Abstract
Metastatic cancer is a heterogeneous entity, some of which could benefit from local consolidative radiotherapy (RT). Although randomized evidence is growing in support of using RT for oligometastatic disease, a highly active area of investigation relates to whether RT could benefit patients with polymetastatic disease. This article highlights the preclinical and clinical rationale for using RT for polymetastatic disease, proposes an exploratory framework for selecting patients best suited for these types of treatments, and briefly reviews potential challenges. The goal of this hypothesis-generating review is to address personalized multimodality systemic treatment for patients with metastatic cancer.
The rationale for utilizing high-dose RT is primarily for local control and immune activation in either oligometastatic or polymetastatic disease. However, the primary application of low-dose RT is to activate distinct antitumor immune pathways and modulate the tumor stroma in efforts to better facilitate T-cell infiltration. We explore clinical cases involving high- and low-dose RT to demonstrate the potential efficacy of such treatment.
We then group patients by extent of disease burden in order to implement high- and/or low-dose RT. Patients with low-volume disease may receive high-dose RT to all sites as part of an oligometastatic paradigm. Subjects with high-volume disease (for whom standard of care remains palliative RT only) could be treated with a combination of high-dose RT to a few sites for immune activation, while receiving low-dose RT to several remaining lesions in order to enhance systemic responses from high-dose RT and immunotherapy. We further discuss how emerging but speculative concepts such as immune function may be integrated into this approach, and examine therapies currently under investigation that may help address immune deficiencies.
The review concludes by addressing challenges in using RT for polymetastatic disease, such as concerns over treatment planning workflows, treatment times, dose constraints for multiple-isocenter treatments, and economic considerations.
Keywords: Multi-site radiotherapy, immunotherapy, cell therapy, personalized medicine, low-dose radiotherapy, immune function
INTRODUCTION
Radiation therapy (RT) is one of the most effective means of providing local tumor control in oncology. Ongoing technologic improvements in radiation planning and delivery have the potential to extend RT as a means for systemic disease control.1 In combination with immunotherapy, the same RT used for local control can potentially convert tumors into a form of in-situ vaccines, as T cells activated by RT can exert effects beyond local disease to enhance systemic control of disease.2 Moreover, advances in radiation planning and delivery facilitate more efficient treatment planning, improved dosimetry, and faster delivery, enabling the simultaneous treatment of multiple lesions and sites.3 As the boundaries begin to blur between using RT for local and systemic disease control, clinical strategies are needed to define the appropriate use of systemic RT, identifying which patients, histologies, and metastatic sites are best suited for safe and effective treatment with multi-site RT. As RT becomes increasingly used for systemic as well as local disease control, the principles of personalized medicine should guide the choice of treatment options for metastatic cancer.
Herein, we propose an approach for the use of multi-site RT based on disease burden and endogenous immune function. The suggested approach reflects our experience to date with multi-site RT to treat disseminated disease, including several clinical trials examining abscopal (out-of-field radiation) effects in patients with metastatic disease.4–6 In this hypothesis-generating article, we explore the role of personalized multi-site RT, in conjunction with immunomodulation, as a means of systemic treatment for disseminated malignancies.
RADIOTHERAPY FOR LOCAL CONTROL AND IMMUNE ACTIVATION
Stereotactic body RT (SBRT, also known as stereotactic ablative therapy [SABR]) is increasingly being used to deliver highly-targeted, ablative doses in fewer fractions and with comparable toxic effects relative to conventionally fractionated RT.7 Over the past decade, SBRT, which was originally developed to treat earlier stages of cancer, has revolutionized the treatment of oligometastatic disease, with high rates of local control and significant benefits in terms of overall survival in several randomized phase II trials.8,9 The advent of targeted agents and immunotherapy has also expanded the treatment options for metastatic cancer patients, with patients living longer and deriving durable benefits from local therapies. Response to immunotherapy given as monotherapy, however, remains low.10,11 Early clinical findings suggest that the addition of radiation, particularly SBRT, has the potential to improve responses to immune checkpoint inhibitors (ICPIs).5,12,136 Irradiation of local tumor sites can also upregulate checkpoint receptors such as PD-L1, thereby enhancing the function of ICPIs.14 Radiation can activate CD8+ T cells by causing the release of tumor antigens, damage-associated molecular patterns (DAMPS), and cytokines.15 The proliferation of antitumor CD8+ T cells, in conjunction with upregulation of PD-L1, enables a more robust response to ICPIs.
Analysis of the PACIFIC trial showed that administering the anti-PD-L1 agent durvalumab to patients who had previously received chemoradiotherapy for stage III non-small cell lung cancer led to significantly improved overall survival, regardless of PD-L1 status.16 In this sequential-therapy approach, radiation may contribute to control of macroscopic sites of disease while T cells and chemotherapy address sites of microscopic, subclinical disease.17 That radiation can improve T-cell priming may explain in part why the PACIFIC trial showed favorable results for patients with either low or high PD-L1 expression.18,19
A significant challenge to achieving systemic responses relates to the entry of activated T cells into unirradiated metastatic sites. As discussed below, one major contributor to this challenge is the inhibitory nature of the tumor microenvironment.
OVERCOMING THE INHIBITORY EFFECTS OF THE TUMOR MICROENVIRONMENT
Tumor Stroma
The tumor-associated stroma is a physical barrier consisting of extracellular matrix and various cells including fibroblasts, regulatory T cells (Tregs), myeloid-derived suppressor cells, and tumor-associated macrophages.20 The stroma remains a significant obstacle in eliciting systemic responses to therapy. Stromal cells create an immunosuppressive environment by secreting inhibitory cytokines and recruiting Tregs.21 The stroma can be directly toxic to T cells, blunting their activity and causing anergy.22,23 The stroma also impedes contact between T cells and cancer cells by forming a physical barrier around tumors and by secreting inhibitory chemokines that prevent T-cell infiltration.24,25 Averting T cell infiltration by the stroma enables cancer cells to continue proliferating despite immune activation. Indeed, the extent of lymphocyte invasion into the tumor microenvironment is essential in eliciting antitumor responses. Meta-analyses of retrospective studies exploring the role of tumor-infiltrating lymphocytes (TILs) show a positive correlation between the presence TILs and overall survival.26,27 Overcoming the immunosuppressive activity of the tumor stroma is important for achieving systemic responses and is one mechanism by which RT contributes to systemic disease control.
