INTRODUCTION
Coronavirus disease 2019 (COVID-19) infection is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Gastrointestinal symptoms and elevated liver enzymes may be observed in 14%–53% of cases.[1,2] It has been reported that an increase in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) is related to the severity of the disease.[1] Angiotensin-converting enzyme 2 (ACE2) receptors play a role in the entry of the virus into the cell, and these receptors are especially expressed in cholangiocytes. In addition, SARS-CoV-2 can cause cholangiocyte dysfunction and liver damage by triggering a systemic inflammatory response.[3]
In this study, we aimed to examine the frequency and pattern of liver enzyme changes and their relation to poor prognosis or mortality in hospitalised COVID-19 patients.
METHODS
Study design and participants
The data of patients hospitalised due to COVID-19 infection between 16 March 2020 and 11 July 2020 were retrospectively analysed. The patients were categorised into two groups — intensive care unit (ICU) and non-ICU.
Detailed medical history, initial physical examination findings and prior medical data of the patients were retrospectively evaluated. Our primary concern was evaluation of the liver enzyme patterns and their relation to mortality. This study protocol was not able to analyse the effect of COVID-19 infection on chronic liver disease and vice versa, and so patients with the following were excluded from the study: any known liver disease, a history of chronic alcohol consumption, imaging and/or laboratory findings suggestive of liver cirrhosis, immunodeficiency, haematological malignancies, solid organ tumour with liver metastases or active chemotherapy.
The diagnosis of COVID-19 infection was established using the World Health Organization (WHO) interim guidance, which is by reverse transcription-polymerase chain reaction (RT-PCR) on nasal swab sample.[4]
This study was approved by the Ministry of Health, Turkey and the local ethics committee.
Data collection
We collected the patients’ initial, peak and last serum biochemistry test results: AST, ALT, alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), serum amylase, total bilirubin, international normalised ratio (INR) and C-reactive protein (CRP), as well as the initial, lowest and last values for albumin. For ICU patients, the admission and discharge values for AST and ALT and the day of peak enzyme detection during the ICU follow-up period were also noted. Normal limits were defined as follows: ALT ≤40 U/L, AST ≤40 U/L, GGT ≤60 U/L, ALP ≤130 U/L and amylase ≤100 U/L. Initial, peak and last (excluding the patients who died) ALT–AST values were grouped into normal, >1×–≤3 × upper limit of normal (ULN), >3×–≤10 × ULN and > 10 × ULN. Initial, peak and last AST to ALT ratios were calculated; patients were subcategorised based on their AST to ALT ratios — those with ratio >1 and those with ratio >1.5. A 20-unit increase in ALT or AST value from the patient’s baseline was considered as an ALT or AST increase.
Statistical analysis
Comparison of qualitative variables was performed using the Pearson’s chi-square test. The only parametric data was age, for which Student’s t-test was used. Kruskal–Wallis test and Mann–Whitney U test were used for independent non-parametric data; Wilcoxon signed rank test was used to compare the follow-up enzyme levels. Parameters found to be related to need for intensive care/mortality in univariate analysis were further evaluated in binary logistic regression analysis. Statistical analysis was performed using IBM SPSS Statistics version 21.0 (IBM Corp, Armonk, NY, USA).
RESULTS
Four hundred and eight-five patients had positive COVID-19 polymerase chain reaction (PCR) test results. There were 362 patients in the non-ICU group and 123 patients in the ICU group. Table 1 shows the distribution of gender, age, average hospitalisation duration and mortality by patient groups. Sixty-one (16.9%) non-ICU inpatients were transferred to ICU. There was a male preponderance in both the ICU and non-ICU groups, with a higher proportion of males in the former. There was no relation between gender and need for intensive care or mortality in the non-ICU group (P = 0.1 and P = 0.14, respectively).
Table 1.
