Abstract
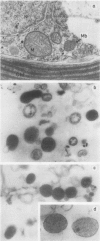
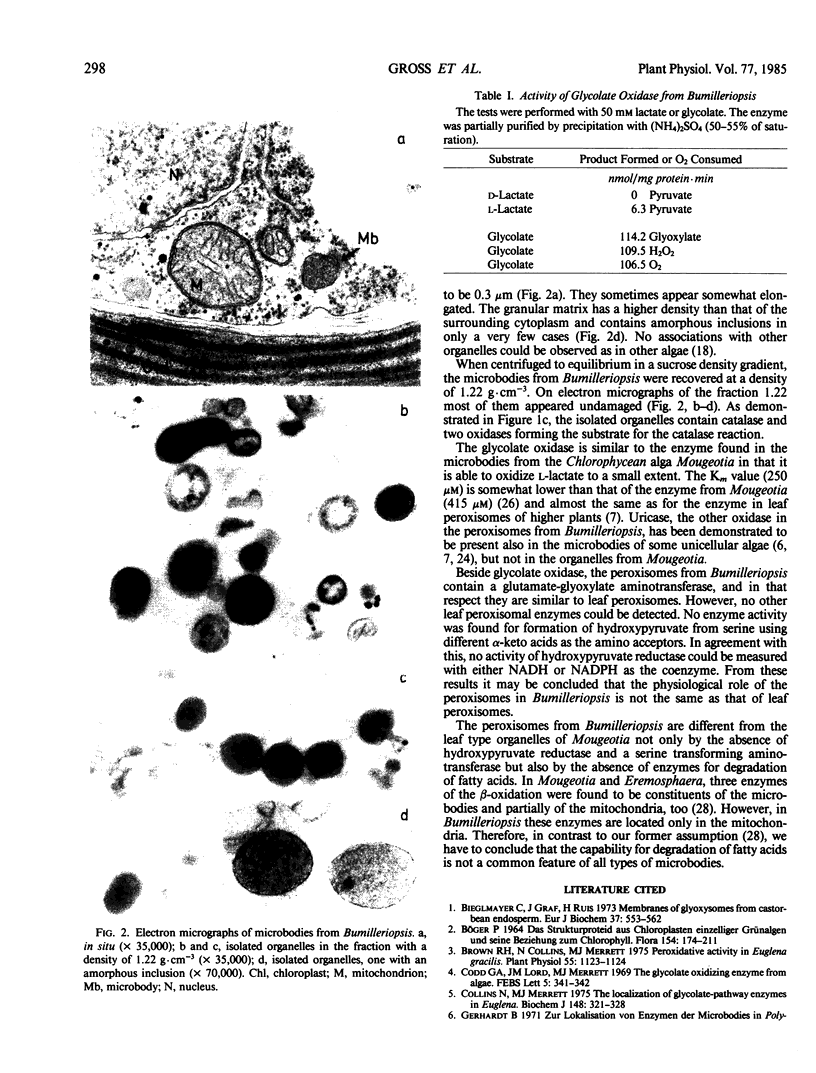
The Xanthophycean alga Bumilleriopsis filiformis possesses peroxisomes which on electron micrographs show a mostly spherical or ovoid shape with a diameter in the range of 0.3 micrometer. Their granular matrix is usually of moderate electron density and in a very few cases contains amorphous inclusions. No associations with other organelles could be observed.
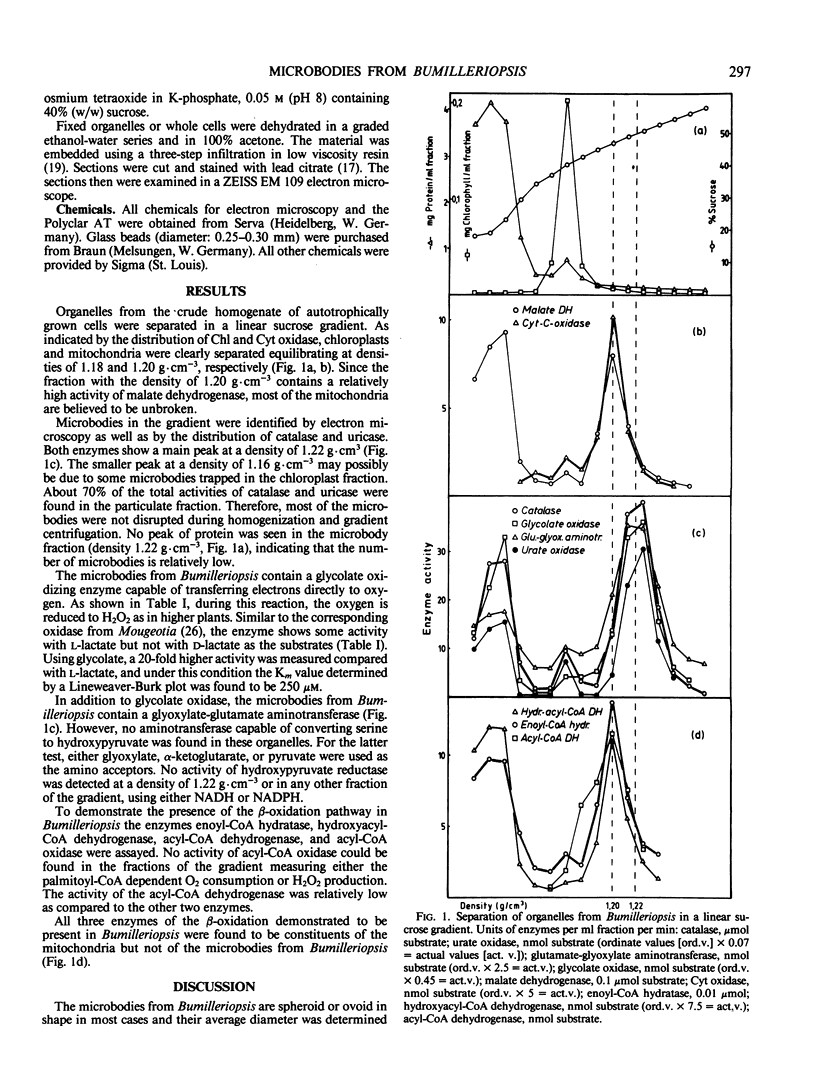
During separation in a sucrose gradient, the peroxisomes from Bumilleriopsis equilibrate at a density of 1.22 grams per cubic centimeter. Glycolate oxidase and glyoxylate-glutamate aminotransferase were found in the isolated organelles along with catalase and uricase. However, no further leaf peroxisomal enzymes were detected. This is the first time that an alga of the group of Xanthophyceae has been demonstrated to possess a glycolate oxidase.