Low-Dose Radiotherapy
Several potential benefits have been proposed in favor of using low-dose RT to modulate the tumor stroma. The first such benefit is that low-dose RT kills relatively few lymphocytes. Lymphocytes are required for antitumor immune responses, and lymphopenia is a negative prognostic factor across several types of cancers.28–30 Various RT dose and fractionation schedules affect the host immune system differentially; low doses are less likely to be destructive to lymphocytes than are high doses.31,32 Macrophages and Tregs, on the other hand, are more resistant to radiation.33 High doses of radiation can cause fibrosis and the increased secretion of TGF-β, which recruits Tregs to tumors.34 Low doses of radiation, however, seem to have the opposite effect by reducing TGF-β,35 which has pleotropic effects on several inhibitory components. Once it is decreased, it creates a ripple effect on several immunosuppressive cell types such as Tregs, MDSCs, M2 macrophages, and cancer associated fibroblasts.36 Depletion of Tregs has been shown to result in improved sensitivity of tumors to radiation and enhanced regression.37 Low doses of radiation may also induce cytokines that have a positive influence on the percentages and activation status of antitumor effector immune cells. For example, delivering whole-body radiation to rodents was shown to increase the number of splenocytes and to boost immunity,38–40 whereas a localized 2-Gy dose polarized macrophages into the proinflammatory, antitumoral iNOS+ M1 phenotype.41
Another study conducted in an immunocompetent murine model of Lewis lung carcinoma (LLC), demonstrated that the antitumor immunity induced by low-dose RT was attributable to the infiltration of activated NK and T cells, through Th2 to Th1 cytokine polarization.42 In a CT26 colon carcinoma model, low-dose RT of 2Gy x 5 has been shown to enhance the outcomes of anti-PD1 checkpoint inhibitor by expanding both tumor-resident T-cells and promoting the recruitment of new effector T-cells from circulation.43 Moreover, researchers have shown that in the 3LL murine lung and 4T1 breast models, delivering 0.5Gy x 4 low-dose RT to primary tumors after a single high-dose of ablative RT helped to slow tumor growth, reduced Tregs, and suppressed pulmonary metastasis.44
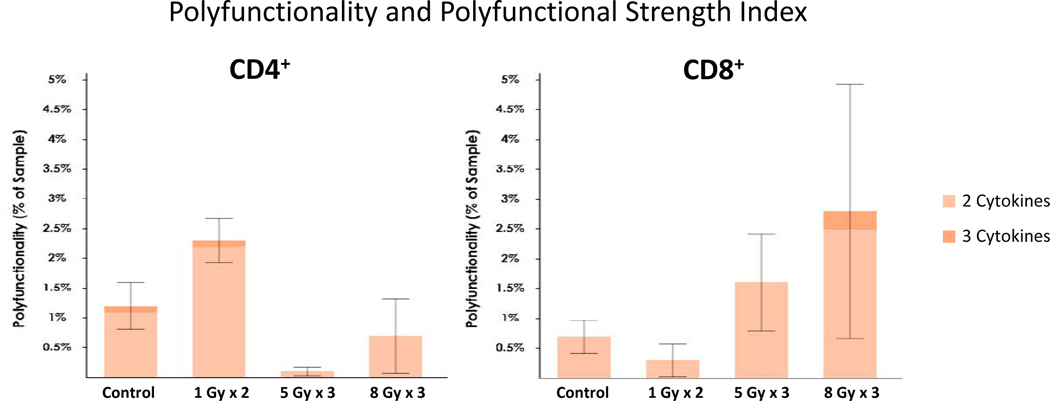
Our group has been exploring the use of low-dose radiation in preclinical models and in early clinical studies as a means of overcoming the inhibitory stroma and promoting systemic responses. In one study of patients with disease progression on immunotherapy, lesions receiving low-dose RT (the majority of which was a result of scatter from high-dose treated lesions) or no RT to distant tumor sites led to response rates of 58%, compared to 18% for lesions at distant tumor sites receiving no dose21 In this study, RT planning techniques included both SBRT and intensity modulated radiotherapy (IMRT), which demonstrated variable dose fall-off geometry with scatter lesions receiving low doses in the range of 1–20 Gy. To study the mechanisms involved in this low-dose effect, we transplanted 344SQ lung adenocarcinoma tumors into 129Sv/Ev mice and irradiated the tumors with low (1 Gy × 2), intermediate (5 Gy x 3), and high (12 Gy x 3) doses of radiation and measured the functionality of CD4+ and CD8+ T cells isolated from tumor tissue with magnetic beads. The 5-Gy and 12-Gy doses initially eliminated most of the local immune cells, and 7 days was needed for the T cells to replenish themselves before another TIL harvest. Using Isoplexis single-cell analysis technology, we assessed the polyfunctional strength index (PSI) of the isolated T cells after treatment with either high or low-dose radiation. PSI is a measure of the percentage of individual cells that secrete two or more effector or regulatory soluble factors, multiplied by the intensity of such factors and has been validated in the immunotherapy setting as a potential predictor of response.45,46 The cells were stimulated in vitro with CD3/CD28 overnight to compare the levels of various cytokines and chemokines produced and to assess the activation status on a single-cell level. The radiation dose used had a quite different effect on the observed immune outcomes. High-dose radiation increased the PSI of effector CD8+ T cells through the production of IFN-γ, Granzyme B and MIP-1α. Low-dose radiation on the other hand favored the polyfunctional stimulation of CD4+ T cells along with upregulation in the activation marker CD137/4–1BB (Figure 1). The primary implication is that high-dose radiation is important for cytotoxic T-cell priming and acquisition of killer effector functions, while low-dose radiation is important to activate and stimulate helper T cells which in turn augment CD8+ T cells and help generate immune memory.
Figure 1.
Average single-cell polyfunctional activated topology principal component analysis (PAT-PCA). Polyfunctionality is defined as 2+ cytokines secreted per cell. PSI, or Polyfunctional Strength Index, is defined as the percentage of polyfunctional cells in the sample, multiplied by the intensities of the secreted cytokines.
The second important function of low-dose RT is in modulating the stroma within neoplastic lesions. CD8+ T cells primed after high-dose radiation can address disseminated tumor cells. However, low-dose irradiation is being tested as a way to augment infiltration of the otherwise hostile tumor stroma. The reduction in TGF-β from low-dose RT reduces the numbers of Tregs in the tumor microenvironment and also seems to address many other immunosuppressive effects of the stroma.35 Moreover, as noted above, stimulating CD4 T cells with low-dose RT can contribute further to the immune response. Indeed, the rationale for using low-dose RT is not necessarily to ablate or kill the tumor but rather to activate the immune system such that it targets these lesions.
Clinically, low-dose RT has several advantages over high-dose RT. First, low doses are much safer in terms of their potential for damaging normal tissues, which would make meeting dose-limiting normal tissue constraints easier if several lesions (isocenters) within that organ are to be treated with SBRT. As such, treating larger volumes such as whole organs with one isocenter (e.g. clinical liver example in the “Low-Dose Radiotherapy Clinical Cases” section) with low-dose RT might be more dosimetrically feasible in addressing microscopic disease than treating individual lesions with SBRT (each with its own isocenter). Our clinical experience suggests that the addition of low-dose radiation has limited impact on lymphocyte counts, although this data is yet to be reported from prospective clinical trials. Second, low-dose RT also seems to be safer for treating patients with previously irradiated tumors, with minimal concern for exceeding normal tissue dose-constraints in the setting of reirradiation. Finally, low-dose RT can be delivered by means of simple 3-dimensional techniques, which could be adapted more easily by treatment centers lacking the specialized imaging or gating capabilities needed for SBRT.
Another safety issue involves the need for combination therapy (e.g., combinatorial immunotherapy agents), as the use of single agents often leads to the development of treatment resistance. While combinations of systemic agents such as anti-CTLA4 and anti-PD-1 have proven to be quite toxic,47 a recent phase I study has demonstrated that high-dose radiation can be safely delivered in patients receiving dual checkpoint inhibition with comparable rates of toxicity.48 Our clinical experience of administering low-dose RT in patients who progressed on combined anti-CTLA4 and anti-PD-1 has also demonstrated safe and tolerable treatment (data under review). While long-term implications of low-dose treatments and the use of radiation in dual checkpoint immunotherapy-naive patients are yet to be evaluated, low-dose RT may represent a safe and effective adjunct to combined systemic regimens in the future.
Although the exact mechanisms underlying acquired resistance to immunotherapy are unclear, poor T-cell function or stromal immunosuppressive activity may both be involved. We have developed a novel technique, named RadScopal™, which combines both high- and low-dose RT to elicit improved systemic responses. With this combination of high- and low-dose RT, we can potentially address two potential mechanisms of resistance simultaneously: high-dose RT stimulates T-cell priming, and low-dose RT modulates the inhibitory stroma, enabling immune cells to enter the tumor environment and elicit an antitumor response.
LOW-DOSE RADIOTHERAPY CLINICAL CASES
In our ongoing phase II clinical trial of high-dose RT with or without low-dose RT in patients progressing on immunotherapy (NCT[XXX BLINDED]), we have now treated over thirty patients with low-doses ranging up to 10 Gy in <2 Gy fractions (most commonly 7 Gy in 5 fractions). Patients continued receiving the same immunotherapy agent (most commonly anti-PD-1) after receiving RT, as it is impossible to independently evaluate the effect of RT on progression if the immunotherapy agent is switched. Preliminary data of lesion-specific responses to low-dose RT (as per immune-related response criteria) have shown response rates of >40%. To offer illustrative examples of the contexts in which low-dose RT may be applicable, brief cases are described below.
