Abstract
Introduction:
The clinical presentation and outcomes of coronavirus disease 2019 (COVID-19) in kidney transplant recipients (KTRs) have not been well studied.
Methods:
We performed a meta-analysis to examine the presenting features, outcomes and the effect of treatment on outcomes of KTRs with COVID-19. Database search was performed up to 5 September 2020 through PubMed, Embase, Web of Science, Scopus and CENTRAL.
Results:
Overall, 23 studies (1,373 patients) were included in the review and meta-analysis. The most common presenting symptoms included fever (74.0%, 95% confidence interval [CI] 65.3–81.1), cough (63.3%, 95% CI 56.5–69.6) and dyspnoea (47.5%, 95% CI 39.6–55.6). Pooled rates of mortality and critical illness were 21.1% (95% CI 15.3–28.4) and 27.7% (95% CI 21.5–34.8), respectively. Acute kidney injury occurred in 38.9% (95% CI 30.6–48.1) and dialysis was required in 12.4% (95% CI 8.3–18.0) of the cases.
Conclusion:
Kidney transplant recipients with COVID-19 have a similar clinical presentation as the general population, but they have higher morbidity and mortality. It is uncertain whether high-dose corticosteroid or hydroxychloroquine reduces the risks of mortality in KTRs with COVID-19.
Keywords: Coronavirus disease 2019, COVID-19, kidney transplantation, meta-analysis, systematic review
INTRODUCTION
Coronavirus disease 2019 (COVID-19) was first detected in China at the end of 2019 and had evolved into a global pandemic[1] with profound impact on transplantation services around the world.[2,3] Older patients and patients with multiple comorbidities are at increased risk of severe complications, including acute respiratory distress syndrome requiring intensive care support, and death.[4] While kidney transplant recipients (KTRs) are likely to be more vulnerable to severe complications given their immunocompromised status and multiple comorbidities,[5] some have argued that immunosuppression may have protective effects against the severe systemic inflammatory response responsible for severe disease in COVID-19.[6] Also, KTRs have been known to present atypically for other viral illnesses due to factors such as immunosuppression and uraemia.[7] Risk factors for severe COVID-19 in this population and the impact of treatment, such as modification of immunosuppression, remain unclear. Similar studies of COVID-19 have been performed in the general population.[8-12] In the KTR population, systematic reviews[13-17] have been performed; however, meta-analysis has rarely been conducted.[18] The present systematic review and meta-analysis examines the clinical, laboratory and radiological features of KTRs diagnosed with COVID-19, and their outcomes.
METHODS
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The protocol of this systematic review is registered at Prospero (Registration ID: CRD42020183896).
A search for studies that examined COVID-19 in KTRs, limited to studies in the English language, was performed on 5 September 2020 on the databases, PubMed, Embase, Web of Science, Scopus and CENTRAL, according to the registered search strategy [see Supplemental Digital Appendix] with the keywords ‘COVID-19’, ‘kidney’ and ‘transplant’ and their related terms. References of retrieved articles were manually screened for additional eligible publications. Publications were screened for duplication using the Mendeley Reference Management Software.
Two investigators (QYH and TLL) independently screened all titles and abstracts and subsequently reviewed all potentially relevant full-text articles for eligibility for inclusion. Only studies with five or more subjects were included. Disagreement about study inclusion was resolved by consensus. If consensus could not be reached, additional reviewers (HH and TK) arbitrated the disagreement.
The quality of studies included in the meta-analysis was assessed using the Joanna Briggs Institute critical appraisal tools for prevalence studies.[19] The methodological quality was categorised into low (score ≤3), moderate (4–6) and high (≥7). The level of evidence for primary outcomes was assessed using the Grades of Recommendation, Assessment, Development and Evaluation approach.[20]
Data on study and patient characteristics, clinical, laboratory and radiological findings, management strategies and outcomes were extracted independently by two reviewers (QYH and TLL) using a standardised data extraction form. Critical illness was defined as the need for intensive care unit admission and/or mechanical ventilation, as per previous similar studies.[9,10] Missing data were requested from corresponding authors.
The primary outcomes (mortality, critical illness and need for dialysis) and secondary outcomes (need for oxygen, acute kidney injury) were treated as dichotomous variables. All continuous and categorical demographic, clinical and treatment variables were summarised as mean with 95% confidence interval (95% CI) and event rate/proportion with corresponding 95% CI using the random-effects model. Subgroup analysis was also conducted based on continents. I2 index and Q statistic were applied to assess heterogeneity among the studies, and I2 ≥75.0% was considered as considerable heterogeneity.[21] The robustness of pooled conclusion was evaluated using a sensitivity analysis including studies with low and moderate risk of bias (ROB). Meta-regression analyses were also performed to examine the effect of high-dose corticosteroids or hydroxychloroquine use on mortality after adjusting for mechanical ventilation. Bubble plot with a fitted meta-regression line of proportion of outcomes was also plotted. Bubbles were sized according to the precision of each estimate, with larger bubbles denoting more precise estimates. Publication bias was assessed using the funnel plot, Egger’s test and Begg’s test.[22,23] Studies that reported individual-level data were first converted to aggregate level data and then all included studies were pooled using random-effects model. All reported P values were two sided, P <0.05 was considered statistically significant. Statistical analyses were performed using Comprehensive Meta–Analysis Version 3.3.07 (Biostat Inc, Englewood, NJ, USA) software. Meta-regression analysis was performed in SAS version 9.2.2 (SAS Institute, Cary, NC, USA).
RESULTS
Our search identified 1,575 records. After removal of duplicates, 945 unique records were identified, of which 636 records were excluded after screening of titles and abstracts. Of the 309 remaining studies, 23 studies (1,373 patients)[24-46] were included for systematic review and meta-analysis after full-text review [Figure 1]. Details of the included studies are reported in Table 1. The methodological quality for studies included for meta-analysis was assessed to be high for five studies, moderate for 14 studies and low for four studies [Table S1, Supplemental Digital Appendix].
Figure 1.
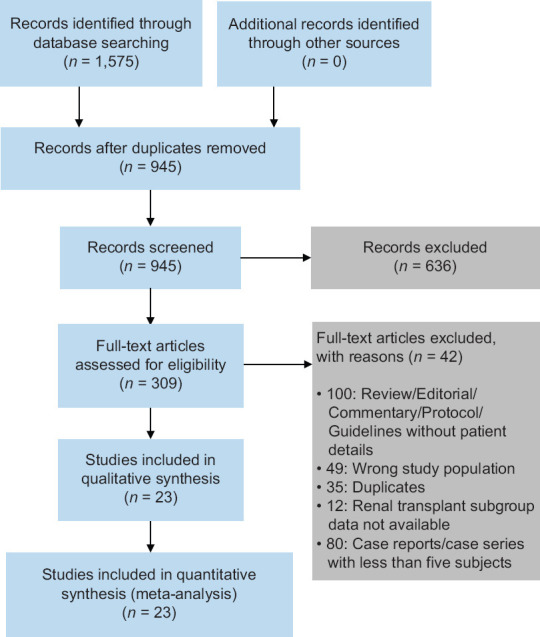
Study selection flow diagram.
