Abstract
Background
Health literacy (HL) is a determinant of health and important for autonomous decision‐making. Migrants are at high risk for limited HL. Improving HL is important for equitable promotion of migrants' health.
Objectives
To assess the effectiveness of interventions for improving HL in migrants. To assess whether female or male migrants respond differently to the identified interventions.
Search methods
We ran electronic searches to 2 February 2022 in CENTRAL, MEDLINE, Embase, PsycInfo and CINAHL. We also searched trial registries. We used a study filter for randomised controlled trials (RCTs) (RCT classifier).
Selection criteria
We included RCTs and cluster‐RCTs addressing HL either as a concept or its components (access, understand, appraise, apply health information).
Data collection and analysis
We used the methodological procedures recommended by Cochrane and followed the PRISMA‐E guidelines. Outcome categories were: a) HL, b) quality of life (QoL), c) knowledge, d) health outcomes, e) health behaviour, f) self‐efficacy, g) health service use and h) adverse events. We conducted meta‐analysis where possible, and reported the remaining results as a narrative synthesis.
Main results
We included 28 RCTs and six cluster‐RCTs (8249 participants), all conducted in high‐income countries. Participants were migrants with a wide range of conditions. All interventions were adapted to culture, language and literacy.
We did not find evidence that HL interventions cause harm, but only two studies assessed adverse events (e.g. anxiety). Many studies reported results for short‐term assessments (less than six weeks after total programme completion), reported here. For several comparisons, there were also findings at later time points, which are presented in the review text.
Compared with no HL intervention (standard care/no intervention) or an unrelated HL intervention (similar intervention but different information topic)
Self‐management programmes (SMP) probably improve self‐efficacy slightly (standardised mean difference (SMD) 0.28, 95% confidence interval (CI) 0.06 to 0.50; 2 studies, 333 participants; moderate certainty). SMP may improve HIV‐related HL (understanding (mean difference (MD) 4.25, 95% CI 1.32 to 7.18); recognition of HIV terms (MD 3.32, 95% CI 1.28 to 5.36)) (1 study, 69 participants). SMP may slightly improve health behaviours (3 studies, 514 participants), but may have little or no effect on knowledge (2 studies, 321 participants) or subjective health status (MD 0.38, 95% CI ‐0.13 to 0.89; 1 study, 69 participants) (low certainty). We are uncertain of the effects of SMP on QoL, health service use or adverse events due to a lack of evidence. HL skills building courses (HLSBC) may improve knowledge (MD 10.87, 95% CI 5.69 to 16.06; 2 studies, 111 participants) and any generic HL (SMD 0.48, 95% CI 0.20 to 0.75; 2 studies, 229 participants), but may have little or no effect on depression literacy (MD 0.17, 95% CI ‐1.28 to 1.62) or any health behaviour (2 studies, 229 participants) (low certainty). We are uncertain if HLSBC improve QoL, health outcomes, health service use, self‐efficacy or adverse events, due to very low‐certainty or a lack of evidence. Audio‐/visual education without personal feedback (AVE) probably improves depression literacy (MD 8.62, 95% CI 7.51 to 9.73; 1 study, 202 participants) and health service use (MD ‐0.59, 95% CI ‐1.11 to ‐0.07; 1 study, 157 participants), but probably has little or no effect on health behaviour (risk ratio (RR) 1.07, 95% CI 0.91 to 1.25; 1 study, 135 participants) (moderate certainty). AVE may improve self‐efficacy (MD 3.51, 95% CI 2.53 to 4.49; 1 study, 133 participants) and may slightly improve knowledge (MD 8.44, 95% CI ‐2.56 to 19.44; 2 studies, 293 participants) and intention to seek depression treatment (MD 1.8, 95% CI 0.43 to 3.17), with little or no effect on depression (SMD ‐0.15, 95% CI ‐0.40 to 0.10) (low certainty). No evidence was found for QoL and adverse events. Adapted medical instruction may improve understanding of health information (3 studies, 478 participants), with little or no effect on medication adherence (MD 0.5, 95% CI ‐0.1 to 1.1; 1 study, 200 participants) (low certainty). No evidence was found for QoL, health outcomes, knowledge, health service use, self‐efficacy or adverse events.
Compared with written information on the same topic
SMP probably improves health numeracy slightly (MD 0.7, 95% CI 0.15 to 1.25) and probably improves print literacy (MD 9, 95% CI 2.9 to 15.1; 1 study, 209 participants) and self‐efficacy (SMD 0.47, 95% CI 0.3 to 0.64; 4 studies, 552 participants) (moderate certainty). SMP may improve any disease‐specific HL (SMD 0.67, 95% CI 0.27 to 1.07; 4 studies, 955 participants), knowledge (MD 11.45, 95% CI 4.75 to 18.15; 6 studies, 1101 participants) and some health behaviours (4 studies, 797 participants), with little or no effect on health information appraisal (MD 1.15, 95% CI ‐0.23 to 2.53; 1 study, 329 participants) (low certainty). We are uncertain whether SMP improves QoL, health outcomes, health service use or adverse events, due to a lack of evidence or low/very low‐certainty evidence. AVE probably has little or no effect on diabetes HL (MD 2, 95% CI ‐0.15 to 4.15; 1 study, 240 participants), but probably improves information appraisal (MD ‐9.88, 95% CI ‐12.87 to ‐6.89) and application (RR 1.51, 95% CI 1.29 to 1.77) (1 study, 608 participants; moderate certainty). AVE may slightly improve knowledge (MD 8.35, 95% CI ‐0.32 to 17.02; low certainty). No short‐term evidence was found for QoL, depression, health behaviour, self‐efficacy, health service use or adverse events.
AVE compared with another AVE
We are uncertain whether narrative videos are superior to factual knowledge videos as the evidence is of very low certainty.
Gender differences
Female migrants' diabetes HL may improve slightly more than that of males, when receiving AVE (MD 5.00, 95% CI 0.62 to 9.38; 1 study, 118 participants), but we do not know whether female or male migrants benefit differently from other interventions due to very low‐certainty or a lack of evidence.
Authors' conclusions
Adequately powered studies measuring long‐term effects (more than six months) of HL interventions in female and male migrants are needed, using well‐validated tools and representing various healthcare systems.
Keywords: Female, Humans, Male, Anxiety, Anxiety/therapy, Diabetes Mellitus, Health Literacy, HIV Infections, Quality of Life, Randomized Controlled Trials as Topic, Transients and Migrants
Plain language summary
What are the benefits and risks of health literacy interventions for migrants?
Health literacy (HL) means the knowledge, motivation and competencies (e.g. reading and writing abilities) that people need to find, understand, evaluate and use health information. Migrants are at risk for difficulties in HL (e.g. when they don't know the country's health system well).
'Generic' HL means that people can find, understand and use general health information to make health decisions. 'Disease‐specific' HL means that people can find, understand and use information about a certain disease or that they know about the symptoms of a disease or understand treatment options.
Key messages
We have moderate to low confidence in these findings that some HL interventions have small to moderate positive effects on migrants' HL. This means that these interventions can help people improve their knowledge, recognition and understanding of medical terms, or use of health information.
There is a need for larger, well‐designed studies that measure long‐term effects of HL interventions in migrant women and men.
What did we want to find out?
Our main goal was to find out whether HL interventions can help migrants to improve their HL. We also wanted to find out if migrant women or migrant men benefit more from these interventions.
What did we do?
We searched for studies that looked at interventions for improving HL in migrants. These interventions were compared with 1) no HL intervention (e.g. standard care), 2) written information on the same health topic (e.g. brief brochure), 3) an unrelated HL intervention (participants received a similar intervention, but the information was on a different health topic), or 4) another HL intervention (participants received a different intervention, but the information was on the same health topic).
The included studies measured HL either as an overall concept or only components of it (e.g. understanding health information). We compared and summarised the results of studies and rated our confidence in the evidence, based on factors like study methods.
What did we find?
We found 34 studies that involved 8249 migrants with a wide range of health conditions. All studies were conducted in high‐income countries. All interventions were adapted to the participants' culture, language and literacy level. None of the studies reported that HL interventions cause harm, but only two studies reported possible harms (anxiety). Many studies reported short‐term results (up to six weeks after the intervention ended, the focus in this summary). There were also several findings at later time points (presented in the main review).
Compared with no or unrelated HL intervention:
Self‐management programmes (SMP)(long‐term programmes including group education and personal support) probably improve self‐efficacy in managing one's disease slightly (which means that the participants had higher beliefs in their abilities to act on health information). SMP may also improve disease‐specific HL and may slightly improve health behaviour, but may have little effect on knowledge or self‐rated health. We do not know if SMP improves quality of life (QoL) or health service use.
HL skills building courses(group education in which participants, for example, learn what to do to prevent a disease) may improve knowledge and generic HL, but they may have little effect on depression literacy or health behaviour. We do not know if they improve QoL, health outcomes, health service use or self‐efficacy.
Audio‐/visual education without personal feedback (AVE)(including video education, interactive computer education or printed educational photo stories)probably improves depression literacy and health service use. AVE may improve self‐efficacy and slightly improve knowledge and intention to seek depression treatment, but may have little effect on health behaviour or depression. No study reported on QoL.
Adapted medical instructions(medical instructions that use simple language, illustrations or pictures) may improve understanding health information, but may have little effect on medication adherence. No study reported on QoL, health outcomes, knowledge, health service use or self‐efficacy.
Compared with written information:
SMP probably improves print literacy and self‐efficacy, and health numeracy slightly. SMP may improve any disease‐specific HL, knowledge and some health behaviours, but may have little effect on health information appraisal. We do not know whether SMP improves QoL, health outcomes or health service use.
AVEprobably has little effect on diabetes HL but probably improves information appraisal and application. AVE may slightly improve knowledge. No study reported on QoL, depression, health behaviour, self‐efficacy or health service use.
AVE compared with another AVE:
We are uncertain if narrative videos are better than factual knowledge videos as the evidence was very uncertain.
Do migrant women or men benefit differently from HL interventions?
Migrant women's diabetes HL may improve slightly more than that of migrant men after receiving AVE. For other comparisons and outcomes we either did not find evidence, or we are uncertain about the results.
What are the limitations of the evidence?
It is possible that people in some studies knew which treatment they were getting. In addition, studies were done in different migrant groups, coming from different regions and with different health conditions, and some studies included few people.
How up‐to‐date is this evidence?
This review is up‐to‐date to 2 February 2022.
Summary of findings
Summary of findings 1. Culturally and literacy adapted self‐management programme versus no health literacy intervention.
| Culturally and literacy adapted self‐management programme versus no health literacy intervention | ||||||
| Patient or population: migrants Setting: all settings Intervention: culturally and literacy adapted self‐management programme (programme length: 6 to 12 months) Comparison: no health literacy intervention (usual care, placebo intervention or wait‐list control) | ||||||
| Outcome category – outcome(s)* | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no health literacy intervention | Risk with self‐management programme | |||||
|
Health literacy – Disease‐specific health literacy Assessed with:
Higher scores are better Time point: short‐term (immediately post‐intervention)** |
One RCT reported that the change from baseline score for understanding of HIV terms was 4.25 points higher (1.32 higher to 7.18 higher) and recognition of HIV terms was 3.32 points higher (1.28 higher to 5.36 higher) in the intervention group. | — | 69 (1 RCT) |
⊕⊕⊝⊝ Lowa | Self‐management programmes compared to no health literacy intervention may improve disease‐specific health literacy (HIV health literacy) immediately post‐intervention. | |
| Quality of life | — | — | — | — | The effect of self‐management programmes is unknown as there was no direct evidence identified. | |
|
Health‐related knowledge – Multiple measures used: (1)Diabetes knowledge
(2) HIV knowledge
Time point: short‐term (immediately post‐intervention) |
(1) Diabetes knowledge One RCT (N = 252) reported that the mean diabetes knowledge score was 5.6 points higher (range = 2.2 higher to 9.0 higher) in the intervention group. The mean knowledge score in the control group was 68; P = 0.001. (2) HIV knowledge One RCT (N = 69) reported that the mean HIV global disease/treatment knowledge was 1.18% lower (9.23 lower to 6.87 higher) in the intervention group, but the CI encompassed values indicating both an improvement and a reduction in knowledge. The same study reported that the mean knowledge of the risk of getting sicker when stopping taking one's HIV medication was higher in the intervention group: 0.33 higher (‐0.01 lower to 0.67 higher) but the CI also encompassed values indicating a null effect. |
— | 321 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | Self‐management programmes compared to no health literacy intervention may have little or no effect on health‐related knowledge immediately post‐intervention. One cluster RCT (n = 230) was missing information about participant numbers but reported that the intervention increased breast cancer‐related knowledge (MD 0.5, P < 0.0001) at 6 months post test (very low certainty)d,e One other RCT (N = 194) was missing data about the control group but reported that knowledge about heart health increased in the intervention group 3 months post‐intervention.4 |
|
|
Health outcome – Self‐reported health status Assessed with:
Higher score is better Time point: short‐term (immediately post‐intervention) |
One RCT reported that the mean subjective health status in the past week was 0.38 points higher (0.13 lower to 0.89 higher) in the intervention group immediately post‐intervention, but the CI encompassed both an improvement and a reduction in subjective health status. | — | 69 (1 RCT) | ⊕⊕⊝⊝ Lowf | Self‐management programmes compared to no health literacy intervention may have little or no effect on subjective health status immediately post‐intervention. | |
|
Health behaviour5– Time point a: short‐term (immediately post‐intervention) Multiple outcomes assessed and multiple measures used: (1) Blood glucose self‐monitoring
(2) Adherence to HIV medication
(3) Physical activity Assessed with:
Higher scores are better |
Time point a: short‐term (1) Blood glucose self‐monitoring: One RCT (n = 252) reported higher odds of self‐reported blood glucose self‐monitoring in the intervention group immediately post‐intervention (RR 1.30, 95% CI 1.11 to 1.52) (2) Adherence to HIV medication: One RCT (n = 69) reported that the proportion of participants who reported > 95% adherence to HIV medication within the last 4 days was higher in the intervention group immediately post‐intervention (IG change score: 1.71%, CG change score: ‐4.85%) (3) Physical activity: One RCT (n = 193) reported that the mean average daily steps was higher in the intervention group, but the CI encompassed both an improvement and a reduction in physical activity immediately post‐intervention (MD 289 daily steps higher, 95% CI 601.41 lower to 1179.41 higher) |
— | 514 (3 RCTs) | ⊕⊕⊝⊝ Lowg,h | Self‐management programmes compared to no health literacy interventions may slightly improve any health behaviour immediately post‐intervention, but outcome measures and effects appear variable. One cluster‐RCT was missing information about the number of participants randomised to each study group, as well as the intensity and length of the programme. In addition, data were not reported in a way in which they could be extracted for meta‐analysis. |
|
|
Self‐efficacy – Self‐efficacy to manage one's disease Multiple measures used:
Higher score is better Time point: short‐term (immediately post‐intervention) |
— | The mean score in the intervention group was 0.28 standard deviations higher (0.06 higher to 0.50 higher) | — | 333 (2 RCTs) | ⊕⊕⊕⊝ Moderateg | Self‐management programmes compared to no health literacy interventions probably improve self‐efficacy to manage one's disease slightly. |
| Health service use – not measured | — | — | — | — | — | The effect of self‐management programmes on health service use is unknown as there was no direct evidence identified. |
| Adverse events – not reported | — | — | — | — | — | The effect of self‐management programmes on adverse events is unknown as there was no direct evidence identified. |
| *More detail on scoring and direction for each outcome measure is provided in Table 2; Table 3; Table 4; Table 5; Table 6; **Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: from 6 weeks up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. ACTG: Adult AIDS Clinical Trials Group; ADKnowl: Audit of Diabetes Knowledge; CG: control group; CI: confidence interval; IG: intervention group; LSESLD: Lifestyle Self‐Efficacy Scale for Latinos with Diabetes; MD: mean difference; n.r.: not reported; RCT: randomised controlled trial; RR: risk ratio; REALM: Rapid Estimate of Adult Literacy in Medicine | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Results for understanding HIV terms and recognition of HIV terms were reported separately in the study, and only change scores were reported.
