Abstract
Although IGF-I has been identified as an important growth factor for the skeleton, the role of IGF-I on embryonic bone development remains unknown. Here we show that, in IGF-I-deficient (IGF-I−/−) mice, skeletal malformations, including short-limbed dwarfism, were evident at days post coitus (dpc) 14.5 to 18.5, accompanied by delays of mineralization in the spinal column, sternum, and fore paws. Reduced chondrocyte proliferation and increased chondrocyte apoptosis were identified in both the spinal ossification center and the growth plate of long bones. Abnormal chondrocyte differentiation and delayed initiation of mineralization was characterized by small size and fewer numbers of type X collagen expressing hypertrophic chondrocytes and lower osteocalcin expression. The Indian hedgehog-PTHrP feedback loop was altered; expression of Indian hedgehog was reduced in IGF-I−/− mice in long bones and in the spine, whereas expression of PTHrP was increased. Our results indicate that IGF-I plays an important role in skeletal development by promoting chondrocyte proliferation and maturation while inhibiting apoptosis to form bones of appropriate size and strength.
Development of the vertebrate skeleton is a highly regulated process. In mammals, the sequential phases of skeletogenesis include the migration of cells to the site of future skeletogenesis, the tissue interactions that result in condensation, and the overt differentiation of chondroblasts or osteoblasts (1, 2). Patterning of individual skeletal elements occurs at the level of the condensation, i.e. the number and adhesion properties of mesenchymal cells regulate the size and shape of the future bone (1). In most condensations, the cells become chondrocytes, the primary cell type of cartilage, which secrete a matrix rich in type II collagen. The cartilage enlarges through chondrocyte proliferation and matrix production. After the initial proliferation, these cells then hypertrophy and change their genetic program to synthesize type X collagen (3). Hypertrophic chondrocytes direct the mineralization of their surrounding matrix, attract blood vessels through the production of vascular endothelial growth factor and other factors, and attract chondroclasts. Hypertrophic chondrocytes also direct adjacent perichondrial cells to become osteoblasts; these secrete a characteristic matrix, forming a bone collar. Hypertrophic chondrocytes then undergo apoptosis (3, 4). While hypertrophic chondrocytes perform these multiple tasks in the center of the cartilage mold, the mold enlarges further through continued proliferation of chondrocytes. Bone lengthening, particularly rapid during fetal life, is driven primarily by the rate of production of hypertrophic chondrocytes from these proliferating chondrocytes (3, 5, 6).
The molecular mechanisms controlling bone development are complicated, and just starting to be elucidated. Indian Hedgehog (Ihh) and PTHrP are two important regulators in controlling chondrogenic and osteogenic programing (7). Ihh is a member of the Hedgehog family. During bone development, Ihh is produced by prehypertrophic chondrocytes and by early hypertrophic chondrocytes (8). PTHrP is a protein secreted during fetal life by perichondrial cells at the ends of cartilage molds and by early proliferative chondrocytes (3). Its receptor, PTH/PTHrP receptor, is expressed at low levels by proliferating chondrocytes and at high levels by prehypertrophic/early hypertrophic chondrocytes (9). PTHrP acts primarily to keep proliferating chondrocytes in the proliferative zone and to block their differentiation (10). Ihh and PTHrP interact in a negative feedback loop to regulate the onset of hypertrophic chondrocyte differentiation (11, 12). IGF-I is an anabolic growth factor required for normal fetal and postnatal development (13, 14). Much of the circulating IGF-I is produced by the liver and is transported to other tissues, acting as an endocrine hormone. IGF-I is also produced by many other tissues, including bone cells and chondrocytes, and acts locally as an autocrine/paracrine hormone (15). Although it is widely accepted that IGF-I is an important regulator of skeletal metabolism (16), the role of IGF-I on embryonic bone development has not been evaluated in detail.
To assess the role of IGF-I in modulating fetal skeletal development, we analyzed the fetal bone tissue of IGF-I-deficient mice with respect to skeletal phenotype and markers of chondrocyte proliferation and differentiation, including levels of Ihh and PTHrP.
Materials and Methods
Mice
The IGF-I-deficient mice used in this study were developed by Lyn Powell-Braxton at Genentech, Inc. (San Francisco, CA) (14) and backcrossed into a CD-1 genetic background. The construct used for the homologous recombination contained a neomycin cassette inserted into exon 3 of the IGF-I gene. Genotyping of the offspring used PCR to identify either the intact exon 3 or the neomycin cassette as described (14). Heterozygous mice were time-mated, with embryonic d 0.5 [0.5 days post coitus (dpc)] defined as noon on the day a vaginal plug was observed. These studies were approved by the Animal Use Committee of the San Francisco Veterans Affairs Medical Center where the animals were raised and studied.
Skeletal preparations and histology
For skeletal preparations, 13.5- to 18.5-dpc embryos were eviscerated and fixed in 99% ethanol overnight and stained with Alcian blue for cartilage and with alizarin red for calcified tissue as described previously (17). For histological analyses, femurs, tibias, and embryos were fixed overnight at 4 C in 4% paraformaldehyde in PBS, rinsed in PBS, dehydrated through an ethanol series, cleared in xylene, embedded in paraffin, and cut into 5-mm sections. The sections were stained with hematoxylin and eosin (H&E) or stained immunohistochemically for proliferating cell nuclear antigen (PCNA) (Zymed Laboratories, Inc., South San Francisco, CA), type II collagen (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), type X collagen (Hdm Diagnostics & Imaging Inc., Toronto, Ontario, Canada), Ihh (Santa Cruz Biotechnology, Inc.), PTHrP (Santa Cruz Biotechnology, Inc.), and osteocalcin (Takara Bio Inc., Otsu, Japan). Apoptotic cells were detected with the TACS 2 TdT-DAB in Situ Apoptosis Detection kit (TREVIGEN, Gaithersburg, MD) according to the manufacturer’s instructions. Sections were stained for mineral with the von Kossa procedure and counterstained with hematoxylin.
Electron microscopy
Tibias were fixed in fixative [2% paraformaldehyde (Polysciences, Inc., Warrington, PA) and 0.5% glutaraldehyde in 0.05 m cacodylate buffer (J. T. Baker, Inc., Phillipsburg, NJ) pH 7.4] for 24 h, then postosmicated for 1–2 h, dehydrated in alcohols, and embedded in Epon resin. Thin sections were collected on water containing brom-thymol blue (pH 8.0), mounted onto copper grids, stained with lead citrate (Reynolds, pH 8.0) and alcoholic uranyl acetate, and viewed by a CM-12 Philips (FEI Co., Hillsboro, OR) transmission electron microscope.
