Abstract
Background
Rarer dementias include Huntington's disease (HD), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), frontotemporal dementia (FTD), dementia in multiple sclerosis (MS) and progressive supranuclear palsy (PSP). Cholinesterase inhibitors, including donepezil, galantamine and rivastigmine, are considered to be the first‐line medicines for Alzheimer's disease and some other dementias, such as dementia in Parkinson's disease. Cholinesterase inhibitors are hypothesised to work by inhibiting the enzyme acetylcholinesterase (AChE) which breaks down the neurotransmitter acetylcholine. Cholinesterase inhibitors may also lead to clinical improvement for rarer dementias associated with neurological conditions.
Objectives
To assess the efficacy and safety of cholinesterase inhibitors for cognitive impairment or dementia associated with neurological conditions.
Search methods
We searched the Cochrane Dementia and Cognitive Improvement Group's Specialised Register, CENTRAL, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS, several trial registries and grey literature sources in August 2013.
Selection criteria
We included randomised, double‐blind, controlled trials assessing the efficacy of treatment of rarer dementias associated with neurological conditions with currently marketed cholinesterase inhibitors.
Data collection and analysis
Two review authors independently assessed eligibility and quality of trials, and extracted data. We used the standard methodological procedures of the Cochrane Collaboration.
Main results
We included eight RCTs involving 567 participants. Six studies used a simple parallel‐group design; the other two consisted of an open‐label treatment period followed by a randomised phase. All trials were well concealed for allocation and double‐blind, however the sample sizes of most trials were small. All trials used placebo as control. We performed meta‐analyses for some outcomes in patients with MS. For all other conditions, results are presented narratively.
Two trials included patients with HD; one found that cholinesterase inhibitor use in the short‐term had no statistically significant impact on the cognitive portion of the Alzheimer Disease Assessment Scale (ADAS‐Cog; 1 study, WMD 1.00, 95% CI ‐1.66 to 3.66, P = 0.46; low quality evidence), Unified Huntington's Disease Rating Scale (UHDRS) Verbal Fluency Test (1 study, WMD ‐1.20, 95% CI ‐7.97 to 5.57, P = 0.73; low quality evidence), UHDRS Symbol Digit Modalities Test (SDMT; 1 study, WMD 2.70, 95% CI ‐0.95 to 6.35, P = 0.15; low quality evidence) and other psychometric tests. The other study found that cholinesterase inhibitor use in the medium‐term improved the results of the verbal fluency test (1 study, WMD 6.43, 95% CI 0.66 to 12.20, P = 0.03; moderate quality evidence) and California Verbal Learning Test ‐ Second Edition (CVLT‐II) Recognition Task (1 study, WMD 2.42, 95% CI 0.17 to 4.67, P = 0.04; moderate quality evidence). There was no statistically significant difference between groups on the SDMT (1 study, WMD ‐0.31, 95% CI ‐7.77 to 7.15, P = 0.94; moderate quality evidence), CVLT‐II trials 1‐5 (1 study, WMD ‐2.09, 95% CI ‐11.65 to 7.47, P = 0.67; moderate quality evidence), short‐delay recall (1 study, WMD 0.35, 95% CI ‐2.87 to 3.57, P = 0.83; moderate quality evidence), or long‐delay recall (1 study, WMD ‐0.14, 95% CI ‐3.08 to 2.80, P = 0.93; moderate quality evidence), and other psychometric tests.
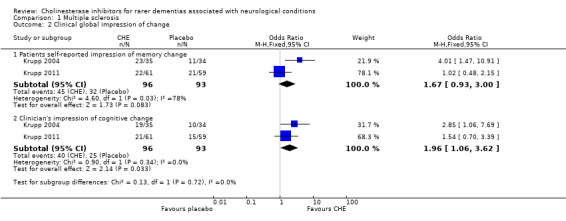
Four trials included patients with MS; one found no differences between the cholinesterase inhibitors (short‐term) and placebo groups on the Wechsler Memory Scales general memory score (1 study, WMD 0.90, 95% CI ‐0.52 to 2.32, P = 0.22; low quality evidence). The three other trials found that, in the medium‐term ‐ cholinesterase inhibitors improved the clinician's impression of cognitive change (2 studies, OR 1.96, 95% CI 1.06 to 3.62, P = 0.03; high quality evidence). However, the treatment effect on other aspects of cognitive change were unclear, measured by the Selective Reminding Test (3 studies, WMD 1.47, 95% CI ‐0.39 to 3.32, P = 0.12; high quality evidence), patient's self‐reported impression of memory change (2 studies, OR 1.67, 95% CI 0.93 to 3.00, P = 0.08; high quality evidence) and cognitive change (1 study, OR 0.95, 95% CI 0.45 to 1.98, P = 0.89; high quality evidence), clinician's impression of memory change (1 study, OR 1.50, 95% CI 0.59 to 3.84, P = 0.39; moderate quality evidence), other psychometric tests, and activities of daily living ‐ patient reported impact of multiple sclerosis activities (1 study, WMD ‐1.18, 95% CI ‐3.02 to 0.66, P = 0.21; low quality evidence).
One study on patients with CADASIL found a beneficial effect of cholinesterase inhibitors on the Executive interview, and Trail Making Test parts A and B. The impact of cholinesterase inhibitors on the Vascular ADAS‐Cog score (1 study, WMD 0.04, 95% CI ‐1.57 to 1.65, P = 0.96; high quality evidence), the Clinical Dementia Rating Scale Sum of Boxes (1 study, WMD ‐0.09, 95% CI ‐0.48 to 0.03, P = 0.65; high quality evidence) Disability Assessment for Dementia scale (1 study, WMD 0.58, 95% CI ‐2.72 to 3.88, P = 0.73; moderate quality evidence), and other measures was unclear
One study included patients with FTD. This trial consisted of an open‐label treatment period followed by a randomised, double‐blind, placebo‐controlled phase. No data of primary outcomes were reported in this study.
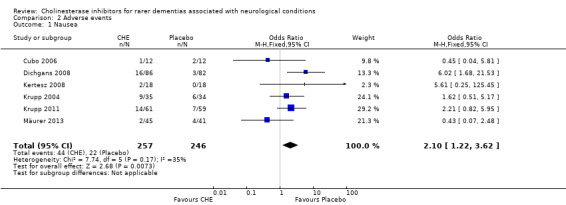
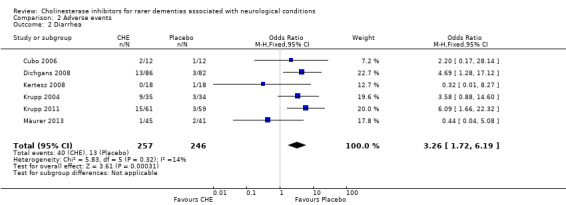
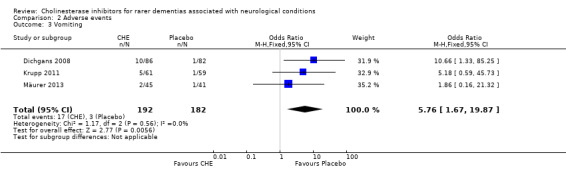
In the included studies, the most common side effect was gastrointestinal symptoms. For all conditions, compared to the treatment group, the placebo group experienced significantly less nausea (6 studies, 44/257 vs. 22/246, OR 2.10, 95% CI 1.22 to 3.62, P = 0.007; high quality evidence), diarrhoea (6 studies, 40/257 vs. 13/246, OR 3.26, 95% CI 1.72 to 6.19, P = 0.0003; moderate quality evidence) and vomiting (3 studies, 17/192 vs. 3/182, OR 5.76, 95% CI 1.67 to 19.87, P = 0.006; moderate quality evidence).
Authors' conclusions
The sample sizes of most included trials were small, and some of the results were extracted from only one study. There were no poolable data for HD, CADASIL and FTD patients and there were no results for patients with PSP. Current evidence shows that the efficacy on cognitive function and activities of daily living of cholinesterase inhibitors in people with HD, CADASIL, MS, PSP or FTD is unclear, although cholinesterase inhibitors are associated with more gastrointestinal side effects compared with placebo.
Plain language summary
[Cholinesterase inhibitors for rarer dementia associated with neurological conditions]
There are various rarer dementias including Huntington's disease (HD), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), frontotemporal dementia (FTD), dementia in multiple sclerosis (MS) and progressive supranuclear palsy (PSP). A group of chemicals known as cholinesterase inhibitors are considered to be the first‐line medicines for Alzheimer's disease and some other dementias. Cholinesterase inhibitors may also lead to clinical improvement for rarer dementias associated with neurological conditions.
We analysed eight randomised controlled trials including 567 participants, which all used a placebo as a control. The methodological quality of most included trials was moderate. Some of the results were extracted from only one study, and there were no results for patients with PSP identified. Furthermore, some studies had small numbers of participants. The beneficial effect of cholinesterase inhibitors on cognitive function was only observed on a few cognitive function tests for patients with HD, CADASIL or MS. Cholinesterase inhibitors had no significant impact on improving cognitive level, activities of daily living and quality of life in patients with these conditions. For all conditions, compared to the treatment group, the placebo group experienced significantly less gastrointestinal side effects (nausea, diarrhoea and vomiting). There is no evidence for the efficacy of cholinesterase inhibitors for these conditions.
Summary of findings
Summary of findings for the main comparison. Cholinersterase inhibitors for Huntington's disease (short‐term).
| Cholinersterase inhibitors for Huntington's disease (short‐term) | ||||||
| Patient or population: Huntington's disease Settings: Intervention: Cholinersterase inhibitors | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cholinersterase inhibitors | |||||
| Cognitive function ‐ the Cognitive portion of the Alzheimer Disease Assessment Scale ADAS‐Cog1 Follow‐up: 12 weeks | The mean cognitive function ‐ the cognitive portion of the ADAS in the control groups was ‐0.1 | The mean cognitive function ‐ the cognitive portion of the ADAS in the intervention groups was 1 higher (1.66 lower to 3.66 higher) | 24 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| Cognitive function ‐ the Unified Huntington's Disease Rating Scale‐Symbol Digit Modalities Test change UHDRS‐SDMT1 Follow‐up: 12 weeks | The mean cognitive function ‐ the UHDRS‐SDMT change in the control groups was ‐1.8 | The mean cognitive function ‐ the UHDRS‐SDMT change in the intervention groups was 2.7 higher (0.95 lower to 6.35 higher) | 24 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| Cognitive function ‐ the Unified Huntington's Disease Rating Scale‐Verbal Fluency Test change UHDRS‐FAS1 Follow‐up: 12 weeks | The mean cognitive function ‐ the UHDRS‐FAS change in the control groups was 3.8 | The mean cognitive function ‐ the UHDRS‐FAS change in the intervention groups was 1.2 lower (7.97 lower to 5.57 higher) | 24 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 An increase in scores indicates improvement 2 Downgraded by 1 point due to risk of bias: 20% of the participants were lost to follow up 3 Downgraded by 1 point due to imprecision: small sample size
Summary of findings 2. Cholinesterase inhibitors for Huntington's disease (medium‐term).
