Abstract
Chemical mutagenesis procedures and two newly developed rapid plate assays were used to identify two Mn(IV) reduction-deficient (Mnr) mutants of Shewanella putrefaciens. All eleven members of a set of previously isolated Fe(III) reduction-deficient (Fer) mutants displayed Mnr-positive phenotypes on the plate assays and were also capable of anaerobic growth on Mn(IV) as the sole terminal electron acceptor.
Dissimilatory Mn(IV) reduction can be one of the major terminal electron-accepting processes in redox-stratified natural water systems (3, 4, 9, 20, 21). Traditional enrichment techniques (5, 11, 17, 18, 22) and 16S rRNA-targeted nucleic acid hybridization analyses (5, 7) indicate that Mn(IV)-reducing bacteria constitute a major fraction of the anaerobic microbial population that resides in organic carbon- and Mn(IV)-rich anaerobic environments. The microbially catalyzed reduction of (insoluble) Mn(IV) oxides to their (soluble) Mn(II) forms may therefore be central not only to the biogeochemical cycling of Mn but also to the oxidation of naturally occurring and contaminant organic compounds. Dissimilatory Mn(IV) reduction also greatly influences the release and subsequent distribution of inorganic phosphate and toxic trace metals that otherwise adsorb to particulate Mn(IV) surfaces (9).
The proteobacteria Shewanella and Geobacter are the most extensively studied of the Mn(IV)-reducing microorganisms (for reviews, see references 9, 10, 20, and 21). Shewanella putrefaciens is particularly well adapted to redox-stratified environments, as it is capable of growing anaerobically on a wide variety of compounds as the sole terminal electron acceptor, including oxygen (O2), nitrate (NO3−), nitrite (NO2−), ferric iron (Fe3+), trimethylamine N-oxide, sulfite (SO32−), thiosulfate (S2O32−), uranyl carbonate (U6+), fumarate, and Mn(IV) oxides (6, 12, 13, 17–19, 21, 23, 24). Results from a variety of biochemical studies confirm that Mn(IV) reduction by S. putrefaciens is respiratory chain linked (14, 15, 17, 19), and recent genetic analyses of S. putrefaciens mutants with multiple respiratory deficiencies indicate that electron transport to Mn(IV) proceeds through a menaquinone pool (14) and at least one c-type cytochrome (16). Despite these findings, a Mn(IV)-specific terminal reductase gene or enzyme has yet to be isolated.
The present study describes the development and application of two rapid, plate-assay-based screening techniques for identifying Mn(IV) reduction-deficient (Mnr) mutants of S. putrefaciens 200. The inability of S. putrefaciens 200 to form anaerobic colonies on Mn(IV)-supplemented solid medium [most likely due to limiting local Mn(IV) concentrations or to toxic effects associated with elevated levels of produced Mn(II) (17)] necessitated the development of alternate plate-assay-based screening methods. The Mnr mutants generated in this study will be employed in subsequent genetic complementation analyses with an S. putrefaciens 200 gene clone bank to identify Mn(IV) reduction-specific genes and gene products. A similar strategy was recently employed in the isolation of wild-type DNA fragments that restore Fe(III) reduction capability to an array of Fe(III) reduction-deficient (Fer) mutants of S. putrefaciens 200 (6).
Chemical basis of Mn(IV)-specific colorimetric assays and plate-assay-based screening techniques.