1. Low-dose RT can be effective for treating large volume disease
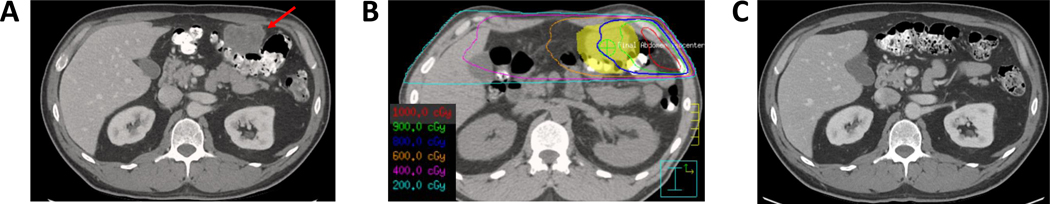
A patient with HPV-positive oropharyngeal squamous cell carcinoma six months status post concurrent chemoradiation developed biopsy-proven metastases in their lung, liver, and peritoneum. The patient was treated with pembrolizumab with initial response, followed by progression in the bilateral lung, mediastinum, and peritoneum (Figure 2a) 10 months after starting treatment. The patient then received 50 Gy in 4 fractions to a 1.9 cm left upper lobe nodule and 6 Gy in 4 fractions to the 5.5 cm peritoneal implant (Figure 2b). Currently, 20 months after treatment, he has no evidence of disease, including a complete response in the large peritoneal implant (Figure 2c). No toxicities were reported.
Figure 2.
Low-dose RT to a 5.5 cm peritoneal lesion. A) CT with contrast before RT. B) Low-dose RT treatment plan (axial). C) CT with contrast after RT.
2. Low-dose RT to large areas of tumor burden can be safe and achieve significant responses
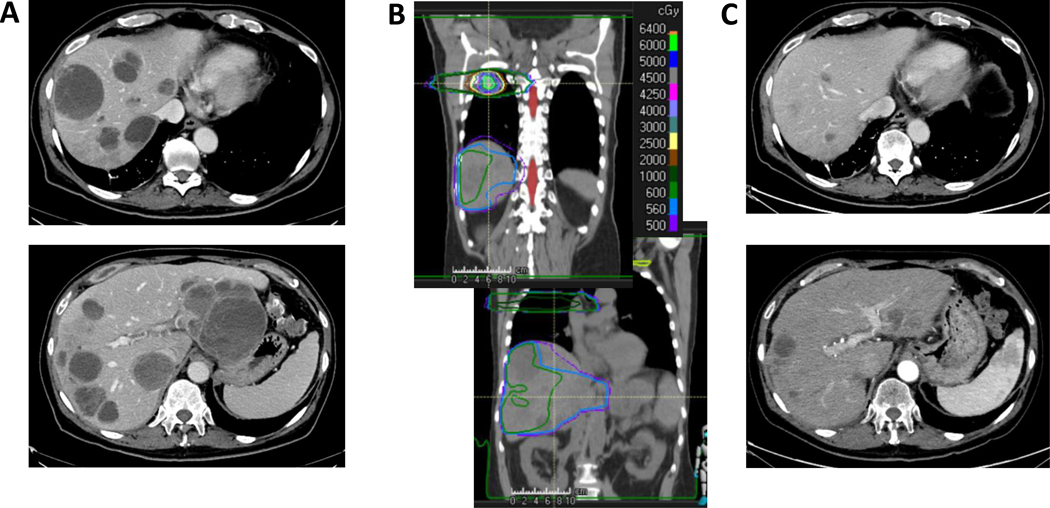
A patient with melanoma metastases in the liver, lung, bone, and brain that progressed over the course of four years on numerous chemo, targeted, and immune therapies, yttrium-90 radioablation to two liver lobes, and T-cell therapy presented to our clinic. Three months after T-cell infusion and one month after resuming combined ipilimumab with nivolumab the patient continued to progress (Figure 3a). He was then treated with 50 Gy in 4 fractions to a lung lesion and 5.6 Gy in 4 fractions to nearly the entire liver (Figure 3b). Given the extent of disease burden (largest liver lesion measuring 9.5 cm) and the risk for radiation-induced hepatopathy, a small portion of the inferior liver was spared, and one fraction was truncated (1.4 Gy x 4 instead of the planned 5). Four months after treatment, the patient had a confirmed partial response in the liver with an 83% reduction in tumor burden. Systemically, this patient also remained stable or responded in non-irradiated regions of the lung. Interestingly, this patient experienced grade 3 lymphopenia and no other grade 2+ toxicities (Common Terminology Criteria for Adverse Events v5.0). ALC decreased from 1.19x103 to 0.46x103/μL after treatment but returned to baseline levels within one month. Liver enzymes increased after RT (AST 66→75 U/L; ALT 74→95 U/L), but returned to lower than baseline levels by 4 months after treatment (AST 42 U/L, ALT 27 U/L). Although the patient did not have follow-up coagulation tests or albumin measurements, the platelets decreased after RT (63x103/uL→33x103/uL), but improved by 4 months after treatment (95x103/uL) demonstrating that synthetic function of the liver may have slightly improved. Whether this patient responded due to the cell therapy three months prior, the low-dose RT with dual checkpoint inhibition, or the combination of therapies is yet to be determined. That being said, low-dose RT can be safely administered to the whole liver. Our group has seen a similar response in one other patient treated to the whole liver prior to cell therapy and only experiencing grade 3 neurotoxicity resolving within two days of cell therapy administration.
Figure 3.
Low-dose RT to nearly the whole liver. A) CT with contrast before RT. B) Low-dose RT treatment plan (coronal). C) CT with contrast after RT.
3. Low dose RT can be effective in treating areas that need re-irradiation, especially for smaller sized lesions
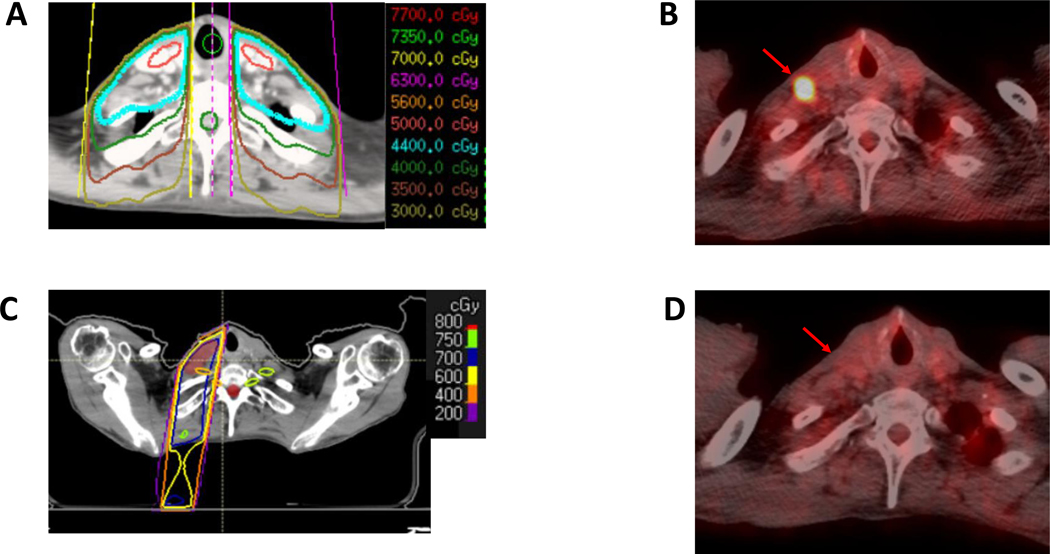
A patient with HPV-negative oropharyngeal squamous cell carcinoma isolated to the primary site underwent initial chemoradiation with 70 Gy in 35 fractions to the primary site and 44 Gy in 22 fractions to the bilateral neck (Figure 4a). A year later, restaging PET-CT demonstrated recurrence in the cervical nodes as well as a left lower lobe pulmonary nodule. He was started on pembrolizumab with complete response to therapy. Two years after response, he progressed in the cervical lymph nodes (largest lesions 1.0 and 1.5 cm in shortaxis) and in a right lower lobe pulmonary nodule (1.1 cm in long-axis), which were confirmed to be progressing on repeat scans (Figure 4b). He was treated with 50 Gy in 4 fractions to the lung and 7 Gy in 5 fractions to the cervical lymph nodes (Figure 4c) and continued on pembrolizumab. Currently, three months after treatment, he has had a complete response in all areas (Figure 4d). Although this patient had low disease burden, this patient was an excellent example of how low-dose can be used in areas sensitive to re-irradiation in patients progressing after response to immunotherapy.
Figure 4.
Low-dose RT to previously irradiated neck lymph nodes. A) Initial prophylactic neck irradiation treatment plan. B) PET-CT before RT. C) Low-dose RT treatment plan (axial). D) PET-CT after RT. Red arrow identifies site of cervical lymph node.
