Abstract
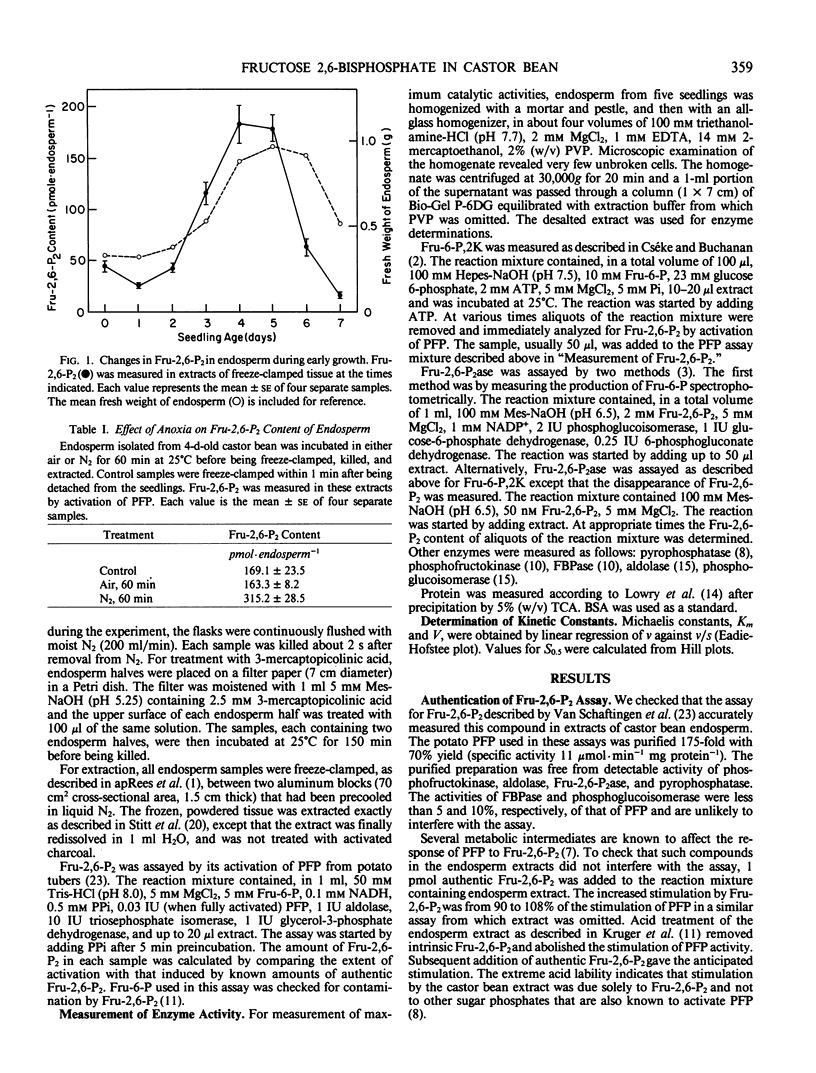
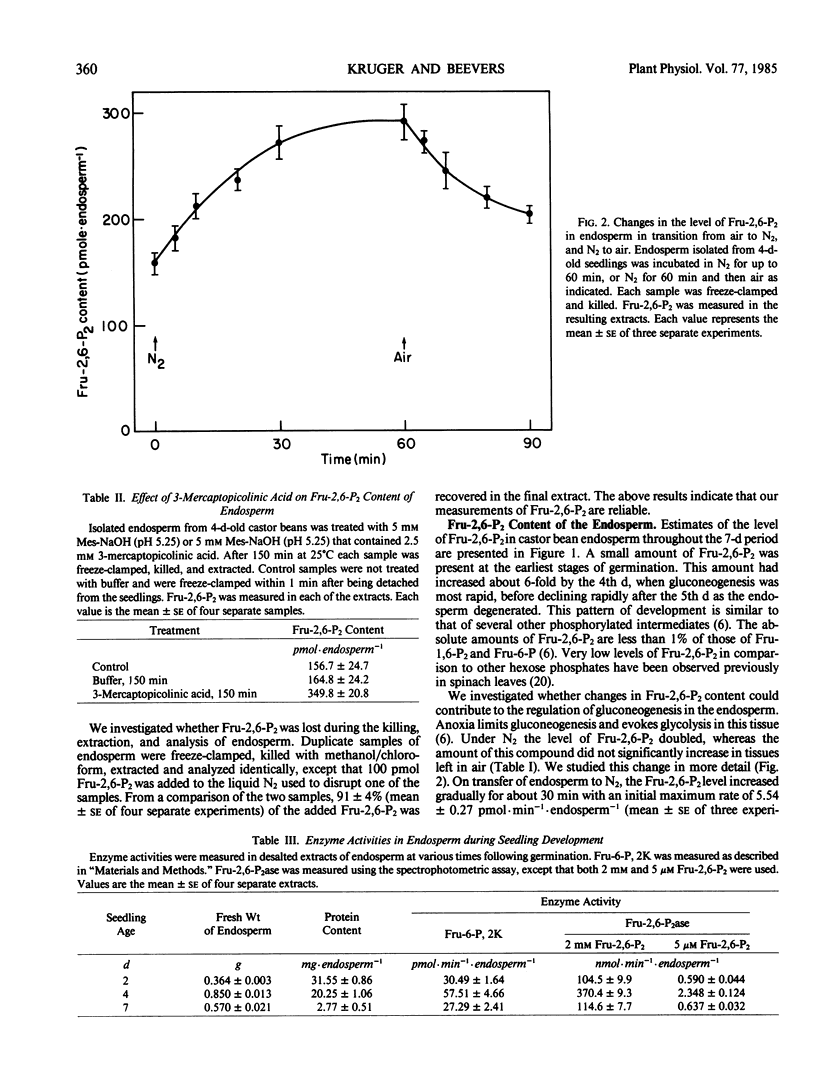
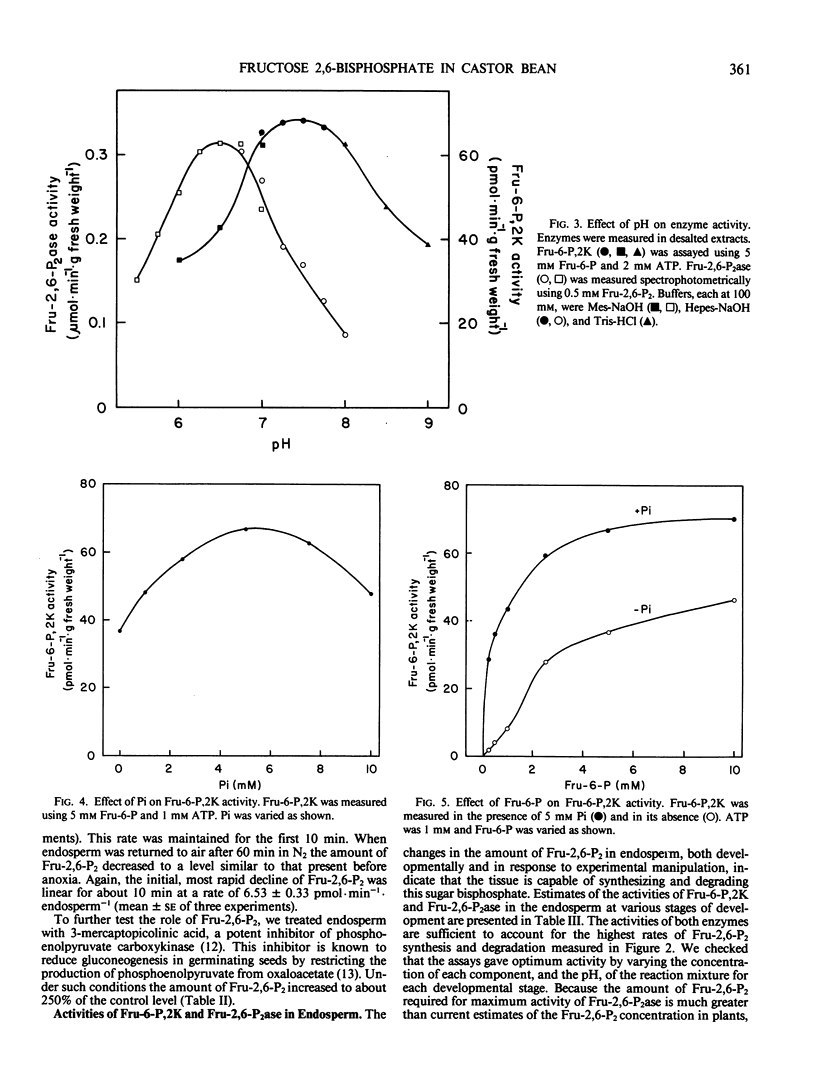
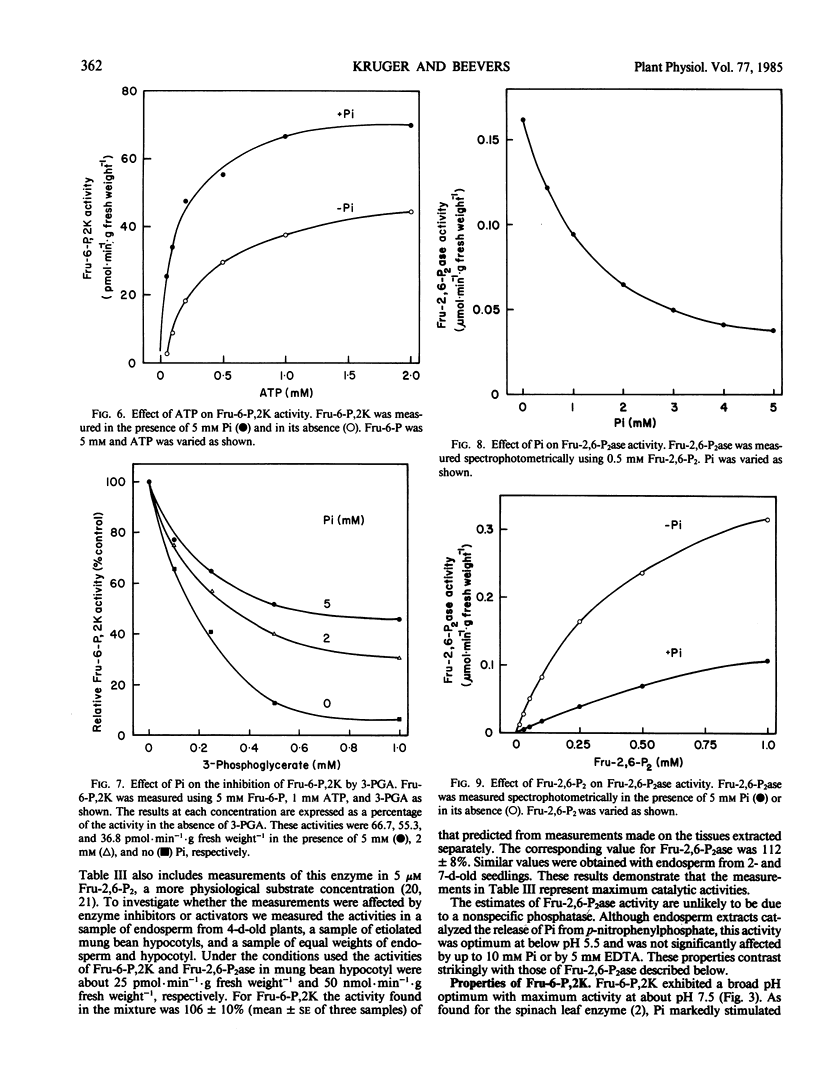
The aim of this work was to examine the possibility that fructose 2,6-bisphosphate (Fru-2,6-P2) plays a role in the regulation of gluconeogenesis from fat. Fru-2,6-P2 is known to inhibit cytoplasmic fructose 1,6-bisphosphatase and stimulate pyrophosphate:fructose 6-phosphate phosphotransferase from the endosperm of seedlings of castor bean (Ricinus communis). Fru-2,6-P2 was present throughout the seven-day period in amounts from 30 to 200 picomoles per endosperm. Inhibition of gluconeogenesis by anoxia or treatment with 3-mercaptopicolinic acid doubled the amount of Fru-2,6-P2 in detached endosperm. The maximum activities of fructose 6-phosphate,2-kinase and fructose 2,6-bisphosphatase (enzymes that synthesize and degrade Fru-2,6-P2, respectively) were sufficient to account for the highest observed rates of Fru-2,6-P2 metabolism. Fructose 6-phosphate,2-kinase exhibited sigmoid kinetics with respect to fructose 6-phosphate. These kinetics became hyperbolic in the presence of inorganic phosphate, which also relieved