Quantitative real-time PCR
RNA was extracted from the spines and growth plates of long bones. Briefly, under a dissection microscope, lumbar spines (L1–L5) or tibia femur joint cartilages from the IGF-I+/+ and IGF-I−/− embryos were identified and isolated and attached tissue was removed. The samples were then frozen in liquid nitrogen and stored in −80 C until processed. mRNA levels of bone and chondrocyte differentiation markers were determined by quantitative real-time PCR as described previously (18). Primers and probes are as follows: glyceraldehyde-3-phosphate dehydrogenase (forward, 5′-TGCACCACCAACTGCTTAG-3′; reverse, 5′-GGATGCAGGGATGATGTTC-3′; probe, 5′-CAGAAGACTGTGGATGGCCCCTC-3′); osteocalcin (forward, 5′-CTCACAGATGCCAAGCCCA-3′; reverse, 5-‘CCAAGGTAGCGCCGGAGTCT-3′; probe, 5′-CCCTGAGTCTCTGACAAAGCCTTCATGTCCA-3′); PTHrP (forward, 5′-AGTGGCCTGCTTGAGGACC-3′; reverse, 5′-AATCCTGTAACGTGTCCTTGGAA-3′; probe, 5′-AGCCCAGCTTAAGGACGCATTGAA-3′); Ihh (forward, 5′-CATCTTCAAGGACGAGGAGAACA-3′; reverse, 5′CAGAGATGGCCAGTGAGTTCAG-3′; probe, 5′-ATGACCCAGCGCTGCAAGGACC-3′); type II collagen (forward, 5′-ACTGGTGGAGCAGCAAGAGC-3′; reverse, 5 ′-GACGTTAGCGGTGTTGGGAG-3′; probe, 5′-ACGGTGGCTTCCACTTCAGCTATGGC-3′); type X collagen (forward, 5′-TTATGCTGAACGGTACCAAACG-3′; reverse, 5′-GTTCTCCTCTTACTGGAATCCCTTTA-3′; probe, 5′-CCAAGACACAATACTTCATCCCATACGCC-3′); aggrecan (forward, 5′-AAACAACCATGTCCCTGACAGAT-3′; reverse, 5′-GTCCACCCCTCCTCACATTG-3′; probe, 5′-AGACGGAGTCAACCGTTGCAGACCAG-3′).
Statistical analysis
The data were analyzed by Student’s t test as appropriate using programs in SigmaStat 3.0 (Systat Software, Inc., Richmond, CA). A value of P < 0.05 was considered significant.
Results
IGF-I, IGF-I receptor (IGF-IR), type II collagen, and aggrecan expression in skeletal development
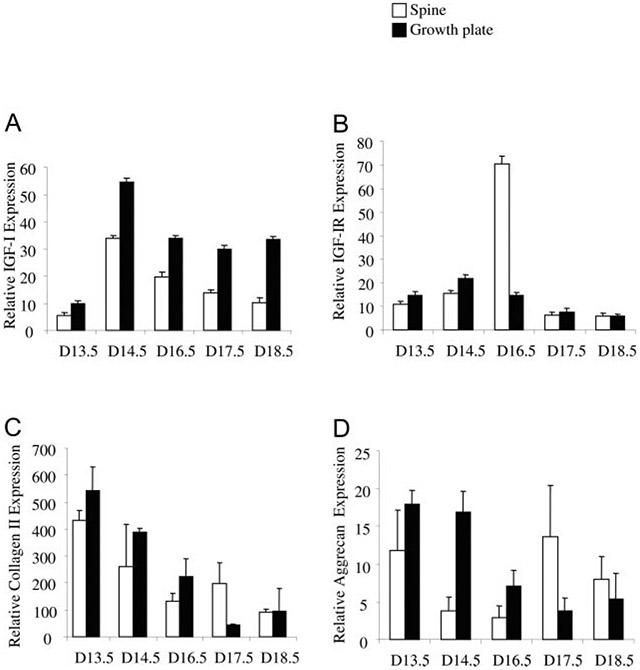
To investigate the expression of IGF-I and its receptor in comparison to chondrocyte differentiation markers type II collagen and aggrecan during bone development, we isolated RNA from the spines and the growth plates of long bones and quantitated their mRNA levels by quantitative real-time PCR. As shown in Fig. 1, expression of IGF-I in spine and growth plate (Fig. 1A) was detected at 13.5 dpc, peaked at 14.5 dpc, and then decreased as gestation continued, whereas expression of IGF-IR (Fig. 1B) was detected at 13.5 dpc, peaked at 16.5 dpc (spine) or 14.5 dpc (growth plate), and then decreased as gestation continued. The expression of type II collagen (Fig. 1C) and aggrecan (Fig. 1D) was detected at 13.5 dpc and then decreased as gestation continued.
Fig. 1.
Expression of IGF-I (A), IGF-IR (B), type II collagen (C), and aggrecan (D) during embryonic bone development. The dpc (D) is shown on the x-axis. Open bar, Spine; solid bar, growth plate of knee. Results are expressed as percentage of glyceraldehyde-3-phosphate dehydrogenase expression (means ± SD) of triplicate determinations.
Skeletal abnormalities in IGF-I−/− embryos
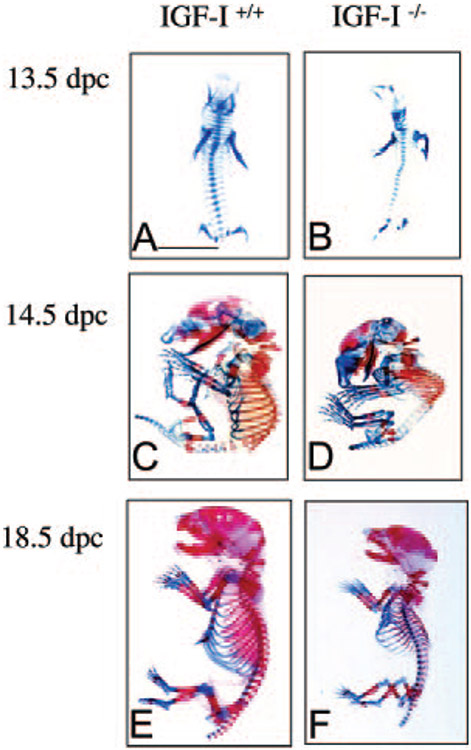
The first overt sign of an embryonic phenotype was an obvious dwarfism, visible as early as 13.5 dpc. To assess whether the lack of IGF-I causes defects in bone development, embryos at 13.5, 14.5, and 18.5 dpc were stained with Alcian blue and Alizarin red to detect cartilage and calcified tissue, respectively. At 13.5 dpc, in the IGF-I−/− embryo, all of the skeletal elements were presented in the right position and right number, but the size of the IGF-I−/− skeleton was smaller than that of the IGF-I+/+ embryo. Both IGF-I−/− and IGF-I+/+ embryos showed no mineralization at 13.5 dpc (Fig. 2, A and B). At 14.5 dpc, the onset of ossification was observed in the calvaria, axial skeleton, limbs, and ribs of IGF-I+/+ embryo (Fig. 2C), whereas ossification was reduced, especially in the axial skeleton, in the IGF-I−/− embryo (Fig. 2D). A normal onset of ossification of long bones was observed in the IGF-I−/− embryo, but both the fore-and hindlimbs were shorter than those in the IGF-I+/+ embryo (Fig. 2D). At 18.5 dpc, ossification was almost completed in the IGF-I+/+ embryo (Fig. 2E), but the skeletal abnormalities, including smaller size (85% of wild type, WT) and large cartilaginous regions in the axial skeleton (spinal column), ribs, and metacarpal and metatarsal bones of front paws, persisted in the IGF-I−/− embryo (Fig. 2F). The undermineralized rib cage may not support the initial effects to breath after birth as the cause for the high perinatal mortality of the IGF-I−/− fetuses (19). The length of the femur ([4333 ± 288 mm in WT vs. 3066 ± 115 mm in knockout (KO), n = 9 in each group] and tibia (5166 ± 288 mm in WT vs. 4163 ± 278 mm in KO, n = 9 in each group) was significantly shorter in the IGF-I−/− embryo.
