Abstract
Interest in the pathophysiology, etiology, management, and outcomes of patients with tricuspid regurgitation (TR) has grown in the wake of multiple natural history studies showing progressively worse outcomes associated with increasing TR severity, even after adjusting for multiple comorbidities. Historically, isolated tricuspid valve surgery has been associated with high in-hospital mortality rates, leading to the development of transcatheter treatment options. The aim of this first Tricuspid Valve Academic Research Consortium document is to standardize definitions of disease etiology and severity, as well as endpoints for trials that aim to address the gaps in our knowledge related to identification and management of patients with TR. Standardizing endpoints for trials should provide consistency and enable meaningful comparisons between clinical trials. A second Tricuspid Valve Academic Research Consortium document will focus on further defining trial endpoints and will discuss trial design options.
Keywords: transcatheter, treatment, tricuspid regurgitation
Highlights.
There is an independent relationship between increasing TR and worse outcomes, but gaps exist in understanding of pathophysiology, diagnosis, and treatment.
Refinement of classification categories, novel methods for assessing and grading TR, and better selection of outcome measures should inform the design of clinical trials.
This document provides recommendations for classification of disease etiology, standardized definitions and methods to assess disease severity, and trial endpoints.
Transcatheter alternatives to surgical intervention are under investigation; yet, more work is needed to define patient selection criteria, goals of therapy, and clinical outcomes.
Abbreviations and Acronyms
- 3D-VCA
3-dimensional vena contracta area
- A-STR
atrial secondary tricuspid regurgitation
- CIED
cardiac implantable electronic device
- CMR
cardiac magnetic resonance
- CT
computed tomography
- EROA
effective regurgitant orifice area
- LTR-A
lead-associated tricuspid regurgitation, type A
- LTR-B
lead-associated tricuspid regurgitation, type B
- PA
pulmonary artery
- PH
pulmonary hypertension
- PISA
proximal isovelocity surface area
- RA
right atrium/atrial
- RV
right ventricle/ventricular
- RVEF
right ventricular ejection fraction
- sPAP
systolic pulmonary artery pressure
- TAPSE
tricuspid annular plane systolic excursion
- TR
tricuspid regurgitation
- T-TEER
tricuspid transcatheter edge-to-edge repair
- TTVI
transcatheter tricuspid valve intervention
- V-STR
ventricular secondary tricuspid regurgitation
Interest in the pathophysiology, etiology, management, and outcomes of patients with tricuspid regurgitation (TR) has grown significantly over the last 10 years given the natural history studies showing a high disease prevalence1–3 and poor outcomes associated with increasing TR severity, even after adjusting for comorbidities.3–5 However, a number of gaps in our understanding of the disease as well as the rapid expansion of transcatheter solutions for structural heart disease have increased the need for further research and trials. Indications for isolated tricuspid valve (TV) surgery have poor penetration into clinical practice, which may be related to reported high in-hospital mortality rates, partly associated with late clinical diagnosis.6 Transcatheter alternatives to surgical intervention are being investigated; yet, appropriate patient selection, goals of therapy, and clinically meaningful outcomes are unknown.
Considering these challenges, standardized clinical trial pathways and endpoint definitions to evaluate treatment outcomes in TR patients are needed. The Heart Valve Collaboratory is a multidisciplinary community of physicians, regulators, industry partners, an d patient advocates who organized a comprehensive meeting to define the knowledge gaps in our understanding of TR, standardize definitions and methods to assess disease severity, and develop feasible and efficient trial designs. This first Tricuspid Valve Academic Research Consortium (TVARC) document describes the current understanding of the disease state, natural history, and treatment options. The paper includes a proposal for general categories of clinical trial endpoints. A second planned TVARC document will focus on further defining trial endpoints and discusses trial design options.
Importantly, TVARC is not a guideline intended to influence clinical practice, but is a document intended to inform clinical research and, in particular, clinical trials. Unlike a guideline, which must rely on evidence-based medicine for recommendations, this document aims to standardize trial endpoints to address gaps in our knowledge related to identification and management of patients with TR. Standardizing endpoints for trials should provide consistency across trials and may strengthen the evidence required for guideline recommendations. Given the dynamic field of TR therapeutics, our understanding of its disease state will likely increase with time, and as treatment options expand, trial design and endpoints will undoubtedly evolve. This document is, therefore, intended to be a “living document,” which will allow periodic updating as our knowledge expands.
Standardizing terminology in valve intervention
In the current literature, various abbreviations are used for the description of transcatheter valve procedures. Table 1 shows standardized common abbreviations within this academic research consortium.
Table 1.
Suggested Standardized Abbreviations in the Context of Tricuspid Valve Interventions
| Abbreviation | Full Name | Description |
|---|---|---|
| TTVI | Transcatheter tricuspid valve intervention | To be used for any tricuspid valve repair or replacement technique |
| TTVR | Transcatheter tricuspid valve replacement | To be used for any tricuspid valve replacement, including orthotopic and heterotopic techniques |
| T-TEER | Tricuspid valve transcatheter edge-to-edge repair | To be used for tricuspid leaflet-based edge-to-edge repair techniques |
| TTV Repair | Transcatheter tricuspid valve repair | To be used for any tricuspid valve repair technique |
| A-STR | Atrial secondary tricuspid regurgitant | To be used to indicate secondary tricuspid regurgitation with atrial/annular dilatation as the main mechanism of regurgitation |
| V-STR | Ventricular secondary tricuspid regurgitation | To be used to indicate secondary tricuspid regurgitation with ventricular dilatation/dysfunction as the main mechanism of regurgitation |
| PTR | Primary tricuspid regurgitation | To be used to indicate tricuspid regurgitation caused by leaflet abnormalities |
| LTR-A | Lead-associated tricuspid regurgitation, type A | To be used to indicate tricuspid regurgitation caused by cardiac implantable electronic device caused regurgitation |
| LTR-B | Lead-associated tricuspid regurgitation, type B | To be used to indicate tricuspid regurgitation not related directly to cardiac implantable electronic device |
Section 1: Disease State Considerations
TR classification and clinical presentation
TR classification
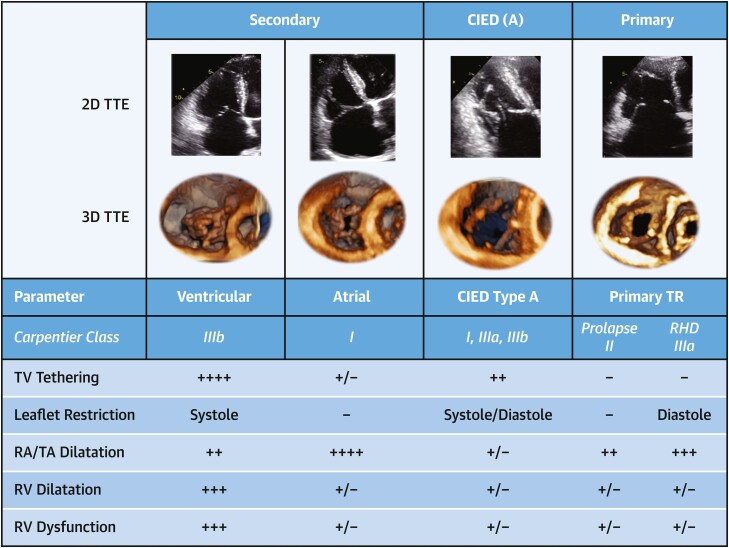
Historically, TR is viewed as a binary classification, which includes primary TR, where leaflet abnormalities are the cause of regurgitation, and secondary diseases, where leaflets are normal but structural changes of the annulus or right ventricle (RV) result in insufficient leaflet coaptation. Currently, a more comprehensive classification system for TR etiologies more appropriately encompasses the pathophysiology of this multifaceted disease (Table 2, Figure 1).7,8 Different TR etiologies may be associated with disparate outcomes,9–12 supporting a refinement of current classification categories. The main changes to the TR mechanistic classification scheme include the following: 1) subdividing secondary TR into atrial secondary tricuspid regurgitation (A-STR) and ventricular secondary tricuspid regurgitation (V-STR); 2) creating a separate category for TR associated with cardiac implantable electronic device (CIED) leads, labeled as lead-associated tricuspid regurgitation (LTR); and 3) subcategorizing LTR into type A (LTR-A), whose CIED lead is causing the TR, and type B (LTR-B), whose CIED lead is incidental (Table 2, Figure 1). Each mechanism of secondary TR may be identified by morphologic changes that occur as a result of the pathophysiologic changes associated with a particular disease process.
Table 2.
Classification of TR by Cause and Presenting Abnormalities
| Causative Disease Process | Etiology | Tricuspid Valve/RV Morphology | Carpentier Classification |
|---|---|---|---|
| Primary TR (5%-10% of patients) | |||
| Degenerative disease | Prolapse or flail leaflet | Abnormal leaflet mobility, normal RV | II |
| Congenital | Apical displacement of leaflet attachment (ie, Ebstein's anomaly) | Abnormal leaflet position, atrialized RV | I or IIIb |
| Acquired (ie, tumors, trauma, carcinoid, RHD, radiation) | Leaflet Injury (ie, tumor, trauma, biopsy, lead extraction) or infiltration/fibrosis (ie, carcinoid, rheumatic disease, radiation valvulopathy) | Abnormal leaflet morphology/mobility, normal RV | I or IIIa |
| Secondary TRa (∼80% of patients) | |||
| Ventricular secondary TR | |||
| Left ventricular disease | Postcapillary PH (HFpEF, HFrEF) | RV dilatation (spherical remodeling)/dysfunctional → leaflet tethering, dilated RA/TA | I or IIIb |
| Left heart valvular disease | Postcapillary PH | I or IIIb | |
| Pulmonary disease | Precapillary PH (chronic lung disease, CTEPH, PAH) | I or IIIb | |
| RV dysfunction/remodeling | RV dilatation and dysfunction (ie, RV infarct, RV dysplasia) | IIIb | |
| Atrial secondary TR | |||
| RA/TA dilatationa | RA/TA dilatation (ie, related to age, atrial fibrillation, HFpEF) | RA dilatation/dysfunction → TA dilatation (minimal leaflet tethering), conical RV remodeling | I |
| CIED-related TR (∼10%-15%) | |||
| LTR-A (causative) | Leaflet impingement, perforation, valvular/subvalvular adhesions/restriction | Tricuspid leaflet tethering/adhesions | I or IIIb |
| LTR-B (incidental)b | CIED present without tricuspid valve apparatus interference | Morphology dependent on primary disease process | I, II, or III |
CIED = cardiovascular implantable electronic device; CTEPH = chronic thromboembolic pulmonary hypertension; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LTR = lead-related tricuspid regurgitation; PAH = pulmonary arterial hypertension; PH = pulmonary hypertension; RA = right atrial; RHD = rheumatic heart disease; RV = right ventricular; TA = tricuspid annular; TR = tricuspid regurgitation.
aChronic TR may result in RV remodeling with subsequent leaflet tethering.
bCIED may be present and although not the causative mechanism of tricuspid regurgitation, may impact transcatheter device choice.
Figure 1.
Classification of TR Etiology
The expanded tricuspid regurgitation (TR) classification by etiology currently separates secondary TR into atrial secondary and ventricular secondary disease. When cardiac implantable electronic device (CIED) leads are present, lead-associated TR may be subcategorized into type A, whose CIED is causing the TR, and type B, whose CIED is incidental. 2D = 2-dimensional; 3D = 3-dimensional; RA = right atrium; RV = right ventricle; TA = tricuspid annulus; TTE = transthoracic echocardiography; RHD = rheumatic heart disease; TV = tricuspid valve. Modified from Hahn RT, Badano LP, Bartko PE, et al. Tricuspid regurgitation: recent advances in understanding pathophysiology, severity grading and outcome. Eur Heart J Cardiovasc Imaging. 2022;21;23(7):913–929.
Given the recent literature suggesting that patients with A-STR have different outcomes compared with V-STR,11,12 defining this etiologic category for trials may be important. Table 3 provides suggested anatomic and functional parameters for defining an A-STR phenotype based on current literature,12,13 understanding the following: 1) normative data from a large registry suggests consideration of sex-based cutoffs14,15; and 2) V-STR is a heterogeneous population, and the primary disease process will determine the combination of findings that define V-STR. For instance, invasive measures of pulmonary vascular hemodynamics will differ depending on whether the patient has precapillary or postcapillary pulmonary hypertension. Likewise, patients with primary RV cardiomyopathies and V-STR may have normal LV function. The main morphologic and hemodynamic characteristics of A-STR include absence of significant leaflet tethering, marked dilatation of the right atrium (RA) in the setting of relatively normal RV size and function, as well as normal LV function and absence of pulmonary hypertension. It is likely there will be a population of patients who have some features of A-STR and some of V-STR, and thus cannot be clearly categorized. Further research may help determine if a “mixed” morphology is a separate population of patients with different outcomes.
Table 3.