Demographics and treatment characteristics of patient groups.a
| Variable | n (%) | |
|---|---|---|
|
| ||
| Non-ICU (n=362) | ICU (n=123) | |
| Ageb (yr) | 54.7±16.4 | 66±15.4 |
|
| ||
| Gender | ||
|
| ||
| Male | 203 (56.1) | 84 (68.3) |
|
| ||
| Female | 159 (43.9) | 39 (31.7) |
|
| ||
| Duration of stayb (day) | 9.8±6.5 | 10.3±9 |
|
| ||
| Medication | ||
|
| ||
| Tociluzmab | 57 (15.7) | 38 (30.9) |
|
| ||
| Favipiravir | 193 (53.5) | 114 (92.7) |
|
| ||
| Died | 42 (11.6) | 61 (49.6) |
aInitial laboratory data of patient groups and their statistical analysis are shown in Table 2. bData presented as mean ± standard deviation. ICU: intensive care unit
First ALT–AST values were normal in approximately 80% of the non-ICU inpatients; only 50% of the non-ICU inpatients and 14% of the ICU patients had normal peak enzyme levels. The distributions of ALT and AST in the non-ICU and ICU patients are shown in Figure S1 [see Supplemental Digital Appendix].
In the non-ICU group, 40% had AST or ALT increase (>20 IU increase); peak enzyme levels in these patients were 4.9 times for AST and 5.7 times for ALT. In ICU patients, the proportion of patients with AST (65%) or ALT (58%) increase and their peak enzyme values were higher. Mean duration for AST to reach the peak value in ICU patients was shorter than for ALT to reach the peak value (6.9–7.8 days). Initial enzyme values were higher in males; this difference disappeared in the peak enzyme values. Male gender, need for intensive care and mortality were more frequent in groups with enzyme increase (P < 0.001). ALT increase and mortality were not related (P = 0.6), but a but significant relationship was found between AST increase and mortality (P = 0.008) in the ICU group. ALT and/or AST increase was found to be associated with a longer duration of stay in both patient groups (P < 0.001).
First and peak AST values were higher in patients who died in the ICU group (P = 0.01 and P = 0.001, respectively), while there was no difference in the first and peak ALT values between patients who died and survived (P = 0.9 and P = 0.3, respectively). In the non-ICU group, first AST, peak AST and peak ALT values were higher in patients who were transferred to ICU and/or died (all P < 0.001), but the first ALT values did not significantly differ between the groups (P = 0.9 and P = 0.5, respectively).
First, peak and last enzyme levels (excluding patients who died) were compared to assess the normalisation patterns of ALT and AST. Although the enzyme levels regressed in the non-ICU group, the last values before discharge were higher than the first hospitalisation values (all P < 0.001). In the ICU group, the last AST values decreased to the initial values (P = 0.2), while ALT remained high (P < 0.001) [Table S1, Supplemental Digital Appendix].
Initial AST to ALT ratios were evaluated. ALT was in the foreground in one-third of the non-ICU inpatients, and 26% of the remaining non-ICU inpatients had an AST to ALT ratio of >1.5. In the ICU group, AST was in the foreground in 78% of the patients, and half of the patients had a rate >1.5 times. First and peak AST to ALT ratio >1.5 was associated with mortality in the ICU group (P = 0.003 and P < 0.001, respectively) and with both mortality and need for intensive care in the non-ICU group (all P < 0.001).
High levels of ALP were seen in 15.7% of non-ICU inpatients (2.5 × ULN) and 32% of ICU patients (1.9 × ULN). GGT was found to be high in 36.2% of non-ICU inpatients and 58.5% of ICU patients (3.1–3.3 × ULN). Total bilirubin levels exceeding 1.2 mg/dL were seen in 7.8% and 30% of non-ICU and ICU patients, respectively. Amylase values were high in one-fourth of the non-ICU inpatients and in 28.5% of the ICU patients 2.0 × ULN and 2.6 × ULN, respectively.
