Abstract
Fifty-nine Borrelia burgdorferi sensu lato culture isolates collected from northeastern China were characterized by 5S-23S rRNA intergenic spacer restriction fragment length polymorphism (RFLP) analysis and reactivity with monoclonal antibodies (MAbs). Among 59 culture isolates, 30 (50.8%) were Borrelia garinii and 17 (28.8%) were Borrelia afzelii, 2 were mixtures composed of B. garinii with RFLP pattern B and B. garinii with pattern C, and 9 were mixtures composed of B. garinii and B. afzelii. One isolate, ChY13p, produced a unique pattern and was identified as B. garinii based on analyses of 16S rRNA gene sequence, flagellin PCR-RFLP typing, and MAb reactivities. No Borrelia burgdorferi sensu stricto or Borrelia japonica isolates were detected. The results indicate that Lyme disease Borrelia species in northeastern China resemble those of Borrelia isolates from far eastern Russia and Japan.
Lyme disease is a multisystemic disorder caused by infection with Borrelia burgdorferi sensu lato, which is transmitted by ticks of the Ixodes ricinus complex (1, 15). Since the etiologic agent was first isolated from Ixodes scapularis in 1982 (6), a large number of B. burgdorferi sensu lato isolates have been obtained from patients, animal reservoirs, and vector ticks from various geographic areas of the world (2, 15, 26, 33, 36). Genetically and immunologically, B. burgdorferi sensu lato, originally regarded as a single species (16), can be subdivided into nine species based on the reference methods for delineation of bacterial species (3, 7, 8, 10, 17, 19, 28, 34): B. burgdorferi sensu stricto, B. garinii, B. afzelii, B. japonica, B. andersonii, B. tanukii, B. turdae, B. valaisiana, and B. lusitaniae. The divergence within B. burgdorferi sensu lato may correlate with epidemiological and clinical features of Lyme disease (2, 31, 32). B. burgdorferi sensu stricto is present in North America and Europe but seems to be absent in Asia (22, 26, 30). Moreover, B. burgdorferi sensu stricto, found in the United States and Europe, is mainly associated with arthritic forms of Lyme disease. B. garinii and B. afzelii are present in Europe and Asia: the former is frequently associated with neurological manifestations, and the latter seems to be the exclusive agent of late cutaneous lesions of acrodermatitis chronica atrophicans (Pick-Herxheimer disease), which occurs mainly in northern Europe. B. japonica is nonpathogenic and seems to be restricted to Japan (17, 28). A simple and useful method for assessing the genetic diversity of Borrelia strains associated with Lyme borreliosis that is based on restriction fragment length polymorphism (RFLP) analysis of the 5S-23S rRNA intergenic spacer amplicon has been developed (27). This method was used to confirm the nine major species defined previously and to identify an additional genomic group among the Borrelia strains. Several papers have described the genetic characteristics and species determination of isolates from North America, Europe, Japan, Korea (18), and Russia (20, 30). Lyme disease is also widespread in China, with endemic foci of the disease discovered and typical cases diagnosed in 11 provinces as well as the suburbs of Beijing (37). Many Lyme Borrelia species have been isolated in China, but few species determination studies have been published. We conducted a survey in northeastern China in May 1996. Fifty-nine Borrelia culture isolates were obtained from Ixodes persulcatus ticks and Apodemus peninsulae rodents. Here we report the genetic characterization and species identification of these Chinese culture isolates by RFLP analysis and sequence analysis of 5S-23S rRNA intergenic spacer, 16S rRNA sequence analysis, flagellin molecular typing, and reactivity with monoclonal antibodies (MAbs).
One hundred twenty-seven I. persulcatus ticks were collected by beating vegetation and two A. peninsulae rodents were captured by snap traps in six different areas of Yakeshi in northeastern China from the end of May 1996 to the beginning of June 1996. The midgut of each tick and the earlobe of each rodent were inoculated into BSKII medium and cultured at 31°C for 4 weeks as previously described (4, 25). Fifty-seven Borrelia culture isolates obtained from the ticks were designated ChY01p to ChY57p, and two Borrelia culture isolates obtained from the rodents were designated ChYAE1 and ChYAE2. B. burgdorferi sensu stricto strain B31, the B. garinii strains 20047, ASF, and FujiP2, the B. afzelii strains VS461 and NT28, B. japonica HO14, and B. hermsii HS1 were used as comparative reference strains.