2The score range was taken from publications cited by the study authors (Rosal 2003; Speight 2001), as it was not reported in the published trial report (Rosal 2011).
3To improve the interpretation of results, we transformed the original scale, which had negative values indicating better performance, into a positive scale with higher values indicating better performance.
4GRADE was not used due to missing control group data.
5All outcomes except physical activity were assessed via self‐report.
aDowngraded by ‐2 for imprecision: result was based on a single study with a small sample size (less than 100) and wide CI.
bDowngraded by ‐1 for imprecision: narrative synthesis conducted and the CI of one study encompassed values indicating both an improvement and a worsening in the outcome. In addition, the sample size was small.
cDowngraded by ‐1 for inconsistency: CI of one study indicated a small improvement in the outcome. The other study reported two measures of knowledge; results of the first measure indicated a reduction in knowledge with a CI encompassing values suggesting both an improvement and a worsening. The second measure indicated an improvement in knowledge with a CI encompassing an improvement and a null effect (lower CI ‐0.01).
dDowngraded by ‐2 for risk of bias: unclear risk of bias in several domains including random sequence generation and allocation concealment.
eDowngraded by ‐1 for imprecision: missing information about the number of participants in the intervention and control groups; the length and intensity of the programme and effect measures were not reported per study group.
fDowngraded by ‐2 for imprecision: result was based on a single study with a small sample size (less than 100) and the CI encompassed values indicating both an improvement and a worsening.
gDowngraded by ‐1 for risk of bias: high risk of bias for blinding in 2 out of 3 studies, unclear risk of bias for allocation concealment in one study.
hDowngraded by ‐1 for inconsistency: Two studies indicated an improvement in health behaviour, but the CI of one study indicated a worsening or an improvement in physical activity.
1. Outcome category: (disease‐specific) health literacy.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm(s) Mean (SD)* |
Control arm(s) Mean (SD) |
Notes |
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | |||||||
| van Servellen 2005 | HIV | HIV health literacy Print literacy (recognition of HIV terms): modified REALM, 0 to 24, higher score is better Functional health literacy (understanding HIV terms): participants had to explain HIV‐relevant terms, 0 to 24, higher score is better |
IG: 34 CG: 35 |
6 months after randomisation (immediately post‐intervention) | 4.66 (4.80) (recognition) |
1.34 (3.76) (recognition) |
Change scores are reported Intervention group: P < 0.001 (both time points) |
| 6.16 (7.97) (understanding) |
1.91 (3.60) (understanding) |
||||||
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Han 2017 | Breast/cervical cancer | Cancer screening health literacy AHL‐C, 52 items, 0 to 52, higher score is better |
IG: 278 CG: 282 |
6 months after randomisation (immediately post‐intervention) | 32.1 (12.7) | 27.2 (13.0) | Cluster‐RCT; data have been re‐analysed for meta‐analysis using the appropriate unit of analysis with the use of the ICC reported by Han 2017 (see Analysis 2.3; Analysis 2.4; Analysis 2.6) |
| Kaur 2019 | Oral health | Oral health literacy TS‐REALD, 27 to 73, higher score is better |
IG: 70 CG: 70 |
3 months after randomisation (immediately post‐intervention) | 6.51 (3.85) | 1.41 (3.69) | Change scores, calculated from reported linear mixed model analysis. MD 5.10 (95% CI 3.85 to 6.34) Group x time P < 0.0001 |
| Kim 2014 | High blood pressure | HBP health literacy HBP health literacy scale, 0 to 43, higher score is better |
IG: 184 CG: 185 |
12 months after randomisation (immediately post‐intervention) | 28.2 (12.1) | 24.9 (13.7) | Cluster‐RCT; data have been re‐analysed for meta‐analysis using the appropriate unit of analysis with the use of the ICC reported by Han 2017 (see Analysis 2.3; Analysis 2.4; Analysis 2.6) |
| 18 months after randomisation (6‐month follow‐up) | 29.4 (11.4) | 25.3 (13.4) | |||||
| Kim 2020 | Type 2 diabetes | Print literacy REALM, 0 to 66, higher score is better |
IG: 105 CG: 104 |
12 months after randomisation (immediately post‐intervention) | 40.5 (SE 2.2) | 31.5 (SE 2.2) | P < 0.01 (all time points) |
| Diabetes‐specific print literacy DM‐REALM, 0 to 82, higher score is better |
12 months after randomisation (immediately post‐intervention) | 62.9 (SE 2.1) | 50.8 (SE 2.7) | P < 0.001 (all time points) | |||
| Functional health literacy TOFHLA, 0 to 7, higher score is better |
12 months after randomisation (immediately post‐intervention) | 4.9 (SE 0.2) | 4.4 (SE 0.3) | No difference | |||
| Health numeracy NVS, 0 to 6, higher score is better |
12 months after randomisation (immediately post‐intervention) | 3.1 (SE 0.2) | 2.4 (SE 0.2) | P < 0.05 | |||
| 3 Culturally adapted health literacy skills building course vs no/unrelated health literacy intervention | |||||||
| Otilingam 2015 | Nutrition/heart and brain health | Health numeracy NVS, 0 to 6, higher score is better |
IG 1: 29 IG 2: 29 CG 1: 16 CG 2: 18 |
Immediately post‐intervention | IG 1: 2.59 (1.92) IG 2: 2.34 (1.99) |
CG: 1.00 (1.63) CG 2: 1.61 (1.79) |
Both IG and both CG were combined for meta‐analysis (see Analysis 3.1). CG 2 was assessed immediately post‐intervention only. Group x time P = 0.0103 |
| At 1‐month follow‐up | IG 1: 2.59 (1.76) IG 2: 2.55 (1.70) Combined: 2.57 (1.72) |
CG 1: 1.38 (1.54) | |||||
| Soto Mas 2018 | Cardiovascular health | Functional health literacy TOFHLA, 0 to 100, higher score is better |
IG: 77 CG: 78 |
Immediately post‐intervention | 72.8 Mean change post‐pre (95% CI): 12.9 (10.4 to 15.3) |
73.7 Mean change post‐pre (95% CI): 8.2 (5.5 to 10.9) |
P = 0.012 |
| 6 weeks after first session | — | — | |||||
| Wong 2020 | Mental health (depression) | Depression literacy D‐Lit, 0 to 22, higher score is better |
IG: 18 CG: 19 |
Immediately post‐intervention | 13.06 (2.10) | 12.89 (2.40) | P = 0.36 |
| At 2‐month follow‐up | 13.38 (2.12) (combined sample) |
— | |||||
| 5 Culturally and literacy adapted media education without personal feedback vs no health literacy intervention | |||||||
| Kiropoulos 2011 | Mental health (depression) | Depression literacy D‐Lit, 0 to 22, higher score is better |
IG: 110 CG: 92 |
Immediately post‐intervention | 17.43 (3.99) | 8.03 (4.33) | P < 0.001 |
| At 1‐week follow‐up | 16.84 (3.58) | 8.22 (4.33) | Pre‐intervention measure of the variable as a covariate P < 0.001 Post‐intervention measure of the variable as a covariate P < 0.01 |
||||
| 6 Culturally and literacy adapted media intervention without personal feedback vs literacy adapted written information | |||||||
| Calderón 2014 | Type 2 diabetes | Diabetes literacy DHLS, 37 items on type 2 diabetes knowledge (21 items) and knowledge application and cultural perceptions about diabetes management (16 items) |
IG: 118 CG: 122 |
Immediately post‐intervention | 0.55 (0.08) | 0.53 (0.09) | Unadjusted values were obtained from study authors |
* Unadjusted mean (SD) if not otherwise reported.
AHL‐C: Assessment of Health Literacy in Cancer Screening; CG: control group; CI: confidence interval; DHLS: Diabetes Health Literacy Survey; D‐Lit: Depression Literacy Questionnaire; DM‐REALM: Diabetes Mellitus‐Rapid Estimate of Adult Literacy in Medicine; HBP: high blood pressure; IG: intervention group; MD: mean difference; NVS: newest vital sign; RCT: randomised controlled trial; REALM: Rapid Estimate of Adult Literacy in Medicine; SD: standard deviation; SE: standard error; TOFHLA: Test of Functional Health Literacy in Adults; TS‐REALD: Two Stage Rapid Estimate of Adult Literacy in Dentistry
2.3. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 3: Any disease‐specific health literacy (short‐term: immediately post‐intervention) ‐ all studies
2.4. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 4: Any disease‐specific health literacy (short‐term: immediately post‐intervention ‐ by subgroup length of programme)
2.6. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 6: Any disease‐specific health literacy (short‐term: immediately post‐intervention) ‐ without Kaur 2019
