Abstract
Background
Keratoconus is a condition of the eye that affects approximately 1 in 2000 people. The disease leads to a gradual increase in corneal curvature and decrease in visual acuity with consequent impact on quality of life. Collagen cross‐linking (CXL) with ultraviolet A (UVA) light and riboflavin (vitamin B2) is a relatively new treatment that has been reported to slow or halt the progression of the disease in its early stages.
Objectives
The objective of this review was to assess whether there is evidence that CXL is an effective and safe treatment for halting the progression of keratoconus compared to no treatment.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 7), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2014), EMBASE (January 1980 to August 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to August 2014), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to August 2014), OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu/), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organisation International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We used no date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 28 August 2014.
Selection criteria
We included randomised controlled trials (RCTs) where CXL with UVA light and riboflavin was used to treat people with keratoconus and was compared to no treatment.
Data collection and analysis
Two review authors independently screened the search results, assessed trial quality, and extracted data using standard methodological procedures expected by Cochrane. Our primary outcomes were two indicators of progression at 12 months: increase in maximum keratometry of 1.5 dioptres (D) or more and deterioration in uncorrected visual acuity of more than 0.2 logMAR.
Main results
We included three RCTs conducted in Australia, the United Kingdom, and the United States that enrolled a total of 225 eyes and analysed 219 eyes. The total number of people enrolled was not clear in two of the studies. Only adults were enrolled into these studies. Out of the eyes analysed, 119 had CXL (all using the epithelium‐off technique) and 100 served as controls. One of these studies only reported comparative data on review outcomes. All three studies were at high risk for performance bias (lack of masking), detection bias (only one trial attempted to mask outcome assessment), and attrition bias (incomplete follow‐up). It was not possible to pool data due to differences in measuring and reporting outcomes. We identified a further three unpublished trials that potentially had enrolled a total of 195 participants.
There was limited evidence on the risk of progression. Analysis of the first few participants followed up to one year in one study suggested that eyes given CXL were less likely to have an increase in maximum keratometry of 1.5 D or more at 12 months compared to eyes given no treatment, but the confidence intervals (CI) were wide and compatible with no effect or more progression in the CXL group (risk ratio (RR) 0.12, 95% CI 0.01 to 2.00, 19 eyes). The same study reported the number of eyes with an increase of 2 D or more at 36 months in the whole cohort with a RR of 0.03 favouring CXL (95% CI 0.00 to 0.43, 94 eyes). Another study reported "progression" at 18 months using a different definition; people receiving CXL were less likely to progress, but again the effect was uncertain (RR 0.14, 95% CI 0.01 to 2.61, 44 eyes). We judged this to be very low‐quality evidence due to the risk of bias of included studies, imprecision, indirectness and publication bias but noted that the size of the potential effect was large.
On average, treated eyes had a less steep cornea (approximately 2 D less steep) (mean difference (MD) ‐1.92, 95% CI ‐2.54 to ‐1.30, 94 eyes, 1 RCT, very low‐quality evidence) and better uncorrected visual acuity (approximately 2 lines or 10 letters better) (MD ‐0.20, 95% CI ‐0.31 to ‐0.09, 94 eyes, 1 RCT, very low‐quality evidence) at 12 months. None of the studies reported loss of 0.2 logMAR acuity. The data on corneal thickness were inconsistent. There were no data available on quality of life or costs. Adverse effects were not uncommon but mostly transient and of low clinical significance. In one trial, 3 out of 12 participants treated with CXL had an adverse effect including corneal oedema, anterior chamber inflammation, and recurrent corneal erosions. In one trial at 3 years 3 out of 50 participants experienced adverse events including mild diffuse corneal oedema and paracentral infiltrate, peripheral corneal vascularisation, and subepithelial infiltrates and anterior chamber inflammation. No adverse effects were reported in the control groups.
Authors' conclusions
The evidence for the use of CXL in the management of keratoconus is limited due the lack of properly conducted RCTs.
Plain language summary
Corneal collagen cross‐linking for thinning of the transparent front part of the eye ('keratoconus')
Review question Is corneal collagen cross‐linking (CXL) a good treatment for slowing down the progression of keratoconus?
Background Keratoconus is a condition where the transparent front of the eye (cornea) gets thinner and begins to bulge. This leads to vision problems, usually short‐sightedness (distant objects appear blurred). The condition is more common in children and young adults and can deteriorate over time. Initially glasses and contact lenses can help. If the disease progresses, the only option may be a corneal transplant.
CXL is a new treatment for keratoconus. The eye doctor removes the outer layer of the cornea, puts in vitamin B2 eye drops, and then treats the eye with ultraviolet A light radiation. This can be done in outpatients and takes about an hour.
Study characteristics The searches are current to August 2014. We found three randomised controlled trials, which were done in the United States, the United Kingdom, and Australia. A total of 219 eyes were randomly allocated to treatment with CXL or no treatment. In all three studies the surgery was done in the same way. None of the studies included children.
Key results Eyes treated with CXL were less likely to have problems with progression of bulging compared to eyes that were not treated. However, the studies were small and there were some concerns about the way they were done. It is therefore difficult to say exactly how much the treatment helped. None of the studies reported the risk of eyesight getting worse but, on average, treated eyes had better vision (about 10 letters better) compared to untreated eyes. None of the studies reported on a change in quality of life for the participant. The main adverse effects were inflammation and swelling; this occurred in approximately one in 10 participants.
Quality of the evidence We judged the quality of the evidence to be very low because of problems in the way the studies were done and reported and the small number of eyes included.
Summary of findings
for the main comparison.
| Corneal collagen cross‐linking compared with no treatment for keratoconus | ||||||
|
Patient or population: people with keratoconus Settings: hospital Intervention: corneal collagen cross‐linking Comparison: no or sham treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No or sham treatment | Corneal collagen cross‐linking | |||||
|
Progression (increase in maximal keratometry of 1.5 dioptres or more) Follow‐up: 12 months |
400 per 1000 | 48 per 1000 (4 to 800) | RR 0.12 (0.01 to 2.0) | 19 eyes (1) | ⊕⊝⊝⊝ very low1‐5 | In the same study, increase of 2 dioptres or more at 36 months gave a RRof 0.03 (95% CI 0.00 to 0.43, 94 eyes). A different study reported RR of "progression" at 18 months of 0.14 (95% CI 0.01 to 2.61, 44 eyes). |
|
Steepness of the cornea (maximal keratometry) (measured using dioptres. A higher dioptre represents steeper cornea and worse outcome) Follow‐up: 12 months |
The mean maximal keratometry increased in the control group by 1.2 dioptres. | The mean maximal keratometry in the intervention group was 1.92 dioptres less (better) (1.30 to 2.54 less). | 94 eyes (1) | ⊕⊝⊝⊝ very low2,4,6 | ||
|
Uncorrected visual acuity (UCVA) (measured using logMAR scale. A score of 0 = good vision, higher score is worse vision) Follow‐up: 12 months |
The mean UCVA increased on average by 0.06 logMAR units in the control group. | The mean UCVA in the intervention groups was 0.20 logMAR units less (i.e. better vision) (0.09 units less to 0.31 units less). | 94 eyes (1) | ⊕⊝⊝⊝ very low2,4,6 | Another study reported mean UCVA at 18 months was 0.33 (Snellen decimal equivalent) in 22 treated eyes and 0.21 in 22 untreated eyes. In the treated eyes, the UCVA on average changed by +0.06 compared to ‐0.01 in the control group. | |
|
Corneal thickness at the thinnest part of the cornea (measured in microns) Follow‐up: 12 months |
The mean corneal thickness decreased on average by 5.4 µm | The mean corneal thickness in the intervention group was 8.93 µm thicker (0.60 µm thinner to 18.46 µm thicker) | 94 eyes (1) |
⊕⊝⊝⊝ very low2,3,4,7 | We have reported the study with the largest numbers. Inconsistent results were seen in the other 2 studies included in this review. In 1 study there was a change of ‐31.4 µm in the treated group at 3 months compared to a change of ‐2.3 µm in the sham treatment group. In the other study, corneal thickness increased by 4 µm in 22 treated eyes compared to 6 µm in 22 untreated eyes (mean difference ‐2 µm, 95% CI ‐33.10 to 29.01). | |
|
Absolute spherical equivalent (measured in dioptres) Follow‐up: 12 months |
The mean absolute spherical equivalent in the control group was ‐0.55 dioptres. | The mean absolute spherical equivalent in the intervention group was 0.65 dioptres higher (‐0.37 to 1.67). | 94 eyes (1) |
⊕⊝⊝⊝ very low2,3,4 | ||
|
Quality of life Follow‐up: 12 months |
Not reported. | |||||
|
Adverse effects Follow‐up: 12 months |
In 1 trial 3/12 participants treated with corneal collagen cross‐linking had an adverse effect including corneal oedema, anterior chamber inflammation, and a recurrent corneal erosion. In 1 trial at 3 years, 3/50 participants experienced adverse events including mild diffuse corneal oedema and paracentral infiltrate, peripheral corneal vascularisation, and subepithelial infiltrates and anterior chamber inflammation. No adverse effects were reported for untreated controls in any of the studies. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded for indirectness (‐1) as the studies reported different cutpoints and definitions of progression. 2 Downgraded for risk of bias (‐1) as the studies were at high or unclear risk of performance, detection, attrition and selective outcome reporting bias. 3 Downgraded for imprecision (‐1) as the confidence intervals were wide and compatible with both benefit and harm. 4 Downgraded for publication bias (‐1) as three unpublished studies (195 participants) identified. 5 Upgraded (+1) because the size of the effect was strong in both studies with approximate 90% relative risk reduction. 6 Downgraded for imprecision (‐1) as results based on information from only 94 eyes. 7 Downgraded for inconsistency (‐1) as different findings seen in the studies.
Background
Description of the condition
Keratoconus means 'conical cornea'. It is a rare condition of the eye that affects approximately 1 in 2000 people (Rabinowitz 1998). The cornea is the main focusing surface of the eye. Keratoconus reduces vision by altering the shape of the cornea so that it becomes stretched and thin, making the vision short‐sighted, irregular, and distorted. The condition can affect one or both eyes and can progress at varying rates.
Presentation
Initially, the patient may present with either a spherical cornea or regular corneal astigmatism. Around the onset of puberty, or earlier in some instances, the cornea begins to thin and protrude, resulting in irregular astigmatism with what is usually a steep curvature. Usually, over a period of the next 10 to 20 years, the process continues until the progression gradually stops. The rate of progression is variable. The severity of the disorder at the time progression stops can range from very mild irregular astigmatism to severe thinning and protrusion with scarring (Krachmer 1984).
Keratoconus is usually diagnosed during the second and third decades of life. The ectasia progresses at a variable rate, although it is more rapid at a younger age. Patients usually have myopic astigmatism and are often suspected of having the condition by their ophthalmologist or optometrist, or both, when a deterioration in visual acuity that is no longer correctable by spectacles occurs.
Hydrops is an acute complication of keratoconus where there is severe photophobia (sensitivity to light) and reduction in visual acuity due to sudden stromal oedema. This is caused by breaks in the Descemet’s membrane (deep layer in the cornea) due to progressive ectasia.
Reported ocular associations of keratoconus include vernal keratoconjunctivitis, retinitis pigmentosa, and Leber's congenital amaurosis. Systemic putative associations include many of the connective tissue disorders (for example Ehlers‐Danlos and Marfan syndromes), mitral valve prolapse, atopic dermatitis, and Down's syndrome (Rabinowitz 1998). The outcomes of the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study showed that keratoconus is not associated with increased risk of connective tissue disease (Wagner 2007).
Identified predisposing factors include atopic history, especially ocular allergies; rigid contact lens wear; and vigorous eye rubbing. In 13.5% of cases there is a family history of the disease (Zadnik 1996). The inheritance in these cases is thought to exhibit variable penetrance (Zadnik 1998). There is no sexual or racial predilection, although the incidence has been found to be higher or more severe in certain ethnic groups (Georgiou 2004).