Fig. 2.
Effects of IGF-I on skeletal development. The skeletons from IGF-I +/+ and IGF-I −/− embryos were stained with alizarin red (for calcified tissue) and Alcian blue (for uncalcified tissue). A and B, Whole mount of skeletons on 13.5 dpc; note cartilage elements form normally in the IGF-I −/− embryos (B), only smaller in size; C and D, whole mount of skeletons on d 14.5; note the onset of mineralization in long bones in both IGF-I +/+ (C) and IGF-I −/− (D), but the initiation of mineralization in the spine of the IGF-I −/− is delayed (D); E and F, whole mount of skeletons on 18.5 dpc; general patterning of the skeleton is normal but all elements of the axial and appendicular skeletons are significantly reduced in size in the IGF-I −/− (F), delay of mineralization in the spine becomes progressively more severe in the IGF-I −/−, and the delay of mineralization in the ribs and metacarpal and metatarsal bones of front paws is now obvious. Bar, 5 mm.
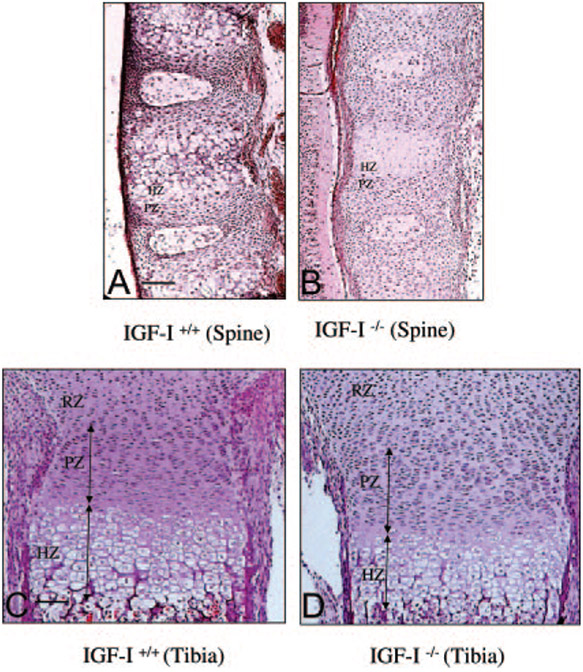
To compare the morphological differences in the spinal ossification center and long bones between the IGF-I−/− embryo and the IGF-I+/+ embryo, H&E staining was performed on sections from these bones (Fig. 3). In these sections, the four chondrocyte subpopulations—the resting, proliferating, prehypertrophic, and hypertrophic chondrocytes—are arranged in distinct zones that are clearly distinguishable by morphological criteria (20). In the spinal ossification center (Fig. 3, A and B), there are no significant differences in the length of the proliferative zone (37.5 ± 3.2 mm in WT vs. 39.2 ± 5.2 mm in KO, n = 6 in each group) and the number of proliferating chondrocytes (108 ± 25 in WT vs. 114 ± 30 in KO, n = 6 in each group) between IGF-I+/+ embryos (Fig. 3A) and the IGF-I−/− embryos (Fig. 3B). Although the number of hypertrophic cells is equal (60.5 ± 6.6 in WT vs. 56.4 ± 7.5 in KO, n = 6 in each group), only prehypertrophic chondrocytes, but no hypertrophic chondrocytes, were observed in the spinal ossification center of the IGF-I−/− embryo. In the sections from the long bones, a similar pattern in the morphology of the chondrocytes and bone structure was observed in the IGF-I+/+ embryo and IGF-I−/− embryo (Fig. 3, C and D). Although there were no significant differences in the number of the proliferating chondrocytes (448 ± 98 in WT vs. 530 ± 96 in KO, n = 6 in each group) and the hypertrophic chondrocytes (240 ± 30 in WT vs. 231 ± 33 in KO, n = 6 in each group) between the IGF-I+/+ embryo (Fig. 3C) and IGF-I−/− embryo (Fig. 3D), the hypertrophic chondrocytes in the IGF-I−/− embryo were smaller. To compare the size of the hypertrophic chondrocytes, we counted cells per high-power field (HPF, ×630). The number of hypertrophic chondrocytes per HPF was significantly less in the IGF-I+/+ embryos (54 ± 6.16/HPF) than that in the IGF-I−/− embryos (81.8 ± 11.03/HPF) (n = 6 in each group, P < 0.05), indicating that the average hypertrophic chondrocyte size was reduced in the IGF-I−/− embryos. This caused a significant reduction in the length of the hypertrophic zone in the IGF-I−/− embryo (140.4 ± 7.8 μm in WT vs. 110.5 ± 8.5 mm in KO, P < 0.05, n = 6). The lengths of the proliferative zones of the growth plates were not significantly different in the IGF-I+/+ embryo and IGF-I−/− embryo (150 ± 35 mm in WT vs. 160 ± 46 mm in KO, n = 6).
Fig. 3.
Bone structure of the spinal column (A and B) and tibias (C and D) in IGF-I +/+ (A and C) and IGF-I −/− (B and D) embryos. Paraffin-embedded nondecalcified sections from 18.5-dpc embryos were stained by H&E staining. Only premature (prehypertrophic) chondrocytes locate in the spinal ossification center of the IGF-I −/− embryo (B). A similar morphological pattern and structure is seen in the long bones of the IGF-I +/+ and the IGF-I −/− embryos, but the size of the chondrocytes is smaller and the length of the hypertrophic zone is shorter in the IGF-I −/− embryo (D) than in the IGF-I +/+ embryo (C). Resting zone (RZ), proliferating zone (PZ), and hypertrophic zone (HZ) were distinguished by morphology of the cells in each zone. Bar, 50 μm.