| Cholinesterase inhibitors for Huntington's disease (medium‐term) | ||||||
| Patient or population: Huntington's disease Settings: Intervention: Cholinesterase inhibitors | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cholinesterase inhibitors | |||||
| Cognitive function ‐ Verbal fluency test Verbal fluency test1 Follow‐up: 24 weeks | The mean cognitive function ‐ verbal fluency test in the control groups was ‐2.16 | The mean cognitive function ‐ verbal fluency test in the intervention groups was 6.43 higher (0.66 to 12.2 higher) | 17 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| Cognitive function ‐Symbol Digit Modalities Test SDMT1 Follow‐up: 24 weeks | The mean cognitive function ‐SDMT in the control groups was ‐3.5 | The mean cognitive function ‐ SDMT in the intervention groups was 0.31 lower (7.77 lower to 7.15 higher) | 17 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| Cognitive function ‐ California Verbal Learning Test‐II trials 1‐5 CVLT‐II trials 1‐51 Follow‐up: 24 weeks | The mean cognitive function ‐ CVLT‐II trials 1‐5 in the control groups was 5.83 | The mean cognitive function ‐ CVLT‐II trials 1‐5 in the intervention groups was 2.09 lower (11.65 lower to 7.47 higher) | 17 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| Cognitive function ‐ California Verbal Learning Test‐II short‐delay recall CVLT‐II SD recall1 Follow‐up: 24 weeks | The mean cognitive function ‐ CVLT‐II SD recall in the control groups was ‐0.17 | The mean cognitive function ‐ CVLT‐II SD recall in the intervention groups was 0.35 higher (2.87 lower to 3.57 higher) | 17 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| Cognitive function ‐ California Verbal Learning Test‐II long‐delay recall CVLT‐II LD recall1 Follow‐up: 24 weeks | The mean cognitive function ‐ CVLT‐II LD recall in the control groups was 0.5 | The mean cognitive function ‐ CVLT‐II LD recall in the intervention groups was 0.14 lower (3.08 lower to 2.8 higher) | 17 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| Cognitive function ‐ California Verbal Learning Test‐II recognition task CVLT‐II recognition task1 Follow‐up: 24 weeks | The mean cognitive function ‐ CVLT‐II recognition task in the control groups was ‐0.33 | The mean cognitive function ‐ CVLT‐II recognition task in the intervention groups was 2.42 higher (0.17 to 4.67 higher) | 17 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 An increase in scores indicates improvement 2 Downgraded by 1 point due to imprecision: small sample size
Summary of findings 3. Cholinesterase inhibitors for multiple sclerosis (short‐term).
| Cholinesterase inhibitors for multiple sclerosis (short‐term) | ||||||
| Patient or population: Multiple sclerosis Settings: Intervention: Cholinesterase inhibitors | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cholinesterase inhibitors | |||||
| Cognitive function ‐ Wechsler Memory Scales WMS‐general memory score1 Follow‐up: 12 weeks | The mean cognitive function ‐ WMS‐general memory score in the control groups was 4 | The mean cognitive function ‐ WMS‐general memory score in the intervention groups was 0.9 higher (0.52 lower to 2.32 higher) | 60 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 An increase in scores indicates improvement 2 Downgraded by 1 point due to risk of bias: using last outcome carried forward analysis 3 Downgraded by 1 point due to imprecision: small sample size
Summary of findings 4. Cholinesterase inhibitors for multiple sclerosis (medium‐term).
| Cholinesterase inhibitors for multiple sclerosis (medium‐term) | ||||||
| Patient or population: Multiple sclerosis Settings: Intervention: Cholinesterase inhibitors | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cholinesterase inhibitors | |||||
| Cognitive function ‐ Selective Reminding Test total SRT1 Follow‐up: 24 weeks | The mean cognitive function ‐ SRT in the control groups was ‐0.76 | The mean cognitive function ‐ SRT in the intervention groups was 1.47 higher (0.39 lower to 3.32 higher) | 270 (3 studies) | ⊕⊕⊕⊕ high | ||
| Clinical global impression of change ‐ patient's self‐reported impression of memory change Follow‐up: 24 weeks | 344 per 1000 | 467 per 1000 (328 to 611) | OR 1.67 (0.93 to 3) | 189 (2 studies) | ⊕⊕⊕⊕ high | |
| Clinical global impression of change ‐ patient's self‐reported impression of cognitive change Follow‐up: 24 weeks | 390 per 1000 | 378 per 1000 (223 to 558) | OR 0.95 (0.45 to 1.98) | 120 (1 study) | ⊕⊕⊕⊕ high | |
| Clinical global impression of change ‐ clinician's impression of memory change Follow‐up: 24 weeks | 237 per 1000 | 378 per 1000 (215 to 572) | OR 1.95 (0.88 to 4.3) | 120 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| Clinical global impression of change ‐ clinician's impression of cognitive change Follow‐up: 24 weeks | 269 per 1000 | 419 per 1000 (280 to 571) | OR 1.96 (1.06 to 3.62) | 189 (2 studies) | ⊕⊕⊕⊕ high | |
| Activities of daily living ‐ Patient reported impact of multiple sclerosis activities Patient reported impact of multiple sclerosis activities1 Follow‐up: 16 weeks | The mean Activities of daily living ‐ Patient reported impact of multiple sclerosis activities in the control groups was 0.55 |
The mean Activities of daily living ‐ Patient reported impact of multiple sclerosis activities in the intervention groups was 1.18 lower (3.02 lower to 0.66 higher) | 81 (1 study) | ⊕⊕⊝⊝ low3,4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 An increase in scores indicates improvement 2 Downgraged by 1 point due to imprecision: wide confidence intervals 3 Downgraded by 1 point due to risk of bias: carry‐over effects 4 Downgraded by 1 point due to imprecision: small sample size
Summary of findings 5. Cholinesterase inhibitors for CADASIL.
| Cholinesterase inhibitors for CADASIL | ||||||
| Patient or population: CADASIL Settings: Intervention: Cholinesterase inhibitors | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cholinesterase inhibitors | |||||
| Cognitive function ‐ the Cognitive portion of the Vascular Alzheimer Disease Assessment Scale change V‐ADAS‐Cog1 Follow‐up: 18 weeks | The mean cognitive function ‐ V‐ADAS‐Cog change in the control groups was 0.81 | The mean cognitive function ‐ V‐ADAS‐Cog change in the intervention groups was 0.04 higher (1.57 lower to 1.65 higher) | 161 (1 study) | ⊕⊕⊕⊕ high | ||
| Clinical global impression of change ‐ the Sum of Boxes of the Clinical Dementia Rating Scale CDR‐SB1 Follow‐up: 18 weeks | The mean clinical global impression of change ‐ CDR‐SB in the control groups was ‐0.1 | The mean clinical global impression of change ‐ CDR‐SB in the intervention groups was 0.09 lower (0.48 lower to 0.3 higher) | 161 (1 study) | ⊕⊕⊕⊕ high | ||
| Activities of daily living ‐ Disability Assessment for Dementia scale DAD1 Follow‐up: 18 weeks | The mean activities of daily living ‐ DAD in the control groups was 1.53 | The mean activities of daily living ‐ DAD in the intervention groups was 0.58 higher (2.27 lower to 3.88 higher) | 161 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 An increase in scores indicates improvement 2 downgraded by 1 point due to imprecision: wide confidence intervals
Summary of findings 6. Adverse events associated with the use of cholinesterase inhibitors for rarer dementias.
| Adverse events associated with the use of cholinesterase inhibitors for rarer dementias | ||||||
| Patient or population: Patients with neurological conditions associated with rarer dementias Settings: Hospital Intervention: Cholinesterase inhibitors | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Adverse event | |||||
| Nausea Follow‐up: 26 weeks | Study population | OR 2.1 (1.22 to 3.62) | 503 (6 studies) | ⊕⊕⊕⊕ high | ||
| 89 per 1000 | 171 per 1000 (107 to 262) | |||||
| Moderate | ||||||
| 108 per 1000 | 203 per 1000 (129 to 305) | |||||
| Diarrhea Follow‐up: 26 weeks | Study population | OR 3.26 (1.72 to 6.19) | 503 (6 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 53 per 1000 | 154 per 1000 (88 to 257) | |||||
| Moderate | ||||||
| 53 per 1000 | 154 per 1000 (88 to 257) | |||||
| Vomiting Follow‐up: 24 weeks | Study population | OR 5.76 (1.67 to 19.87) | 374 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 16 per 1000 | 88 per 1000 (27 to 250) | |||||
| Moderate | ||||||
| 17 per 1000 | 91 per 1000 (28 to 256) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraged by 1 point due to imprecision: wide confidence intervals
Summary of findings 7. Study funding/ Financial support.
| Study name | Funding name/Financial support resource |
| Cubo 2006 | Pfizer‐Eisai Inc. |
| Krupp 2004 | the National Institutes of Health (grant HD38107‐01); the National Institutes for Disability and Rehabilitation Research (grant H133G990058); the National Multiple Sclerosis Society (grant RG3042‐A‐2); the National Center for Research Resources (grant M01‐RR10710‐02). |
| Krupp 2011 | the National Institutes of Health (2 R01 HD38107); the National Center for Research Resources (M01 RR10710). |
| Mäurer 2013 | Novartis Pharma GmbH. |
| Dichgans 2008 | Eisai Medical Research (Ridgefield Park, NJ, USA). |
| Kertesz 2008 | Janssen‐Ortho Inc., Canada; Johnson & Johnson Pharmaceutical Research and Development. |
| Sešok 2014 | Pharmacy Brod, Ljubljana, Slovenia. |
Background
Description of the condition
Dementia is a chronic or progressive syndrome that results from diseases of the brain. Dementia is characterised by "disturbances of multiple higher cortical functions, including memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgment" (WHO 2010). There were estimated to be 36 million people with dementia in the world in 2010, and this is predicted to rise to 76 million in 2030, and to 135 million by 2050 (Martin 2013). Dementia due to Alzheimer's disease (AD) is the most common (Cacabelos 2008), accounting for 50% to 70% of the cases, followed by vascular dementia (30% to 40%) and mixed Alzheimer's/vascular cases (15% to 20%). Lewy body dementia (LBD) and Parkinson's disease dementia (PDD) together account for 5% to 10% of dementia cases (Mollenhauer 2010). There are also various rarer dementias due to other neurodegenerative disease which are a small percentage of dementias. These include frontotemporal dementia (FTD), Huntington's disease (HD), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), dementia in multiple sclerosis (MS) and progressive supranuclear palsy (PSP).
FTDs are group of disorders caused by progressive degeneration of the frontal or temporal lobes of the brain, characterised by personality changes and deterioration of the language skills (WHO 2010). Some FTDs are familial disorders associated with single gene mutation. The prevalence of FTD in population‐based studies has varied between 2.7 per 100,000 inhabitants in the Netherlands to 17.6 per 100,000 inhabitants in Northern Italy (Premi 2012). Behavioural variant frontotemporal dementia (bvFTD) is the most common clinical presentation, with prominent personality changes and impaired social behaviour. The most common symptoms of cognitive decline are "poor judgment, inattentiveness and distractibility, loss of planning ability and disorganisation" (Rabinovici 2010).
HD is a hereditary neurodegenerative disorder caused by an expansion of a repeating CAG triplet series in the huntington gene. It is characterised by the onset of progressive chorea and dementia in the fourth or fifth decade of life (WHO 2010). The worldwide service‐based prevalence of HD, based on a meta‐analysis (including 13 studies), was 2.71 per 100,000 (95% confidence interval (CI) 1.55 to 4.72) (Pringsheim 2012). The cognitive and behavioural symptoms and signs of HD have been shown to be evident at least 15 years prior to the time at which motor diagnosis is typically given (Paulsen 2011). Thus, the "cognitive and behavioural impairments have been growing in prominence in HD diagnosis and treatment".
CADASIL is a single‐gene disorder (mutation in the NOTCH3 gene) directly affecting the cerebral small blood vessels (Joutel 1996). The main clinical manifestations of CADASIL are migraine, recurrent subcortical stroke, mood disturbance, and a progressive cognitive decline leading to dementia (Chabriat 1995). Estimates of the population prevalence of CADASIL vary from 1.98 per 100,000 in West Scotland to 4.10 per 100,000 in North‐East England (Narayan 2012; Razvi 2005). Cognitive deficits can be found in about 60% of people with CADASIL. Typically these are frontal lobe cognitive deficits, including problems with executive function, working memory, and verbal fluency (Choi 2010; Fukutake 2011). As the disease progresses, people begin to show cognitive deficits typical of subcortical vascular dementia (Choi 2010).
Multiple sclerosis (MS) is a progressive autoimmune disorder affecting the central nervous system (CNS) resulting in demyelination (WHO 2010). Depending on the extent and location of damage in the CNS, people with MS may experience a wide variety of symptoms, including motor, cognitive, and neuropsychiatric problems (WHO 2010; Chiaravalloti 2008). MS affects more than 600,000 people in the United States and more than two million people worldwide, and 40% to 65% of these people experience some degree of cognitive impairment (Rahn 2012). MS detrimentally affects various aspects of cognitive functioning, including attention, information processing efficiency, executive functioning, processing speed and long‐term memory; processing speed, visual learning and memory seem to be most commonly affected (Chiaravalloti 2008).