There is a general observation that elderly patients with keratoconus are not seen as often in clinics, and some have postulated that the co‐existing connective tissue disease may be contributory to a decreased life expectancy. However, a study showing that people with keratoconus do not have an increased mortality rate has disputed this (Moodaley 1992).
Diagnosis
Keratoconus is unique among eye diseases in that it is typically diagnosed during peak education, income‐earning, and childbearing years (Wagner 2007). Keratoconus is diagnosed based on clinical examination and corneal topographic/tomographic analysis. There are several clinical signs for which the presence or absence of each is determined by the severity of the condition. An acute angulation, made by the ectatic cornea, can be seen in the lower lid on downgaze (Munson’s sign). Fleischer’s ring is a ring of epithelial iron deposition around the base of the cone. Vertical striae (Vogt’s), which are fine stress lines in the Descemet’s membrane, are detectable posteriorly. With time there is progressive thinning of the corneal stroma, and the ectasia may be clinically detectable. An 'oil droplet' sign is often visible by direct ophthalmoscopy on viewing the red reflex in a dilated eye. Scissoring and distortion of the reflex can be seen on retinoscopy (Zadnik 1996). After repeated attacks of hydrops, stromal scarring may be visible.
Computer‐assisted videophotokeratoscopy or Scheimpflug imaging are sensitive means for detecting subtle changes in topography on the anterior and posterior corneal surface and allow detailed qualitative and quantitative analysis of corneal shape. Corneal topography measures the steepening in terms of a dioptric power map of the cornea. Various topographic indices have been proposed for preclinical diagnosis of keratoconus (forme fruste) or the diagnosis of keratoconus and grading of the severity of the disease.
Classification of keratoconus can be based on morphology, disease evolution, ocular signs, and index‐based systems of keratoconus. Smolek, et al. have developed the Keratoconus Severity Index using a neural network approach to data collected from corneal topography (Smolek 1997). Another method used to classify the disease is the Amsler‐Krumeich classification of keratoconus, which depends on mean keratometry readings on the anterior curvature sagittal map, thickness at the thinnest location, and the refractive error of the patient. This classification is useful in choosing the best approach for treating keratoconus. The keratoconus percentage index, which combines many of the earlier indices, has been shown to have a high sensitivity in the videokeratoscopic identification of keratoconus (Li 2009). Keratoconus has three characteristics seen by videokeratoscopy that are not present in normals: an increased area of corneal power surrounded by concentric areas of decreasing power, inferior‐superior power asymmetry, and skewing of the steepest radial axes above and below the horizontal meridian (Rabinowitz 1998). Keratoconus Severity Score is another simple tool that was developed using common clinical markers in addition to two corneal topographic indices: average corneal power and higher‐order first corneal surface wavefront root mean square error, resulting in severity score (0 to 5) (McMahon 2006). Belin/Ambrósio Enhanced Ectasia Display is an integrated application in Pentacam system (Oculus Optikgeräte GmbH, Wetzlar, Germany) that combines data from maximal keratometry, tomographic thickness distribution, and enhanced elevation to facilitate the detection of keratoconus (Ambrósio 2003; Belin 2007). Additional metrics, such as epithelial thickness mapping with very high frequency ultrasound or high‐resolution optical coherence tomography, can detect early keratoconus (Li 2012; Reinstein 2009; Reinstein 2010). Newer techniques include analysing biomechanical properties of the cornea using the Ocular Response Analyzer (Reichert, Depew, NY), which so far is limited in its ability to screen for keratoconus (Fontes 2010).
To date, the best and safest method of screening/diagnosing keratoconus is to use as many data as possible in combination with established clinical parameters.
The differential diagnosis of keratoconus includes keratoglobus, pellucid marginal degeneration, and posterior keratoconus.
Aetiology and pathogenesis
Keratoconus has been reported in various clinical settings. It is most commonly an isolated sporadic disorder, or it may be associated with other rare genetic disorders, Down's syndrome, Leber’s congenital amaurosis, connective tissue disorders, atopy, hard contact lens wear, eye rubbing, and a positive family history of the disorder. Several theories have been postulated regarding the aetiology of keratoconus.
The biomechanical characteristics of the normal cornea result from the collagen scaffold and collagen compound and their bonding with the collagen fibrils. The three‐dimensional configuration of the collagen lamella fundamentally codetermines the cornea’s resistance. Biochemical and immunohistochemical studies of the matrix’s proteoglycans show differences between normal and keratoconic corneas (Meek 2005; Raiskup‐Wolf 2008).
Despite intensive biochemical investigation into the pathogenesis of keratoconus, the underlying biochemical process and its aetiologic basis remain poorly understood. Corneal thinning appears to result from loss of structural components in the cornea; the reason this occurs is not clear. Theoretically, the cornea can thin because it has fewer collagen lamellae than normal, fewer collagen fibrils per lamellae, closer packing of collagen fibrils, or various combinations of these factors. These conditions may result from defective formation of extracellular constituents of corneal tissue, a destruction of previously formed components, an increased distensibility of corneal tissue with sliding collagen fibres or collagen lamellae, or a combination of these mechanisms (Akhtar 2008; Hayes 2008). However, some biochemical studies have demonstrated that collagen composition in corneas with keratoconus was unaltered. Biochemical assays and immunohistological studies of corneas with keratoconus suggest that the loss of corneal stroma after digestion by proteolytic enzymes could be caused by increased levels of proteases and other catabolic enzymes (Rabinowitz 1998).
Knowing the natural course of keratoconus is important in order to understand the rate and severity of visual change. However, it is difficult to fully appreciate the natural course of the disorder, as usually the corneal changes have begun before the patient is first seen and, after that, treatment may modify the natural course (Krachmer 1984). CLEK study findings revealed that age appears to be a factor in severity‐related outcomes in keratoconus (Wagner 2007).
Disease progression
There is a general trend for the disease to progress, leading to a gradual increase in corneal curvature and decrease in visual acuity with consequent impact on quality of life (dependency, driving, mental health, near activities, and role difficulties) (Wagner 2007). There are no definitive criteria for progression, but parameters to consider are change in refraction (both sphere and cylinder), uncorrected and best‐corrected visual acuity, and corneal topographical changes. The increase of the maximum keratometry reading by 1 dioptre or more (≥ 1 D) remains the most frequently reported index of disease progression (Caporossi 2010; Hersh 2011; Raiskup‐Wolf 2008; Wittig‐Silva 2008).
Treatment
It is possible in the early stages to use spectacles to improve vision, but as the disease progresses, rigid gas permeable contact lenses often offer the best vision. Various contact lens designs and fittings have been developed to adapt to the challenging needs of this disease, which typically progresses. The presence of corneal scarring, significant thinning, and intolerance to contact lens wear are indications for corneal transplantation (keratoplasty). In high‐income countries, keratoconus is often the most common indication for keratoplasty in young adults.
Several new therapeutic options have emerged, including refractive, optical, and tectonic interventions, which slow the progression of disease and/or delay more invasive treatment.
There are several methods for corneal transplantation. Penetrating keratoplasty (replacement of the full thickness of the cornea) and, more recently, deep anterior lamellar keratoplasty (replacement of the front layers of the cornea only) are the most commonly performed surgical interventions (Shimmura 2006).
Intrastromal rings (Intacs, Ferrara, Kerarings) are small devices that can be implanted into the cornea in an attempt to flatten the corneal profile to achieve a better uncorrected visual acuity and to enhance contact lens tolerance (Rabinowitz 2007). However, this procedure has its own limitations. Firstly, it does not affect the underlying biochemical properties of the cornea. Secondly, there is a limit to how much corneal flattening can be achieved. Most complications of intrastromal ring implantation can be reversed by removing the segment, but serious complications can occur, such as intraoperative corneal perforation, infectious keratitis (corneal infection), damage to the central visual axis, or corneal melt (Boxer Wachler 2003; Miranda 2003). Conductive keratoplasty has been used in an attempt to reduce the severity of astigmatism (Naoumidi 2005). 'Bioptics' is a sequential method of treating large and complex refractive errors by several methods often involving intraocular lens implants. It has been used in keratoconus with treatment algorithms that involve various combinations of intracorneal rings, phacoemulsification, in‐bag implants, iris clipped phakic lenses, and posterior chamber phakic lenses (Leccisotti 2006). It is likely that bioengineered corneas will be available in the future for transplantation and may offer superior optical results to currently available treatments (Carlsson 2003).
Collagen cross‐linking (CXL) with ultraviolet A (UVA) light and riboflavin (vitamin B2) is a relatively new treatment that has been reported to slow the progression of the disease in its early stages (Spoerl 1998; Wollensak 2003; Wollensak 2006). The improvement in vision when combined with intracorneal ring segments has been found to be greater than when using the segments alone (Chan 2007).
Description of the intervention
CXL with UVA and topical riboflavin is carried out in sterile conditions. There are two main established methods of performing the procedure: corneal epithelium off or corneal epithelium on, but different methods are currently being developed.
Corneal epithelium off
In this method (Wollensak 2003; Baiocchi 2009; Hayes 2008), the epithelium of the central 7 mm of cornea is removed after installing topical anaesthesia (for example proxymetacaine 0.5%). The surface is then treated by the application of riboflavin 0.1% solution (10 mg riboflavin‐5‐phosphate in 10 ml dextran 20% solution which is iso‐osmolar 0.1% riboflavin solution) for 30 minutes beginning 5 minutes before the start of irradiation. UVA radiation of 370 nm wavelength and an irradiance of 3 mW/cm2 at distance of 1 cm from the cornea is applied for a period of 30 minutes, delivering a dose of 5.4 J/cm2 (Wollensak 2006). Antibiotic drops are instilled as prophylaxis and a bandage contact lens is inserted, which is then removed at the follow‐up visit once epithelial healing is complete.
Variations of this protocol include the use of pilocarpine 1% pre‐operatively, a treatment area of 9 mm (Vinciguerra 2009), and the selective use of steroids in the postoperative regimen to prevent corneal haze (Vinciguerra 2012). Additionally, in eyes with a corneal thickness less than 400 microns after epithelial removal, there is a risk of corneal endothelial, lenticular, or intraocular UVA damage (Kymionis 2012). To counter this, prior to application of UVA a hypo‐osmolar (hypotonic) solution of riboflavin is used to swell the corneal stroma and hence increase the corneal thickness through a denuded epithelium. This technique was tried on corneal thickness (after epithelial removal) between 320 to 400 microns (Hafezi 2009)
Corneal epithelium on
In this method, the corneal epithelium is kept on (Chan 2007; Pinelli 2007). In 2003, Boxer Wachler, et al. proposed a slight modification of the treatment using pre‐operative anaesthetic eyedrops containing benzalkonium chloride to loosen the tight junctions of the corneal epithelial cells (Boxer Wachler 2003). The use of benzalkonium chloride may allow transepithelial cross‐linking treatment without removal of the epithelium. This technique was designed to reduce postoperative pain and improve patient comfort. This modification is known as C3‐R (Baiocchi 2009; Vicente 2010). In this technique, 30 minutes before the UVA treatment, 1 drop of pilocarpine 2% is installed and riboflavin solution is started (1 drop every 2 minutes with minimum 16 drops over 30 minutes). Topical anaesthetic drop is started 20 minutes before the treatment (1 drop every 4 minutes, repeated 4 times). Treatment with UVA irradiation lasts for a total of 30 minutes, adding 1 drop riboflavin every 5 minutes.
In both techniques, a bandage contact lens is inserted at the end of the procedure and removed after five days. A number of modified riboflavin formulations have been introduced to facilitate diffusion through the corneal epithelium (Caporossi 2013; Koppen 2011).
Different techniques
A hybrid technique currently used in some centres is to perform epithelial disruption in the 9 mm zone using a special disrupter to create pockmarks in the corneal epithelium (Rechichi 2013). The primary goal is to maintain as much live epithelium as possible but also promote riboflavin penetration. The secondary goal is to reduce eye inflammation and to get the contact lens out of the patient within 48 hours. Riboflavin eye drops are instilled after disruption and every 2 to 5 minutes for at least 30 minutes before UVA radiation treatment.