Reduced chondrocyte proliferation in the IGF-I−/− embryos
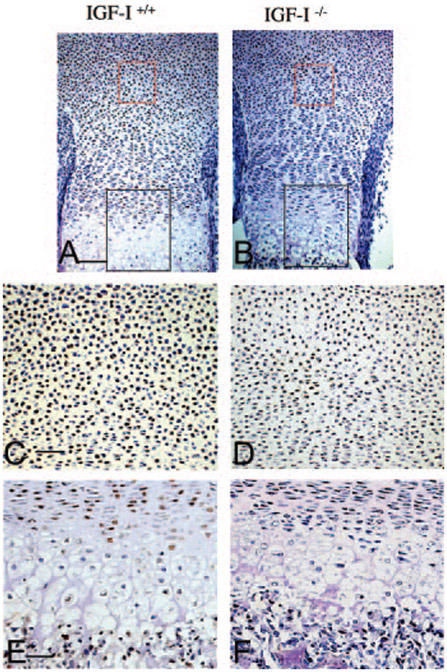
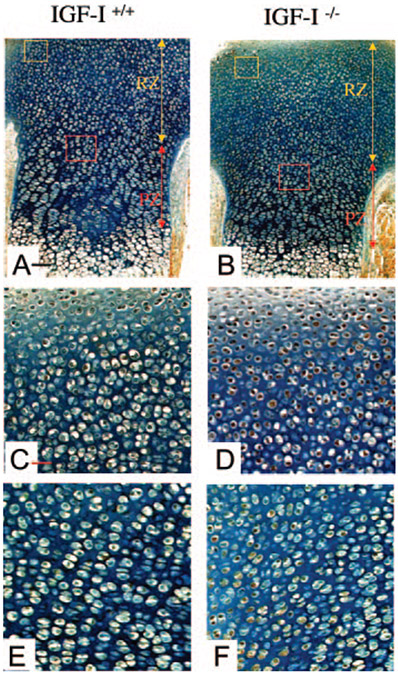
Growth within the cartilage element is largely dependent on proliferation of chondrocytes. To address proliferation in the IGF-I−/− embryos, we performed PCNA staining of sections of the spine and the long bones from IGF-I+/+ and IGF-I−/− embryos. As shown in Fig. 4, a marked reduction in the percentage of PCNA-positive nuclei (brown) in the resting zone and proliferative zone of the long bones (Fig. 4, B, D, and F) and spines (data not shown) of the IGF-I−/− embryos was observed compared with IGF-I+/+ embryos (Fig. 4, A, C, and E). The proportion of PCNA-positive nuclei in the long bones of the IGF-I−/− embryos was 25% of that in the IGF-I+/+ embryos (65 ± 4% in WT vs. 17 ± 3% in KO, P < 0.05, n = 5 in each group). Thus, IGF-I signaling is required to maintain the high rate of chondrocyte proliferation observed in rapidly growing long bones and spinal columns in mouse skeletal development.
Fig. 4.
Effects of IGF-I on chondrocyte proliferation. Paraffin-embedded nondecalcified sections from tibias of 18.5-dpc IGF-I+/+ (A, C, and E) and IGF-I−/− (B, D, and F) embryos were immunostained for PCNA. PCNA-positive nuclei are stained brown. Representative regions of the resting zone and proliferating and hypertrophic zones are labeled by a red box and black box, respectively. C and D, High magnification of the resting zone (red box in A and B, respectively). E and F, High magnification of the proliferating and hypertrophic zone (black box in A and B, respectively). Magnification, ×10 in A and B; ×20 in C–F. Bar, 50 mm in A and B; 100 mm in C–F.
Increase of chondrocyte apoptosis in the IGF-I−/− embryos
To test whether the reduced size of the skeleton in the IGF-I−/− embryos is associated with reduced survival of chondrocytes, we examined the apoptosis of chondrocytes in the IGF-I−/− embryos by TUNEL (terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick end labeling) assay. In the IGF-I−/− embryos, most of the resting chondrocytes under the perichondrium and proliferative chondrocytes close to the bone collar were undergoing apoptosis (Fig. 5B, yellow and red boxes, respectively). Apoptotic chondrocytes (stained brown in Fig. 5) were found to be significantly increased in the resting zone (6.7-fold, 67% in KO vs. 10% in WT, n = 4) (Fig. 5, C and D), and in the proliferative zone (3.8-fold, 31% in KO vs. 8% in WT n = 4) (Fig. 5, E and F) of the long bones. Apoptotic chondrocytes were also found to be increased in the disc of the spine (5.6-fold, 60% in KO vs. 11% in WT, n = 4, data not shown).
Fig. 5.
Effects of IGF-I on chondrocyte apoptosis. Apoptotic chondrocytes are detected in paraffin-embedded nondecalcified sections from tibias of 18.5-dpc IGF-I+/+ (A, C, and E) and IGF-I−/− (B, D, and F) embryos by TUNEL staining. Representative regions of the resting zone (RZ) and proliferating zone (PZ) are labeled by a yellow box and red box, respectively. C and D, High magnification of the resting zone (yellow box in A and B, respectively); E and F, high magnification of the proliferating zone (red box in A and B, respectively). Apoptotic nuclei are stained brown. More apoptotic chondrocytes can be detected in the resting zone and proliferating zone in the IGF-I−/− embryos (D and F) than in the IGF-I+/+ (C and E) embryos. Magnification, ×10 in A and B; ×40× in C–F. Bar, 200 μm in A and B; 50 μm in C–F.
Abnormal mineralization in the IGF-I−/− embryos
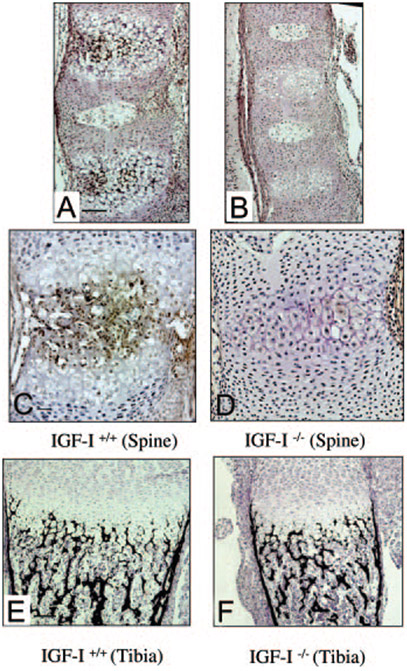
As described above, IGF-I−/− embryos showed a significant reduction of mineralization identified by alizarin red staining in the spinal column (Fig. 2, A-F). To confirm this observation, we performed von Kossa staining on bone sections from the spine and tibia of 18.5-dpc embryos. Black-stained mineralized bone was observed in the spinal ossification center in IGF-I+/+ embryos (Fig. 6A) but not in the spinal ossification center in IGF-I−/− embryos (Fig. 6B). As also noted by alizarin red staining (Fig. 2, A-F), von Kossa staining in the sections from the long bones showed no significant differences in the mineralization between IGF-I+/+ (Fig. 6E) and IGF-I−/− (Fig. 6F) embryos (mineralized area/total area, 40 ± 11% in WT vs. 43 ± 9% in KO, n = 4). To address the failure of bone mineralization in the spine of IGF-I−/− embryos, we examined the expression of osteocalcin, a marker of mineralization, in the sections from the spinal ossification center by immunohistochemistry and analyzed the mRNA levels of osteocalcin by quantitative real-time PCR using RNA isolated from the spines. As shown in Fig. 6C, osteocalcin was expressed in the hypertrophic chondrocytes and osteoblasts (brown stained) in the spinal ossification center of the IGF-I+/+ embryos but was expressed much less in the spinal ossification center of the IGF-I−/− embryos (Fig. 6D). Analyzed by quantitative real-time PCR, the mRNA levels of osteocalcin of the IGF-I−/− embryos were decreased to 32% of the IGF-I+/+ embryos (Table 1).
