Abstract
The novel sucrose derivative 1′-fluorosucrose (α-d-glucopyranosyl-β- d-1-deoxy-1-fluorofructofuranoside) was synthesized in order to help define mechanisms of sucrose entry into plant cells. Replacement of the 1′-hydroxyl by fluorine very greatly reduces invertase hydrolysis of the derivative (hydrolysis at 10 millimolar 1′-fluorosucrose is less than 2% that of sucrose) but does not reduce recognition, binding, or transport of 1′-fluorosucrose by a sucrose carrier. Transport characteristics of 1′-fluorosucrose were studied in three different tissues. The derivative is transported by the sucrose carrier in the plasmalemma of developing soybean cotyledon protoplasts with a higher affinity than sucrose (Km 1′-fluorosucrose 0.9 millimolar, Km sucrose 2.0 millimolar). 1′-Fluorosucrose is a competitive inhibitor of sucrose uptake with an apparent Ki also of 0.9 millimolar, while the Ki of sucrose competition of 1′-fluorosucrose uptake was 2.0 millimolar. Thus, both sugars are recognized at the same binding site in the plasmalemma. Both sucrose and 1′-fluorosucrose show very similar patterns of phloem translocation from an abraded leaf surface through the petiole indicating that recognition of 1′-fluorosucrose by sucrose carriers involved in phloem loading is likely as well.
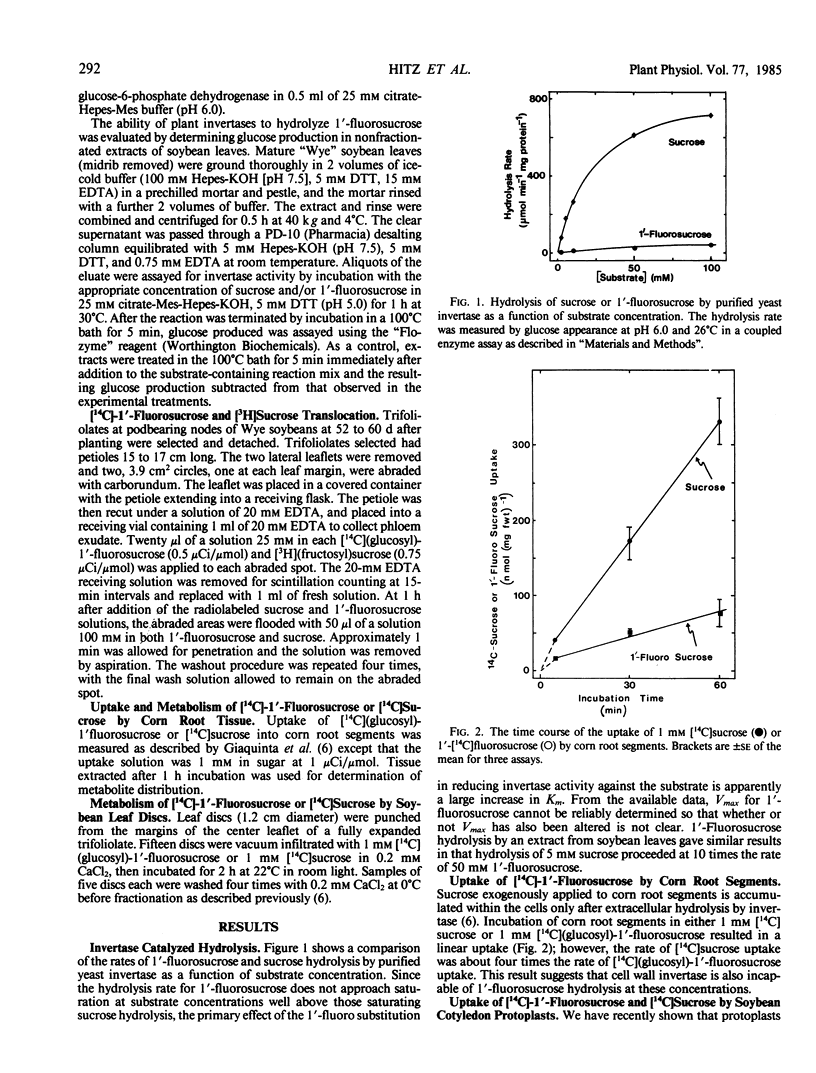
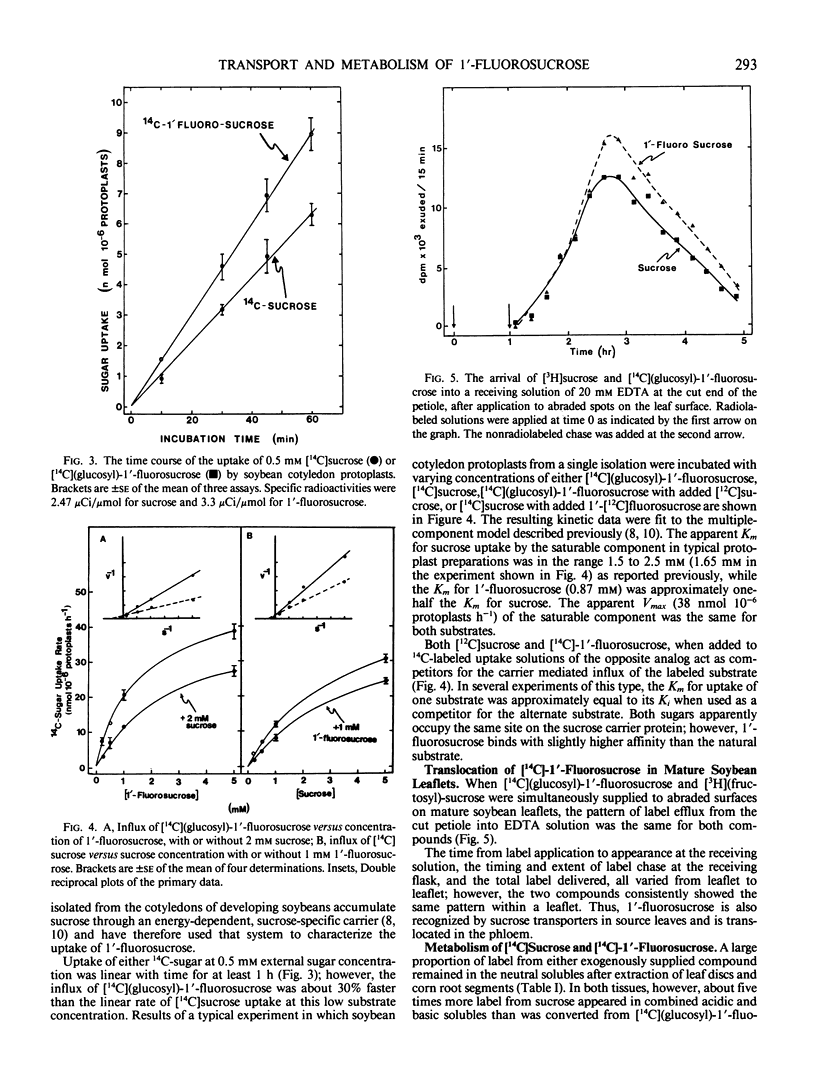
1′-Fluorosucrose is a very poor substrate for invertase and as such is absorbed only slowly by corn root segments, a tissue in which sucrose hydrolysis by a cell wall invertase is required prior to active hexose uptake.