The 5S-23S rRNA intergenic spacer was amplified by using primers RS1 (5′-CTGCGAGTTCGCGGGAGA-3′) and RS2 (5′-TCCTAGGCATTCACCATA-3′) (27), and RFLP analysis was accomplished by digestion of the PCR products with MseI and DraI as described previously (22). The PCR product of Chinese culture isolate ChY13p was cloned into plasmid pGEM-5Zf by using a pGEM-T vector system kit (Promega Corporation, Madison, Wis.) and sequenced with a PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing Kit (The Perkin-Elmer Corporation, Norwalk, Conn.). The intergenic spacer sequence of isolate ChY13p has been assigned accession no. AB003785. The accession numbers of reference strains used in this study are as follows: strain B31, accession no. L30127; 20047, L30119; ASF, D84403; VS461, L30135; NT28, D84405; and HO14, L30128.
The 16S rRNA gene of Borrelia isolate ChY13p was amplified by primers 5′-GCTGGCAGTGCGTCTTAAGCATGC-3′ and 5′-GTGACGGGCGGTGTGTACAAGGCCC-3′ as described previously (12) and was sequenced as described above. Phylogenetic analyses of the 16S rRNA gene sequences were performed by the DNASTAR (Madison, Wis.) program with the CLUSTAL method (13). The 16S rRNA gene sequence of isolate ChY13p determined in this study has been assigned accession no. AB007450. The accession numbers of sequences used for phylogenetic analysis have been assigned as follows: strain B31, accession no. M88329; 20047, D67018; 935T, L39081; G1, M64311; G2, M60967; HT61, D67019; J1, L46697; IP3, M75149; HO14, L40597; IKA2, L40598; 20004, M64310; 1352, M64309; SH-2-82, M60969; and HS1, M60968. Flagellin PCR-RFLP analysis was carried out as described previously (11). The amplified DNAs were digested with HapII, HhaI, HincII (Takara, Tokyo, Japan), CelII (Boehringer GmbH, Mannheim, Germany), and DdeI (Toyobo, Osaka, Japan). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were carried out as described before (24). The monoclonal antibodies (MAbs) used were H9724, which is reactive to the flagellin antigen (5); H5332, reactive to the outer surface protein A (OspA) (14); P62a, reactive to the 62-kDa heat shock protein (21); P3134, raised to the outer surface protein B (OspB) and cross-reactive with OspA of some isolates (23); G7, reactive to the outer surface protein C (OspC) (20); D6, specific to the 12-kDa protein of B. garinii (3); I.17.3, specific to the OspB of B. afzelii (7); and O1441b, specific to the flagellin protein of B. japonica (21).
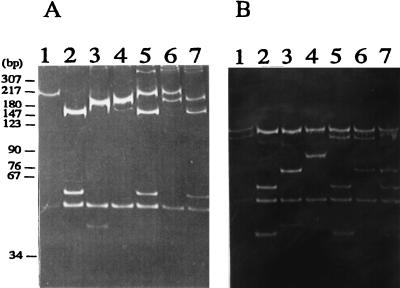
Table 1 summarizes the 5S-23S rRNA intergenic spacer RFLP patterns and species identified in this study. The representative RFLP patterns observed among the 59 Borrelia culture isolates from northeastern China are shown in Fig. 1. The RFLP patterns found previously among nine species and one genomic group of Lyme disease-related Borrelia are as follows: pattern A, B. burgdorferi sensu stricto; patterns B and C, B. garinii; patterns D and N, B. afzelii; pattern E, B. japonica; pattern F, B. valaisiana; patterns G and H, B. lusitaniae; patterns L and M, B. andersonii; pattern O, B. tanukii; pattern P, B. turdae; and patterns I, J, and K, group DN127 (22, 27). In this study, 6 and 24 Borrelia culture isolates generated patterns B and C, respectively, and consequently were identified as B. garinii. Seventeen culture isolates generated pattern D and were identified as B. afzelii. One isolate, ChY13p, showed a pattern never seen before in Borrelia strains. We designated this RFLP pattern pattern R. No B. burgdorferi sensu stricto or B. japonica isolates were detected. Eleven Borrelia culture isolates (about 20%) produced unique RFLP patterns. Each of these isolates was identified as a mixture of two Borrelia strains based on the visible bands. The patterns for seven culture isolates were identified as mixtures of patterns C and D, those for two were identified as mixtures of patterns B and C, and those for two were identified as mixtures of patterns B and D. Patterns representing mixed culture isolates were also observed for Russian isolates (20, 26). This indicated that the Borrelia isolate from one tick culture, originally regarded as one isolate, may be composed of two different species or subspecies. The coinfection with two Borrelia species may explain the complicated manifestations related to Lyme spirochetosis.