Progressive supranuclear palsy (PSP) is a neurodegenerative disease which falls in the general class of tauopathies (Boeve 2012). It classically presents with early postural instability and falls; PSP usually occurs in middle‐aged or elderly people. Symptoms may include personality changes, speech, vision and swallowing problems (WHO 2010). The prevalence of PSP ranges from 3.1 to 6.5 per 100,000 in the United Kingdom (Hoppitt 2011). The age‐standardised incidence of PSP in Sweden (2004 to 2007) is 1.2 (95% CI 0.4 to 2.6) per 100,000 (Linder 2010).
Description of the intervention
The first cholinesterase inhibitors were introduced into clinical practice in 1993 and the three currently licensed cholinesterase inhibitors ‐ donepezil, rivastigmine and galantamine ‐ are now considered to be the first‐line medicines for dementia due to AD. They are usually recommended for dementia of mild to moderate severity. Cholinesterase inhibitors inhibit the enzyme acetylcholinesterase which functions to break down acetylcholine (a neurotransmitter in both the peripheral and central nervous systems). The cholinergic system is known to play an important role in cognition.
Donepezil is a selective reversible inhibitor of acetylcholinesterase. Donepezil is produced by Eisai Ltd and co‐marketed with Pfizer. It is given orally, usually starting at a dose of 5 mg per day, increased after several weeks to 10 mg per day. Rivastigmine is an inhibitor of both acetylcholinesterase and butylcholinesterase. It can be administered orally or transdermally. Both oral formulation and transdermal system are produced by Novartis Pharmaceuticals. Oral rivastigmine treatment is initiated at 1.5 mg twice daily, and is increased gradually over weeks to 6 mg twice daily. Rivastigmine patch is initiated at 4.6 mg/day for four weeks, then is increased to 9.6 mg/day. Kurz observed that "The rivastigmine patch provides continuous drug delivery over 24 hours and similar efficacy to the highest recommended dose of oral rivastigmine with improved tolerability" (Kurz 2009). Galantamine can stimulate nicotinic acetylcholine receptors as well as inhibiting cholinesterase activity. The manufacturer of galantamine is Janssen Pharmaceutica. It is administered orally in once‐ or twice‐daily formulations. Galantamine treatment is usually initiated at 8 mg daily, and can be increased gradually up to 24 mg daily (Lanctôt 2009). These agents have the same principal mechanism of action‐inhibiting acetylcholinesterase. There is insufficient evidence to differentiate between the three cholinesterase inhibitors in terms of clinical effectiveness for AD (NICE 2011). Side effects of all cholinesterase inhibitors include gastrointestinal symptoms such as nausea, diarrhoea and vomiting, as well as leg cramps, abnormal dreams, dizziness and weight loss (Hansen 2008; Tayeb 2012).
There is evidence for the efficacy of cholinesterase inhibitors in dementias due to Parkinson's disease. These medications have been shown to significantly improve Global Assessment of Functioning, cognitive function, behavioural disturbance and activities of daily living in people with dementia or cognitive impairment in Parkinson's disease (Rolinski 2012). They are also reported to produce small cognitive improvements in people with vascular dementia (Baskys 2012) and vascular cognitive impairment (Levine 2011). Cholinesterase inhibitors have also recently been tested in rare dementias. Some studies (Krupp 2004; Greene 2000) reported that cholinesterase inhibitors improved memory performance in patients with MS, while another study (Dichgans 2008) found that donepezil improved some executive function tests in patients with CADASIL.
How the intervention might work
Cholinesterase inhibitors are thought to improve cognitive function primarily by preventing the breakdown of acetylcholine and hence boosting cholinergic neurotransmission in forebrain regions (Tayeb 2012).
CADASIL is a genetic form of subcortical ischaemic vascular dementia. Several recent studies have suggested that subcortical ischaemic lesions disrupt cholinergic pathways. Hence, cholinergic deficits may play a role in the dementia of CADASIL (Keverne 2007; Mesulam 2003). Reductions in cerebrospinal fluid (CSF) markers of cholinergic activity, which may reflect reductions in global brain cholinergic activity, have been found in MS, possibly due to disruption of cholinergic pathways by demyelination and axonal transection (Ruberg 1987). Depletions of postsynaptic cholinoreceptors have been found in the temporal cortex of people with FTD (Odawara 2003). Cholinergic neurons may also be indirectly affected in other neurodegenerative conditions associated with cognitive decline and dementia, including HD (Cubo 2006) and PSP (Litvan 2001). The cholinesterase inhibitors may therefore have an impact on cognitive impairment in these rarer dementias.
Why it is important to do this review
Cognitive impairment is one of the major causes of disability in the neurological conditions in which it occurs, resulting in a serious burden for individuals and their carers (Nunnemann 2012; Rahn 2012). It is therefore important to evaluate the treatment of the rarer dementias. Some studies (Krupp 2004; Dichgans 2008) have suggested that cholinesterase inhibitors might be associated with improved cognition in people with rarer dementias, but there are conflicting reports in the literature (Krupp 2011; Shaygannejad 2008; Mäurer 2013). A systematic review to focus on the efficacy of cholinesterase inhibitors for rarer dementias is therefore needed.
Objectives
The objectives of this review were:
To assess the efficacy of cholinesterase inhibitors for the treatment of rarer dementias associated with neurological conditions.
To assess the adverse effects of cholinesterase inhibitors in these conditions.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised double‐blind controlled trials assessing the efficacy of treatment with currently marketed cholinesterase inhibitors of rarer dementias associated with neurological conditions.
Types of participants
Participants of any age and either gender with FTDs, HD, CADASIL, MS, or PSP. The diagnostic criteria for these conditions was considered to be the globally accepted criteria, for example, Lund–Manchester criteria (Brun 1994; Neary 1998) or recent consensus criteria (Rascovsky 2011) for FTD; genetically‐confirmed or positive family history, and clinical motor disorders for HD; genetic or biopsy diagnosis for CADASIL; Poser or McDonald criteria for MS (Poser 1983, Polman 2011) and Litvan criteria for PSP (Litvan 1996). Participants could have any level of cognitive function at inclusion.
Types of interventions
Cholinesterase inhibitors versus placebo.
Cholinesterase inhibitors versus no intervention.
Cholinesterase inhibitors plus other therapy (or therapies) versus placebo plus same other therapy (or therapies).
Cholinesterase inhibitors plus other therapy (or therapies) versus other therapy (or therapies).
Cholinesterase inhibitors could be given at any dose for any duration. The currently marketed cholinesterase inhibitors are galantamine, donepezil and rivastigmine.
Types of outcome measures
The outcomes were measured at different time points from baseline: short‐term (three months or less), medium‐term (three to 12 months) and long‐term (more than12 months). We analysed the outcome measures according to these time groupings.
Primary outcomes
Cognition (measured by psychometric tests, including tests of single cognitive domains as well as multi‐domain scales and neuropsychological test batteries).
Clinical global impression of change (measured by scales or by the physician's or participant's self‐reported impression of any change).
Global severity of dementia.
Activities of daily living (measured by scales such as Alzheimer's Disease Co‐operative Study ‐ Activities of Daily Living Scale).
Adverse effects.
Secondary outcomes
Quality of life.
Caregiver burden.
Behavioural disturbance and/or neuropsychiatric symptoms.
Dependency (such as institutionalisation).
Mortality.
Tolerability (all drop‐outs, and those due to adverse drug reactions).
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois): the Cochrane Dementia and Cognitive Improvement Group’s (CDCIG) Specialised Register. The search terms used were: frontal lobe dementia, primary progressive aphasia, Huntington's disease, CADASIL, multiple sclerosis, progressive supranuclear palsy, motor neurone disease, amyotrophic lateral sclerosis, combined with terms for the interventions (galantamine, donepezil, rivastigmine).
ALOIS is maintained by the CDCIG Trials Search Co‐ordinator and contains dementia and cognitive improvement studies identified from the following:
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO and LILACS.
Monthly searches of a number of trial registers: metaRegister of Controlled Trials; Umin (Japan's Trial Register); ICTRP/WHO portal (which covers ClinicalTrials.gov; International Standard Randomised Controlled Trial Number (ISRCTN) register; Chinese Clinical Trials Register; German Clinical Trials Register; Australian New Zealand Clinical Trials Registry (ANZCTR); Iranian Registry of Clinical Trials and the Netherlands National Trials Regsiter, plus others).
Quarterly search of the Cochrane Central Register of Controlled Trials (CENTRAL).
Monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Additional separate searches were run in many of the above sources to ensure that the most up‐to‐date results were retrieved. The search strategy that was used for the retrieval of reports of trials from MEDLINE (via the Ovid SP platform, 1950 to August week 1, 2013) can be seen in Appendix 1.
Searching other resources
We searched identified publications for additional trials. We contacted the first author of identified trials for additional references and unpublished data. We also checked the US Food and Drug Administration website (FDA) for more information, and sent e‐mails to the manufacturers of the marketed cholinesterase inhibitors requesting any unpublished trial data.
Data collection and analysis
Selection of studies
Two review authors (Li and Zhou) screened all identified trials for relevance based on the title and abstract before retrieving the papers. Both review authors independently read the abstract and methods sections of these papers to select the trials for inclusion in this review. Both review authors independently assessed all the references to identify additional potentially relevant trials. We resolved any disagreement regarding differences in opinion by discussion and the decision was referred to the third author (Dong) when there were unresolved differences.
Data extraction and management
Two review authors (Li and Zhou) independently extracted data for the trials including study characteristics, methods, interventions, participant demographic characteristics, enrolment criteria, outcomes, adverse effects, and number and reasons for drop‐out. We resolved any disagreements through discussion or by consulting the third review author.
For binary data, we recorded the number in each treatment group and the number experiencing the outcome of interest. For continuous data, we extracted the mean change from baseline, the standard deviation of the mean change, and the number of participants for each treatment group in individual studies. When the studies used different measurement scales for the same outcome, we calculated standardised mean differences. For cross‐over trials, because of potential for carry‐over effects and progressive nature of dementia condition, we only extracted data from the first treatment period.
Assessment of risk of bias in included studies
Two review authors (Li and Zhou) independently assessed studies for quality according to the Cochrane 'Risk of bias' tool described in the Cochrane Handbook (Chapter 8). The following criteria formed the main evaluation of methodological quality.
Generation of random sequence.
Concealment of allocation schedule.
Blinding of: clinician (person delivering treatment), participant, and outcome assessor to treatment allocation.
The proportion of included randomised participants in the main analysis, noting particularly where more than 20% were 'lost to follow‐up'. We report the proportion of differing levels of losses to follow‐up affecting the validity of the results for different outcomes to different degrees.
All the study's prespecified outcomes and all expected outcomes of interest to the review have been reported.
For each criterion, we made a judgment of low, high or unclear risk of bias. We recorded the judgment and supporting evidence in a study‐linked table and discuss it in the text of the review where relevant.
We also evaluated other problems that could put the study at risk of bias, including possible conflicts of interest.
We used the quality assessment to explore differences in the results of studies as part of any investigations of heterogeneity or in sensitivity analyses to explore the robustness of summary estimates.
Measures of treatment effect
For binary outcomes, we used the risk ratio (RR) to measure treatment effect, with a 95% confidence interval (CI). We expressed continuous data as mean difference (MD) or standardised mean difference (SMD), if different scales had been used.
Unit of analysis issues
We did not anticipate finding cluster‐randomised trials for this review. We planned to only use first‐period data from cross‐over trials and to avoid double‐counting of participants in case of multiple interventions in the same trial.
Dealing with missing data
Where there were missing data, we attempted to seek the necessary information from the study authors. We analysed the outcome measures on an intention to treat (ITT) basis (i.e. we considered participants who dropped out of a study along with those who continued).
Assessment of heterogeneity
For pooled effects, we calculated the I² statistic. An I² value greater than 40% was taken to mean possible heterogeneity (Cochrane Handbook; Higgins 2003).
Assessment of reporting biases
It is acknowledged that funnel plots are difficult to interpret with a small numbers of studies (i.e. less than 10) in systematic reviews. Therefore, we did not assess the presence of publication bias for all included trials. If there are more studies included in future updates, a funnel plot will be used to assess the presence of publication bias.
Data synthesis
We analysed studies of the different neurological conditions separately. We combined data in a meta‐analysis using Review Manager 5, provided they were of sufficient quality and were from studies which were sufficiently similar clinically, using a fixed‐effect method.
Subgroup analysis and investigation of heterogeneity
Heterogeneity would have been explored by conducting subgroup analyses, however there were insufficient data in each condition to undertake subgroup analyses.