Table 1.
Characteristics of included studies.
| Cohort study | Country | Sample size, n | Time frame (in 2020) | Median follow-up durationa (day) | Mean ageb (yr) | Male, n (%) |
|---|---|---|---|---|---|---|
| Asia | ||||||
|
| ||||||
| Zhang et al.[24] | China | 5 | 1 Jan–28 Feb | 29 (range 22–32) | 44.8±11.5 | 4 (80.0) |
|
| ||||||
| Zhu et al.[25] | China | 10 | 17 Jan–5 Feb | 35.5 (range 6–49) | 45.0±14.0 | 8 (80.0) |
|
| ||||||
| Molaei et al.[36] | Iran | 10 | 8 Feb–28 Mar | 20.4±12.9 | 59.6±7.72 | 8 (80.0) |
|
| ||||||
| Monfared et al.[40] | Iran | 22 | 20 Feb–19 Apr | 8.5 (IQR 5.25–13.5) | 52 (IQR 40.75–62.75) | 15 (68.2) |
|
| ||||||
| Abolghasemi et al.[41] | Iran | 24 | 20 Mar–20 May | 6.6 (range 5–9) | 49 (range 29–64) | 15 (62.5) |
|
| ||||||
| Europe | ||||||
|
| ||||||
| Silva et al.[42] | Portugal | 5 | 28 Feb–27 Apr | 30 (range 10–37) | 50.8±13.8 | 5 (100) |
|
| ||||||
| Banerjee et al.[43] | UK | 7 | 2 Mar–17 Mar | 25 (range 5–27) | 57.4±9.6 | 4 (57.1) |
|
| ||||||
| Tschopp et al.[44] | Switzerland | 13 | 9 Mar–6 Apr | 33 (range 8–44) | 59.2±13.6 | 9 (69.2) |
|
| ||||||
| Meziyerh et al.[45] | Netherlands | 15 | 1 Mar–4 May | 30 | 56 (IQR 49–72) | 9 (60.0) |
|
| ||||||
| Devresse et al.[46] | Belgium | 22 | 14 Mar–15 Apr | 18 (range 5–30) | 57 (range 41–73) | 8 (44.4) |
|
| ||||||
| Felldin et al.[26] | Sweden | 35 | 21 Feb–22 Jun | NR | 53.1±12.3 | 23 (65.7) |
|
| ||||||
| Maritati et al.[27] | Italy | 5 | 17 Mar–6 May | 34.8 | 66±9.27 | 3 (60.0) |
|
| ||||||
| Cavagna et al.[28] | Italy | 6 | 1 Feb–28 Apr | NR | 57.5 (IQR 51–64) | 5 (83.3) |
|
| ||||||
| Mella et al.[29] | Italy | 6 | 4 Mar–26 Apr | NR | 55.5±9.3 | 6 (100) |
|
| ||||||
| Bossini et al.[30] | Italy | 53 | 1 Mar–16 Apr | NR | 60 (IQR 50–67) | 42 (79.2) |
|
| ||||||
| Demir et al.[31] | Turkey | 40 | 1 Feb–4 May | 32 (IQR 14–51) | 44.9±14.8 | 20 (50.0) |
|
| ||||||
| Caillard et al.[32] | France | 279 | 4 Mar–21 Apr | 22 | 61.6 (range 50.8–69.0) | 182 (65.2) |
|
| ||||||
| Crespo et al.[33] | Spain | 414 | 18 Mar–16 May | 44 | 62 (IQR 52–71) | 265 (64.0) |
|
| ||||||
| North America | ||||||
|
| ||||||
| Columbia University Kidney Transplant Program[34] | USA | 15 | Until 27 Mar | 7 (range 3–11) | 50.6±21.4 | 10 (66.6) |
|
| ||||||
| Nair et al.[35] | USA | 10 | 1 Mar–27 Mar | 25 (IQR 11–26) | 56.3±15.8 | 6 (60.0) |
|
| ||||||
| Lubetzky et al.[37] | USA | 54 | 13 Mar–20 Apr | 37 | 57 (IQR 29–83) | 38 (70.4) |
|
| ||||||
| Others | ||||||
|
| ||||||
| De Sandes-Freitas et al.[38] | Brazil | 5 | 10–30 Apr | 14 | 39.2±24.0 | 4 (80.0) |
|
| ||||||
| Kates et al.[39] | International | 318 | 1 Mar–15 Apr | >28 | 56 (IQR 46–66) | 186 (58.5) |
aData presented as median unless otherwise specified. bData presented as mean ± standard deviation, unless otherwise specified. IQR: interquartile range, NR: not reported
A total of 1,373 KTRs diagnosed with COVID-19 from 23 studies were included in the meta-analysis. The characteristics, outcomes and management strategies for cases included for meta-analysis are summarised in Table 2. The pooled mean age (23 studies, 1,337 participants) was 55.3 (95% CI 53.0–57.6) years and the proportion of males (23 studies, 1,369 participants) was 63.6% (95% CI 60.7–66.4). The common comorbidities reported included hypertension (18 studies, 916 participants: 76.1%; 95% CI 68.3–82.5) and diabetes mellitus (18 studies, 916 participants: 31.5%; 95% CI 23.9–40.3). Baseline immunosuppression used consisted mainly of calcineurin inhibitors (CNIs) (18 studies, 927 participants: 84.8%; 95% CI 82.2–87.1), mycophenolate (18 studies, 1,016 participants: 75.7%; 95% CI 69.8–80.8) and corticosteroids (19 studies, 1,330 participants: 74.8%; 95% CI 67.7–80.8).
Table 2.
Characteristics, treatments and outcomes of patients in included studies.