Recent studies aiming to reduce the procedure time to 9 minutes have used higher power (up to 30 mW/cm2 compared to 3 mW/cm2 in the standard procedure). The goal is to achieve a rapid treatment protocol by using higher intensity UVA and shorter irradiation time. This technique (known as flash‐linking or rapid cross‐linking) aims to keep an equivalent energy dose to the standard irradiation of 3 mW/cm2 whilst reducing the treatment time from 30 minutes to 9 minutes (Schumacher 2011).
Another technique currently being investigated is iontophoresis transcorneal delivery technique, which is a method of facilitating the penetration of riboflavin through the cornea through the use of a low‐intensity electrical current. Compared to the classic technique, iontophoresis shortens the time needed for riboflavin penetration and the duration of exposure to UVA radiation and does not require epithelium removal (Arboleda 2014; Mastropasqua 2014).
How the intervention might work
CXL employs the photosensitiser riboflavin (vitamin B2), which when exposed to longer wavelength ultraviolet light (370 nm UVA), will induce chemical reactions (free radical production) in the corneal stroma and ultimately result in the formation of covalent bonds between the collagen molecules, fibres, and microfibrils. This increase in collagen bonding is thought to prevent further thinning and ectasia and as such slow or halt the progression of keratoconus. Some pre‐clinical investigations, including biochemical and biophysical measurements, have demonstrated enhanced corneal rigidity and greater biomechanical stability of the cornea following this treatment (Raiskup‐Wolf 2008; Wollensak 2003).
Wollensak, et al. have further demonstrated a significant increase in collagen fibre diameter as the underlying histopathologic correlate after CXL. Increased resistance to pepsin digestion after cross‐linking has been found, which might be important for keratoconus, as a significantly elevated activity of collagenases has previously been noted (Wollensak 2004).
Why it is important to do this review
As mentioned above, CXL is the only treatment that claims to slow down the progression of keratoconus. CXL is carried out largely unregulated with very few standardised criteria available for identification of the ideal patient to benefit from the treatment. Keratoconus is very asymmetrical and at times a very slowly progressive disease; in particular, it is well known that the rate of progression slows with age (Hovakimyan 2012; Krachmer 1984). Short‐term data from trials are available, but robust evidence on the long‐term efficacy and safety of CXL is not and is needed. Keratometric indices are at present the main indicators of treatment effect. Changes in corneal biomechanics, which this treatment purports to induce, have not been studied in vivo (Ashwin 2009). This review examined evidence from the current literature (and will examine future trial results as and when they become available) to provide clinicians with answers about what they can expect of this relatively new treatment. This review also provided an evidence‐based reference point for people with keratoconus who seek validated information regarding the efficiency of the specific treatment.
Objectives
The objective of this review was to assess whether there is evidence that CXL is an effective and safe treatment for halting the progression of keratoconus compared to no treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
We included all studies in which participants had been diagnosed with keratoconus. We have excluded no participants according to age. We had planned to exclude studies where participants had previous treatment, but in the event we did not identify any such studies.
Types of interventions
We included trials that compared collagen cross‐linking (CXL) (with riboflavin and ultraviolet A (UVA)) to no treatment. We excluded trials that compared different ways of doing CXL and did not have a control (no treatment) group. We excluded trials examining the use of this treatment for conditions other than keratoconus.
Types of outcome measures
Primary outcomes
Two indicators were used to measure disease progression at 12 months after treatment:
Increase in maximal keratometry (Kmax) of more than 1.5 D
Worsening in uncorrected visual acuity of more than 0.2 logMAR
Secondary outcomes
Other indicators of disease progression:
Mean maximal keratometry in dioptres at 12 months after treatment
Mean uncorrected visual acuity at 12 months after treatment
Mean average corneal power in dioptres at 12 months after treatment
Mean corneal thickness at the thinnest part of the cornea 12 months after treatment
Mean absolute spherical equivalent at 12 months after treatment
Contact lens intolerance developed within 12 months of treatment
Safety measures: We looked at all adverse outcomes related to CXL reported in the included studies including the following:
Infectious keratitis
Corneal haze and scarring
Glare and halo
Reduction in uncorrected or best‐corrected visual acuity
Corneal epithelial defect
Anisometropia
Diplopia
Induced astigmatism
Reduction in contrast sensitivity
Fluctuating vision (during the day or from day to day)
Increased or decreased light sensitivity
Endothelial cell damage as indicated by fall in endothelial cell density
Quality‐of‐life outcomes
One aim of this review was to summarise any validated quality‐of‐life measures used.
Economic data
One aim of this review was to summarise any data on cost or economics reported in the included studies.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (which contains the Cochrane Eyes and Vision Group Register) (CENTRAL; 2014, Issue 7), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2014), EMBASE (January 1980 to August 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to August 2014), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to August 2014), OpenGrey (System for Information on Grey Literature in Europe) (www.opengrey.eu/), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organisation International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We used no date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 28 August 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), CINAHL (Appendix 5), OpenGrey (Appendix 6), mRCT (Appendix 7), ClinicalTrials.gov (Appendix 8) and the ICTRP (Appendix 9).
Searching other resources
We searched other resources by handsearching book chapters, contacting clinical experts, and searching the reference lists of all included studies. We emailed all contact authors from the included studies asking for further information on their methodology and results.
Data collection and analysis
Selection of studies
Two review authors (ES, RK) independently assessed all retrieved citations regarding eligibility for inclusion. The same two review authors obtained full‐text copies of definitely or potentially relevant studies and assessed them carefully. We had non‐English trial reports translated to determine whether they met the inclusion criteria. We also recorded the reasons for excluding studies. In cases where there were disagreements between the two review authors, a third review author was involved.
Data extraction and management
Two review authors (ES, JE) independently extracted data. We recorded on a form information about the methods used in the trial along with the following:
Details of participants (age, gender, setting, number in each group, grade of keratoconus, and comparability at baseline).
Details of intervention(s).
Outcomes (including adverse effects). For dichotomous data, the number of participants assigned to each intervention was collected and the number of participants who experienced the event. For continuous data, the mean and standard deviations were collected or calculated, or the median and interquartile range if the data were skewed, for each study.
Percentage of participants for whom no outcome data could be obtained.
Disagreements were resolved by discussion.
Assessment of risk of bias in included studies
Two review authors (ES, RK) independently assessed for risk of bias based on the following parameters and using Cochrane’s tool for assessing risk of bias as specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Method of randomisation: We considered the method used to generate the random sequence within the trials at low risk of bias if it involved a computer random number generator, random number tables, shuffled cards, or envelopes.
Allocation concealment: Low risk would be central allocation or numbered, sealed, opaque envelopes.
Masking of participants, personnel, and outcome assessment.
Participant attrition: Were rates of follow‐up similar in comparison groups? Was the analysis based on an intention‐to‐treat?
Selective reporting in terms of outcomes, time points, subgroups, or analyses.
We assessed and graded each parameter as follows: low risk of bias, high risk of bias, and unclear risk of bias.
We emailed all first authors of included trials twice for clarification where we judged risk of bias to be unclear, but received no response.
Measures of treatment effect
The primary outcome variables (increase in maximum keratometry of 1.5 D or more and loss of 0.2 logMAR visual acuity or more) were dichotomous, and we used the risk ratio (RR) as the measure of treatment effect. Secondary outcomes were continuous; for these, we used the mean difference as a measure of treatment effect. We planned to report medians in the event that data were skewed, but where data were reported, means were available.
Unit of analysis issues
We included both within‐person and parallel group studies. We did not do any meta‐analysis. For within‐person studies, we calculated confidence intervals for the measures of effect without taking into account the pairing (due to lack of appropriately reported data). This is a conservative assumption. We requested raw data from the authors via email but the authors did not supply.
In future editions of this review that may include meta‐analyses, we will analyse within‐person and parallel group studies separately and then combine estimates using the generic inverse variance method. If data are not adequately analysed in the published reports, we will ask for raw data from the authors. If paired studies have been used, we will assess whether or not eyes were symmetric at baseline to see whether or not a paired approach seemed plausible. We will also examine whether or not effect estimates from paired studies seem consistent with those from unpaired studies.
Dealing with missing data
We contacted authors regarding missing data. We did an available case analysis and judged the extent to which attrition bias might be a problem in the 'Risk of bias' table. We did not look at the potential impact of missing data in a sensitivity analysis (as planned in our protocol (Hamada 2013)) due to the sparcity of data and lack of meta‐analysis.
Assessment of heterogeneity
We planned to assess heterogeneity by looking at the clinical and methodological diversity of the studies and by examining the forest plots and I2 statistic as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011) . However, this was not required as we did not do any meta‐analysis.
Assessment of reporting biases
To assess publication bias, we performed a search of clinical trials registers to identify studies that have been completed but may not have been published. We addressed selective outcome reporting as part of the 'Risk of bias' assessment (Assessment of risk of bias in included studies).
Data synthesis
We did not conduct any meta‐analysis because we found only three eligible studies, and these studies did not report the required outcomes in the correct format to enable the data to be pooled.
In future updates of this review, we will meta‐analyse studies provided it is sensible to do so, for example, we find no evidence of substantial heterogeneity (I2 less than 50%), or the effect estimates are in the same direction. We will use a fixed‐effect model if there are three or fewer studies and a random‐effects model if more studies are available.
Subgroup analysis and investigation of heterogeneity
If sufficient data are available in future updates of this review, we will perform a subgroup analysis to assess whether severity of keratoconus has an effect on response to treatment. We will use keratometry‐based classification of disease severity (mild less than 45 D, moderate 45 D to 52 D, severe greater than 52 D) (Zadnik 1996).
Sensitivity analysis
In future updates, providing sufficient data are available, we will do two sensitivity analyses: first, excluding studies at high risk of bias in one or more domains and second, excluding studies where missing data were imputed.
Summary of findings table
We prepared a summary of findings table presenting relative and absolute risks. One author (JE) graded the overall quality of the evidence for each outcome using the GRADE classification (www.gradeworkinggroup.org/) and the other authors checked this grading. We included the following outcomes in the summary of findings table.
Progression
Steepness of the cornea
Uncorrected visual acuity
Corneal thickness
Absolute spherical equivalent
Quality of life
Adverse effects
These outcomes were not selected a priori as the summary of findings tables was not planned at the protocol stage.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 670 references (Figure 1). After removing duplicates, we reviewed 482 references and discarded 431 as not relevant to the scope of the review. We obtained 51 full‐text reports to assess for potential inclusion in the review and included eight reports of three studies (see Characteristics of included studies) and excluded 40 reports of 38 studies (see Characteristics of excluded studies). We sought two additional reports of studies originally found on clinicaltrials.gov to help ascertain if these studies should be excluded. We also identified one ongoing trial (see Characteristics of ongoing studies) and aim to include this study in the review when completed, if appropriate. There were also four reports of three potentially relevant studies for which results were not currently available. If we are able to access the results for these studies, we could include them in further updates of this review (see Characteristics of studies awaiting classification).
1.
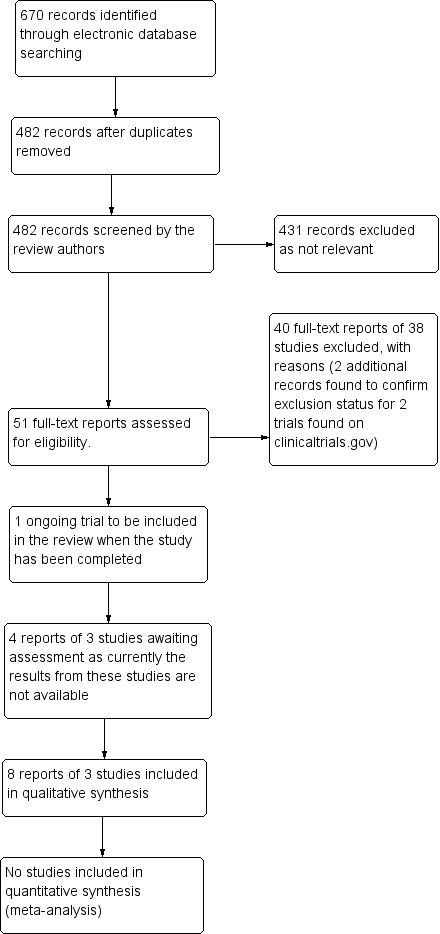
Results from searching for studies for inclusion in the review
Included studies
Study populations
We included three RCTs that enrolled a total of 225 eyes and analysed 219 eyes. Out of these eyes, 119 had CXL with the epithelium off technique and 100 served as controls. The three trials were conducted in Australia (Wittig‐Silva 2008), the United Kingdom (O'Brart 2011), and the United States (Hersh 2011). The number of eyes in each study was 100, 48, and 71, respectively. It was not clear how many participants were involved in Wittig‐Silva 2008 or Hersh 2011; 24 people were included in O'Brart 2011.