TABLE 1.
5S-23S rRNA intergenic spacer RFLP patterns of Chinese Borrelia culture isolates
| Species | No. of culture isolates (% of total no.) | RFLP patterna from digestion by:
|
No. (%) of culture isolates reactive to MAb P3134 with OspA and OspB | |
|---|---|---|---|---|
| DraI | MseI | |||
| B. garinii | 6 (10.2) | B′ | B | 1 (1.7) |
| 24 (40.7) | C′ | C | 15 (25.4) | |
| B. garinii (pattern B) + B. garinii (pattern C) | 2 (3.4) | B′ + C′ | B + C | 1 (1.7) |
| B. afzelii | 17 (28.8) | D′ | D | 0 |
| B. garinii + B. afzelii | 2 (3.4) | B′ + D′ | B + D | 0 |
| 7 (11.9) | C′ + D′ | C + D | 3 (5.1) | |
| B. garinii (tentative) | 1 (1.7) | R′ | R | 0 |
RFLP patterns are shown in Fig. 1, and their designations are given in the corresponding legend.
FIG. 1.
Representative RFLP patterns of 5S-23S rRNA intergenic spacer observed among Chinese Borrelia culture isolates. The PCR products were digested by DraI (A) or MseI (B). DNA was electrophoresed on a 16% polyacrylamide gel and stained with ethidium bromide. The molecular size standards are indicated on the left of the gel. Lane 1, pattern B (isolate ChY02p); lane 2, pattern C (ChY50p); lane 3, pattern D (ChY55p); lane 4, pattern R (ChY13p); lane 5, patterns B and C (ChY27p); lane 6, patterns B and D (ChY28p); lane 7, patterns C and D (ChY15p).
Until now, all Borrelia species isolated from I. persulcatus were identified as either B. garinii or B. afzelii. In the present study, all 57 Borrelia culture isolates from I. persulcatus in China were also identified as either B. garinii or B. afzelii. It was considered that I. persulcatus was not a vector for B. burgdorferi sensu stricto strains and other Borrelia species. Furthermore, three isolates obtained from a rat and from ticks which were feeding on the rat were determined to be B. afzelii with pattern D, and one isolate from another rat was B. garinii with pattern C. This finding might support the role of rodents in maintaining Lyme Borrelia spp.
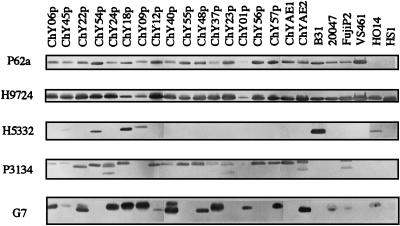
The representative MAb reactivities of Chinese Borrelia culture isolates are shown in Fig. 2. All Chinese Borrelia culture isolates in this study reacted with MAb H9724, which is specific to the 41-kDa flagellin protein of the genus Borrelia, and MAb P62a, which is reactive to the 62-kDa heat shock protein of B. burgdorferi sensu lato but not B. japonica. The reference strains HS1 (B. hermsii) and HO14 (B. japonica) showed a negative reactivity with MAb P62a. Thus, there were no B. japonica isolates among these 59 Chinese cultures. We identified all culture isolates as B. burgdorferi sensu lato with genus-specific MAb G7 reactive to OspC. Five Borrelia culture isolates showed two OspC bands which might have resulted from mixtures of two isolates. These five culture isolates were also identified as mixtures by RFLP analysis. Twenty of 59 culture isolates showed cross-reactivity of both OspA and OspB to MAb P3134, including 15 B. garinii isolates with pattern C, 1 B. garinii isolate with pattern B, 1 isolate mixture of B. garinii with patterns B and C, and 3 isolate mixtures of B. garinii with pattern C and B. afzelii with pattern D (Table 1). Previous studies had reported that some B. garinii isolates from Japan and Russia showed cross-reactivity of both OspA and OspB with MAb P3134 (9, 17). Sequence analysis revealed that the ospA and ospB genes of these isolates share a conserved 282 bp sequence at their 3′ ends (35). To date, these isolates have been observed only in eastern Asia, not in North America or Europe.