3.1. Analysis.

Comparison 3: Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention, Outcome 1: Any generic health literacy (short‐term: up to 1 month post‐intervention)
2. Outcome category: health‐related knowledge.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm(s) Mean (SD)* |
Control arm(s) Mean (SD)* |
Notes |
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | |||||||
| Bloom 2014 | Breast health/breast cancer | Not reported | N: 230 | 6 months post‐intervention | — | — | MD 0.5 (P < 0.0001) Cluster‐RCT; "GEE were used to account for clustering (sample and analysis)" (Bloom 2014) Increased knowledge did not increase mammography |
| Koniak‐Griffin 2015 | Cardiovascular disease | Heart knowledge questionnaire, adapted from a previous survey by Mosca et al (2004) (10 items, true/false format, 0 to 10, higher score is better) |
IG: 98 CG: 95 |
6 months after randomisation (immediately post‐intervention) |
7.9 (2.6) | Not reported | — |
| IG: 100 CG: 94 |
9 months after randomisation (at 3‐month follow‐up) |
9.4 (1.9) | |||||
| Rosal 2011 | Type 2 diabetes | ADKnowl, adapted version (23 item‐sets (104 items), 0 to 104, higher score is better) |
IG: 124 CG: 128 |
12 months after randomisation (immediately post‐intervention) | 0.089 (range ‐0.065 to 0.113) | 0.033 (range 0.009 to 0.057) | Intervention effect 0.056 (0.022 to 0.090) P = 0.001 |
| van Servellen 2005 | HIV | (1) HIV Illness and Treatment Knowledge and Misconceptions Scale (17 items, 0 to 17, higher score is better) (2) Knowledge of risk of getting sicker 1 item, 1 = very high risk to 4 = nonexistent risk, lower score is better |
IG: 34 CG: 35 |
6 months after randomisation (immediately post‐intervention) | (1) 1.20 (3.19) (2) ‐0.24 (0.78) |
(1) 1.40 (2.59) (2) 0.09 (0.67) |
Change scores are reported To improve the interpretation of results, the original scale has been transformed into a positive scale with higher values indicating better performance (see Analysis 1.4) |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Han 2017 | Cervical/breast cancer | Breast Cancer Knowledge Test (0 to 18, higher score is better) |
IG: 278 CG: 282 |
6 months after randomisation (immediately post‐intervention) | 11.0 (3.9) | 10.4 (3.8) | Cluster‐RCT; data have been re‐analysed for meta‐analyses using the appropriate unit of analysis with the use of the ICC reported by Han 2017. In addition, combined scores for breast cancer knowledge and cervical cancer knowledge were calculated (see Analysis 2.10; Analysis 2.11). Estimated MD 0.7 (95% CI ‐0.1 to 1.6) MD estimated from linear mixed‐effects models adjusted for baseline knowledge, age, insurance, English proficiency, years of US residence, years of education, employment and family history of breast cancer. |
| Cervical Cancer Knowledge Test (0 to 20, higher score is better) |
5.6 (2.4) | 5.3 (2.6) | Estimated MD –0.1 (95% CI –0.3 to 0.1) | ||||
| Kaur 2019 | Oral health | Questionnaire on oral self‐care knowledge and oral self‐care behaviour (0 to 15, higher score is better) |
IG: 70 CG: 70 |
3 months after randomisation | 4.389 (2.15) | 0.82 (2.013) (95% CI 0.34 to 1.31) | MD 3.57 (2.88 to 4.26) Group x time P < 0.0001 Mean (SD) was calculated from reported linear mixed model analysis |
| Kim 2009 | Type 2 diabetes | DKT (14 items, 0 to 14 (general test, knowledge I), 9 items insulin subscale (knowledge II)1, higher score is better) |
IG: 40 CG: 39 |
30 weeks after randomisation | Knowledge (I) 2.4 (2.3) Knowledge (II) 0.3 (3.7)1 |
Knowledge (I) 0.7 (2.4) Knowledge (II) 0.4 (0.8)1 |
Change scores are reported Knowledge (I) P = 0.00 Knowledge (II) P = 0.27 |
| Kim 2014 | High blood pressure | HBP knowledge questionnaire (0 to 26, higher score is better) |
IG: 184 CG: 185 |
12 months after randomisation | 20.8 (2.7) | 19.3 (3.7) | Cluster‐RCT; data have been re‐analysed for meta‐analysis using the appropriate unit of analysis with the use of the ICC reported by Han 2017. Group x time P = 0.001 (see Analysis 2.10; Analysis 2.11; Analysis 2.14; Analysis 2.13) |
| 18 months after randomisation (6‐month follow‐up) | 20.8 (2.8) | 20.1 (3.2) | |||||
| Kim 2020 | Type 2 diabetes | DKT (14 items, 0 to 14 (general test), 9 items insulin subscale (results not reported), higher score is better) |
IG: 105 CG: 104 |
12 months after randomisation | 10.3 (SE 0.2) | 8.3 (SE 0.3) | Group P < 0.001 |
| Rosal 2005 | Type 2 diabetes | ADKnowl, adapted version (23 item‐sets (104 items), 0 to 104), higher score is better |
IG: 15 CG: 10 |
3 months after randomisation (immediately post‐intervention) | 0.05 (0.15) | ‐0.02 (0.11) | Change scores are reported Group x time P = 0.27 |
| 6 months after randomisation (4.5 months post‐intervention) | 0.05 (0.13) | ‐0.03 (0.08) | |||||
| 3 Culturally adapted health literacy skills building course vs no/unrelated health literacy intervention | |||||||
| Elder 1998 | Nutrition/cardiovascular health | Nutrition knowledge test (0 to 12, higher score is better) |
IG: 134 CG: 157 |
3 months after randomisation (immediately post‐intervention) | 6.76 | 6.04 | Cluster‐RCT; unadjusted values are reported Group x time P ≤ 0.001 |
| At 6‐month follow‐up | 6.90 | 6.11 | |||||
| Otilingam 2015 | Nutrition/heart and brain health | US Department of Agriculture's Diet and Health Knowledge Survey (0 to 9, higher score is better) |
IG 1: 32 IG 2: 33 CG 1: 16 CG 2: 18 |
Immediately post‐intervention | IG 1: 6.86 (1.27) IG 2: 7.03 (0.91) Combined: 6.95 (1.10) |
CG 1: 5.94 (1.12) CG 2: 6.22 (0.94) Combined: 6.09 (1.02) |
Group x time P = 0.0293 (combined IGs vs CG 1) Both IGs and CGs were combined for meta‐analyses (see Analysis 3.3) CG 2 was assessed post‐test only |
| IG 1: 29 IG 2: 29 CG 1: 16 CG 2: 18 |
At 1‐month follow‐up | IG 1: 6.72 (1.33) IG 2: 6.66 (1.11) IG 1, 2*: 6.69 (1.21) |
CG 1: 5.56 (1.71) | ||||
| Taylor 2011 | Hepatitis B prevention, no specific health problem of participants reported | Questionnaire (0 to 5, higher score is better) |
IG: 80 CG: 100 |
At 6‐month follow‐up | 3.68 (1.12) | 2.87 (1.38) | Cluster‐RCT; data have been re‐analysed for meta‐analysis using the appropriate unit of analysis with the use of the ICC reported by Han 2017. |
| Immigrants are more likely
to be infected with HBV
AOR 2.12 (1.12 to 4.03) HBV can be spread during childbirth AOR 2.10 (0.96 to 4.62) HBV can be spread during sexual intercourse AOR 2.58 (1.29 to 5.15) HBV can be spread by sharing razors AOR 5.42 (1.91 to 15.39) HBV infection can cause liver cancer AOR 2.08 (1.08 to 4.02 |
AOR estimated through GEE models were used to account for clustering; adjusted for ESL organisation, class time, country of origin, years since immigration, gender, age group, years of education and marital status | ||||||
| Tong 2017 | Colorectal cancer | Questionnaire (0 to 5, higher score is better) |
IG: 161 CG: 168 |
6 months after first session (at 3‐month follow‐up) | Knowledge of colon polyps: 23.6% to 78.3%, MD 54.7% Screening start age at 50 years: 14.3% to 36.0%, MD 21.7% FOBT yearly: 10.6% to 38.5%, MD 27.9% Sigmoidoscopy every 5 years: 3.7% to 24.2%, MD 20.5% Colonoscopy every 10 years: 2.5% to 20.5%, MD 18% |
Knowledge of colon polyps: 19.6% to 37.5%, MD 17.9% Screening start age at 50 years: 11.9% to 14.3%, MD 2.4% FOBT yearly: 11.9% to 17.3%, 5.4% Sigmoidoscopy every 5 years: 1.2% to 4.2%, MD 3% Colonoscopy every 10 years: 3.6% to 6.5%, MD 2.9% |
MD 36.8%, P < 0.0001 MD 19.3%, P = 0.0056 MD 22.5%, P = 0.0001 MD 17.5%, P < 0.0001 MD 15.1%, P = 0.012 Cluster‐RCT. No composite score reported. Authors state that GEE models were used to account for clustering. "For every point increase on the knowledge score (0‐5), the odds of ever‐screening and being up to date with screening were significantly increased, supporting knowledge as a mediator of the intervention effect." (Tong 2017 |
| Wong 2020 | Mental health (depression) | CBT‐Q (0 to 9, higher score is better) |
IG: 18 CG: 19 |
Immediately post‐intervention | 5.06 (0.10) | 4.33 (1.24) | P = 0.07 |
| At 2‐month follow‐up | — | ||||||
| 4 Culturally and literacy adapted telephone education vs unrelated culturally and literacy adapted telephone education | |||||||
| Lepore 2012 | Prostate cancer screening | Questionnaire (0 to 14, higher score is better) |
IG: 215 CG: 216 |
Approx. 7 months post‐intervention | 61.6 (SE 0.009) | 54.7 (SE 0.009) | P < 0.001 Adjusted for education, any PSA claim prior to pretest, and percent correct on knowledge index at pretest |
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| DeCamp 2020 | Child health | Questionnaire (0 to 5, higher score is better) |
IG: 72 CG: 63 |
10 to 13 months after randomisation (immediately to 3 months post‐intervention) | 0.67 (0.15) | 0.52 (0.15) | Change scores are reported P = 0.52 |
| Hernandez 2013 | Depression | Depression Knowledge Scale (0 to 17, higher score is better) | IG: 72 CG: 64 |
Immediately post‐intervention | 2.44 (2.24) | 0.02 (1.79) | Change scores are reported |
| Thompson 2012 | Child nutrition and feeding | Questionnaire (0 to 19, higher score is better) |
IG: 80 CG: 78 |
Immediately post‐intervention | 17.25 (1.7) | 13.7 (2.1) | P < 0.001 |
| 90.8 (9) | 72.3 (11.2) | ||||||
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Gwede 2019 | Colorectal cancer | Awareness of colorectal cancer and screening tests (Questionnaire based on NCI’s Health Information National Trends Survey and on literature, 0 to 11, higher score is better) |
IG: 32 CG: 27 |
At 3‐month follow‐up | 7.9 (2.0) | 6.4 (2.2) | — |
| Payán 2020 | Breast cancer | Questionnaire (0 to 16, higher score is better) |
IG 1: 79 (Cuidarse brochure) IG 2: 79 (Cuidarse brochure, CHW delivered) CG: 82 (standard brochure) |
Immediately post‐intervention | IG 1: 11.7 (2.7) IG 2: 11.5 (2.6) IG 1, 2: 11.6 (2.64) |
CG: 11.5 (3.0) | 10 to 13 months after randomisation; and IGs were combined for meta‐analysis (see, Analysis 6.6; Analysis 6.7; Analysis 6.8; Analysis 6.9) |
| IG 1: 67 IG 2: 61 CG: 65 |
At 3‐month follow‐up | IG 1: 10.3 (3.1) IG 2: 10.2 (2.8) IG 1, 2: 10.25 (2.95) |
CG: 10.7 (2.7) | ||||
| Poureslami 2016a | Asthma | Functional knowledge of asthma symptoms, triggers and factors that could make asthma worse (5‐point Likert scale, range not reported, higher score is better) |
Group 1: 22 Group 2: 21 Group 3: 20 Group 4: 22 |
At 3‐month follow‐up | Knowledge of asthma symptoms Group 1: ‐0.19, 95% CI ‐0.78 to 0.40 Group 2: 0.33, 95% CI ‐0.30 to 0.97 Group 3: 0.88, 95% CI ‐0.02 to 1.79 Knowledge of asthma triggers Group 1: 0.50, 95% CI ‐0.62 to 1.62 Group 2: 1.29, 95% CI ‐0.03 to 2.54) Group 3: 0.29, 95% CI ‐0.99 to 1.58 Knowledge of triggers that could make asthma worse Group 1: ‐0.18, 95% CI ‐2.37 to 2.01 Group 2: 0.86, 95% CI ‐0.51 to 2.22 Group 3: 0.35, 95% CI ‐1.12 to 1.94 |
Knowledge of asthma symptoms Group 4: 0.17, 95% CI ‐0.62 to 0.95 Knowledge of asthma triggers Group 4: 1.22, 95% CI 0.38 to 2.07 Knowledge of triggers that could make asthma worse Group 4: 0.45, 95% CI ‐1.41 to 2.31 |
6‐month assessment not reported No composite score reported, data were not combined as no score range was reported; the scale could not be standardised on a scale ranging from 0 to 100 Results reported are adjusted for age, gender, educational level and ethnicity Data have been extracted from the secondary reference (see Poureslami 2016a for all trial reports related to this study) |
| Poureslami 2016b | COPD | "Some" questions of BCKQ | — | ||||
| Unger 2013 | Depression | Depression Knowledge Scale (0 to 17, higher score is better) | IG: 69 CG: 70 |
Immediately post‐intervention | 2.37 (SE 0.32) | 0.86 (SE=0.27) | |
| 1‐month follow‐up | t = 5.09, P < 0.05 | t = 2.64, P < 0.05 | "[T]he data collectors reported that several students shared their photonovel with students in the text pamphlet group after the posttest." (Unger 2013, p. 405) | ||||
| Valdez 2015 | Cervical cancer | Questionnaire (0 to 12, higher score is better) |
IG: 290 CG: 318 |
At 1‐month follow‐up | 8.9 (1.6) | 7.1 (2.0) | P < 0.0001 |
| Valdez 2018 | Cervical Cancer | Questionnaire (0 to 5, higher score is better) |
IG: 383 CG: 344 |
At 6‐month follow‐up | 3.7 (1.6) | 3.1 (1.4) | P < 0.0001 |
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally and literacy adapted audio‐/visual education without personal feedback | |||||||
| Ochoa 2020 | Cervical cancer | Questionnaire (0 to 8, higher score is better) |
IG: 61 CG: 48 |
At 2‐week follow‐up | 5.10 (1.45) | 4.44 (1.15) | P = 0.011 |
| At 6‐month follow‐up | 5.38 (1.27) | 5.29 (1.17) | P = 0.718 | ||||
| Poureslami 2016a | Asthma | Functional knowledge of asthma symptoms, triggers, and factors that could make asthma worse (5‐point Likert scale, range not reported, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative, peer‐led video): 21 |
At 3‐month follow‐up | Knowledge of asthma symptoms Group 1: ‐0.19, 95% CI ‐0.78 to 0.40 Knowledge of asthma triggers Group 1: 0.50, 95% CI ‐0.62 to 1.62 Knowledge of triggers that could make asthma worse Group 1: ‐0.18, 95% CI ‐2.37 to 2.01 |
Knowledge of asthma symptoms Group 2: 0.33, 95% CI ‐0.30 to 0.97 Knowledge of asthma triggers Group 2: 1.29, 95% CI ‐0.03 to 2.54) Knowledge of triggers that could make asthma worse Group 2: 0.86, 95% CI ‐0.51 to 2.22 |
6‐month assessment not reported No composite score reported Results are adjusted for age, gender, educational level and ethnicity |
| Poureslami 2016b | COPD | "Some" questions from BCKQ | A 3‐month follow‐up | — | |||
*Unadjusted mean (SD) if not otherwise reported.