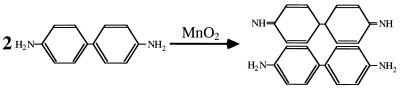
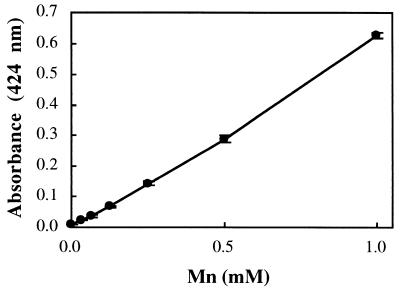
Mn(IV) oxides are known to oxidize benzidine hydrochloride ([1,1′-biphenyl]-4,4′-diamine · 2HCl) to a blue-colored, meroquinoid oxidation product known as “benzidine blue.” Benzidene blue consists of one molecule of benzidine in the amine form, one molecule of benzidine in the imine form, and two equivalents of a monobasic acid (8) (Fig. 1). The Mn(IV)-benzidine hydrochloride reaction was used as the chemical basis for development of a colorimetric assay for determining Mn(IV) concentrations during anaerobic growth experiments. A pronounced maximum at 424 nm was observed when Mn(IV) stock solutions (synthesized as ∂MnO2 according to previously described procedures [1, 2]) were quenched in a 2 mM benzidine hydrochloride solution (10% acetic acid) and analyzed spectrophotometrically (data not shown). A linear correlation (ɛ = 15.5 cm−1) between Mn(IV) concentration and A424 values was observed in the 0 to 100 μM Mn(IV) range (Fig. 2). The Mn(IV)-benzidine hydrochloride reaction was also used as the chemical basis for development of plate-assay-based Mnr mutant screening techniques. Benzidine blue was readily detectable on the surface of Mn(IV)-supplemented nutrient agar [5 mM Mn(IV), pH 8.5 (Difco, Detroit, Mich.)] at approximately 10 min after flooding with 2 mM benzidine hydrochloride solution (Fig. 3). It was hypothesized that S. putrefaciens colonies expressing mutant (or wild-type) Mn(IV) reduction activity could be identified by the presence (or absence) of benzidine blue beneath the colony surface.
FIG. 1.
Products and reactants of Mn(IV)-catalyzed oxidation of benzidine hydrochloride to benzidine blue (adapted from reference 8).
FIG. 2.
Standard curve of Mn(IV) concentration as a function of absorbance at 424 nm, measured after quenching in 2 mM benzidine hydrochloride.
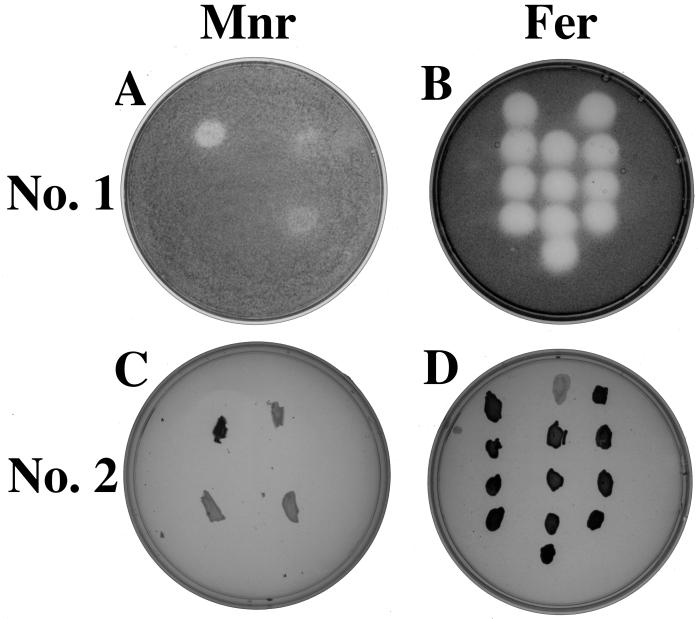
FIG. 3.
(A and C) Plate images of Mnr mutants after application of Mnr screening techniques 1 (anaerobic) (A) and 2 (aerobic) (C) with strains oriented as follows: S. putrefaciens wild type, upper left; anaerobic respiratory mutant T121, upper right; Mnr mutant 48-4, bottom left; Mnr mutant 10-10, bottom right. (B and D) Plate images of Fer mutants after application of Mnr screening techniques 1 (anaerobic) (B) and 2 (aerobic) (D) with strains oriented as follows (from top to bottom): wild type, B29, B41, and A5 (lefthand column); T121, B31, B43, A27, and C23 (middle column); B25, B39, B45, and B101 (righthand column). Note that black color intensity corresponds to benzidine blue color intensity with each screening technique.