Sensitivity analysis
We did not perform sensitivity analyses since only a few studies were included in each subgroup.
Results
Description of studies
Results of the search
We retrieved 577 records from MEDLINE (148 records), EMBASE (170 records), PsycINFO (36 records), CINAHL (70 records), CENTRAL (26 records), Web of Knowledge (117 records), LILACS (5 records), Clinicaltrials.gov (2 records), ICTRP Search Portal (3 records) from our electronic literature searches. We removed duplicates and did first assessment, leaving 111 records. We excluded most records which were not related to our question by further scanning the title and the abstract. We identified 14 full texts of clinical trials for further assess (see Figure 1).
1.
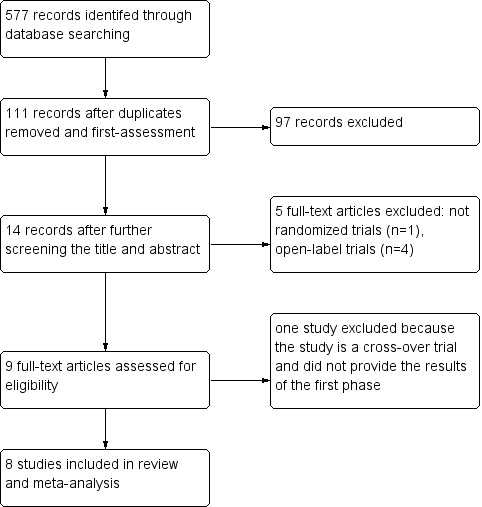
Flow diagram
Included studies
Eight included studies which involved 567 participants were identified for inclusion: Cubo 2006; Krupp 2004; Shaygannejad 2008; Krupp 2011; Mäurer 2013; Dichgans 2008; Kertesz 2008; Sešok 2014. Six studies (Cubo 2006; Krupp 2004;Dichgans 2008;Krupp 2011; Shaygannejad 2008;Sešok 2014) used a simple parallel‐group design. One study Kertesz 2008 consisted of an open‐label treatment period followed by a randomised, double‐blind, placebo‐controlled phase, while another Mäurer 2013 began with a four‐week titration period followed by a double‐blind, randomised, placebo‐controlled phase and an optional one‐year open‐label treatment phase; we analysed data from the double‐blind randomised phases.
The funding sources for each study are shown in Table 7.
The detailed inclusion criteria and exclusion criteria of each study are displayed in Characteristics of included studies.
Huntington's disease (HD)
Cubo 2006 was a double‐blind, randomised, placebo‐controlled trial including 30 HD patients. Fifteen patients received oral donepezil 5 mg/day for six weeks and then 10 mg/day for another six weeks, and 15 received placebo. Six participants were lost to follow up. The following tests and scales were used to evaluate cognitive function: the cognitive portion of the Alzheimer's Disease Assessment Scale (ADAS‐Cog), Unified Huntington's Disease Rating Scale (UHDRS; Verbal Fluency Test (FAS) and Symbol Digit Modalities Test (SDMT)), Stroop black and white and Stroop interference tests and Wechsler Adult Intelligence Survey III symbol searching raw score (WAIS‐III‐SSRS). Quality of life was evaluated by using a modified Sickness Impact Profile (SIP) scale.
Sešok 2014 was a double‐blind, randomised, placebo‐controlled trial including 18 HD patients. Participants were all from the Republic of Slovenia. Twelve patients received oral rivastigmine 1.5 mg twice daily for three months and then 3 mg twice daily for another three months. Six patients received placebo. One treatment participant was lost to follow up. The following tests and scales were used to evaluate cognitive function: SDMT, Stroop Colour and Word Test, Comprehensive Trail‐Making Test (CTMT), FAS, Ruff Figural Fluency Test (RFFT), Tower of London test, Rey‐Osterrieth Complex Figure Test (ROCF) and the California Verbal Learning Test ‐ Second Edition (CVLT‐II).
Multiple sclerosis (MS)
Krupp 2004 was a single‐centre, double‐blind, randomised, placebo‐controlled parallel‐group clinical trial including 69 MS patients with cognitive impairment. Participants were all from the United States. The 35 subjects in the active treatment group received oral donepezil 5 mg/day for four week, increasing to 10 mg/day for another 20 weeks. Two participants were lost to follow up. The study used an ITT analysis and a last‐observation‐carried‐forward (LOCF) imputation strategy for missing data. The primary outcome measure was the change score in total recall on the Selective Reminding Test (SRT). The secondary outcomes are shown in Characteristics of included studies.
Krupp 2011 was a multi‐centre, double‐blind, randomised, placebo‐controlled clinical trial on MS patients with cognitive impairment. The participants were from five Northeastern United States hospital‐based MS centres. Of the 120 enrolled patients, 61 patients received oral donepezil treatment. The initial donepezil dose was 5 mg donepezil daily, increased to 10 mg daily at week four. The duration of the trial was 24 weeks. One hundred and thirteen participants completed their final visit and data collection (55 in placebo, 58 in treatment). The study used an ITT analysis and a LOCF imputation strategy for missing data. The primary outcomes included total recall on the SRT and the patient self‐reported impression of memory change. The secondary outcomes are shown in Characteristics of included studies.
Shaygannejad 2008 was a single‐centre, double‐blind, randomised, placebo‐controlled clinical trial including 60 MS patients. These participants were from a hospital in Iran. Sixty patients were randomised; thirty in the rivastigmine group, thirty in the placebo group. The active treatment group had oral rivastigmine 1.5 mg once daily, increased after four weeks to 3 mg twice daily for a further eight weeks. All the patients completed the trial. The outcome measures were the Wechsler Memory Scales (WMS) consisting of seven sub‐tests: Information, Orientation, Mental Control, Logical Memory, Digit Span, Visual Reproduction and Associative Learning.
Mäurer 2013 was a multi‐centre, double‐blind, randomised, placebo‐controlled clinical trial on MS patients. Participants were from 30 investigational sites in Germany. A total of 86 MS patients were randomised to either rivastigmine (n = 45) or placebo (n = 41). Participants entered a four‐week titration period (rivastigmine patches 4.6 mg/day), followed by a 12‐week double‐blind maintenance period (rivastigmine patches 9.5 mg/day) and an optional 12‐month open‐label treatment phase. A total of 34 patients in each group completed the double‐blind phase. The study used a modified ITT analysis. The primary outcome measure was total recall on the SRT. Secondary outcome measures included a variety of other cognitive measures (see Characteristics of included studies).
CADASIL
Dichgans 2008 was a multi‐centre, double‐blind, randomised, placebo‐controlled, parallel‐group trial on CADASIL patients, which was undertaken in 10 countries. One hundred and sixty‐eight patients were randomly assigned to receive placebo (n = 82) or donepezil (n = 86). The treatment group had oral donepezil 5 mg/day for 6 weeks, and 10 mg/day for the remaining 12 weeks. The primary analysis was an ITT analysis at week 18 with LOCF. The ITT population included 77 patients in the placebo group and 84 patients in the donepezil group. One hundred and forty‐six patients completed the trial (73 in placebo, 73 in donepezil). The primary outcome measure was the vascular ADAS‐Cog (V‐ADAS‐Cog) score. The secondary outcomes are shown in Characteristics of included studies.
FTD: behaviouralvariant and primary progressive aphasia
Kertesz 2008 was a clinical trial on patients with the behavioural variant of FTD (bvFTD) and primary progressive aphasia (PPA). There was an open‐label period of 18 weeks in which all participants received galantamine, followed by an eight‐week randomised, double‐blind phase comparing continued galantamine with placebo. In the open label phase, the patients were on 8 mg oral galantamine daily for the first four weeks and received 16‐24 mg for the rest of the 18 weeks. In the double blind phase, they were then randomised in equal numbers to an additional eight weeks of 16‐24 mg galantamine daily or eight weeks of placebo treatment. Thirty‐nine patients received at least one dose of galantamine and 36 completed the 18 weeks of open label treatment. Of these 36 patients, 34 completed the eight weeks of double blind treatment. The analysis of the study was based on the ITT population. LOCF was not used for the double blind phase. The primary outcomes included the Frontal Behavioural Inventory (FBI), Aphasia Quotient (AQ) of the Western Aphasia Battery (WAB) and Clinical Global Impressions of Improvement (CGI‐I) and of Severity (CGI‐S). The secondary outcomes are shown in Characteristics of included studies.
Excluded studies
We excluded six trials (see Characteristics of excluded studies). Four of these trials were open‐label trials. One was a double‐blind, randomised, placebo‐controlled, crossover design trial not reporting results of the first phase, and one was not a double‐blind, randomised trial.
Risk of bias in included studies
In general, the methodological quality of the included trials was moderate. See the 'Risk of bias' table for more details. The overall risk of bias is presented graphically in Figure 2 and summarised in Figure 3.
2.
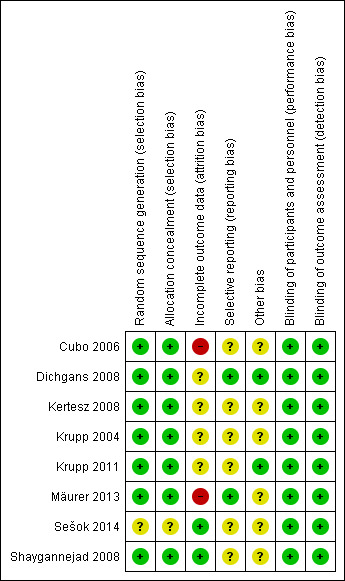
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
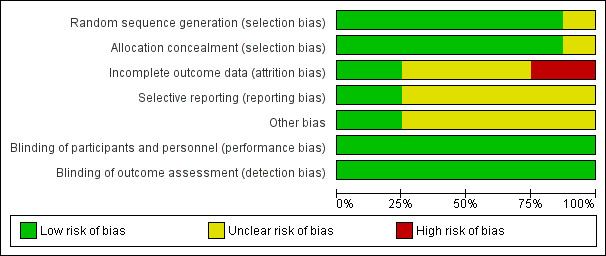
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sešok 2014 mentioned "randomly allocate participants" but did not provide a detailed description of the randomisation method. The other seven included trials clearly described the methods of randomised sequence generation and concealment for allocation of participants.
Blinding
Six double‐blind designed trials reported double‐blinding. In the double‐blind period of the other two trials, double‐blinding was also reported. Four studies (Krupp 2004; Krupp 2011; Mäurer 2013; Sešok 2014) described the details.
Incomplete outcome data
All included trials provided sufficient information for the incomplete outcome data to be calculated, or else described the withdrawal rate. The dropout rate of Cubo 2006 and Mäurer 2013 were more than 20%, therefore these two studies had a high risk of attrition bias. Four studies (Krupp 2004; Krupp 2011; Dichgans 2008; Kertesz 2008) used an ITT analysis with LOCF which might cause bias, therefore these four studies had a unclear risk of this bias. In Shaygannejad 2008, all the participants completed the trial and the statistical analysis was based on an ITT principle, which had a low risk of attrition bias.
Selective reporting
Dichgans 2008 and Mäurer 2013 reported well all the outcomes described in the protocol registered on ClinicalTrials.gov. The other six studies did not have available protocols.
Other potential sources of bias
Most of the included studies had small sample sizes which might have led to other potential sources of bias. Kertesz 2008 and Mäurer 2013 were open‐label to begin with, followed by a double‐blind period, which might cause a carry‐over effect.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
There were four included trials that focused on MS; we were able to conducted meta‐analyses for some results from these studies. Two trials focused on HD, but the results were to heterogeneous to pool. Only one study was identified for each of the other conditions, therefore it was impossible to pool results for meta‐analyses. Instead, we describe the results of these studies.
Huntington's disease (HD)
One study (Cubo 2006) evaluated the short‐term (12 weeks) efficacy of a cholinesterase inhibitor and one study Sešok 2014 evaluated the medium‐term (24 weeks) efficacy in patients with HD.The main results are summarised in Table 1 and Table 2.