CHARACTERIZING IMMUNE FUNCTION
Historically, RT has been used for local control of certain malignancies and for eradicating localized microscopic disease. In addition to providing these benefits, multi-site RT has the potential to be used in conjunction with immunotherapies to achieve abscopal responses (i.e., to address systemic, disseminated disease49). Consequently, the relative strength of the immune system may be an important factor in determining the appropriate use of multi-site RT. Indeed, preclinical models have shown that mice with suppressed or depleted immune cells before tumor implantation show a lesser response to combined immunoradiotherapy than mice with intact immune systems.50 We propose that considering the following aspects of immune function can help to indicate which patients would experience benefit from multi-site RT.
Absolute lymphocyte count (ALC) can be readily obtained from peripheral blood samples and is relatively well standardized. ALC has been shown to predict clinical response to immunotherapy in several types of cancers.51–53 Our clinical experience also suggests that ALC is an independent predictor of abscopal responses and progression-free survival after immunoradiotherapy.54 In this study, patients with an ALC greater than 1,300/μL prior to RT, 560/μL after RT, or a decrease in ALC of less than 740/μL were associated with improved abscopal responses, although higher than median post-RT ALC was predictive of improved progression-free survival.54 Our clinical trial of patients with non-small cell lung cancer demonstrated better abscopal responses in patients receiving 50 Gy in 4 fractions of SBRT than in patients receiving 45 Gy in 15 fractions.55 A secondary analysis showed that this difference might reflect the effects of RT on the ALC, in that SBRT did not significantly decrease ALC, but moderately hypofractionated treatment did.53 This finding was likely related to larger margins required for, along with larger treatment volumes in, the latter. Because lymphopenia has been linked with worse prognosis after immunotherapy, presumably patients with low baseline ALC would benefit from treatments associated with minimal reduction in lymphocytes, or from therapies that account for weakened immune function (e.g., cell therapy).
There are several factors that relate to anti-tumor immunity. Characterizing T cell function and strength may offer unique ways of stratifying immune function. This can be done in several ways. First, cell surface markers like ICOS, GITR, OX40, 4–1BB, CD40L and CD44 can indicate T-cell activation.56–60 Markers of exhaustion, or attenuated T-cell effector function, include PD-1, Tim-3, Lag3, and TIGIT.61–64 Furthermore, the extent of proliferation after repeated or frequent activation by stimulatory molecules like CD3 and CD28 may offer insight into T-cell strength.65
Second, information on T-cell cytokine expression from single-cell polyfunctionality analysis may be another useful predictor of immune status. While the PSI score itself may offer insight into the polyfunctionality of T-cells and has been shown to correlate with response to immunotherapies,45,46 characterizing polyfunctional T cell populations by the amount and type of cytokines released matters (e.g. pro- vs anti-inflammatory, stimulatory vs inhibitory).66,67
Lastly, an interesting way of measuring T-cell “fitness”, or capability for expansion, is through assessing mitochondrial function. In chimeric antigen receptor (CAR) T cells, high mitochondrial biomass (measured by microscopy) correlates with T-cell fitness, and therefore proliferative capability.68 Other potential means of assessing mitochondrial function are by measuring peroxisome proliferated-activated receptor gamma, coactivator 1 alpha expression in T cells, which correlates with mitochondrial biogenesis and oxidative metabolism69 or by targeting key elements of the electron transport chain to assess oxygen consumption rate, which may approximate mitochondrial functional status.70 The several aforementioned measures for T-cell function are feasible to test in clinical settings, as T cells can readily be isolated from peripheral blood samples; however, this may add to costs as described in the subsequent “Feasibility and Challenges” section.
While prospectively testing these approximators of anti-tumor immunity is needed, they may offer future potential for quantifying the strength of an individual patient’s immune system. Further efforts will involve finding other factors that more accurately reflect T-cell fitness and can easily and inexpensively be attained in clinical settings. However, at this time a general index of a patient’s immune status (e.g. ALC) can be useful as a decision-making tool for the choice of single-site versus multi-site RT. However, before applying specific cutoffs when constructing randomized trials in these patients, validation is required; as a result, no single ALC cutoff will be suggested herein owing to the overly speculative nature of doing so.
HYPOTHESIS-GENERATING TREATMENT APPROACH
Our experience treating patients with multi-site RT to enhance systemic responses has led us to define a general approach for stratifying patients for such therapy. In this approach, patients are grouped according to extent of disease burden. We will then briefly discuss how more emerging notions such as immune function may be incorporated into this paradigm.
Extent of Disease
Patients classified as having low-volume or oligometastatic disease (although this term is continuing to be defined, currently it most often refers to 1–5 metastatic sites (with the vast majority of data referring to 1–3 lesions) after up-front systemic therapy)10,11 may be candidates for high-dose RT delivery to all affected areas for immune priming and local control. Such patients may derive further benefit from receiving RT combined with immunotherapy to enhance the ability of T cells to address microscopic disease throughout the body.
An important consideration in this population is the extent to which radiation doses are delivered. Radiation doses could be pushed at each site with the goal of achieving ablation while staying within organ-at-risk dose constraints (or some percentage of the dose constraint, e.g., 85%).76 Treatments intended to be “ablative” have classically been defined as a biologic effective dose (BED) in excess of 100 Gy. Whether this BED threshold also applies to the metastatic setting remains unknown, given the low quality and quantity of data.77,78 79
In patients with high-volume or polymetastatic disease, systemic therapies remain the standard of care with radiation administered only for palliation. It is less realistic to give high-dose RT to all lesions owing to the risk of toxicity, although this notion is being tested in randomized trials such as SABR-COMET10 (NCT03721341). However, those with a substantial disease burden could be considered for high-dose RT to one or a few larger lesions to stimulate immune priming, followed by low-dose RT to all other lesions (if feasible) for stromal modulation. Immune priming doses could range from 6–12 Gy per fraction to activate CD8 T cells71 and should remain below doses (e.g. 12–18 Gy) that would cause DNA degradation and potentially attenuate immunogenicity.72,73 Such patients would also receive immunotherapy to promote T-cell activity in tumor sites as well as other areas of the body.
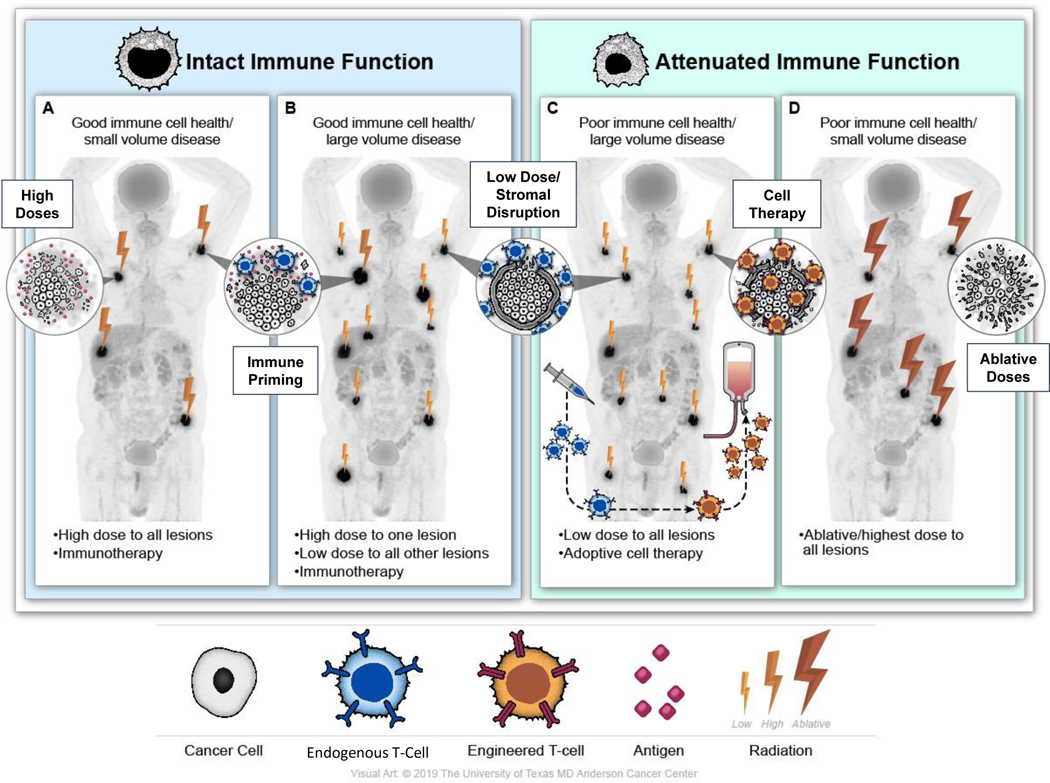
Immune function
The up-and-coming (yet still speculative) concept of immune function could also be used to inform treatment (Figure 5). Harboring relatively stronger endogenous immune function (the particular definition of which remains unvalidated to date as mentioned above) may be more compatible with immune priming doses as opposed to more ablative RT in oligometastatic patients (Figure 5a). Conversely, because immunotherapy-related outcomes in patients with poor immune function may be worse51,52,54, and given that immune cell function plays a role in tumor response to radiation75, these patients may benefit from more ablative RT for tumor control (Figure 5d).