| Characteristic | No. of studies | No. of patients | Pooled mean/event rate (95% CI) | I2 (%) |
|---|---|---|---|---|
| Demographic/comorbidity | ||||
|
| ||||
| Age (yr) | 23 | 1,337 | 55.28 (53.00, 57.56) | 83.52 |
|
| ||||
| Male | 23 | 1,369 | 63.59 (60.67, 66.41) | 4.51 |
|
| ||||
| Diabetes mellitus | 18 | 916 | 31.52 (23.87, 40.33) | 74.81 |
|
| ||||
| Hypertension | 18 | 916 | 76.10 (68.28, 82.49) | 67.96 |
|
| ||||
| Cardiac disease | 11 | 495 | 19.64 (14.20, 26.52) | 25.50 |
|
| ||||
| Malignancy | 11 | 711 | 8.26 (4.13, 15.85) | 63.35 |
|
| ||||
| Time after transplant (mth) | 20 | 1,304 | 82.88 (70.66, 95.09) | 82.14 |
|
| ||||
| Baseline Immunosuppression | ||||
|
| ||||
| Calcineurin inhibitors (CNI) | 18 | 927 | 84.81 (82.23, 87.07) | 0.0 |
|
| ||||
| Mycophenolate | 18 | 1,016 | 75.72 (69.79, 80.81) | 49.08 |
|
| ||||
| Corticosteroids | 19 | 1,330 | 74.83 (67.74, 80.81) | 69.50 |
|
| ||||
| mTOR inhibitors | 17 | 1,270 | 10.22 (6.57, 15.55) | 0.0 |
|
| ||||
| Symptoms | ||||
|
| ||||
| Fever | 20 | 1,314 | 73.98 (65.25, 81.14) | 81.91 |
|
| ||||
| Cough | 19 | 900 | 63.28 (56.49, 69.58) | 53.81 |
|
| ||||
| Dyspnoea | 20 | 1,314 | 47.52 (39.58, 55.59) | 75.41 |
|
| ||||
| Sputum | 8 | 79 | 12.10 (4.98, 26.55) | 27.79 |
|
| ||||
| Rhinorrhoea | 8 | 336 | 9.23 (6.55, 12.87) | 0.00 |
|
| ||||
| Sore throat | 9 | 132 | 11.69 (7.10, 18.68) | 0.00 |
|
| ||||
| Myalgia | 11 | 474 | 33.15 (24.71, 42.83) | 39.04 |
|
| ||||
| Fatigue | 10 | 407 | 36.75 (21.86, 54.70) | 66.64 |
|
| ||||
| Vomiting | 10 | 162 | 12.07 (7.76, 18.30) | 0.00 |
|
| ||||
| Diarrhoea | 13 | 487 | 29.65 (23.63, 36.47) | 23.59 |
|
| ||||
| Gastrointestinal symptoms | 13 | 885 | 33.21 (25.30, 42.21) | 66.07 |
|
| ||||
| Laboratory features | ||||
|
| ||||
| White blood cell (×109/L) | 14 | 504 | 6.02 (5.63, 6.42) | 38.16 |
|
| ||||
| Lymphocyte (×109/L) | 17 | 702 | 0.69 (0.62, 0.76) | 69.72 |
|
| ||||
| C-reactive protein (mg/dL) | 15 | 398 | 72.37 (57.32, 87.42) | 78.66 |
|
| ||||
| Chest X-ray findings | ||||
|
| ||||
| Normal | 6 | 381 | 81.18 (70.41, 88.66) | 46.76 |
|
| ||||
| Bilateral/multifocal | 5 | 111 | 65.21 (51.98, 76.46) | 34.97 |
|
| ||||
| Unilateral | 4 | 106 | 20.45 (12.22, 32.19) | 28.86 |
|
| ||||
| Treatment | ||||
|
| ||||
| Increased corticosteroids | 16 | 1,001 | 41.44 (24.61, 60.53) | 92.13 |
|
| ||||
| Discontinued antimetabolite | 16 | 465 | 84.61 (73.69, 91.52) | 74.67 |
|
| ||||
| Reduced or discontinued CNI | 14 | 253 | 76.62 (57.36 88.87) | 79.34 |
|
| ||||
| Discontinued CNI | 16 | 491 | 29.02 (16.55, 45.74) | 81.49 |
|
| ||||
| Hydroxychloroquine | 20 | 1,266 | 65.25 (48.05, 79.22) | 93.26 |
|
| ||||
| Protease inhibitor | 15 | 1,163 | 20.22 (9.39, 38.27) | 92.54 |
|
| ||||
| Anti-influenza agents | 11 | 371 | 28.17 (8.53, 62.27) | 90.26 |
|
| ||||
| Tocilizumab | 12 | 1,178 | 13.03 (7.97, 20.59) | 77.72 |
|
| ||||
| Intravenous immunoglobulin | 10 | 426 | 15.98 (5.68, 37.56) | 82.20 |
|
| ||||
| Remdesivir | 3 | 615 | 2.27 (1.07, 4.76) | 32.74 |
|
| ||||
| Convalescent plasma | 5 | 987 | 2.68 (1.80, 3.96) | 0.00 |
|
| ||||
| Outcomes | ||||
|
| ||||
| Acute kidney injury | 17 | 859 | 38.94 (30.54, 48.06) | 9.26 |
|
| ||||
| Need for dialysis | 16 | 857 | 12.37 (8.3, 18.04) | 19.65 |
|
| ||||
| Required oxygen | 13 | 463 | 61.71 (27.79, 87.09) | 83.38 |
|
| ||||
| Required mechanical ventilation | 22 | 1,331 | 24.50 (20.35, 29.20) | 45.94 |
|
| ||||
| Required ICU/mechanical ventilation | 22 | 1,331 | 27.65 (21.49, 34.8) | 64.08 |
|
| ||||
| Death | 23 | 1,373 | 21.08 (15.27, 28.37) | 49.58 |
I2 represents heterogeneity statistics. Age and all laboratory results are expressed as mean with 95% confidence interval (CI). All other variables are expressed as event rate with 95% CI. ICU: intensive care unit, mTOR: mammalian target of rapamycin
The most common presenting symptoms on meta-analysis were fever (20 studies, 1,314 participants: 74.0%; 95% CI 65.3–81.1), cough (19 studies, 900 participants: 63.3%; 95% CI 56.5–69.6) and dyspnoea (20 studies, 1,314 participants: 47.5%; 95% CI 39.6–55.6) [Table 2]. Diarrhoea was reported in 13 studies (487 participants: 29.7%; 95% CI 23.6–36.5), while gastrointestinal symptoms were reported in 13 studies (885 participants: 33.2%; 95% CI 25.3–42.2).
The pooled mean white blood cell count, lymphocyte count and C-reactive protein level on presentation were 6.02 × 109/L (95% CI 5.63–6.42), 0.69 × 109/L (95% CI 0.62–0.76) and 72.4 mg/dL (95% CI 57.3–87.4), respectively [Table 2].
Chest X-rays on admission were commonly reported to be normal (six studies, 381 participants: 81.2%; 95% CI 70.4–88.7), had bilateral or multifocal infiltrates (five studies, 111 participants: 65.2%; 95% CI 52.0–76.5) or unilateral infiltrates (four studies, 106 participants: 20.5%; 95% CI 12.2–32.2).
Common strategies for modification of immunosuppressants included discontinuation of antimetabolite (16 studies, 465 participants: 84.6%; 95% CI 73.7–91.5) and reduction or discontinuation of CNI (14 studies, 253 participants: 76.62%; 95% CI 57.4–88.9). The CNI was completely discontinued in 16 studies (491 participants: 29.0%; 95% CI 16.6–45.7). On the other hand, corticosteroid dose was increased in 16 studies (1,001 participants: 41.4%; 95% CI 24.6–60.5).
Hydroxychloroquine use was reported in 20 studies (1,266 participants: 65.3%; 95% CI 48.1–79.2), while protease inhibitors were used in 15 studies (1,163 participants: 20.2%; 95% CI 9.4–38.3). Interleukin-6 receptor antagonists, such as tocilizumab, were used in 12 studies (1,178 participants: 13.0%; 95% CI 8.0–20.6).
