Abstract
Background and aim:
Androgenetic alopecia (AGA) is a common chronic, hereditary, cutaneous, and androgen-dependent condition. Low self-esteem and negative impact on quality of life are often consequences of AGA. Clinical treatment of AGA using SVF (Stromal Vascular Fraction) has been effective. The hair follicle is affected by various environmental factors and one of the most important factors is the vascularity of the scalp which is itself affected by SVF.
Methods:
In October 2022, we conducted a systematic review to identify all scientific publications discussing hair loss treatment with stromal vascular fraction or adipose stem cells. We selected 140 articles. After a screening process, we kept 9 articles complying with inclusion criteria.
Results:
No serious adverse events were reported in all studies. Despite a standardized protocol was not found, all studies reported a statistically significant increase in the number (density) of hair after SVF treatment. Two studies found a significant improvement in the pull test. An increase in hair diameter was noticed after treatment. The combination of medical therapy and SVF proved to be advantageous.
Conclusions:
SVF is nowadays at the center of studies in the field of regenerative medicine due to its potential applications in many branches of medicine and surgery. The initial results are very promising but furthermore, studies are necessary to establish a methodical and systematic research capable of demonstrating its real benefits and the creation of homogenous treatment protocols. (www.actabiomedica.it)
Keywords: AGA, androgenic alopecia, SVF, adipose-derived stromal vascular fraction, PRP, regenerative medicine
Introduction
Androgenetic alopecia (AGA) is a common, chronic, and cutaneous condition. AGA is an androgen-dependent pathology and it is characterized by a hereditary inheritance pattern, beginning with the advent of puberty. In predisposed males and females, scalp hair progressively thins in a defined pattern, with non-scarring, progressive miniaturization of the hair follicle and shaft (1).
In men, hair loss has its characteristic “horseshoe” pattern involving the temporal and vertex region, sparing the occipital region.
Age and race influence the incidence and prevalence of AGA. We know that up to 30% of Caucasian men will have AGA by the age of 30 years, up to 50% by 50 years, and 80% by 70 years (1).
Chinese, Japanese, and African American people are less affected than Caucasian ones (2).
Low self-esteem and negative impact on quality of life are often consequences of AGA.
Despite its prevalence, finasteride and topical minoxidil are the unique therapeutic options approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (3).
In the past, due to the absence of other therapeutic modalities, practitioners could use surgical hair transplants, but this was associated with risks such as bleeding and infection (4).
Nowadays regenerative medicine is a sphere of great interest for scientific research. Stromal Vascular Fraction (SVF) isolated from the adipose tissue is one of the latest innovative solutions in this research field (5).
According to recent academic papers, the result of clinical treatment of AGA with SVF has been statistically effective and those with adipose-derived stem cells or its culture fluid have been extensively reported (6).
The uses of SVF from fat are reported to be effective in the regeneration of damaged tissue, degenerative arthritis, and wound care. Many clinical cases reported the stability of the treatment, while several studies showed the effectiveness of neovascularization stimulation and inflammatory change reduction (7,8).
SVF is composed of stem cells (ADSC) and immune cells but it can activate surrounding tissues by secreting various cytokines depending on the environment (9).
Hair follicle is affected by various environmental factors (10) and one of the most important is the vascularity of the scalp (11). For this reason, SVF is supposed to contribute to restoring the hair cycle and improving vascularization with stem and vascular cells.
This review will provide a summary of past and current clinical studies investigating the use of SVF in AGA treatment.
Materials and method
In October 2022 we carried out a systematic review to identify all scientific publications discussing hair loss treatment with stromal vascular fraction or adipose stem cells. We followed PRISMA guidelines and searched as keywords (“alopecia” or “hair loss”) AND (“stromal vascular fraction” or “adipose stem cell”). We selected 135 articles, and we added 5 more due to citations. After a screening process, we keep 9 articles complying with inclusion criteria. Figure 1 represents the “PRISMA” flow chart.
Figure 1.
