 48 h for postoperative pain relief. We will further validate our results in the future by extending these periods. Fifth, sufentanil, rocuronium, and muscle relaxation monitoring were not considered in the original design, which might affect the primary outcomes. Finally, we did not check sensory block dermatomes after local anesthetic injection to confirm the effectiveness of SQLB before the start of surgery, which probably affected the accuracy of the outcomes.
Conclusion
SQLB with and without DEX could attenuate postoperative pain, reduce opioids requirement and side effects, as well as facilitate early postoperative rehabilitation. More interesting, SQLB with DEX could confer kidney protection. This composite technology could be a safe and effective anesthesia for LRS.
Acknowledgments
The authors thank all the research assistants and patients for their time and efforts in this prospective study.
Funding Statement
This work was supported by the Horizontal funding of Shandong University (No.6010220014).
Abbreviations
ANOVA, analysis of variance; AKI, acute kidney injury; ASA, American Society of Anesthesiologists; BIS, bispectral index; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; DEX, dexmedetomidine; dSAPB, deep serratus anterior plane block; ES, erector spinae; GA, general anesthesia; LA, local anesthetic; LD, latissimus dorsi; LRS, laparoscopic renal surgery; NRS, numeric rating scale; P, probability; PCIA, patient-controlled intravenous analgesia; PM, psoas major; PONV, postoperative nausea and vomiting; QL, quadratus lumborum; QLB, quadratus lumborum block; SD, standard deviation; SQLB, subcostal anterior quadratus lumborum block; TAPB, transversus Abdominis Plane block; TF, transversalis fascia; TPVB, thoracic paravertebral block.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sung H, Ferlay J, Rebecca L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Nasrallah G, Souki FG. Perianesthetic management of laparoscopic kidney surgery. Curr Urol Rep. 2018;19(1):1. doi: 10.1007/s11934-018-0757-4 [DOI] [PubMed] [Google Scholar]
- 3.Jimenez-Romero ME, Moreno-Cortes JC, Canelon-Castillo EY, Diez-Farto S, Santotoribio JD. Predictive factors of renal function in partial laparoscopic nephrectomy in patients with a kidney tumor. Curr Urol. 2019;13(3):150–156. doi: 10.1159/000499277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei X, Hu X, Xu Z, et al. Clinical effect of retroperitoneal laparoscopic radical nephrectomy on renal cell carcinoma, the influence of renal function, and the influencing factors of recurrence. Evid-Based Compl Alt. 2022;2022:4182853. doi: 10.1155/2022/4182853 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Guglielmetti GB, Dos Anjos GC, Sawczyn G, et al. A prospective, randomized trial comparing the outcomes of open vs laparoscopic partial nephrectomy. J UROLOGY. 2022;208(2):259–267. doi: 10.1097/JU.0000000000002695 [DOI] [PubMed] [Google Scholar]
- 6.Franzén S, Semenas E, Taavo M, et al. Renal function during sevoflurane or total intravenous propofol anaesthesia: a single-centre parallel randomised controlled study. Brit J Anaesth. 2022;128(5):838–848. doi: 10.1016/j.bja.2022.02.030 [DOI] [PubMed] [Google Scholar]
- 7.Bai Y, He H, Zhang P, Huang L, Huang L. Effects of dexmedetomidine on immune function, renal function and inflammatory factors of patients undergoing percutaneous nephrolithotomy under general anesthesia. Exp Ther Med. 2021;21(4):406. doi: 10.3892/etm.2021.9837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franzén S, DiBona G, Frithiof R. Anesthesia and the renal sympathetic nervous system in perioperative AKI. Semin Nephrol. 2022;42(3):151283. doi: 10.1016/j.semnephrol.2022.10.009 [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Wang HW, Zhong TD. Influence of different anesthesia on liver and renal function in elderly patients undergoing laparoscopic colon or rectal resection. Hepato-Gastroenterol. 2013;60(121):79–82. doi: 10.5754/hge12478 [DOI] [PubMed] [Google Scholar]
- 10.Kwon O, Lee JM, Park J, et al. Influence of anesthesia methods on surgical outcomes and renal function in retrograde intrarenal stone surgery: a prospective, randomized controlled study. BMC Anesthesiol. 2019;19(1):239. doi: 10.1186/s12871-019-0901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng M, Wang L, Sun J, et al. Thoracic paravertebral block combined with general anaesthesia or general anaesthesia alone for thoracoscopic lung adenocarcinoma surgery: a retrospective study. Cancer Manag Res. 2022;14:953–965. doi: 10.2147/CMAR.S346285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coviello A, Golino L, Maresca A, Vargas M, Servillo G. Erector spinae plane block in laparoscopic nephrectomy as a cause of involuntary hemodynamic instability: a case report. Clin Case Rep. 2021;9(5):e04026. doi: 10.1002/ccr3.4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao R, Peng S, Wang L, et al. Ultrasound-guided quadratus lumborum block combined with general anaesthesia or general anaesthesia alone for laparoscopic radical gastrectomy for gastric adenocarcinoma: a monocentric retrospective study. Int J Gen Med. 2022;15:7739–7750. doi: 10.2147/IJGM.S382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco R, Ansari T, Riad W, Shetty N. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain after cesarean delivery: a randomized controlled trial. Region Anesth Pain M. 2016;41(6):757–762. doi: 10.1097/AAP.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 15.Huang D, Song L, Li Y, et al. Posteromedial quadratus lumborum block versus transversus abdominal plane block for postoperative analgesia following laparoscopic colorectal surgery: a randomized controlled trial. J Clin Anesth. 2020;62:109716. doi: 10.1016/j.jclinane.2020.109716 [DOI] [PubMed] [Google Scholar]
- 16.Pang M, Sun G, Yao W, et al. Ultrasound-guided transmuscular quadratus lumborum block reduced postoperative opioids consumptions in patients after laparoscopic hepatectomy: a three-arm randomized controlled trial. BMC Anesthesiol. 2021;21(1):45. doi: 10.1186/s12871-021-01255-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Q, Cui X, Fei Y, Xu Z, Huang Y. Transmuscular quadratus lumborum block versus thoracic paravertebral block for acute pain and quality of recovery after laparoscopic renal surgery: study protocol for a randomized controlled trial. Trials. 2019;20(1):276. doi: 10.1186/s13063-019-3359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie BQ, Niu LX, Yang E, Yao SL, Yang L. Effect of subcostal anterior quadratus lumborum block vs. oblique subcostal transversus abdominis plane block after laparoscopic radical gastrectomy. Curr Med Sci. 2021;41(5):974–980. doi: 10.1007/s11596-021-2429-8 [DOI] [PubMed] [Google Scholar]
- 19.Li H, Shi R, Shi D, et al. Anterior quadratus lumborum block at the lateral supra-arcuate ligament versus transmuscular quadratus lumborum block for postoperative analgesia in patients undergoing laparoscopic nephrectomy: a randomized controlled trial. J CLIN ANESTH. 2021;75:110561. doi: 10.1016/j.jclinane.2021.110561 [DOI] [PubMed] [Google Scholar]
- 20.Zhu M, Y Q, He H, Zhang S, Mei Y. Effect of quadratus lumborum block on postoperative cognitive function in elderly patients undergoing laparoscopic radical gastrectomy: a randomized controlled trial. BMC Geriatr. 2021;21(1):238. doi: 10.1186/s12877-021-02179-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M, Qi Y, He H, et al. Analgesic effect of the ultrasound-guided subcostal approach to transmuscular quadratus lumborum block in patients undergoing laparoscopic nephrectomy: a randomized controlled trial. BMC Anesthesiol. 2019;19(1):154. doi: 10.1186/s12871-019-0825-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsharkawy H, Ahuja S, DeGrande S, Maheshwari K, Chan V. Subcostal approach to anterior quadratus lumborum block for pain control following open urological procedures. J Anesth. 2019;33(1):148–154. doi: 10.1007/s00540-018-02605-1 [DOI] [PubMed] [Google Scholar]
- 23.Wu HH, Wang HT, Jin J, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9(3):e93114. doi: 10.1371/journal.pone.0093114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Sherif FA, Abdel-Ghaffar H, Othman A, et al. Pharmacokinetics and pharmacodynamics of dexmedetomidine administered as an adjunct to bupivacaine for transversus abdominis plane block in patients undergoing lower abdominal cancer surgery. J Pain Res. 2022;15:1–12. doi: 10.2147/jpr.S335806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Kang Y, Li Y, Fu B. Dexmedetomidine as an adjuvant to ropivacaine inpatient quality of recovery scores undergoing modified radical mastectomy: a randomized controlled trial. Front Oncol. 2022;12:858030. doi: 10.3389/fonc.2022.858030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang P, Luo Y, Lin L, et al. The efficacy of transversus abdominis plane block with or without dexmedetomidine for postoperative analgesia in renal transplantation. A randomized controlled trial. Int J Surg. 2020;79:196–201. doi: 10.1016/j.ijsu.2020.05.073 [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Fang PP, Yu YQ, et al. Effect of intraoperative dexmedetomidine on recovery of gastrointestinal function after abdominal surgery in older adults: a randomized clinical trial. JAMA Netw Open. 2021;4(10):e2128886. doi: 10.1001/jamanetworkopen.2021.28886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loomba RS, Villarreal EG, Dhargalkar J, et al. The effect of dexmedetomidine on renal function after surgery: a systematic review and meta-analysis. J Clin Pharm Ther. 2022;47(3):287–297. doi: 10.1111/jcpt.13527 [DOI] [PubMed] [Google Scholar]