All three trials randomly allocated eyes to treatment. O'Brart 2011 was a within‐person study, but for the other two trials it was not clear how eyes within person were allocated. In Hersh 2011, apart from keratoconics, participants with iatrogenic corneal ectasia were studied, but the reports from the two groups were reported separately, and also of note is the fact that the control group was only half the size of the intervention group, and all controls were treated at three months.
All three trials included participants where there was topographic or refractive evidence of progression of keratoconus, but while O'Brart 2011 and Wittig‐Silva 2008 excluded eyes where corneal pachymetry was less than 400μm, Hersh 2011 used as exclusion criterion corneal pachymetry of less than 300μm.
Interventions and comparators
All three trials used the same technique for CXL with minimal variations. Epithelium was removed under topical anaesthesia, topical isotonic riboflavin solution of 0.1% was applied, and then the cornea was treated with UVA radiation (370 nm at 3 mW/cm2) for 30 minutes. In the trials by Wittig‐Silva 2008 and O'Brart 2011, there was no sham treatment for controls, while in Hersh 2011, the following sham treatment was used for controls: riboflavin was applied without epithelial debridement, followed by sham treatment in which the UVA light was not turned on.
Outcomes and follow‐up
Wittig‐Silva 2008 reported 12‐months results for a subset of the whole cohort and subsequently published 12‐months and 36‐months results for a larger group of participants. Hersh 2011 reported findings at 12 months, and O'Brart 2011 at 18 months follow‐up.
Funding sources
Hersh 2011 was supported in part by Peschke Meditrade GmbH, Zurich, Switzerland, and an unrestricted grant to the Department of Ophthalmology, University of Medicine and Dentistry of New Jersey–New Jersey Medical School from Research to Prevent Blindness, Inc., New York, New York, USA.
O'Brart 2011 was funded by Guy's and St. Thomas' NHS Foundation Trust, own account, NHS R&D Support Funding.
Wittig‐Silva 2008 was funded by the Royal Victorian Eye and Ear Hospital Research Fund, Eye Research Australia Foundation, Scholarship for Postgraduate Studies (Faculty of Medicine and University of Melbourne), and Contact Lens Society of Australia
Excluded studies
We excluded 38 studies. See Characteristics of excluded studies.
Risk of bias in included studies
2.
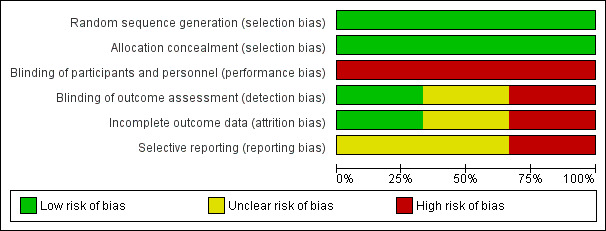
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
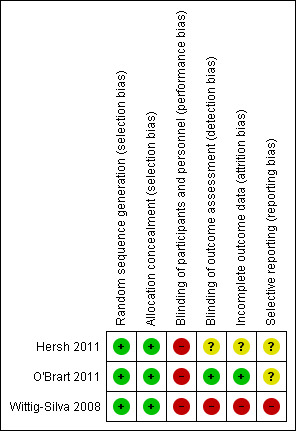
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Overall the trials were judged to be at low risk of selection bias.
O'Brart 2011 and Wittig‐Silva 2008 described computer‐generated allocation schedules, and O'Brart 2011 used shuffled envelopes.
All three trials described methods to conceal the allocation. Hersh 2011 and O'Brart 2011 used sealed envelopes; Wittig‐Silva 2008 described how the schedule was kept secure and managed by staff not involved in the trial.
Blinding
All three trials were judged to be at high risk of performance bias. In two trials (O'Brart 2011; Wittig‐Silva 2008), no attempt was made to mask participants or caregivers to the treatment (and indeed this would have been difficult due to the invasive nature of the treatment). Hersh 2011 had a sham treatment arm but reported that participants were aware of their randomly assigned group, so the significance of the sham treatment was unclear.
O'Brart 2011 was judged to be at low risk for detection bias as the visual acuity measurements were done by a masked observer who was not otherwise involved in the trial. For Hersh 2011, it was unclear the extent to which the outcome assessments were masked, as they did have a sham treatment group. We judged Wittig‐Silva 2008 to be at high risk of detection bias, as they described the trial as "unmasked", and the treatment groups were quite different (treated/not treated).
Incomplete outcome data
O'Brart 2011 was judged to be at low risk of attrition bias because it was a within‐person study and therefore follow‐up was equal between the two treatment groups.
Overall Wittig‐Silva 2008 was judged to be at high risk of attrition bias due to differential follow‐up: At one year 46 (92%) treated and 41 (82%) control eyes were followed up. Using last observation carried forward, data for 46 treated and 48 control eyes were reported. Over 3 years, 21 eyes in the control group left the trial, of which 12 were treated with CXL, 5 had corneal transplants, and 4 withdrew for personal reasons. In the treatment group, five people (eyes) withdrew for personal reasons. This unequal loss to follow‐up meant that the control group was followed up, on average, for less time than the treatment group.
For Hersh 2011 it was unclear.
Selective reporting
We did not have access to study protocols and in general felt we could not accurately judge the extent of selective reporting.
Effects of interventions
See: Table 1
Primary outcomes
See Analysis 1.1
1.1. Analysis.
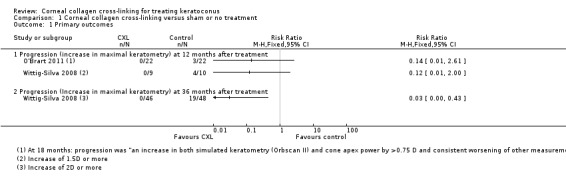
Comparison 1 Corneal collagen cross‐linking versus sham or no treatment, Outcome 1 Primary outcomes.
Increase in maximal keratometry of more than 1.5 D at 12 months after treatment
Wittig‐Silva 2008 reported an interim analysis after 19 eyes (out of a total of 100 eyes) had been followed up to 1 year. Zero out of 9 eyes treated with CXL experienced an increase in maximal keratometry of 1.5 D or more at 12 months compared to 4 out of 10 eyes in the control group (risk ratio (RR) 0.12, 95% confidence interval (CI) 0.01 to 2.00).
Wittig‐Silva 2008 did not report 12‐months data for this outcome for the whole cohort, but at 36 months no treated eyes had increased by 2 D or more (out of 46 treated eyes), compared to 19 out of 48 eyes in the control group (RR 0.03, 95% CI 0.00 to 0.43). It must be noted that in Wittig‐Silva 2008, 26 eyes were not followed up for the full 36 months, and last observation carried forward was used. The loss to follow‐up was different between the two groups: 21 eyes in the control group left the trial, of which 12 were treated with CXL, 5 had corneal transplants, and 4 withdrew for personal reasons. In the treatment group, five people (eyes) withdrew for personal reasons. This unequal loss to follow‐up meant that the control group was followed up, on average, for less time than the treatment group. The effect of this bias will probably be in favour of the control group (as keratoconus is a progressive disease, and the control group did not have so much time to progress). This means that the measure of effect is likely to be an underestimate of the size of the effect.
O'Brart 2011 did not report this outcome but did report "progression" at 18 months. Progression was defined as "an increase in both simulated keratometry (Orbscan II) and cone apex power by >0.75 D and consistent worsening of other measurements". According to this definition, 0 out of 22 treated eyes compared to 3 out of 22 untreated eyes progressed over the time period (RR 0.14, 95% CI 0.01 to 2.61).
Hersh 2011 did not report this outcome.
Deterioration in uncorrected visual acuity of more than 0.2 logMAR at 12 months after treatment
None of the studies reported this outcome.
Secondary outcomes
See Analysis 1.2.
1.2. Analysis.
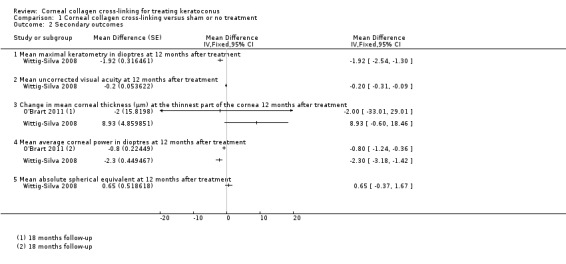
Comparison 1 Corneal collagen cross‐linking versus sham or no treatment, Outcome 2 Secondary outcomes.
Mean maximal keratometry in dioptres at 12 months after treatment
Wittig‐Silva 2008 reported that maximal keratometry in treated eyes decreased on average by 0.72 D (standard deviation (SD) 1.0) over 12 months in contrast to control eyes, where maximal keratometry increased on average by 1.2 D (SD 1.94), (mean difference (MD) ‐1.92 D, 95% CI ‐2.54 D to ‐1.30 D).
O'Brart 2011 did not report this outcome.
Hersh 2011 reported "no significant differences" between treated and control eyes at three months but did not report the data.
Mean uncorrected visual acuity at 12 months after treatment
Wittig‐Silva 2008 reported that uncorrected visual acuity (UCVA) logMAR score in treated eyes decreased on average by 0.14 (SD 0.3) over 12 months in contrast to control eyes, where UCVA logMAR increased on average by 0.06 (SD 0.21) (MD ‐0.20, 95% CI ‐0.31 to ‐0.09).
O'Brart 2011 reported that mean UCVA at 18 months was 0.33 (Snellen decimal equivalent) in 22 treated eyes and 0.21 in 22 untreated eyes. In the treated eyes, the UCVA on average changed by +0.06 compared to ‐0.01 in the control group. This gives a MD of 0.07 Snellen decimal equivalent (95% CI ‐0.04 to 0.18, estimated from P value of 0.2)
Hersh 2011 reported "no significant differences" between treated and control eyes at three months but did not report the data.
Mean average corneal power in dioptres at 12 months after treatment
Wittig‐Silva 2008 reported preliminary data on a subset of the trial followed up to one year. Average corneal power in treated eyes decreased on average by 1.2 D (estimate from graph, SD not reported, 9 eyes) over 12 months in contrast to control eyes, where corneal power increased on average by 1.10 D (SD not reported, 11 eyes). If we assume a SD of 1 D in both groups (based on other data in the paper), then this gives a MD of ‐2.30, 95% CI ‐3.18 to ‐1.42. This outcome was not reported for the whole cohort at one year.
O'Brart 2011 reported that average corneal power decreased in the treated eyes over 18 months, but there was a discrepancy in the paper such that it was not clear whether the decrease was 0.62 D or 0.66 D. There was an increase of 0.14 D in the control group. As a P value was provided for the comparison between ‐0.66 D and 0.14 D (less than 0.001), we have estimated the CIs for the difference between ‐0.66 D and 0.14 D. This gives a mean difference of ‐0.8 D (95% CI ‐0.36 to ‐1.24).
Hersh 2011 reported "no significant differences" between treated and control eyes at three months but did not report the data.
Mean corneal thickness at the thinnest part of the cornea 12 months after treatment
Wittig‐Silva 2008 reported that corneal thickness at the thinnest part of the cornea in treated eyes increased on average by 3.53 µm (SD 23.7, 46 eyes) over 12 months in contrast to control eyes, where corneal thickness decreased on average by 5.4 µm (SD 23.4, 48 eyes) (MD 8.93, 95% CI ‐0.60 to 18.46).