Other drugs reported included remdesivir (three studies, 13 participants), convalescent plasma (two studies, 11 participants), anti-influenza agents (e.g. oseltamivir, umifenovir or favipiravir) (11 studies, 68 participants), intravenous immunoglobulin (10 studies, 28 participants), ribavirin (three studies, nine participants) and anakinra (one study, three participants). Concomitant antibiotic use was reported in 577 out of 1,106 cases with available data, including azithromycin (393 of 1,056 cases with available data).
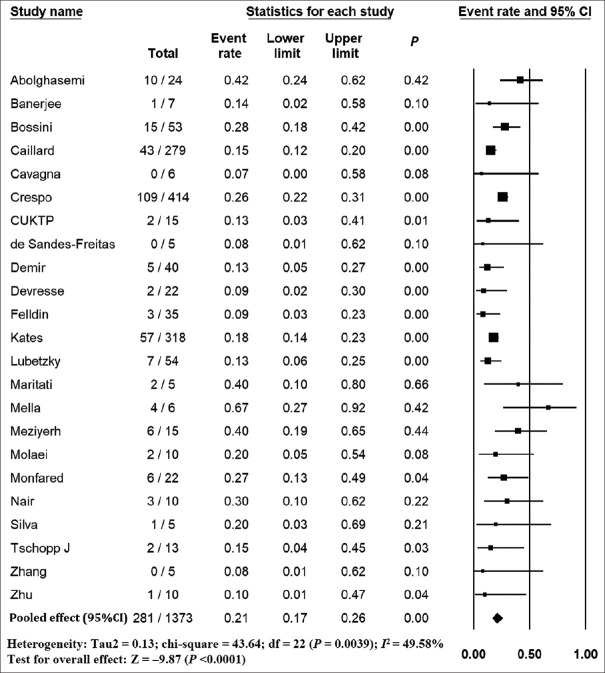
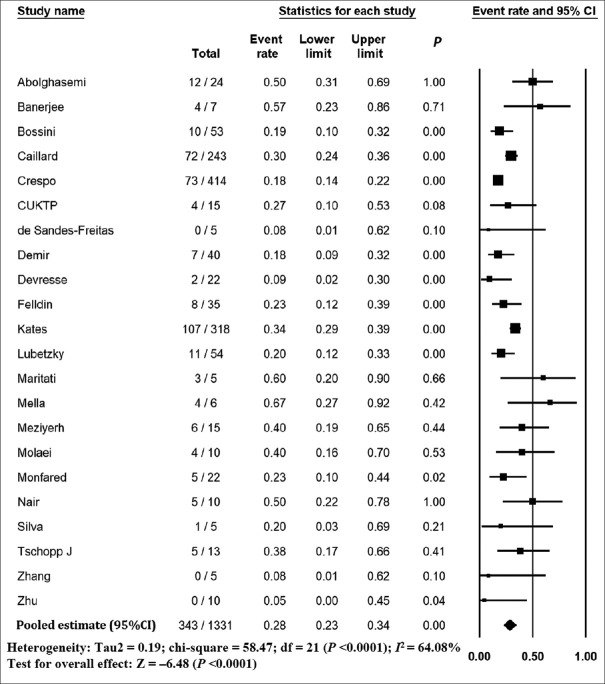
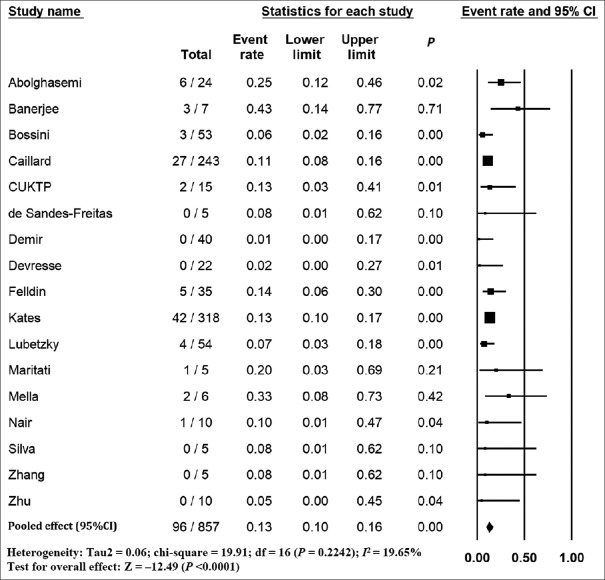
The pooled mortality rate from meta-analysis of 23 studies (1,373 participants) was 21.1% (95% CI 15.3–28.4) [Figure 2]. Similar findings were observed after excluding studies with high ROB [Figure S1, Supplemental Digital Appendix]. Meta-analysis of 13 studies (412 participants) showed a pooled rate of critical illness of 27.7% (95% CI 21.5–34.8) [Figure 3]. There was substantial heterogeneity observed in both outcomes of death (I2 = 49.6%) and critical illness (I2 = 64.1%). In the subgroup analysis based on the geographic distribution of studies, heterogeneity was found in studies from Asia and Europe [Table S2, Supplemental Digital Appendix]. In sensitivity analysis, after excluding studies with high ROB, similar findings were observed [Figure S2, Supplemental Digital Appendix]. The need for oxygen was reported in 13 studies (463 participants: 61.7; 95% CI 27.8–87.1) with substantial heterogeneity. In subgroup analysis, according to the geographic distribution of studies, moderate heterogeneity was observed in Europe and North America [Table S2, Supplemental Digital Appendix]. The rate of acute kidney injury was reported in 17 studies (859 participants: 38.9%; 95% CI 30.5–48.1), while the need for dialysis was reported in 16 studies (857 participants: 12.4%; 95% CI 8.3–18.0) [Figure 4]. Similar findings were observed after excluding studies with high ROB [Figure S3, Supplemental Digital Appendix]. Publication bias for the primary outcomes based on funnel plots and Egger’s regression test did not demonstrate evidence of publication bias [Figures S4–S6, Supplemental Digital Appendix]. In very low-certainty evidence, the use of high-dose corticosteroids or hydroxychloroquine was not associated with mortality outcomes in meta-regression after adjusting for the need for mechanical ventilation [Table S3 and Figure S7, Supplemental Digital Appendix].
Figure 2.
Forest plot shows the incidence of mortality in kidney transplant recipients with COVID-19. CI: confidence interval
Figure 3.
Forest plot shows the incidence of critical illness in kidney transplant recipients with COVID-19. CI: confidence interval
Figure 4.
Forest plot shows the incidence of need for dialysis in kidney transplant recipients with COVID-19. CI: confidence interval
DISCUSSION
This review demonstrated that fever, cough and dyspnoea were the common presenting symptoms in KTRs with COVID-19. In addition, approximately one-third of patients presented with diarrhoea or gastrointestinal symptoms. Furthermore, KTRs with COVID-19 may have higher risk of developing acute kidney injury, higher requirement of dialysis and increased acceptable mortality as compared to the general population It is uncertain whether different treatments (high-dose corticosteroids or hydroxychloroquine) reduce the risks of mortality in KTRs with COVID-19, given the suboptimal quality of included studies in the review.