Identification of putative Mnr mutants.
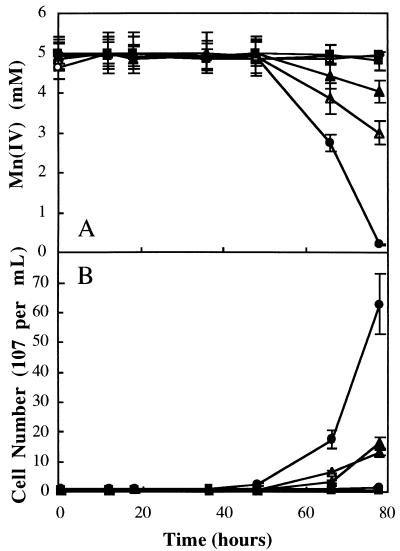
Screening technique 1 consisted of transferring mutagenized colonies (arising from ethyl methane sulfonate-treated cells, as described previously [6]) to Mn(IV)-supplemented nutrient agar and incubating them anaerobically for 24 h. Putative Mnr mutant phenotypes could be differentiated from the wild type at 10 min after flooding of the agar surface with benzidine hydrochloride; Mn(IV) was visually detectable as benzidine blue beneath putative Mnr mutant colonies (and anaerobic respiratory mutant T121) yet was not detectable beneath wild-type colonies (Fig. 3A). Approximately 1,000 mutagenized colonies were visually scored for the Mnr mutant phenotype, and four putative Mnr mutants were identified (Table 1). Three of the four putative Mnr mutants were deficient in the ability to grow anaerobically on a broad spectrum of compounds as the sole terminal electron acceptor and were not studied further (synthetic growth medium, terminal electron acceptor concentrations, and anaerobic growth conditions were identical to those described previously [6]). Cell numbers were determined via direct counts of acridine orange-stained cells according to previously described procedures (11). Particulate Mn(IV) was reduced to soluble Mn(II) prior to counting via addition of sodium dithionite (17). The fourth putative Mnr mutant (10-10) was proficient in anaerobic growth on all terminal electron acceptors except Mn(IV). During anaerobic growth on Mn(IV), strain 10-10 exhibited a lag phase nearly identical to that of the wild-type strain yet reached a final cell density that was only 20% of that reached by the wild-type strain (Fig. 4). In addition, strain 10-10 depleted Mn(IV) to levels that were only 39% of that depleted by the wild-type strain [as detected via the Mn(IV)-specific colorimetric assay described above]. Anaerobic respiratory mutant T121 (capable only of aerobic growth [25]) was unable to grow anaerobically [or to deplete Mn(IV)] under these experimental conditions. The wild-type strain was unable to grow anaerobically [or to deplete Mn(IV)] in anaerobic control experiments in which Mn(IV) (or lactate) was omitted. Mn(IV) was not depleted in abiotic control experiments (Fig. 4).
TABLE 1.
Isolation techniques and anaerobic growth capabilities of wild-type S. putrefaciens and Mnr mutants on Mn(IV) and alternate compounds as the sole terminal electron acceptor
| Strain | Isolation technique | Growtha
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O2 | Mn(IV)b | Mn(IV) | Fumarate | NO2− | NO3− | TMAOc | Fe3+ | S2O32− | SO32− | ||
| 200 | + | + | + | + | + | + | + | + | + | + | |
| T121 | + | − | − | − | − | − | − | − | − | − | |
| 48-4 | 1 | + | − | − | + | + | + | + | + | + | + |
| 10-10 | 2 | + | − | − | + | + | + | + | + | + | + |
| 8-2 | 2 | + | − | ND | + | + | − | + | − | + | − |
| 10-22 | 2 | + | − | ND | + | + | − | + | − | − | − |
| 3-3 | 2 | + | − | ND | + | + | − | + | + | + | − |
| 9-19 | 1 | + | − | ND | + | + | − | + | + | + | − |
| 3-8 | 1 | + | − | ND | + | + | + | + | + | + | − |
| 19-10 | 1 | + | − | ND | + | + | − | + | − | − | + |
| 13-12 | 1 | + | − | ND | + | + | + | + | − | − | + |
−, unable to grow anaerobically on defined medium with the indicated compound as the sole terminal electron acceptor; +, able to grow anaerobically on defined medium with the indicated compound as the sole terminal electron acceptor; ND, not determined.