Figure 5.
Schematic illustration of a hypothesis-generating treatment approach in patients with metastatic disease, incorporating concepts of disease burden and immune function as well as therapeutic strategies under investigation.
The high- with low-dose RT approach in patients with polymetastatic disease has shown early promise, but it could be further refined by immune function. Patients with stronger endogenous immune function may benefit from this approach, as they have the immune functionality to clear disease (Figure 5b). Conversely, those with poor immune function may not. One modality currently being studied in polymetastatic solid tumors is cell therapy (Figure 5c). Unlike cell therapies for hematologic malignancies, the challenge with respect to using cell therapies in solid tumors is the entry of immune cells into the immunosuppressive tumor environment.81 As such, a treatment approach currently under investigation in prospective studies (NCT03132922, NCT[XXX BLINDED]) combines adoptive T-cell therapy with low-dose RT, the latter for the purpose of stromal modulation. For patients receiving CAR T cells as opposed to unmodified expanded endogenous T cells, high-dose RT should be limited to areas that require local control, as it would not be needed for immune priming. Essentially, patients without intact immune function, whether from prior therapy or T-cell dysfunction, may benefit from engineering and/or expansion of endogenous immune cells. Natural killer (NK) cell therapy may also be considered, as NK cells do not exclusively rely on tumor antigens and can intrinsically identify tumor cells via their native receptors.82 Moreover, low-dose RT activates NK cells, which may further contribute to the effectiveness of this type of combined therapy.83
FEASIBILITY AND CHALLENGES
At present, several limitations complicate bringing multi-site RT into widespread use. The first such limitation is the lack of knowledge of appropriate dose-volume histogram (DVH) (i.e., organ dose-volume) constraints when high RT doses are delivered to multiple isocenters. 91 Current DVH measurements and constraints provide great detail on the point doses to organs at risk, but in plans with multiple isocenters, scatter dose, and the low-dose cloud (i.e., volumes receiving 5 Gy) become significant issues. These low-dose volumes would be expected to affect circulating immune cells, particularly highly radiosensitive lymphocytes. A patient with good immune function before multi-site RT could experience a significant ALC drop depending on the isocenters being treated, which could lead to T cell depletion; thus, concepts such as total bone marrow dose will need to be defined. While toxicities related to low-dose irradiation of large tumor volumes is yet to be defined, prior studies examining lower-dose irradiation of large tumors volumes may offer insight. For example, conventionally fractionated whole liver irradiation (ranging from 17–30 Gy) is relatively safe with complication rates of less than 16%.92–94 Our understanding of the extent to which we can safely irradiate lesions is still somewhat limited, in that little information is available as to whether it is better to irradiate a few lesions, most lesions, or all lesions to prompt adequate release of antigens or TILs or to appropriately overcome the tumor stroma.
A second limitation pertains to workflow constraints. Creating and executing a radiation treatment plan for a single site can take hours to days. When three or more sites with several isocenters are to be treated, even with low-dose RT (which limits organ-toxicity concerns), creating and executing treatment plans for several sites becomes even more time-consuming because of the need for repeated simulation, contouring, reviewing, and treatment as well as image review during treatment. Several tools and techniques are being developed for this purpose that can aid in creating a suitable workflow. Tools for auto-contouring, auto-planning, and auto-quality assurance substantially reduce the number of on-hands hours in treatment planning, especially when several sites are to be treated with low-dose RT.95–99 Indeed, bringing multi-site RT to areas with fewer resources further emphasizes the need for auto-planning tools. Automation, which can enable standardization of treatment parameters, doses, and dose constraints, can make this paradigm practical. Artificial-intelligence-based automation is increasingly being used in the RT setting 100. With the ability to train and refine algorithms over time such technology may offer significant aid to multi-site treatment planning.
A third limitation in the implementation of multi-site RT is treatment time. Treating 5–10 unique isocenters with high-dose RT or 10+ lesions with low-dose RT is currently not feasible, given the need for about 30 minutes for treatment set-up for each site when onboard imaging is being used; under these conditions, our current linear accelerators are limited to treating around 3–4 unique isocenters per day at most. Moreover, patients may not be able to maintain a particular position for extended periods. Further, for lesions that move with respiration or cardiac activity, the time spent “on the table” can be even longer due to use of gating or breath-hold approaches to limit lung and heart involvement in treatment fields. New technologies in development may allow more rapid treatment of multiple sites including linear accelerators that can treat multiple sites at once, which would reduce planning and treatment time as well as reducing the effects of target motion and uncertainty.
A final consideration in the widespread use of multi-site RT, with or without immunotherapy, is cost. US healthcare spending, particularly for oncologic care, remains disproportionally high relative to the total gross domestic product, and is only projected to increase.101 For example, the annual cost of new cancer medications (such as ICPIs) routinely exceeds $100,000 per patient.102–106 Regarding the proposed Group 3 patients, novel cellular therapies (such as CAR-T) are even more expensive, with prices of $375,000–475,000 per infusion itself plus $550,000 of associated care (including $50,000 per adverse event), resulting in total expenses of $1 million per patient.107–109 Furthermore, significant costs would be associated with patient stratification into personalized therapy classes based on immune function and tumor-specific antigen presentation.110,111
Nonetheless, and amidst the shift towards evaluating therapies based on healthcare value (defined as outcomes per cost), immunotherapy remains cost-effective for many malignancies.112–115 Similarly, CAR-T cellular therapies justify their value from both the healthcare and societal perspectives for hematologic malignancies,107,116,117 due to the extensive survival benefit relative to standard-of-care. These costs associated with treatment delivery are also expected to decrease with the reduction in admission lengths and treatment complications.108,118,119 However, while these prior data offer hope, new models will need to be generated to clarify the cost-effectiveness of these novel treatment paradigms, incorporating data on clinical effectiveness and response durability upon long-term follow-up.
Multi-site RT could further improve the value of these systemic therapies by enhancing response and minimizing relapse through synergistic mechanisms. To put the previous figures into perspective, the cost of an SBRT course is roughly $15,000;120,121 and despite use in ≥30% of all cancer patients,122 RT accounts for <5% of the cumulative cost of oncologic care.123,124 Indeed, published analyses support the cost-effectiveness of consolidative RT for oligometastatic disease121,125 126,127 relative to expensive systemic therapies.121 Hypofractionated approaches, such as SBRT, further strengthen the high-value proposition of adjunctive multi-site RT to economically improve outcomes.120,121,126–128 Furthermore, as personnel expenses are the primary cost driver behind RT delivery,129–133 novel advancements in auto-contouring95,96 and auto-planning97–99 technologies will result in further cost savings with multi-site RT.
CONCLUSIONS
The goal of this hypothesis-generating review aims to address personalized multimodality systemic treatment for patients with metastatic cancer. Emerging clinical and preclinical evidence support the shift from using RT as only a means of local control to one of systemic disease control. Low dose radiation is a potentially meaningful application of RT that can overcome inhibitory stroma and contribute positive immunomodulatory effects, with minimal toxicity. Combined with immunotherapies, RT is integral to creating in-situ vaccines that harness patient immune systems to fight cancer. Patient immune status is an important stratifying factor for deciding whether or not to harness patient immune systems via immunoradiotherapy and/or use ablative RT for systemic control. This approach will evolve over the next few years and is likely to expand the benefits of radiation to many patients as new immunotherapy options become incorporated with radiation oncology. Oncology as a whole has undergone a rapid transformation over the past few decades with the incorporation of genomics and personalized medicine to the current focus on reinvigorating patient immune systems to clear cancer. Radiation oncology is poised to make a similar paradigm shift as advances in molecular immunology are incorporated with automation to bring RT into an era of personalized multimodality systemic treatment. Multi-site RT in combination with immunotherapy is possible but requires consideration of patient immune function, tumor biology, and workflow.