a strong inhibition of the enzyme by 3-phosphoglycerate. Fructose 2,6-bisphosphatase was inhibited by both phosphate and fructose 6-phosphate, the products of the reaction. The properties of the two enzymes suggest that in vivo the amounts of fructose-6-phosphate, 3-phosphoglycerate, and phosphate could each contribute to the control of Fru-2,6-P2 level. Variation in the level of Fru-2,6-P2 in response to changes in the levels of these metabolites is considered to be important in regulating flux between fructose 1,6-bisphosphate and fructose 6-phosphate during germination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Kobr M. J., Beevers H. Gluconeogenesis in the castor bean endosperm: I. Changes in glycolytic intermediates. Plant Physiol. 1971 Jan;47(1):48–52. doi: 10.1104/pp.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Kruger N. J., Beevers H. Kinetic properties of pyrophosphate:fructose-6-phosphate phosphotransferase from germinating castor bean endosperm. Plant Physiol. 1984 Feb;74(2):395–401. doi: 10.1104/pp.74.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger N. J., Beevers H. Effect of fructose 2,6-bisphosphate on the kinetic properties of cytoplasmic fructose 1,6-bisphosphatase from germinating castor bean endosperm. Plant Physiol. 1984 Sep;76(1):49–54. doi: 10.1104/pp.76.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger N. J., Kombrink E., Beevers H. Fructose 2,6-bisphosphate as a contaminant of commercially obtained fructose 6-phosphate: effect on PPi:fructose 6-phosphate phosphotransferase. Biochem Biophys Res Commun. 1983 Nov 30;117(1):37–42. doi: 10.1016/0006-291x(83)91537-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leegood R. C., ap Rees T. Identification of the regulatory steps in gluconeogenesis in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1978 Aug 3;542(1):1–11. doi: 10.1016/0304-4165(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Leegood R. C., ap Rees T. Phosphoenolpyruvate carboxykinase and gluconeogenesis in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1978 May 11;524(1):207–218. doi: 10.1016/0005-2744(78)90119-5. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Isoenzymes of sugar phosphate metabolism in endosperm of germinating castor beans. Plant Physiol. 1981 Jun;67(6):1255–1258. doi: 10.1104/pp.67.