1 Assessed only for those injecting insulin (intervention, n = 5; control, n = 7). Data were not included in the meta‐analyses.
ADKnowl: Audit of Diabetes Knowledge; AOR: adjusted odds ratio; BCKQ: Bristol COPD Knowledge Questionnaire; CBT: cognitive behavioural therapy; CBT‐Q: Knowledge of CBT questionnaire; CG: control group; CI: confidence interval; COPD: chronic obstructive pulmonary disease; DKT: Diabetes Knowledge Test; ESL: English as a second language; GEE: generalised estimating equations; HBP: high blood pressure; HBV: hepatitis B virus; IG: intervention group; NCI: National Cancer Institute; OR: odds ratio; PSA: prostate‐specific antigen; SD: standard deviation; SE: standard error
1.4. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 4: Health‐related knowledge: HIV knowledge, risk of getting sicker (short‐term: immediately post‐intervention)
2.10. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 10: Any health‐related knowledge, 0 to 100 (short‐term: immediately post‐intervention) ‐ all studies
2.11. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 11: Any health‐related knowledge, 0 to 100 (short‐term: immediately post‐intervention) ‐ by subgroup length of programme
2.14. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 14: Any health‐related knowledge, 0 to 100 (medium‐term: up to 6 months post‐intervention)
2.13. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 13: Any health‐related knowledge, 0 to 100 (short‐term: immediately post‐intervention) ‐ without Kaur 2019
3.3. Analysis.

Comparison 3: Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention, Outcome 3: Any health‐related knowledge, 0 to 100 (short‐term: up to 1 month post‐intervention) ‐ all studies
6.6. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 6: Any health‐related knowledge, 0 to 100 (short‐term: up to 1 month post‐intervention) ‐ all studies
6.7. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 7: Any health‐related knowledge, 0 to 100 (short‐term: up to 1 month post‐intervention) ‐ by subgroup audiovisual (multimedia)/visual (print only)
6.8. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 8: Any health‐related knowledge, 0 to 100 (medium‐term: 3 to 6 months post‐intervention) ‐ all studies
6.9. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 9: Any health‐related knowledge, 0 to 100 (medium‐term: 3 to 6 months post‐intervention) ‐ by subgroup audiovisual (multimedia)/visual (print only)
3. Outcome category: health outcomes.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD)* |
Notes |
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | |||||||
| van Servellen 2005 | HIV | Self‐reported health status (1 item assessing general health status in the past week) |
IG: 34 CG: 35 |
6 months after randomisation (immediately post‐intervention) | 0.47 (1.21) | 0.09 (0.95) | Change scores are reported No differences between study groups |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Kim 2009 | Depression | KDSKA (21 items with 4 subscales, 0 to 75, lower score is better) |
IG: 40 CG: 39 |
30 weeks after randomisation | ‐0.5 (4.5) | ‐1.0 (4.3) | P = 0.70 |
| Kim 2014 | Depression | PHQ‐9 (9 items, 0 to 27, lower score is better) |
IG: 184 CG: 185 |
12 months after randomisation | 2.1 (2.9) | 3.0 (3.0) | Group x time P = 0.04 |
| 18 months after randomisation (at 6‐month follow‐up) | 2.5 (3.3) | 2.9 (3.3) | |||||
| Kim 2020 | Depression | PHQ‐9K (9 items, 0 to 27, lower score is better) |
IG: 105 CG: 104 |
12 months after randomisation | 4.8 (SE 0.5) | 4.1 (SE 0.4) | — |
| Rosal 2005 | Depression | CES‐D (20 items, 0 to 60, lower score is better) |
IG: 15 CG: 10 |
3 months after randomisation (immediately post‐intervention) | ‐3.7 (7.6) | 7.6 (8.9) | Change scores are reported Group x time P = 0.03 |
| 6 months after randomisation (4.5 months post‐intervention) | 1.4 (9.8) | 9.57 (11.0) | |||||
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| DeCamp 2020 | (Parent) depression | PHQ‐8 (8 items, 0 to 24, lower score is better) |
IG: 72 CG: 63 |
Immediately to 3 months post‐intervention (10 to 13 months after randomisation) | 0.68 (3.82) | 0.70 (4.18) | P = 0.97 |
| Kiropoulos 2011 | Depression | BDI‐II (0 to 63, lower score is better) |
IG: 110 CG: 92 |
Immediately post‐intervention | 7.26 (7.64) | 8.13 (7.53) | P = 0.87 |
| 1 week post‐intervention | 6.36 (6.60) | 8.26 (7.88) | P = 0.181 P = 0.192 |
||||
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Sudore 2018 | Depression | PHQ‐8 (0 to 24) referred to as adverse events, lower score is better |
IG: 219 CG: 226 |
At 12‐month follow‐up | 3.9 (95% CI 3.3 to 4.4) | 4.5 (95% CI 4.0 to 5.1) | P = 0.10 Adjusted for baseline depression and anxiety scores |
*Unadjusted mean (SD) if not otherwise reported.
1ANCOVA employed the pre‐intervention measure of the variable as a covariate.
2ANCOVA employed the postintervention measure of the variable as a covariate.
BDI‐II: Beck Depression Inventory‐II; CES‐D: Center for Epidemiological Studies‐Depression Scale; CG: control group; IG: intervention group; KDSKA: Kim Depression Scale for Korean Americans; PHQ‐8: Patient Health Questionnaire‐8; PHQ‐9: Patient Health Questionnaire‐9; PHQ‐9K: Korean version of PHQ‐9; SD: standard deviation; SE: standard error
4. Outcome category: health behaviour.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD) |
Notes |
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | |||||||
| Bloom 2014 | Breast health/breast cancer | Self‐report, mammography | N: 230 | 6 months after randomisation (no further details) | 56% | 10% | P < 0.0001 Cluster‐RCT; authors state that general linear models with GEE used to account for clustering (sample and analysis) |
| Koniak‐Griffin 2015 | Cardiovascular health | Physical activity; Lenz Lifecorder Plus Accelerometer, assesses vertical acceleration and counts movements that are correlated with steady‐state oxygen consumption | IG: 98 CG: 95 |
6 months after randomisation (immediately post‐intervention) | 8769 (2747) | 8480 (3506) | Number of average daily steps is reported "[T]here was a statistically significant decrease in the control group, approaching a 1000‐step decline, whereas intervention participants maintained their activity level." (Koniak‐Griffin 2015, p.82 f) |
| IG: 100 CG: 94 |
9 months after randomisation (at 3‐month follow‐up) | 8577 (2872) | 7241 (2764) | ||||
| Rosal 2011 | Diabetes type 2 | Self‐monitoring of blood glucose 3 recalls per time point (oral assessment), 3 questions on physical activity and 3 questions on self‐monitoring of blood glucose, higher score is better |
IG: 124 CG: 128 |
12 months after randomisation (immediately post‐intervention) | 102/124; 81.5% | 81/128; 63.6% | P = 0.023 Values reflect blood glucose self‐monitoring 2 or more times per day; absolute numbers were calculated from reported percentages |
| van Servellen 2005 | HIV | HIV medication adherence, adherence behaviours baseline questionnaire (Proportion of > 95% adherence within last 4 days) |
IG: 34 CG: 35 |
6 months after randomisation (immediately post‐intervention) | 1.71% | ‐4.85% | Change scores are reported |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Han 2017 | Breast cancer | Adherence to mammogram, pap test, or both tests (Medical record review) |
Mammograma IG: 198 CG: 201 |
6 months after randomisation (immediately post‐intervention) | n: 111 (56.1%)b | n: 20 (10.0%)b | Cluster‐RCT AOR (95% CI)b (1) 18.5 (9.2 to 37.4) (2) 13.3 (7.9 to 22.3) (3) 17.4 (7.5 to 40.3) aWomen who were missing screening status were assumed to have not undergone screening bEstimated from GEE model accounting for clustering, adjusted for age, insurance, English proficiency, years in US, years of education, employment and family history of breast cancer |
| Pap testa IG: 246 CG: 251 |
Immediately post‐intervention | n: 134 (54.5%)b | n: 23 (9.2%)b | ||||
| Both testsa IG: 166 CG: 170 |
Immediately post‐intervention | 77/166 (46.4%)b | 11/170 (6.5%)b | ||||
| Kaur 2019 | Oral hygiene | Questionnaire on oral self‐care behaviour (higher score is better) |
IG: 70 CG: 70 |
3 months after randomisation (immediately post‐intervention) | 3.10 (95% CI 2.50 to 3.69) | Group x time P < 0.0001 |
|
| Kim 2009 | Diabetes type 2 | Diabetes self‐care activities, SDSCA (higher score is better) |
IG: 40 CG:39 |
30 weeks after randomisation (immediately post‐intervention) | 17.5 (16.9) | 2.5 (15.4) | Change scores are reported P = 0.00 |
| Kim 2014 | High blood pressure | Non‐adherence to blood pressure medication, HB‐MAS (8 items, 4‐point Likert‐scale, 1 = none of the time to 4 = all of the time, 8 to 32, lower score is better) |
IG: 184 CG: 185 |
12 months after randomisation | 9.1 (1.7) | 9.5 (2.0) | Cluster‐RCT; data have been re‐analysed for meta‐analyses using the appropriate unit of analysis with the use of the ICC reported by Han 2017 |
| 18 months after randomisation (at 6‐month follow‐up) | 8.8 (1.4) | 9.2 (1.6) | |||||
| Rosal 2005 | Diabetes type 2 | Blood glucose self‐monitoring; 24‐hour recall of self‐monitoring of blood glucose by asking individuals whether they had checked their blood sugar level in the previous 24 hours, at what time, and the value, higher score is better | IG: 15 CG: 8 |
3 months after randomisation (immediately post‐intervention) | No./day capped at 2; 2/day both calls 0.63 (0.26); 12/15 (80%) |
No./day capped at 2; 2/day both calls 0.19 (0.35); 4/8 (50%) |
No difference |
| 6 months after randomisation (4.5 months post‐intervention) | No./day capped at 2; 2/day both calls 0.63 (0.24); 11/15 (74%) |
No./day capped at 2; 2/day both calls 0.06 (0.27); 3/8 (38%) |
|||||
| 3 Culturally adapted health literacy skill building course vs no/unrelated health literacy intervention | |||||||
| Otilingam 2015 | Behaviours to reduce dietary fat | Fat‐Related Diet Habits Questionnaire (12 items, 4‐point Likert scale, rarely/never, sometimes, often, usually, 1 to 4, higher score is better) |
IG 1: 32 IG 2: 33 CG 1: 16 CG 2: 18 |
Immediately post‐intervention | IG 1: 3.18 (0.46) IG 2: 3.25 (0.27) |
CG 1: 3.16 (0.39) CG 2: 3.12 (0.50) |
IGs were combined to create a single score (see Analysis 3.5). CG 2 was assessed immediately post‐intervention only. Group x time P = 0.0140 |
| At 1‐month follow‐up | IG 1: 3.43 (0.40) IG 2: 3.38 (0.30) Combined: 3.41 (0.35) |
CG 1: 3.16 (0.47) | |||||
| Taylor 2011 | Hepatitis B | Medical record of HBV testing | IG: 80 CG: 100 |
At 6‐month follow‐up | 5/80 (6.25%) | 0/100 (0%) | Cluster‐RCT; data have been re‐analysed for meta‐analyses using the appropriate unit of analysis with the use of the ICC reported by Han 2017 (see Analysis 3.6) |
| Tong 2017 | Colorectal cancer | Up‐to‐date colorectal cancer screening* FOBT, S/C; self‐report of test receipt and when the test was obtained | IG: 161 CG: 168 |
6 months after first intervention session | 92/161 (57.1%) | 73/168 (43.5%) | Cluster‐RCT. Unadjusted values are reported. |
| Soto Mas 2018 | Cardiovascular health |
Cardiovascular health behaviour; CSC (34 items, 4‐point Likert scale, 1 = never to 4 = always, 34 to 136, higher score is better) |
IG: 77 CG: 78 |
Immediately post‐intervention | 59.1 | 57.9 | P = 0.067 |
| 4 Culturally and literacy adapted telephone education vs unrelated culturally and literacy adapted telephone education | |||||||
| Lepore 2012 | Prostate cancer | Prostate cancer screening behaviour; verified PSA test (Medical claims scanned for PSA procedure codes using an expert system, 0 = no, 1 = yes) |
IG: 244 CG: 246 |
1‐year follow‐up 2‐year follow‐up |
110/244 (45.1%) 153/244 (62.7%) |
113/246 (45.9%) 165/246 (66.7%) |
Absolute numbers were calculated from reported percentages |
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| DeCamp 2020 | Child's health | Prostate cancer screening behaviour; electronic medical record | IG: 72 CG: 63 |
3 months post‐intervention (15 months after child's birth) | n: 61 (85%) | n: 50 (79%) | No difference Percentages‐only are reported |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Gwede 2019 | Colorectal cancer | Colorectal cancer screening uptake; Return of completed FIT kit within 90 days of intervention delivery, yes/no | IG: 40 CG: 36 |
3 months post‐intervention | n: 36 (90%) | n: 30 (83%) | P = 0.379 Percentages‐only are reported |
| Sudore 2018 | Advance care planning, no specific | Documentation of new advance care planning (Legal forms and documented discussions with clinicians and/or surrogates) |
IG: 219 CG: 226 |
At 12‐month follow‐up | 84/219 | 58/226 | — |
| Valdez 2018 | Cervical cancer | Pap test screening behaviour (Self‐report, having had a Pap test or made an appointment in the interval between pre‐test and post‐test, yes/no) |
IG: 383 CG: 344 |
At 6‐month follow‐up | n: 195 (51%) | n: 165 (48%) | Absolute numbers were calculated from reported percentages |
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally and literacy adapted audio‐/visual education without personal feedback | |||||||
| Ochoa 2020 | Cervical cancer | Pap testing behaviour (1 item, "Since you saw the film, have you had a Pap test?", yes/no/do not know) |
IG: 61 CG: 48 |
At 6‐month follow‐up | n: 23 (37.9%) | n: 14 (29.2%) | Absolute numbers were calculated from reported percentages Results of the 2‐week post‐intervention assessment are not reported |
| 8 Culturally and literacy adapted medical instruction vs no health literacy intervention | |||||||
| Mohan 2014 | No specific | Medication adherence; ARMS, patients' self‐reported adherence under various circumstances (sub‐scale to medication refills) (8 items, 8 to 32, lower, score is better) |
IG: 99 CG: 101 |
At 1‐week follow‐up | 10.3 | 9.9 | No variance per study group reported, but MD of change scores: MD 0.5 (95% CI ‐0.1 to 1.1) "Each 1‐point increase in BHLS score was associated with a decrease of 0.1 (95% CI, –0.2 to 0.0) in the ARMS score." (Mohan 2014) |
*Results are unadjusted mean (SD) if not otherwise reported.