This review also demonstrated that KTRs with COVID-19 had similar presenting symptoms as the general population with COVID-19,[8,9,11,12] including fever (74.0% vs. 72.4%–87.3%), cough (63.3% vs. 53.9%–60.3%) and dyspnoea (44.4% vs. 18.8%–38.3%). However, diarrhoea was more common in KTRs than in the general population (29.7% vs. 6.8%–9.5%). A previous systematic review of COVID-19 in KTRs including 12 case reports with 204 patients reported similar findings.[13] The presence of gastrointestinal symptoms may reflect more severe COVID-19 in the KTR cohorts[12,47,48] or alterations in the gut immune response and microbiota from uraemia or immunosuppression.[49,50]
In terms of outcomes, the need for oxygen (61.7% vs. 62.6%–71.5%) and intensive care unit admission (27.7% vs. 10.6%–25.6%) for KTRs may be similar to the general population.[8-12] However, KTRs with COVID-19 may have higher risk of acute kidney injury (38.9% vs. 5.7%–7.1%) and higher need for dialysis (12.4% vs. 4.7%–8.3%)[12,51,52] than the general population.
The mortality rate for the general population with COVID-19 was reported to be 3.6%–6.8% in previous meta-analyses.[8,9] However, the present review demonstrated that the mortality rate for KTRs with COVID-19 (21.1%) was higher than for general population with COVID-19. Previous systematic reviews also reported similarly high mortality rates (21.2%–23%)[13,14,16,17,37] in KTRs with COVID-19, which was even higher (46%) for hospitalised patients.[16] The mortality rate reported in the large dialysis cohort studies[53-55] ranged between 14% and 24.9%, which was higher than that of the general population and was slightly lower than or similar to that of KTRs.
Worse outcomes in KTR patients have been shown in studies comparing KTR and non-transplant cohorts.[24,55] Outcomes in the KTR cohort may be worse due to a higher prevalence of risk factors such as advanced age, comorbidities and chronic kidney disease.[56,57] The impact of immunosuppression on the outcomes of KTRs with COVID-19 is uncertain.[58] While immunosuppression may exacerbate COVID-19 by inhibiting appropriate antiviral immune responses, it may also attenuate detrimental hyperinflammatory responses and inhibit viral replication directly.[58-60] Studies on immunosuppression in the non-transplant populations have also not reported worse outcomes.[61,62]
Previous studies in the general population with COVID-19 reported that advanced age, presence of comorbidities,[9,57] laboratory findings such as leucocytosis, lymphopenia and transaminitis, raised lactate dehydrogenase, C-reactive protein and procalcitonin,[10,63] and presence of ground-glass opacities on computed tomography[64] may predict worse outcomes. Analysis of available KTR individual patient-level data from case reports and case series suggested that longer transplant vintage, hypoxaemia and higher lactate dehydrogenase levels are associated with mortality.[17]
Studies on corticosteroids[65,66] and tocilizumab[67,68] showed that they may be useful for the treatment of severe COVID-19, while studies on hydroxychloroquine,[69] protease inhibitors[70,71] and azithromycin[72] did not demonstrate benefit. While remdesivir may shorten the time to recovery in patients with COVID-19[73,74] and vaccines are being developed,[75,76] their efficacy in the KTR subgroup is unclear. Moreover, participants with kidney disease are frequently excluded from COVID-19 clinical trials.[77] In our study, it is uncertain whether different treatments, including increased dose of corticosteroids or use of hydroxychloroquine, reduced the risk of mortality in KTRs with COVID-19 because the quality of evidence was graded as very low, given that all included studies were observational studies and had small sample size with imprecision.
To the best of our knowledge, this is one of the largest systematic reviews and meta-analyses performed on the outcomes of KTRs with COVID-19. However, there are several limitations in this review. Given that new COVID-19 studies are published at a high rate, unindexed or recently published studies that were excluded may have altered the outcomes of our analysis. Excluding non-English studies may also have introduced selection bias. All studies included were observational studies and had relatively small sample sizes. There were some missing data for which we attempted, but were unable, to obtain responses from the primary authors. Moreover, the follow-up duration in many studies was inadequate, with multiple patients still admitted at the time of publication. There was also significant heterogeneity in the reporting and definition of parameters and outcomes. Of note, the largest study, which contributed more than half the cases, was a national registry study that relied on reporting of cases and data by individual centres and collected only limited data. Individual patient data, which may have been able to provide clearer information regarding prognostic factors and response to therapy, were not available.
In conclusion, KTRs with COVID-19 may have similar clinical presentation to the general population, except in the case of diarrhoea. In addition, KTRs with COVID-19 may have higher risk of developing acute kidney injury, higher requirement of dialysis and increased mortality compared to the general population. More high-quality studies and international collaboration are required to investigate the impact of various clinical factors and management strategies on the outcomes of COVID-19 in KTR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Supplemental digital content
Appendix at http://links.lww.com/SGMJ/A52
APPENDIX
Table S1.
Methodological Quality Assessment for Cohort Studies
| Author (Country) | Q1. Was the sample frame appropriate to address the target population? | Q2. Were study participants sampled in an appropriate way? | Q3. Was the sample size adequate? | Q4. Were the study subjects and the setting described in detail? | Q5. Was the data analysis conducted with sufficient coverage of the identified sample? | Q6. Were valid methods used for the identificatio n of the condition? | Q7. Was the condition measured in a standard, reliable way for all participant? | Q8. Was there appropriat e statistical analysis? | Q9. Was the response rate adequate, and if not, was the low response rate managed appropriately ? | Overall Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Asia | ||||||||||
| Zhang et al [24] | Yes | Yes | No | No | Yes | Yes | No | No | Yes | 5 |
| Zhu et al [25] | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | 6 |
| Monfared et al [40] | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Abolghase et al [41] | No | No | No | Yes | Yes | No | No | No | Yes | 3 |
| Molaei et al [36] | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | 6 |
| Europe | ||||||||||
| Banerjee et a I [43] | Yes | Yes | No | No | Yes | Yes | No | No | Yes | 5 |
| Tschopp et al [44] | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | 6 |
| Devresse et al [46] | Yes | Yes | No | No | Yes | No | Yes | No | Yes | 5 |
| Maritati et al [27] | No | No | No | Yes | Yes | Yes | No | No | Yes | 4 |
| Cavagna et al [28] | Yes | No | No | No | No | No | No | No | Yes | 2 |
| Mella et al [29] | No | No | No | No | Yes | Yes | No | No | Yes | 3 |
| Silva et al [42] | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | 7 |
| Bossini et al [30] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Demir et al [31] | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| Caillard et al [32] | No | No | Yes | No | Yes | Yes | No | Yes | Yes | 5 |
| Crespo et al [33] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7 |
| Felldin et al [26] | Yes | Yes | No | No | Yes | Yes | No | No | Yes | 5 |
| Meziyerh et al [45] | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | 6 |
| North America | ||||||||||
| Nair et al [35] | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | 7 |
| Columbia University Kidney Transplant Program [34] | No | No | No | No | Yes | Yes | No | No | Yes | 3 |
| Lubetzky et al [37] | No | No | No | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Others | ||||||||||
| □e Sandes- Freitas et al [38] | Yes | Yes | No | No | Yes | Yes | No | No | Yes | 5 |
| Kates et al [39] | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | 6 |
Table S2.