Fig. 6.
Effects of IGF-I on mineralization of spinal columns (A–D) and tibias (E and F). Paraffin-embedded nondecalcified sections from 18.5-dpc embryos were stained by von Kossa staining (A, B, E, and F) or immunostained with osteocalcin (C and D). Mineralized bone is stained black. Note little mineralization in the spine in the IGF-I −/− embryos (B) but not in the long bones (F). Osteocalcin (brown) is expressed in the hypertrophic chondrocytes and osteoblasts adjacent to the hypertrophic zone in the spinal ossification center of the IGF-I +/+ embryo (C), but very little expression was observed in the spinal ossification center of the IGF-I −/− embryo (D). A, C, and E, IGF-I +/+; B, D, and F, IGF-I −/−. Bar, 50 μm.
TABLE 1.
IGF-I regulates chondrocyte differentiation markers (spine)
| Group | OCN | AGG | Col.II | Col.X | Ihh | PTHrP |
|---|---|---|---|---|---|---|
| IGF-I+/+ | 100 ± 21 | 100 ± 30 | 100 ± 43 | 100 ± 45 | 100 ± 46 | 100 ± 36 |
| IGF-I−/− | 32 ± 11a | 15 ± 6a | 48 ± 20a | 45 ± 18a | 50 ± 15a | 152 ± 30a |
Results were assessed by real-time PCR and are expressed as percentage of WT control (means ± SD), and all values have been normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA levels in the same sample. OCN, Osteocalcin; AGG, aggrecan; Col.II, type II collagen; Col.X, type X collagen.
P < 0.05 IGF-I−/− vs. IGF-I+/+ embryos (n = 6 in each group).
Chondrocyte maturation is abnormal in IGF-I−/− embryos
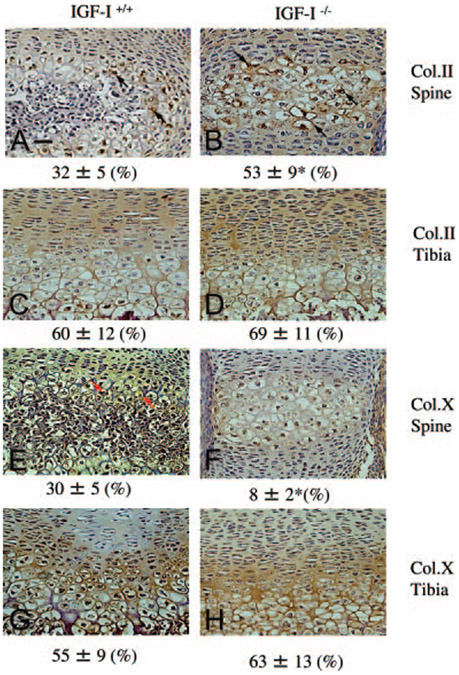
Hypertrophic chondrocytes play an important role in mineralization. To determine whether the failure of mineralization in IGF-I−/− embryos is due to the failure of chondrocyte maturation, we performed immunohistochemistry in sections of the spinal ossification center and proximal tibia to compare the expression of collagen type II, which is expressed by less mature chondrocytes, and collagen type X, a marker of hypertrophic chondrocytes. At 18.5 dpc, in the spinal ossification center (Fig. 7A) and the growth plate of the long bones (Fig. 7C) of the IGF-I+/+ embryos, type II collagen (brown stain) was expressed primarily in the proliferative region, including proliferative chondrocytes and extracellular matrix (ECM). It was also expressed slightly in the hypertrophic zone, limited to some of the prehypertrophic chondrocytes, and the ECM around them. As cells matured into hypertrophic chondrocytes, they switched from expressing type II collagen to type X collagen. Type X collagen expression was observed in the hypertrophic region, including prehypertrophic chondrocytes, hypertrophic chondrocytes, and the ECM around them (Fig. 7, E and G). Only the prehypertrophic chondrocytes expressed both types of collagen. In contrast, in the spinal ossification center of the IGF-I−/− embryos, type II collagen expression was observed throughout the proliferative region and into the hypertrophic region (Fig. 7B). The number of type II collagen-expressing prehypertrophic chondrocytes was more than that in the ossification center in the IGF-I+/+ embryos. On the other hand, collagen X expression was much reduced (Fig. 7F). The data indicate a reduction in chondrocyte maturation in the spine of the IGF-I−/− embryo. In the growth plates of long bones, no difference in type II collagen (Fig. 7D) and type X collagen expression (Fig. 7H) between the IGF-I−/− and IGF-I+/+ embryos was observed.
Fig. 7.
Effects of IGF-I on chondrocyte differentiation. Paraffin-embedded nondecalcified sections from spinal columns (A, B, E, and F) and tibias (C, D, G, and H) of 18.5-dpc IGF-I +/+ (A, C, E, and G) and IGF-I −/− embryos (B, D, F, and H) were immunostained for collagen II (Col.II; A, B, C, and D) or collagen X (Col.X; E, F, G, and H). Collagen II is expressed in the prehypertrophic chondrocytes (black arrows) and matrix, whereas collagen X is expressed in hypertrophic chondrocytes (red arrows) and matrix in the spinal ossification center (A) and tibia (C) from IGF-I +/+ embryos. In the spinal ossification center from IGF-I −/− embryos, most of the cells are collagen II-expressing prehypertrophic chondrocytes (B, black arrows), but almost no collagen X-expressing hypertrophic chondrocytes are observed. In the long bones from the IGF-I −/− embryos, collagen II and collagen X are expressed in the pattern similar to that in the IGF-I +/+ embryos. Quantitation refers to the percent area immunostained for the collagen being assessed. Results are expressed as means ± SD *, P < 0.05 vs. IGF-I +/+ embryos. n = 5 in each group. Bar, 100 μm.