In Hersh 2011 there was a change in pachymetry (thinnest part of the cornea) of ‐31.4 µm (SD not reported) in the treated group at three months compared to a change of ‐2.3 µm (no SD reported) in the control group.
In O'Brart 2011 ultrasonic central corneal pachymetry increased by 4 µm in 22 treated eyes compared to 6 µm in 22 untreated eyes. (MD ‐2 µm, 95% CI ‐33.01 to 29.01 (CI estimated from p‐value of 0.9)).
Mean absolute spherical equivalent at 12 months
Wittig‐Silva 2008 reported change in absolute spherical equivalent in treated eyes of 0.1 D (SD 2.6, 46 eyes) over 12 months compared to control eyes, where absolute spherical equivalent changed by ‐0.55 D (SD 2.42, 48 eyes). This gives a MD of 0.65 D, 95% CI ‐0.37 to 1.67.
Contact lens intolerance developed within 12 months of treatment
Only one study mentioned contact lens intolerance (O'Brart 2011). Three out of 22 participants were contact lens intolerant pre‐operatively. Two of these three participants ended up having intrastromal corneal ring segment insertion.
Adverse outcomes
Only O'Brart 2011 and Wittig‐Silva 2008 described adverse effects in their papers. In O'Brart 2011, overall three participants were noted to have an adverse effect, including corneal oedema, anterior chamber inflammation, and a recurrent corneal erosion. In Wittig‐Silva 2008, 36‐month results document 3 participants with adverse events: 1 case with mild diffuse corneal oedema and a paracentral infiltrate, 1 case with peripheral corneal vascularisation, and 1 case with subepithelial infiltrates and anterior chamber inflammation. No adverse effects were reported for any controls in any of the studies.
Quality‐of‐life outcomes
Hersh 2011 reported this for the cohort of treated participants but did not provide a comparison between treated and untreated participants. The other studies did not report quality of life.
Economic data
The included studies did not report economic data.
Discussion
Summary of main results
We found three eligible trials that compared CXL versus no treatment, but one of these trials reported very little useable data. It was not possible to pool data due to differences in measuring and reporting outcomes. All three trials were at high risk of bias. We identified a further three unpublished trials.
There was indirect information on the risk of progression (which we defined as increase of 1.5 D or more in maximum keratometry). The available data suggest that there may be in the order of an 80% to 90% relative risk reduction in progression over 12 months, but we are very uncertain as to the size of the effect (Table 1).
Other data reported suggested that on average treated eyes had a less steep cornea (approximately 2 D less steep) and better uncorrected visual acuity (approximately 2 lines or 10 letters better), but again we judged the quality of the evidence to be very low, as it was largely derived from one trial at high risk of bias and there was the possibility of publication bias. The data on corneal thickness were inconsistent.
There were no data available on quality of life.
Adverse effects were not uncommon. In 1 trial, 3 out of 12 participants treated with CXL had an adverse effect, including corneal oedema, anterior chamber inflammation, and a recurrent corneal erosion. In 1 trial at 3 years, 3 out of 50 participants had experienced adverse events, including mild diffuse corneal oedema and a paracentral infiltrate, peripheral corneal vascularisation, and subepithelial infiltrates and anterior chamber inflammation.
Overall completeness and applicability of evidence
Overall the evidence is incomplete, with only three trials reported with small sample sizes and at high risk of bias. Important outcomes, such as risk of visual acuity loss and quality‐of‐life measures, have not been reported. Only one trial followed up participants for longer than one year. As keratoconus is a slowly progressing disease, longer follow‐up may be important.
With respect to applicability, the participants included in these studies were reasonably representative of patients likely to be interested in this treatment. We did not find any studies in children, which is an important omission in the evidence. The surgical techniques used are similar to those used in clinical practice.
Quality of the evidence
The overall quality of the evidence was judged to be very low (Table 1). The main reasons for downgrading the evidence included risk of bias in the included studies, imprecision, indirectness and publication bias.
Potential biases in the review process
We used standard methods expected by Cochrane. Search screening and data extraction were done independently by two review authors. We have clarified and amended the protocol for this review a little‐‐in particular clarifying the comparison group and refining the outcomes. We do not believe that this will have introduced any bias into the review but note that the outcomes selected for presentation in the summary of findings table were not planned a priori but were selected while the review was in progress. We have documented all changes in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
Although there are not many RCTs available, we did find a few systematic reviews and meta‐analyses in the literature. The major one is performed by NICE (NICE 2013; Craig 2014), which includes RCTs and prospective studies. This review concluded that there is evidence for the effectiveness of CXL in halting the progression of keratoconus. Performing meta‐analysis by comparing qualitatively different maximal keratometry (Kmax) (NICE 2013; Gore 2013) is clinically questionable, as different topography instruments have been used in different studies. Although most studies have used increase in Kmax of more than or equal to 1 D to report progression, we have chosen 1.5 D, as repeatability variations and limitations in measurement accuracy of most topographers is a well‐known factor (Szalai 2012). In fact, in their meta‐analysis, Gore 2013, by using 1 D difference in Kmax to measure progression and regression, found that 10% of controls were found to have regression, something that does not normally happen in keratoconic corneas, confirming that 1.5 D is more appropriate clinically. Another meta‐analysis, by Chunyu 2014, concluded that further research from randomised trials is necessary to confirm whether CXL is an effective treatment. The difficulty in performing meta‐analysis of RCTs is once more highlighted as we can see that the way studies are reported can be misleading for review authors. For example, the study by Henriquez 2011 identified by NICE as an RCT does not fit the strict criteria to be included in our review, as controls were recruited separately and their methodology did not include random allocation of treatment. Additionally, Chunyu 2014 did not identify the fact that Hersh 2011 was published in multiple publications which may have introduced bias.
Authors' conclusions
Implications for practice.
Despite the numerous prospective and retrospective studies available in the literature and the fact that CXL seems to be accepted worldwide as a breakthrough treatment in the management of keratoconus, evidence is limited due to the lack of properly conducted RCTs. If strict criteria are used and only data from RCTs accepted, then there is a lack of evidence that CXL is indeed an effective treatment in halting the progression of keratoconus.
Implications for research.
Higher‐quality studies are needed before an appropriate meta‐analysis can be conducted to confirm the importance of this treatment. However, things look promising, as there seem to be multiple ongoing registered studies looking into the effectiveness of CXL, as well as studies looking into modifications of the treatment protocols.
Acknowledgements
The Cochrane Eyes and Vision Group (CEVG) created and ran the electronic search strategies. We thank Alex Shortt, Mayank Nanavaty and Gianni Virgili for comments on the review and Stephanie Watson for comments on the protocol. We thank Anupa Shah, Managing Editor for CEVG, for her assistance throughout the review process, and we acknowledge Pammal Ashwin's contribution to earlier versions of this protocol.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Keratoconus #2 keratoconus #3 ectasia #4 (#1 OR #2 OR #3) #5 MeSH descriptor Collagen #6 collagen cross near/2 link* #7 collagen crosslink* #8 CCL #9 CXL #10 C3R #11 (#5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 (#4 AND #11)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp keratoconus/ 14. keratoconus.tw. 15. ectasia.tw. 16. or/13‐15 17. exp collagen/ 18. (collagen cross adj2 link$).tw. 19. collagen crosslink$.tw. 20. CCL.tw. 21. CXL.tw. 22. C3R.tw. 23. or/17‐22 24. 16 and 23 25. 12 and 24
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp keratoconus/ 34. keratoconus.tw. 35. ectasia.tw. 36. or/33‐35 37. exp collagen/ 38. (collagen cross adj2 link$).tw. 39. collagen crosslink$.tw. 40. CCL.tw. 41. CXL.tw. 42. C3R.tw. 43. or/37‐42 44. 36 and 43 45. 32 and 44
Appendix 4. LILACS search strategy
keratoconus or ectasia and collagen cross link$ or collagen crosslink or CCL or CXL
Appendix 5. CINAHL (EBSCO) search strategy
S24 S12 and S23 S23 S15 and S22 S22 S16 or S17 or S18 or S19 or S20 or S21 S21 C3R S20 CXL S19 CCL S18 collagen crosslink* S17 collagen cross link* S16 (MH "Collagen") S15 S13 or S14 S14 ectasia S13 keratoconus S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 TX allocat* random* S10 (MM "Quantitative Studies") S9 (MM "Placebos") S8 TX placebo* S7 TX random* allocat* S6 (MM "Random Assignment") S5 TX randomi* control* trial* S4 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) S3 TX clinic* n1 trial* S2 PT Clinical trial S1 (MH "Clinical Trials+")
The search filter for trials at the beginning of the CINAHL strategy was developed by the Scottish Intercollegiate Guidelines Network (SIGN 2010).
Appendix 6. OpenGrey search strategy
keratoconus AND collagen cross
Appendix 7. metaRegister of Controlled Trials search strategy
(keratoconus or ectasia) and (collagen cross or CCL or CXL)
Appendix 8. ClinicalTrials.gov search strategy
(keratoconus OR ectasia) AND (collagen cross OR CCL OR CXL)
Appendix 9. ICTRP search strategy
keratoconus OR ectasia = Condition AND collagen cross OR CCL OR CXL = Intervention
Data and analyses
Comparison 1. Corneal collagen cross‐linking versus sham or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcomes | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Progression (increase in maximal keratometry) at 12 months after treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Progression (Increase in maximal keratometry) at 36 months after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcomes | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2.1 Mean maximal keratometry in dioptres at 12 months after treatment | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mean uncorrected visual acuity at 12 months after treatment | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Change in mean corneal thickness (µm) at the thinnest part of the cornea 12 months after treatment | 2 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Mean average corneal power in dioptres at 12 months after treatment | 2 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Mean absolute spherical equivalent at 12 months after treatment | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hersh 2011.
| Methods | Parallel group RCT Results reported by eye, but more eyes enrolled than people and unclear whether there was also within‐person randomisation. Study recruited participants with ectasia due to keratoconus and iatrogenic (after surgery for myopia). Only the keratoconus participants were included in this review. |
|
| Participants | Country: USA Number of people randomised: not reported, (77 eyes) Average age: Not reported % women: Not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
"The sham control group received riboflavin 0.1% ophthalmic solution alone. In this group, the epithelium was not removed. Riboflavin was administered topically every 2 minutes for 30 minutes. Next, the cornea was exposed to a sham treatment in which the UVA light was not turned on, during which time riboflavin was administered topically every 2 minutes for an additional 30 minutes. The sham control patients were followed for 3 months postoperatively, at which point the study eye crossed over to the treatment group and received full CXL treatment." Hersh 2011 page 150 |
|
| Outcomes | From clinical trials registry entry: Primary outcome:
No secondary outcomes listed. The following outcomes were reported in various publications:
Follow‐up: 1, 3, 6, and 12 months Note: As the sham control group was treated at 3 months, comparisons are valid up to 3 months only. |
|
| Notes | Date study conducted: December 2007 to April 2011 (from trials register), but main publication accepted for publication in 2010. Funding: "Supported in part by Peschke Meditrade GmbH, Zurich, Switzerland, and an unrestricted grant to the Department of Ophthalmology, UMDNJ–New Jersey Medical School from Research to Prevent Blindness, Inc., New York, New York, USA" Conflict of interest: "No author has a financial or proprietary interest in any material or method mentioned." "Dr. Hersh is a paid medical consultant to Avedro, Inc." Trial registration: NCT00647699 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was computer generated ..." Hersh 2011 page 150 |
| Allocation concealment (selection bias) | Low risk | "... on the procedure day, a sealed envelope was opened revealing whether the eye would be in the sham or treatment group" Hersh 2011 page 150 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
"Patients were aware of their randomly assigned group." Hersh 2011 page 150 but the control group was given a sham procedure, it is not clear why if participants were told which group they were in. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Control group was given a sham procedure, but participants were aware of their status (see above). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol and could not accurately judge the extent of selective reporting. |
O'Brart 2011.