FIG. 2.
Western blot analysis of Chinese Borrelia culture isolates with MAbs P62a, H9724, H5332, P3134, and G7. B. burgdorferi B31, B. garinii 20047, ASF, and FujiP2, B. afzelii VS461 and NT28, B. japonica HO14, and B. hermsii HS1 were used as comparative reference strains.
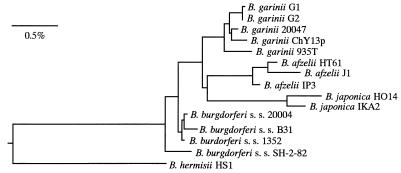
One isolate, ChY13p, was observed to have an RFLP pattern never found before among Borrelia strains. To further confirm this characteristic of ChY13p, the 5S-23S rRNA intergenic spacer sequence was determined and compared with those of other representative strains (Fig. 3). ChY13p produced a 237-bp 5S-23S rRNA spacer amplicon that was similar in size to that of B. japonica (236 bp). Two fragments, 185 bp and 52 bp in size, were generated by digestion with DraI, and three fragments, of 105, 79, and 53 bp, were observed after digestion with MseI. Although the MseI pattern of ChY13p was almost identical to that of B. japonica, the DraI patterns of these strains were quite different (19). The sequence between nucleotide 73 and nucleotide 97 differed among the different Borrelia species. Compared with the sequences of B. burgdorferi sensu stricto and B. garinii, 10 nucleotides were missing from the sequences of B. afzelii VS461 and NT28 and 18 nucleotides were missing from those of ChY13 and B. japonica. Furthermore, ChY13p has the nucleotide sequence AAAACA, was found specifically in the sequences of B. afzelii with RFLP pattern D and B. japonica. The sequence of ChY13p showed the highest similarity to those of B. afzelii with pattern D and B. japonica. To identify the species of isolate ChY13p, the 16S rRNA gene sequence of isolate ChY13p was determined to assess the phylogenetic divergence. About 90% of the whole 16S rRNA gene sequence was aligned and compared with previously published sequences of Borrelia species. A neighbor-joining phylogenetic tree (29) was constructed on the basis of the sequence similarity matrix. Phylogenetic analysis placed the strains into a coherent cluster of the Lyme disease Borrelia and related genomic groups. According to this tree, ChY13p was clustered into the group of B. garinii strains (Fig. 4). The isolate ChY13p was further characterized by flagellin PCR-RFLP typing method. The following Borrelia strains have been previously determined as producing the flagellin RFLP types indicated: type I, B. burgdorferi sensu stricto; II, B. garinii; III, B. afzelii; IV, B. tanukii; V, B. turdae; VI, B. valaisiana; VII, B. japonica; VIII, B. lusitaniae; IX, group DN127; and X, B. andersonii (11). ChY13p produced pattern II. Western blot analysis reveals that isolate ChY13p reacted with MAb D6, which is specific to the 12-kDa protein of B. garinii, but was nonreactive to MAb I.17.3, which is specific to the OspB of B. afzelii, and MAb O1441b, which is specific to the flagellin protein of B. japonica. In this study, 58 of 59 Borrelia culture isolates were identified on the basis of 5S-23S rRNA intergenic spacer RFLP analysis. It suggests that PCR-RFLP analysis is a useful and reliable method for the species determination of Lyme disease-related Borrelia spp. One isolate, ChY13p, was observed to have an RFLP pattern never found before among Borrelia strains. Further analyses of 16S rRNA gene sequence, flagellin gene typing, and MAb reactivities identified this isolate as B. garinii. ChY13p showed the highest 5S-23S rRNA intergenic spacer sequence homology to B. afzelii with pattern D and also to B. japonica. It was hypothesized that B. afzelii and B. japonica seem to have evolved from B. garinii (27). Compared with typical B. garinii isolates, ChY13p might be evolutionally closer to B. afzelii or B. japonica or it may be an intermediate strain in the course of evolution.