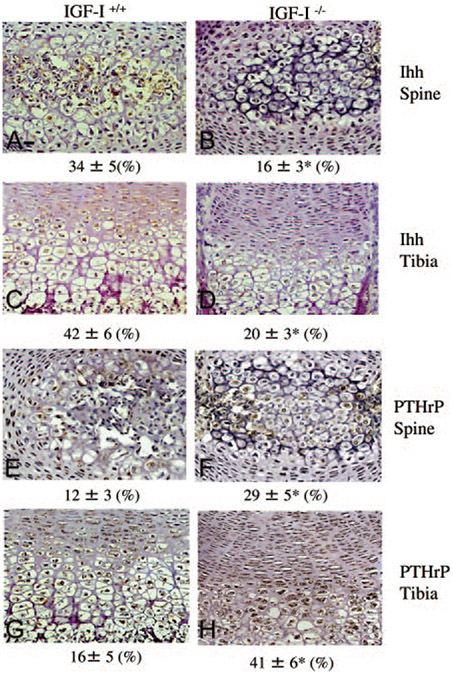
To address potential molecular mechanisms controlling chondrocyte differentiation, we evaluated Ihh and PTHrP by immunohistochemistry (Fig. 8) and real-time PCR (Table 1). In the IGF-I+/+ embryo, Ihh (brown stain) was expressed in the prehypertrophic chondrocytes and hypertrophic chondrocytes in the spinal ossification center (Fig. 8A) and the growth plates of long bones (Fig. 8C), whereas PTHrP (brown stain) was expressed in proliferative chondrocytes and prehypertrophic chondrocytes (Fig. 8, E and G). In the IGF-I−/− embryo, the expression of Ihh was decreased in the spinal ossification center (Fig. 8B) and the growth plates of long bones (Fig. 8D). On the other hand, PTHrP was expressed throughout all stages of the chondrocytes, and its expression was increased in the spinal ossification center (Fig. 8F) and the growth plates of long bones (Fig. 8H) of the IGF-I −/− embryos. These results were confirmed by quantitative real-time PCR analysis using RNA isolated from the spine. As shown in Table 1, in the IGF-I−/− embryos, the mRNA levels of aggrecan, type II collagen, type X collagen, and Ihh were significantly decreased (15, 48, 45, and 50%, respectively, n = 6), whereas the mRNA levels of PTHrP were significantly increased (152%) compared with these mRNA levels in the IGF-I+/+ embryos. The increase in type II collagen protein levels (Fig. 7B) but decrease in mRNA levels (Table 1) suggests that the delay in maturation may lead to a prolonged half life of type II collagen in the IGF-I−/− embryos. On the other hand, quantitative real-time PCR analysis using RNA isolated from the growth plate showed no significant differences in the mRNA levels of osteocalcin (82% of WT) and type II collagen (97% of WT) between IGF-I−/− and IGF-I+/+ embryos, confirming the immunohistochemistry results.
Fig. 8.
Effects of IGF-I on chondrocyte differentiation. Paraffin-embedded nondecalcified sections from spinal columns (A, B, E, and F) and tibias (C, D, G, and H) of 18.5-dpc IGF-I +/+ (A, C, E, and G) and IGF-I −/− (B, D, F, and H) embryos were immunostained for Ihh (A, B, C, and D) or PTHrP (E, F, G,and H). Ihh (brown) is expressed in similar pattern in the spinal ossification center and the growth plates of long bones in both IGF-I +/+ and IGF-I −/− embryos, but the expression of Ihh in the IGF-I −/− embryos is much lower than that in the IGF-I +/+ embryos. Unlike that in the IGF-I +/+ embryos, PTHrP is highly expressed throughout all the cell types in the growth plates of long bones and the spinal ossification of the IGF-I −/− embryos. Numbers refer to the percentage of Ihh- or PTHrP-positive chondrocytes of the total number of cells evaluated. Results are expressed as means ± SD *, P < 0.05 vs. IGF-I +/+ embryos. n = 5 in each group.
Abnormal ultrastructure of chondrocytes in the in the IGF-I−/− embryos
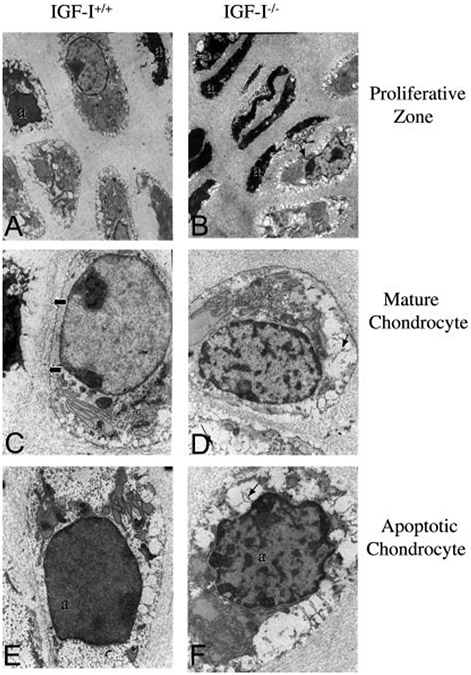
To more closely examine the morphological differences in chondrocytes from the IGF-I−/− embryos compared with their WT littermates, we performed ultrastructural studies of the growth plates from these mice (Fig. 9). Compared with the IGF-I+/+ embryos (Fig. 9A), more apoptotic cells were observed in the proliferative zone in the IGF-I−/− embryos (Fig. 9B), confirming the histological observations using TUNEL staining (see Fig. 5). The mature chondrocytes from the IGF-I−/− embryos had more clumped chromatin (Fig. 8D) compared with the IGF-I+/+ embryos (Fig. 9C). In the apoptotic chondrocytes from the IGF-I+/+ embryos (Fig. 9E, labeled as “a”), the cell cytoplasm was sparse, glycogen was present and surrounded the remaining organelles (endoplasmic reticulum and mitochondria), and the nucleus was dense but with uniform chromatin. In contrast, in the apoptotic chondrocytes from the IGF-I−/− embryos (Fig. 9F, a), the cytoplasm was disrupted, and the nucleus was dense with clumped chromatin. Fibrous material was observed only in the chondrocytes of the IGF-I−/− embryos (Fig. 9, B, D and F, arrows). It was present intracellularly in the mature chondrocytes (Fig. 9D) and released to the extracellular matrix adjacent to the apoptotic chondrocytes (Fig. 9, B and F). The nature of this fibrous material remains under investigation. These results indicate that the abnormalities in chondrocyte function are accompanied by abnormalities in chondrocyte ultra structure during skeletal development.
Fig. 9.
Effects of IGF-I on chondrocyte ultrastructure. Chondrocytes from 18.5-dpc IGF-I +/+ (A, C, and E) and IGF-I −/− (B, D, and F) embryos were examined by electron microscopy. More apoptotic proliferating chondrocytes (labeled as “a”) were observed in the IGF-I −/− (B) than in the IGF-I +/+ (A) embryos. The mature chondrocytes from the IGF-I+/+ embryos actively synthesized collagenous matrix (C, arrows), unlike those of the IGF-I −/− embryos. The mature chondrocytes from the IGF-I −/− embryos (D) had more clumped chromatin in the nucleus than their IGF-I +/+ counterparts. A “fibrous” material was only observed in the IGF-I −/− embryos (arrows in B, D, and F), which was present intracellularly (arrows in D), and apparently released to the extracellular matrix adjacent to apoptotic chondrocytes (B and F). Magnifications: ×8,000 in A and B; ×21,000 in C–F.