| Methods | Within‐person RCT Eyes randomly allocated to treatment, treated and untreated groups compared using unpaired t tests. |
|
| Participants | Country: UK Number of people randomised: 24 (48 eyes) Average age: 30 years (range 21 to 42) % women: 21 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
Follow‐up: 1 week; 3, 6, 12, 18 months (outcomes reported at 18 months) |
|
| Notes | Date study conducted: June 2006 to December 2007 (anticipated recruitment dates as reported on trials register entry) Funding: Guy's and St. Thomas' NHS Foundation Trust, own account, NHS R&D Support Funding Conflict of interest: reported "none" Trial registration: ISRCTN08013636 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eyes were randomised to receive treatment using a shuffled closed envelope system by a member of staff not involved in the study. There were 36 envelopes (18 for right and 18 left). Sixteen right eyes and eight left eyes were randomly selected for treatment." Page 1520 |
| Allocation concealment (selection bias) | Low risk | "Eyes were randomised to receive treatment using a shuffled closed envelope system by a member of staff not involved in the study. There were 36 envelopes (18 for right and 18 left). Sixteen right eyes and eight left eyes were randomly selected for treatment." Page 1520 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not masked due to the nature of the intervention. Subjective measurements (visual‐acuity assessment and refraction) in participants were undertaken by a masked observer (PP) not involved with either the randomisation process or the surgical procedure. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Subjective measurements (visual‐acuity assessment and refraction) were undertaken by a masked observer (PP), not involved with either the randomisation process or the surgical procedure." Page 1520 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Within‐person study, so follow‐up equal between treatment groups |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol and could not accurately judge the extent of selective reporting. |
Wittig‐Silva 2008.
| Methods | Parallel group RCT If both eyes of a participant were eligible, each eye was randomised independently. |
|
| Participants | Country: Australia Number of people randomised: Not stated. 100 eyes Average age: 26 years % women: 43 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Eyes randomised to control were offered compassionate CXL (no earlier than 6 months after randomisation) during the course of study if continuing and significant progression was noted. |
|
| Outcomes | As reported on clinical trials register entry: Primary outcome:
Secondary outcomes:
Follow‐up: 5 years (on clinical trial register). Outcomes reported to date: BSCVA and maximum and average keratometry values at 3, 6, 12, 24, and 36 months. At 12 months, results from 46 treated and 48 control eyes reported using LOCF. |
|
| Notes | Date study conducted: June 2006 to June 2009 (as reported on trials register entry) Funding: Royal Victorian Eye and Ear Hospital Research Fund, Eye Research Australia Foundation, Scholarship for Postgraduate Studies (Faculty of Medicine and University of Melbourne), and Contact Lens Society of Australia Conflict of interest: reported "The authors have no financial interest in the materials presented herein." Trial registration: ACTRN12613000143729 Paper was published in 2011. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363630 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Enrolled eyes were randomized separately to either the treatment or control groups using a computer generated randomization plan with blocks of 10. If both eyes of 1 patient qualified for participation in the study, each eye was randomized independently" Page 813, Wittig‐Silva 2014 |
| Allocation concealment (selection bias) | Low risk | "The randomisation plan was maintained in a secure location by a staff member in another hospital department not involved with the recruitment or conduct of the study" Page 813, Wittig‐Silva 2014 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was described as open label (masking not used) on clinical trials registry entry. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was described as open label (masking not used) on clinical trials registry entry. "All images were acquired and analyzed in an unmasked manner". Page 813, Wittig‐Silva 2014 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential follow‐up: At one year 46 (92%) treated and 41 (82%) control eyes followed up to 12 months. Using LOCF, data for 46 treated and 48 control eyes were reported. Over 3 years, 21 eyes in the control group left the trial, of which 12 were treated with CXL, 5 had corneal transplants, and 4 withdrew for personal reasons. In the treatment group, five people (eyes) withdrew for personal reasons. This unequal loss to follow‐up meant that the control group was followed up, on average, for less time than the treatment group. |
| Selective reporting (reporting bias) | High risk | Different cut‐points used at different time periods: 1.5D or more reported at one year and 2D or more reported at 36 months. |
BSCVA: best spectacle‐corrected visual acuity CXL: Collagen cross‐linkage D: dioptre Kmax: maximal keratometry LOCF: last observation carried forward logMAR: logarithm of the minimum angle of resolution MRSE: manifest refraction spherical equivalent RCT: randomised controlled trial UVA: ultraviolet A
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alessio 2013 | Not an RCT |
| Caporossi 2010 | Not an RCT |
| Caporossi 2013 | Not an RCT |
| Coskunseven 2009 | Not an RCT |
| Doors 2009 | Not an RCT |
| Gkika 2012 | Not an RCT |
| Goldich 2010 | Not an RCT |
| Greenstein 2013 | Not an RCT |
| Grewal 2009 | Not an RCT |
| Hallahan 2014 | Not an RCT |
| Henriquez 2011 | Not an RCT |
| Holopainen 2011 | Not an RCT |
| ISRCTN04451470 | RCT comparing epithelium on or off |
| ISRCTN29378493 | RCT comparing use of contact lenses before surgery or not |
| Jordan 2014 | Not an RCT |
| Kilic 2012 | Not an RCT |
| Koller 2009 | Not an RCT |
| Kránitz 2014 | Not an RCT |
| Mastropasqua 2013 | RCT comparing two different cross‐linking methods |
| Mazzotta 2007 | Not an RCT |
| NCT01143389 | RCT comparing two different riboflavin regimens |
| NCT01152541 | RCT comparing two different riboflavin regimens |
| NCT01181219 | RCT comparing epithelium on or off |
| NCT01325298 | RCT comparing two different UVA dosing regimens |
| NCT01344187 | RCT comparing riboflavin versus placebo |
| NCT01459679 | RCT comparing three different treatment regimens |
| NCT01464268 | RCT comparing two different riboflavin regimens |
| NCT01643226 | RCT comparing riboflavin versus placebo |
| NCT01708538 | RCT comparing epithelium on or off |
| NCT01868620 | RCT comparing iontophoretic CXL with standard CXL |
| NCT01972854 | RCT comparing riboflavin versus placebo |
| NCT02009709 | RCT comparing two different irradiance levels |
| Poli 2013 | Not an RCT |
| Razmjoo 2014 | RCT comparing total and partial removal of the epithelium |
| Rechichi 2013 | Not an RCT |
| Salman 2013 | Not an RCT |
| Viswanathan 2013 | Not an RCT |
| Wisse 2014 | Not an RCT |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00626717.
| Methods | RCT |
| Participants | 30 |
| Interventions | CXL with riboflavin and UVA light versus sham treatment |
| Outcomes | Keratoconus progression and endothelial cell loss |
| Notes | Primary completion date: December 2012 Principal investigator confirmed publication under review March 2015 |
NCT00841386.
| Methods | RCT |
| Participants | Target:150 |
| Interventions | CXL with riboflavin and UVA irradiation versus sham treatment |
| Outcomes |
|
| Notes | Primary completion date: December 2011 Email sent to principal investigator October 2014 and March 2015 with no response. |
Serapicos 2009.
| Methods | RCT |
| Participants | Target: 15 |
| Interventions | CXL with riboflavin and UVA light versus control |
| Outcomes | Stop progression of keratoconus in cornea imaging exams |
| Notes | Primary completion date: August 2009 Abstract reporting first two cases published 2009 Email sent to principal investigator October 2014 and February 2015 with no response. |
CXL: Collagen cross‐linkage RCT: randomised controlled trial UVA: ultraviolet A
Characteristics of ongoing studies [author‐defined order]
NCT01604135.
| Trial name or title | Collagen crosslinking for keratoconus‐‐a randomized controlled clinical trial (CXL‐RCT) |
| Methods | RCT |
| Participants | Target: 200 |
| Interventions | CXL with riboflavin and UVX |
| Outcomes | Kmax (non‐progression) |
| Starting date | May 2012 |
| Contact information | Madeleine Zetterberg, 00463431000, madeleine.zetterberg@anatcell.gu.se |
| Notes | NCT01604135 |
BSCVA: best spectacle‐corrected visual acuity CXL: Collagen cross‐linkage Kmax: maximal keratometry RCT: randomised controlled trial UVA: ultraviolet A
Differences between protocol and review
The protocol for this review was not explicit about how modifications of the CXL technique were to be handled. We decided to include only trials of treatment versus control in this review. There are a large number of ongoing studies that compare modifications of the technique and they will be included in future separate Cochrane reviews.
In this review's original protocol we defined mainly dichotomous secondary outcome measures using various cutpoints. However, these cutpoints were not reported. Trials in this field generally report these outcomes as continuous measures. Given the sparcity of the data, we felt that we would lose potentially relevant information, so we amended the secondary outcome measures to allow collection of continuous data.
The protocol did not specify the methods to be used when compiling the summary of findings table. We have prepared a summary of findiings table but note the outcomes for presenting were selected at the same time as the review was being prepared.
Contributions of authors
Conceiving the review: Cochrane Eyes and Vision Group
Protocol: Designing the review protocol: SH, PJM, SP, KAA Providing general advice on the protocol: PJM Writing the protocol: SH, SP, KAA, CB
Review: Co‐ordinating the review: ES, SH Statistical advice: CB Screening included and excluded studies: ES, RK Screening ongoing studies: ES, KAA, SP Downloading of full text articles: RK, ES Assessment of risk of bias: RK, ES, JE Data extraction: ES, RK, JE Analysis: RK, ES, JE Critical review of the manuscript: PJM Writing up the manuscript: ES, JE, RK, SH
Sources of support
Internal sources
-
National Institute for Health Research (NIHR), UK.
Catey Bunce acknowledges financial support from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
External sources
-
Royal College of Surgeons of Edinburgh, UK.
Research funding has been applied for.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
ES: None known RK: None known JE: None known CB is a co‐applicant of an National Institute for Health Research (NIHR) Efficacy and Mechanism Evaluation (EME) application to assess the efficacy and safety of cross‐linking in children with keratoconus. KAA: None known SP: None known PJM: None known SH: None known
New
References
References to studies included in this review
Hersh 2011 {published data only}
- Brooks N, Greenstein S, Fry K, Hersh P. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. Journal of Cataract and Refractive Surgery 2012;33(4):615‐9. [DOI] [PubMed] [Google Scholar]
- Greenstein S, Fry K, Hersh P. Effect of topographic cone location on outcomes of corneal collagen cross‐linking for keratoconus and corneal ectasia. Journal of Refractive Surgery 2012;28(6):397‐405. [DOI] [PubMed] [Google Scholar]
- Greenstein S, Fry K, Hersh P. In vivo biomechanical changes after corneal collagen cross‐linking for keratoconus and corneal ectasia: 1‐year analysis of a randomized, controlled, clinical trial. Cornea 2012;31(1):21‐5. [DOI] [PubMed] [Google Scholar]
- Greenstein S, Shah V, Fry K, Hersh P. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one‐year results. Journal of Cataract and Refractive Surgery 2011;37(4):691–700. [DOI] [PubMed] [Google Scholar]
- Hersh P, Greenstein S, Fry K. Corneal collagen crosslinking for keratoconus and corneal ectasia: One‐year results. Journal of Cataract and Refractive Surgery 2011;37(1):149‐60. [DOI] [PubMed] [Google Scholar]
O'Brart 2011 {published data only}
- O’Brart D, Chan E, Samaras K, Patel P, Shah S. A randomised, prospective study to investigate the efficacy of riboflavin/ultraviolet A (370 nm) corneal collagen cross‐linkage to halt the progression of keratoconus. British Journal of Ophthalmology 2011;95(11):1519‐24. [DOI] [PubMed] [Google Scholar]
Wittig‐Silva 2008 {published data only}
- Wittig‐Silva C, Chan E, Islam FM, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross‐linking in progressive keratoconus: three‐year results. Ophthalmology 2014;121(4):812‐21. [DOI] [PubMed] [Google Scholar]
- Wittig‐Silva C, Whiting M, Lamoureux E, Lindsay R, Sullivan L, Snibson GR. A randomized controlled trial of corneal collagen cross‐linking in progressive keratoconus: preliminary results. Journal of Refractive Surgery 2008;24(7):S720‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alessio 2013 {published data only}
- Alessio G, L'abbate M, Sborgia C, Tegola MG. Photorefractive keratectomy followed by cross‐linking versus cross‐linking alone for management of progressive keratoconus: two‐year follow‐up. American Journal of Ophthalmology 2013;155(1):54‐65. [DOI] [PubMed] [Google Scholar]
Caporossi 2010 {published data only}
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long‐term results of riboflavin ultraviolet a corneal collagen cross‐linking for keratoconus in Italy: the Siena eye cross study. American Journal of Ophthalmology 2010;149(4):585‐93. [DOI] [PubMed] [Google Scholar]
Caporossi 2013 {published data only}
Coskunseven 2009 {published data only}
Doors 2009 {published data only}
Gkika 2012 {published data only}
- Gkika M, Labiris G, Giarmoukakis A, Koutsogianni A, Kozobolis V. Evaluation of corneal hysteresis and corneal resistance factor after corneal cross‐linking for keratoconus. Graefe's Archive for Clinical and Experimental Ophthalmology 2012;250(4):565‐73. [DOI] [PubMed] [Google Scholar]
Goldich 2010 {published data only}
- Goldich Y, Marcovich AL, Barkana Y, Avni I, Zadok D. Safety of corneal collagen cross‐linking with UV‐A and riboflavin in progressive keratoconus. Cornea 2010;29(4):409‐11. [DOI] [PubMed] [Google Scholar]
Greenstein 2013 {published data only}
Grewal 2009 {published data only}
- Grewal DS, Brar GS, Jain R, Sood V, Singla M, Grewal SP. Corneal collagen crosslinking using riboflavin and ultraviolet‐A light for keratoconus: one‐year analysis using Scheimpflug imaging. Journal of Cataract and Refractive Surgery 2009;35(3):425‐32. [DOI] [PubMed] [Google Scholar]
Hallahan 2014 {unpublished data only}
- Hallahan KM, Rocha K, Roy AS, Randleman JB, Stulting RD, Dupps WJ Jr. Effects of corneal cross‐linking on ocular response analyzer waveform‐derived variables in keratoconus and postrefractive surgery ectasia. Eye and Contact Lens 2014;40(6):339‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00567671. Safety and effectiveness of the UV‐X system for corneal collagen cross‐linking in eyes with corneal ectasia or progressive keratoconus. clinicaltrials.gov/show/NCT00567671 (accessed 22 September 2014).