Acknowledgments:
The authors thank Christine Wogan, MS, of MD Anderson Cancer Center for reviewing and editing this manuscript and Jordan Pietz, MA, CMI, of MD Anderson Cancer Center for the illustration.
Funding:
This work was supported by the Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center; the Goodwin family research fund; the family of M. Adnan Hamed and the Orr Family Foundation to MD Anderson Cancer Center’s Thoracic Radiation Oncology program; and the MD Anderson Knowledge Gap award.
Outside of the submitted work, SGC reports personal fees from AstraZeneca; CT reports personal fees from RefleXion Medical, AstraZeneca, and Wolter Kluwer; JYC reports grants from Bristol-Meyers Squibb, personal fees from Varian and AstraZeneca, and other from Global Oncology One; PL reports grants, personal fees, and nonfinancial support from Viewray, AstraZXeneca, and Varian; PB reports other from Raysearch and Varian; SG reports grants from Bristol-Meyers Squibb, Astrazeneca, and Nanobiotix, as well as personal fees and other from Novocure; JWW reports grants from Bristol-Meyers Squibb, personal fees and other from Alpine Immune Sciences, Legion Healthcare Partners, Molecular Match, Nanorobotix, OncoResponse, and RefleXion Medical, grants and personal fees from Nanobiotics and Varian, and grants, personal fees, and other from Checkmate Pharmaceuticals.
Footnotes
Conflict of interest:
All other authors declare no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing:
Research data are stored in an institutional repository and will be shared upon reasonable request.
References:
- 1.Loi M, Desideri I, Greto D, et al. Radiotherapy in the age of cancer immunology: Current concepts and future developments. Critical reviews in oncology/hematology. 2017;112:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Cadena A, Cushman TR, Anderson C, Barsoumian HB, Welsh JW, Cortez MA. Radiation and Anti-Cancer Vaccines: A Winning Combination. Vaccines. 2018;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garibaldi C, Jereczek-Fossa BA, Marvaso G, et al. Recent advances in radiation oncology. Ecancermedicalscience. 2017;11:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.XXX [BLINDED]
- 5.XXX [BLINDED]
- 6.XXX [BLINDED]
- 7.Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. The Lancet Oncology. 2019;20(4):494–503. [DOI] [PubMed] [Google Scholar]
- 8.Gomez DR, Blumenschein GR Jr., Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. The Lancet Oncology. 2016;17(12):1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non–Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA oncology. 2018;4(1):e173501-e173501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 11.Zatloukal P, Heo D, Park K-S, Kang J, Butts C, Bradford D. Randomized phase II clinical trial comparing tremelimumab (CP-675, 206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2009;27. [Google Scholar]
- 12.Theelen W, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nature medicine. 2018;24(12):1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation. 2014;124(2):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine. 2018;379(24):2342–2350. [DOI] [PubMed] [Google Scholar]
- 17.Cushman TR, Gomez D, Kumar R, et al. Combining radiation plus immunotherapy to improve systemic immune response. Journal of thoracic disease. 2018;10(Suppl 3):S468–s479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agustoni F, Hirsch FR. PACIFIC trial: new perspectives for immunotherapy in lung cancer. Translational lung cancer research. 2018;7(Suppl 1):S19–s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Deng W, Li N, et al. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Frontiers in pharmacology. 2018;9:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon H, Ramapriyan R, Cushman TR, et al. Role of Radiation Therapy in Modulation of the Tumor Stroma and Microenvironment. Frontiers in immunology. 2019;10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon H, Chen D, Ramapriyan R, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. Journal for immunotherapy of cancer. 2019;7(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CH, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nature immunology. 2016;17(4):364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molon B, Cali B, Viola A. T Cells and Cancer: How Metabolism Shapes Immunity. Frontiers in immunology. 2016;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science (New York, NY). 2015;348(6230):74–80. [DOI] [PubMed] [Google Scholar]
- 25.Molon B, Ugel S, Del Pozzo F, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. The Journal of experimental medicine. 2011;208(10):1949–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Q, Chen N, Ge C, et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: a systematic review and meta-analysis. Oncoimmunology. 2019;8(7):1593806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang C, Hobbs B, Amer A, et al. Development of an Immune-Pathology Informed Radiomics Model for Non-Small Cell Lung Cancer. Scientific reports. 2018;8(1):1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild AT, Ye X, Ellsworth SG, et al. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. American journal of clinical oncology. 2015;38(3):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho O, Oh YT, Chun M, Noh OK, Hoe JS, Kim H. Minimum absolute lymphocyte count during radiotherapy as a new prognostic factor for nasopharyngeal cancer. Head & neck. 2016;38 Suppl 1:E1061–1067. [DOI] [PubMed] [Google Scholar]
- 30.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(16):5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janiak MK, Wincenciak M, Cheda A, Nowosielska EM, Calabrese EJ. Cancer immunotherapy: how low-level ionizing radiation can play a key role. Cancer immunology, immunotherapy : CII. 2017;66(7):819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falcke SE, Ruhle PF, Deloch L, Fietkau R, Frey B, Gaipl US. Clinically Relevant Radiation Exposure Differentially Impacts Forms of Cell Death in Human Cells of the Innate and Adaptive Immune System. International journal of molecular sciences. 2018;19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Sun X, Luo J, et al. Effects of radiation on T regulatory cells in normal states and cancer: mechanisms and clinical implications. American journal of cancer research. 2015;5(11):3276–3285. [PMC free article] [PubMed] [Google Scholar]
- 34.Kachikwu EL, Iwamoto KS, Liao Y-P, et al. Radiation enhances regulatory T cell representation. International journal of radiation oncology, biology, physics. 2011;81(4):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barsoumian HB, Younes AI, Ramapriyan R, et al. Low dose radiotherapy promotes immune-mediated anti-tumor responses. The Journal of Immunology. 2019;202(1 Supplement):136.111. [Google Scholar]
- 36.Jayaraman P, Parikh F, Newton JM, et al. TGF-β1 programmed myeloid-derived suppressor cells (MDSC) acquire immune-stimulating and tumor killing activity capable of rejecting established tumors in combination with radiotherapy. Oncoimmunology. 2018;7(10):e1490853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kachikwu EL, Iwamoto KS, Liao YP, et al. Radiation enhances regulatory T cell representation. International journal of radiation oncology, biology, physics. 2011;81(4):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashimoto S, Shirato H, Hosokawa M, et al. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiation research. 1999;151(6):717–724. [PubMed] [Google Scholar]
- 39.Kojima S, Matsumori S, Ishida H, Yamaoka K. Possible role of elevation of glutathione in the acquisition of enhanced proliferation of mouse splenocytes exposed to small-dose gammarays. International journal of radiation biology. 2000;76(12):1641–1647. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Zhou J, Wu M, et al. Low-Dose Total Body Irradiation Can Enhance Systemic Immune Related Response Induced by Hypo-Fractionated Radiation. Frontiers in immunology. 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer cell. 2013;24(5):589–602. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L, Zhang X, Li H, et al. Validating the pivotal role of the immune system in low-dose radiation-induced tumor inhibition in Lewis lung cancer-bearing mice. Cancer medicine. 2018;7(4):1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(18):5514–5526. [DOI] [PubMed] [Google Scholar]
- 44.Savage T, Pandey S, Guha C. Postablation Modulation after Single High-Dose Radiation Therapy Improves Tumor Control via Enhanced Immunomodulation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020;26(4):910–921. [DOI] [PubMed] [Google Scholar]