Suggested Anatomic and Functional Parameters to Define Atrial and Ventricular Secondary Tricuspid Regurgitationa
| A-STR Phenotypeb | V-STR Phenotypeb | |
|---|---|---|
| Leaflet morphologyc | ||
| Tenting height (4Ch), mm | ≤9 | > 9 |
| Tenting area (4Ch), cm2 | <2.1 | ≥2.1 |
| Tenting volume, mL | <2.5d | ≥2.5 |
| Right heart chamber sizec | ||
| RV midventricular diameter, mm | ≤38d | >38 |
| RV midventricular diameter index, mm/m2 | <21 | ≥21 |
| RV end-diastolic volume index, mL/m2 | <80 | ≥80 |
| RV end-systolic volume index, mL/m2 | <21 | ≥21 |
| 2D sphericity indexe | <55 | ≥55 |
| End-systolic RA to RV area ratioe | ≥1.5 | <1.5 |
| Right ventricular systolic functionc | ||
| TAPSE, mm | >17 | ≤17 |
| FAC, % | ≥35 | <35 |
| RVFWS, % | ≥20 | <20 |
| RV TDI S’, cm/s | ≥9 | <9 |
| 3D RVEF, % | ≥50 | <50 |
| LVEF | ≥50d | Variablef |
| Invasive pulmonary vascular hemodynamicsc | ||
| PCWP, mm Hg | ≤15 | Variablef |
| mPAP, mm Hg | <20 | Usually >20f |
| PVR, WU | <2.0 | Variablef |
2D = 2-dimensional; 3D = 3-dimensional; 4Ch = 4-chamber view; A-STR = atrial secondary tricuspid regurgitation; FAC = fractional area change; LVEF = left ventricular ejection fraction; mPAP = mean pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RA = right atrium/atrial; RV = right ventricle; RVEF = right ventricular ejection fraction; RVFWS = right ventricular free wall strain; TAPSE = tricuspid annular plane systolic excursion; TDI = tissue Doppler imaging; V-STR = ventricular secondary tricuspid regurgitation.
aThis is a ctoonsensus recommendation of TVARC and the PCR Tricuspid Focus Group.
bAssumes no primary TR or CIED-causative TR.
cIn the setting of discordant measures within the anatomic or functional categories, an integrative approach should be used to define A-STR (absence of significant leaflet tethering in the setting of a dilated right atrium, and normal RV size and function) and V-STR (significant leaflet tethering with dilated RV). Note: within each category, the volumetric assessment and the indexed values may be preferred for research studies when available.
dFrom Schlotter et al.12
eFrom Florescu et al.49
fCritieria cannot be strictly defined given the heterogeneous etiologies of V-STR (ie, precapillary, postcapillary or combined precapillary/postcapillary pulmonary hypertension, and primary RV cardiomyopathies).
TR severity assessment
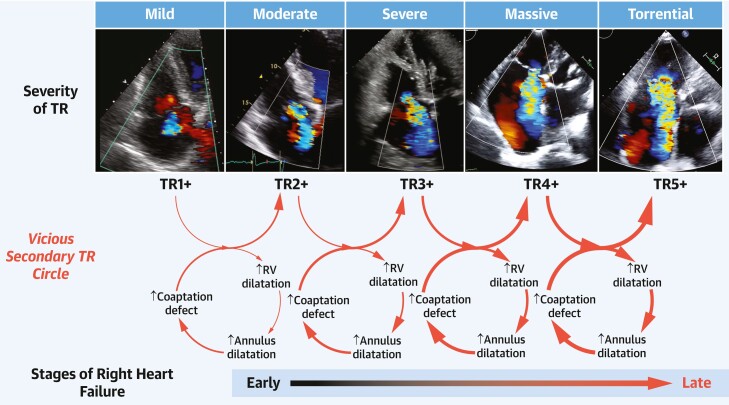
Echocardiography is the most widely used imaging modality to assess TR severity, TV anatomy, and RV function. The American Society of Echocardiography Guidelines recommend a multiparametric and hierarchical approach to TR assessment, which culminates in a 3-class grading scheme (mild [1+], moderate [2+], and severe [3+])16 that continues to be used clinically. The increasing mortality risk associated with increasing TR severity17,18 supports an extended grading scheme.19 Initial early feasibility studies20–22,23 and a recent randomized controlled trial of transcatheter device therapy24 show utility to the subclassification or extension of the “severe” grade of TR to severe (3+), massive (4+), and torrential (5+). The degree of symptom improvement parallels the TR grade reduction when using the 5-grade scale.24 The European Society of Cardiology and European Association of Cardiovascular Imaging guidelines state that the further subclassification of patients referred for transcatheter interventions with “severe” disease into “severe, massive and torrential,” may have prognostic significance.8,25 TVARC supports the use of the 5-grade scheme in this context, noting that the extended grading scheme was specifically developed for patients entering into transcatheter device therapy trials to refine TR reduction endpoint criteria. Table 4 shows qualitative, semiquantitative, and quantitative echocardiographic parameters with relevant cutoff values for the 5-grade TR severity.
Table 4.
Echocardiographic Parameters and Relative Cutoffs for TR 5-Tier Grading
| Mild (1+) | Moderate (2+) | Severe (3+) | Massive (4+) | Torrential (5+) | |
|---|---|---|---|---|---|
| Qualitative | |||||
| Tricuspid morphology | Normal or mildly abnormal | Moderately abnormal | Severely abnormal (flail leaflet, large coaptation gap, marked tethering) | ||
| Color-flow jet area | Small, narrow, central | Moderate central | Large central, or eccentric, wall impinging | ||
| Flow convergence zone | Not visible, transient, or small | Intermediate in size and duration | Large throughout systole | ||
| CWD contour | Faint, partial, parabolic | Dense, parabolic | Dense, parabolic or triangular | Dense, often triangular, may have low peak velocity | Dense, usually triangular, often low peak velocity |
| Right heart dimensions | Usually normal | Normal or mild dilatation | Usually dilated | Dilated | |
| Semiquantitative | |||||
| VCW (biplane), mma | <3 | 3–6.9 | 7–13.9 | 14–20.9 | ≥21 |
| PISA radius, mmb | ≤5.4 | 5.5–8.9 | ≥9 | ||
| Hepatic vein flowc | Systolic dominant | Systolic blunting | Systolic flow reversal | ||
| Tricuspid inflow (PWD) | A-wave dominant | Variable | E-wave dominant (≥1 m/s) | ||
| Quantitative | |||||
| PISA EROA, mm2 | <20 | 20–39 | 40–59 | 60–79 | ≥80 |
| Regurgitant volume (2D PISA), mL | <30 | 30–44 | 45–59 | 60–74 | ≥75 |
| New quantitative methods | |||||
| Regurgitant fraction, % | ≤15 | 16–49 | ≥50 | ||
| 3D VCA, mm2 | — | — | 75–94.9 | 95–114.9 | ≥115 |
| 2D Doppler EROA, mm2 | — | — | 75–94.9 | 95–114.9 | ≥115 |
CWD = continuous-wave Doppler; EROA = effective regurgitant orifice area; PISA = proximal isovelocity surface area; PWD = pulsed-wave Doppler; VCA = vena contracta area; VCW = vena contracta width; other abbreviations as in Table 3.
aAt a color Doppler scale between 40 and 60 cm/s. Note that some studies suggest an average VCW of >9 mm should define severe TR.
bColor Doppler Nyquist shift down toward 20 cm/s, until the hemispherical flow convergence zone is clearly visualized.
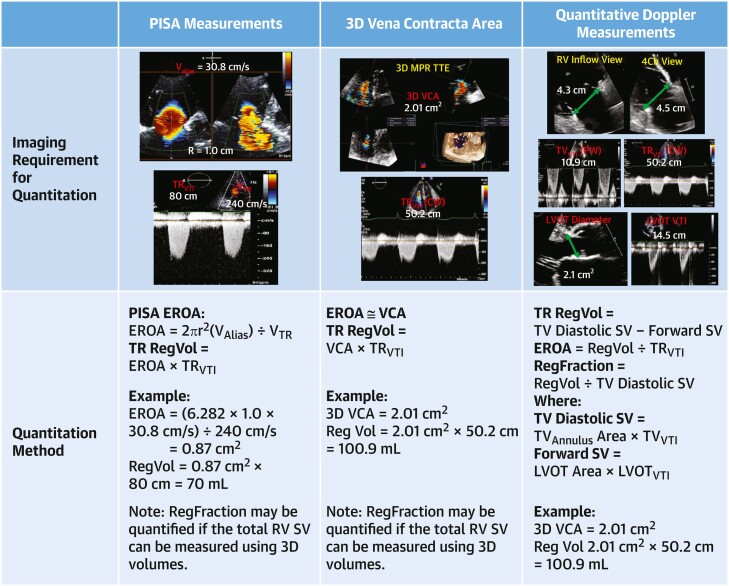
Current imaging guidelines recommend quantifying TR severity whenever possible.8 The proximal isovelocity surface area (PISA) flow convergence method is most commonly used and is associated with outcomes.4,17,26 Several methodologic limitations of this calculation are known to result in underestimation of the effective regurgitant orifice area (EROA).27–29 The new 5-grade TR severity scheme, which may be used for clinical trials, suggests different cutoffs reflecting the underestimation of EROA by the PISA method compared with direct measurement of 3-dimensional vena contracta area (3D-VCA). This underestimation may be reduced by correcting for the tethering angle of the valve leaflet and the relative low velocity of the tricuspid regurgitant jet.30 In addition, studies of quantitative Doppler methods for measuring regurgitant volume and calculating EROA have shown good correlation with 3D-VCA27,31 but require further validation. The current recommended methods of quantifying TR, as well as a proposed method by quantitative Doppler, are shown in Figure 2.
Figure 2.
Echocardiographic Quantitative Measures of TR Severity
Assessment of tricuspid regurgitation (TR) severity includes quantitative assessment of regurgitant orifice area (EROA), regurgitant volume (RegVol) and regurgitant fraction (RegFraction). Three methods of quantitation include: proximal isovelocity surface area (PISA) method, planimetry of the 3-dimensional vena contracta area (3D VCA), and quantitative Doppler method. The last method is not used clinically at this time, but allows quantitation of RegFraction as RegVol/Diastolic SV, because diastolic SV should equal the total right ventricular (RV) SV. Investigators have also used 3D echocardiography to quantify total RV SV. CW = continuous wave; LVOT = left ventricular outflow tract; PW = pulsed wave; SV = stroke volume; TTE = transthoracic echocardiogram; VTI = velocity time integral.
Although limitations of individual echocardiographic methods exist, the multiparametric and hierarchical approach proposed by imaging guidelines performs well when compared with cardiac magnetic resonance (CMR).8,25,32 Limitations of CMR and quantitation of RV stroke volume raise questions about the accuracy of the method.16 Risk stratification by CMR quantitative indexes of TR severity33 require further validation. Cardiac computed tomography (CT) angiography–based chamber volumes are similar to those derived from CMR,34 and CT may offer confirmatory evidence of TR severity.
Due to the dynamic nature of TR during respiration, echocardiographic measurements should ideally be made at end-expiration in spontaneously breathing patients, when alveolar pressure is near zero. The dependency on volume load as well as the responsiveness to diuretic therapy also should be considered when grading TR by any imaging modality. Accordingly, TR assessment should ideally be performed when patients are euvolemic, on stable diuretic therapy, with optimized pulmonary pressure and normal systemic blood pressure. The role of exercise testing remains an unexplored tool. An important consequence of significant TR is the reduction of forward stroke volume, which may then affect the accurate assessment of concomitant valvular diseases.35,36 Significant TR is more common with low-flow, low-gradient aortic stenosis and is associated with poor outcomes.37 Low flow may also cause underestimation of the echocardiographic assessment of mitral regurgitation and mitral stenosis.38 Following device therapy of TR, there may be an improvement of forward flow,39 which may change the hemodynamic quantitation of concomitant valvular disease.
Because the tricuspid annulus is much larger than the mitral annulus, tricuspid stenosis (TS) is much less likely to occur after surgical or transcatheter annuloplasty or tricuspid transcatheter edge-to-edge repair (T-TEER), and gradients have not been associated with outcomes following T-TEER.40 In contrast, TS can occur after transcatheter valve replacement, because of leaflet thrombosis, structural valve deterioration, or possibly prosthesis-patient mismatch. The minimum echocardiographic parameters that should be assessed are continuous wave Doppler measurements of peak and mean transtricuspid diastolic gradients, peak and mean transtricuspid systolic (eg, regurgitant) gradients, and the diastolic velocity time integral (VTI). Although for native TVs, an effective orifice area (EOA) by continuity equation of ≤1 cm2, velocity time integral > 60 cm, mean gradient ≥5 mm Hg, and pressure half-time ≥190 ms are suggestive of TS, there are different cutoffs reported for surgical bioprostheses as well as transcatheter valve-in-surgical valve. Multimodality imaging assessment and follow-up recommendations are listed in Supplemental Table 1.
TR clinical presentation
Clinically, right heart failure associated with TR can be characterized by the following: 1) systemic fluid retention, which may lead to elevated jugular venous pressure, peripheral edema, ascites, hepatic distention, reduced intestinal absorption, and anasarca; 2) decreased systolic reserve and low cardiac output, resulting in exercise intolerance, dyspnea, and fatigue; and 3) atrial or ventricular arrhythmias. Significant chronic TR is associated with signs and symptoms of profound reduction in cardiac output including malnutrition, anemia, and reduced cognitive function.41 The downstream consequences of chronic severe TR and right heart failure include chronic kidney disease (cardiorenal syndrome) and liver disease (cardiohepatic syndrome) (Supplemental Table 2), and, less commonly, protein-losing enteropathy.