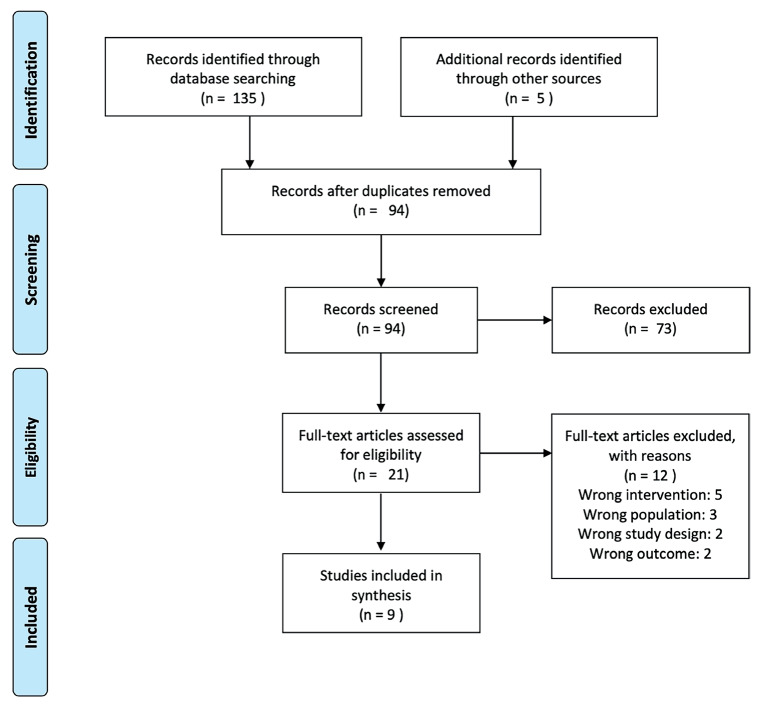
PRISMA flow chart.
We included prospective, observational, or experimental studies on the use of SVF for the treatment of androgenic alopecia (AGA) or FPHL (female pattern hair loss). Non-English language studies, animal model ones, papers using non-autologous SVF or acellular SVF, and studies lacking objective outcome data were excluded. The nine selected studies comprehended 3 randomized clinical trials (RCT) and 6 case series, as reported in Table 1.
Table 1.
Demographic data, AGA severity, and treatments of studies included in this review.
| Author (first listed), Year | Study Design | No. of Patients | Geographic Location | Age (y), | Sex | Severity AGA | Isolation SVF/ADSC | Other pharma treatment | Intervention/ frequency | Comparator |
|---|---|---|---|---|---|---|---|---|---|---|
| Fukuoka al, 2015 | Case series | 10 | Japan | 20-73 | 8M 2W |
- | Enzymatic (AAPE) | none | SVF, 1/month for 6 months | saline |
| Perez-Mesa al, 2017 | Case series | 5 | Spain | 18-55 | 4M 1W |
M II-VI W I-III |
Enzymatic | none | Fat enriched with SVF, single treatment | - |
| Stevens al, 2018 | Case series | 10 | Netherland | 25-72 | 10M | II-VII | Mechanical | none | SVF+PRP, single treatment | - |
| Kadry al, 2018 | RCT | 60 | Egypt | 20-35 | 28M 32F |
M II-IV F II-III |
Enzymatic | none | SVF, 1/month for 3 months | PRP, 1/month for 3 months |
| Narita al, 2019 | Case series | 40 | Japan | 23-74 | 21M 19W |
M II-VI W I-II |
Enzymatic (AAPE) |
Sub-group finasteride 1mg/day | SVF, 1/month for 6 months | - |
| Nilforoushzadeh al, 2020 | Case series | 9 | Iran | 25-40 | 4M 5W |
- | No isolation, whole fat | none | Whole fat, single treatment | - |
| Butt al, 2020 | RCT | 22 | Pakistan | 15+ | 18M 4W |
M III-VI W I-III |
Enzymatic | PRP | SVF+PRP 1 treatment/month for 2 months | PRP 1 treatment/month for 2 months |
| Kuka al, 2020 | RCT | 71 | USA | 24-73 | 54M 17F |
M III-IV F I-II |
Mechanical and Enzymatic | none | Autologous fat enriched with either low or high-dose SVF, single treatment | Saline or fat, single treatment |
| Kim al. 2021 | Case series | 9 | South Korea | 43-64 | 4M 5W |
M IV-V W I-III |
Enzymatic (HuriCell) |
1mg finasteride, 0.5mg dutasteride M Minoxidil 3% W |
SVF, single treatment |
Abbreviations: RCT: randomized clinical trial; M: man; W: woman; AGA: androgenic alopecia; SVF: stromal vascular fraction; ADSC: adipose derived stem cells; PRP: platelet-rich plasma.