- 45.Parisi G, Saco JD, Salazar FB, et al. Persistence of adoptively transferred T cells with a kinetically engineered IL-2 receptor agonist. Nature communications. 2020;11(1):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu D, Paczkowski P, Mackay S, Ng C, Zhou J. Single-Cell Multiplexed Proteomics on the IsoLight Resolves Cellular Functional Heterogeneity to Reveal Clinical Responses of Cancer Patients to Immunotherapies. Methods in molecular biology (Clifton, NJ). 2020;2055:413–431. [DOI] [PubMed] [Google Scholar]
- 47.Khair DO, Bax HJ, Mele S, et al. Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma. Frontiers in immunology. 2019;10(453). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Postow MA, Knox SJ, Goldman DA, et al. A Prospective, Phase 1 Trial of Nivolumab, Ipilimumab, and Radiotherapy in Patients with Advanced Melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nature Reviews Clinical Oncology. 2019;16(2):123–135. [DOI] [PubMed] [Google Scholar]
- 50.Crittenden MR, Zebertavage L, Kramer G, et al. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Scientific reports. 2018;8(1):7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer. 2018;6(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soyano AE, Dholaria B, Marin-Acevedo JA, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung Cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer. 2018;6(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.XXX [BLINDED]
- 54.XXX [BLINDED]
- 55.XXX [BLINDED]
- 56.Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–266. [DOI] [PubMed] [Google Scholar]
- 57.Kanamaru F, Youngnak P, Hashiguchi M, et al. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. Journal of immunology (Baltimore, Md : 1950). 2004;172(12):7306–7314. [DOI] [PubMed] [Google Scholar]
- 58.Paterson DJ, Jefferies WA, Green JR, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24(12):1281–1290. [DOI] [PubMed] [Google Scholar]
- 59.Kim H-D, Park S, Jeong S, et al. 4–1BB Delineates Distinct Activation Status of Exhausted Tumor-Infiltrating CD8(+) T Cells in Hepatocellular Carcinoma. Hepatology. 2019: 10.1002/hep.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schumann J, Stanko K, Schliesser U, Appelt C, Sawitzki B. Differences in CD44 Surface Expression Levels and Function Discriminates IL-17 and IFN-γ Producing Helper T Cells. PloS one. 2015;10(7):e0132479-e0132479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. [DOI] [PubMed] [Google Scholar]
- 62.Golden-Mason L, Palmer BE, Kassam N, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. Journal of virology. 2009;83(18):9122–9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong Y, Li X, Zhang L, et al. CD4+ T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunology. 2019;20(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong Y, Zhu L, Schell TD, et al. T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8<sup>+</sup> T-Cell Exhaustion and Poor Clinical Outcome in AML Patients. Clinical Cancer Research. 2016;22(12):3057. [DOI] [PubMed] [Google Scholar]
- 65.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. Journal of immunological methods. 2003;275(1–2):251–255. [DOI] [PubMed] [Google Scholar]
- 66.Berraondo P, Sanmamed MF, Ochoa MC, et al. Cytokines in clinical cancer immunotherapy. British journal of cancer. 2019;120(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Groot R, Van Loenen MM, Guislain A, et al. Polyfunctional tumor-reactive T cells are effectively expanded from non-small cell lung cancers, and correlate with an immune-engaged T cell profile. Oncoimmunology. 2019;8(11):e1648170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jena B, Rushworth D, McNamara GT, Cooper LJ. Mitochondrial Biomass As a Measure of Fitness for T Cells Expressing Chimeric Antigen Receptors. Blood. 2015;126(23):3242–3242. [Google Scholar]
- 69.Scharping NE, Menk AV, Moreci RS, et al. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016;45(2):374–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bond ST, McEwen KA, Yoganantharajah P, Gibert Y. Live Metabolic Profile Analysis of Zebrafish Embryos Using a Seahorse XF 24 Extracellular Flux Analyzer. Methods in molecular biology (Clifton, NJ). 2018;1797:393–401. [DOI] [PubMed] [Google Scholar]
- 71.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(17):5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature communications. 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Durante M, Formenti SC. Radiation-Induced Chromosomal Aberrations and Immunotherapy: Micronuclei, Cytosolic DNA, and Interferon-Production Pathway. Frontiers in oncology. 2018;8:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89(5):1084–1091. [DOI] [PubMed] [Google Scholar]
- 75.Liang H, Deng L, Chmura S, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. Journal of immunology (Baltimore, Md : 1950). 2013;190(11):5874–5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salama JK, Chmura SJ, Mehta N, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(16):5255–5259. [DOI] [PubMed] [Google Scholar]
- 77.Foster CC, Sher DJ, Rusthoven CG, et al. Overall survival according to immunotherapy and radiation treatment for metastatic non-small-cell lung cancer: a National Cancer Database analysis. Radiation oncology (London, England). 2019;14(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kok END, Jansen EPM, Heeres BC, et al. High versus low dose Stereotactic Body Radiation Therapy for hepatic metastases. Clinical and translational radiation oncology. 2020;20:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park S, Urm S, Cho H. Analysis of biologically equivalent dose of stereotactic body radiotherapy for primary and metastatic lung tumors. Cancer research and treatment : official journal of Korean Cancer Association. 2014;46(4):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan Q, Nanduri A, Yang J, et al. Toward a planning scheme for emission guided radiation therapy (EGRT): FDG based tumor tracking in a metastatic breast cancer patient. Medical physics. 2013;40(8):081708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang BL, Qin DY, Mo ZM, et al. Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Science China Life sciences. 2016;59(4):340–348. [DOI] [PubMed] [Google Scholar]
- 82.Sotillo E, Barrett DM, Black KL, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer discovery. 2015;5(12):1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang G, Kong Q, Wang G, et al. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer biotherapy & radiopharmaceuticals. 2014;29(10):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pompos A, Durante M, Choy H. Heavy Ions in Cancer Therapy. JAMA oncology. 2016;2(12):1539–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazar AA, Schulte R, Faddegon B, Blakely EA, Roach M 3rd. Clinical trials involving carbon-ion radiation therapy and the path forward. Cancer. 2018;124(23):4467–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Au-Yeung BB, Smith GA, Mueller JL, et al. IL-2 Modulates the TCR Signaling Threshold for CD8 but Not CD4 T Cell Proliferation on a Single-Cell Level. Journal of immunology (Baltimore, Md : 1950). 2017;198(6):2445–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. Journal of immunology (Baltimore, Md : 1950). 2011;187(3):1157–1165. [DOI] [PubMed] [Google Scholar]
- 88.Strauss J, Heery CR, Kim JW, et al. First-in-Human Phase I Trial of a Tumor-Targeted Cytokine (NHS-IL12) in Subjects with Metastatic Solid Tumors. Clinical Cancer Research. 2019;25(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang C, Zhang J, Niu J, Zhang J, Tian Z. Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine. 2008;42(1):128–136. [DOI] [PubMed] [Google Scholar]
- 90.Conlon KC, Potter EL, Pittaluga S, et al. IL15 by Continuous Intravenous Infusion to Adult Patients with Solid Tumors in a Phase I Trial Induced Dramatic NK-Cell Subset Expansion. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(16):4945–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milano MT, Mihai A, Kang J, et al. Stereotactic body radiotherapy in patients with multiple lung tumors: a focus on lung dosimetric constraints. Expert review of anticancer therapy. 2019;19(11):959–969. [DOI] [PubMed] [Google Scholar]
- 92.Greco C, Catalano G, Di Grazia A, Orecchia R. Radiotherapy of liver malignancies. From whole liver irradiation to stereotactic hypofractionated radiotherapy. Tumori. 2004;90(1):73–79. [DOI] [PubMed] [Google Scholar]
- 93.Borgelt BB, Gelber R, Brady LW, Griffin T, Hendrickson FR. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. International journal of radiation oncology, biology, physics. 1981;7(5):587–591. [DOI] [PubMed] [Google Scholar]
- 94.Edyta WR, Jakub L, Jerzy W. Whole Liver Palliative Radiotherapy for Patients with Massive Liver Metastases. Asian Pacific journal of cancer prevention : APJCP. 2015;16(15):6381–6384. [DOI] [PubMed] [Google Scholar]
- 95.Shao Y, Wang H, Chen H, et al. Dosimetric comparison and biological evaluation of PET- and CT-based target delineation for LA-NSCLC using auto-planning. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics (AIFB). 2019;67:77–84. [DOI] [PubMed] [Google Scholar]
