Abstract
The ATP-dependent proton-pumping activity of soybean (Glycine max L.) root microsomes is predominantly nitrate sensitive and presumably derived from the tonoplast. We used microsomes to characterize anion effects on proton pumping of the tonoplast vesicles using two distinctly different techniques.
Preincubation of the vesicles with nitrate caused inhibition of proton pumping and ATPase activity, with similar concentration dependence. Fluoride, which preferentially inhibits the plasma membrane ATPase, inhibited ATPase activity strongly at concentrations which did not affect proton pumping activity.
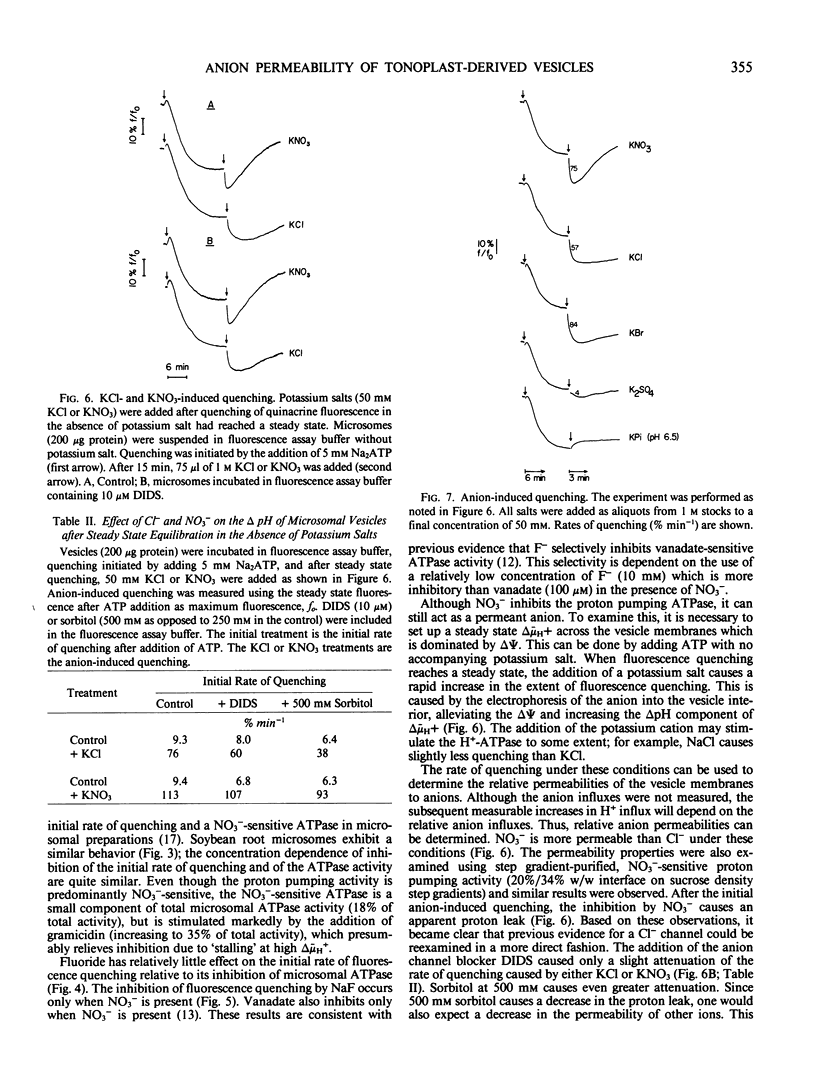
Addition of potassium salts, after a steady-state pH gradient is established in the absence of such salts, caused an increased pH gradient which was due to alleviation of Δ Ψ and subsequent increased influx of H+ into these vesicles. This anion-induced increase in the pH gradient could be used as a measure of the relative anion permeabilities, which were of the order Br− = NO3− > Cl− ≫ SO42−. Phosphate and fluoride caused no increase in the pH gradient. Since the concentration dependence of KCl- and KNO3-induced quenching exhibited a saturable component, and since H+ uptake was increased by only certain anions, the data suggest that there may be a relatively specific anion channel associated with tonoplast-derived vesicles.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Prince R. C., Crofts A. R. The response of fluorescent amines to pH gradients across liposome membranes. Biochim Biophys Acta. 1972 Aug 9;274(2):323–335. doi: 10.1016/0005-2736(72)90180-0. [DOI] [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Yoder O. C., Filner P. Anaerobic nitrite production by plant cells and tissues: evidence for two nitrate pools. Plant Physiol. 1973 Mar;51(3):423–431. doi: 10.1104/pp.51.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Mettler I. J., Taiz L. Localization of the proton pump of corn coleoptile microsomal membranes by density gradient centrifugation. Plant Physiol. 1982 Dec;70(6):1743–1747. doi: 10.1104/pp.70.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Mandala S., Taiz L. Characterization of in vitro proton pumping by microsomal vesicles isolated from corn coleoptiles. Plant Physiol. 1982 Dec;70(6):1738–1742. doi: 10.1104/pp.70.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Bennett A. B., Spanswick R. M. Characterization of a NO(3)-Sensitive H-ATPase from Corn Roots. Plant Physiol. 1983 Jul;72(3):837–846. doi: 10.1104/pp.72.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Kasamo K., Brooker R. J., Slayman C. W. Electrogenic H+ translocation by the plasma membrane ATPase of Neurospora. Studies on plasma membrane vesicles and reconstituted enzyme. J Biol Chem. 1984 Jun 25;259(12):7884–7892. [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., de Michelis M. I., Pugliarello M. C. Evidence for an electrogenic ATPase in microsomal vesicles from pea internodes. Biochim Biophys Acta. 1981 Mar 20;642(1):37–45. doi: 10.1016/0005-2736(81)90135-8. [DOI] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Evidence for a Cl-Stimulated MgATPase Proton Pump in Oat Root Membranes. Plant Physiol. 1982 Apr;69(4):798–803. doi: 10.1104/pp.69.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewicz W. K. Effect of osmotic gradient on the physical properties of membrane lipids in liposomes. Chem Phys Lipids. 1983 Jul;33(1):81–85. doi: 10.1016/0009-3084(83)90010-5. [DOI] [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]