Short‐term efficacy
Cognitive function
In Cubo 2006, cholinesterase inhibitor use had no statistically significant impact on ADAS‐Cog Score (WMD 1.00, 95% CI ‐1.66 to 3.66, P = 0.46), UHDRS‐FAS (WMD ‐1.20, 95% CI ‐7.97 to 5.57, P = 0.73), UHDRS‐SDMT (WMD 2.70, 95% CI ‐0.95 to 6.35, P = 0.15), Stroop black and white (WMD ‐0.50, 95% CI ‐8.37 to 7.37, P = 0.90), Stroop interference (WMD ‐0.70, 95% CI ‐4.41 to 3.01, P = 0.71) and WAIS‐III‐SSRS (WMD 1.70, 95% CI ‐1.94 to 5.34, P = 0.36).
Quality of life
In Cubo 2006, cholinesterase inhibitor use had no statistically significant impact on the combined SIP subscales (WMD 7.10, 95% CI ‐4.22 to 18.42, P = 0.22).
Medium‐term efficacy
Cognitive function
In Sešok 2014, cholinesterase inhibitor use improved the results of the FAS (WMD 6.43, 95% CI 0.66 to 12.20, P = 0.03) and CVLT‐II Recognition Task (WMD 2.42, 95% CI 0.17 to 4.67, P = 0.04). There was no statistically significant improvement in the cholinesterase inhibitors group on the other psychometric tests: SDMT (WMD ‐0.31, 95% CI ‐7.77 to 7.15, P = 0.94), Stroop Colour and Word Test (WMD ‐3.74, 95% CI ‐13.42 to 5.94, P = 0.45), CTMT (WMD ‐10.07, 95% CI ‐48.60 to 28.46, P = 0.61), RFFT (WMD 5.14, 95% CI ‐20.29 to 30.57, P = 0.69), Tower of London (WMD 30.15, 95% CI ‐121.41 to 181.71, P = 0.70), ROCF recognition test (WMD 1.28, 95% CI ‐1.14 to 3.70, P = 0.30), ROCF immediate recall test (WMD ‐4.34, 95% CI ‐11.48 to 2.80, P = 0.23), ROCF delayed recall test (WMD ‐4.49, 95% CI ‐11.67 to 2.69, P = 0.22), CVLT‐II tasks 1‐5 (WMD ‐2.09, 95% CI ‐11.65 to 7.47, P = 0.67), CVLT‐II short‐delay recall task (WMD 0.35, 95% CI ‐2.87 to 3.57, P = 0.83) and CVLT‐II long‐delay recall task (WMD ‐0.14, 95% CI ‐3.08 to 2.80, P = 0.93).
Multiple sclerosis (MS)
One study (Shaygannejad 2008) evaluated the short‐term (12 weeks) efficacy of cholinesterase inhibitors and three studies (Krupp 2004; Krupp 2011; Mäurer 2013) evaluated the medium‐term (16 to 24 weeks) efficacy in patients with MS. The main results are summarised in Table 3 and Table 4.
Short‐term efficacy
Cognitive function
In Shaygannejad 2008, there were no differences between the cholinesterase inhibitor group and placebo group on the WMS overall score (WMD 0.90, 95% CI ‐0.52 to 2.32, P = 0.22). In the sub‐test analyses, the treatment group showed some improvement in Logical Memory (WMD 0.70, 95% CI 0.02 to 1.38, P = 0.04) and Associative Learning (WMD 2.10, 95% CI 1.41 to 2.79, P < 0.001). However, the cholinesterase inhibitors group showed no significant improvement in Information (WMD ‐0.20, 95% CI ‐0.44 to 0.04, P = 0.10), Orientation (WMD ‐0.10, 95% CI ‐0.26 to 0.06, P = 0.21), Mental Control (WMD ‐0.80, 95% CI ‐1.28 to ‐0.32, P = 0.001), Digit Span (WMD ‐0.40, 95% CI ‐0.75 to ‐0.05, P = 0.02) and Visual Reproduction (WMD ‐0.40, 95% CI ‐0.88 to 0.08, P = 0.10).
Medium‐term efficacy
Cognitive function
Cholinesterase inhibitors showed no significant treatment effect on the cognitive function of MS patients assessed by the SRT (3 studies, WMD 1.47, 95% CI ‐0.39 to 3.32, P = 0.12), the 10/36 Spatial Recall Test (10/36 SRT; 3 studies, WMD ‐1.07, 95% CI ‐2.23 to 0.09, P = 0.07), SDMT (3 studies, WMD ‐1.30, 95% CI ‐2.96 to 0.37, P = 0.13), Paced Auditory Serial Addition Test (PASAT) ‐ total corrected sum of the two‐ and three‐ second forms of the task (2 studies, WMD 2.14, 95% CI ‐1.15 to 5.43, P = 0.20), PASAT ‐ 3 seconds (1 study, WMD 1.71, 95% CI ‐1.41 to 4.83, P = 0.28), Controlled Oral Word Association (2 studies, WMD ‐0.16, 95% CI ‐1.48 to 1.17, P = 0.82), Tower of Hanoi performance (1 studies, WMD ‐0.40, 95% CI ‐3.49 to 2.69, P = 0.80), Delis‐Kaplan Executive Function System Sorting total (1 study, WMD 0.10, 95% CI ‐0.78 to 0.98, P = 0.82), Judgment of Line Orientation total (1 study, WMD 0.00, 95% CI ‐1.15 to 1.15, P = 1.00), Faces Symbol Test (1 study, WMD ‐0.01, 95% CI ‐0.30 to 0.28, P = 0.95) and Modified Fatigue Impact Scale (1 study, WMD ‐5.16, 95% CI ‐11.81 to 1.49, P = 0.13).
Clinical global impression of change
Cholinesterase inhibitors improved the clinician's impression of cognitive change of MS patients (2 studies, OR 1.96, 95% CI 1.06 to 3.62, P = 0.03). However, they had no significant impact on patient's self‐reported impression of memory change (2 studies, OR 1.67, 95% CI 0.93 to 3.00, P = 0.08), patient's self‐reported impression of cognitive change (1 study, OR 0.95, 95% CI 0.45 to 1.98, P = 0.89), clinician's impression of memory change (1 study, OR 1.50, 95% CI 0.59 to 3.84, P = 0.39), significant other's impression of memory change (1 study, OR 1.40, 95% CI 0.65 to 3.02, P = 0.40), significant other's impression of cognitive change (1 study, OR 1.50, 95% CI 0.71 to 3.21, P = 0.29) and global rating of change of condition (1 study, OR 0.80, 95% CI 0.38 to 1.68, P = 0.56).
Activities of daily living
Only Mäurer 2013 assessed activities of daily living, and there was no statistically significant improvement in the cholinesterase inhibitors group measured by the patient reported impact of multiple sclerosis activities (WMD ‐1.18, 95% CI ‐3.02 to 0.66, P = 0.21).
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)
One study (Dichgans 2008) evaluated the efficacy of cholinesterase inhibitors in CADASIL patients. The main results are summarised in Table 3.
Cognitive function
Cholinesterase inhibitor use had no statistically significant impact on V‐ADAS‐Cog score improvement (WMD 0.04, 95% CI ‐1.57 to 1.65, P = 0.96). A beneficial effect of cholinesterase inhibitors on cognitive function was observed on the Executive interview (WMD 1.47, 95% CI 0.15 to 2.79, P = 0.03), CTMT part A (WMD 8.05, 95% CI 1.65 to 14.45, P = 0.01) and part B (WMD 23.34, 95% CI 6.39 to 40.29, P = 0.007). No significant difference between the treatment group and the placebo group was observed on the ADAS‐Cog (WMD ‐0.06, 95% CI ‐1.45 to 1.33, P = 0.93), Mini‐Mental State Examination (MMSE; WMD 0.34, 95% CI ‐0.31 to 0.99, P = 0.31), Stroop Colour and Word Test (WMD 0.75, 95% CI ‐1.68 to 3.18, P = 0.54) and two clock drawing tasks: CLOX1 (WMD 0.67, 95% CI ‐0.12 to 1.46, P = 0.10) and CLOX2 (WMD 0.47, 95% CI ‐0.07 to 1.01, P = 0.09).
Clinical global impression of change
Assessed by the Clinical Dementia Rating Scale Sum of Boxes of the (CDR‐SB), there was no statistically significant difference between the two groups (WMD ‐0.09, 95% CI ‐0.48 to 0.03, P = 0.65).
Activities of daily living
Cholinesterase inhibitors resulted in no improvement on the Disability Assessment for Dementia (DAD) scale (WMD 0.58, 95% CI ‐2.72 to 3.88, P = 0.73).
Frontotemporal dementia (FTD): behavioural variant and primary progressive aphasia
Kertesz 2008 evaluated the efficacy of cholinesterase inhibitors in patients with FTD. The study provided the primary outcomes in figures and we could not extract the exact data, therefore we only analysed the secondary outcome measures.
Cognitive function
Cholinesterase inhibitor use had no statistically significant impact on MMSE (WMD 4.40, 95% CI ‐3.27 to 12.07, P = 0.26), Mattis Dementia Rating Scale (WMD 22.00, 95% CI ‐3.38 to 47.38, P = 0.09), Frontal Assessment Battery (WMD 2.50, 95% CI ‐0.99 to 5.99, P = 0.16) or Neuropsychiatric Inventory (WMD 5.80, 95% CI ‐7.26 to 18.86, P = 0.38).
Activities of daily living
Data from Kertesz 2008 showed no difference between the two groups on the Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living scale (WMD 7.00, 95% CI ‐7.55 to 21.55, P = 0.35).
Adverse events
In all included studies, the most common side effect was gastrointestinal symptoms. For all conditions, compared to the treatment group, the placebo group experienced significantly less nausea (6 studies, 44/257 vs. 22/246, OR 2.10, 95% CI 1.22 to 3.62, P = 0.007), diarrhoea (6 studies, 40/257 vs. 13/246, OR 3.26, 95% CI 1.72 to 6.19, P = 0.0003) and vomiting (3 studies, 17/192 VS. 3/182, OR 5.76, 95% CI 1.67 to 19.87, P = 0.006).
Krupp 2004 and Krupp 2011 reported that abnormal dreams were more common in the treatment groups (2 studies, 24/96 vs. 8/93, OR 3.55, 95% CI 1.50 to 8.37, P = 0.004).
Discussion
Summary of main results
We analysed eight RCTs including 567 participants. Two of these trials included patients with HD. In these trials, cholinesterase inhibitors had no significant impact on cognitive level or quality of life, but improved results on the FAS and CVLT‐II Recognition Task. Four trials included patients with MS. In these trials, the beneficial effect of cholinesterase inhibitors on cognitive function was only observed on "clinician's impression of cognitive change." One study included patients with CADASIL. In this study, cholinesterase inhibitor use had no statistically significant improvement on primary cognitive scales and other measurements, but improved some executive function tests. One study included patients with FTD; we could only analyse the secondary outcomes of this trial. Cholinesterase inhibitors resulted in no significant improvements in these secondary outcomes. There were no trials examining the efficacy of cholinesterase inhibitors in patients with PSP. In addition, in all conditions, the placebo groups experienced significantly fewer gastrointestinal side effects.
The results for the included outcomes were unsatisfactory because some studies had small sample sizes and most of the results in this review are based on single trials. In addition, some studies did not provide satisfactory data for the main results.
Overall completeness and applicability of evidence
We searched all possible sources of published articles related to our question and included seven studies. The participants had cognitive impairment associated with the following conditions: HD, CADASIL, MS, or FTD. The results on HD, CADASIL and FTD were only extracted from one trial each, and some trials had small sample sizes.
Quality of the evidence
Allocation concealment was described in seven of the eight included studies. Double‐blinding was reported in eight studies and the details of the blinding methods were reported in three of them. In our review, the cut‐off of "small size study" is a sample size of 45 in each group. Most of the included trials had a small sample size, which might be a source of bias. Four studies used an ITT analysis with LOCF. The LOCF analyses in these small studies are more susceptible to large outlier effects. Two studies were open‐label to begin with, followed by a double‐blind period, which might cause a carry‐over effect.
Potential biases in the review process
We were unable to analyse the primary outcomes of the FTD study; this may cause bias. Furthermore, we identified one double‐blind, randomised, placebo‐controlled, crossover design trial on patients with PSP. We sent emails to the study authors to get first phase data, but we did not receive a reply, therefore the first phase data from the study were unavailable. This may result in reporting bias.