Discussion
Our studies demonstrate that, in embryonic skeletal development, IGF-I deficiency results in markedly reduced chondrocyte proliferation, increased chondrocyte apoptosis, and abnormal chondrocyte differentiation and maturation, resulting in severe dwarfism of the axial and appendicular skeleton and reduction in mineralization of the spinal column. Thus, IGF-I plays a fundamental role in skeletal development by regulating and coordinating chondrocyte proliferation, maturation, and apoptosis to form endochondral bones of appropriate size and strength.
IGF-I is required for skeletogenesis but is not necessary for patterning
The size, shape, and position of each bone in the vertebrate skeleton depends both on patterning processes acting on mesenchymal cells and on differentiation of these cells along the lines of chondrogenesis and osteogenesis (2). Our study shows in the IGF-I−/− embryos that all of the skeletal elements are presented in the right position, right number ,and right shape, suggesting that IGF-I does not act on cell patterning required for skeletal morphogenesis. Smaller bone size and delay of mineralization may be explained by the requirement of IGF-I for a later function in chondrocyte proliferation and maturation. A recent study (21) investigated chondrogenesis of primary bone marrow-derived mesenchymal stem cells in aggregate cultures after genetic modification with adenoviral vectors encoding IGF-I and found that overexpression of IGF-I failed to induce chondrogenesis of mesenchymal stem cells. However, our results indicate that at least some IGF-I is required for normal chondrocyte differentiation.
IGF-I signaling pathway maintains bone size by regulating chondrocyte proliferation, differentiation, and apoptosis
Bone lengthening is driven primarily by the rate of production of hypertrophic chondrocytes from the proliferating chondrocytes and then subsequent differentiation into bone. Previous studies have reported that IGF-I stimulates chondrocyte proliferation and increases bone length in cultured metatarsals (22), suggesting that the small bone size in IGF-I−/− embryos is associated with reduced proliferation of chondrocytes (23). Because cell division is not arrested in its absence, IGF-I is not an essential signaling molecule for the occurrence of chondrocyte proliferation per se, but rather is an important efficiency factor necessary for the maintenance of proliferation at a normal rate (13). In addition, IGF-I also has been reported to inhibit chondrocyte apoptosis (24, 25). During normal skeletogenesis, hypertrophic chondrocytes undergo apoptosis with the onset of calcification and transformation of cartilage into bone. In IGF-I−/− embryos, the number of apoptotic chondrocytes was increased in the resting and proliferative zones. Given that proliferation of these chondrocytes is also reduced in the IGF-I−/− embryo, the maintenance of the proliferative zone indicates that fewer proliferative chondrocytes differentiated into hypertrophic chondrocytes. Breur et al. (26) noted a positive linear relationship between the rate of longitudinal bone growth and the volume of hypertrophic chondrocytes. Our results indicate that hypoproliferation and increased apoptosis in the zone of actively dividing chondrocytes results in fewer mature hypertrophic chondrocytes, leading in turn to decreased bone formation. As a consequence, the IGF-I−/− embryos have shorter bones.
IGF-I signaling regulates mineralization by stimulating chondrocyte differentiation
A striking phenotype in the IGF-I−/− embryos is an obvious delay of mineralization in the spinal ossification center (19). It has been demonstrated in previous studies that, during the final steps of chondrocyte differentiation, hypertrophic chondrocytes produce a mineralized matrix. This mineralized cartilaginous matrix provides a rigid scaffold on which osteoblasts deposit and mineralize bone matrix (27, 28). The maturation of chondrocytes from resting cells to hypertrophic cells is a critical event in regulating mineralization (29, 30). Factors regulating chondrocyte maturation may affect mineralization of cartilage matrix and bone calcification (30). Our data indicate that IGF-I is involved in chondrocyte maturation and cartilage mineralization. First, during skeletal development, the expression of IGF-I mRNA peaked at 14.5 dpc, paralleling the onset of mineralization. Second, in the IGF-I−/− embryos, staining with H&E showed smaller hypertrophic chondrocytes and a shorter hypertrophic zone in the growth plate of long bones. Third, the onset of type II collagen expression is normal in the IGF-I−/− embryos, but the expression of collagen X, a marker specific for hypertrophic chondrocytes, was reduced in the spinal ossification center. Finally, “fibrous” material observed in the chondrocytes from the IGF-I−/− embryos suggested a change in chondrocyte synthetic activity. This might have an impact on the collagenous matrix and its subsequent mineralization. These data demonstrate that IGF-I is essential for chondrocyte maturation, and abnormal chondrocyte maturation leads to a delay of mineralization.
Previous studies showed matrix adjacent to hypertrophic chondrocytes mineralized, indicating that chondrocyte differentiation affects mineralization of cartilaginous matrix in a non-cell-autonomous fashion and that matrix mineralization requires a critical mass of adjacent hypertrophic chondrocytes. Presumably some combination of secreted stimulators or inhibitors of mineralization requires a critical mass of hypertrophic chondrocytes to provide an appropriate milieu for mineralization (10). The patterns of chondrocyte mineralization of the IGF-I−/− embryos are consistent with these observations that the small hypertrophic chondrocyte mass in IGF-I−/− embryos cannot initiate matrix mineralization. The detailed mechanism involved will require further analysis. However, in the IGF-I−/− embryos, expression of the bone mineralization-related gene osteocalcin was lower than that in the IGF-I+/+ embryos, consistent with a failure of these chondrocytes to initiate mineralization. The degree of the disturbance of chondrocyte maturation in IGF-I−/− embryos is site specific, severe in the spinal column, ribs, and digits, but limited in the long bones.
IGF-I signaling is required to maintain the Ihh-PTHrP loop during skeletogenesis
Ihh and PTHrP form a negative feedback loop regulating the onset of hypertrophic differentiation of chondrocytes. Disruption of this signaling pathway can result in abnormal chondrocyte differentiation. In our study, lack of IGF-I alters the Ihh-PTHrP loop, dissociating the regulation of these two molecules, resulting in down-regulation of Ihh expression and up-regulation of PTHrP expression. These results suggest that, in the IGF-I−/− embryos, Ihh and PTHrP may act independently to regulate skeletogenesis. Previous studies showed that Ihh is essential for normal chondrocyte proliferation, differentiation, and bone formation (8). Ihh drives chondrocyte proliferation, stimulates periarticular chondrocyte differentiation, and regulates growth plate length through PTHrP-independent pathways (12, 31). In the IGF-I−/− embryos, the lower proliferation rate of chondrocytes, the delay of chondrocyte maturation, and the lower endochondral bone formation may result from lower Ihh expression. Consistent with previous studies (9), we observed that PTHrP was expressed at the interface of proliferating and hypertrophic zones, where it colocalized with the PTH/PTHrP receptor in the growth plate of long bones and spinal ossification center in the IGF-I+/+ embryos. In contrast, in IGF-I−/− embryos, PTHrP is expressed throughout the proliferative zone as well as within hypertrophic chondrocytes in the growth plate of long bones and the spinal ossification center, indicating that lack of IGF-I leads to an abnormal high expression of PTHrP. This would be expected to delay the rate of differentiation of chondrocytes, and delay the mineralization, similar to overexpression of PTHrP in transgenic mice (32).