AOR: adjusted odds ratio; ARMS: Adherence to Refills and Medications Scale; CI: confidence interval; CSC: Cardiovascular Health Questionnaire; EMR: Electronic Medical Record; FOBT: faecal occult blood test; GEE: generalised estimating equations; HB‐MAS: Hill‐Bone Medication Adherence Scale; HBV: hepatitis B virus; MD: mean difference; OR: odds ratio; Pap test: Papanicolaou test; PSA: prostate‐specific antigen; S/C: sigmoidoscopy or colonoscopy; SD: standard deviation; SDSCA: Summary of Diabetes Self‐Care Activities
3.5. Analysis.

Comparison 3: Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention, Outcome 5: Health behaviour: fat‐related dietary habits, self‐report (short‐term: 1‐month post‐intervention)
3.6. Analysis.

Comparison 3: Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention, Outcome 6: Health behaviour: any screening adherence, odds ratio short‐/medium‐term: up to 6 months post‐intervention)
5. Outcome category: self‐efficacy.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD)* |
Notes |
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | |||||||
| Rosal 2011 | Diabetes type 2 | Self‐efficacy in diabetes management; LSESLD (17 items, 17 to 68, higher score is better) |
IG: 124 CG: 128 |
4 months after randomisation | 0.448 (0.362 to 0.534) | 0.132 (0.040 to 0.219) | Mean (range) is reported P < 0.001 For meta‐analysis, the final SD was substituted with the reported baseline SD (Analysis 1.9) |
| 12 months after randomisation | 0.448 (0.0348 to 0.548) | 0.213 (0.113 to 0.313) | P = 0.001 | ||||
| van Servellen 2005 | HIV | Self‐efficacy for HIV medication adherence; adherence behaviours baseline questionnaire (item from the ACTG) (1 question on certainty to take medications correctly, 0 = not at all sure to 3 = extremely sure, higher scores are better) |
IG: 41 CG: 40 |
At 6 weeks after randomisation | 0.27 (0.92) | ‐0.08 (0.92) | Intervention group: P ≥ 0.10 Change scores are reported |
| IG: 34 CG: 35 |
At 6 months after randomisation | 0.12 (0.95) | ‐0.06 (0.59) | Change scores are reported | |||
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Kim 2009 | Diabetes type 2 | Adapted Stanford Chronic Disease Self‐Efficacy Scale (8 x 10‐point Likert items, 0 to 80, 1 = not confident at all, 4 = very confident, higher scores are better) |
IG: 40 CG: 39 |
18 weeks after randomisation | 8.7 (11.4) | 2.6 (15.0) | Change scores are reported P = 0.02 |
| 30 weeks after randomisation | 6.6 (14.4) | ‐0.9 (15.1) | Change scores are reported P = 0.01 |
||||
| Kim 2014 | HBP | Self‐efficacy in managing high blood pressure; questionnaire adapted from the HBP belief scale (8 items, 4‐point Likert scale, 1 = not confident at all, 4 = very confident, 8 to 32, higher scores are better) |
IG: 184 CG: 185 |
12 months after randomisation (immediately post‐intervention) | 26.6 (3.2) | 25.4 (3.7) | Cluster‐RCT; data have been re‐analysed for meta‐analysis using the appropriate unit of analysis with the use of the ICC reported by Han 2017 (see Analysis 2.23; Analysis 2.25) Group x time P = 0.001 (at 12 months) |
| 18 months after randomisation (6‐month follow‐up) | 25.9 (3.7) | 26.1 (3.9) | |||||
| Kim 2020 | Diabetes type 2 | Adapted Stanford Chronic Disease Self‐Efficacy Scale (8 items, 10‐point Likert scale, 0 to 80, 1 = not confident at all, 4 = very confident, higher scores are better) |
IG: 105 CG: 104 |
12 months after randomisation | 58.6 (SE 1.2) | 46.5 (SE 1.6) | P < 0.001 |
| Rosal 2005 | Diabetes type 2 | IMDSES (26 items, 4‐point Likert‐scale, 1 = "low confidence" to 4 = "high confidence", 26 to 104, higher scores are better) |
IG: 15 CG: 10 |
3 months after randomisation (immediately post‐intervention) | Self‐efficacy for
(1) Diet 0.03 (0.4) (2) Exercise 0.11 (0.9) (3) Self‐monitoring 0.3 (1.0) (4) Oral glycaemic agents ‐0.1 (0.3) (5) Insulin ‐0.14 (1.3) |
Self‐efficacy for
(1) Diet 0.44 (0.3)* (2) Exercise 0.24 (0.6) (3) Self‐monitoring –0.3 (0.7) (4) Oral glycaemic agents 0 (0) (5) Insulin –0.2 (0.5) |
Change scores are reported No composite score reported. For meta‐analysis, a single score was calculated (see Analysis 2.23) |
| 6 months after randomisation (4.5 months post‐intervention) | (1) Diet 0.10 (0.6) (2) Exercise 0.04 (0.6) (3) Self‐monitoring 0.30 (1.0) (4) Oral glycaemic agents 0.04 (0.1) (5) Insulin 0.01 (0.6) |
(1) Diet 0.13 (0.4) (2)Exercise –0.14 (1.0) (3) Self‐monitoring –0.07 (0.7) (4) Oral glycaemic agents –0.25 (0.5) (5) Insulin –0.27 (0.4) |
|||||
| 3 Culturally adapted health literacy skills building course vs unrelated health literacy intervention | |||||||
| Elder 1998 | Nutrition/cardiovascular health | Self‐efficacy to change one's diet (5 items, 1 to 3, higher score is better) |
IG: 133 CG: 157 |
3 months post‐intervention | 2.29 | 2.25 | No difference Cluster‐RCT; unadjusted values are reported |
| At 6‐month follow‐up | 2.30 | 2.27 | |||||
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| Hernandez 2013 | Depression | Self‐efficacy to identify the need for treatment scale (3 items, 5‐point Likert scale, 1 = not sure, 5 = very sure, 0 to 15, higher scores are better) |
IG: 70 CG: 63 |
Immediately post‐intervention | 3.64 (3.36) | 0.13 (2.35) | Change scores are reported P < 0.001 |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Gwede 2019 | Colorectal cancer | Self‐efficacy for screening using FIT (6 items, 6 to 30, higher scores indicating higher levels of self‐efficacy) |
IG: 27 CG: 36 |
At 3‐month follow‐up | 29.7 (1.0) | 29.5 (1.3) | P = 0.039 |
| Poureslami 2016b (4‐arms, COPD) | COPD | COPD Self‐Efficacy Scale (short version) (5 items, 5‐point Likert‐scale, 1 = not at all confident to 5 = totally confident, higher scores are better) |
Group 3: 29 Group 4: 14 |
3 months post‐intervention | (1) Prepared to manage COPD Group 3 vs Group 4 0.87 (0.04 to 1.71), P < 0.05 (2) Perception of being informed about COPD Group 3 vs Group 4 0.12 (‐0.65 to 0.90), P < 0.05 (3) Remain calm when facing a worsening of COPD Group 3 vs Group 4 0.28 (‐0.54 to 1.11), N/S (4) Ability to achieve goals in managing COPD Group 3 vs Group 4 1.05 (0.08 to 2.02), P < 0.05 (5) Ability to self‐manage COPD symptoms Group 3 vs Group 4 0.38 (‐1.18 to 0.41), P < 0.05 |
No composite score reported MD (95% CI), P values are reported No difference between female and male participants |
|
| Payán 2020 | Breast cancer | Self‐efficacy in accessing breast cancer‐related advice or information (1 item, "Overall, how confident are you that you could get advice or information about breast cancer if you needed it?”, 5‐point Likert scale 1 = "completely confident" to 3 = "not confident at all" (3), higher scores are better) |
IG 1: 79 IG 2: 79 CG: 82 |
Immediately post‐intervention | IG 1: 0.87 (0.34) IG 2: 0.89 (0.32) IG 1, 2: 0.88 (0.33) |
0.80 (0.40) | Final values were obtained from study authors IG 1 and IG 2 were combined to create a single pairwise comparison |
| IG 1: 67 IG 2: 61 CG: 65 |
At 3‐month follow‐up | IG 1: 0.67 (0.47) IG 2: 0.88 (0.33) IG 1, 2: 0.77 (0.42) |
0.75 (0.44) | ||||
| Unger 2013 | Depression | Self‐efficacy to identify depression (2 items, 10‐point Likert scale, 1 = "not at all confident" to 10 = "very confident", higher scores are better) |
IG: 69 CG: 70 |
Immediately post‐intervention | t = 4.54, P < 0.05 | t = 3.16, P < 0.05 | — |
| At 1‐month follow‐up | t = 3.31, P < 0.05 | t = 3.00, P < 0.05 | "[T]he data collectors reported that several students shared their photonovel with students in the text pamphlet group after the posttest." (Unger 2013, p. 405). | ||||
| Valdez 2018 | Cervical cancer/Pap testing | Self‐efficacy regarding Pap smear (1 item, "Can get a pap smear if needed", yes/no) |
IG: 383 CG: 344 |
6‐month follow‐up | n: 356, 93 % | n: 314, 91 % | P = 0.40 |
* Unadjusted mean (SD) if not otherwise reported.
ACTG: Adult AIDS Clinical Trials Group; CG: control group; COPD: chronic obstructive pulmonary disease; FIT: faecal immunochemical test; HBP: high blood pressure; IG: intervention group; IMDSES: Insulin Management Self‐Efficacy Scale; LSESLD: Lifestyle Self‐Efficacy Scale for Latinos with Diabetes; MD (95% CI): mean difference (95% confidence interval); N/S: not significant; SD: standard deviation; SE: standard error ; Pap: Papanicolaou
1.9. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 9: Self‐efficacy to manage one's disease (short‐term: immediately post‐intervention)
2.23. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 23: Self‐efficacy to manage one's disease (short‐term: immediately post‐intervention) ‐ all studies
2.25. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 25: Self‐efficacy to manage one's disease (medium‐term: 6 months post‐intervention)
Summary of findings 2. Culturally and literacy adapted self‐management programme versus written information on the same topic.