Agreements and disagreements with other studies or reviews
In an open‐label study on patients with frontotemporal dementia (Moretti 2004), cholinesterase inhibitors showed a "general amelioration of behavioural changes", a reduction of caregiver burden and improvement of executive function. Another prospective, open‐label, randomised, controlled study on twenty‐one HD patients (de Tommaso 2004, de Tommaso 2007) reported that patients treated with rivastigmine showed a significant improvement of global motor performances and chorea in comparison with the control group, with a trend toward a reduction of functional disability and cognitive impairment. However, open‐label studies are prone to performance bias.
Authors' conclusions
Implications for practice.
The effects of cholinesterase inhibitors on cognitive function, activities of daily living and quality of life for patients with HD, CADASIL, MS, PSP, or FTD were evaluated in this review. The current evidence is unclear as there are small effects on some outcomes and insufficient evidence on many outcomes to draw firm conclusions. The evidence shows that cholinesterase inhibitors were associated with more gastrointestinal side effect compared with placebo. There is no clear evidence to support the efficacy of cholinesterase inhibitors in these conditions.
Implications for research.
This review included eight randomised controlled trials comparing cholinesterase inhibitors with placebo which found no significant efficacy for improving cognitive function. Future randomised controlled trials should consider: 1) Employing a study design with a large sample size; 2) Including common assessment of cognitive level as outcomes (for example, the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change (ADCS‐CGIC) score); 3) Using a longer duration of intervention (more than 6 months) and long‐term (more than 12 months) follow‐up (see Additional Table 8).
1. PICO Table.
| E (Evidence) | One review included seven small randomised controlled trials comparing cholinesterase inhibitors with placebo which found no significant efficacy for improving cognitive function. |
| P (Population) | Patients with Hungtington's disease, CADASIL, multiple sclerosis, progressive supranuclear palsy, or frontotemporal dementia |
| Suggesting large sample size (adequately powered studies able to show clinically relevant differences on patient relevant outcomes). | |
| I (Intervention) | Currently marketed cholinesterase inhibitors |
| Suggesting longer duration of intervention (more than 6 months) and long‐term (more than 12 months) to follow up. | |
| C (Comparison) | Placebo |
| O (Outcome) | Suggesting common assessment of cognitive level (for example, ADCS‐CGIC score). |
| T (Time stamp) | June 2014 |
| Study type | Randomised controlled trial |
| Methods: concealment clear | |
| Blinding: patients, therapists, assessors blinded |
ADCS‐CGIC = Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change
Acknowledgements
Sponsored by the Chinese Cochrane Center. We wish to acknowledge the consumer referee, Shirley Hall.
Appendices
Appendix 1. MEDLINE search strategy
| Source | Search strategy |
| MEDLINE (Ovid SP) | 1.Cholinesterase Inhibitors/ 2.cholinesterase inhibitor*.mp. 3.Galantamine/ 4.(galantamine OR galanthamin*).mp. 5.reminyl*.mp. 6.(donepezil OR donezepil).mp. 7.aricept*.mp. 8.rivastigmine.mp. 9.exelon*.mp. 10.6 or 3 or 7 or 9 or 2 or 8 or 1 or 4 or 5 11.Frontal Lobe/ 12."frontal lobe dementia*" OR FTD.mp. 13.("frontotemporal dement*" OR "frontotemporal lobar degeneration" OR FTLD).mp. 14.Aphasia, Primary Progressive/ 15."primary progressive aphasia*".mp. 16.HuntingtonDisease/ 17.Huntington*.mp. 18.CADASIL/ 19.CADASIL.mp. 20."cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy".mp. 21."subcortical vascular cognit* impair*".mp. 22.Multiple Sclerosis/ 23.multiple sclerosis.mp. 24.MS.ti. 25.Supranuclear Palsy, Progressive/ 26.progressive supranuclear palsy.mp. 27.Motor Neuron Disease/ 28."motor neuron* disease*".mp. 29.Amyotrophic Lateral Sclerosis/ 30.amyotrophic lateral sclerosis.mp. 31.or/11‐19 32.10 and 31 33.randomized controlled trial.pt. 34.controlled clinical trial.pt. 35.randomized.ab. 36.placebo.ab. 37.drug therapy.fs. 38.randomly.ab. 39.trial.ab. 40.groups.ab. 41.35 or 33 or 39 or 40 or 36 or 38 or 34 or 37 42.(animals not (humans and animals)).sh. 43.41 not 42 44.32 and 43 |
Data and analyses
Comparison 1. Multiple sclerosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive function (medium‐term) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Selective Reminding Test total | 3 | 270 | Mean Difference (IV, Fixed, 95% CI) | 1.47 [‐0.39, 3.32] |
| 1.2 10/36 Spatial Recall Test total | 3 | 270 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐2.23, 0.09] |
| 1.3 Symbol‐Digit Modalities Test (SDMT) | 3 | 270 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.96, 0.37] |
| 1.4 Paced Auditory Serial Addition Test total correct sum of the two and three second forms of the task (PASAT 2+3 sec) | 2 | 189 | Mean Difference (IV, Fixed, 95% CI) | 2.14 [‐1.15, 5.43] |
| 1.5 Controlled Oral Word Association (COWA) | 2 | 189 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.48, 1.17] |
| 2 Clinical global impression of change | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Patients self‐reported impression of memory change | 2 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.93, 3.00] |
| 2.2 Clinician's impression of cognitive change | 2 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.06, 3.62] |
1.1. Analysis.
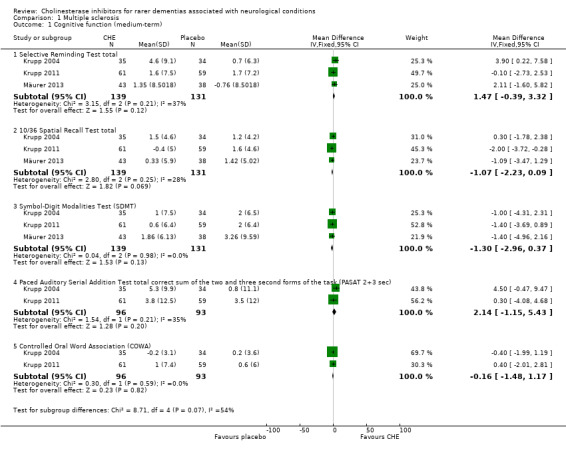
Comparison 1 Multiple sclerosis, Outcome 1 Cognitive function (medium‐term).
1.2. Analysis.
Comparison 1 Multiple sclerosis, Outcome 2 Clinical global impression of change.
Comparison 2. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 6 | 503 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.22, 3.62] |
| 2 Diarrhea | 6 | 503 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.26 [1.72, 6.19] |
| 3 Vomiting | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.76 [1.67, 19.87] |
| 4 Dizzness | 4 | 314 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.43, 4.01] |
| 5 Abnormal dreams | 2 | 189 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.55 [1.50, 8.37] |
2.1. Analysis.
Comparison 2 Adverse events, Outcome 1 Nausea.
2.2. Analysis.
Comparison 2 Adverse events, Outcome 2 Diarrhea.
2.3. Analysis.
Comparison 2 Adverse events, Outcome 3 Vomiting.
2.4. Analysis.
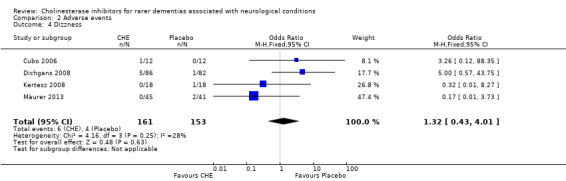
Comparison 2 Adverse events, Outcome 4 Dizzness.
2.5. Analysis.
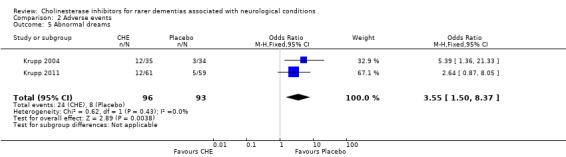
Comparison 2 Adverse events, Outcome 5 Abnormal dreams.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cubo 2006.
| Methods | Study design: a double‐blind, placebo‐controlled, randomised clinical study with 2 parallel arms Method of randomisation: using a random‐length permuted blocks design. The study biostatistician provided the randomisation assignment for each subject to the drug preparer. Blinding: DB. Duration: 12 weeks Exclusions post‐randomisation: 0 Losses to follow up: 6; 3 in donepezil group; 3 in placebo group |
|
| Participants | No. of participants: 30; 15 in the donepezil group, 15 in the placebo group Age: older than 18 years Inclusion criteria: patients who had either a positive test result for HD or a positive family history of chorea and psychiatric disorder, and had a minimum total score of 6 in chorea items of the UHDRS. Exclusion criteria: patients with dementia or a MMSE score below 24; pregnant or breast feeding women, sensitivity to donepezil, depression Hamilton Rating Scale for Depression score ≥ 15), history of stereotaxic brain surgery for HD, and use of cholinergic/anticholinergic/antidopaminergic drugs within 4 weeks before enrolment. |
|
| Interventions | Treatment group: oral donepezil 5 mg daily for 6 weeks, increasing to10 mg for 6 more weeks. Control group: placebo Length of follow up: 12 weeks |
|
| Outcomes | Primary outcomes: 1. ADAS‐Cog; 2. UHDRS‐verbal fluency; 3. UHDRS‐symbol digit modalities; 4. Stroop black and white test; 5. Stroop interference tests; 6. Wechsler Adult Intelligence Survey III symbol searching raw score; 7. Quality of life using a modified Sickness Impact Profile scale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a random‐length permuted blocks design |
| Allocation concealment (selection bias) | Low risk | The study biostatistician provided the randomisation assignment for each subject to the drug preparer. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 participants lost to follow up (20% dropout) |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the protocol of the study, so there was not enough information to assess selective reporting bias. |
| Other bias | Unclear risk | The sample size of study was small. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | DB |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DB |
Dichgans 2008.
| Methods | Study design: a multi‐centre, placebo‐controlled, DB, randomised, parallel‐group trial undertaken in 10 countries. Method of randomisation: using a computer‐generated randomisation protocol. The randomisation ratio was 1:1. Blinding: DB. Pre‐prepared allocation was sent out to centres. Duration: 18 weeks Losses to follow up: 15 patients (4 in placebo, 11 in donepezil), using an ITT analysis with LOCF. The ITT LOCF population included all patients who were randomised, had received at least one dose of study medication, had a baseline assessment, and had at least one post‐baseline assessment from which the last post‐baseline observation for each patient was used. |
|
| Participants | No. of participants: 168; 86 in the donepezil group, 82 in the placebo group Age: 25–70 years Inclusion criteria: 1) having a diagnosis of CADASIL documented by a typical mutation in the NOTCH3 gene, or by the presence of characteristic electron‐dense granular osmiophilic material in blood vessels obtained from biopsy material. 2) having cognitive impairment as defined by both of two criteria: (a) a description of cognitive problems given by patients or their study partners; and (b) an MMSE score of 10‐27 (inclusive), or a TMT B time score 1.5 SD below the mean, after adjustment for age and education. Exclusion criteria: disorders other than CADASIL that may affect cognition or the ability to assess it; new stroke within the past 12 weeks; clinically relevant conditions affecting absorption, distribution, or metabolism of the study medication; clinically significant, active gastrointestinal, renal, hepatic, respiratory, infectious, endocrine, or cardiovascular system disease; left bundle block; pregnancy; history of chronic alcohol or illegal drug use; known hypersensitivity to cholinesterase inhibitors or piperidine‐containing drugs; or unapproved prior or concomitant drugs. |
|
| Interventions | Treatment group: oral donepezil 5 mg/day for 6 weeks, 10 mg/day thereafter. Control group: placebo Length of follow up: 18 weeks |
|
| Outcomes | Primary outcome: 1. Vascular ADAS‐Cog. Secondary outcomes: 1. ADAS‐Cog; 2. MMSE; 3. executive function tests: (1) TMT A and B time, which scores the time needed to complete a specific task; (2) Executive Interview, a 25‐item interview scored from 0 to 50; (3) Stroop Colour and Word Test; (4) CLOX, an executive clock‐drawing test; 4. the Disability Assessment for Dementia scale, which assesses the patient's ability to perform basic ADL and instrumental ADL; 5. Clinical Dementia Rating scale Sum of Boxes. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | using a computer‐generated randomisation protocol |
| Allocation concealment (selection bias) | Low risk | Pre‐prepared allocation was sent out to centres. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 146 patients completed the trial and the analysis of the study was an ITT analysis with LOCF at week 18. |
| Selective reporting (reporting bias) | Low risk | Study well reported all the outcomes described in the protocol registered in register ClinicalTrials.gov. |
| Other bias | Low risk | ‐ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | DB |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DB |
Kertesz 2008.