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Subcellular distribution of gluconeogenetic enzymes in germinating castor bean endosperm. Plant Physiol. 1979 Jul;64(1):31–37. doi: 10.1104/pp.64.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. D-Fructose 2,6-bisphosphate: a naturally occurring activator for inorganic pyrophosphate:D-fructose-6-phosphate 1-phosphotransferase in plants. Biochem Biophys Res Commun. 1981 Dec 15;103(3):848–855. doi: 10.1016/0006-291x(81)90888-3. [DOI] [PubMed] [Google Scholar]
- Stitt M., Cseke C., Buchanan B. B. Regulation of fructose 2,6-bisphosphate concentration in spinach leaves. Eur J Biochem. 1984 Aug 15;143(1):89–93. doi: 10.1111/j.1432-1033.1984.tb08345.x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Gerhardt R., Kürzel B., Heldt H. W. A role for fructose 2,6-bisphosphate in the regulation of sucrose synthesis in spinach leaves. Plant Physiol. 1983 Aug;72(4):1139–1141. doi: 10.1104/pp.72.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- ap Rees T., Fuller W. A., Wright B. W. Measurements of glycolytic intermediates during the onset of thermogenesis in the spadix of Arum maculatum. Biochim Biophys Acta. 1977 Aug 10;461(2):274–282. doi: 10.1016/0005-2728(77)90177-3. [DOI] [PubMed] [Google Scholar]