Comparison of non-ICU and ICU patients in terms of various laboratory parameters is shown in Table 2. In the ICU group, the mean age was higher and there were more males. While there was no difference between the groups in terms of final AST and ALT values, the median enzyme levels, AST–ALT increase frequencies and AST to ALT ratios, CRP and albumin values were found to be significantly different. Also, the peak INR and total bilirubin levels during follow-up were found to be higher in the ICU group compared to the non-ICU group.
Table 2.
Comparison of some parameters in ICU versus non-ICU patients.
| Parameter | Mean (median)/% | P | |
|---|---|---|---|
|
| |||
| ICUa | Non-ICUa | ||
| Ageb (yr) | 66±15.4 | 52.7±15.7 | <0.001 |
|
| |||
| Male gender | 67.9 | 54.1 | 0.007 |
|
| |||
| AST (U) | |||
|
| |||
| First | 52.2 (38) | 28.9 (24) | <0.001 |
|
| |||
| Peak | 207 (93) | 59.7 (37) | <0.001 |
|
| |||
| Lastc | 30.7 (24) | 30 (24) | 0.89 |
|
| |||
| ALT (U) | |||
|
| |||
| First | 40.1 (25) | 26.3 (21) | 0.06 |
|
| |||
| Peak | 184 (80) | 62.2 (37) | <0.001 |
|
| |||
| Lastc | 43 (27) | 42 (27) | 0.96 |
|
| |||
| Peak ALP (U) | 176.5 (138) | 97.1 (71) | <0.001 |
|
| |||
| Peak GGT (U) | 154.5 (128) | 67.8 (33) | <0.001 |
|
| |||
| Lowest albumin (g/dL) | 2.8 (2.4) | 3.6 (3.6) | <0.001 |
|
| |||
| Peak total bilirubin (mg/dL) | 1.8 (0.9) | 0.7 (0.5) | <0.001 |
|
| |||
| CRP (mg/L) | |||
|
| |||
| First | 164.7 (139) | 42.2 (20) | <0.001 |
|
| |||
| Peak | 222.3 (253) | 71.5 (43) | <0.001 |
|
| |||
| High amylase (>100 U/L) | 28.5 | 20.1 | 0.02 |
|
| |||
| High ALP (>130 U/L) | 33.1 | 11.0 | <0.001 |
|
| |||
| High GGT (>60 U/L) | 60.3 | 30.9 | <0.001 |
|
| |||
| With AST increased | 65.1 | 36.2 | <0.001 |
|
| |||
| With ALT increased | 58.0 | 37.9 | <0.001 |
|
| |||
| First AST to ALT ratio >1 | 78.1 | 66.0 | 0.014 |
|
| |||
| First AST to ALT ratio >1.5 | 55.3 | 26.2 | <0.001 |
|
| |||
| Peak AST to ALT ratio >1 | 63.4 | 48.1 | 0.004 |
|
| |||
| Peak AST to ALT ratio >1.5 | 43.1 | 23.4 | <0.001 |
|
| |||
| Died | 49.6 | 3.2 | <0.001 |
aPatients with both ICU and non-ICU follow-up are evaluated in the ICU group only. bData presented as mean ± standard deviation. cLast AST and ALT values are only evaluated in nondeceased patients. d20unit increase from the patient’s baseline. ALP: alkaline phosphatase, ALT: alanine aminotransferase, AST: aspartate aminotransferase, CRP: Creactive protein, GGT: gammaglutamyl transpeptidase, ICU: intensive care unit
Age, AST and/or ALT increase and admission AST to ALT ratio >1.5 times were found to be associated with mortality in the whole group in univariate analysis. Again, first AST, peak AST and peak ALT values were significantly higher in the patients who died. Results of the logistic regression analysis of parameters related to mortality in the whole group are summarised in Table 3. In the non-ICU group, AST increase was independently associated with the need for ICU transfer (P = 0.006, relative risk [RR] [1.6–15.1]).
Table 3.