The organelles from Bumilleriopsis differ from leaf peroxisomes also by the absence of enzymes of the β-oxidation pathway. All enzymes for the degradation of fatty acids which were tested are located solely in the mitochondria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieglmayer C., Graf J., Ruis H. Membranes of glyoxysomes from castor-bean endosperm. Enzymes bound to purified-membrane preparations. Eur J Biochem. 1973 Sep 3;37(3):553–562. doi: 10.1111/j.1432-1033.1973.tb03018.x. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Collins N., Merrett M. J. Peroxidative Activity in Euglena gracilis. Plant Physiol. 1975 Jun;55(6):1123–1124. doi: 10.1104/pp.55.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Lord J. M., Merrett M. J. The glycollate oxidising enzyme of algae. FEBS Lett. 1969 Dec 30;5(5):341–342. doi: 10.1016/0014-5793(69)80352-2. [DOI] [PubMed] [Google Scholar]
- Collins N., Merrett M. J. The localization of glycollate-pathway enzymes in Euglena. Biochem J. 1975 May;148(2):321–328. doi: 10.1042/bj1480321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. Zur Lokalisation von Enzymen der Microbodies in Polytomella caeca. Arch Mikrobiol. 1971;80(3):205–218. [PubMed] [Google Scholar]
- Godel H., Graser T., Földi P., Pfaender P., Fürst P. Measurement of free amino acids in human biological fluids by high-performance liquid chromatography. J Chromatogr. 1984 Aug 3;297:49–61. doi: 10.1016/s0021-9673(01)89028-2. [DOI] [PubMed] [Google Scholar]
- Graves L. B., Jr, Becker W. M. Beta-oxidation in glyoxysomes from Euglena. J Protozool. 1974 Nov;21(5):771–774. doi: 10.1111/j.1550-7408.1974.tb03750.x. [DOI] [PubMed] [Google Scholar]
- Hryb D. J., Hogg J. F. Chain length specificities of peroxisomal and mitochondrial beta-oxidation in rat liver. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1200–1206. doi: 10.1016/s0006-291x(79)80034-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. The intracellular localization of glycollate oxidoreductase in Euglena gracilis. Biochem J. 1971 Sep;124(2):275–281. doi: 10.1042/bj1240275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg B. A. An ultrastructural and cytochemical characterization of microbodies in the green algae. Protoplasma. 1975;83(3):269–295. doi: 10.1007/BF01282559. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stabenau H., Beevers H. Isolation and Characterization of Microbodies from the Alga Chlorogonium elongatum. Plant Physiol. 1974 Jun;53(6):866–869. doi: 10.1104/pp.53.6.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenau H. Localization of Enzymes of Glycolate Metabolism in the Alga Chlorogonium elongatum. Plant Physiol. 1974 Dec;54(6):921–924. doi: 10.1104/pp.54.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenau H. Microbodies from spirogyra: organelles of a filamentous alga similar to leaf peroxisomes. Plant Physiol. 1976 Nov;58(5):693–695. doi: 10.1104/pp.58.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenau H., Winkler U., Säftel W. Enzymes of beta-Oxidation in Different Types of Algal Microbodies. Plant Physiol. 1984 Jul;75(3):531–533. doi: 10.1104/pp.75.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. E., Brody M. Enzymatic characterization of sucrose-gradient microbodies of dark-grown, greening and continuously light-grown Euglena gracilis. FEBS Lett. 1974 Apr 1;40(2):325–330. doi: 10.1016/0014-5793(74)80255-3. [DOI] [PubMed] [Google Scholar]
- Winkler U., Säftel W., Stabenau H. Studies on the aminotransferases participating in the glycolate metabolism of the alga mougeotia. Plant Physiol. 1982 Aug;70(2):340–343. doi: 10.1104/pp.70.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]