Henriquez 2011 {published data only}
- Henriquez M, Izquierdo L, Bernilla C, Zakrzewski P, Mannis M. Riboflavin/ultraviolet a corneal collagen cross‐linking for the treatment of keratoconus: Visual outcomes and scheimpflug analysis. Cornea 2011;30(3):281‐6. [DOI] [PubMed] [Google Scholar]
Holopainen 2011 {published data only}
- Holopainen JM, Krootila K. Transient corneal thinning in eyes undergoing corneal cross‐linking. American Journal of Ophthalmology 2011;152(4):533‐6. [DOI] [PubMed] [Google Scholar]
ISRCTN04451470 {unpublished data only}
- ISRCTN04451470. Trans‐epithelial corneal cross‐linking to halt the progression of keratoconus. www.controlled‐trials.com/ISRCTN04451470/ (accessed 23 March 2014).
ISRCTN29378493 {unpublished data only}
- ISRCTN29378493. Corneal collagen cross‐linking with riboflavin (C3R) with orthokeratology. www.controlled‐trials.com/ISRCTN29378493 (accessed 23 March 2014).
Jordan 2014 {published data only}
- Jordan C, Patel DV, Abeysekera N, McGhee CN. In vivo confocal microscopy analyses of corneal microstructural changes in a prospective study of collagen cross‐linking in keratoconus. Ophthalmology 2014;121(2):469‐74. [DOI] [PubMed] [Google Scholar]
Kilic 2012 {published data only}
- Kilic A, Kamburoglu G, Akinci A. Riboflavin injection into the corneal channel for combined collagen crosslinking and intrastromal corneal ring segment implantation. Journal of Cataract and Refractive Surgery 2012;38(5):878‐83. [DOI] [PubMed] [Google Scholar]
Koller 2009 {published data only}
- Koller T, Iseli HP, Hafezi F, Vinciguerra P, Seiler T. Scheimpflug imaging of corneas after collagen cross‐linking. Cornea 2009;28(5):510‐5. [DOI] [PubMed] [Google Scholar]
Kránitz 2014 {published data only}
- Kránitz K, Kovacs I, Mihaltz K, Sandor GL, Juhasz E, Gyenes A, et al. Changes of corneal topography indices after CXL in progressive keratoconus assessed by Scheimpflug camera. Journal of Refractive Surgery 2014;30(6):374‐8. [DOI] [PubMed] [Google Scholar]
Mastropasqua 2013 {published data only}
- Mastropasqua L, Nubile M, Lanzini M, Calienno R, Mastropasqua R, Agnifili L, et al. Morphological modification of the cornea after standard and transepithelial corneal cross‐linking as imaged by anterior segment optical coherence tomography and laser scanning in vivo confocal microscopy. Cornea 2013;32(6):855–61. [DOI] [PubMed] [Google Scholar]
Mazzotta 2007 {published data only}
- Mazzotta C, Balestrazzi A, Traversi C, Baiocchi S, Caporossi T, Tommasi C, et al. Treatment of progressive keratoconus by riboflavin‐UVA‐induced cross‐linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea 2007;26(4):390‐7. [DOI] [PubMed] [Google Scholar]
NCT01143389 {unpublished data only}
- NCT01143389. Evaluation of two riboflavin dosing regimens for corneal collagen cross‐linking in eyes with progressive keratoconus or ectasia. clinicaltrials.gov/show/NCT01143389 (accessed 23 March 2014).
NCT01152541 {unpublished data only}
- NCT01152541. Corneal collagen crosslinking for progressive keratoconus and ectasia using riboflavin/dextran and hypotonic riboflavin. clinicaltrials.gov/show/NCT01152541 (accessed 23 March 2014).
NCT01181219 {unpublished data only}
- NCT01181219. Transepithelial corneal collagen cross‐linking (CXL) in treatment of keratoconus [Behandling av keratoconus med "cornea collagen cross‐linking" uten hornhinneepitelfjerning]. clinicaltrials.gov/show/NCT01181219 (accessed 23 March 2014).
NCT01325298 {published and unpublished data}
- NCT01325298. UVA‐riboflavin crosslinking treatment of corneal ectasia. clinicaltrials.gov/show/NCT01325298 (accessed 23 March 2014).
NCT01344187 {unpublished data only}
- NCT01344187. Safety and efficacy study of corneal collagen cross‐linking in eyes with keratoconus. clinicaltrials.gov/ct2/show/NCT01344187 (accessed 23 March 2014).
NCT01459679 {unpublished data only}
- NCT01459679. A multi‐center, randomized, controlled evaluation of the safety and efficacy of the KXL system with vibeX (riboflavin ophthalmic solution) for corneal collagen cross‐linking in eyes with keratoconus or corneal ectasia after refractive surgery. clinicaltrials.gov/show/NCT01459679 (accessed 23 March 2014).
NCT01464268 {unpublished data only}
- NCT01464268. Transepithelial corneal collagen crosslinking for keratoconus and corneal ectasia. clinicaltrials.gov/show/NCT01464268 (accessed 23 March 2014).
NCT01643226 {unpublished data only}
- NCT01643226. A multi‐center, randomized, placebo‐controlled evaluation of the safety and efficacy of the KXL system with vibeX (riboflavin ophthalmic solution) for corneal collagen cross‐linking in eyes with keratoconus. clinicaltrials.gov/show/NCT01643226 (accessed 23 March 2014).
NCT01708538 {unpublished data only}
- NCT01708538. Phase III study of corneal collagen cross‐linking using two different techniques. clinicaltrials.gov/show/NCT01708538 (accessed 23 March 2014).
NCT01868620 {unpublished data only}
- NCT01868620. Non‐inferiority trial of iontophoretic corneal collagen crosslinking (CXL) compared to standard corneal collagen crosslinking in progressive keratoconus. clinicaltrials.gov/show/NCT01868620 (accessed 23 March 2014).
NCT01972854 {published data only}
- NCT01972854. A multi‐center, randomized, placebo‐controlled evaluation of the safety and efficacy of the KXL system with vibeX (riboflavin ophthalmic solution) for corneal collagen cross‐linking in eyes with keratoconus. clinicaltrials.gov/show/NCT01972854 (accessed 23 March 2014).
NCT02009709 {unpublished data only}
- NCT02009709. Study of the safety and effectiveness of photochemically induced collagen cross‐linking at an irradiance of 9 mW/cm2 and 18 mW/cm2 in eyes with keratoconus or ectasia. clinicaltrials.gov/show/NCT02009709 (accessed 23 March 2014).
Poli 2013 {published data only}
- Poli M, Cornut PL, Balmitgere T, Aptel F, Janin H, Burillon C. Prospective study of corneal collagen cross‐linking efficacy and tolerance in the treatment of keratoconus and corneal ectasia: 3‐year results. Cornea 2013;32(5):583‐90. [DOI] [PubMed] [Google Scholar]
Razmjoo 2014 {published data only}
- NCT01809977. Study of surgical methods for keratoconus. clinicaltrials.gov/show/NCT01809977 (accessed 22 September 2014).
- Razmjoo H, Rahimi B, Kharraji M, Koosha N, Peyman A. Corneal haze and visual outcome after collagen crosslinking for keratoconus: A comparison between total epithelium off and partial epithelial removal methods. Advanced Biomedical Research 2014;3:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rechichi 2013 {published data only}
Salman 2013 {published data only}
- Salman AG. Transepithelial corneal collagen crosslinking for progressive keratoconus in a pediatric age group. Journal of Cataract and Refractive Surgery 2013;39(8):1164‐70. [DOI] [PubMed] [Google Scholar]
Viswanathan 2013 {published data only}
- Viswanathan D, Males J. Prospective longitudinal study of corneal collagen cross‐linking in progressive keratoconus. Clinical and Experimental Ophthalmology 2013;41(6):531–6. [DOI] [PubMed] [Google Scholar]
Wisse 2014 {published data only}
- Wisse RP, Godefrooij DA, Soeters N, Imhof SM, Lelij A. A multivariate analysis and statistical model for predicting visual acuity and keratometry one year after cross‐linking for keratoconus. American Journal of Ophthalmology 2014;157(3):519‐25. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00626717 {unpublished data only}
- NCT00626717. Riboflavin mediated corneal crosslinking for stabilizing progression of keratoconus. clinicaltrials.gov/show/NCT00626717 (accessed 22 September 2014).
NCT00841386 {unpublished data only}
- NCT00841386. Treatment of keratoconus using collagen cross‐linking by means of topical riboflavin and UV light. clinicaltrials.gov/show/NCT00841386 (accessed 23 March 2014).
Serapicos 2009 {unpublished data only}
- NCT00815256. The safety and effectiveness of collagen cross linking with riboflavin and ultraviolet‐A light in progressive mild and moderate grades of keratoconus. clinicaltrials.gov/show/NCT00815256 (accessed 22 September 2014).
- Serapicos PC, Bottos KM, Kurauchi MP, Sakai V, Schor P, Chamon W, et al. The safety and effectiveness of the collagen cross linking with riboflavin and ultraviolet‐A light in progressive mild and moderate grades of keratoconus. Investigative Ophthalmology and Visual Science 2009; Vol. 50:ARVO E‐Abstract 5494.
References to ongoing studies
NCT01604135 {unpublished data only}
- NCT01604135. Collagen crosslinking for keratoconus ‐ a randomized controlled clinical trial. clinicaltrials.gov/show/NCT01604135 (accessed 23 March 2014).