Independent risk factors of mortality in the whole group.
| Risk parameter | RR (95% CI) | P |
|---|---|---|
| Age | 1.05 (1.02–1.08) | <0.001 |
|
| ||
| First AST | 1.02 (1.01–1.03) | 0.001 |
|
| ||
| Peak AST | 1.01 (1.00–1.01) | 0.019 |
|
| ||
| Peak ALT | 1.01 (0.99–1.01) | 0.102 |
|
| ||
| With AST increase | 3.93 (1.4–10.8) | 0.008 |
|
| ||
| With ALT increase | 2.07 (0.7–5.9) | 0.171 |
|
| ||
| First AST to ALT ratio >1.5 | 4.3 (1.9–9.6) | <0.001 |
ALT: alanine aminotransferase, AST: aspartate aminotransferase, CI: confidence interval
DISCUSSION
In this study, we have examined the incidence of liver enzyme alterations, their pattern and relation to need for intensive care and mortality among hospitalised COVID-19 patients. Previous studies have indicated that age >50 years may be associated with severe course.[5-7] In our study, mean age and frequency of male gender were higher in ICU patients.
It has been shown in the literature that SARS-CoV can infect hepatocytes. The genetic similarity of the COVID-19 agent, SARS-CoV-2, to SARS-CoV suggests that COVID-19 infection may also involve the liver.[8,9] It has been shown that SARS-CoV-2 requires the ACE2 receptor to enter the cell.[10,11] This receptor is more abundant on cholangiocytes, while it is also expressed in small amounts on hepatocytes.[3] There are different reports about how the liver enzymes change during COVID-19 infection. The incidence of high AST and/or ALT has been reported to be between 4% and 50% in the literature.[12,13] While in the non-ICU group, the rate of normal AST–ALT at baseline was approximately 80%, nearly 75% of the initial ALT values and only half of the initial AST values of ICU patients were within the normal range. In the literature, the reported peak enzyme level is around 5 × ULN.[14,15] In our patient population, peak enzyme values were 6 × ULN and 7 × ULN in the non-ICU and ICU groups, respectively.
Higher amount of ACE2 receptors in cholangiocytes suggests that cholestatic-type enzyme elevation would be the prominent biochemical presentation of liver damage.[3,10,11] However, AST and ALT elevation is more frequently reported.[16,17] Cholestatic enzyme elevation was less prominent when compared to AST and ALT elevation in our study. GGT elevation was more common and prominent than ALP elevation, and the prevalence of cholestatic enzyme elevation was higher in ICU patients. This is parallel to the higher GGT levels seen in severe cases in the literature.[13,18,19]
Although it is theoretically known that COVID-19 infection can cause liver damage, it is difficult to say that these enzyme elevations are caused only by direct viral hepatotoxicity. Hepatotoxicity may arise from four different possible mechanisms: viral; drug induced; overactive immune response; and circulatory impairment or microthrombi-induced hypoxia.[2,20-22] Even the physiopathology has not been clearly demonstrated; AST–ALT elevation is more common in patients with severe infection.[18,23] In our study, the frequency of enzyme increases and the first and peak AST–ALT values were found to be higher in the ICU group. However, there was no difference between the final enzyme values. Secondary infections, multiple antibiotic usage, sepsis or shock in patients under intensive care make it difficult to interpret the real cause of the enzyme elevation, and the association of high cytokine levels with severe disease may indicate immune overaction among the causes of enzyme elevation.[24]
Studies have shown that during severe COVID-19 infection, AST increases earlier, more frequently and progresses to higher values than ALT. Also, AST increase is associated with mortality.[1,16,17,25-27] In univariate analysis, we found that AST or ALT increase during follow-up, first AST, peak AST and peak ALT values were associated with mortality, and in multivariate regression analysis, AST increase and first and peak AST values were found to be related to mortality. AST, which is a less liver-specific enzyme, was also associated with poor prognosis in our study. AST elevation may not be merely due to hepatocyte damage; it may also be caused by widespread tissue damage that could be experienced during sepsis, circulatory dysfunction or immune activation syndrome.