| Methods | Study design: an open‐label treatment period followed by a randomised, DB, placebo‐controlled phase. Method of randomisation: using a computer‐generated randomisation code. Blinding: DB Duration: 26 weeks (18 weeks for open‐label period and 8 weeks for DB phase) Losses to follow up: 2 in DB phase (1 in galantamine group; 1 in placebo group); using an ITT analysis without LOCF in DB phase. |
|
| Participants | No. of participants: 36 in DB phase, 18 in the galantamine group, 18 in the placebo group Age: 30‐80 years Inclusion criteria: either with documented (≥ one year) primary progressive aphasia, using Mesulam's criteria of predominantly aphasic symptoms at onset and when first seen, or predominantly behavioural variant FTD; a recent (within the year) MRI or CT scan confirming frontotemporal lobar atrophy consistent with Pick Complex/FTD; an MMSE score of more than 5, able to complete neuropsychometric tests. Exclusion criteria: other neurodegenerative disorders, traumatic brain injury, cerebrovascular disease, hypoxic cerebral damage, vitamin deficiency, infection, cerebral neoplasia, uncontrolled epilepsy, clinically significant psychiatric, cardiovascular, renal, pulmonary, metabolic or endocrine disease, history of alcohol or drug abuse, and treatment with agents for dementia or other cognitive impairment. |
|
| Interventions | In the open label phase, the patients were on 8 mg oral galantamine daily for the first four weeks and received 16‐24 mg for the rest of the 18 weeks. In the DB phase: treatment group: 16‐24 mg galantamine daily; control group: placebo; length of follow up: 8 weeks. |
|
| Outcomes | Primary outcomes: 1. Frontal Behavioural Inventory ; 2. Aphasia Quotient of the Western Aphasia Battery; 3. Clinical Global Impressions of Improvement and of Severity. Secondary outcomes: 1. MMSE; 2. Mattis Dementia Rating Scale‐2; 3. Frontal Assessment Battery; 4. Neuropsychiatric Inventory; 5. Alzheimer's Disease Cooperative Study‐ADL scale. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | using a computer‐generated randomisation code. |
| Allocation concealment (selection bias) | Low risk | the DB study medications were provided in numbered blister cards with placebo and galantamine in identical format. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants lost to follow up, using an ITT analysis without LOCF |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the protocol of the study, so there was not enough information to assess selective reporting bias. |
| Other bias | Unclear risk | Carry‐over effects and the sample size of study was small. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | DB |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DB |
Krupp 2004.
| Methods | Study design: a single‐center, DB, placebo‐controlled parallel‐group trial Method of randomisation: the study biostatistician using a computerised random number generator. Blinding: DB ‐ ll clinical staff and patients were masked regarding treatment assignment Duration: 24 weeks Exclusions post‐randomisation: 0 Losses to follow up: 2 (1 in donepezil group; 1 in placebo group); using an ITT population analysis with LOCF. |
|
| Participants | No. of participants: 69; 35 in the donepezil group, 34 in the placebo group Age: from 18 to 55 years Inclusion criteria: having an MMSE score of 26 or more, Montgomery–Åsberg Depression Rating Scale scores of 14 or less, and Expanded Disability Status Scale scores of 6.5 or less. Exclusion criteria: currently taking benzodiazepines and these medications may affect cognition, current alcohol or substance abuse, history of head injury, or other medical condition known to affect cognition. |
|
| Interventions | Treatment group: Oral donepezil 5 mg daily for 4 weeks, increasing to10 mg for 20 more weeks. Control group: placebo Length of follow up: 24 weeks |
|
| Outcomes | Primary outcome: 1. total recall on the SRT Secondary outcomes: 1. patient self‐reported impression of memory change; 2. physician global impression of cognitive change; 3. 10/36 SRT; 4. SDMT; 5. PASAT total correct sum of the two and three second forms of the task; 6. COWA; 7. Tower of Hanoi. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computerized random number generator |
| Allocation concealment (selection bias) | Low risk | The pharmacist was responsible for randomisation assignments and labelling the study drug. All clinical staff and patients were blinded regarding treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants lost to follow up and the analysis of the study was based on the ITT population with LOCF. |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the protocol of the study, so there was not enough information to assess reporting bias. |
| Other bias | Unclear risk | The sample size of the study was small. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The blinding of the active and placebo treatment groups was preserved by creating treatments that looked identical. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DB |
Krupp 2011.
| Methods | Study design: a multi‐centre, randomised, DB, placebo‐controlled trial. Method of randomisation: using a random number generator. Participants were assigned by the pharmacist. All other research staff and participants were blinded regarding treatment assignment. Blinding: Blinding of active and placebo treatments was preserved by creating capsules that appeared identical. Duration: 24 weeks Losses to follow up: 7 (4 in placebo group, 3 in donepezil group). The study used an ITT analysis with LOCF. |
|
| Participants | No. of participants: 120; 61 in the donepezil group, 59 in the placebo group Age: from 18 to 59 years Settings: from 5 Northeastern United States hospital‐based MS centres Inclusion criteria: EDSS scores of 7.0 or less and a score of 0.5 SD below age‐ and gender‐corrected normative data on the Rey Auditory Verbal Learning Test. Exclusion criteria: benzodiazepine use, prior use of donepezil, a current diagnosis of major depression, current alcohol or substance abuse, and history of any other neurologic or medical condition that could adversely affect cognition. |
|
| Interventions | Treatment group: the initial donepezil dose was 5 mg donepezil daily, increased to 10 mg daily at week 4. Control group: placebo Length of follow up: 24 weeks |
|
| Outcomes | Primary outcomes: 1. SRT total recall 2. patients self‐reported impression of memory change. Secondary outcomes: 1.10/36 SRT; 2. SDMT; 3. PASAT total correct sum of the two and three second forms of the task; 4. COWA; 5. Delis‐Kaplan Executive Function System Sorting total; 6. Judgment of Line Orientation total; 7. patient's self‐reported impression of cognitive change; 8. clinician's impression of memory change; 9. clinician's impression of cognitive change; 10. significant other's impression of memory change; 11. significant other's impression of cognitive change. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | using a random number generator |
| Allocation concealment (selection bias) | Low risk | participants were assigned by the pharmacist, all other research staff and participants were blinded regarding treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 participants lost to follow up and the analysis of the study was an ITT analysis with LOCF. |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the protocol of the study, so there was not enough information to assess reporting bias. |
| Other bias | Low risk | ‐ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of active and placebo treatments was preserved by creating capsules that appeared identical. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DB |
Mäurer 2013.
| Methods | Study design: a multi‐center, randomised, DB, placebo‐controlled, parallel‐group trial. Method of randomisation: Randomisation lists were generated by an external CRO using a validated system that automates the random assignment of the treatment arms under the responsibility of the GCP officer. Blinding: DB Duration: 16 weeks (4 weeks for titration period, 12 weeks for DB phase) Losses to follow up: 18; 11 in rivastigmine group, 7 in placebo group. The study used a modified ITT analysis. |
|
| Participants | No. of participants: 86, 45 in rivastigmine group, 41 in placebo group Age: from 18 to 65 years Settings: from 30 investigational sites in Germany Inclusion criteria: FST score of ≥ 3.0 and/or a Multiple Sclerosis Inventarium Cognition score of ≤ 19 Exclusion criteria: used Alzheimer's disease medication, started taking psychoactive medication, or used muscle relaxants or lithium at different time points before randomisation, pregnancy or breastfeeding, diabetes mellitus, malignancy, any cognition‐affecting medical condition, drug addiction, alcohol abuse and depression, subjected to cognitive testing with Brief Repeatable Battery of Neuropsychological Tests within the last year before randomisation, attended any cognitive rehabilitation study or program in the three months prior to the screening visit. |
|
| Interventions | In titration period, the patients received rivastigmine patches 4.6 mg/day for 4 weeks. In DB phase: Treatment group: rivastigmine patches 9.5 mg/day Control group: placebo Length of follow up: 12 weeks |
|
| Outcomes | Primary outcome: total recall on the SRT. Secondary outcomes: 1. 10/36 SRT; 2. SDMT; 3. PASAT‐3 seconds 4. FST; 5. Global rating of change of condition (CGI score) 6. Patient reported impact of multiple sclerosis activities (ADL); 7. Modified Fatigue Impact Scale; |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization lists were generated by an external CRO using a validated system. |
| Allocation concealment (selection bias) | Low risk | The random assignment of the treatment arms was under the responsibility of the GCP officer. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20.9% dropout (24% rivastigmine vs. 17% placebo). |
| Selective reporting (reporting bias) | Low risk | We do not have access to the protocol of the study, so there was not enough information to assess selective reporting bias. |
| Other bias | Unclear risk | Carry‐over effects and the sample size of study was small. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, investigator staff, persons performing the assessments and data analysts remained blinded throughout the entire study period. Study drugs were identical in packaging, labelling, schedule of administration, appearance and odour. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients, investigator staff, persons performing the assessments and data analysts remained blinded throughout the entire study period. |
Sešok 2014.
| Methods | Study design: a DB, placebo‐controlled, randomised clinical study Method of randomisation: no description of the randomisation method in detail. Blinding: DB. Duration: 6 months Exclusions post‐randomisation: 0 Losses to follow up: 1 in treatment group |
|
| Participants | No. of participants: 18; 12 in the rivastigmine group, 6 in the placebo group Age: between 18 and 65 years of age Inclusion criteria: clinically diagnosed and genetically confirmed HD with mild motor impairment, as measured by the Slovenian version of the UHDRS. Mild motor impairment is reflected in the UHDRS score range of 5 ‐ 25. Exclusion criteria: contraindication to rivastigmine, history or presence of neurological disease other than HD; traumatic brain injury; brain surgery; psychiatric disease and all cognitive‐function‐affecting diseases, as well as all life‐threatening states, such as heart rhythm disorder, heart failure, severe and uncontrolled hypertension, severe chronic obstructive pulmonary disease, liver or kidney failure, endocrine disorder and all other study obstructive conditions (severe eyesight loss, language incompatibility, illiteracy). |
|
| Interventions | Treatment group: oral rivastigmine 1.5 mg twice daily for 3 months, increasing to 3 mg twice daily for 3 more months. Control group: placebo Length of follow up: 6 months |
|
| Outcomes | 1. SMDT; 2. Stroop Colour and Word Test; 3. Comprehensive TMT; 4. verbal fluency; 5. Ruff Figural Fluency Test; 6. Tower of London, 7. Rey‐Osterrieth Complex Figure Test, 8. California Verbal Learning Test‐II |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocate participants" was mentioned, but no description of the randomisation method in detail. |
| Allocation concealment (selection bias) | Unclear risk | No information about how the allocation was performed and whether the sequence was concealed or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was lost to follow up |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the protocol of the study, so there was not enough information to assess reporting bias. |
| Other bias | Unclear risk | The sample size of the study was small. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo group was given the inactive ingredient in alike capsules and at same time intervals as the treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DB |
Shaygannejad 2008.