Results
SVF harvesting
In all the studies, adipose tissue was harvested by liposuction technique. Seven studies (77,77%) (5,12–17), including RCT, performed enzymatic SVF isolation, while Stevens et al. (6) used the mechanical way and Nilforoushzadeh et al. used not manipulated fat (18). Regardless of the isolation method, the SVF was injected into the scalp, either as a single treatment or as a monthly cycle for 2 months, 3 months, or 6 months.
Hair density
All 9 studies (100%) reported a statistically significant increase in the hair count after SVF treatment compared to the pre-treatment baseline. Fukuoka et al. described a mean increase of 29 ± 4.1 hairs/cm2 after 7 months, with no statistically significant differences between the group taking finasteride and the control group. Instead, a statistically significant difference was found in the subgroup treated with SVF compared to the subgroup treated with saline (p < .05) (17), with a major increase in the first group. Perez-Meza et al. reported a mean increase of 31.2 hairs/cm2 (15) by administering SVF + adipose tissue after 6 months; Stevens et al. found an increase of 30.7 hairs/cm2 after 3 months from a single SVF + PRP treatment (6). Kadry et al. noticed an average increase of 19.30 hairs/cm2 after 6 months in the SVF group patients (high statistical significance p < .001), while an average increase of 10.73 (low statistical significance p = 0.037) in PRP group patients (12). In Narita’s paper, an average increase of 15 terminal hairs/cm2 was observed after a follow-up of 6 months, with no statistically significant differences between the group in therapy with finasteride and the control group (16). Nilforoushzadeh obtained an average increase of 145 hairs/cm2 in female patients and 117 hairs/cm2 in male patients after 6 months with a single treatment of decanted adipose tissue (18). Butt’s group reported an average increase of 19.51 hairs/cm2 at six months in the group treated with SVF + PRP, while 4.67 hairs/cm2 in the PRP-only group. In the group treated with SVF + PRP, the increase was significantly greater than in the PRP-only group (p = 0.006) (14). In the STYLE study, the best average increase at 6 months was in the group treated with adipose tissue + low concentration SVF (average increase 16.56 hair/cm2), while in the other groups (adipose tissue only, adipose tissue + high SVF concentration, saline) the increase from baseline was not statistically significant (13). The combination of systemic/topical therapy (finasteride in male patients, minoxidil 3% in female patients) proved to be advantageous in Kim’s study which observed an average increase of 46.67 hairs/cm2, while in patients who did not take any medications, the mean increase at 6 months was 24.44 hairs/cm2 (5).
Hair diameter
Four studies evaluated the variation of hair diameter after treatment: two found a significant increase in hair diameter (12,18), while two observed a non-statistically significant increase (5,13). Kadry’s group found a significant increment in the diameter of the hair compared to the baseline state (average increase of 50 μm) after 6 months. The increase was not significant in the group treated with PRP instead of SVF (12). Nilforoushzadeh et al. found a mean augment of 44.6 μm after 6 months (p < 0.001), more significant in female patients (18).
Neither Kuka’s article reported any statistically significant increased diameters in any group (fat, fat + low concentration SVF, fat + high concentration SVF, physiological solution) (13) nor the Kim’s study at 6 months follow-up (5).
Pull test
This simple test measures the severity of hair loss. During a pull test, a physician grasps small portions of hair, about 40 strands, from different areas of the scalp and gently tugs. An active hair loss is diagnosed if six or more strands fall out.
Two studies evaluated changes in hair removed with the pull test (14,18) and, in both, a significant reduction 6 months after the treatment was found.
Table 2 summarizes the results of the studies included in the review.
Table 2.
Results of studies included in the review.
| Author (first listed), Year | Hair Count (Hair/cm2 – mean increase) 6 months |
Hair Diameter (μm) (mean change) 6 months |
Pull Test (6 months) |
|---|---|---|---|
| Fukuoka al, 2015 | 18.4±9.4 SVF 6.5±11.7 saline |
- | - |
| Perez-Mesa al, 2017 | 31.2±17.7 (p=0.017) Fat + SVF | - | - |
| Stevens al, 2018 | 30.7±30.4 (range 5-59) (p<0.001) SVF + PRP | - | - |
| Kadry al, 2018 | 19.30 ± 13.65 (p<0.001) SVF 10.73 ± 3.66 (p=0.037) PRP |
50 (p<0.001) SVF 20.05 ± 10.5 (p=0.145) PRP |
- |
| Narita al, 2019 | 15 (p<0.01) SVF | - | - |
| Nilforoushzadeh al, 2020 | 117 M (p<0.0001) 145 F (p<0.0001) |
38.2 M (p<0.0001) 50.4 F (p< 0.0001) |
-3.7 F (p<0.001) - 4.1 M (p<0.001) |
| Butt al, 2020 | 19.51 SVF+PRP 4.67 PRP |
- | -80.78% SVF+PRP -34.01% PRP |
| Kuka al, 2020 | 16.56 ± 14.68 (p<0.05) fat + low-dose SVF | - | - |
| Kim al. 2021 |
46.67 (p=0.009) SVF + medication 24.44 SVF |
- | - |
Abbreviations: SVF: stromal vascular fraction; PRP: platelet-rich plasma; M: male; F: female.