[28] Another parameter we evaluated was the AST to ALT ratio. In the ICU group, the incidence of first or peak AST to ALT ratio >1.5 was higher than in the non-ICU group. In a similar patient population, AST to ALT ratio >1 was associated with severe course.[29] Since AST has been shown to be associated with poor prognosis, we think that it may be more valuable to have an AST to ALT ratio cutoff of >1.5. This ratio was significant in terms of the need for intensive care and was associated with mortality in the whole group in logistic regression analysis. As we mentioned earlier, it is difficult to interpret the real cause of the liver enzyme elevation; contribution of extrahepatic (muscle, kidney and cardiac) injury to the AST increase, especially during the late hospital stay, is possible. Higher need for intensive care and mortality of patients with predominant AST increase may be because of the underlying multi-organ injury. However, our study was neither designed to differentiate between the hepatic–extrahepatic sources of enzyme elevation nor to evaluate the contribution of associated complications to liver enzyme elevations. Nevertheless, it is important to emphasise that ALT was high in almost all cases where AST was high at the foreground, suggesting a non-ignorable contribution of ongoing liver injury to high AST levels. Despite these, an initial (at the point where clinical complications are still scarce) AST to ALT ratio >1.5, which we have shown to be associated with morbidity and mortality, may be an early warning regardless of the underlying physiopathology.
We observed that both AST and ALT did not return to basal levels in the non-ICU group, while only the last ALT values were higher in the ICU group. This suggests that although there is a reduction in enzyme levels, normalisation may be achieved after clinical improvement. The normalisation of AST values with still relatively high ALT levels in the ICU group may be related to the shorter half-life of AST and the possible longer disease duration of these patients. Still, long-term enzyme change pattern after COVID-19 infection is another issue open to evaluation.
It has been reported that hypoalbuminaemia (<4 g/dL) can be seen in half of patients with COVID-19 infection and is associated with severity and mortality.[19,30,31] In our study, albumin level was lower in the ICU group (2.4–3.6 g/dL, P < 0.001). Studies report that during COVID-19 infection, pre-albumin levels and hepatic synthesis capacity decrease and malnutrition may contribute to the process.[19] However, albumin is also a negative acute phase reactant and cytokine levels are higher in severe infection.[32] In addition to malnutrition, this situation probably influences the frequency of hypoalbuminaemia.
Our study has some limitations. Firstly, it has a retrospective design. Another limitation is that although the first and follow-up enzyme values were evaluated, it is difficult to reveal the primary cause (COVID-19 infection, secondary infection, drug effect, adult respiratory distress syndrome, disseminated Iintravascular coagulation or cytokine storm) leading to the enzyme elevations. It is reported that obesity and non-alcoholic fatty liver disease (NAFLD) may be associated with higher incidence of enzyme elevation during COVID-19 infection.[33] Since data on NAFLD frequency in our patients were not available, we cannot comment on this relationship. We evaluated liver enzyme levels only in hospitalised patients. The fact that AST elevation is associated with poor outcomes evoked the question whether this pattern could predict hospitalisation or be incorporated to screen suspected COVID-19 infection. Further studies should assess this potential.
In conclusion, our study showed that elevated liver enzymes with AST in the foreground can be frequently seen in hospitalised COVID-19 patients. Increase in AST during hospitalisation and higher AST levels may be a sign that a patient needs intensive care. Increasing AST and an initial AST to ALT ratio >1.5 are associated with mortality and may be an early warning.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Supplemental digital content
Appendix at https://links.lww.com/SMJ/XXX
APPENDIX
Table S1.