Mnr phenotype after application of screening techniques 1 and 2.
TMAO, trimethylamine N-oxide.
FIG. 4.
Mn(IV) depletion (A) and anaerobic growth (B) of wild-type S. putrefaciens (•), anaerobic respiratory mutant T121 (○), and Mnr mutants 10-10 (▴) and 48-4 (▵) on Mn(IV) as the sole terminal electron acceptor. Anaerobic control experiments included incubations in which lactate ( ), Mn(IV) (□), or cells (⧫) were omitted. The initial cell density was (1 ± 0.2) × 107 cells per ml for each culture. Mn(IV) was determined colorimetrically, and acridine orange-stained cells were counted directly. Values are means of three parallel but independent anaerobic incubations; error bars represent standard deviations. Some of the error bars cannot be seen due to the small standard deviations.
Screening technique 2 was based on the observation that wild-type S. putrefaciens colonies accumulated Mn(IV) on the surface after prolonged aerobic incubation (7 days, 30°C) on solid synthetic medium (6) supplemented with 1 mM Mn(IV). Mn(IV) accumulation was detected after flooding the agar plates with 2 mM benzidine hydrochloride solution (Fig. 3C) and noting the formation of benzidine blue on the colony surface. It was hypothesized that Mn(IV) accumulated as the result of a stepwise process in which Mn(IV) in the underlying agar was first reduced (in the anaerobic colony interior) to its soluble Mn(II) form. The microbially produced Mn(II) then diffused to the colony surface, where it was oxidized under aerobic conditions to Mn(IV). The oxidized Mn(IV) precipitated and was immobilized in the uppermost colony layers, where it reacted with benzidine hydrochloride to form benzidine blue. This process effectively acted as a Mn pump, facilitating rapid detection of putative Mnr mutant strains that were unable to catalyze the initial Mn(IV) reduction step. Application of screening technique 2 to a set of 1,000 mutagenized colonies resulted in the identification of five putative Mnr mutants that visually displayed negative (or weak) benzidine blue signals (Table 1). Four of the five putative Mnr mutants were deficient in the ability to grow anaerobically on a broad spectrum of compounds as the sole terminal electron acceptor and were not studied further (Table 1). The fifth putative Mnr mutant (48-4) was proficient in anaerobic growth on all terminal electron acceptors except Mn(IV). Mnr mutant 48-4 exhibited a lag phase nearly identical to that of the wild-type strain yet reached a final cell density of only 25% of that reached by the wild-type strain and depleted only 20% of the available Mn(IV) (Fig. 4). Mnr mutants 10-10 (isolated via technique 1) and 48-4 (isolated via technique 2) displayed Mnr mutant phenotypes after application of the alternate screening technique. This finding suggests that the two screening techniques identify Mnr mutants with similar anaerobic growth deficiencies.
To check for the possibility that some Mnr mutants may have passed through the two screening techniques undetected, 10 randomly selected, mutagenized colonies from each screening technique [that visually scored positive for Mn(IV) reduction capability] were tested for anaerobic growth on Mn(IV). All 20 strains displayed lag phases, final cell densities, and Mn(IV) depletion rates that were nearly identical to that of the wild-type strain (data not shown). These results suggest that Mnr mutants with false-positive phenotypes most likely do not pass through the two screening techniques undetected.