| Methods | Study design: a single‐centre randomised, DB, placebo‐controlled clinical trial. Method of randomisation: using a computer generator Blinding: DB Duration: 12 weeks Exclusions post‐randomization: 0 Losses to follow up: 0 |
|
| Participants | No. of participants: 60 patients were randomised; 30 in the rivastigmine group, 30 in the placebo group. Age: 16‐55 years Settings: hospital Inclusion criteria: stable neurological functioning for at least one month prior to study entry and an EDSS score of 6 or less. All patients displayed at least mild verbal memory impairment as indicated by the WMS. Exclusion criteria: currently taking benzodiazepines, current alcohol or substance abuse, history of head injury, or other medical condition known to affect cognition. Women of childbearing potential had to practice a clinically accepted method of contraception. |
|
| Interventions | Treatment group: 1.5 mg once daily increased over 4 weeks to 3 mg twice daily for a total of 12 weeks Control group: placebo Length of follow up: 12 weeks. |
|
| Outcomes | Primary outcome measure: WMS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | using a computer generator |
| Allocation concealment (selection bias) | Low risk | The hospital pharmacist was responsible for labelling the study drug, maintaining a master list linking the patients and their treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the participants completed the trial and statistical analysis was based on an ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | We do not have access to the protocol of the study, so there was not enough information to assess selective reporting bias. |
| Other bias | Unclear risk | The sample size of the study was small. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | DB |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy assessments of all patients were administered by a single rater in the same sequence who did not know which patients had received which treatment. |
DB = double blind; HD = Huntington’s disease; MMSE = Mini Mental State Examination; UHDRS = Unified Huntington's Disease Rating Scale; ADAS‐Cog = cognitive portion of the Alzheimer Disease Assessment Scale; UHDRS = Unified Huntington's Disease Rating Scale; ITT = intention to treat; LOCF = last outcome carried forward; CADASIL = Cerebral Autosomal‐Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy; TMT = trail‐making test; SD = standard deviation; ADL = activities of daily living; FTD = frontotemporal degeneration; MRI = magnetic resonance imaging; CT = computerised tomography; SRT = Selective Reminding Test; 10/36 SRT = 10/36 Spatial Recall Test; SDMT = Symbol‐Digit Modalities Test; PASAT = Paced Auditory Serial Addition Test; COWA = Controlled Oral Word Association; MS = Multiple Sclerosis; EDSS = Expanded Disability Status Scale; CRO = clinical research organization; GCP = good clinical practice; FST = Faces Symbol Test; WMS = Wechsler Memory Scales.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| de Tommaso 2004 | A open‐label trial |
| de Tommaso 2007 | An open‐label trial |
| Fabbrini 2001 | Not RCT |
| Greene 2000 | A open‐label pilot study |
| Litvan 2001 | The study was a crossover trial and did not provided the data of the first phase. |
| Moretti 2004 | An open‐label study |
Differences between protocol and review
We used "quality of life" as one of the secondary outcome measures in the review.
Contributions of authors
All correspondence: Ying Li and Bi Rong Dong Drafting of review versions: Ying Li, Shan Hai and Yan Zhou Search for trials: Cochrane Dementia and Cognitive Improvement Group Obtaining copies of trial reports: Ying Li and Yan Zhou Selection of trials for inclusion/exclusion: Ying Li, Yan Zhou and Bi Rong Dong Extraction of data: Ying Li and Yan Zhou Entry of data: Ying Li and Yan Zhou Interpretation of data analyses: Yan Zhou and Bi Rong Dong
Sources of support
Internal sources
Chinese Cochrane Centre, China.
External sources
No sources of support supplied
Declarations of interest
Ying Li ‐ None known Shan Hai ‐ None known Yan Zhou ‐ None known Bi Rong Dong ‐ None known
New
References
References to studies included in this review
Cubo 2006 {published data only}
- Cubo E, Shannon KM, Tracy D, Jaglin JA, Bernard BA, Wuu J, et al. Effect of donepezil on motor and cognitive function in Huntington disease. Neurology 2006;67(7):1268‐71. [DOI] [PubMed] [Google Scholar]
Dichgans 2008 {published data only}
Kertesz 2008 {published data only}
Krupp 2004 {published data only}
Krupp 2011 {published data only}
- Krupp LB, Christodoulou C, Melville P, Scherl WF, Pai L‐Y, Muenz LR, et al. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology 2011; Vol. 76, issue 17:1500‐7. [DOI] [PMC free article] [PubMed]
Mäurer 2013 {published data only}
Sešok 2014 {published data only}
- Sešok S, Bolle N, Kobal J, Bucik V, Vodušek DB. Cognitive function in early clinical phase huntington disease after rivastigmine treatment. Psychiatria Danubina 2014;26(3):239‐48. [PubMed] [Google Scholar]
Shaygannejad 2008 {published data only}
References to studies excluded from this review
de Tommaso 2004 {published data only}
- Tommaso M, Specchio N, Sciruicchio V, Difruscolo O, Specchio LM. Effects of rivastigmine on motor and cognitive impairment in Huntington's disease. Movement Disorders 2004;19(12):1516‐18. [DOI] [PubMed] [Google Scholar]
de Tommaso 2007 {published data only}
- Tommaso M, Difruscolo O, Sciruicchio V, Specchio N, Livrea P. Two years' follow‐up of rivastigmine treatment in Huntington disease. Clinical Neuropharmacology 2007;30(1):43‐6. [DOI] [PubMed] [Google Scholar]
Fabbrini 2001 {published data only}
- Fabbrini G, Barbanti P, Bonifati V, Colosimo C, Gasparini M, Vanacore N, Meco G. Donepezil in the treatment of progressive supranuclear palsy. Acta Neurologica Scandinavica 2001;103(2):123‐5. [DOI] [PubMed] [Google Scholar]
Greene 2000 {published data only}
- Greene YM, Tariot PN, Wishart H, Cox C, Holt CJ, Schwid S, et al. A 12‐week, open trial of donepezil hydrochloride in patients with multiple sclerosis and associated cognitive impairments. Journal of clinical psychopharmacology 2000;20(3):350‐6. [DOI] [PubMed] [Google Scholar]
Litvan 2001 {published data only}
Moretti 2004 {published data only}
- Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Rivastigmine in frontotemporal dementia: an open‐label study. Drugs & aging 2004;21(14):931‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Baskys 2012
- Baskys A, Cheng JX. Pharmacological prevention and treatment of vascular dementia: approaches and perspectives. Experimental Gerontology 2012;47(11):887‐91. [DOI] [PubMed] [Google Scholar]
Boeve 2012
- Boeve BF. Progressive supranuclear palsy. Parkinsonism Related Disorders 2012;18(Suppl 1):S192‐4. [DOI] [PubMed] [Google Scholar]
Brun 1994
- Brun A, Englund B, Gustafson L, Passant U, Mann DMA, Neary D, et al. Clinical and neuropathological criteria for frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry 1994;57(4):416‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cacabelos 2008
- Cacabelos R. Pharmacogenomics and therapeutic prospects in dementia. European Archives of Psychiatry and Clinical Neuroscience 2008;258(Suppl 1):28‐47. [DOI] [PubMed] [Google Scholar]
Chiaravalloti 2008
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurology 2008;7(12):1139‐51. [DOI] [PubMed] [Google Scholar]
Choi 2010
- Choi JC. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: a genetic cause of cerebral small vessel disease. Journal of Clinical Neurology (Seoul, Korea) 2010;6(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Fukutake 2011
- Fukutake T. Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL): from discovery to gene identification. Journal of Stroke and Cerebrovascular Diseases 2011;20(2):85‐93. [DOI] [PubMed] [Google Scholar]
Hansen 2008
- Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer's disease: a systematic review and meta‐analysis. Clinical interventions in Aging 2008;3(2):211‐25. [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SJ, Deeks JJ, Altman DJ. Measuring inconsistencies in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoppitt 2011
- Hoppitt T, Pall H, Calvert M, Gill P, Yao G, Ramsay J, et al. A systematic review of the incidence and prevalence of long‐term neurological conditions in the UK. Neuroepidemiology 2011;36(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joutel 1996
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult‐onset condition causing stroke and dementia. Nature 1996;383(6602):707‐10. [DOI] [PubMed] [Google Scholar]
Keverne 2007
- Keverne JS, Low WC, Ziabreva I, Court JA, Oakley AE, Kalaria RN. Cholinergic neuronal deficits in CADASIL. Stroke 2007;38(1):188‐91. [DOI] [PubMed] [Google Scholar]
Kurz 2009
- Kurz A, Farlow M, Lefèvre G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer's disease: a review. International journal of clinical practice 2009;63(5):799‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanctôt 2009
- Lanctôt KL, Rajaram RD, Herrmann N. Therapy for Alzheimer's Disease: How effective are current treatments?. Therapeutic Advances in Neurological Disorders 2009;2(3):163‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2011
- Levine DA, Langa KM. Vascular cognitive impairment: disease mechanisms and therapeutic implications. Neurotherapeutics 2011;8(3):361‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linder 2010
- Linder J, Stenlund H, Forsgren L. Incidence of Parkinson's disease and parkinsonism in northern Sweden: a population‐based study. Movement Disorders 2010;25(3):341‐8. [DOI] [PubMed] [Google Scholar]
Litvan 1996
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): report of the NINDS‐SPSP international workshop. Neurology 1996;47(1):1‐9. [DOI] [PubMed] [Google Scholar]
Martin 2013
- Martin P, Maëlenn G, Matthew P, Alzheimer’s Disease International. Policy Brief for Heads of Government: The Global Impact of Dementia 2013–2050. http://www.alz.co.uk/research/GlobalImpactDementia2013.pdf December 2013.
Mesulam 2003
- Mesulam M, Siddique T, Cohen B. Cholinergic denervation in a pure multi‐infarct state: observations on CADASIL. Neurology 2003;60(7):1183‐5. [DOI] [PubMed] [Google Scholar]
Mollenhauer 2010
- Mollenhauer B, Forstl H, Deuschl G, Storch A, Oertel W, Trenkwalder C. Lewy body and parkinsonian dementia: common, but often misdiagnosed conditions. Deutsches Ärzteblatt International 2010;107(39):684‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narayan 2012
- Narayan SK, Gorman G, Kalaria RN, Ford GA, Chinnery PF. The minimum prevalence of CADASIL in northeast England. Neurology 2012;78(13):1025‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neary 1998
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51(6):1546‐54. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence (NICE). Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease.. London, UK: National Institute for Health and Clinical Excellence (NICE), 2011. [Google Scholar]
Nunnemann 2012
- Nunnemann S, Kurz A, Leucht S, Diehl‐Schmid J. Caregivers of patients with frontotemporal lobar degeneration: a review of burden, problems, needs, and interventions. International Psychogeriatrics/IPA 2012;24(9):1368‐86. [DOI] [PubMed] [Google Scholar]
Odawara 2003
- Odawara T, Shiozaki K, Iseki E, Hino H, Kosaka K. Alterations of muscarinic acetylcholine receptors in atypical Pick's disease without Pick bodies. Journal of Neurology, Neurosurgery and Psychiatry 2003;74(7):965‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paulsen 2011
- Paulsen JS. Cognitive impairment in Huntington disease: diagnosis and treatment. Current Neurology and Neuroscience Reports 2011;11(5):474‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polman 2011
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69(2):292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. [DOI] [PubMed] [Google Scholar]
Premi 2012
- Premi E, Padovani A, Borroni B. Frontotemporal Lobar Degeneration. Advances in Experimental Medicine and Biology 2012;724:114‐27. [DOI] [PubMed] [Google Scholar]
Pringsheim 2012
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington's disease: a systematic review and meta‐analysis. Movement Disorders 2012;27(9):1083‐91. [DOI] [PubMed] [Google Scholar]
Rabinovici 2010
- Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs 2010;24(5):375‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahn 2012
- Rahn K, Slusher B, Kaplin A. Cognitive impairment in Multiple Sclerosis: a forgotten disability remembered. Cerebrum 2012 Nov [Epub ahead of print]; Vol. 14. [PMC free article] [PubMed]
Rascovsky 2011
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(Pt 9):2456‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Razvi 2005
- Razvi SS, Davidson R, Bone I, Muir KW. The prevalence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) in the west of Scotland. Journal of Neurology, Neurosurgery and Psychiatry 2005;76(5):739‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rolinski 2012
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD006504.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruberg 1987
- Ruberg M, Villageois A, Bonnet AM, Pillon B, Rieger F, Agid Y. Acetylcholinesterase and butyrylcholinesterase activity in the cerebrospinal fluid of patients with neurodegenerative diseases involving cholinergic systems. Journal of Neurology, Neurosurgery and Psychiatry 1987;50(5):538‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tayeb 2012
- Tayeb HO, Yang HD, Price BH, Tarazi FI. Pharmacotherapies for Alzheimer's disease: beyond cholinesterase inhibitors. Pharmacology & Therapeutics 2012;134(1):8‐25. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision Version (ICD‐10). Geneva,Switzerland: WHO Press, 2010. [Google Scholar]
References to other published versions of this review
Chabriat 1995
- Chabriat H, Vahedi K, Iba‐Zizen MT, Joutel A, Nibbio A, Nagy TG, et al. Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Lancet 1995;346(8980):934‐9. [DOI] [PubMed] [Google Scholar]