Safety
No serious adverse events were reported in all studies. The most frequently reported adverse events were injection site pain and puncture site ecchymosis.
Discussion
This review identified nine published studies concerning the treatment of androgenetic alopecia with SVF. Despite the heterogenicity in the administration of treatments, in the measurement of results, and in follow-up times, all studies reported some improvement in hair density, although not in all degrees of alopecia. Evidence for the efficacy of SVF on hair diameter was less robust, with 50% of studies showing a significant increase. Instead, the studies that evaluated the pull test observed an improvement in hair strength.
Importantly, no serious adverse events were reported in any study. These results suggest that SVF can be a valid treatment against a pathology that has no etiological therapy, also considering the risk-benefit ratio. Probably the effectiveness of the treatment depends on some specific characteristics of the patient, such as the severity of the hair loss, as well as on the characteristics and modalities of the treatment, including methods of preparation, frequency, and adjunctive therapies (19). The treatment protocols are extremely varied, but a common point of the procedure is certainly the method of isolating the SVF. Most of the studies (78%) utilize an enzymatic method, which is characterized by the use of proteases that determine the digestion of the adipose tissue and subsequent cell culture to expand the ADSC present in the SVF. This procedure has advantages and disadvantages. The advantage is the certainty of the vitality of the stem component being used. The first disadvantage is the juridical aspect which equates treatment to tissue engineering procedures, and in this way, it restricts and regulates this procedure’s use. In fact, EMA considers the autologous tissues as tissue engineering procedures if they have undergone a substantial manipulation.(20) Substantial manipulation is when the tissue(s) have been manipulated during the manufacturing process so that their structural properties, biological characteristics or physiological functions have been modified to be relevant for their intended function. Enzymatic digestion is one example of substantial manipulation.(21) Finally, tissue engineering procedures (such as BTM bone) are registered in Italy as medical devices, must have a National Device Code (CND) and a repertoire number, must have the CE mark, follow the legislation of medical devices.(22) The last negative aspect of the enzymatic technique is the laboratory times necessary for the execution and completion of the biochemical reactions, that it is a not modifiable waiting time. Considering these aspects and the preparatory works by Tonnard on nanofat (23) and the more recent studies by Stevens(6), and Vestita (24), our group believes that research should move towards the use of a reliable and reproducible mechanic method of SVF isolation. This trend is supported by the research of Nilforoushzadeh et al. (18) who proved that a significant effective treatment can be obtained even with decantation of the fat alone and by the findings of Kuka et al. (13) who demonstrated that adipose tissue + SVF with a low cellular concentration was more effective of adipose tissue + SVF at high cellular concentration. Copcu et al. suggest using the term TOST (Total Stromal cells), for mechanical stromal cells, instead of SVF or nanofat. In their opinion, the most important convenience of the mechanical isolating method is that no dissolving chemical is used, such as an enzyme, so the integrity and presence of stromal cells are maximized (25).
Complementary therapies, such as finasteride/dutasteride orally or minoxidil topically (the drugs traditionally used for AGA), may provide an additional treatment benefit, but they are often poorly tolerated due to the side effects and the strong compliance they require (26–28).
SVF treatment demonstrated efficacy both in subjects on therapy and in the control group, thus demonstrating an independent beneficial effect. The severity of alopecia is a fundamental aspect to be evaluated. Studies show that the best results are obtained in the initial stages, even with a single treatment (13). Therefore, this type of treatment in a single administration can be suggested in the initial phase, while in the more advanced phases, several interventions may be necessary monthly to obtain the same results.
The relationship with the PRP is still under study. Stevens demonstrated the synergic effect, safety, and effectiveness of the combination of PRP and SVF for AGA treatment (6), so PRP could be used as a support tool when the alopecia area is too big to be treated with only SVF.