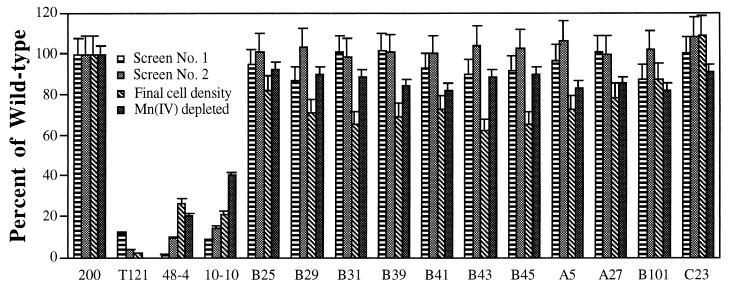
Application of the two screening techniques to a set of 11 previously isolated Fer mutants (6) indicated that each expressed Mnr-positive phenotypes (Fig. 3). The Mnr-positive phenotypes were confirmed in anaerobic growth experiments on Mn(IV) as the sole terminal electron acceptor (Fig. 5). These results demonstrate that the Fer mutants were capable of near-wild-type Mn(IV) reduction activity and that the two newly developed Mnr screening techniques were able to discern between Mnr and Fer mutant strains.
FIG. 5.
Comparison of digitized colony image intensities (detected after application of screening techniques 1 and 2), final cell densities, and Mn(IV) depletion after anaerobic growth on Mn(IV) by wild-type S. putrefaciens 200, anaerobic mutant T121, Mnr mutants 48-4 and 10-10, and the 11 Fer mutants. Values of image intensities are means of pixel intensities obtained from 10 individual colonies screened on separate plates (0.40-mm2 demarcated area per colony). Values for final cell densities and Mn(IV) depletion are means of three parallel but independent anaerobic growth experiments. Error bars represent standard deviations. All values were normalized to wild-type levels and expressed as percentages.
Analysis of digitized images of the Mnr and Fer mutant strains.
Images displayed by colonies after application of the two screening techniques were recorded as 8-bit TIFF files with the UVP 7500 Gel Documentation System (UVP, Inc., Upland, Calif.). Image intensity (reported as mean pixel value in Table 2) was measured with NIH Image software, version 1.58 (anonymous FTP [zippy.nimh.nih.gov]; National Institutes of Health, Bethesda, Md.). Colony boundaries were demarcated with the circular selection tool, and image intensity was noted as the mean pixel value within each selected area. Errors associated with mean pixel differences within each colony (i.e., as a function of the demarcated area), between colonies of identical strains on the same plate, or between colonies of identical strains on different plates were minimal (Table 2). Analysis of digitized images of the 11 Fer mutants (Fig. 5) and the 20 randomly selected Mnr-positive control strains (Table 2) revealed that these strains produced images whose intensities were nearly identical to that of the wild-type strain. The near-wild-type images produced by these strains correlated with their ability to grow anaerobically on Mn(IV) as the sole terminal electron acceptor (Fig. 5). Analysis of digitized images of Mnr mutants 10-10 and 48-4 (as well as anaerobic respiratory mutant T121) after application of each screening technique revealed that our visual inspection methods were discerning Mnr mutants with image intensities of <14% of the wild-type strain (Fig. 3; Table 2). It is possible that Mnr mutants which display intensities of >14% exist and are therefore not detected by our visual inspection methods. Current work in the development of Mnr plate-assay-based screening techniques centers on automation of Mnr mutant detection methods via computer-assisted analysis of digitized colony images.
TABLE 2.