Outcomes of untreated TR
Although TR was considered benign for decades, multiple studies now suggest that increasing severity of TR is associated with progressively worse outcomes regardless of pulmonary artery (PA) pressure and left ventricular ejection fraction.5 A recent large retrospective analysis suggests that even mild TR is associated with worse outcomes compared with none/trace TR.3 However, assessments of comorbidities and RV function were limited, raising concern that TR may simply be a surrogate marker for other cardiac or systemic comorbidities. Recent studies have attempted to isolate TR from potential confounders and to assess outcomes for individual etiologies separately. For primary etiologies, TR associated with rheumatic disease,42 flail leaflets, and pacemaker leads43 is associated with adverse outcomes, even when adjusted for RV dysfunction. Although A-STR is associated with excess mortality in adjusted analyses,44,45 recent studies show that outcomes are worse for patients with V-STR.11,12
Current guidelines give a Class I recommendation for concomitant TV intervention at the time of left heart surgery when severe TR or annular dilatation is present.13,25 A recent randomized trial questions this practice showing that at the time of mitral valve repair, concomitant repair of moderate TR but not mild TR with annular dilatation is associated with reduction in TR progression.46
Right heart response to TR
Significant TR causes right heart volume overload leading to dilatation of the RA, tricuspid annulus,47 and RV.48 The resulting papillary muscle displacement and change in the right heart axis further worsen TR and lead to a vicious cycle characterized by progressive RV dilation, RV dysfunction, and clinical deterioration (Figure 3).44
Figure 3.
Progression of TR and Right Heart Failure
Tricuspid regurgitation (TR) of any etiology results in maladaptive dilatation of the right ventricle, which can subsequently result in further annular dilatation and tethering of the leaflets. A vicious cycle then ensues until, late in the disease, the reduced cardiac output results in signs and symptoms of right heart failure.
Assessment of RV size, shape, and function
The assessment of RV size and function is helpful in distinguishing the different morphologic etiologies of STR: A-STR is associated with conical RV remodeling compared with V-STR, which is associated with spherical remodeling and midventricular dilatation.49 RV function and size are usually assessed by transthoracic echocardiography (Tables 5 and 6, respectively). Normal values of RV size from current guidelines are shown in Table 6; however, recently, the World Alliance of Societies of Echocardiography study has published normative values for RV size and function based on age, sex, and ethnicity, which may be important considerations in clinical trials.14,15 One of the most useful correlates of RV function remains tricuspid annular plane systolic excursion (TAPSE), which characterizes the longitudinal shortening of the RV which typically accounts for ∼80% of RV stroke volume. Three-dimensional echocardiography assessment of RV ejection fraction can predict outcomes in patients with cardiovascular disease50 and may be superior to TAPSE in patients following transcatheter tricuspid valve intervention (TTVI).51 Both RV and RA strain may also be prognostically important in patients with significant TR.52 Although current imaging guidelines do not define grades of severity in RV dysfunction, a proposal for possible cutoffs to be used in clinical trials is shown in Table 7, and is based on guideline-reported normal mean ± SD,53 multinational reports of normative data,15 as well as outcomes studies,54 and will require validation, particularly given the load dependence of these measurements.
Table 5.
Echocardiographic Assessment of Right Ventricular Function
| Mean ± SD | Lower Limit of Normal | |
|---|---|---|
| Longitudinal systolic function | ||
| TAPSE, mm | 21.8 ± 3.8 | 15.2 |
| RV TDIs’ | 13.2 ± 2.3 | 9.4 |
| RV GLS, |%| | 25.4 ± 3.8 | 18.2 |
| RVFWS, |%| | 28.3 ± 4.3 | 20.0 |
| Circumferential systolic function | ||
| RV FAC, % | 42.8 ± 4.3 | 35.3 |
| RVEF (3D echo), % | 56 ± 6 | 44 |
| Systolic and diastolic function | ||
| RIMP (PW Doppler) | 0.25 ± 0.085 | 0.0 |
| RIMP (TDI) | 0.38 ± 0.08 | 0.22 |
FWS = free wall strain; GLS = global longitudinal strain; PW = pulsed wave; RIMP = right ventricular index of myocardial performance; TAPSE = tricuspid annular plane systolic excursion; TDI = tissue Doppler imaging; other abbreviations as in Table 3.
Table 6.
Echocardiographic Assessment of Right Ventricular Size
| RV Size Parameter | Mean ± SD | Upper Limit of Normal |
|---|---|---|
| RV apical focused view | ||
| RV basal diameter, mm | 32.8 ± 5.3 | 44.2 |
| RV basal diameter index, mm/m2 | 18.6 ± 2.7 | 24.5 |
| RV mid diameter, mm | 26.4 ± 5.7 | 38.6 |
| RV mid diameter index, mm/m2 | 15.0 ± 2.9 | 20.9 |
| RV length (4Ch view), mm | 73.7 ± 8.7 | 91.8 |
| RV length index, mm/m2 | 41.9 ± 5.1 | 52.5 |
| Parasternal view | ||
| RVOT PLAX diameter, mm | 27.9 ± 4.2 | 36.4 |
| RVOT PLAX diameter index, mm/m2 | 15.8 ± 2.3 | 20.8 |
| RVOT PSAX proximal diameter, mm | 28.3 ± 4.9 | 38.1 |
| RVOT PSAX proximal diameter index, mm/m2 | 16.1 ± 2.8 | 22.1 |
| Tricuspid annulus, mm | 28.6 ± 5.1 | 39.4 |
| Tricuspid annulus index, mm/m2 | 16.4 ± 2.6 | 22.0 |
| Subcostal RV wall thickness, mm | 3 ± 1 | 1–5 |
| RV volume | ||
| RV EDV, mL | 137 ± 44 | 179 |
| RV EDVi, mL/m2 | 76 ± 20 | 95 |
| RV ESV, mL | 61 ± 22 | 84 |
| RV ESV, mL | 76 ± 24 | 43 |
| RV ESVi, mL/m2 | 34 ± 11 | 37 |
4Ch = 4-chamber view; EDV = end-diastolic volume; EDVi = end-diastolic volume indexed to body surface area; ESV = end-systolic volume; ESVi = end-systolic volume indexed to body surface area; RV = right ventricle; RVOT = right ventricular outflow tract; PLAX = parasternal long-axis; PSAX = parasternal short-axis view.
Table 7.
Proposed Echocardiographic Cutoffs for Right Ventricular Function for Clinical Trials
| Mild Dysfunction | Moderate Dysfunction | Severe Dysfunction | |
|---|---|---|---|
| TAPSE, mm | 14–17 | 10–13 | <10 |
| RV TDIs’, cm/s | 9–11 | 6–8 | <6 |
| RV GLS, |%|a | 18–21 | 14–17 | <14 |
| RV FWS, |%|a | 20–23 | 15–19 | < 15 |
| FAC, %b | 34–37 | 30–33 | <30 |
| RVEF (3DE), % | 45–50 | 35–45 | <35 |
aFrom Ancona F, Melillo F, Calvo F, et al. Right ventricular systolic function in severe tricuspid regurgitation: prognostic relevance of longitudinal strain. Eur Heart J Cardiovasc Imaging. 2021;22(8):868–875.
bFrom Anavekar NS, Skali H, Bourgoun M, et al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study). Am J Cardiol. 2008;101(5):607–612.
CT provides a more precise anatomic visualization of cardiac anatomy and adjacent structures. CT imaging of the right heart, vena cava, and right coronary artery are prerequisites for the appropriate selection and sizing of several transcatheter devices. CMR remains the most reproducible method for assessing chamber volumes, ejection fraction, as well as myocardial structural changes, and may have utility in patients with TR. Multimodality imaging assessment of the right heart is summarized in Supplemental Table 3.
Assessment of pulmonary vascular pathophysiology
An assessment of PA pressures is essential for understanding TR pathophysiology. Precapillary pulmonary hypertension (PH) in patients with severe TR has also been associated with worse outcomes following T-TEER.55 Echocardiography estimates of PA systolic pressure correlate well with invasive measurement in patients with nonsevere TR, but may underestimate PA systolic pressure when there is rapid equilibration of RA and RV pressures, which may occur with severe TR with or without a noncompliant RA.
Recent PH guidelines recommend confirmation of PA pressures with invasive right heart catheterization.56 The routine assessment of invasive pulmonary vascular hemodynamics in patients with TR is even more important given the discordance that may occur in some patients with severe disease. A recent study showed that high PA pressures by invasive measurement with discordant low estimated pressures by echocardiography is associated with poor outcomes following T-TEER.57 Variables that should be obtained by right heart catheterization are listed in Supplemental Table 4. The pulmonary artery pulsatility index, defined as the ratio of PA pulse pressure to RA pressure, has emerged as a predictor of RV failure and worse survival in patients with moderate or greater TR without pulmonary hypertension.58
Because of the sensitivity of the RV to afterload, an index of ventricular contractility to afterload may further characterize RV function compensation to specific loading conditions. RV-PA coupling describes a hemodynamic state where mechanical stroke work is most efficiently transferred to the pulmonary vasculature, whereas uncoupling suggests the RV can no longer maintain forward cardiac output.59 Multiple noninvasive measures of RV function have been coupled to estimates of PA pressure; however, TAPSE/systolic pulmonary artery pressure (sPAP) has been the most frequently studied. In contrast to TAPSE60 or sPAP alone,61 TAPSE/sPAP is associated with outcomes among patients undergoing transcatheter TV repair.57,62 Thus, despite the inaccuracies of sPAP measurements in some patients with TR, these studies suggest that TAPSE/sPAP by echocardiography may predict outcomes. The use of invasively measured sPAP in assessing RV-PA coupling may have additional predictive benefit.63 Another estimate of RV-PA coupling is RV stroke volume indexed to RV end-systolic volume, whether measured by computed tomography64 or by 3-dimensional echocardiography.65 The latter method avoids the use of echocardiographic estimates of sPAP. Which measures and what cutoffs of RV function should be used to determine a patient's suitability for surgical or transcatheter therapy is unknown.
Current treatment options for TR
Medical therapy for right heart failure secondary to TR
There is limited data to define appropriate medical therapy for TR, with no Class I recommendations in current guidelines.13,25 The use of diuretic agents is a Class IIa recommendation in the current American College of Cardiology/American Heart Association valve guidelines, but robust clinical trial evidence is lacking.13,66–68 An understanding of renal physiology and diuretic pharmacokinetics is essential to the thoughtful use of diuretic agents in the management of heart failure. According to the recent position statement of the European Society of Cardiology,67 diuretic response should always be interpreted considering the dose and type of the diuretic agent administered (Supplemental Table 5) and the degree of volume overload, body composition, and kidney function. Loop diuretic agents are recommended in chronic heart failure to prevent signs and symptoms of congestion; however, these drugs must be secreted into the proximal convoluted tubule requiring adequate dosing with sufficient plasma levels. Thiazide and thiazide-like diuretic agents and sodium-glucose cotransporter-2 inhibitors may offer an additional therapeutic option because of their diuretic effects. Other diuretic agents are listed in Supplemental Table 5.
Besides the effects of reduced renal perfusion and changes in pharmacokinetics, diuretic resistance occurs in the late-stages of right heart failure caused by multiple neurohormonal changes and reduced intestinal absorption. European guidelines suggest a stepped pharmacologic approach focused on achieving successful decongestion with alterations in diuretic therapy based on frequent treatment reassessment.67 Notwithstanding these guidelines, medical therapy remains a significant challenge in the presence of diuretic resistance, variable renal function, and associated electrolyte disturbances. Because of the vicious cycle of TR begetting more TR (Figure 3), the goals of optimal medical therapy should likely be the same as the goal of interventional therapy, which is to reduce TR to mild or less.
Current valve guidelines also state that the primary cause of heart failure should be treated (eg, pulmonary vasodilators to reduced elevated PA pressures, guideline-directed medical therapy for heart failure with reduced or preserved left ventricular ejection fraction, or rhythm control of atrial fibrillation).13,25,68,69 Optimal medical and device therapy for left heart failure should follow published guidelines68,70 and consensus documents.71 Importantly, all medical therapies should be recorded at baseline and after management changes (Supplemental Table 6), to improve our understanding of the role of medical therapy in the management of patients with TR.
Outcomes of surgical therapies for TR
A referral for surgical or percutaneous intervention has often been delayed until significant signs or symptoms of advanced stages of right heart failure occur. In addition to poor RV function, right heart failure is associated with worse outcomes after isolated TV surgery.72 In addition, comorbidities, advanced heart failure, rapid equalization of RV to RA gradients,73 and large systolic RV areas74 have been identified as independent predictors of poor outcome. Thus, in-hospital mortality for isolated TR has been as high as 9% to 11% with a morbidity rate of ∼30%.75 Consequently, utilization of surgery remains low compared with the prevalence of TR.76 Dedicated surgical risk scores have only recently been described,72,77 with other studies finding utility in the use of standard surgical risk scores or hepatorenal scores78 (Supplemental Table 7). All surgical risk scores require further validation.
Outcomes of transcatheter therapies for TR
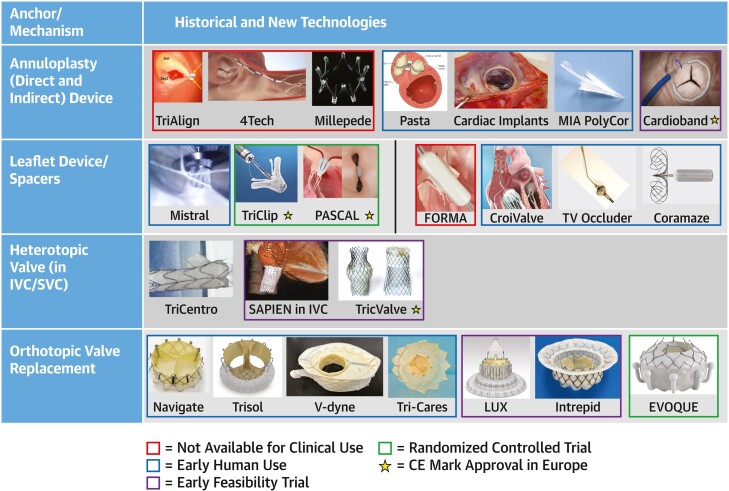
Given the constantly evolving landscape for TTVI devices (Figure 4), specific devices will not be discussed individually. Current clinical trials are listed in Supplemental Table 8. A propensity-matched registry study showed an improvement in survival and heart failure hospitalizations in patients treated with TTVI compared with medical therapy.79 The recent report from the Triluminate Pivotal Trial demonstrated an improved outcome in the composite endpoint after TTVI, which was driven by an improvement in quality of life.24 However, the study failed to show a benefit in mortality and heart failure hospitalizations over medical therapy. The rates for preceding heart failure hospitalizations and the observed rates for 1-year mortality in both arms were very low (∼10%), and further analysis in the patient population is warranted.
Figure 4.
Transcatheter Tricuspid Valve Investigational Device
In this snapshot of the landscape of transcatheter device therapy, the 4 classic anchoring mechanisms are listed in the left column with examples of devices in each row. Devices of historical interest (red outline), devices in early human use (blue outline), devices in early feasibility studies (purple outline), and devices in randomized controlled trials (green outline) are shown. Of note, devices with a star have received the Conformité Européenne (CE) mark in Europe. IVC = inferior vena cava; SVC = superior vena cava.
The impact of TTVI on RV function is unclear so far. Multiple studies have, however, shown no significant acute changes in RV function following T-TEER,80,81 or even short-term improvement.82 A meta-analysis of TTVI studies shows that in the setting of TR reduction following TTVI, a reduction in RV function may nonetheless be associated with an improvement in cardiac output.39
Section 2: endpoint definitions
Inherent in any discussion of endpoint definitions is the timing and duration of follow-up for each endpoint. Duration of follow-up should be long enough to allow reliable ascertainment of the effectiveness and safety of TR therapy. The timing of endpoint assessment must be considered when interpreting the periprocedural and the early and late risks and benefits of the TR therapy (Table 8). At a minimum, the occurrence of clinical outcomes should be reported in-hospital, at 30 days, and at 1 year. Common safety endpoints are frequent at the in-hospital and 30-day time points, while less common safety endpoints and device failures may be identified during longer follow-up. Premarket studies should continue follow-up through 5 years, as for other implantable devices. Imaging efficacy endpoints should be reported at postprocedure or predischarge, 30 days, and 1 year at minimum, and yearly up to 5 years in premarket studies. Powered clinical efficacy endpoints may be best assessed at a minimum of 1-year follow-up. Finally, the duration of follow-up must be sufficient to ascertain whether device durability is acceptable for the intended patient population and comparable to alternative therapies.
Table 8.
Timing of Events With Respect to Procedure
| Type | Definition |
|---|---|
| Periprocedural | ≤30 d or before discharge (whichever comes last) after a procedure |
| Acute | <24 h from index procedure |
| Subacute | ≥24 h and ≤30 d |
| Early | >30 d but ≤1 y after index hospitalization |
| Late | >1 y after index hospitalization |
1. Efficacy endpoints: clinical, patient-centered, and surrogate outcomes
All-cause and cardiovascular mortality
Although cardiovascular mortality is an important contributor to total mortality in TR, differentiating between cardiovascular and noncardiovascular mortality may be challenging in TR patients, because right heart failure is often accompanied by cardiorenal and cardiohepatic syndromes. It is therefore recommended that all-cause mortality is used as the primary mortality endpoint in TR therapy trials.
Cardiovascular mortality can be adjudicated as a secondary endpoint given the previously mentioned considerations and subcategorized as indicated in Table 9. Noncardiovascular mortality is adjudicated only as a death clearly related to a noncardiovascular cause. Procedure or device relatedness should be ascertained in view of existing recommendations.83 In TR trials, relatedness to TR for both cardiovascular and noncardiovascular deaths (eg, hepatic failure, endocarditis) must also be ascertained.
Table 9.
Mortality Endpoints
| All-Cause Mortality | Cardiovascular Mortality and Noncardiovascular Mortality |
|---|---|
| Cardiovascular mortality | Death classified and adjudicated as 1 of the mutually exclusive categories below: |
| Cardiac mortality—death related to: | |
| |
| |
| |
| |
| |
| |
| |
| Vascular mortality—death caused by noncardiac vascular causes classified and adjudicated as one category below | |
| |
| |
| |
| |
| |
| |
| |
| |
| Valve-related mortalitya—death presumed to be related to | |
| |
| |
| |
| Cardiohepatic: hepatic failure related to tricuspid valve dysfunction | |
| Cardiorenal: renal failure related to tricuspid valve dysfunction | |
| Noncardiovascular mortality | Death clearly related to a noncardiovascular cause such as respiratory failure not related to heart failure (eg, pneumonia), noncardiac renal failure, noncardiac liver failure, infection (eg, urosepsis), cancer, trauma, and suicide |
aRelatedness to device therapy should be adjudicated for each endpoint.
bTiming with respect to device therapy to be classified as per Table 8.
Hospitalization endpoints
TR and right heart failure are associated with a high hospitalization rate. As per the Heart Failure Collaboratory and Academic Research Consortium,71 trials should report both all-cause hospitalizations as well as cardiovascular and heart failure hospitalizations (Table 10). Hospitalizations should also be adjudicated as valve (both native or device) and/or procedure-related. Particularly in the COVID-19 era, it should be recognized that heart failure may be treated more aggressively in the outpatient setting (including ED visits) with either intravenous or more intense oral diuretics. In the PARADIGM-HF (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial such “heart failure hospitalization equivalent” events had similar prognostic implications for both total and cardiovascular mortality as traditional inpatient hospitalizations.84 Including these events as heart failure events controls for variability between sites in threshold for hospitalization and increases the power of studies by increasing the number of events.
Table 10.
Hospitalization Endpoints
| All-cause hospitalization | Hospitalization is defined as an unplanned admission to an inpatient unit or ward in the hospital for ≥24 h or as measured by a change in calendar date, including an emergency department stay. Preplanned hospitalizations for pre-existing conditions or for planned procedures are excluded unless theses are arranged for a condition related to the tricuspid valve dysfunction such as worsening heart failure. |
| Cardiovascular hospitalization | Heart failure hospitalization |
| |
| Other cardiovascular hospitalization | |
| |
| Valve- or procedure-related hospitalization | |
| |
| Unknown | |
| |
| Noncardiovascular hospitalization | Noncardiovascular hospitalization - Hospitalization not caused by cardiovascular causes as listed in the previous text |
| Heart failure exacerbation | Heart failure exacerbation without hospitalization |
|
Relatedness to device therapy should be adjudicated for each endpoint. Timing with respect to device therapy to be classified as per Table 8.
Patient-centered outcomes
Disease-state comparisons and endpoint definitions
The optimization of a patient's health status (ie, symptoms, functional status, and quality of life) is a central goal in the treatment of disease. Accordingly, patient-reported outcomes measures are being incorporated more frequently into the assessment of new devices, and the U.S. Food and Drug Administration has recognized that a treatment may demonstrate effectiveness based on improvement in health status alone85 (Supplemental Table 9).
Disease-specific assessment of health status
Disease-specific measures are designed to evaluate the effects of a specific disease process on health status. The most commonly used disease-specific instruments for patients with heart failure include the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the Minnesota Living with Heart Failure Questionnaire. KCCQ changes of 5, 10, and 20 points represent small, moderate, and large improvements in a patient's quality of life.86 Unfortunately, the placebo effect in unblinded as well as sham-control trials remains a major pitfall for this measurement, with up to 10- to 12-point improvements seen in the control arms of heart failure trials.87 The novel design of the Triluminate Pivotal trial included KCCQ as a coprimary composite endpoint along with mortality or TV intervention, and heart failure hospitalization; only KCCQ showed a significant difference between treatment groups.24 A ≥15-point increase in KCCQ (considered significant) was reached in patients with moderate or less TR at 1 year.24 Importantly, to attribute a change in patient symptoms to TR reduction, patients should be on a stable medical regimen before and following device therapy. Increasing diuretic agents in the “control” group, as was seen in the Triluminate Pivotal trial, as well as increasing diuretic agents just before transcatheter device, which has been an approach to management suggested by some investigators,82,88 result in differences in “treatment” between the groups that will confound results of device therapy.
Generic assessment of health status
In contrast to disease-specific measures, generic health status measures are designed to be used in patients with any disease or condition, thereby allowing for comparisons across disease states.
Other assessments of functional capacity
In addition to measuring a patient's perception of their functional status and quality of life, objective performance measures (which are not true patient-reported outcomes) can also be used to further quantify a patient's physical function and health status. The most commonly used measure is the 6-minute walk test (6MWT), which can be affected by age and sex.89 In general, a 25- to 50-m increase in the 6MWT is considered a clinically significant improvement for an individual patient, although these thresholds may vary for patients with differing functional levels at baseline.90,91
Although the 6MWT provides clinicians with a snapshot of a patient's abilities, this assessment may not reflect a patient's usual activities at home. Continuous actigraphy may allow for a more complete assessment of a patient's functional status.92 Actigraphy involves use of a wearable accelerometer to measure acceleration along multiple axes and can be used to estimate energy expenditure based on validated algorithms.
Imaging endpoints
Reduction of TR and absence of TS
Comprehensive echocardiography, using a multiparametric approach but relying on quantitative measures, remains the test of choice in preprocedural and postprocedural TR grading.93 Imaging endpoints followed serially from baseline to 5 years postintervention for TV repair or replacement in early feasibility and pivotal clinical trials are best assessed via 2- and 3-dimensional echocardiography given its broad availability. CMR imaging is an acceptable alternative for quantification of TR severity given recent data on prognostication with using CMR parameters, although it may have different severity cutoffs.33 All measures of TR severity are more robust in the setting of isolated disease and are limited by the absence of a true gold standard and the dynamic nature of TR severity in terms of both volume status and beat-to-beat variation with respiration or arrhythmia/atrial fibrillation.
The assessment of postdevice TR currently relies predominantly on TTE, based on integration of information from qualitative and semiquantitative parameters of color and spectral Doppler. The type of device significantly affects the utility of particular parameters for TR assessment (Table 11). The multiple residual regurgitant jets following T-TEER and deformation of the proximal flow convergence by the device limit the applicability of semiquantitative and quantitative measures of severity.94 Nonetheless, moderate or more (≥2+) residual TR following device therapy is associated with adverse outcomes.39,95 Given the pitfalls of postdevice quantitative methods depending on device type, 3D-VCA may be an important method for assessing both baseline and postdevice efficacy; however, significant limitations of this method also exist. Precise algorithms to integrate discordant parameters need to be developed and validated. Ongoing advances in echocardiographic platforms and automation of quantitation should improve the quantification of both TR severity and RV function.
Table 11.
Echocardiographic Parameters for Assessing TR Severity Pre- and Post-TTVR
| Baseline | Post-TEER | Post-Annular Repair | Post-TTVR (Orthotopic) | |
|---|---|---|---|---|
| Qualitative parameters | ||||
| Flow convergence zone | + | + | + | + |
| CWD jet density/shape | + | + | + | + |
| Semiquantitative parameters | ||||
| Color flow jet area | ++ | + | + | + |
| VC width (average of orthogonal views) | +++ | + (adding multiple jets has not been validated) | +++ | +++ |
| PISA radius | +++ | ± (abnormal shape of proximal flow may result in overestimation) | +++ | +++ (for central TR) |
| Hepatic vein flow pattern | ++ | ± (abnormal RA compliance may affect specificity) | ± (abnormal RA compliance may affect specificity) | ± (abnormal RA compliance may affect specificity) |
| Quantitative parameters | ||||
| PISA EROA | +++ | ± (abnormal shape of proximal flow may result in overestimation) | + | +++ (for central TR) |
| 2D Doppler quantitative EROA | ++ | - | + | - |
| 3D vena contracta area | +++ | +++ | +++ | +++ |
| Regurgitant volume | +++ | + | + | + |
| Regurgitant fraction | +++ | (+)a | +++ | (+)a |
PISA = proximal isovelocity surface area; VC = vena contracta; other abbreviations as in Tables 3 and 4.
aGiven the diastolic flow restriction by the device, diastolic stroke volume will be overestimated. Regurgitant fraction may be performed if a total RV stroke volume is obtained by other methods (ie, 3D echocardiography or cardiac magnetic resonance) and regurgitant volume is quantified by 3D VCA. + indicates the utility of the parameter with multiple plus signs indicating greater utility; − indicates no utility or significant limitations of the parameter; ± indicates possible utility.
Continuous wave Doppler measurements to assess for TS include pressure half-time, peak and mean transtricuspid diastolic gradients, peak and mean transtricuspid systolic (eg, regurgitant) gradients, and the diastolic VTI. The left ventricular outflow VTI should also be measured to calculate the Doppler velocity (or VTI) index. Current normal hemodynamic performance values for surgical bioprosthetic and mechanical prosthetic valve are listed in Supplemental Table 10, which may inform shared decision making when considering surgical or transcatheter replacement options. If TS is suspected after TV replacement, leaflet excursion, thickening, and valve area can be assessed by 4-dimensional transesophageal echocardiography or full-cycle CT imaging to confirm whether the valve is functioning normally or whether there is evidence of leaflet thrombus or restriction.
Cardiac output
Multiple transcatheter TV therapy studies have reported an increase in stroke volume and cardiac output, presumably caused by reduction in TR.21,22,60,96 Cardiac output should be measured at baseline and following TTVI using invasive or noninvasive (echocardiography, inert gas rebreathing spirometry, or CMR) methods.
Hepatic vein flow reversal
Hepatic vein flow reversal is a specific sign of clinically significant TR,16 which in the original studies, included both moderate and severe disease. Hepatic vein flow reversal may be affected by not only TR, but also by RA chamber compliance, and peak TR pressure gradients.
RV function and RV-PA coupling
Noninvasive parameters of RV function are inherently load-dependent, and, in conditions of altered preload and/or afterload or regional RV dysfunction, may not provide an accurate representation of RV intrinsic or overall performance. Nonetheless, these measurements have been associated with outcomes for native TR postsurgical and post-TTVI, and thus should be reported. The quantification of RV-PA coupling provide important insights into the mechanism of adaptation of RV contractility to afterload in patients with TR.97 In addition to noninvasive measures of RV afterload, invasively measured PA pressures (systolic, diastolic, and mean) as well as pulmonary capillary wedge pressures, pulmonary vascular resistance, and transpulmonary gradients allow differentiation of precapillary, postcapillary, and combined precapillary and postcapillary PH. The differentiation of these entities may determine the most appropriate medical and transcatheter therapies.
RV/RA reverse remodeling after TV intervention
The effects of transcatheter TV repair procedures on the RV and RA size and function remain to be clarified. Available studies have reported conflicting results in terms of RV and RA size reduction and RV function improvement.39 Current trials should routinely report measures of right heart size and function during follow-up.
Circulating biomarkers and end-organ function
Although there has been extensive research to identify, characterize, and determine the prognostic significance of biomarkers related to left-sided heart disease and failure, comparatively little data is available for the right heart and more specifically for TR. Significant TR produces volume overload of the RV, causing stretch and stress of the myocardium with an accompanying release of cardiac proteins, most notably natriuretic peptides.98–100 Although circulating natriuretic peptide levels tend to be more influenced by left-sided cardiac disease, ventricular interdependence results in elevations of these markers in the setting of right heart failure. Natriuretic peptide levels have been shown to be predictors of outcomes in primary PH,101,102 and a reduction in septal shift may improve left heart function and reduce pulmonary congestion. Because congestion is a dominant feature of right-sided heart failure associated with significant TR, CA125 may turn out to be a useful biomarker responsive to reductions in TR.103,104 Liver and renal function are also linked to the venous congestion and reduced forward flow associated with significant TR. Accordingly, circulating markers of liver and renal function may respond to treatment of TR, particularly if they are abnormal before the procedure.105 To identify and evaluate known and novel biomarkers of procedural success and predictors of clinical outcome, it will be helpful to include biobanks in trials testing TV therapies. It should be noted that their association with TR severity, responsiveness to reduction in TR, and clinical outcomes are currently unknown. Table 12 outlines the circulating biomarkers that should be routinely included in TR trials.
Table 12.
Circulating Biomarkers and End-Organ Function
| Biobanks | Recommended for inclusion in trials testing therapies for TR to identify and characterize the association between known and novel circulating biomarkers and procedural success and longer-term clinical outcomes |
| Cardiac | N-terminal pro-hormone B-type natriuretic peptide |
| Renal | BUN |
| Creatinine | |
| eGFR | |
| Liver | Albumin |
| Transaminases (AST, ALT) | |
| Alkaline phosphatase | |
| Gamma-glutamyl transferase (GGT) | |
| Bilirubin | |
| INR | |
| MELD score (requires creatinine, bilirubin, INR, sodium) | |
| Hematologic | WBC (with differential) |
| Hemoglobin and hematocrit | |
| Platelets |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; eGFR = estimated glomerular filtration rate; INR = international normalized ratio; MELD = model for end-stage liver disease; WBC = white blood cell.
2. Safety endpoints, including device-related complications and success endpoints
Safety endpoints
TV reintervention
A repeat intervention for TV disease is defined as a transcatheter or surgical procedure that targets new-onset or persistent valve or device dysfunction following a prior TV procedure. To be classified as a safety endpoint, reinterventions should be unplanned (ie, planned staged interventions are excluded) and may be related to a prior unsuccessful procedure, a device-related complication, either acute (eg, embolization) or chronic (eg, paravalvular leak) events, or device failure. Device explants should be classified as reinterventions as well. Transcatheter or surgical procedures targeting procedural complications should also be noted as safety endpoints.
Bleeding
Bleeding is a central safety outcome in cardiovascular trials and can be challenging to ascertain and grade. The use of consistent standardized bleeding scales across different types of cardiovascular studies will avoid confusion among clinicians and investigators and facilitate comparison of bleeding risk across patient population and procedure types. TVARC has therefore aligned its bleeding endpoints with the Bleeding Academic Research Consortium scale,106 which has become widely used in cardiovascular trials (Table 13).
Table 13.
TVARC Bleeding Classification
Type 1
|
Type 2
|
| Type 3 |
Type 3a
|
Type 3b
|
Type 3c
|
Type 4: Thoracotomy or percutaneous entry-site related bleeding
|
| Type 5: Life-threatening bleeding |
Type 5a
|
aCorrected for transfusion (1-U packed red blood cells or 1-U whole blood = 1 g/dL hemoglobin).
bCell saver products are not counted. Adapted with permission from the Bleeding Academic Research Consortium.106
Vascular, access-related, and cardiac injury
Table 14 shows definitions and severity of vascular, nonvascular access, and cardiac injury complications. TVARC leverages prior definitions from MVARC (Mitral Valve Academic Research Consortium) and VARC-3 (Valve Academic Research Consortium 3), with minor differences derived from the point of access (eg, venous vs arterial) and cardiac chamber involvement (eg, right-sided vs left-sided structures).
Table 14.
Vascular, Nonvascular Access, and Cardiac Complications
| Vascular complications |
Definitions
|
| Nonvascular access complications |
Definition
|
| Cardiac complications |
Definitions
|
Adapted with permission from VARC-3 (Valve Academic Research Consortium 3).110
AV = atrioventricular.
Conduction disturbances and complications involving CIEDs
Preprocedural and postprocedural conduction disturbances, and the resultant need for ventricular pacing, are key considerations in the management of TR. Postprocedural conduction disturbances are frequently observed following surgical TV intervention.46 In early feasibility studies, pacemaker rates following transcatheter TV intervention appear to be low following T-TEER and annuloplasty repair,21,22,82,107 but are at least 11% following transcatheter TV replacement.108 In patients at high risk of postprocedural conduction system disturbance, preprocedural planning for potential pacing strategies, including His-bundle, left bundle, coronary sinus, and leadless RV pacing, should be considered as part of a heart team approach. Table 15 presents an adaptation of conduction-related disturbances from VARC-3 that considers adverse effects of the procedure on the conduction system as well as on pre-existing CIEDs.
Table 15.
Rhythm and Conduction Disturbances
| Preindex procedure | |
| Rhythm or conduction disturbance | First-degree AV block |
| Second-degree AV block | |
| Right bundle branch block | |
| Left bundle branch block | |
| Left anterior hemiblock | |
| Left posterior hemiblock | |
| IVCD with QRS ≥ 120 ms | |
| Bradycardia (heart rate <60 beats/min) or sick sinus syndrome | |
| Atrial fibrillation/flutter (indicate whether paroxysmal or persistent, long-standing, or recent) | |
| CIEDs | Type of implanted CIED should be recorded (eg, single chamber, dual chamber, resynchronization therapy, physiological pacing (ie, HIS and left bundle), leadless pacemaker, transvenous, or subcutaneous defibrillator) |
| Implantation indication and date (eg, pre-existing or new in planning before tricuspid intervention) | |
| Pre-existing device revision or extraction (indicate which) | |
| During or after index procedurea | |
| New-onsetb rhythm or conduction disturbance | First-, second-, third-degree AV block |
| Right bundle branch block | |
| Left bundle branch blockc Left anterior hemiblockc Left posterior hemiblockc | |
| IVCD with QRS ≥ 120 ms | |
| Bradycardia (heart rate <60 beats/min) or sick sinus syndrome | |
| Atrial fibrillation/flutter | |
| Nonfatal ventricular arrhythmia (indicate nonsustained [<30 s] or sustained [ ≥ 30 s]) | |
| Timing of rhythm or conduction disturbance | Periprocedural: ≤30 d after the index procedure |
| Early: > 30 d but ≤1 y after index hospitalization | |
| Late: > 1 y after index hospitalization | |
| Duration of rhythm or conduction disturbance | Atrial fibrillation or flutter:
|
Bradycardia and conduction abnormalities:
| |
| New CIED | Indication: atrioventricular block, sick sinus syndrome, ventricular tachycardia or fibrillation, and so on |
| Type: Pacemaker: single chamber, dual chamber, resynchronization, physiological pacing (ie, His-bundle or left bundle), leadless, epicardial. Defibrillator: single, dual, resynchronization | |
| Location of leads (eg, transannular, intraventricular, epicardial, coronary sinus, extravascular [subcutaneous, substernal, epicardial]). | |
| Timing: number of days after the index procedure | |
| Pre-existing CIED | Change in pacing capture threshold ( ≥ 1 V at 0.5 ms) |
| Change in pacing lead impedance (increase or decrease of > 200 Ω) | |
| Change in atrial or ventricular sensing not amenable to reprogramming | |
| Lead dislodgement | |
| Requirement for device revision after the tricuspid intervention | |
| Entrapment of transannular lead by the device | |
AV = atrioventricular; CIED = cardiac implantable electronic device; ECG = electrocardiogram; IVCD = intraventricular conduction delay.
aThe calculation of new pacemaker rates should exclude patients with pre-existing pacemaker. The same principle applies to reporting of rates of new conduction disturbances and arrhythmias.
bDefined as any arrhythmia or conduction abnormality that was not present at baseline and lasts sufficiently long to be recorded on a 12-lead ECG or at least 30 s on a rhythm strip.
cLeft-sided conduction disturbances less likely in tricuspid valve procedures.
Neurological events
Although less frequent than in left heart interventions, neurological events may still occur because of paradoxical emboli in the presence of patent foramen ovale or other intracardiac shunts, as well as in the setting of periprocedural hemodynamic compromise. TVARC leverages neurological event definitions from NeuroARC and VARC-3 in Table 16, which are recommended for adjudication of cerebrovascular events in tricuspid intervention trials.
Table 16.
Categories of Neurological Events
| Overt CNS injury (NeuroARC Type 1) All strokea
|
| Covert CNS injury (NeuroARC Type 2) Covert CNS infarctionc or hemorrhage
|
| Neurological dysfunction (acutely symptomatic) without CNS injury (NeuroARC Type 3) TIA (NeuroARC Type 3a or 3aH)
|
| Stroke Gradinga |
Acute stroke severityd
|
Stroke disabilitye
|
aIn general, all studies should report at a minimum all stroke and stroke disability.
bIncludes hemorrhagic conversions when ischemic infarction is the primary mechanism.
cWhen central nervous system (CNS) infarction location does not match transient (<24 h) symptoms, the event should be classified as covert CNS infarction (NeuroARC Type 2a) and transient ischemic attack (TIA) (NeuroARC Type 3a), not as an ischemic stroke.
dSeverity assessment should be performed at the time of stroke diagnosis using the National Institutes of Health Stroke Scale (NIHSS).
eDisability assessment using the modified Rankin Scale (mRS) should be performed between 30–90 days, with 90 days being optimal. Reproduced with permission from VARC-3.110
Pulmonary embolism and deep vein thrombosis
Large-bore venous access and right-sided device therapy predispose patients undergoing TTVI to venous thromboembolic complications. TVARC identifies pulmonary embolism and deep vein thrombosis as 2 events that require careful ascertainment and reporting in tricuspid trials. For standardization, TVARC proposes definitions of these outcomes, as well as for right atrium/right ventricle thrombus, which are summarized in Table 17, including a proposed classification based on severity and clinical relevance.
Table 17.
Venous Thromboembolic Complications
Definitions
|
Severity
|
CT = computed tomography; V/Q = ventilation/perfusion scan.
Acute kidney injury
The rate of newly initiated renal replacement therapy appears to be infrequent in early feasibility studies following transcatheter device therapies for TR.82,107–109 TVARC recommends assessing chronic kidney disease according to the definitions in the Heart Failure Collaboratory and Academic Research Consortium71 (Table 18), TVARC recommends assessing acute kidney injury (AKI) according to KDIGO criteria with the addition of stage 4 AKI, defined as AKI requiring new temporary or permanent renal replacement therapy. The TVARC AKI criteria are consistent with VARC-3.110 Importantly, reduction in TR with resulting improvement in renal perfusion pressure may be associated with an improvement in renal function.
Table 18.
Definition of Changes in Renal Function in Heart Failure
| Chronic Kidney Injury |
| General definition: abnormal renal function present for > 3 mo |
| CKD Stage 1 |
|
| CKD stage 2 |
|
| CKD Stage 3a |
|
| CKD Stage 3b |
|
| CKD stage 4 |
|
| CKD stage 5 |
|
| Acute Kidney Injury |
| AKI Stage 1 |
AKI that fulfils at least 1 of the following criteria:
|
| AKI Stage 2 |
AKI that fulfils the following criterion:
|
| AKI Stage 3 |
AKI that fulfils at least one of the following criteria:
|
| AKI Stage 4 |
|
AKI definitions adapted with permission from KDIGO. Clinical Practice Guidelines for Acute Kidney Injury 2012. Accessed August 15, 2023. https://kdigo.org/guidelines/acute-kidney-injury/. After Mullens et al67 and Abraham et al.71
AKI = acute kidney injury; CKD = chronic kidney disease.
Device- and procedure-related complications
Defining device-related vs procedure-related complications can be challenging, particularly in the periprocedural setting. For example, device embolization may be the result of a procedural error, but device design itself may be a key contributor. Even events beyond the periprocedural time period may be influenced by procedural events; eg, paravalvular regurgitation may be the result of undersizing of the device. Complications related to device delivery systems should be distinguished from complications related to the transcatheter valve repair or replacement device itself. ARC has recently established general recommendations83 with regards to cardiovascular device trials. When there is not a clear distinction between device-related vs procedure-related complications, ARC recommends that attribution be assigned to both, with relative probabilities (definitely related, probably related, possibly related, unrelated) helping to define the respective contributions of the device and procedure. In procedures where more generic ancillary devices are used (such as off the shelf vascular access devices), complications attributed to those procedures would typically be considered procedure-related but not device-related, because the assessment of device relatedness applies to the investigational device only.
Device-related complication endpoints may be specific to the device or a group of devices. For example, single leaflet device attachment is relevant only to T-TEER, whereas paravalvular regurgitation is relevant to TV replacement. TVARC has therefore defined device- and procedure-related complications by category of procedure (repair vs replacement, edge to edge vs annuloplasty, and so on) (Table 19). Hypoattenuated leaflet thickening or restricted leaflet motion are well-described complications of transcatheter aortic valve replacement111 and may be of particular concern for transcatheter TV replacement given the lower pressure right heart hemodynamics as well as the frequency of atrial fibrillation in this patient population.
Table 19.
Device-Related Complications
| Dislodgement/dehiscence |
| Embolization |
| Migration from intended location |
Partial detachment/dehiscence
|
| Device dysfunction |
Residual/recurrent tricuspid regurgitation, including central and/or paravalvular regurgitation
|
| Tricuspid stenosis (mean gradient > 5 mm Hg) |
| Leaflet thickening, reduced leaflet motion, and leaflet thrombosis |
| Definitions: HALT
|
Severity
|
| Device erosion |
| Endocarditis |
Frame fracture (replacement devices)
|
| Other device-specific endpoints |
| Number of devices used by intent to achieve the desired reduction in tricuspid regurgitation |
| Need for unplanned use of additional devices (eg, valves, clips, bands) as a result of failed implant delivery, detachment, fracture, or other failure |
| Inability to rerepair (defined as inability to maintain functional native tissue without prosthetic replacement performed via transcatheter or surgical means) |
HALT = hypo-attenuated leaflet thickening; RLM = reduced leaflet motion.
aComputed tomography (CT) with high spatial and temporal resolution is required to accurately assess leaflet thickness and motion and a > 64 detector scanner is recommended. Typical CT acquisition parameters include: intravenous contrast-enhancement, submillimeter slice thickness, ECG gating with full cardiac cycle coverage and without dose modulation, target heart rate ≤70 beats/min. If CT is of either low quality, contraindicated, or inconclusive, transesophageal echocardiography may be used for the evaluation of leaflet thickness and motion.
bAdditional leaflet assessments may include the following: 1) systolic measurements of maximal affected leaflet thickness and area on longitudinal and axial projections of the tricuspid valve, respectively; and 2) affected prosthetic leaflet(s) should be identified relative to the positions of the native commissures. Additional stent/frame assessments includes the following: 1) implant depth; 2) stent expansion and eccentricity at multiple levels; and 3) stent strut-separation at the annular level.
Adjudication of complications and associated device and procedure relatedness according to the TVARC definitions should ideally be performed by an independent clinical events committee to ensure objectivity and consistency across sites.
Success endpoints
TVARC distinguishes between immediate intraprocedure technical success vs short- and long-term device and/or procedure-related outcomes and valve performance. Specifically, TVARC defines intraprocedural success as successful deployment and adequate immediate performance of the device in the absence of serious complications, and clinical success as the proper positioning of the device with adequate device function and the absence of procedure-related complications, need for reintervention, or readmissions for the underlying condition (Table 20). Clinical success (measured at 30 days and beyond) incorporates ongoing valve performance as well as major adverse events, clinical outcomes, functional status, and quality-of-life metrics. This simplification of success endpoints, compared with more complex MVARC criteria in mitral therapies, will ease their implementation and interpretation in future clinical trials in TTVI.
Table 20.
Success Endpoints (Formerly Technical, Device, and Procedural Success)
| I. Intraprocedural success |
All of the following must be present:
|
| II. Clinical success (assessed at 30 d and 1 y) |
All of the following must be present at 30 d:
|
The following must be present at 1 y:
|
BMI = body mass index; DVI = Doppler velocity index; NYHA = New York Heart Association; TR = tricuspid regurgitation; TVA = tricuspid valve area; TVAi = tricuspid valve area index; TVARC = Tricuspid Valve Academic Research Consortium.
Conclusions
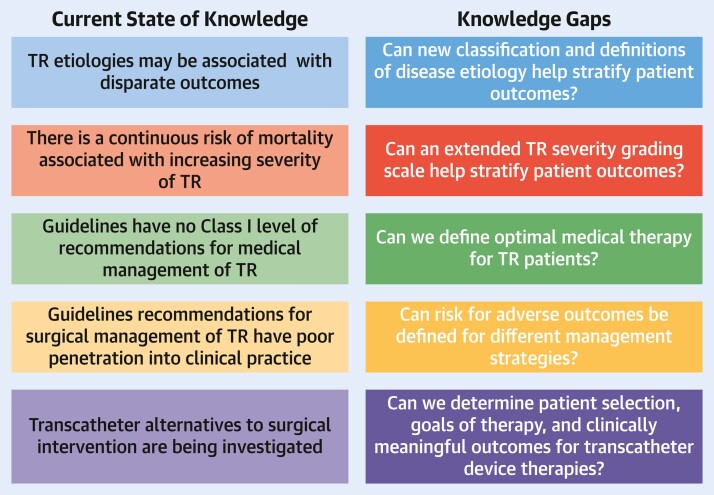
Interest in pathophysiology, etiology, management, and outcomes of patients with TR has grown as a consequence of natural history studies showing progressively worse outcomes independently associated with increasing TR severity. This first TVARC document proposes standardized definitions of disease etiology and severity, as well as endpoints for trials that aim to address the gaps in our knowledge related to identification and management of patients with TR (Central Illustration). Standardizing endpoints for trials should provide consistency and enable meaningful comparisons between clinical trials. A second TVARC document will focus on further defining trial endpoints and discuss trial design options.
Central Illustration.
TVARC Definitions for Tricuspid Regurgitation Disease State and Trial Endpoints
Using consensus definitions of disease etiology and severity and adopting well-defined endpoints for trial design may reduce the knowledge gaps in our understanding of tricuspid regurgitation (TR). TVARC = Tricuspid Valve Academic Research Consortium.
Supplementary Material
Appendix
For a complete list of the TVARC Steering Committee as well as supplemental tables, please see the online version of this paper.
Contributor Information
Rebecca T Hahn, Department of Cardiology, Columbia University Irving Medical Center, New York, New York, USA; Cardiovascular Research Foundation, New York, New York,USA.
Matthew K Lawlor, Department of Cardiology, Columbia University Irving Medical Center, New York, New York, USA.
Charles J Davidson, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Vinay Badhwar, Department of Cardiovascular and Thoracic Surgery, West Virginia University, Morgantown, West Virginia, USA.
Anna Sannino, Baylor Research Institute, The Heart Hospital Baylor Plano, Plano, Texas, USA; Department of Advanced Biomedical Sciences, Division of Cardiology, Federico II University, Naples, Italy.
Ernest Spitzer, Cardialysis, Rotterdam, the Netherlands; Department of Cardiology, Thoraxcenter, Erasmus University Medical Center, Rotterdam, the Netherlands.
Philipp Lurz, Heart Center Leipzig at University of Leipzig, Leipzig, Germany.
Brian R Lindman, Structural Heart and Valve Center, Cardiovascular Division, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Yan Topilsky, Department of Cardiology, Tel Aviv Sourasky Medical Center and Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Suzanne J Baron, Division of Cardiology, Lahey Hospital and Medical Center, Burlington, Massachusetts, USA; Baim Institute of Clinical Research, Boston, Massachusetts, USA.
Scott Chadderdon, Knight Cardiovascular Institute, Oregon Health and Science University, Portland, Oregon, USA.
Omar K Khalique, Division of Cardiology, Saint Francis Hospital and Catholic Health, Roslyn, New York, USA.
Gilbert H L Tang, Department of Cardiovascular Surgery, Mount Sinai Health System, New York, New York, USA.
Maurizio Taramasso, Herzzentrum Hirslanden Zürich, Zürich, Switzerland; University of Zürich, Zürich, Switzerland.
Paul A Grayburn, Baylor Scott and White Heart and Vascular Hospital at Plano, Plano, Texas, USA.
Luigi Badano, Department of Medicine and Surgery, University of Milano Bicocca, Milan, Italy; Department of Cardiology, Istituto Auxologico Italiano, IRCCS, Milan, Italy.
Jonathon Leipsic, Department of Radiology and Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
JoAnn Lindenfeld, Vanderbilt Heart and Vascular Institute, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Stephan Windecker, Department of Cardiology, University Cardiovascular Center, Bern University Hospital, Inselspital, Bern, Switzerland.
Sreekanth Vemulapalli, Duke Clinical Research Institute, Durham, North Carolina, USA; Duke University School of Medicine, Durham, North Carolina, USA.
Bjorn Redfors, Cardiovascular Research Foundation, New York, New York,USA; Department of Cardiology, Sahlgrenska University Hospital, Gothenburg, Sweden.
Maria C Alu, Cardiovascular Research Foundation, New York, New York,USA.
David J Cohen, Cardiovascular Research Foundation, New York, New York,USA; Division of Cardiology, Saint Francis Hospital and Catholic Health, Roslyn, New York, USA.
Josep Rodés-Cabau, Quebec Heart and Lung Institute, Laval University, Quebec City, Quebec, Canada.
Gorav Ailawadi, Department of Cardiac Surgery, University of Michigan, Ann Arbor, Michigan, USA.
Michael Mack, Baylor Scott and White Health, Dallas, Texas, USA.
Ori Ben-Yehuda, Cardiovascular Research Foundation, New York, New York,USA; University of California-San Diego, San Diego, California, USA.
Martin B Leon, Department of Cardiology, Columbia University Irving Medical Center, New York, New York, USA; Cardiovascular Research Foundation, New York, New York,USA.
Jörg Hausleiter, Medizinische Klinik und Poliklinik I, Klinikum der Universität München, Munich, Germany; DZHK (German Center for Cardiovascular Research), Partner Site Munich Heart Alliance, Munich, Germany.
Funding Support and Author Disclosures
Dr Hahn has received speaker fees from Abbott Structural, Baylis Medical, Edwards Lifesciences, and Philips Healthcare; has institutional consulting contracts for which she receives no direct compensation with Abbott Structural, Boston Scientific, Edwards Lifesciences, Medtronic, and Novartis; and is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation. Dr Lawlor has received grant support from Edwards Lifesciences. Dr Davidson has received research grant support from Edwards Lifesciences and Abbott; has received consulting fees from Philips Healthcare; and acts as an uncompensated consultant for Edwards Lifesciences. Dr Badhwar has received institutional research support for clinical trials from Abbott. Dr Spitzer has institutional contracts for which he receives no direct compensation with Boston Scientific, Cardiawave, Edwards Lifesciences, Medtronic, Shanghai Microport Medical Co Ltd, NVT GmBH, Pie Medical Imaging, and Siemens Healthcare GmBH. Dr Lurz has received institutional grants from Edwards Lifesciences. Dr Lindman has received investigator-initiated research grants from Edwards and Roche Diagnostics. Dr Topilsky has received consulting fees from Edwards, Mitralix, and Mitraltech. Dr Baron has received research support from Abiomed and Boston Scientific Corp; and has served as a consultant, on an advisory board, or as a paid speaker for Zoll Medical, Medtronic, Biotronik, Boston Scientific Corp, and Shockwave. Dr Chadderdon has served as a consultant for Edwards Lifesciences and Medtronic Inc. Dr Khalique has received consulting fees from Edwards Lifesciences, Abbott Structural, Cardiac Implants, Triflo, and Restore Medical. Dr Tang is a consultant for Medtronic and Abbott Structural Heart. Dr Taramasso has served as a consultant for Abbott Vascular, Edwards Lifescience, Boston Scientific, Shenqi Medical, MEDIRA, CoreMedic, Simulands, MTEX, Occlufit, Ventrimend, and Hi-D Imaging. Dr Grayburn has received research grants from Abbott Vascular, Boston Scientific, Cardiovalve, Edwards Lifesciences, W.L. Gore, Medtronic, Neochord, and 4C Medical; and has served as a consultant or on the advisory board of Abbott Vascular, Cardiovalve, Edwards Lifesciences, W.L. Gore, Medtronic, Neochord, and 4C Medical. Dr Badano has served as a member of the Speakers Bureau of GE Healthcare, Philips Medical Systems, and EsaOte SpA; has served as a member of the clinical event committee valve prosthesis trials for Edwards Lifesciences; and has received research grants from GE Healthcare, and EsaOte SpA. Dr Leipsic has served as a consultant for MVRX, CIRCLE CVI, and HeartFlow; has had an Institutional Core Lab with Edwards, Medtronic, PI Cardia, MVRX, Boston, Abbott, and NEO VASC; has received CT core laboratory grants from Edwards, Medtronic, Abbott, Boston, and PI Cardia; and has served on the Speakers Bureau for GE Healthcare and Philips. Dr Lindenfeld has received investigator-initiated research grants from AstraZeneca and Volumetrix; and has received consulting fees from AstraZeneca, Abbott, Alleviant, Boehringer Ingelheim, CVRx, Edwards Lifesciences, Merck, Medtronic, Boston Scientific, VWave, Vascular Dynamics, and Whiteswell. Dr Windecker has received research, travel, or educational grants to the institution from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardinal Health, CardioValve, Corflow Therapeutics, CSL Behring, Daiichi-Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Janssen-Cilag, Johnson and Johnson, Medicure, Medtronic, Merck Sharp and Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pfizer, Polares, Regeneron, Sanofi, Servier, Sinomed, Terumo, Vifor, and V-Wave; has served as an advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol Myers Squibb, Edwards Lifesciences, Janssen, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, V-Wave, and Xeltis with payments to the institution but no personal payments; and has been a member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. Dr Vemulapalli has received grants and contracts from the American College of Cardiology, Society of Thoracic Surgeons, National Institutes of Health (R01 and SBIR), Abbott Vascular, Boston Scientific, Food and Drug Administration (Nest cc), and Cytokinetics; and has received advisory board/consulting fees for Medtronic, Edwards Lifesciences, American College of Physicians, and Total CME. Dr Alu has received institutional research support (no direct financial compensation) from Edwards Lifesciences and Abbott. Dr Cohen has received research grant support from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific; and has received consulting fees from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. Dr Rodés-Cabau has received institutional research grants and speaker fees from Edwards Lifesciences. Dr Ailawadi has served as a consultant for Medtronic, Edwards, Abbott, Gore, Anteris, AtriCure, Cryolife, Johnson and Johnson, Philips, and Jena. Dr Mack has served as uncompensated Co-PI of the COAPT trial, funded by Abbott, Co-PI of the PARTNER trial, funded by Edwards Lifesciences, and study chair of the Apollo trial, funded by Medtronic. Dr Leon has received institutional clinical research grants from Abbott, Boston Scientific, Edwards Lifescience, and Medtronic. Dr Hausleiter has received speaker honoraria from and served as a consultant for Edwards Lifesciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplementary data
Supplementary data are available at European Heart Journal online.
References
- 1. Topilsky Y, Maltais S, Medina Inojosa J, et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. J Am Coll Cardiol Img. 2019;12:433–442. [DOI] [PubMed] [Google Scholar]
- 2. Tung M, Nah G, Tang J, Marcus G, Delling FN. Valvular disease burden in the modern era of percutaneous and surgical interventions: the UK Biobank. Open Heart. 2022;9(2):e002039. 10.1136/openhrt-2022-002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Offen S, Playford D, Strange G, Stewart S, Celermajer DS. Adverse prognostic impact of even mild or moderate tricuspid regurgitation: insights from the National Echocardiography Database of Australia. J Am Soc Echocardiogr. 2022;35:810–817. [DOI] [PubMed] [Google Scholar]
- 4. Chorin E, Rozenbaum Z, Topilsky Y, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020;21:157–165. [DOI] [PubMed] [Google Scholar]
- 5. Wang N, Fulcher J, Abeysuriya N, et al. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J. 2019;40:476–484. [DOI] [PubMed] [Google Scholar]
- 6. Scotti A, Sturla M, Granada JF, et al. Outcomes of isolated tricuspid valve replacement: a systematic review and meta-analysis of 5,316 patients from 35 studies. EuroIntervention. 2022;18:840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Praz F, Muraru D, Kreidel F, et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention. 2021;17:791–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lancellotti P, Pibarot P, Chambers J, et al. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC Council of Valvular Heart Disease position paper. Eur Heart J Cardiovasc Imaging. 2022;23(5):e171–e232. 10.1093/ehjci/jeab253. [DOI] [PubMed] [Google Scholar]
- 9. Prihadi EA, Delgado V, Leon MB, Enriquez-Sarano M, Topilsky Y, Bax JJ. Morphologic types of tricuspid regurgitation: characteristics and prognostic implications. J Am Coll Cardiol Img. 2019;12:491–499. [DOI] [PubMed] [Google Scholar]
- 10. Dietz MF, Prihadi EA, van der Bijl P, et al. Sex-specific differences in etiology and prognosis in patients with significant tricuspid regurgitation. Am J Cardiol. 2021;147:109–115. [DOI] [PubMed] [Google Scholar]
- 11. Gavazzoni M, Heilbron F, Badano LP, et al. The atrial secondary tricuspid regurgitation is associated to more favorable outcome than the ventricular phenotype. Front Cardiovasc Med. 2022;9: 1022755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schlotter F, Dietz MF, Stolz L, et al. Atrial functional tricuspid regurgitation: novel definition and impact on prognosis. Circ Cardiovasc Interv. 2022;15: e011958. [DOI] [PubMed] [Google Scholar]
- 13. Otto CM, Nishimura RA, Bonow RO, et al. 2020. ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:e25–e197. [DOI] [PubMed] [Google Scholar]
- 14. Addetia K, Miyoshi T, Citro R, et al. Two-dimensional echocardiographic right ventricular size and systolic function measurements stratified by sex, age, and ethnicity: results of the world alliance of societies of echocardiography study. J Am Soc Echocardiogr. 2021;34:1148–1157.e1. [DOI] [PubMed] [Google Scholar]
- 15. Addetia K, Miyoshi T, Amuthan V, et al. Normal values of three-dimensional right ventricular size and function measurements: results of the World Alliance Societies of Echocardiography Study. J Am Soc Echocardiogr. 2023;36(8):858–866.e1. 10.1016/j.echo.2023.04.011. [DOI] [PubMed] [Google Scholar]
- 16. Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 17. Fortuni F, Dietz MF, Prihadi EA, et al. Prognostic implications of a novel algorithm to grade secondary tricuspid regurgitation. J Am Coll Cardiol Img. 2021;14(6):1085–1095. [DOI] [PubMed] [Google Scholar]
- 18. Peri Y, Sadeh B, Sherez C, et al. Quantitative assessment of effective regurgitant orifice: impact on risk stratification, and cut-off for severe and torrential tricuspid regurgitation grade. Eur Heart J Cardiovasc Imaging. 2020;21:768–776. [DOI] [PubMed] [Google Scholar]
- 19. Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. 2017;18:1342–1343. [DOI] [PubMed] [Google Scholar]
- 20. Nickenig G, Weber M, Lurz P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–2011. [DOI] [PubMed] [Google Scholar]
- 21. Nickenig G, Weber M, Schüler R, et al. Tricuspid valve repair with the Cardioband system: two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention. 2021;16:e1264–e1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kodali S, Hahn RT, Eleid MF, et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. 2021;77:345–356. [DOI] [PubMed] [Google Scholar]
- 23. Hahn RT, Meduri CU, Davidson CJ, et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J Am Coll Cardiol. 2017;69:1795–1806. [DOI] [PubMed] [Google Scholar]
- 24. Sorajja P, Whisenant B, Hamid N, et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. 2023;388(20):1833–1842. 10.1056/NEJMoa2300525. [DOI] [PubMed] [Google Scholar]
- 25. Vahanian A, Beyersdorf F, Praz F, et al. 2021. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 26. Benfari G, Antoine C, Essayagh B, et al. Functional mitral regurgitation outcome and grading in heart failure with reduced ejection fraction. J Am Coll Cardiol Img. 2021;14:2303–2315. [DOI] [PubMed] [Google Scholar]
- 27. Dahou A, Ong G, Hamid N, Avenatti E, Yao J, Hahn RT. Quantifying tricuspid regurgitation severity: a comparison of proximal isovelocity surface area and novel quantitative Doppler methods. J Am Coll Cardiol Img. 2019;12:560–562. [DOI] [PubMed] [Google Scholar]
- 28. Utsunomiya H, Harada Y, Susawa H, et al. Comprehensive evaluation of tricuspid regurgitation location and severity using vena contracta analysis: a color Doppler three-dimensional transesophageal echocardiographic study. J Am Soc Echocardiogr. 2019;32:1526–1537.e2. [DOI] [PubMed] [Google Scholar]
- 29. Öztürk C, Schueler R, Weber M, Nickenig G, Hammerstingl C. Comparison of different imaging modalities for the quantification of tricuspid valve geometry and regurgitation: a retrospective, single-center study. Health Sci Rep. 2020;3:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomaselli M, Badano LP, Menè R, et al. Impact of correcting the 2D PISA method on the quantification of functional tricuspid regurgitation severity. Eur Heart J Cardiovasc Imaging. 2022;23(11):1459–1470. 10.1093/ehjci/jeac104. [DOI] [PubMed] [Google Scholar]
- 31. Praz F, Khalique OK, Dos Reis Macedo LG, et al. Comparison between three-dimensional echocardiography and computed tomography for comprehensive tricuspid annulus and valve assessment in severe tricuspid regurgitation: implications for tricuspid regurgitation grading and transcatheter therapies. J Am Soc Echocardiogr. 2018;31:1190–1202.e3. [DOI] [PubMed] [Google Scholar]
- 32. Zhan Y, Senapati A, Vejpongsa P, Xu J, Shah DJ, Nagueh SF. Comparison of echocardiographic assessment of tricuspid regurgitation against cardiovascular magnetic resonance. J Am Coll Cardiol Img. 2020;13:1461–1471. [DOI] [PubMed] [Google Scholar]
- 33. Zhan Y, Debs D, Khan MA, et al. Natural history of functional tricuspid regurgitation quantified by cardiovascular magnetic resonance. J Am Coll Cardiol. 2020;76:1291–1301. [DOI] [PubMed] [Google Scholar]
- 34. Khalique OK, Cavalcante JL, Shah D, et al. Multimodality imaging of the tricuspid valve and right heart anatomy. J Am Coll Cardiol Img. 2019;12:516–531. [DOI] [PubMed] [Google Scholar]
- 35. Tribouilloy C, Bohbot Y, Kubala M, et al. Characteristics, management, and outcomes of patients with multiple native valvular heart disease: a substudy of the EURObservational Research Programme Valvular Heart Disease II Survey. Eur Heart J. 2022;43:2756–2766. [DOI] [PubMed] [Google Scholar]
- 36. Mantovani F, Fanti D, Tafciu E, et al. When aortic stenosis is not alone: epidemiology, pathophysiology, diagnosis and management in mixed and combined valvular disease. Front Cardiovasc Med. 2021;8: 744497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dahou A, Magne J, Clavel MA, et al. Tricuspid regurgitation is associated with increased risk of mortality in patients with low-flow low-gradient aortic stenosis and reduced ejection fraction: results of the multicenter TOPAS Study (True or Pseudo-Severe Aortic Stenosis). J Am Coll Cardiol Intv. 2015;8:588–596. [DOI] [PubMed] [Google Scholar]
- 38. El Sabbagh A, Reddy YNV, Barros-Gomes S, et al. Low-gradient severe mitral stenosis: hemodynamic profiles, clinical characteristics, and outcomes. J Am Heart Assoc. 2019;8:e010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sannino A, Ilardi F, Hahn RT, et al. Clinical and echocardiographic outcomes of transcatheter tricuspid valve interventions: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:919395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orban M, Orban MW, Braun D, et al. Clinical impact of elevated tricuspid valve inflow gradients after transcatheter edge-to-edge tricuspid valve repair. EuroIntervention. 2019;15:e1057–e1064. [DOI] [PubMed] [Google Scholar]
- 41. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. [DOI] [PubMed] [Google Scholar]
- 42. Sagie A, Schwammenthal E, Newell JB, et al. Significant tricuspid regurgitation is a marker for adverse outcome in patients undergoing percutaneous balloon mitral valvuloplasty. J Am Coll Cardiol. 1994;24:696–702. [DOI] [PubMed] [Google Scholar]
- 43. Hoke U, Auger D, Thijssen J, et al. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart (British Cardiac Society). 2014;100:960–968. [DOI] [PubMed] [Google Scholar]
- 44. Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. J Am Coll Cardiol Img. 2014;7:1185–1194. [Google Scholar]
- 45. Bar N, Schwartz LA, Biner S, et al. Clinical outcome of isolated tricuspid regurgitation in patients with preserved left ventricular ejection fraction and pulmonary hypertension. J Am Soc Echocardiogr. 2018;31:34–41. [DOI] [PubMed] [Google Scholar]
- 46. Gammie JS, Chu MWA, Falk V, et al. Concomitant tricuspid repair in patients with degenerative mitral regurgitation. N Engl J Med. 2022;386:327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muraru D, Addetia K, Guta AC, et al. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: a three-dimensional echocardiographic study. Eur Heart J Cardiovasc Imaging. 2021;22:660–669. [DOI] [PubMed] [Google Scholar]
- 48. Rana BS, Robinson S, Francis R, et al. Tricuspid regurgitation and the right ventricle in risk stratification and timing of intervention. Echo Res Practice. 2019;6:R25–R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Florescu DR, Muraru D, Florescu C, et al. Right heart chambers geometry and function in patients with the atrial and the ventricular phenotypes of functional tricuspid regurgitation. Eur Heart J Cardiovasc Imaging. 2022;23:930–940. [DOI] [PubMed] [Google Scholar]
- 50. Muraru D, Badano LP, Nagata Y, et al. Development and prognostic validation of partition values to grade right ventricular dysfunction severity using 3D echocardiography. Eur Heart J Cardiovasc Imaging. 2020;21:10–21. [DOI] [PubMed] [Google Scholar]
- 51. Orban M, Wolff S, Braun D, et al. Right ventricular function in transcatheter edge-to-edge tricuspid valve repair. J Am Coll Cardiol Img. 2021;14:2477–2479. [DOI] [PubMed] [Google Scholar]
- 52. Vely M, L'Official G, Galli E, et al. Functional tricuspid regurgitation: a clustering analysis and prognostic validation of three echocardiographic phenotypes in an external cohort. Int J Cardiol. 2022;365:140–147. [DOI] [PubMed] [Google Scholar]
- 53. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 54. Muraru D, Previtero M, Ochoa-Jimenez RC, et al. Prognostic validation of partition values for quantitative parameters to grade functional tricuspid regurgitation severity by conventional echocardiography. Eur Heart J Cardiovasc Imaging. 2021;22:155–165. [DOI] [PubMed] [Google Scholar]
- 55. Stocker TJ, Hertell H, Orban M, et al. Cardiopulmonary hemodynamic profile predicts mortality after transcatheter tricuspid valve repair in chronic heart failure. J Am Coll Cardiol Intv. 2021;14:29–38. [DOI] [PubMed] [Google Scholar]
- 56. Humbert M, Kovacs G, Hoeper MM, et al. 2022. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2023;61(1):2200879. 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 57. Lurz P, Orban M, Besler C, et al. Clinical characteristics, diagnosis, and risk stratification of pulmonary hypertension in severe tricuspid regurgitation and implications for transcatheter tricuspid valve repair. Eur Heart J. 2020;41:2785–2795. [DOI] [PubMed] [Google Scholar]
- 58. Kane CJ, Lara-Breitinger KM, Alabdaljabar MS, et al. Pulmonary artery pulsatility index in patients with tricuspid valve regurgitation: a simple non-invasive tool for risk stratification. Eur Heart J Cardiovasc Imaging. 2023;24(9):1210–1221. [DOI] [PubMed] [Google Scholar]
- 59. Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. [DOI] [PubMed] [Google Scholar]
- 60. Kresoja KP, Rommel KP, Lücke C, et al. Right ventricular contraction patterns in patients undergoing transcatheter tricuspid valve repair for severe tricuspid regurgitation. J Am Coll Cardiol Intv. 2021;14:1551–1561. [DOI] [PubMed] [Google Scholar]
- 61. Karam N, Mehr M, Taramasso M, et al. Value of echocardiographic right ventricular and pulmonary pressure assessment in predicting transcatheter tricuspid repair outcome. J Am Coll Cardiol Intv. 2020;13:1251–1261. [DOI] [PubMed] [Google Scholar]
- 62. Brener MI, Lurz P, Hausleiter J, et al. Right ventricular-pulmonary arterial coupling and afterload reserve in patients undergoing transcatheter tricuspid valve repair. J Am Coll Cardiol. 2022;79:448–461. [DOI] [PubMed] [Google Scholar]
- 63. Stolz L, Weckbach LT, Karam N, et al. Invasive right ventricular to pulmonary artery coupling in patients undergoing transcatheter edge-to-edge tricuspid valve repair. J Am Coll Cardiol Img. 2023;16(4):564–566. 10.1016/j.jcmg.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 64. Tello K, Dalmer A, Axmann J, et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail. 2019;12: e005512. [DOI] [PubMed] [Google Scholar]
- 65. Gavazzoni M, Badano LP, Cascella A, et al. Clinical value of a novel three-dimensional echocardiography derived index of right ventricle-pulmonary artery coupling in tricuspid regurgitation. J Am Soc Echocardiogr. Published online July 3, 2023. 10.1016/j.echo.2023.06.014 [DOI] [PubMed] [Google Scholar]
- 66. Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic therapy for patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1178–1195. [DOI] [PubMed] [Google Scholar]
- 67. Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137–155. [DOI] [PubMed] [Google Scholar]
- 68. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. [DOI] [PubMed] [Google Scholar]
- 69. Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. [DOI] [PubMed] [Google Scholar]
- 70. McDonagh TA, Metra M, Adamo M, et al. 2021. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 71. Abraham WT, Psotka MA, Fiuzat M, et al. Standardized definitions for evaluation of heart failure therapies: scientific expert panel from the Heart Failure Collaboratory and Academic Research Consortium. J Am Coll Cardiol HF. 2020;8:961–972. [DOI] [PubMed] [Google Scholar]
- 72. Dreyfus J, Audureau E, Bohbot Y, et al. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J. 2022;43(7):654–662. 10.1093/eurheartj/ehab67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Topilsky Y, Khanna AD, Oh JK, et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929–1939. [DOI] [PubMed] [Google Scholar]
- 74. Kim YJ, Kwon DA, Kim HK, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. 2009;120:1672–1678. [DOI] [PubMed] [Google Scholar]
- 75. Dreyfus J, Flagiello M, Bazire B, et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J. 2020;41:4304–4317. [DOI] [PubMed] [Google Scholar]
- 76. Enriquez-Sarano M, Messika-Zeitoun D, Topilsky Y, Tribouilloy C, Benfari G, Michelena H. Tricuspid regurgitation is a public health crisis. Prog Cardiovasc Dis. 2019;62:447–451. [DOI] [PubMed] [Google Scholar]
- 77. LaPar DJ, Likosky DS, Zhang M, et al. Development of a risk prediction model and clinical risk score for isolated tricuspid valve surgery. Ann Thorac Surg. 2018;106:129–136. [DOI] [PubMed] [Google Scholar]
- 78. Wang TKM, Akyuz K, Kirincich J, et al. Comparison of risk scores for predicting outcomes after isolated tricuspid valve surgery. J Cardiac Surg. 2022;37:126–134. [DOI] [PubMed] [Google Scholar]
- 79. Taramasso M, Benfari G, van der Bijl P, et al. Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:2998–3008. [DOI] [PubMed] [Google Scholar]
- 80. Rommel KP, Besler C, Noack T, et al. Physiological and clinical consequences of right ventricular volume overload reduction after transcatheter treatment for tricuspid regurgitation. J Am Coll Cardiol Intv. 2019;12:1423–1434. [DOI] [PubMed] [Google Scholar]
- 81. Orban M, Rommel KP, Ho EC, et al. Transcatheter edge-to-edge tricuspid repair for severe tricuspid regurgitation reduces hospitalizations for heart failure. J Am Coll Cardiol HF. 2020;8:265–276. [DOI] [PubMed] [Google Scholar]
- 82. Lurz P, Stephan von Bardeleben R, Weber M, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. 2021;77:229–239. [DOI] [PubMed] [Google Scholar]
- 83. Cutlip DE, Mehran R, Spitzer E, Morice MC, Krucoff MW. Device and procedure relatedness: viewpoint from members of the ARC Steering Group. J Am Coll Cardiol Intv. 2022;15:783–788. [DOI] [PubMed] [Google Scholar]
- 84. Okumura N, Jhund PS, Gong J, et al. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF). Circulation. 2016;133:2254–2262. [DOI] [PubMed] [Google Scholar]
- 85. U.S. Food and Drug Administration . Center for Biologics Evaluation and Research Center for Drug Evaluation and Research. Treatment for Heart Failure: Endpoints for Drug Development - Guidance for Industry. June 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/treatment-heart-failure-endpoints-drug-development-guidance-industry.
- 86. Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. [DOI] [PubMed] [Google Scholar]
- 87. Shah SJ, Borlaug BA, Chung ES, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399:1130–1140. [DOI] [PubMed] [Google Scholar]
- 88. Lawlor MK, Hamid N, Kampaktsis P, et al. Incidence and predictors of cardiogenic shock following surgical or transcatheter tricuspid valve intervention. Catheter Cardiovasc Interv. 2022;99:1668–1678. [DOI] [PubMed] [Google Scholar]
- 89. Casanova C, Celli BR, Barria P, et al. Six Minute Walk Distance Project (ALAT). The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150–156. [DOI] [PubMed] [Google Scholar]
- 90. Ferreira JP, Duarte K, Graves TL, et al. Natriuretic peptides, 6-min walk test, and quality-of-life questionnaires as clinically meaningful endpoints in HF trials. J Am Coll Cardiol. 2016;68:2690–2707. [DOI] [PubMed] [Google Scholar]
- 91. Jain SS, Cohen DJ, Zhang Z, et al. Defining a clinically important change in 6-minute walk distance in patients with heart failure and mitral valve disease. Circ Heart Fail. 2021;14:e007564. [DOI] [PubMed] [Google Scholar]
- 92. Stocker TJ, Cohen DJ, Arnold SV, et al. Durability of benefit after transcatheter tricuspid valve intervention: insights from actigraphy. Eur J Heart Fail. 2022;24:1293–1301. [DOI] [PubMed] [Google Scholar]
- 93. Hahn RT, Thomas JD, Khalique OK, Cavalcante JL, Praz F, Zoghbi WA. Imaging Assessment of Tricuspid Regurgitation Severity. J Am Coll Cardiol Img. 2019;12:469–490. [DOI] [PubMed] [Google Scholar]
- 94. Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2019;32:431–475. [DOI] [PubMed] [Google Scholar]
- 95. Sala A, Beneduce A, Maisano F. Transcatheter and surgical treatment of tricuspid regurgitation: Predicting right ventricular decompensation and favorable responders. Front Cardiovasc Med. 2022;9: 980639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stocker TJ, Orban M, Braun D, et al. Physical activity and noninvasive cardiac output as novel clinical endpoints after transcatheter valve repair for severe tricuspid regurgitation. J Am Coll Cardiol Intv. 2018;11:2127–2129. [DOI] [PubMed] [Google Scholar]
- 97. Hahn RT, Waxman AB, Denti P, Delhaas T. Anatomic relationship of the complex tricuspid valve, right ventricle, and pulmonary vasculature: a review. JAMA Cardiol. 2019;4(5):478–487. 10.1001/jamacardio.2019.0535. [DOI] [PubMed] [Google Scholar]
- 98. Spinka G, Bartko PE, Heitzinger G, et al. An integrated imaging and circulating biomarker approach for secondary tricuspid regurgitation. J Pers Med. 2020;10(4):233. 10.3390/jpm10040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang B, Xu H, Zhang H, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide in elderly patients with valvular heart disease. J Am Coll Cardiol. 2020;75:1659–1672. [DOI] [PubMed] [Google Scholar]
- 100. Yoon CH, Zo JH, Kim YJ, et al. B-type natriuretic peptide in isolated severe tricuspid regurgitation: determinants and impact on outcome. J Cardiovasc Ultrasound. 2010;18:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wronski SL, Mordin M, Kelley K, et al. The role of noninvasive endpoints in predicting long-term outcomes in pulmonary arterial hypertension. Lung. 2020;198:65–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nunez J, Bayes-Genis A, Revuelta-Lopez E, et al. Clinical role of CA125 in worsening heart failure: a BIOSTAT-CHF study subanalysis. J Am Coll Cardiol HF. 2020;8:386–397. [DOI] [PubMed] [Google Scholar]
- 104. Soler M, Minana G, Santas E, et al. CA125 outperforms NT-proBNP in acute heart failure with severe tricuspid regurgitation. Int J Cardiol. 2020;308:54–59. [DOI] [PubMed] [Google Scholar]
- 105. Karam N, Braun D, Mehr M, et al. Impact of transcatheter tricuspid valve repair for severe tricuspid regurgitation on kidney and liver function. J Am Coll Cardiol Intv. 2019;12:1413–1420. [DOI] [PubMed] [Google Scholar]
- 106. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 107. Davidson CJ, Lim DS, Smith RL, et al. Early feasibility study of cardioband tricuspid system for functional tricuspid regurgitation: 30-day outcomes. J Am Coll Cardiol Intv. 2021;14:41–50. [DOI] [PubMed] [Google Scholar]
- 108. Kodali S, Hahn RT, George I, et al. Transfemoral tricuspid valve replacement in patients with tricuspid regurgitation: TRISCEND study 30-day results. J Am Coll Cardiol Intv. 2022;15:471–480. [DOI] [PubMed] [Google Scholar]
- 109. Nickenig G, Weber M, Schueler R, et al. 6-month outcomes of tricuspid valve reconstruction for patients with severe tricuspid regurgitation. J Am Coll Cardiol. 2019;73:1905–1915. [DOI] [PubMed] [Google Scholar]
- 110. Généreux P, Piazza N, Alu MC, et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77:2717–2746. [DOI] [PubMed] [Google Scholar]
- 111. Blanke P, Weir-McCall JR, Achenbach S, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol Img. 2019;12:1–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.