Subgroup Analysis of Outcomes Based on the Geographical Distribution of Studies
| Outcomes | Event rates (95% Confidence Interval), l2(%) | |||
|---|---|---|---|---|
| Asia | Europe | North America | Overall | |
| Mortality | 26.79(15.37,42.44), 16.58 | 21.05 (15.48, 27.96), 59.89 | 16.55 (8.09, 30.88), 0.00 | 21.08 (15.27, 28.37), 48.46 |
| Critical illness | 31.45(17.96,49.03), 50.49 | 26.83 (19.87, 35.16), 65.34 | 28.51 (15.45, 46.54), 44.76 | 27.65 (21.49, 34.8), 58.38 |
| Dialysis | 17.79 (6.77, 39.19), 7.13 | 12.45 (7.56, 19.81), 44.10 | 9.41 (3.83, 21.33), 0.00 | 12.37 (8.3, 18.04), 23.89 |
| AKI | 46.3(32.99, 60.16), 0.00 | 37.99 (31.27, 45.2), 20.40 | 36.33 (24.7, 49.81), 24.11 | 38.94 (30.54,48.06), 14.38 |
| O2 | 89.63 (65.73, 97.49), 46.06 | 68.76 (49.34, 83.26), 81.85 | 42.09(14.17, 76.19), 68.68 | 61.71 (27.79, 87.09), 83.39 |
Table S3.
Meta-regression of Factors Predicting Mortality
| Variables | Adjusted β estimate | 95% Confidence Interval | p-value |
|---|---|---|---|
| Increased corticosteroids | 0.61 | -0.07-1.30 | 0.07 |
| Hydroxychloroquine | 0.94 | -0.04-1.93 | 0.06 |
| Mechanical ventilation | 3.15 | 0.98-5.31 | 0.009 |
Figure S1.
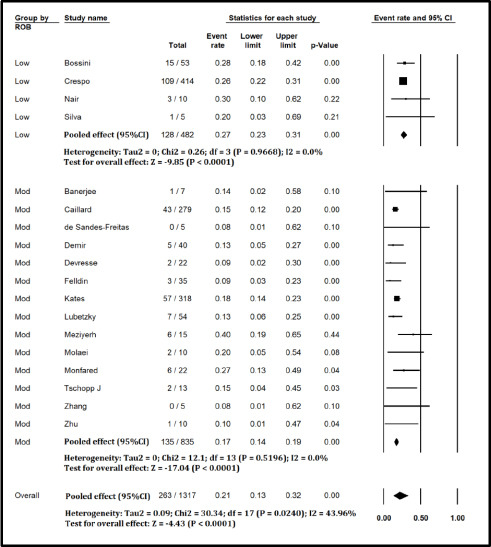
Forest plot showing Incidence of Mortality in Kidney Transplant Recipients with COVID-19 (excluding studies with high risk of bias)
Figure S2.
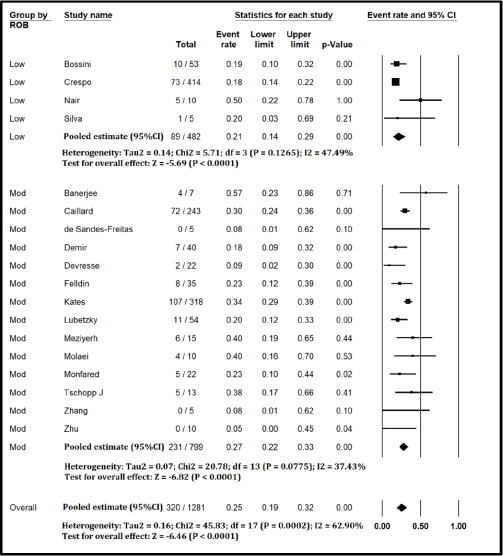
Forest plot showing Incidence of critical Illness in Kidney Transplant Recipients with COVID-19 (excluding studies with high risk of bias)
Figure S3.
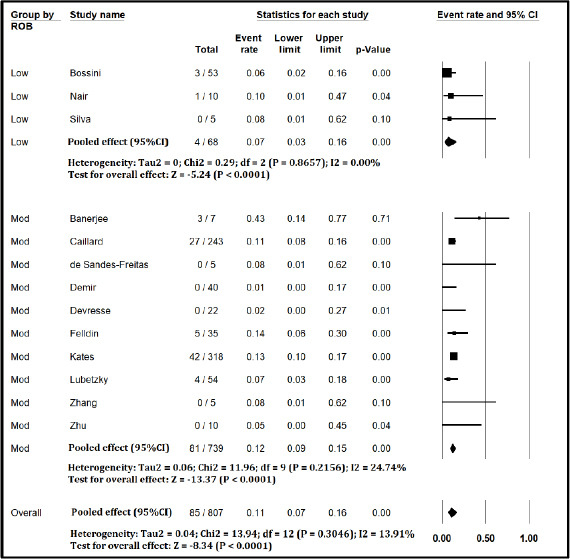
Forest plot showing Incidence of Need for Dialysis in Kidney Transplant Recipients with COVID-19 (excluding studies with high risk of bias)
Figure S4.
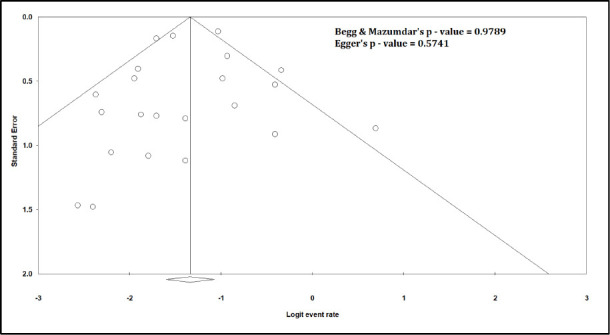
Funnel Plot for Mortality
Figure S5.
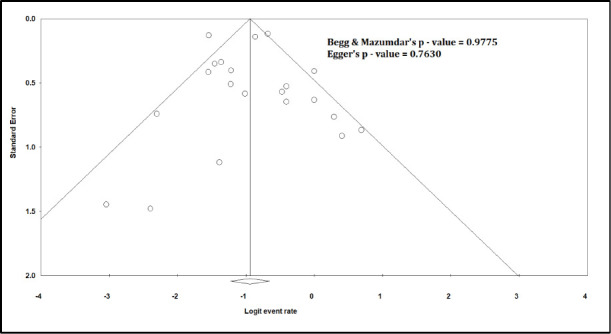
Funnel Plot for Critical illness
Figure S6.