Comparison of mean pixel intensities measured after application of screening techniques 1 and 2 to colonies of the wild type (WT), Mnr mutants 48-4 and 10-10, anaerobic respiratory mutant T121, and 20 randomly selected Mnr-positive strains
| Images compared | Strain | Screening technique
1
|
Screening technique 2
|
nd | ||
|---|---|---|---|---|---|---|
| Pixel intensitya | Normalizedb | Pixel intensityc | Normalizedb | |||
| Within one WT colonye | WT | 114 ± 2 | NAf | 204 ± 10 | NA | 10 |
| Between 50 colonies of a single strain, each on the same plate | WT | 114 ± 3 | 100 | 201 ± 12 | 100 | 50 |
| T121 | 12 ± 1 | 11 | 10 ± 1 | 5 | 50 | |
| 48-4 | 2 ± 0 | 2 | 15 ± 1 | 7 | 50 | |
| 10-10 | 8 ± 0 | 7 | 25 ± 2 | 12 | 50 | |
| Between 50 colonies of a single strain, each on a separate plate | WT | 108 ± 8 | 100 | 192 ± 17 | 100 | 50 |
| T121 | 13 ± 1 | 12 | 8 ± 0 | 4 | 50 | |
| 48-4 | 2 ± 0 | 2 | 17 ± 0 | 9 | 50 | |
| 10-10 | 9 ± 0 | 8 | 27 ± 2 | 14 | 50 | |
| Between single colonies of 20 randomly selected Mnr-positive strains on the same plate | NA | 113 ± 3 | 105 | 199 ± 7 | 104 | 20 |
Mean pixel intensity ± standard deviation (SD) of 0.40-mm2 area (1,394 pixels), except where noted. Total white assigned a value of 256.
Mean pixel intensity of indicated strain divided by mean pixel intensity of wild-type strain (reported as a percentage).
Mean pixel intensity ± SD of 0.40-mm2 area (1,394 pixels), except where noted. Total black assigned a value of 256.
Number of samples analyzed.
Mean pixel intensity of a series of 10 decreasing-size areas demarcated within the wild-type colony shown in either Fig. 3A (screening technique 1) or Fig. 3C (screening technique 2). Demarcated areas were successively decreased in size from 0.40 mm2 (1,394 pixels) to 0.04 mm2 (139 pixels) every 0.04 mm2.
NA, not applicable.
In summary, the application of two newly developed rapid screening techniques resulted in identification of two specific respiratory (Mnr) mutants of S. putrefaciens that were unable to respire anaerobically on Mn(IV) yet retained the ability to respire anaerobically on a suite of eight other compounds as the sole terminal electron acceptor. In addition, each member of a set of 11 previously isolated Fer mutants scored positive for Mn(IV) reduction on the two plate assays and were capable of near-wild-type growth on Mn(IV) as the sole terminal electron acceptor. Future genetic and biochemical analyses of the Mnr mutants will provide information on the genes and gene products required for anaerobic Mn(IV) reduction by S. putrefaciens.
Acknowledgments
Financial support for this study was provided by the Office of Naval Research Biological and Biomedical Science and Technology Program (grant N000149510206) and an accompanying AASERT award (grant N000149510716).
We thank Lee Perry for technical assistance.