In summary, our study demonstrates that IGF-I promotes chondrocyte proliferation and differentiation while inhibiting chondrocyte apoptosis. IGF-I is shown for the first time to be an important regulator of the Ihh-PTHrP feedback loop, and many of the changes observed in the IGF-I−/− embryos can be attributed to the disruption of this feedback loop. We conclude IGF-I is essential for embryonic bone development.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant RO1 DK 54793, the National Aeronautic and Space Administrations Grant, NN04CC67G, and NIH Grant RO1 AG 21353.
Abbreviations:
- dpc
Days post coitus
- ECM
extracellular matrix
- H&E
hematoxylin and eosin
- HPF
high-power field
- IGF-IR
IGF-I receptor
- Ihh
Indian Hedgehog
- KO
knockout
- PCNA
proliferating cell nuclear antigen
- TUNEL
terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick end labeling
- WT
wild type
Footnotes
Disclosure Summary: The authors have nothing to disclose.
Contributor Information
Yongmei Wang, Department of Medicine, Endocrine Unit, Veterans Affairs Medical Center, and University of California, San Francisco, California 94121.
Shigeki Nishida, Department of Medicine, Endocrine Unit, Veterans Affairs Medical Center, and University of California, San Francisco, California 94121.
Takeshi Sakata, Department of Medicine, Endocrine Unit, Veterans Affairs Medical Center, and University of California, San Francisco, California 94121.
Hashem Z. Elalieh, Department of Medicine, Endocrine Unit, Veterans Affairs Medical Center, and University of California, San Francisco, California 94121
Wenhan Chang, Department of Medicine, Endocrine Unit, Veterans Affairs Medical Center, and University of California, San Francisco, California 94121.
Bernard P. Halloran, Department of Medicine, Endocrine Unit, Veterans Affairs Medical Center, and University of California, San Francisco, California 94121
Steven B. Doty, Mineralized Tissues Research Section, Hospital for Special Surgery, New York, New York 10021
Daniel D. Bikle, Department of Medicine, Endocrine Unit, Veterans Affairs Medical Center, and University of California, San Francisco, California 94121
References
- 1.Hall BK, Miyake T 2000. All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22:138–147 [DOI] [PubMed] [Google Scholar]
- 2.Horton WA 2003. Skeletal development: insights from targeting the mouse genome. Lancet 362:560–569 [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg HM 2003. Developmental regulation of the growth plate. Nature 423:332–336 [DOI] [PubMed] [Google Scholar]
- 4.Olsen BR, Reginato AM, Wang W 2000. Bone development. Annu Rev Cell Dev Biol 16:191–220 [DOI] [PubMed] [Google Scholar]
- 5.Zelzer E, Olsen BR 2003. The genetic basis for skeletal diseases. Nature 423:343–348 [DOI] [PubMed] [Google Scholar]
- 6.Karsenty G, Wagner EF 2002. Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2:389–406 [DOI] [PubMed] [Google Scholar]
- 7.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ 1996. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273:613–622 [DOI] [PubMed] [Google Scholar]
- 8.St-Jacques B, Hammerschmidt M, McMahon AP 1999. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13:2072–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K, Deeds JD, Segre GV 1995. Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology 136:453–463 [DOI] [PubMed] [Google Scholar]
- 10.Chung UI, Lanske B, Lee K, Li E, Kronenberg H 1998. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci US 95:13030–13035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM 2002. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 129:2977–2986 [DOI] [PubMed] [Google Scholar]
- 12.Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP 2000. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 127:543–548 [DOI] [PubMed] [Google Scholar]
- 13.Baker J, Liu JP, Robertson EJ, Efstratiadis A 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73–82 [PubMed] [Google Scholar]
- 14.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA 1993. IGF-I is required for normal embryonic growth in mice. Genes Dev 7:2609–2617 [DOI] [PubMed] [Google Scholar]
- 15.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A 2001. The somatomedin hypothesis: 2001. Endocr Rev 22:53–74 [DOI] [PubMed] [Google Scholar]
- 16.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B 2001. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res 16:2320–2329 [DOI] [PubMed] [Google Scholar]
- 17.Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R 1994. The role of Pax-1 in axial skeleton development. Development 120:1109–1121 [DOI] [PubMed] [Google Scholar]
- 18.Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP 2002. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res 17:1570–1578 [DOI] [PubMed] [Google Scholar]
- 19.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- 20.Wagner EF, Karsenty G 2001. Genetic control of skeletal development. Curr Opin Genet Dev 11:527–532 [DOI] [PubMed] [Google Scholar]
- 21.Palmer GD, Steinert A, Pascher A, Gouze E, Gouze JN, Betz O, Johnstone B, Evans CH, Ghivizzani SC 2005. Gene-Induced Chondrogenesis of Primary Mesenchymal Stem Cells in vitro. Mol Ther 12:219–228 [DOI] [PubMed] [Google Scholar]
- 22.Mushtaq T, Bijman P, Ahmed SF, Farquharson C 2004. Insulin-like growth factor-I augments chondrocyte hypertrophy and reverses glucocorticoid-mediated growth retardation in fetal mice metatarsal cultures. Endocrinology 145:2478–2486 [DOI] [PubMed] [Google Scholar]
- 23.Fisher MC, Meyer C, Garber G, Dealy CN 2005. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone 37:741–750 [DOI] [PubMed] [Google Scholar]
- 24.Vincent AM, Feldman EL 2002. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res 12:193–197 [DOI] [PubMed] [Google Scholar]
- 25.Lo MY, Kim HT 2004. Chondrocyte apoptosis induced by collagen degradation: inhibition by caspase inhibitors and IGF-1. J Orthop Res 22:140–144 [DOI] [PubMed] [Google Scholar]
- 26.Breur GJ, VanEnkevort BA, Farnum CE, Wilsman NJ 1991. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res 9:348–359 [DOI] [PubMed] [Google Scholar]
- 27.Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R 1995. Toward a molecular understanding of skeletal development. Cell 80:371–378 [DOI] [PubMed] [Google Scholar]
- 28.Kirsch T, Nah HD, Shapiro IM, Pacifici M 1997. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol 137:1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karsenty G 2003. The complexities of skeletal biology. Nature 423:316–318 [DOI] [PubMed] [Google Scholar]
- 30.Johnson KA, Terkeltaub RA 2005. External GTP-bound transglutaminase 2 is a molecular switch for chondrocyte hypertrophic differentiation and calcification. J Biol Chem 280:15004–15012 [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, Kronenberg HM 2005. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest 115:1734–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir EC, Philbrick WM, Amling M, Neff LA, Baron R, Broadus AE 1996. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc Natl Acad Sci USA 93:10240–10245 [DOI] [PMC free article] [PubMed] [Google Scholar]