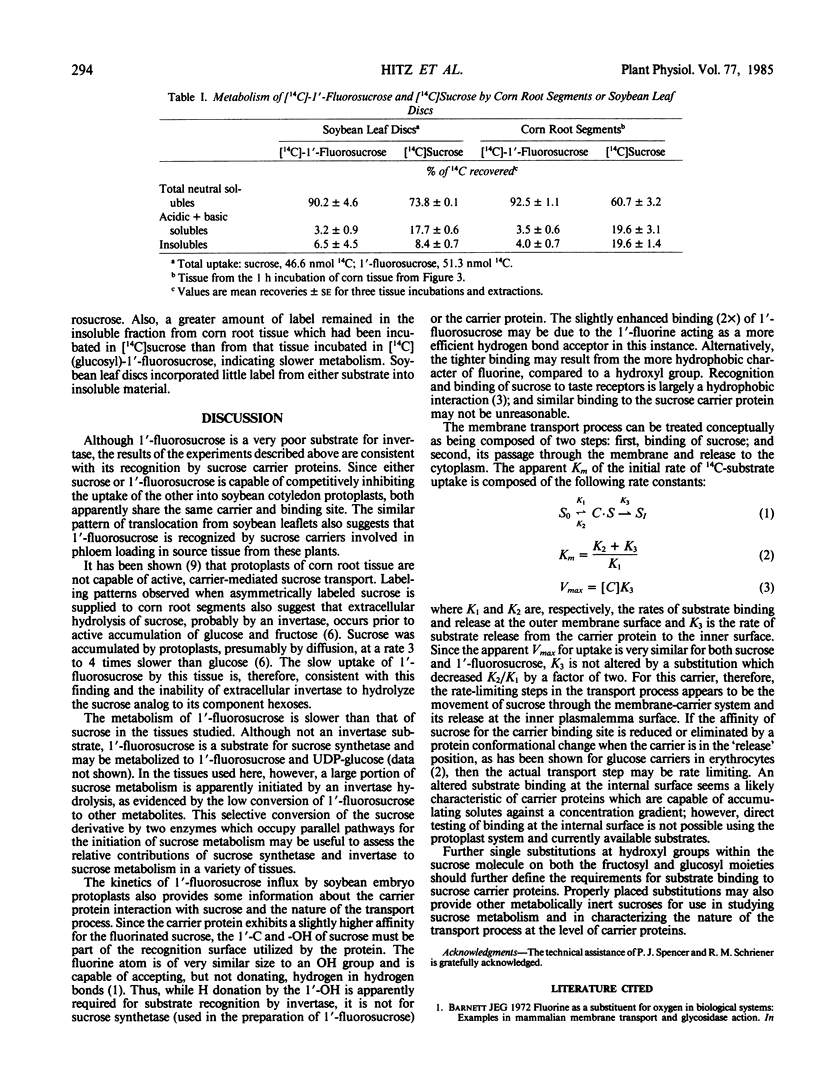
The kinetics of 1′-fluorosucrose uptake by soybean cotyledon protoplasts indicate that membrane passage and substrate release to the protoplast interior are rate limiting to transport. Recognition of sucrose at the inner membrane surface of the carrier protein is apparently different than recognition and binding at the external surface.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Holman G. D., Chalkley R. A., Munday K. A. Evidence for two asymmetric conformational states in the human erythrocyte sugar-transport system. Biochem J. 1975 Mar;145(3):417–429. doi: 10.1042/bj1450417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T., Lin W., Sadler N. L., Franceschi V. R. Pathway of Phloem unloading of sucrose in corn roots. Plant Physiol. 1983 Jun;72(2):362–367. doi: 10.1104/pp.72.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Schmitt M. R., Hitz W. D., Giaquinta R. T. Sugar transport in isolated corn root protoplasts. Plant Physiol. 1984 Dec;76(4):894–897. doi: 10.1104/pp.76.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Schmitt M. R., Hitz W. D., Giaquinta R. T. Sugar transport into protoplasts isolated from developing soybean cotyledons : I. Protoplast isolation and general characteristics of sugar transport. Plant Physiol. 1984 Aug;75(4):936–940. doi: 10.1104/pp.75.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. R., Hitz W. D., Lin W., Giaquinta R. T. Sugar Transport into Protoplasts Isolated from Developing Soybean Cotyledons : II. Sucrose Transport Kinetics, Selectivity, and Modeling Studies. Plant Physiol. 1984 Aug;75(4):941–946. doi: 10.1104/pp.75.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]