Additional references
Akhtar 2008
- Akhtar S, Bron AJ, Salvi SM, Hawkesworth NR, Tuft SJ, Meek KM. Ultrastructural analysis of collagen fibrils and proteoglycans in keratoconus. Acta Ophthalmologica 2008;86(7):764‐72. [DOI] [PubMed] [Google Scholar]
Ambrósio 2003
- Ambrósio R Jr, Klyce SD, Wilson SE. Corneal topographic and pachymetric screening of keratorefractive patients. Journal of Refractive Surgery 2003;19(1):24–9. [DOI] [PubMed] [Google Scholar]
Arboleda 2014
- Arboleda A, Kowalczuk L, Savoldelli M, Klein C, Ladraa S, Naud MC, et al. Evaluating in vivo delivery of riboflavin with coulomb‐controlled iontophoresis for corneal collagen cross‐linking: a pilot study. Investigative Ophthalmology and Vision Sciences 2014;55(4):2731‐8. [DOI] [PubMed] [Google Scholar]
Ashwin 2009
- Ashwin PT, McDonnell PJ. Collagen cross‐linkage: a comprehensive review and directions for future research. British Journal of Ophthalmology 2010;94(8):965‐70. [DOI] [PubMed] [Google Scholar]
Baiocchi 2009
- Baiocchi S, Mazzotta C, Cerretani D, Caporossi T, Caporossi A. Corneal crosslinking: riboflavin concentration in corneal stroma exposed with and without epithelium. Journal of Cataract and Refractive Surgery 2009;35(5):893‐9. [DOI] [PubMed] [Google Scholar]
Belin 2007
- Belin MW, Khachikian SS. Corneal diagnosis and evaluation with the OCULUS Pentacam. Highlights of Ophthalmology 2007;35:5‐8. [Google Scholar]
Boxer Wachler 2003
- Boxer Wachler BS, Christie JP, Chandra NS, Chou B, Korn T, Nepomuceno R. Intacs for keratoconus. Ophthalmology 2003;110(5):1031‐40. [DOI] [PubMed] [Google Scholar]
Carlsson 2003
- Carlsson DJ, Li F, Shimmura S, Griffith M. Bioengineered corneas: how close are we?. Current Opinion in Ophthalmology 2003;14(4):192‐7. [DOI] [PubMed] [Google Scholar]
Chan 2007
- Chan CC, Sharma M, Boxer BS. Effect of inferior segment Intacs with and without C3R on keratoconus. Journal of Cataract and Refractive Surgery 2007;33(1):75‐80. [DOI] [PubMed] [Google Scholar]
Chunyu 2014
- Chunyu T, Xiujun P, Zhengjun F, Xia Z, Feihu Z. Corneal collagen cross‐linking in keratoconus: A systematic review and meta‐analysis. Scientific Reports 2014;4:Article number 5652. [DOI: 10.1038/srep05652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Craig 2014
- Craig JA, Mahon J, Yellowlees A, Barata T, Glanville J, Arber M, et al. Epithelium‐off photochemical corneal collagen cross‐linkage using riboflavin and ultraviolet a for keratoconus and keratectasia: a systematic review and meta‐analysis. Ocular Surface 2014;12(3):202‐14. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Fontes 2010
- Fontes BM, Ambrósio R Jr, Salomao M, Velarde GC, Nosé W. Biomechanical and tomographic analysis of unilateral keratoconus. Journal of Refractive Surgery 2010;26(9):677–81. [DOI] [PubMed] [Google Scholar]
Georgiou 2004
- Georgiou T, Funnell CL, Cassels‐Brown A, O'Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye 2004;18(4):379‐83. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gore 2013
- Gore D, Shortt A, Allan B. New clinical pathways for keratoconus. Eye 2013;27(3):329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hafezi 2009
- Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet‐A and hypoosmolar riboflavin solution in thin corneas. Journal of Cataract and Refractive Surgery 2009;35(4):621‐4. [DOI] [PubMed] [Google Scholar]
Hayes 2008
- Hayes S, O'Brart DP, Lamdin LS, Doutch J, Samaras K, Marshall J, et al. Effect of complete epithelial debridement before riboflavin‐ultraviolet‐A corneal collagen crosslinking therapy. Journal of Cataract and Refractive Surgery 2008;34(4):657‐61. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons Ltd.
Hovakimyan 2012
- Hovakimyan M, Guthoff R, Stachs O. Collagen cross‐linking: current status and future directions. Journal of Ophthalmology 2012;2012:Article ID 406850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koppen 2011
- Koppen C, Wouters K, Mathysen D, Rozema S, Tassignon M. Refractive and topographic results of benzalkonium chloride–assisted transepithelial crosslinking. Journal of Cataract and Refractive Surgery 2011;38(6):1000‐5. [DOI] [PubMed] [Google Scholar]
Krachmer 1984
- Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Survey of Ophthalmology 1984;28(4):293‐322. [DOI] [PubMed] [Google Scholar]
Kymionis 2012
- Kymionis GD, Portaliou DM, Diakonis VF, Kounis GA, Panagopoulou SI, Grentzelos MA. Corneal collagen cross‐linking with riboflavin and ultraviolet‐A irradiation in patients with thin corneas. American Journal of Ophthalmology 2012;153(1):24‐8. [DOI] [PubMed] [Google Scholar]
Leccisotti 2006
- Leccisotti A. Bioptics: where do things stand?. Current Opinion in Ophthalmology 2006;17(4):399‐405. [DOI] [PubMed] [Google Scholar]
Li 2009
- Li X, Yang H, Rabinowitz YZ. Keratoconus: classification scheme based on videokeratography and clinical signs. Journal of Cataract and Refractive Surgery 2009;35(9):1597‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2012
- Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier‐domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology 2012;119(12):2425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mastropasqua 2014
- Mastropasqua L, Lanzini M, Curcio C, Calienno R, Mastropasqua R, Colasante M, et al. Structural modifications and tissue response after standard epi‐off and iontophoretic corneal crosslinking with different irradiation procedures. Investigative Ophthalmology and Vision Science 2014;55(4):2526‐33. [DOI] [PubMed] [Google Scholar]
McMahon 2006
- McMahon TT, Szczotka‐Flynn L, Barr JT, Anderson RJ, Slaughter ME, Lass JH, et al. A new method for grading the severity of keratoconus: the Keratoconus Severity Score (KSS). Cornea 2006;25(7):794‐800. [DOI] [PubMed] [Google Scholar]
Meek 2005
- Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, et al. Changes in collagen orientation and distribution in keratoconus corneas. Investigative Ophthalmology and Visual Science 2005;46(6):1948‐56. [DOI] [PubMed] [Google Scholar]
Miranda 2003
- Miranda D, Sartori M, Francesconi C, Allemann N, Ferrara P, Campos M. Ferrara intrastromal corneal ring segments for severe keratoconus. Journal of Refractive Surgery 2003;19(6):645‐53. [DOI] [PubMed] [Google Scholar]
Moodaley 1992
- Moodaley LC, Woodward EG, Liu CS, Buckley RJ. Life expectancy in keratoconus. British Journal of Ophthalmology 1992;76(10):590‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naoumidi 2005
- Naoumidi TL, Pallikaris IG, Naoumidi II, Astyrakakis NI. Conductive keratoplasty: histological study of human corneas. American Journal of Ophthalmology 2005;140(6):984‐92. [DOI] [PubMed] [Google Scholar]
NICE 2013
- NICE International Procedure Advisory Committee. Photochemical corneal collagen cross‐linkage using riboflavin and ultraviolet A for keratoconus: a systematic review. www.nice.org.uk/guidance/ipg466/resources/photochemical‐corneal‐collagen‐crosslinkage‐using‐riboflavin‐and‐ultraviolet‐a‐for‐keratoconus‐systematic‐review2 (accessed 30 August 2014).
Pinelli 2007
- Pinelli R. Corneal collagen cross‐linking with riboflavin (C3‐R) treatment opens new frontiers for keratoconus and corneal ectasia. www.eyeworld.org/article.php?sid=3797 (accessed 22 May 2011).
Rabinowitz 1998
- Rabinowitz YS. Keratoconus. Survey of Ophthalmology 1998;42(4):297‐319. [DOI] [PubMed] [Google Scholar]
Rabinowitz 2007
- Rabinowitz YS. Intacs for keratoconus. Current Opinion in Ophthalmology 2007;18(4):279‐83. [DOI] [PubMed] [Google Scholar]
Raiskup‐Wolf 2008
- Raiskup‐Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet‐A light in keratoconus: long‐term results. Journal of Cataract and Refractive Surgery 2008;34(3):796‐801. [DOI] [PubMed] [Google Scholar]
Reinstein 2009
- Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. Journal of Refractive Surgery 2009;25(7):604–10. [DOI] [PubMed] [Google Scholar]
Reinstein 2010
- Reinstein DZ, Gobbe M, Archer TJ, Silverman RH, Coleman DJ. Epithelial, stromal, and total corneal thickness in keratoconus: three‐dimensional display with Artemis very‐high frequency digital ultrasound. Journal of Refractive Surgery 2010;26(4):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2011
- Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross‐linking, using riboflavin and ultraviolet radiation. Investigative Ophthalmology and Visual Science 2011;52(12):9048‐52. [DOI] [PubMed] [Google Scholar]
Shimmura 2006
- Shimmura S, Tsubota K. Deep anterior lamellar keratoplasty. Current Opinion in Ophthalmology 2006;17(4):349‐55. [DOI] [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters for randomised controlled trials. www.sign.ac.uk/methodology/filters.html#random (accessed 4 March 2011).
Smolek 1997
- Smolek MK, Klyce SD. Current keratoconus detection methods compared with a neural network approach. Investigative Ophthalmology and Visual Science 1997;38(11):2290–9. [PubMed] [Google Scholar]
Spoerl 1998
- Spoerl E, Huhle M, Seiler T. Induction of cross‐links in corneal tissue. Experimental Eye Research 1998;66(1):97‐103. [DOI] [PubMed] [Google Scholar]
Szalai 2012
- Szalai E, Berta A, Hassan Z, Módis L Jr. Reliability and repeatability of swept‐source Fourier‐domain optical coherence tomography and Scheimpflug imaging in keratoconus. Journal of Cataract and Refractive Surgery 2012;38(3):485–94. [DOI] [PubMed] [Google Scholar]
Vicente 2010
- Vicente LL, Boxer Wachler BS. Factors that correlate with improvement in vision after combined Intacs and trans‐epithelial corneal crosslinking. British Journal of Ophthalmology 2010;94(12):1597‐601. [DOI] [PubMed] [Google Scholar]
Vinciguerra 2009
- Vinciguerra P, Albe E, Trazza S, Seiler T, Epstein D. Intraoperative and postoperative effects of corneal collagen cross‐linking on progressive keratoconus. Archives of Ophthalmology 2009;127(10):1258–65. [DOI] [PubMed] [Google Scholar]
Vinciguerra 2012
- Vinciguerra P, Albé E, Frueh BE, Trazza S. Two‐year corneal cross‐linking results in patients younger than 18 years with documented progressive keratoconus. American Journal of Ophthalmology 2012;154(3):520‐6. [DOI] [PubMed] [Google Scholar]
Wagner 2007
- Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Contact Lens and Anterior Eye 2007;30(4):223‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wollensak 2003
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet‐a‐induced collagen crosslinking for the treatment of keratoconus. American Journal of Ophthalmology 2003;135(5):620‐7. [DOI] [PubMed] [Google Scholar]
Wollensak 2004
- Wollensak G, Wilsch M, Spoerl E, Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea 2004;23(5):503‐7. [DOI] [PubMed] [Google Scholar]
Wollensak 2006
- Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Current Opinion in Ophthalmology 2006;17(4):356‐60. [DOI] [PubMed] [Google Scholar]
Zadnik 1996
- Zadnik K, Barr J, Gordon M, Edrington T. Biomicroscopic signs and disease severity in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Cornea 1996;15(2):139–46. [DOI] [PubMed] [Google Scholar]
Zadnik 1998
- Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, et al. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Investigative Ophthalmology and Visual Science 1998;39(13):2537‐46. [PubMed] [Google Scholar]
References to other published versions of this review
Hamada 2013
- Hamada S, Patwary S, Amissah‐Arthur KN, Bunce C, McDonnell PJ. Corneal collagen cross‐linking for treating keratoconus. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010621] [DOI] [PMC free article] [PubMed] [Google Scholar]