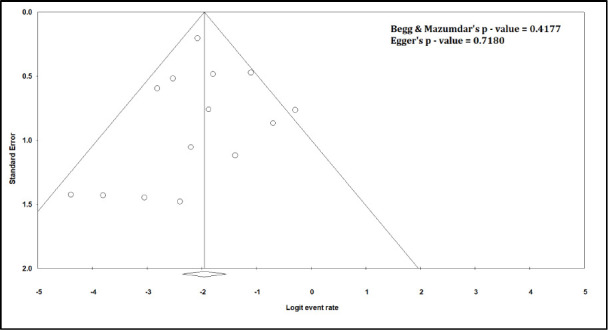
Funnel Plot for Need for Dialysis
Figure S7.
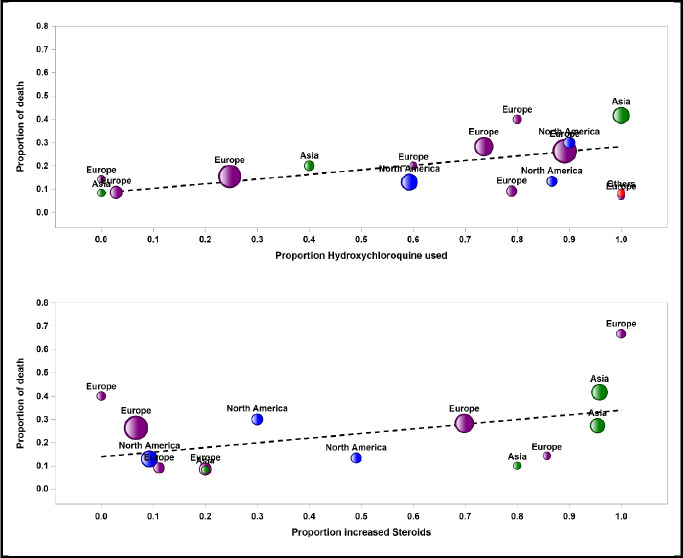
Bubble plots for meta-regression of mortality rate against hydroxychloroquine use and increased corticosteroids
REFERENCES
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyarsky BJ, Chiang TPY, Werbel WA, Durand CM, Avery RK, Getsin SN, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809–18. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395:e95–6. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martino F, Plebani M, Ronco C. Kidney transplant programmes during the COVID-19 pandemic. Lancet Respir Med. 2020;8:e39. doi: 10.1016/S2213-2600(20)30182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma A, Khorsandi SE, Dolcet A, Prachalias A, Suddle A, Heaton N, et al. Low prevalence and disease severity of COVID-19 in post-liver transplant recipients-A single centre experience. Liver Int. 2020;40:1972–6. doi: 10.1111/liv.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karuthu S, Blumberg E. Common infections in kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:2058–70. doi: 10.2215/CJN.04410512. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J Med Virol. 2020;92:1449–59. doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80:656–65. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JJY, Lee KS, Ang LW, Leo YS, Young BE. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: A systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. 2020;71:2199–206. doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CKH, Wong JYH, Tang EHM, Au CH, Wai AKC. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: A systematic review and meta-analysis. Sci Rep. 2020;10:19765. doi: 10.1038/s41598-020-74988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449–58. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oltean M, Søfteland J, Bagge J, Ekelund J, Felldin M, Schult A, et al. Covid-19 in kidney transplant recipients: A systematic review of the case series available three months into the pandemic. Infect Dis (Lond) 2020;52:830–7. doi: 10.1080/23744235.2020.1792977. [DOI] [PubMed] [Google Scholar]
- 14.Aziz F, Mandelbrot D, Singh T, Parajuli S, Garg N, Mohamed M, et al. Early report on published outcomes in kidney transplant recipients compared to nontransplant patients infected with coronavirus disease 2019. Transplant Proc. 2020;52:2659–62. doi: 10.1016/j.transproceed.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marinaki S, Tsiakas S, Korogiannou M, Grigorakos K, Papalois V, Boletis I. A systematic review of COVID-19 infection in kidney transplant recipients: A universal effort to preserve patients’ lives and allografts. J Clin Med. 2020;9:2986. doi: 10.3390/jcm9092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahalingasivam V, Craik A, Tomlinson L, Ge L, Hou L, Wang Q, et al. A systematic review of COVID-19 and kidney transplantation. Kidney Int Rep. 2021;6:24–45. doi: 10.1016/j.ekir.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan I, Rashid T, Suliman S, Amer H, Chirila RM, Mai ML, et al. Predictors of disease severity and outcome of hospitalized renal transplant recipients with COVID-19 infection: A systematic review of a globally representative sample. Rom J Intern Med. 2021;59:10–42. doi: 10.2478/rjim-2020-0034. [DOI] [PubMed] [Google Scholar]
- 18.Phanish M, Ster IC, Ghazanfar A, Cole N, Quan V, Hull R, et al. Systematic review and meta-analysis of COVID-19 and kidney transplant recipients, the South West London Kidney Transplant Network experience. Kidney Int Rep. 2021;6:574–85. doi: 10.1016/j.ekir.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–53. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 24.Zhang H, Chen Y, Yuan Q, Xia QX, Zeng XP, Peng JT, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77:742–7. doi: 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Gong N, Liu B, Lu X, Chen D, Chen S, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: A summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–54. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felldin M, Søfteland JM, Magnusson J, Ekberg J, Karason K, Schult A, et al. Initial report from a Swedish high-volume transplant center after the first wave of the COVID-19 pandemic. Transplantation. 2021;105:108–14. doi: 10.1097/TP.0000000000003436. [DOI] [PubMed] [Google Scholar]
- 27.Maritati F, Cerutti E, Zuccatosta L, Fiorentini A, Finale C, Ficosecco M, et al. SARS-CoV-2 infection in kidney transplant recipients: Experience of the Italian marche region. Transpl Infect Dis. 2020;22:e13377. doi: 10.1111/tid.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavagna L, Seminari E, Zanframundo G, Gregorini M, Di Matteo A, Rampino T, et al. Calcineurin inhibitor-based immunosuppression and COVID-19: Results from a multidisciplinary cohort of patients in Northern Italy. Microorganisms. 2020;8:977. doi: 10.3390/microorganisms8070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mella A, Mingozzi S, Gallo E, Lavacca A, Rossetti M, Clari R, et al. Case series of six kidney transplanted patients with COVID-19 pneumonia treated with tocilizumab. Transpl Infect Dis. 2020;22:e13348. doi: 10.1111/tid.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bossini N, Alberici F, Delbarba E, Valerio F, Manenti C, Possenti S, et al. Kidney transplant patients with SARS-CoV-2 infection: The Brescia renal COVID task force experience. Am J Transplant. 2020;20:3019–29. doi: 10.1111/ajt.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demir E, Uyar M, Parmaksiz E, Sinangil A, Yelken B, Dirim AB, et al. COVID-19 in kidney transplant recipients: A multicenter experience in Istanbul. Transpl Infect Dis. 2020;22:e13371. doi: 10.1111/tid.13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L, et al. An initial report from the French SOT COVID registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–58. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crespo M, Mazuecos A, Rodrigo E, Gavela E, Villanego F, Sánchez-Alvarez E, et al. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplantation. 2020;104:2225–33. doi: 10.1097/TP.0000000000003413. [DOI] [PubMed] [Google Scholar]
- 34.Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–6. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair V, Jandovitz N, Hirsch J, Nair G, Abate M, Bhaskaran M, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1819–25. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molaei H, Khedmat L, Nemati E, Rostami Z, Saadat SH. Iranian kidney transplant recipients with COVID-19 infection: Clinical outcomes and cytomegalovirus coinfection. Transpl Infect Dis. 2021;23:e13455. doi: 10.1111/tid.13455. [DOI] [PubMed] [Google Scholar]