- 96.Zhou R, Liao Z, Pan T, et al. Cardiac atlas development and validation for automatic segmentation of cardiac substructures. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2017;122(1):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gallio E, Giglioli FR, Girardi A, et al. Evaluation of a commercial automatic treatment planning system for liver stereotactic body radiation therapy treatments. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics (AIFB). 2018;46:153–159. [DOI] [PubMed] [Google Scholar]
- 98.Krayenbuehl J, Zamburlini M, Ghandour S, et al. Planning comparison of five automated treatment planning solutions for locally advanced head and neck cancer. Radiation oncology (London, England). 2018;13(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marrazzo L, Meattini I, Arilli C, et al. Auto-planning for VMAT accelerated partial breast irradiation. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2019;132:85–92. [DOI] [PubMed] [Google Scholar]
- 100.Thompson RF, Valdes G, Fuller CD, et al. Artificial intelligence in radiation oncology: A specialty-wide disruptive transformation? Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2018;129(3):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. Journal of the National Cancer Institute. 2011;103(2):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mailankody S, Prasad V. Five Years of Cancer Drug Approvals: Innovation, Efficacy, and Costs. JAMA oncology. 2015;1(4):539–540. [DOI] [PubMed] [Google Scholar]
- 103.Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the Market for Anticancer Drugs. The journal of economic perspectives : a journal of the American Economic Association. 2015;29(1):139–162. [DOI] [PubMed] [Google Scholar]
- 104.Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum Pretium--the just price. J Clin Oncol. 2013;31(28):3600–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saltz LB. Perspectives on Cost and Value in Cancer Care. JAMA oncology. 2016;2(1):1921. [DOI] [PubMed] [Google Scholar]
- 106.Chang CL, Schabert VF, Munakata J, et al. Comparative healthcare costs in patients with metastatic melanoma in the USA. Melanoma research. 2015;25(4):312–320. [DOI] [PubMed] [Google Scholar]
- 107.Fiorenza S, Ritchie DS, Ramsey SD, Turtle CJ, Roth JA. Value and affordability of CAR T-cell therapy in the United States. Bone marrow transplantation. 2020. [DOI] [PubMed] [Google Scholar]
- 108.Zhu F, Wei G, Zhang M, et al. Factors Associated with Costs in Chimeric Antigen Receptor T-Cell Therapy for Patients with Relapsed/Refractory B-Cell Malignancies. Cell transplantation. 2020;29:963689720919434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin JK, Muffly LS, Spinner MA, Barnes JI, Owens DK, Goldhaber-Fiebert JD. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Multiply Relapsed or Refractory Adult Large B-Cell Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019;37(24):2105–2119. [DOI] [PubMed] [Google Scholar]
- 110.Endris V, Buchhalter I, Allgäuer M, et al. Measurement of tumor mutational burden (TMB) in routine molecular diagnostics: in silico and real-life analysis of three larger gene panels. International journal of cancer. 2019;144(9):2303–2312. [DOI] [PubMed] [Google Scholar]
- 111.Sun Y, Yu W, Guan W, et al. Integrated assessment of PD-L1 expression and molecular classification facilitates therapy selection and prognosis prediction in gastric cancer. Cancer management and research. 2019;11:6397–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang M, Lopes GL, Insinga RP, et al. Cost-effectiveness of pembrolizumab versus chemotherapy as first-line treatment in PD-L1-positive advanced non-small-cell lung cancer in the USA. Immunotherapy. 2019;11(17):1463–1478. [DOI] [PubMed] [Google Scholar]
- 113.Huang M, Lou Y, Pellissier J, et al. Cost Effectiveness of Pembrolizumab vs. Standard-of-Care Chemotherapy as First-Line Treatment for Metastatic NSCLC that Expresses High Levels of PD-L1 in the United States. PharmacoEconomics. 2017;35(8):831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weng X, Luo S, Lin S, et al. Cost-utility analysis of pembrolizumab versus chemotherapy as first-line treatment for metastatic non-small cell lung cancer with different PD-L1 expression levels. Oncology research. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. Journal for immunotherapy of cancer. 2018;6(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thornton Snider J, Brauer M, Kee R, et al. The potential impact of CAR T-cell treatment delays on society. The American journal of managed care. 2019;25(8):379–386. [PubMed] [Google Scholar]
- 117.Thielen FW, van Dongen-Leunis A, Arons AMM, Ladestein JR, Hoogerbrugge PM, Uylde Groot CA. Cost-effectiveness of Anti-CD19 chimeric antigen receptor T-Cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. A societal view. European journal of haematology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lyman GH, Nguyen A, Snyder S, Gitlin M, Chung KC. Economic Evaluation of Chimeric Antigen Receptor T-Cell Therapy by Site of Care Among Patients With Relapsed or Refractory Large B-Cell Lymphoma. JAMA network open. 2020;3(4):e202072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith D, Heathman TRJ, Klarer A, LeBlon C, Tada Y, Hampson B. Towards Automated Manufacturing for Cell Therapies. Current hematologic malignancy reports. 2019;14(4):278–285. [DOI] [PubMed] [Google Scholar]
- 120.Shah A, Hahn SM, Stetson RL, Friedberg JS, Pechet TT, Sher DJ. Cost-effectiveness of stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. Cancer. 2013;119(17):3123–3132. [DOI] [PubMed] [Google Scholar]
- 121.Lester-Coll NH, Rutter CE, Bledsoe TJ, Goldberg SB, Decker RH, Yu JB. Cost-Effectiveness of Surgery, Stereotactic Body Radiation Therapy, and Systemic Therapy for Pulmonary Oligometastases. International journal of radiation oncology, biology, physics. 2016;95(2):663–672. [DOI] [PubMed] [Google Scholar]
- 122.Royce TJ, Qureshi MM, Truong MT. Radiotherapy Utilization and Fractionation Patterns During the First Course of Cancer Treatment in the United States From 2004 to 2014. Journal of the American College of Radiology : JACR. 2018;15(11):1558–1564. [DOI] [PubMed] [Google Scholar]
- 123.Milliman. A multi-year look at the cost burden of cancer care - Milliman Insight.: Milliman;2017. [Google Scholar]
- 124.Milliman. Cost drivers of cancer care: A retrospective analysis of Medicare and commercially insured population claim data 2004–2014 - Milliman Insight.: Milliman;2016. [Google Scholar]
- 125.Panje CM, Dedes KJ, Matter-Walstra K, et al. A cost-effectiveness analysis of consolidative local therapy in oligometastatic non-squamous non-small cell lung cancer (NSCLC). Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2018;129(2):257–263. [DOI] [PubMed] [Google Scholar]
- 126.Bijlani A, Aguzzi G, Schaal DW, Romanelli P. Stereotactic radiosurgery and stereotactic body radiation therapy cost-effectiveness results. Frontiers in oncology. 2013;3:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lester-Coll NH, Sher DJ. Cost-Effectiveness of Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy: a Critical Review. Current oncology reports. 2017;19(6):41. [DOI] [PubMed] [Google Scholar]
- 128.Boyce-Fappiano D, Ning MS, Thaker NG, et al. Time-Driven Activity-Based Cost Analysis of Radiation Treatment Options for Spinal Metastases. Journal of oncology practice. 2019:Jop1900480. [DOI] [PubMed] [Google Scholar]
- 129.Su L, Dutta SW, Sanders JC, et al. Time-driven activity-based costing of adjuvant vaginal cuff brachytherapy for uterine cancer in an integrated brachytherapy suite. Brachytherapy. 2019. [DOI] [PubMed] [Google Scholar]
- 130.Ning MS, Klopp AH, Jhingran A, et al. Quantifying institutional resource utilization of adjuvant brachytherapy and intensity-modulated radiation therapy for endometrial cancer via time-driven activity-based costing. Brachytherapy. 2019;18(4):445–452. [DOI] [PubMed] [Google Scholar]
- 131.Ning MS, Venkatesan AM, Stafford RJ, et al. Developing an intraoperative 3T MRI-guided brachytherapy program within a diagnostic imaging suite: Methods, process workflow, and value-based analysis. Brachytherapy. 2019. [DOI] [PubMed] [Google Scholar]
- 132.Ilg AM, Laviana AA, Kamrava M, et al. Time-driven activity-based costing of low-dose-rate and high-dose-rate brachytherapy for low-risk prostate cancer. Brachytherapy. 2016;15(6):760–767. [DOI] [PubMed] [Google Scholar]
- 133.Schutzer ME, Arthur DW, Anscher MS. Time-Driven Activity-Based Costing: A Comparative Cost Analysis of Whole-Breast Radiotherapy Versus Balloon-Based Brachytherapy in the Management of Early-Stage Breast Cancer. Journal of oncology practice. 2016;12(5):e584–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon reasonable request.