| Culturally and literacy adapted self‐management programme versus written information on the same topic | ||||||
| Patient or population: migrants Setting: all settings Intervention: culturally and literacy adapted self‐management programme Comparison: written information on the same topic (standard brochure, or written pamphlet) | ||||||
| Outcome category – outcome(s)* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with written information on the same topic | Risk with self‐management programme | |||||
|
Health literacy – Time point a: short‐term (immediately post‐intervention)*** (1) Any generic health literacy Multiple outcomes assessed and multiple measures used:
Higher score is better (2) Any disease‐specific health literacy Multiple measures used:
Higher score is better (3) Appraising health information Assessed with:
Higher score is better Time point b: medium‐term (6 months post‐intervention) (1) Disease‐specific health literacy
Higher score is better |
Time point a: short‐term | — | 209 (1 RCT) |
⊕⊕⊕⊝ Moderatea | Self‐management programmes compared to written information on the same topic probably improve health numeracy slightly and probably improve print literacy immediately post‐intervention. | |
|
(1) Any generic health literacy One RCT reported that the intervention slightly increased health numeracy (NVS) immediately post‐intervention (MD 0.7 points higher (0.15 higher to 1.25 higher)). The same RCT reported that the intervention increased generic print literacy (REALM) immediately post‐intervention (MD 9.00 points higher (2.90 higher to 15.10 higher)). | ||||||
|
(2) Any disease‐specific health literacy The mean disease‐specific health literacy score across intervention groups was 0.67 standard deviations higher (0.27 higher to 1.07 higher) immediately post‐intervention. |
— | 955 (2 RCTs, 2 cluster‐RCTs1) | ⊕⊕⊝⊝ Lowb,c | Self‐management programmes compared to written information on the same topic may improve any disease‐specific health literacy immediately post‐intervention.2 | ||
|
(3) Appraising health information (decisional balance for using mammography or Pap testing) The mean decisional balance score in the intervention group was MD 1.15 points higher (0.23 lower to 2.53 higher) than in the control group immediately post‐intervention.3 |
— | 329 (1 cluster‐RCT1) |
⊕⊕⊝⊝ Lowd,e | Self‐management programmes compared to written information may have little or no effect on the appraisal of health information (decisional balance) immediately post‐intervention. | ||
| Time point b: medium‐term | — | (242) (1 cluster‐RCT1) |
⊕⊕⊝⊝ Lowa,d | Self‐management programmes compared to written information on the same topic may slightly improve high blood pressure health literacy 6 months after the programme was completed. | ||
| The mean high blood pressure health literacy in the control group was 25.3 | The mean high blood pressure health literacy in the self‐management group was MD 4.10 higher (0.97 higher to 7.23 higher) than in the control group | |||||
|
Quality of life – Diabetes‐related quality of life standardised on score 0 (no quality of life) to 100 (perfect quality of life) Time point: short‐term (immediately post‐intervention) |
The mean score for diabetes‐related quality of life ranged from 66.5% to 96.2% | The mean diabetes‐related quality of life score in the intervention groups was MD 9.06 points higher (2.85 higher to 15.27 higher) | — | 288 (2 RCTs)3 | ⊕⊝⊝⊝ Very lowa,f,g | We are uncertain whether self‐management programmes compared to written information on the same topic improve diabetes‐specific quality of life immediately post‐intervention. |
|
Health‐related knowledge – Time point a: short‐term (immediately post‐intervention) Any health‐related knowledge standardised on score 0 (no knowledge) to 100 (perfect knowledge) Time point b: medium‐term (up to 6 months post‐intervention) Any health‐related knowledge standardised on score 0 (no knowledge) to 100 (perfect knowledge) |
Time point a: short‐term | — | 1101 (4 RCTs, 2 cluster‐RCTs1) | ⊕⊕⊝⊝ Lowh,i | Self‐management programmes compared to written information on the same topic may improve health‐related knowledge immediately post‐intervention. | |
| The mean health‐related knowledge score across control groups ranged from 24.4% to 74.2% | The mean score in the intervention groups was MD 11.45 points higher (4.75 higher to 18.15 higher) | |||||
| Time point b: medium‐term | — | 298 (2 RCTs) |
⊕⊕⊝⊝ Lowd,e | Self‐management programmes compared to written information on the same topic may have little or no effect on health‐related knowledge up to 6 months post‐intervention. | ||
| The mean health‐related knowledge score across control groups was 73.7% | The mean knowledge score in the intervention groups was MD 3.87 points higher (0.46 lower to 8.19 higher) | |||||
|
Health outcome – Any depression Time point a: short‐term (immediately post‐intervention) Multiple measures used:
Lower score is better Time point b: medium‐term (up to 6 months post‐intervention) Multiple measures used:
Lower score is better |
Time point a: short‐term | — | 555 (3 RCTs, 1 cluster‐RCT1) | ⊕⊝⊝⊝ Very lowj,k,l | We are uncertain whether self‐management programmes compared to written information on the same topic improve depression immediately post‐intervention. | |
| — | The mean depression score in the intervention group was 0.19 standard deviations lower (0.62 lower to 0.23 higher) | |||||
| Time point b: medium‐term | — | 267 (1 RCT, 1 cluster‐RCT1) |
⊕⊕⊝⊝ Lowe,m | Self‐management programmes compared to written information on the same topic may have little or no effect on depression 6 months post‐intervention.2 | ||
| — | The mean depression score in the intervention group was 0.32 standard deviations lower (0.90 lower to 0.27 higher) | |||||
|
Health behaviour – Multiple outcomes assessed and multiple measures used Time point a: short‐term (immediately post‐intervention) (1) Diabetes self‐care activities
(2) Oral self‐care behaviour
(3) Cervical/breast cancer screening adherence
(4) Non‐adherence to blood pressure medication:
Time point b: medium‐term (up to 6 months post intervention) (1) Non‐adherence to blood pressure medication
(2) Blood glucose self‐monitoring:
|
Time point a: short‐term (1) Diabetes self‐care activities One RCT (n = 79) reported that the self‐management programme improved diabetes self‐care activities (MD 15 points higher (7.87 higher to 22.13 higher) (2) Oral self‐care behaviour One RCT (n = 140) found that the intervention improved self‐reported oral self‐care behaviour (MD 3.1 points higher (2.5 higher to 3.7 higher) (3) Cervical/breast cancer screening adherence One cluster RCT (n = 336) that properly accounted for the cluster design, found that the intervention improved cervical/breast cancer screening adherence (RR 7.17, 95% CI 3.96 to 12.99)8 (4) Non‐adherence to blood pressure medication One cluster‐RCT (N = 242) reported that the mean non‐adherence to blood pressure medication was 0.4 points lower (0.87 lower to 0.07 higher) in the intervention group. The mean non‐adherence score in the control group was 9.2. |
— | 797 (2 RCTs, 2 cluster‐RCTs)6,7 | ⊕⊕⊝⊝ Lowm,n | Self‐management programmes compared to written information on the same topic may improve health behaviour immediately post‐intervention, but measures and sizes of effects appear variable. | |
|
Time point b: medium‐term (1) Non‐adherence to blood pressure medication One cluster‐RCT (n = 242) reported that the intervention had slightly lower scores on non‐adherence to blood pressure medication (MD 0.40 points lower (0.78 lower to 0.02 lower)). The mean non‐adherence score in the control group was 8.8. (2) Blood glucose self‐monitoring One RCT (n = 23) reported greater self‐reported blood glucose‐self‐monitoring in the intervention groups 4.5 months post‐intervention (RR 1.96, 95% CI 0.76 to 5.03). |
— | 265 (1 RCT, 1 cluster‐RCT1) |
⊕⊕⊝⊝ Lowl,o | Self‐management programmes compared to written information on the same topic may slightly improve health behaviour 6 months post‐intervention, but outcome measures and size of effects appear variable. | ||
|
Self‐efficacy– Self‐efficacy to manage one's disease Time point a: short‐term (immediately post‐intervention) Multiple measures used:
Higher score is better Time point b: medium‐term (up to 6 months post‐intervention) Self‐efficacy to manage high blood pressure
Higher score is better |
Time point a: short‐term | — | 552 (4 RCTs) | ⊕⊕⊕⊝ Moderatej | Self‐management programmes probably improve self‐efficacy immediately post‐intervention, when compared to written information on the same topic.9 | |
| — | The mean self‐efficacy score in the intervention group was 0.47 standard deviations higher (0.30 higher to 0.64 higher) | |||||
| Time point b: medium‐term | — | 242 (1 cluster‐RCT1) |
⊕⊕⊝⊝ Lowm,p | Self‐management programmes compared to written information may have little or no effect on high blood pressure self‐efficacy 6 months post‐intervention. | ||
| The mean self‐efficacy score in the control group was 26.1 | The mean self‐efficacy score was MD 0.20 lower in the intervention group (1.16 lower to 0.76 higher) 6 months post‐intervention | |||||
| Health service use – not reported | — | — | — | — | — | The effect of self‐management programmes on health service use is unknown as there was no direct evidence identified. |
| Adverse events – not reported | — | — | — | — | — | The effect of self‐management programmes on adverse events is unknown as there was no direct evidence identified. |
| * More detail on scoring and direction for each outcome measure is provided in Table 2; Table 8; Table 5; Table 9; Table 4; Table 6; Table 10; **The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ***Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: from 6 weeks up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. ACTG: Adult AIDS Clinical Trials Group; AHL‐C: Assessment of Health Literacy in Cancer Screening; CG: control group; CI: confidence interval; DM‐REALM: Diabetes Mellitus‐Rapid Estimate of Adult Literacy in Medicine; GEE: generalised estimating equations; HB‐MAS: Hill‐Bone Medication Adherence Scale; HBP: high blood pressure; ICC: intra‐cluster correlation IG: intervention group; IMDSES: Insulin Management Self‐Efficacy Scale; KDSKA: Kim Depression Scale for Korean Americans; LSESLD: Lifestyle Self‐Efficacy Scale for Latinos with Diabetes; MD: mean difference; NVS: Newest Vital Sign; PHQ: Patient Health Questionnaire; RCT: randomised controlled trial; REALM: Rapid Estimate of Adult Literacy in Medicine; RR: risk ratio; SDSCA: Summary of Diabetes Self‐Care Activities Scale; TS‐REALD: Two Stage Rapid Estimate of Adult Literacy in Dentistry | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Data have been re‐analysed using the appropriate unit of analysis by considering the ICCs reported by Han 2017. For more details, see Unit of analysis issues.
2We applied the following rule of thumb to rate SMD effect sizes: 0.2 = small effect, SMD ≥ 0.5 = moderate effect, 0.8 = large effect; variation to this rule is SMD < 0.40 = small effect, SMD 0.4 to 0.7 = moderate effect, and SMD > 0.7 large effect (Higgins 2022). The effect size of this SMD was rated as being moderate. Although it was close to a 'large effect', the CI was wide with a lower CI indicating a possible small effect and an upper CI indicating a very large effect.
3Data for decisional balance of using mammography for breast cancer screening or Pap testing for cervical cancer screening were combined to create a single MD. Results for both scales are reported separately in Table 11.
4One RCT (n = 25) reported on diabetes‐related quality of life but due to incomplete reporting, the direction and size of the effect was unclear.
5The validated SDSCA encompasses 11 core‐items and 14 optional items (7‐point Likert scale reflecting days per week) to assess self‐reported diabetes‐related self‐care activities.
6Estimated from GEE model accounting for clustering within a church and adjusting for age, insurance, English proficiency, years in US, years of education, employment and family history of breast cancer; results for use of both tests are reported; results of separate analyses for breast cancer screening and cervical cancer screening are shown in Table 4.
7One RCT reported having assessed self‐care activities, but did not report the results.
8The study also reported results for breast cancer screening adherence and cervical cancer screening adherence separately. Details are shown in additional Table 4.
9Effect size was rated as being moderate due to relatively narrow CI and an SMD near threshold (rule of thumb: 0.2 = small effect, SMD ≥ 0.5 = moderate effect, 0.8 = large effect; variation to this rule is SMD < 0.40 = small effect, SMD 0.4 to 0.7 = moderate effect and SMD > 0.7 = large effect; Higgins 2022).
aDowngraded by ‐1 for imprecision: result was based on a single study with a small sample size and/or CI was wide.
bDowngraded by ‐1 for risk of bias: unclear risk of bias for allocation concealment and/or random sequence generation in three out of four studies.
cDowngraded by ‐1 for inconsistency: considerable statistical heterogeneity (I² > 75%).
dDowngraded by ‐1 for risk of bias: unclear risk of bias for random sequence generation and/or for allocation concealment.
eDowngraded by ‐1 for imprecision: result was based on two studies with a small sample size and the CIs encompassed values indicating both an improvement and a worsening in the outcome. In addition, the CI of one study was large.
fDowngraded by ‐1 for inconsistency: substantial statistical heterogeneity (I² > 50% to 75%), the direction of effect was generally consistent but one of the two CIs encompassed both an improvement and a worsening in this outcome.
gDowngraded by ‐1 for risk of bias: high risk of bias for blinding and outcomes were subjectively measured. One study was also at unclear risk of bias for allocation concealment.
hDowngraded by ‐1 for risk of bias: unclear risk of bias for random sequence generation and/or allocation concealment in five studies.
iDowngraded by ‐1 for inconsistency: substantial statistical heterogeneity (I2 > 90%). The direction of effect was generally consistent but CIs for two out of six effect estimates encompassed both an improvement and a worsening in knowledge.
jDowngraded by ‐1 for risk of bias: high risk of bias for blinding in all studies and outcome was subjectively measured, unclear risk of bias for allocation concealment and/or random sequence generation in three studies.
kDowngraded by ‐1 for inconsistency: substantial statistical heterogeneity (I² = 79%), two out of four studies favoured written information (but CIs included both an improvement and a worsening in the outcome). The other two studies favoured the self‐management programme.
lDowngraded by ‐1 for imprecision: CI encompassed values indicating both an improvement and a worsening in this outcome.
mDowngraded by ‐1 for risk of bias: unclear risk of bias for random sequence generation and/or allocation concealment, high risk of bias for blinding and outcome was subjectively measured.
nDowngraded by ‐1 for inconsistency: one study indicated little or no effect with a CI encompassing both an improvement and a small reduction in the outcome. The results of two studies indicated a large effect.
oDowngraded by ‐1 for imprecision: one CI encompassed both an improvement and a worsening in the outcome, the upper limit of the other CI was close to a null effect (‐0.02).
pDowngraded by ‐1 for imprecision: the result was based on a single study with a small sample size and the CI encompassed values indicating both an improvement and a worsening.
6. Outcome category: quality of life.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD) |
Notes |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Kim 2009 | Diabetes‐related quality of life | DQOL, modified version (4 dimensions of QOL, 46 items, lower score is better) |
IG: 40 CG: 39 |
30 weeks after randomisation (immediately post‐intervention) | 84 ‐4.6 (16.5) |
96.8 0.3 (16.4) |
P = 0.03 |
| Kim 2020 | Diabetes‐related quality of life | DQOL (4 dimensions of QOL, 15 items, 0 to 75, higher score indicates higher level of quality of life) |
IG: 105 CG: 104 |
12 months after randomisation | 57.6 (SE 1.0) Change from baseline: 7.5 (SE 0.9) |
49.9 (SE 1.0) Change from baseline: ‐1.1 (0.9) |
P < 0.001 P < 0.001 |
| Rosal 2005 | Diabetes‐related quality of life | ADDQoL, adapted version, modified for telephone administration (13 items) |
IG: 15 CG: 10 |
3 months after randomisation (immediately post‐intervention) | ‐0.35 (1.4) | ‐0.8 (1.0) | No differences between study groups We do not know which effect indicates a higher level of quality of life |
| 6 months after randomisation (4.5 months post‐intervention) | ‐2.4 (2.0) | ‐1.3 (2.3) | |||||
*Unadjusted mean (SD) if not otherwise reported.