- 37.Lubetzky M, Aull MJ, Craig-Schapiro R, Lee JR, Marku-Podvorica J, Salinas T, et al. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: A report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35:1250–61. doi: 10.1093/ndt/gfaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Sandes-Freitas TV, Canito Brasil IR, Oliveira Sales MLMB, Studart E Neves Lunguinho MS, Pimentel ÍRS, Josino da Costa LAT, et al. Lessons from SARS-CoV-2 screening in a Brazilian organ transplant unit. Transpl Infect Dis. 2020;22:e13376. doi: 10.1111/tid.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. COVID-19 in solid organ transplant: A multi-center cohort study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1097. [Google Scholar]
- 40.Monfared A, Dashti-Khavidaki S, Jafari R, Jafari A, Ramezanzade E, Lebadi MK, et al. Clinical characteristics and outcome of COVID-19 pneumonia in kidney transplant recipients in Razi hospital, Rasht, Iran. Transpl Infect Dis. 2020;22:e13420. doi: 10.1111/tid.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abolghasemi S, Mardani M, Sali S, Honarvar N, Baziboroun M. COVID-19 and kidney transplant recipients. Transpl Infect Dis. 2020;22:e13413. doi: 10.1111/tid.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva F, Cipriano A, Cruz H, Tavares J, Fragoso J, Malheiro J, et al. SARS-CoV-2 infection in kidney transplant recipients: Early report of five cases. Transpl Infect Dis. 2021;23:e13394. doi: 10.1111/tid.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–82. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschopp J, L’Huillier AG, Mombelli M, Mueller NJ, Khanna N, Garzoni C, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20:2876–82. doi: 10.1111/ajt.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meziyerh S, van der Helm D, de Vries APJ. Vulnerabilities in kidney transplant recipients with COVID-19: A single center experience. Transpl Int. 2020;33:1557–61. doi: 10.1111/tri.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devresse A, Belkhir L, Vo B, Ghaye B, Scohy A, Kabamba B, et al. COVID-19 infection in kidney transplant recipients: A single-center case series of 22 cases from Belgium. Kidney Med. 2020;2:459–66. doi: 10.1016/j.xkme.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng SC, Tilg H. COVID-19 and the gastrointestinal tract: More than meets the eye. Gut. 2020;69:973–4. doi: 10.1136/gutjnl-2020-321195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma C, Cong Y, Zhang H. COVID-19 and the digestive system. Am J Gastroenterol. 2020;115:1003–6. doi: 10.14309/ajg.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–70. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibson CM, Childs-Kean LM, Naziruddin Z, Howell CK. The alteration of the gut microbiome by immunosuppressive agents used in solid organ transplantation. Transpl Infect Dis. 2021;23:e13397. doi: 10.1111/tid.13397. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–18. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J, Li J, Zhu G, Zhang Y, Bi Z, Yu Y, et al. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol. 2020;15:1139–45. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.COVID-19, Task Force Committee of the Japanese Association of Dialysis Physicians;Japanese Society for Dialysis Therapy;Japanese Society of Nephrology. Kikuchi K, Nangaku M, Ryuzaki M, et al. COVID-19 in dialysis patients in Japan: Current status and guidance on preventive measures. Ther Apher Dial. 2020;24:361–5. doi: 10.1111/1744-9987.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sánchez-Álvarez JE, Pérez Fontán M, Jiménez Martín C, Blasco Pelícano M, Cabezas Reina CJ, Sevillano Prieto ÁM, et al. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN) Nefrologia (Engl Ed) 2020;40:272–8. doi: 10.1016/j.nefro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu D, Yang B, Xu J, Mao Z, Zhou C, Xue C. COVID-19 infection in a patient with end-stage kidney disease. Nephron. 2020;144:245–7. doi: 10.1159/000507261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nandy K, Salunke A, Pathak SK, Pandey A, Doctor C, Puj K, et al. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab Syndr. 2020;14:1017–25. doi: 10.1016/j.dsx.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kronbichler A, Gauckler P, Windpessl M, Il Shin J, Jha V, Rovin BH, et al. COVID-19: Implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020;16:365–7. doi: 10.1038/s41581-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–42. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: Current state of the science. Immunity. 2020;52:910–41. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: A systematic review and meta-analysis. J Infect. 2020;81:e93–5. doi: 10.1016/j.jinf.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thng ZX, De Smet MD, Lee CS, Gupta V, Smith JR, McCluskey PJ, et al. COVID-19 and immunosuppression: A review of current clinical experiences and implications for ophthalmology patients taking immunosuppressive drugs. Br J Ophthalmol. 2021;105:306–10. doi: 10.1136/bjophthalmol-2020-316586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang ZL, Hou YL, Li DT, Li FZ. Laboratory findings of COVID-19: A systematic review and meta-analysis. Scand J Clin Lab Invest. 2020;80:441–7. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:748–55. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahase E. Covid-19: Low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ. 2020;369:m2422. doi: 10.1136/bmj.m2422. [DOI] [PubMed] [Google Scholar]
- 66.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alzghari SK, Acuña VS. Supportive treatment with tocilizumab for COVID-19: A systematic review. J Clin Virol. 2020;127:104380. doi: 10.1016/j.jcv.2020.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernández-Ruiz M, López-Medrano F, Pérez-Jacoiste Asín M, Maestro de la Calle G, Bueno H, Caro-Teller JM, et al. Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: A single-center cohort study. J Med Virol. 2021;93:831–42. doi: 10.1002/jmv.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease. 2019: Open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–99. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–52. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furtado RHM, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR, Lapa MG, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): A randomised clinical trial. Lancet. 2020;396:959–67. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19-final report. N Engl J Med. 2020;383:1813–26. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–78. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–92. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramasamy M, Minassian A, Ewer K, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–93. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chewcharat A, Chang YT, Sise ME, Bhattacharyya RP, Murray MB, Nigwekar SU. Phase-3 randomized controlled trials on exclusion of participants with kidney disease in COVID-19. Kidney Int Rep. 2021;6:196–9. doi: 10.1016/j.ekir.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]