- 29.Coviello A, Esposito D, Galletta R, Maresca A, Servillo G. Opioid-free anesthesia-dexmedetomidine as adjuvant in erector spinae plane block: a case series. J Med Case Rep. 2021;15(1):276. doi: 10.1186/s13256-021-02868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Yu Y, Miao S, et al. Effects of peri-operative intravenous administration of dexmedetomidine on emergence agitation after general anesthesia in adults: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2019;13:2853–2864. doi: 10.2147/DDDT.S207016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29(6):564–575. doi: 10.1016/j.rapm.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 32.Børglum J, Jensen K, Christensen AF, et al. Distribution patterns, dermatomal anesthesia, and ropivacaine serum concentrations after bilateral dual transverse abdominis plane block. Reg Anesth Pain Med. 2012;37(3):294–301. doi: 10.1097/AAP.0b013e31824c20a9 [DOI] [PubMed] [Google Scholar]
- 33.Murouchi T, Iwasaki S, Yamakage M. Quadratus lumborum block Analgesic effect and chronological ropivacaine concentrations after laparoscopic surgery. Reg Anesth Pain Med. 2016;41(2):146–150. doi: 10.1097/AAP.00000000000003 [DOI] [PubMed] [Google Scholar]
- 34.Mizota T, Yamamoto Y, Hamada M, et al. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br J Anaesth. 2017;119(6):1127–1134. doi: 10.1093/bja/aex255 [DOI] [PubMed] [Google Scholar]
- 35.Biteker M, Dayan A, Tekkes A, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg. 2014;207(1):53–59. doi: 10.1016/j.amjsurg.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 36.Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. 2016;67(5):742–752. doi: 10.1053/j.ajkd.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwasaki M, Edmondson M, Sakamoto A, Ma D. Anesthesia, surgical stress, and “long-term” outcomes. Acta Anaesthesiol Taiwan. 2015;53(3):99–104. doi: 10.1016/j.aat.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 38.Moraca RJ, Sheldon DG, Thirlby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg. 2003;238(5):663–673. doi: 10.1097/01.sla.0000094300.36689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YC, Wang MJ, Lee CY, et al. Effects of perioperative dexmedetomidine infusion on renal function and microcirculation in kidney transplant recipients: a randomised controlled trial. Ann Med. 2022;54(1):1233–1243. doi: 10.1080/07853890.2022.2067351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X, Feng Z. Analysis of risk factors for perioperative acute kidney injury and management strategies. Front Med. 2021;8:751793. doi: 10.3389/fmed.2021.751793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng M, Feng Q, Chen Y, et al. Effect of dezocine on the ratio of Th1/Th2 cytokines in patients receiving postoperative analgesia following laparoscopic radical gastrectomy: a prospective randomised study. Drug Des Devel Ther. 2021;15:2289–2297. doi: 10.2147/DDDT.S306120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alansary AM, Badawy A, Elbeialy MAK. Dexmedetomidine added to bupivacaine versus bupivacaine in transincisional ultrasound-guided quadratus lumborum block in open renal surgeries: a randomized trial. Pain Physician. 2020;23(3):271–282. PMID: 32517393. [PubMed] [Google Scholar]
- 43.Borys M, Szajowska P, Jednakiewicz M, et al. Quadratus lumborum block reduces postoperative opioid consumption and decreases persistent postoperative pain severity in patients undergoing both open and laparoscopic nephrectomies-a randomized controlled trial. J Clin Med. 2021;10(16):3590. doi: 10.3390/jcm10163590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y, Li Z, Qin S, et al. Ultrasound-guided posterior quadratus lumborum block can reduce postoperative opioid consumption and promote rapid recovery in patients undergoing sutureless laparoscopic partial nephrectomy: a triple-blind, randomized, controlled study. Front Oncol. 2022;12:969452. doi: 10.3389/fonc.2022.969452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Hu H, Feng C, et al. Effect of ultrasound-guided quadratus lumborum block preemptive analgesia on postoperative recovery of patients with open radical colon cancer surgery: a retrospective study. Cancer Manag Res. 2021;13:6859–6867. doi: 10.2147/CMAR.S322678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zha J, Ji S, Wang C, et al. Thoracic paravertebral nerve block with ropivacaine and adjuvant dexmedetomidine produced longer analgesia in patients undergoing video-assisted thoracoscopic lobectomy: a randomized trial. J Healthc Eng. 2021;62:1846886. doi: 10.1155/2021/1846886 [DOI] [PMC free article] [PubMed] [Google Scholar]