ADDQoL: Audit of Diabetes Dependent Quality of Life; CG: control group; DQOL: Diabetes Quality of Life measure; IG: intervention group; SD: standard deviation; SE: standard error
7. Outcome category: adverse events.
| Study ID | Health topic | Measure | No. of participants | Time point(s) | Intervention arm Mean (SD)* | Control armMean (SD) | Notes |
| 4 Culturally and literacy adapted telephone education vs unrelated culturally and literacy adapted telephone education | |||||||
| Lepore 2012 | Prostate cancer | Anxiety HADS, 7 items subscale for assessing anxiety, 0 to 21, lower score is better |
IG: 215 CG: 216 |
Approx. 7 months post‐intervention | 2.02 (SE 0.147) | 2.16 (SE 0.146) | P = 0.42 Adjusted for education, any PSA claim prior to pretest and state anxiety level at pretest |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Sudore 2018 | Advance care planning, no specific | Anxiety GAD‐7, 0 to 21, cut‐point > 10 (moderate anxiety), lower score is better |
IG: 219 CG: 226 |
At 12‐month follow‐up | 3.0 (95% CI 2.5 to 3.5) | 3.7 (95% CI 3.2 to 4.2) | P = 0.05 Adjusted for baseline depression and anxiety scores |
*Unadjusted mean (SD) if not otherwise reported.
CI: confidence interval; CG: control group; GAD‐7: Generalised Anxiety Disorder‐7; HADS: Hospital Anxiety and Depression Scale; IG: intervention group; SD: standard deviation; SE: standard error
8. Outcome category: health literacy ‐ applying health information.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD)* |
Notes |
| 3 Culturally adapted health literacy skills building course vs no/unrelated health literacy | |||||||
| Elder 1998 | Cardiovascular health/nutrition | Intention to change nutritional habits, questionnaire (3 items, 1 to 3, higher score is better) |
IG: 131 CG: 156 |
Immediately post‐intervention | 2.71 | 2.69 | Condition x time: P = 0.06 Cluster‐RCT "Results showed the intraclass correlations were negligible and so mixed model analysis of variance (ANOVA) procedures were conducted to test intervention effects." |
| At 6‐month follow‐up | 2.71 | 2.66 | |||||
| 4 Culturally and literacy adapted telephone education vs unrelated health literacy intervention | |||||||
| Lepore 2012 | Testing intention | Testing intention for prostate cancer (Participants were asked whether they had "decided to get tested in the future for prostate cancer", 0 = no, 1 = yes) |
IG: 215 CG: 216 |
Approx. 7 months post‐intervention | n = 215 80.9% |
n = 216 81.0% |
(95% CI 0.614 to 1.610) Adjusted for education level and claims‐verified PSA test prior to pretest |
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| Hernandez 2013 | Depression | Intention to seek treatment for depression Intention to seek treatment for depression scale, 0 to 32, higher score is better |
IG: 63 CG: 57 |
Immediately post‐intervention | 1.10 (2.99) | ‐0.70 (4.46) | Change scores are reported P = 0.012 "[...] groups’ mean increase in intent to seek treatment, [...] used to control for alpha inflation, yielded a more conservative a‐level of.01, rendering the above p value marginally significant in favour of greater intention to seek treatment on the part of experimental participants exposed to the fotonovela" |
| Thompson 2012 | Behaviour intent/behaviour change | Planned changes in behaviour, questionnaire (3 questions on behaviour change based on what was learned through programme) |
IG: 80 CG: 78 |
Immediately post‐intervention | Planned behaviour change (1) 71% Planned to talk to child's doctor 80% Planned to talk to family or friends 100% |
— | Data available for intervention group only 50.9% of those who planned to change behaviour planned to change something related to the milk module |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Unger 2013 | Depression | Willingness to seek help for depression Modified intention to seek help for depression care scale (4 items, 1 = no, 2 = yes, higher score is better) |
IG: 69 CG: 70 |
Immediately post‐intervention | "76 % of the respondents (...) answered "yes" to all of the questions in this scale at baseline, (...) this increased to 83 % at posttest and 86 % at 1‐month follow‐up" | "There were no significant differences between the fotonovela group and the text pamphlet group in willingness to seek help for depression at baseline, posttest, or follow‐up, and neither group changed significantly on this variable." | "[T]he data collectors reported that several students shared their photonovel with students in the text pamphlet group after the posttest." (Unger 2013, p. 405). |
| Valdez 2015 | Informed decision regarding HPV vaccination | Made informed decision regarding HPV vaccination ((1) making a vaccination choice, (2) affirming that the decision was an informed choice, and (3) having a knowledge score of at least 7 out of 12 knowledge items, higher score is better) |
IG: 290 CG: 318 |
At 1‐month follow‐up | 182/290 (62.8%) | 132/318 (41.5%) | P < 0.0001 |
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally and literacy adapted audio‐/visual education without personal feedback | |||||||
| Ochoa 2020 | Behavioural intentions regarding cervical cancer | Pap testing intention ("Since you saw the film, did you make an appointment for a Pap test?", "yes", "no" or "do not know") |
IG: 61 CG: 48 |
2 weeks post‐intervention | Not reported | Not reported | There "was no statistical difference in behavioural intentions at 2 weeks based on the film condition; however, there were trends that the narrative film had a greater effect." (Ochoa 2020, p. 739) |
| At 6‐month follow‐up | 15/61 (24.1%) | 6/48 (12.5%) | Absolute numbers were calculated from reported percentages | ||||
* Unadjusted mean (SD) if not otherwise reported.
CG: control group; CI: confidence interval; HPV: human papillomavirus; IG: intervention group; PSA: prostate‐specific antigen; RCT: randomised controlled trial; SD: standard deviation
9. Outcome category: health literacy ‐ appraising health information.
| Study ID | Domain | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD)* |
Notes |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Han 2017 | Cervical/breast cancer |
Decisional balance measure (weighing pros and cons for mammography and Pap testing) (5 pros and 9 cons on 5‐point Likert scale) |
Breast cancer IG: 278 CG: 282 |
At 6 months after randomisation (immediately post‐intervention) | 50.0 (6.0) | 49.0 (6.0) | Cluster‐RCT; data have been re‐analysed for meta‐analysis using the appropriate unit of analysis with the use of the ICC reported by Han 2017. In addition, outcome data for decisional balance for mammography and decisional balance for Pap testing were combined to create a single score (see Analysis 2.8) Estimated MD 1.3 (95% CI 0.4 to 2.1) Estimated MD adjusted for baseline decisional balance, age, insurance, English proficiency, years of US residence, years of education, employment and family history of breast cancer |
| Cervical cancer IG: 278 CG: 282 |
54.4 (6.1) | 53.1 (6.0) | Estimated MD 1.1 (95% CI 0.5 to 1.6) Estimated MD adjusted for baseline decisional balance, age, insurance, English proficiency, years of US residence, years of education, employment and family history of breast cancer. |
||||
| 3 Culturally adapted health literacy skills building course vs no health literacy intervention | |||||||
| Valdez 2015 | Cervical cancer/HPV vaccine | Decisional Conflict Scale (Subscales (1) informed decision, (2) values clarity, (3) support, 0 to 100 (each scale), lower score is better) |
IG: 290 CG: 318 |
1 month post‐intervention | (1) 19.7 (15.8) (2) 20.3 (15.1) (3) 22.8 (17.1) |
(1) 32.3 (21.4) (2) 32.8 (22.1) (3) 30.0 (20.4) |
Difference between intervention and control in pre‐post change (1) P < 0.0001 (2) P < 0.0001 (3) P = 0.0023 |
| 4 Culturally adapted telephone education vs unrelated culturally adapted telephone education | |||||||
| Lepore 2012 | Prostate cancer | Decisional Conflict Scale (Subscales (1) informed decision, (2) values clarity, (3) support (1 out of 3 items), 0 to 100, lower score is better) |
IG: 215 CG: 216 |
Approximately 7 months post‐intervention | 34.15 (SE 1.639) | 39.85 (SE 1.636) | P = 0.14 Measured post‐test only Adjusted for education and any PSA claim prior to pretest |
* Unadjusted mean (SD) if not otherwise reported.
CG: control group; ICC: intraclass correlation; IG: intervention group; MD: mean difference; PSA: prostate‐specific antigen; SD: standard deviation
2.8. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 8: Health literacy ‐ appraise: decisional balance for using mammography and Pap testing (short‐term: immediately post‐intervention)
Summary of findings 3. Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention.
| Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention | ||||||
| Patient or population: migrants Setting: all settings Intervention: culturally adapted health literacy skills building course Comparison: no health literacy intervention (standard language course, or no additional intervention)/unrelated health literacy intervention (language course plus information on different health topic, or another skills building course plus information on different health topic) | ||||||
| Outcome category – outcome(s)* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no health literacy intervention | Risk with health literacy skills building course | |||||
|
Health literacy – Time point a: short‐term (up to 1 month post‐intervention)*** (1) Any generic functional health literacy Multiple measures used:
Higher score is better (2) Disease‐specific health literacy Depression literacy (i.e. depression knowledge) Assessed with:
Higher score is better Time point b: medium‐term (6 months post‐intervention (1) Applying health information Intention to change nutritional habits Assessed with:
Higher score is better |
Time point a: short‐term (up to 1 month post‐intervention) | — | 229 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Health literacy skills building courses may improve any generic functional health literacy up to 1 month post‐intervention, when compared to no or unrelated health literacy intervention.1 | |
| — | The mean functional health literacy score in the intervention group was 0.48 SD higher (0.20 higher to 0.75 higher) | |||||
| The mean depression literacy score in the control group was 12.89 | The mean depression literacy score in the intervention group was 0.17 points higher (1.28 lower to 1.62 higher) | — | 37 (1 RCT) |
⊕⊕⊝⊝ Lowc | Health literacy skills building courses may have little or no effect on depression literacy immediately post‐intervention, when compared to no or unrelated health literacy intervention.2 | |
|
Time point b: medium‐term (6 months post‐intervention (1) Applying health information One cluster‐RCT reported that the health literacy skills building course had little or no effect on the intention to change nutritional habits (MD 0.05, P > 0.05) |
— | 287 (1 RCT) |
⊕⊝⊝⊝ Very lowd,e |
We are uncertain whether health literacy skills building courses improve the intention to change nutritional habits 6 months post‐intervention, when compared to no or unrelated health literacy intervention. | ||
| Quality of life – not measured | — | — | — | — | — | The effect of the intervention on quality of life is unknown as there was no direct evidence identified. |
|
Health‐related knowledge – Time point a: short‐term (up to 1 month post‐intervention) Any health‐related knowledge standardised on score 0 (no knowledge) to 100 (perfect knowledge) Time point b: medium‐term (6 months post‐intervention) Multiple measures used: (1) Hepatitis b knowledge
(2) Nutrition knowledge
(3) Colorectal cancer screening knowledge
Higher scores are better |
Time point a: short‐term | — | 111 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Health literacy skills building courses may improve health‐related knowledge immediately post‐intervention, when compared to no or unrelated health literacy intervention. | |
| The mean knowledge score across the control groups was 57 | The mean knowledge score was 69 (63 to 73) points out of 100 with the intervention (MD 10.87 (95% CI 5.69 to 16.06) immediately post‐intervention3 | |||||
|
Time point b: medium‐term (1) Hepatitis b knowledge One cluster‐RCT (n = 168) reported that the mean knowledge score in the intervention group was 0.81 higher (0.43 higher to 1.18 higher)4 (2) Nutrition knowledge One cluster‐RCT (n = 291) reported that the intervention improved nutrition knowledge slightly (MD 0.79, P ≤ 0.001)5 (3) Colorectal cancer knowledge One cluster‐RCT (n = 329) that did not report a composite knowledge score (5 questions), found that the proportion of correct answers was higher in the intervention group in all 5 knowledge domains, with MDs ranging from 15.1% to 36.8% and P values ranging from < 0.0001 to 0.0126 |
— | 788 (3 cluster‐RCTs) |
⊕⊕⊝⊝ Lowf,g,h |
Health literacy skills building courses may slightly improve health‐related knowledge 6 months post‐intervention, when compared to no or unrelated health literacy intervention. | ||
| Health outcome – not measured | — | — | — | — | — | The effect of the intervention on health outcomes is unknown as there was no direct evidence identified. |
|
Health behaviour – Time point a: short‐term (up to 1 month post‐intervention) Multiple outcomes assessed and multiple measures used: (1) Fat‐related dietary habits
(2) Cardiovascular health behaviour
Higher scores are better Time point b: medium‐term (6 months post‐intervention) Any screening adherence Multiple measures used:
|
Time point a: short‐term (1) Fat‐related dietary habits One RCT (n = 74) found little or no difference in self‐reported fat‐related dietary habits (MD 0.25 points higher (0.00 higher to 0.50 higher)) 1 month post‐intervention (2) Cardiovascular health behaviour One RCT (n = 155) found little to no effect of the intervention on self‐reported cardiovascular health behaviour (MD 1.2, P = 0.067) |
— | 229 (2 RCTs) | ⊕⊕⊝⊝ Lowi,j | Health literacy skills building courses may have little or no effect on any health behaviour up to 3 months post‐intervention, when compared to no or unrelated health literacy intervention. | |
| Time point b: medium‐term | RR 2.68 (0.33 to 21.83) | 440 (2 cluster‐RCTs) |
⊕⊕⊝⊝ Lowk |
Health literacy skills building courses may improve or reduce screening adherence 6 months post‐intervention, when compared to no or unrelated health literacy intervention; the effect sizes appear to vary considerably. | ||
| 259 per 1000 | 694 per 1000 | |||||
|
Self‐efficacy– Self‐efficacy to change one's diet Assessed with:
Higher scores indicate higher levels of self‐efficacy Time point: medium‐term (6 months post‐intervention) |
One cluster‐RCT found that disease prevention and health literacy skills building courses had little to no effect on self‐efficacy to change one's diet 6 months post‐intervention (MD 0.03, P = 0.64). | — | 290 (1 RCT) |
⊕⊝⊝⊝ Very lowd,e |
We are uncertain whether health literacy skills building courses improve self‐efficacy to change one's diet 6 months post‐intervention, compared to no or unrelated health literacy intervention. | |
| Health service use – not reported | — | — | — | — | The effect of the intervention on health service use is unknown as there was no direct evidence identified. | |
| Adverse events – not reported | — | — | — | — | — | The effect of the intervention on adverse events is unknown as there was no direct evidence identified. |
| *More detail on scoring and direction for each outcome measure is provided in Table 2; Table 5; Table 9; Table 4; Table 13; **The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI), ***Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: from 6 weeks up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. AHL‐C: Assessment of Health Literacy in Cancer Screening; CI: confidence interval; CSC: Cardiovascular Health Questionnaire; D‐Lit: Depression Literacy Questionnaire; GEE: generalised estimating equations; ICC: intra‐class correlation coefficient; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; TOFHLA: TS‐REALD: Two Stage Rapid Estimate of Adult Literacy in Dentistry | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1The effect size was rated as being moderate as the SMD was near the threshold (rule of thumb: SMD ≥ 0.5 represents a moderate effect; variation to this rule is SMD 0.4 to 0.7 = moderate effect; Higgins 2022).