Conclusion
SVF is nowadays at the center of studies of regenerative medicine due to its potential applications in many branches of medicine and surgery and can be considered the “next PRP”. The favorable benefit/risk ratio must not be the prerequisite for a disproportionate indiscriminate use of this treatment. This review must lay the foundations for methodical and systematic research capable of demonstrating its real benefits and the creation of homogenous treatment protocols.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement, etc.) that might pose a conflict of interest in connection with the submitted article.
Authors Contribution:
Conceptualization: Giorgio De Santis, Gian Piero Mantovani, Valentina Pinto; Writing original draft preparation: Caterina Marra, Federico De Maria; Data analysis Gian Piero Mantovani, Caterina Marra, Federico De Maria. All authors reviewed, discussed, and agreed with the final version of the manuscript.
References
- Lolli F, Pallotti F, Rossi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9–17. doi: 10.1007/s12020-017-1280-y. doi: 10.1007/s12020-017-1280-y. [DOI] [PubMed] [Google Scholar]
- Otberg N, Finner AM, Shapiro J. Androgenetic Alopecia. Endocrinol Metab Clin North Am. 2007;36(2):379–98. doi: 10.1016/j.ecl.2007.03.004. doi: 10.1016/j.ecl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lee SW, Juhasz M, Mobasher P, Ekelem C, Mesinkovska NA. A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. J Drugs Dermatol. 2018 Apr 1;17(4):457–63. PMID: 29601622. [PMC free article] [PubMed] [Google Scholar]
- Jimenez F, Alam M, Vogel JE, Avram M. Hair transplantation: Basic overview. J Am Acad Dermatol. 2021;85(4):803–14. doi: 10.1016/j.jaad.2021.03.124. doi: 10.1016/j.jaad.2021.03.124. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim MJ, Lee YJ, et al. Innovative method of alopecia treatment by autologous adipose-derived SVF. Stem Cell Res Ther. 2021;12(1):486. doi: 10.1186/s13287-021-02557-6. doi: 10.1186/s13287-021-02557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HP, Donners S, de Bruijn J. Introducing Platelet-Rich Stroma: Platelet-Rich Plasma (PRP) and Stromal Vascular Fraction (SVF) Combined for the Treatment of Androgenetic Alopecia. Aesthet Surg J. 2018;38(8):811–22. doi: 10.1093/asj/sjy029. doi: 10.1093/asj/sjy029. [DOI] [PubMed] [Google Scholar]
- Chu DT, Nguyen Thi Phuong T, Tien NLB, et al. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J Clin Med. 2019;8(7):917. doi: 10.3390/jcm8070917. doi: 10.3390/jcm8070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Concise Review: The Use of Adipose-Derived Stromal Vascular Fraction Cells and Platelet Rich Plasma in Regenerative Plastic Surgery. Stem Cells. 2017;35(1):117–34. doi: 10.1002/stem.2498. doi: 10.1002/stem.2498. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-Renewal, Multipotency, and the Existence of Two Cell Populations within an Epithelial Stem Cell Niche. Cell. 2004;118(5):635–48. doi: 10.1016/j.cell.2004.08.012. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Di Mascio D, Sapino G, De Maria F. Telogen Effluvium as a complication of scalp reconstruction with tissue expander: a case report. Acta Biomed. 2021;92(S1):e2021431. doi: 10.23750/abm.v92iS1.12066. doi: 10.23750/abm.v92iS1.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Meza D, Niedbalski R. Complications in Hair Restoration Surgery. Oral Maxillofac Surg Clin North Am. 2009;21(1):119–48. doi: 10.1016/j.coms.2008.10.010. doi: 10.1016/j.coms.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Kadry MH, El-Kheir WA, El-Sayed Shalaby M, El Shahid AR, Metwally HG. Autologous Adipose Derived Stem Cell versus Platelet Rich Plasma Injection in the Treatment of Androgentic Alopecia: Efficacy, Side Effects and Safety. J Clin Exp Dermatol Res. 2018;09(03) doi: 10.4172/2155-9554.1000447. [Google Scholar]