FIG. 3.
Nucleotide sequence alignment of 5S-23S rRNA intergenic spacer amplicons of Chinese isolate ChY13p and the reference Borrelia isolates. The 3′ region of the 5S rRNA gene and the 5′ region of the 23S rRNA gene are indicated in boldface type. The corresponding primers are underlined. The asterisks and boxes indicate identical nucleotides and the motif sequence, respectively. s. s., sensu stricto.
FIG. 4.
Phylogenetic tree for the Lyme disease borreliae and their relatives constructed by using 16S rRNA gene sequences. Bar = 0.5% difference between sequences, as determined by measuring the length of the horizontal lines connecting two isolates. s. s., sensu stricto.
The results indicated that Lyme disease Borrelia species in northeastern China resemble those isolated from far eastern Russia (20, 30) and Japan (22, 26, 27). B. garinii with RFLP patterns B and C and B. afzelii with RFLP pattern D were common in China. Particularly, B. garinii with RFLP pattern C, found in Japan and far eastern Russia (20) but not in Europe (22, 27), was detected with high frequency. This suggested that B. garinii with patterns B and C and B. afzelii with pattern D are commonly distributed in eastern Asia. It might provide an important basis for revealing the interrelation between the clinical manifestation of Lyme disease and Borrelia species. This study may also provide an important basis for developing a vaccine for strains from eastern Asia.
Acknowledgments
We thank A. G. Barbour, O. Peter, and D. Postic for providing MAbs.
This work was supported in part by a grants-in-aid for Encouragement of Young Scientists (no. 06772143), for Scientific Research (no. 07670320, 08670312, and 09670294), and for International Cooperative Research (no. 06044191, 08044310, and 08041181) from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Anderson J F. Epizootiology of Borrelia in Ixodes tick vectors and reservoir hosts. Rev Infect Dis. 1989;11:1451–1459. doi: 10.1093/clinids/11.supplement_6.s1451. [DOI] [PubMed] [Google Scholar]
- 2.Assous M V, Postic D, Paul G, Névot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 3.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme Disease, a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 7.Canica M M, Nato F, du Merle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 8.Fleche A L, Postic D, Girardet K, Peter O, Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 9.Fukunaga M, Hamase A. Outer surface protein C gene sequence analysis of Borrelia burgdorferi sensu lato isolates from Japan. J Clin Microbiol. 1995;33:2415–2420. doi: 10.1128/jcm.33.9.2415-2420.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukunaga M, Hamase A, Okada K, Nakao M. Borrelia tanukii sp. nov. and Borrelia turdae sp. nov. found from Ixodid ticks in Japan: rapid species identification by 16S rRNA gene-targeted PCR analysis. Microbiol Immunol. 1996;40:877–881. doi: 10.1111/j.1348-0421.1996.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 11.Fukunaga M, Okada K, Nakao M, Konishi T, Sato Y. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int J Syst Bacteriol. 1996;46:898–905. doi: 10.1099/00207713-46-4-898. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga M, Sohnaka M, Yanagihara Y. Analysis of Borrelia species associated with Lyme disease by rRNA gene restriction fragment length polymorphism. J Gen Microbiol. 1993;139:1141–1146. doi: 10.1099/00221287-139-6-1141. [DOI] [PubMed] [Google Scholar]
- 13.Higgins D G, Bleasby A J, Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 14.Howe T R, Mayer L W, Barbour A G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985;227:645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- 15.Jaenson T G T. The epidemiology of Lyme borreliosis. Parasitol Today. 1991;7:39–45. doi: 10.1016/0169-4758(91)90187-s. [DOI] [PubMed] [Google Scholar]
- 16.Johnson R C, Schmid G P, Hyde F W, Steigerwalt A G, Brenner D J. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 17.Kawabata H, Masuzawa T, Yanagihara Y. Genomic analysis of Borrelia japonica sp. nov. isolated from Ixodes ovatus in Japan. Microbiol Immunol. 1993;37:843–848. doi: 10.1111/j.1348-0421.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 18.Kee S H, Yoon J H, Oh H B, Park Y H, Kim Y W, Cho M K, Park K S, Chang W H. Genetic analysis of Borrelia burgdorferi sensu lato in Korea using genomic hybridization and 16S rRNA gene sequence determination. Microbiol Immunol. 1996;40:599–605. doi: 10.1111/j.1348-0421.1996.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 19.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuzawa T, Iwaki A, Sato Y, Miyamoto K, Korenberg E I, Yanagihara Y. Genetic diversity of Borrelia burgdorferi sensu lato isolated in far eastern part of Russia. Microbiol Immunol. 1997;41:595–600. doi: 10.1111/j.1348-0421.1997.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 21.Masuzawa T, Kawabata H, Beppu Y, Miyamoto K, Nakao M, Sato N, Muramatsu K, Sato N, Johnson R C, Yanagihara Y. Characterization of monoclonal antibodies for identification of Borrelia japonica, isolates from Ixodes ovatus. Microbiol Immunol. 1994;38:393–398. doi: 10.1111/j.1348-0421.1994.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 22.Masuzawa T, Komikado T, Iwaki A, Suzuki H, Kaneda K, Yanagihara Y. Characterization of Borrelia sp. isolated from Ixodes tanuki, I turdus, and I. columnae in Japan by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. FEMS Microbiol Lett. 1996;142:77–83. doi: 10.1111/j.1574-6968.1996.tb08411.x. [DOI] [PubMed] [Google Scholar]
- 23.Masuzawa T, Kaneda K, Suzuki H, Wang J H, Yamada K, Kawabata H, Johnson R C, Yanagihara Y. Presence of common antigenic epitope in outer surface protein (Osp) A and OspB of Japanese isolates identified as Borrelia garinii. Microbiol Immunol. 1996;40:455–458. doi: 10.1111/j.1348-0421.1996.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 24.Masuzawa T, Okada Y, Yanagihara Y, Sato N. Antigenic properties of Borrelia burgdorferi isolated from Ixodes ovatus and Ixodes persulcatus in Hokkaido, Japan. J Clin Microbiol. 1991;29:1568–1573. doi: 10.1128/jcm.29.8.1568-1573.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto K, Nakao M, Sato N, Mori M. Isolation of Lyme disease spirochetes from an ixodid tick in Hokkaido, Japan. Acta Trop. 1991;49:65–68. doi: 10.1016/0001-706x(91)90031-e. [DOI] [PubMed] [Google Scholar]
- 26.Nakao M, Miyamoto K, Uchikawa K, Fujita H. Characterization of Borrelia burgdorferi isolated from Ixodes persulcatus and Ixodes ovatus ticks in Japan. Am J Trop Med Hyg. 1992;47:505–511. doi: 10.4269/ajtmh.1992.47.505. [DOI] [PubMed] [Google Scholar]
- 27.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 28.Postic D, Belfaiza J, Isogai E, Saint Girons I, Grimont P A D, Baranton G. A new genomic species in Borrelia burgdorferi sensu lato isolated from Japanese ticks. Res Microbiol. 1993;144:467–473. doi: 10.1016/0923-2508(93)90054-6. [DOI] [PubMed] [Google Scholar]
- 29.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 30.Sato Y, Miyamoto K, Iwaki A, Masuzawa T, Yanagihara Y, Korenberg E I, Gorelova N B, Volkov V I, Ivanov L I, Liberova R N. Prevalence of Lyme disease spirochetes in Ixodes persulcatus and wild rodents in Far Eastern Russia. Appl Environ Microbiol. 1996;62:3887–3889. doi: 10.1128/aem.62.10.3887-3889.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 32.Van Dam A P, Kuiper H, Vos K, Widjojokusumo A, de Jongh B M, Spanjaard L, Ramselaar A C P, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 33.Wallich R, Helmes C, Schaible U E, Lobet Y, Moter S E, Kramer M D, Simon M M. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992;60:4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G Q, van Dam A P, Fleche A L, Postic D, Peter O, Baranton G, de Boer R, Spanjaard L, Dankert J. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19) Int J Syst Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- 35.Wang J H, Masuzawa T, Komikado T, Yanagihara Y. Consensus sequence on the genes encoding the major outer surface proteins (OspA and OspB) of Borrelia garinii isolate. Microbiol Immunol. 1997;41:83–91. doi: 10.1111/j.1348-0421.1997.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 36.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z F. Proceedings of the International Symposium on Lyme disease in Japan. 1994. Epidemiological features of Lyme disease in China; pp. 84–91. [Google Scholar]