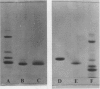
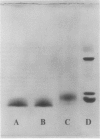
Abstract
Calmodulin has been isolated from the root of Zea mays. It activates the bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase and has electrophoretic mobility very similar to that of bovine brain calmodulin. Ophiobolin A, a fungal toxin, interacts with the maize calmodulin. The interaction is not reversed by dilution or denaturation in SDS and results in the loss of ability of the calmodulin to activate the phosphodiesterase. The inhibition is much faster in the presence than in the absence of Ca2+. The electrophoretic mobility of ophiobolin A-treated calmodulin is less than that of untreated calmodulin. Several similarities are found between the inhibition of maize calmodulin by ophiobolin A in vitro and the effects of ophiobolin A on excised roots. Both are irreversible and time-dependent. The concentration of ophiobolin A for half-maximal inhibition of calmodulin in the phosphodiesterase assay is similar to that for phytotoxicity. In both cases ophiobolin A derivatives behave similarly, i.e. 18-bromo-19-methoxyophiobolin A is as potent as ophiobolin A, while 3-anhydro-ophiobolin A and 6-epi-ophiobolin A are less potent. A smaller amount of active calmodulin was measured in the extract from ophiobolin A-treated roots than in those from untreated roots. The present study suggests that calmodulin is a target molecule in the root for the toxicity of ophiobolin A.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter S. G., Karl D. W. Inorganic phosphate assay with malachite green: an improvement and evaluation. J Biochem Biophys Methods. 1982 Dec;7(1):7–13. doi: 10.1016/0165-022x(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Cormier M. J. Purification of plant calmodulin by fluphenazine-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1039–1047. doi: 10.1016/0006-291x(79)91931-4. [DOI] [PubMed] [Google Scholar]
- Ho C., Teo T. S., Desai R., Wang J. H. Catalytic and regulatory properties of two forms of bovine heart cyclic nucleotide phosphodiesterase. Biochim Biophys Acta. 1976 Apr 8;429(2):461–473. doi: 10.1016/0005-2744(76)90294-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung P. C., Taylor W. A., Wang J. H., Tipton C. L. Ophiobolin A. A natural product inhibitor of calmodulin. J Biol Chem. 1984 Mar 10;259(5):2742–2747. [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Preparation and assay of the Ca2+--dependent modulator protein. Adv Cyclic Nucleotide Res. 1979;10:187–198. [PubMed] [Google Scholar]
- Tipton C. L., Paulsen P. V., Betts R. E. Effects of ophiobolin a on ion leakage and hexose uptake by maize roots. Plant Physiol. 1977 May;59(5):907–910. doi: 10.1104/pp.59.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]