Comparison of first and last AST and ALT values according to enzyme increase among non-deceased patients.
| Variable | Non-ICU group | ICU group | ||||||
|---|---|---|---|---|---|---|---|---|
| First valuea | Last valuea | P | n | First valuea | Last valuea | P | n | |
| With AST increase | 28(32) | 40 (47) | <0.001 | 101 | 35 (38) | 34 (35) | 0.261 | 32 |
| Without AST increase | 22 (27) | 21 (23) | <0.001 | 215 | 36 (44) | 20 (25) | 0.001 | 27 |
| With ALT increase | 25 (30) | 57(77) | <0.001 | 121 | 31 (38) | 57 (58) | <0.001 | 35 |
| Without ALT increase | 19 (24) | 19 (23) | 0.016 | 195 | 21 (27) | 20 (28) | 0.483 | 26 |
aData presented as median (mean) U. ALT: alanine aminotransferase, AST: aspartate aminotransferase, ICU: intensive care unit
Figure S1.
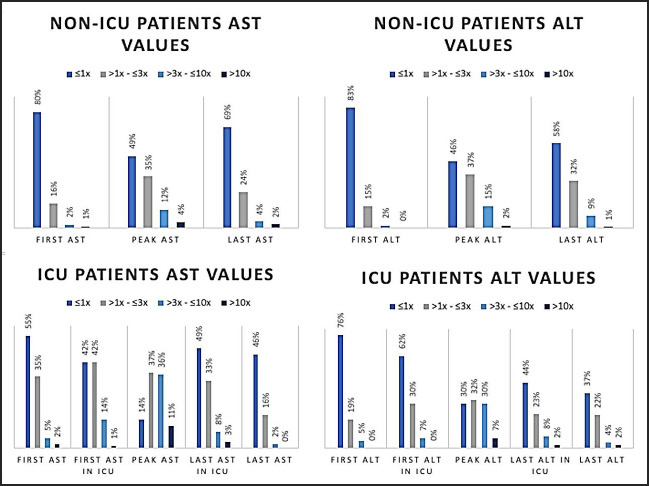
Distribution of first, peak and last ALT & AST values in ICU and Non.ICU patients. ALT: alanine aminotransferase, AST: aspartate aminotransferase, ICU: intensive care unit
REFERENCES
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–30. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020:20200203931766. doi: 10.1101/2020.02.03.931766. [Google Scholar]
- 4.Lin L, Li TS. [Interpretation of “Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Trial Version 5)”. Zhonghua Yi Xue Za Zhi. 2020;100:E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. Chinese. [DOI] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kragholm K, Andersen MP, Gerds TA, Butt JH, Ostergaard L, Polcwiartek C, et al. Association between male sex and outcomes of Coronavirus Disease 2019 (Covid-19)-A Danish nationwide, register-based study. Clin Infect Dis. 2021;73:e4025–30. doi: 10.1093/cid/ciaa924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham JW, Vaduganathan M, Claggett BL, Jering KS, Bhatt AS, Rosenthal N, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020;181:379–81. doi: 10.1001/jamainternmed.2020.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–60. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao SR, Zhang SY, Lian JS, Jin X, Ye CY, Cai H, et al. Liver enzyme elevation in coronavirus disease. 2019: A multicenter, retrospective, cross-sectional study. Am J Gastroenterol. 2020;115:1075–83. doi: 10.14309/ajg.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali N. Relationship between COVID-19 infection and liver injury: A review of recent data. Front Med (Lausanne) 2020;7:458. doi: 10.3389/fmed.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with Coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–77. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–30. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, et al. COVID-19 and liver injury: A meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990–5. doi: 10.1097/MEG.0000000000001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72:389–98. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–74. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 20.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92:2409–11. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–52. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–16. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–81. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–30. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medetalibeyoglu A, Catma Y, Senkal N, Ormeci A, Cavus B, Kose M, et al. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol. 2020;19:614–21. doi: 10.1016/j.aohep.2020.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: A systematic review and meta-analysis. Crit Care. 2020;24:255. doi: 10.1186/s13054-020-02995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92:2152–8. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm'in COVID-19. J Infect. 2020;80:607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertolini A, van de Peppel IP, Bodewes F, Moshage H, Fantin A, Farinati F, et al. Abnormal liver function tests in COVID-19 patients: Relevance and potential pathogenesis. Hepatology. 2020;72:1864–72. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]