REFERENCES
- 1.Balistrieri L S, Murray J W, Paul B. The cycling of iron and manganese in the water column of Lake Sammamish, Washington. Limnol Oceanogr. 1992;37:510–528. [Google Scholar]
- 2.Burns R G, Burns V M. Manganese oxides. In: Burns R G, editor. Marine minerals. Washington, D.C: Mineralogical Society of America; 1979. pp. 1–40. [Google Scholar]
- 3.Canfield D E. Sulfate reduction and oxic respiration in marine sediments: implications for organic carbon preservation in euxinic environments. Deep-Sea Res. 1989;36:121–138. doi: 10.1016/0198-0149(89)90022-8. [DOI] [PubMed] [Google Scholar]
- 4.Christensen J P, Murray J W, Devol A H, Codispoti L A. Denitrification in coastal shelf sediments has major impact on the oceanic nitrogen budget. Global Biogeochem Cycles. 1987;1:97–116. [Google Scholar]
- 5.Coates J D, Phillips E P, Lonergan D J, Jenter H, Lovley D R. Isolation of Geobacterspecies from diverse sedimentary environments. Appl Environ Microbiol. 1996;62:1531–1536. doi: 10.1128/aem.62.5.1531-1536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiChristina T J, DeLong E F. Isolation of anaerobic respiratory mutants of Shewanella putrefaciens and genetic analysis of mutants deficient in anaerobic growth on Fe3+ J Bacteriol. 1994;176:1468–1474. doi: 10.1128/jb.176.5.1468-1474.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiChristina T J, DeLong E F. Design and application of rRNA-targeted oligonucleotide probes for the dissimilatory iron- and manganese-reducing bacterium Shewanella putrefaciens. Appl Environ Microbiol. 1993;59:4152–4160. doi: 10.1128/aem.59.12.4152-4160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiegl F. Spot tests in organic analysis. New York, N.Y: Elsevier Publishing Company; 1962. [Google Scholar]
- 9.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovley D R, Giovannoni S J, White D C, Champine J E, Phillips E J P, Gorby Y A, Goodwin S. Geobacter metallireducensgen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159:336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- 11.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovley D R, Phillips E J P, Lonergan D J. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl Environ Microbiol. 1989;55:700–706. doi: 10.1128/aem.55.3.700-706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers C R, Nealson K H. Iron mineralization by bacteria: metabolic coupling of iron reduction to cell metabolism in Alteromonas putrefaciens strain MR-1. In: Frankel R B, Blakemore R P, editors. Iron biominerals. New York, N.Y: Plenum Press; 1990. pp. 131–149. [Google Scholar]
- 14.Myers C R, Myers J M. Role of menaquinone in the reduction of fumarate, nitrate, iron(III), and manganese(IV) by Shewanella putrefaciensMR-1. FEMS Microbiol Lett. 1993;114:215–222. [Google Scholar]
- 15.Myers C R, Myers J M. Ferric iron reduction-linked growth yields of Shewanella putrefaciensMR-1. J Appl Bacteriol. 1994;76:253–258. doi: 10.1111/j.1365-2672.1994.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 16.Myers C R, Myers J M. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciensMR-1. J Bacteriol. 1997;179:1143–1152. doi: 10.1128/jb.179.4.1143-1152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers C R, Nealson K H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988;240:1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- 18.Myers C R, Nealson K H. Microbial reduction of manganese oxides: interactions with iron and sulfur. Geochim Cosmochim Acta. 1988;52:2727–2732. [Google Scholar]
- 19.Myers C R, Nealson K H. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciensMR-1. J Bacteriol. 1990;172:6232–6238. doi: 10.1128/jb.172.11.6232-6238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nealson K H, Saffarini D. Iron and manganese in anerobic respiration: environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- 21.Nealson K H, Myers C. Microbial reduction of manganese and iron: new approaches to carbon cycling. Appl Environ Microbiol. 1992;58:439–443. doi: 10.1128/aem.58.2.439-443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nealson K H, Myers C R, Wimpee B B. Isolation and identification of manganese-reducing bacteria and estimates of microbial Mn(IV)-reducing potential in the Black Sea. Deep-Sea Res. 1991;38:S907–S920. [Google Scholar]
- 23.Obuekwe C O, Westlake D W S, Plambeck J A, Cook F D. Corrosion of mild steel in cultures of a ferric iron reducing bacterium isolated from crude oil. II. Mechanism of anodic depolarization. Corrosion. 1981;37:632–637. [Google Scholar]
- 24.Obuekwe C O, Westlake D W S, Cook F D. Effect of nitrate on reduction of ferric iron by a bacterium isolated from crude oil. Can J Microbiol. 1981;27:692–697. doi: 10.1139/m81-107. [DOI] [PubMed] [Google Scholar]
- 25.Saffarini D A, DiChristina T J, Bermudes D, Nealson K H. Anaerobic respiration of Shewanella putrefaciensrequires both chromosomal and plasmid-borne genes. FEMS Microbiol Lett. 1994;119:271–278. [Google Scholar]