- Kuka G, Epstein J, Aronowitz J, et al. Cell Enriched Autologous Fat Grafts to Follicular Niche Improves Hair Regrowth in Early Androgenetic Alopecia. Aesthet Surg J. 2020 doi: 10.1093/asj/sjaa037. doi: 10.1093/asj/sjaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt G, Hussain I, Ahmad FJ, Choudhery MS. Stromal vascular fraction-enriched platelet-rich plasma therapy reverses the effects of androgenetic alopecia. J Cosmet Dermatol. 2020;19(5):1078–85. doi: 10.1111/jocd.13149. doi: 10.1111/jocd.13149. [DOI] [PubMed] [Google Scholar]
- Perez-Meza D, Ziering C, Sforza M, Krishnan G, Ball E, Daniels E. Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells Cloning. 2017;Volume 10:1–10. doi: 10.2147/SCCAA.S131431. doi: 10.2147/SCCAA.S131431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Fukuoka H, Sekiyama T, Suga H, Harii K. Sequential Scalp Assessment in Hair Regeneration Therapy Using an Adipose-Derived Stem Cell–Conditioned Medium. Dermatologic Surgery. 2020;46(6):819–25. doi: 10.1097/DSS.0000000000002128. doi: 10.1097/DSS.0000000000002128. [DOI] [PubMed] [Google Scholar]
- Fukuoka H, Suga H. Hair Regeneration Treatment Using Adipose-Derived Stem Cell Conditioned Medium: Follow-up With Trichograms. Eplasty. 2015;15:e10. PMID: 25834689. [PMC free article] [PubMed] [Google Scholar]
- Nilforoushzadeh MA, Lotfi E, Heidari-Kharaji M, Torkamaniha E, Hanifnia AR. Autologous whole fat injection stimulates hair growth in resistant Androgenetic Alopecia: Report of nine cases. J Cosmet Dermatol. 2021;20(8):2480–5. doi: 10.1111/jocd.13907. doi: 10.1111/jocd.13907. [DOI] [PubMed] [Google Scholar]
- Kang BY, Li AW, Lee MH, et al. The safety and efficacy of autologous adipose-derived stromal vascular fraction for nonscarring alopecia: A systematic review. Arch Dermatol Res. 2022;314(4):349–56. doi: 10.1007/s00403-021-02238-7. doi: 10.1007/s00403-021-02238-7. [DOI] [PubMed] [Google Scholar]
- Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004 (Text with EEA relevance) [Google Scholar]
- Raposio E, Ciliberti R. Clinical use of adipose-derived stem cells: European legislative issues. Ann Med Surg (Lond) 2017;24:61–64. doi: 10.1016/j.amsu.2017.11.002. doi: 10.1016/j.amsu.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach del Prever EM, Donati DM, Fiorentino S, Macrì E. Regulatory aspects of regenerative medicine products and informed consent. G.I.O.T. 2010;36(5):223–242. [Google Scholar]
- Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat Grafting. Plast Reconstr Surg. 2013;132(4):1017–26. doi: 10.1097/PRS.0b013e31829fe1b0. doi: 10.1097/PRS.0b013e31829fe1b0. [DOI] [PubMed] [Google Scholar]
- Vestita M, Filoni A, Bonamonte D, Elia R, Giudice G. Abstract: The Use of Nanofat in Androgenic Alopecia. a Prospective Blinded Study. Plast Reconstr Surg Glob Open. 2017;5(9S):90–90. doi: 10.1097.GOX.0000526293.77976.7f. [Google Scholar]
- Copcu HE, Oztan S. Not Stromal Vascular Fraction (SVF) or Nanofat, but Total Stromal-Cells (TOST): A New Definition. Systemic Review of Mechanical Stromal-Cell Extraction Techniques. Tissue Eng Regen Med. 2021;18(1):25–36. doi: 10.1007/s13770-020-00313-0. doi: 10.1007/s13770-020-00313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee Y, Choe S, Lee W. Adverse Sexual Effects of Treatment with Finasteride or Dutasteride for Male Androgenetic Alopecia: A Systematic Review and Meta-analysis. Acta Dermato Venereologica. 2019;99(1):12–17. doi: 10.2340/00015555-3035. doi: 10.2340/00015555-3035. [DOI] [PubMed] [Google Scholar]
- Carreño-Orellana N, Moll-Manzur C, Carrasco-Zuber JE, et al. Efectos adversos de finasteride: mitos y realidades. Una revisión actualizada. Rev Med Chil. 2016;144(12):1584–90. doi: 10.4067/S0034-98872016001200010. doi: 10.4067/S0034-98872016001200010. [DOI] [PubMed] [Google Scholar]
- Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. 2019;13:2777–86. doi: 10.2147/DDDT.S214907. doi: 10.2147/DDDT.S214907. [DOI] [PMC free article] [PubMed] [Google Scholar]