2We do not report the results of the 2‐month follow‐up assessment, as the data were not reported separately for the intervention groups in the identified publications.
3The knowledge score across control groups ranged from 48.1% to 61.8%.
4The results were adjusted for the cluster design by reducing the sample size by the design effect with the use of the ICC reported by Han 2017. Adjusted odds ratios estimated from GEE models are reported separately for each question in Table 3.
5Results reflect unadjusted values as we had insufficient information to re‐analyse the data using the appropriate unit of analysis. According to the authors, the "intraclass correlations were negligible" (Elder 1998, p. 571).
6GEE models were used to account for clustering but only proportions of correct answers per item were reported. Thus, we do not know if the appropriate unit of analysis was used. Details are shown in Table 3.
aDowngraded by ‐1 for risk of bias: all studies at unclear risk of bias for random sequence generation, one study at unclear risk of bias for allocation concealment.
bDowngraded by ‐1 for imprecision: wide CI and small sample size.
cDowngraded by ‐2 for imprecision: result was based on a single study with a very small sample size (fewer than 50) and wide CI that encompassed values indicating both an improvement and a worsening in the outcome.
dDowngraded by ‐2 for risk of bias: unclear risk of bias for random sequence generation and allocation concealment, high risk of bias for blinding and outcome was subjectively measured, and the results were not adjusted for the cluster design, indicating a possible unit of analysis error.
eDowngraded by ‐1 for imprecision: result was based on a single study with a small sample size and data were not reported in a way in which an MD and a measure of spread could be calculated.
fDowngraded by ‐1 for risk of bias: unclear risk of bias for random sequence generation and allocation concealment in one study. In addition, in one study, the results were not adjusted to account for the cluster design and the information was insufficient to re‐analyse the data, which indicates a unit of analysis error. For one study, we do not know whether the appropriate unit of analysis was used as only proportions of correct answers per item were reported.
gDowngraded by ‐1 for imprecision: pooling data was not possible. Two out of three studies did not report the data in a way in which an MD and a measure of spread could be calculated.
hNot downgraded for inconsistency: although two studies found little or no effect on knowledge scores, one study found a large effect, but there was consistency in the direction of effects.
iDowngraded by ‐1 for risk of bias: high risk of bias for blinding and outcomes were subjectively measured in all studies; all studies at unclear risk of bias for random sequence generation, one study at unclear risk of bias for allocation concealment.
jDowngraded by ‐1 for imprecision: data from one study are not reported in a way in which an MD and a measure of spread could be calculated. In addition, the sample size was small.
kDowngraded by ‐2 for imprecision: rare events in one study and the CI of the pooled effect estimate was very wide, including values indicating both a large improvement but also the possibility of a worsening in the outcome.
10. Outcome category: health service use.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD) |
Notes |
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| DeCamp 2020 | Child health | Emergency department visits (EMR) | IG: 79 CG: 78 |
1 to 3 months post‐intervention (12 to 15 months after child's birth) | 1.23 (1.66) | 1.82 (1.64) | P = 0.03 |
*Results are unadjusted mean (SD) if not otherwise reported.
CG: control group; CI: confidence interval; EMR: electronic medical record; ER: emergency room; GEE: generalised estimating equations; IG: intervention group; RR: risk ratio; SD: standard deviation
Summary of findings 4. Culturally and literacy adapted telephone education versus unrelated health literacy intervention.
| Culturally and literacy adapted telephone education versus unrelated health literacy intervention | ||||||
| Patient or population: migrants Setting: participant's home Intervention: culturally and literacy adapted telephone education Comparison: unrelated health literacy intervention (telephone education on healthy nutrition) | ||||||
| Outcome category – outcome(s)* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with unrelated health literacy intervention | Risk with telephone education | |||||
|
Health literacy – (1) Appraising health information Assessed with:
Lower score is better (2) Applying health information (prostate cancer screening intention) Assessed with:
Time point: long‐term (approx. 7 months post‐intervention post‐intervention)*** |
The mean decisional conflict in the control group was 39.891 | The mean decisional conflict in the intervention group was 5.70 points lower (10.24 lower to 1.16 lower) | — | 431 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Culturally and literacy adapted telephone education compared to unrelated health literacy intervention probably improves appraising health information by reducing decisional conflict, when assessed 7 months post‐intervention. |
| 806 per 1000 | 806 per 1000 (741 to 887) |
RR 1.00 (0.92 to 1.10) | 431 (1 RCT) |
⊕⊕⊕⊝ Moderatea | Culturally and literacy adapted telephone education compared to unrelated health literacy intervention probably has little or no effect on applying health information (prostate cancer screening intention) 7 months post‐intervention. | |
| Quality of life – not measured | — | — | — | — | — | The effect of telephone education on quality of life is unknown as there was no direct evidence identified. |
|
Health‐related knowledge – Prostate cancer knowledge Standardised on score 0 (no knowledge) to 100 (perfect knowledge) Time point: long‐term (approx. 7 months post‐intervention) |
The mean prostate cancer knowledge in the control group was 55% | The mean prostate cancer knowledge score was 62% (from 62 to 62) with the intervention (MD 6.9, 95% CI 6.88 to 6.92) | — | 431 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Culturally and literacy adapted telephone education compared to unrelated health literacy intervention probably improves prostate cancer knowledge slightly 7 months post intervention. |
| Health outcome – not measured | — | — | — | — | — | The effect of telephone education on health outcomes is unknown as there was no direct evidence identified. |
Health behaviour – PSA testing
Assessed with:
Time point: long‐term (2 years post‐intervention) |
671 per 1000 | 624 per 1000 (550 to 718) |
RR 0.93 (0.82 to 1.07) | 490 (1 RCT) |
⊕⊕⊕⊝ Moderatea | Telephone education compared to an unrelated health literacy intervention probably has little or no effect on PSA testing 2 years post‐intervention. |
| Self‐efficacy – not measured | — | — | — | — | — | The effect of telephone education on self‐efficacy is unknown as there was no direct evidence identified. |
| Health service use – not measured | — | — | — | — | — | The effect of telephone education on health service use is unknown as there was no direct evidence identified. |
|
Adverse events – Anxiety Assessed with:
Lower score is better Time point: long‐term (approx. 7 months post‐intervention) |
The mean anxiety score in the control group was 2.022 | The mean anxiety score in the intervention group was 0.14 points lower (0.55 lower to 0.27 higher) | — | 431 (1 RCT) |
⊕⊕⊕⊝ Moderatea | Telephone education compared to unrelated health literacy intervention probably has little or no effect on anxiety approximately 7 months post‐intervention. |
| *More detail on scoring and direction for each outcome measure is provided in Table 8; Table 11; Table 4); **The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ***Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: from 6 weeks up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. CI: confidence interval; HADS: Hospital Anxiety and Depression Scale; RCT: randomised controlled trial; RR: risk ratio; PSA: prostate‐specific antigen | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Scores ≤ 25 are associated with following through on decisions; scores > 37.5 are associated with delay in decision‐making or feeling unsecure about its implementation (O'Connor 1993).
2Scores 0 to 7 represent no clinically meaningful anxiety or depression (Zigmond 1983).
aDowngraded by ‐1 for imprecision: result was based on a single study and/or the CI was wide or encompassed values indicating both an improvement and a worsening in the outcome.
Summary of findings 5. Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention.
| Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention | ||||||
| Patient or population: migrants Setting: all settings Intervention: culturally and literacy adapted audio‐/visual education without personal feedback Comparison: no health literacy intervention (usual care, wait‐list control or placebo intervention) | ||||||
| Outcome category – outcome(s)* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no health literacy intervention | Risk with audio‐/visual education | |||||
|
Health literacy – (1) Depression literacy Assessed with:
Higher scores are better (2) Applying health information Multiple measures used:
Higher scores are better Time point: short‐term (immediately up to 1 week post‐intervention)*** |
The mean depression literacy score in the control group was 8.22 points | The mean depression literacy score in the intervention group was 8.62 points higher (7.51 higher to 9.73 higher) | — | 202 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Audio‐/visual education without personal feedback compared to no health literacy intervention probably improves depression literacy 1 week post‐intervention, when compared to no health literacy intervention. |
| One study reported that the intervention improved the intention to seek treatment for depression (MD 1.8 points higher (0.43 higher to 3.17 higher)) | — | 120 (1 RCT)1 | ⊕⊕⊝⊝ Lowb,c | Audio‐/visual education without personal feedback may slightly improve the intention to seek treatment for depression immediately post‐intervention, when compared to no health literacy intervention. | ||
| Quality of life – not measured | — | — | — | — | — | The effect of audio‐/visual education without personal feedback on quality of life is unknown, as there was no direct evidence identified. |
|
Health‐related knowledge – Any health‐related knowledge standardised on score 0 (no knowledge) to 100 (perfect knowledge) Time point: short‐term (up to 1 month post‐intervention) |
The mean knowledge score across control groups ranged from 61.8% to 67.4%2 | The mean knowledge score in the intervention groups was 8.44 higher (2.56 lower to 19.44 higher) | — | 293 (2 RCTs) | ⊕⊕⊝⊝ Lowd,e | Audio‐/visual education without personal feedback compared to no health literacy intervention may slightly improve health‐related knowledge up to 1 month post‐intervention, but the effect sizes appear to vary considerably. |
|
Health outcome ‐ Depression Multiple measures used:
Lower score is better Time point: immediately up to 3 months post‐intervention |
— | The mean depression score in the intervention groups was 0.15 SMD lower (0.40 lower to 0.10 higher) than in the control groups | — | 337 (2 RCTs) |
⊕⊕⊝⊝ Lowf,g | Audio‐/visual education without personal feedback compared to no health literacy intervention may have little or no effect on any depression immediately up to 3 months post‐intervention. |
|
Health behaviour – Child's up‐to‐date immunisation Assessed with:
Time point: short‐term (immediately up to 3 months post‐intervention) |
794 per 1000 | 849 per 1000 (722 to 992) | RR 1.07 (0.91 to 1.25) | 135 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Audio‐/visual education without personal feedback probably has little or no effect on child's up‐to‐date immunisation immediately up to 3 months post‐intervention, when compared to no health literacy intervention. |
|
Self‐efficacy – Self‐efficacy to identify need for treatmentfor depression Assessed with:
Higher score is better Time point: short‐term (immediately post‐intervention) |
One RCT reported that audio‐/visual education improved self‐efficacy to identify the need for treatment for depression (MD 3.51 higher (2.53 higher to 4.49 higher)) immediately post‐intervention | — | 133 (1 RCT) |
⊕⊕⊝⊝ Lowa,c | Audio‐/visual education without personal feedback may improve self‐efficacy to identify the need for treatment for depression immediately post‐intervention, when compared to no health literacy intervention. | |
|
Health service use – Child's emergency room visits Assessed with:
Higher scores indicate higher levels of emergency room visits Time point: short‐‐term (immediately up to 3 months post‐intervention) |
The mean rate of emergency room visits in the control group was 1.82 | The mean rate of child's emergency room visits in the intervention group was 0.59 points lower (1.11 lower to 0.07 lower) | — | 157 (1 RCT) |
⊕⊕⊕⊝ Moderateh |
Audio‐/visual education without personal feedback compared to no health literacy intervention probably reduces child's emergency room visits up to 3 months post‐intervention. |
| Adverse events – not measured | — | — | — | — | — | The effect of audio‐/visual education without personal feedback on adverse events is unknown, as there was no direct evidence identified. |
| *More detail on scoring and direction for each outcome measure is provided in Table 2; