Abstract
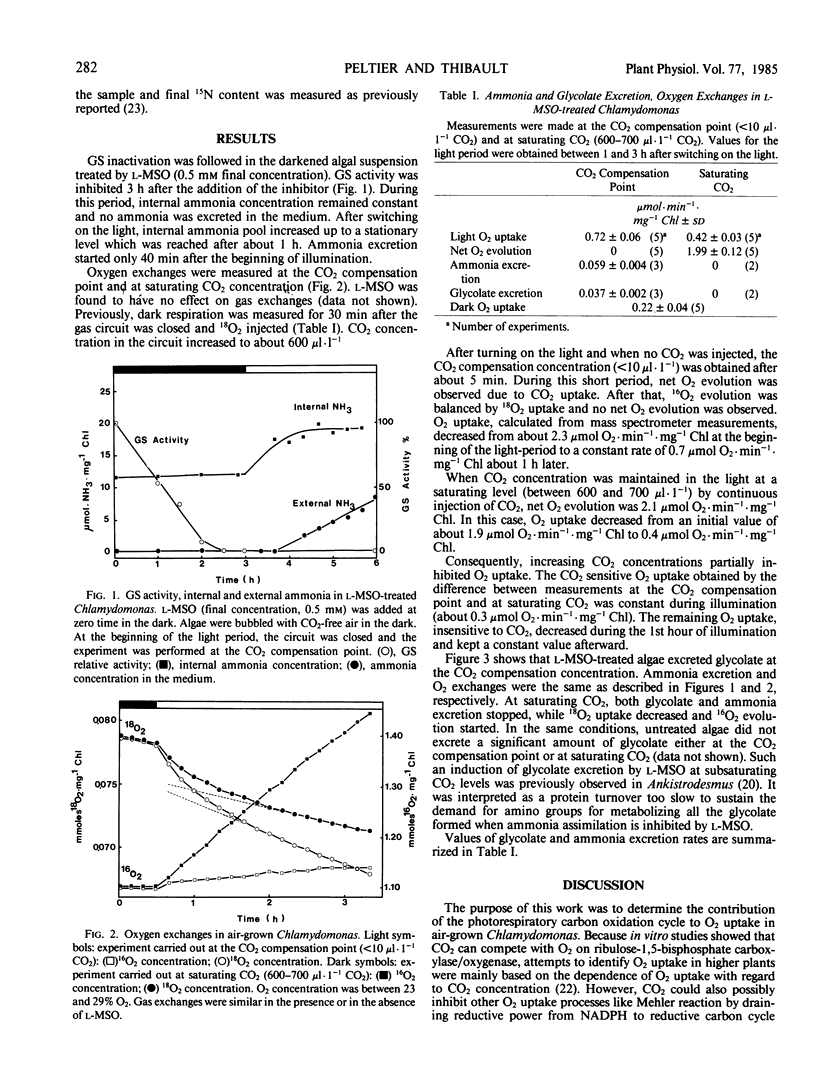
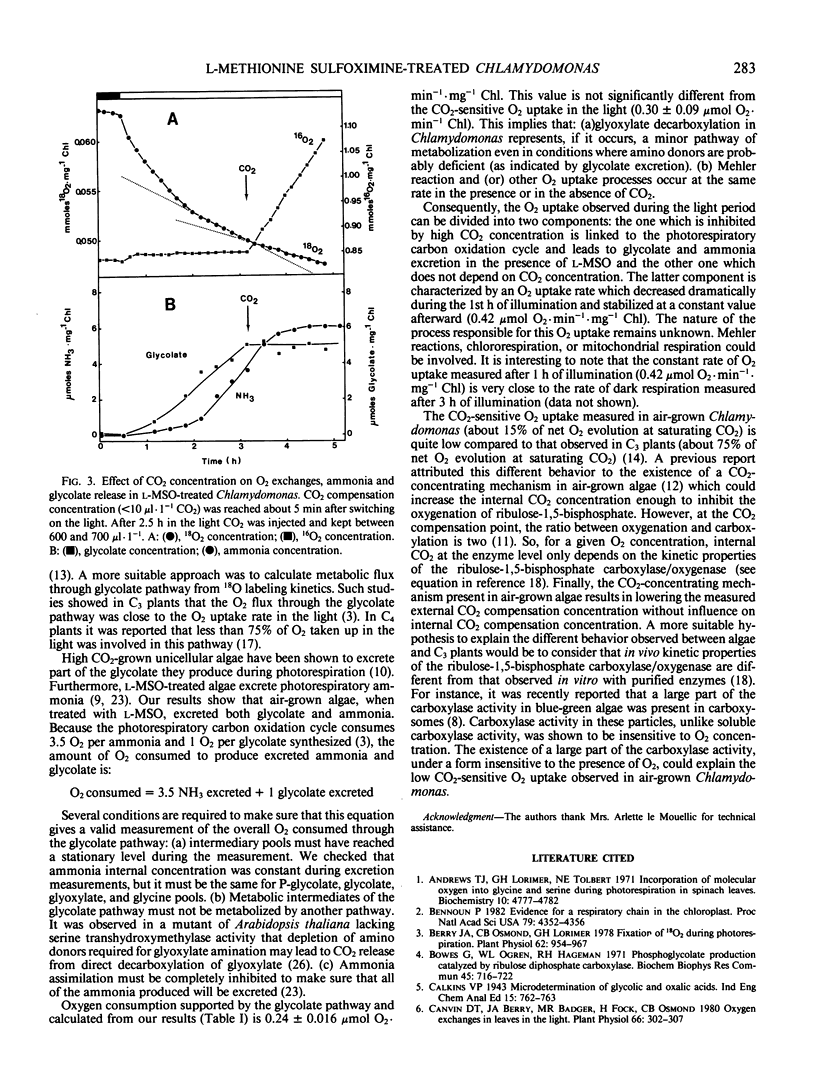
Glycolate and ammonia excretion plus oxygen exchanges were measured in the light in l-methionine-dl-sulfoximine treated air-grown Chlamydomonas reinhardii. At saturating CO2 (between 600 and 700 microliters per liter CO2) neither glycolate nor ammonia were excreted, whereas at the CO2 compensation concentration (<10 microliters per liter CO2) treated algae excreted both glycolate and ammonia at rates of 37 and 59 nanomoles per minute per milligram chlorophyll, respectively. From the excretion values we calculate the amount of O2 consumed through the glycolate pathway. The calculated value was not significantly different from the component of O2 uptake sensitive to CO2 obtained from the difference between O2 uptake of the CO2 compensation point and at saturating CO2. This component was about 40% of stationary O2 uptake measured at the CO2 compensation point. From these data we conclude that glyoxylate decarboxylation in air-grown Chlamydomonas represents a minor pathway of metabolism even in conditions where amino donors are deficient and that processes other than glycolate pathway are responsible for the O2 uptake insensitive to CO2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Lorimer G. H., Tolbert N. E. Incorporation of molecular oxygen into glycine and serine during photorespiration in spinach leaves. Biochemistry. 1971 Dec 7;10(25):4777–4782. doi: 10.1021/bi00801a027. [DOI] [PubMed] [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., Andre M. Photosynthesis and photorespiration in whole plants of wheat. Plant Physiol. 1979 Nov;64(5):735–738. doi: 10.1104/pp.64.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaud A., André M. Effect of CO(2), O(2), and Light on Photosynthesis and Photorespiration in Wheat. Plant Physiol. 1980 Dec;66(6):1032–1036. doi: 10.1104/pp.66.6.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- Jolivet-Tournier P., Gerster R. Incorporation of Oxygen into Glycolate, Glycine, and Serine during Photorespiration in Maize Leaves. Plant Physiol. 1984 Jan;74(1):108–111. doi: 10.1104/pp.74.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M., Larsson C. M., Ullrich W. R. Regulation by amino acids of photorespiratory ammonia and glycolate release from ankistrodesmus in the presence of methionine sulfoximine. Plant Physiol. 1982 Dec;70(6):1637–1640. doi: 10.1104/pp.70.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch Biochem Biophys. 1951 Dec;34(2):339–351. doi: 10.1016/0003-9861(51)90012-4. [DOI] [PubMed] [Google Scholar]
- Peltier G., Thibault P. Ammonia exchange and photorespiration in chlamydomonas. Plant Physiol. 1983 Apr;71(4):888–892. doi: 10.1104/pp.71.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration-deficient Mutants of Arabidopsis thaliana Lacking Mitochondrial Serine Transhydroxymethylase Activity. Plant Physiol. 1981 Apr;67(4):666–671. doi: 10.1104/pp.67.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk R. J., Jackson W. A. Photorespiratory phenomena in maize: oxygen uptake, isotope discrimination, and carbon dioxide efflux. Plant Physiol. 1972 Feb;49(2):218–223. doi: 10.1104/pp.49.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]


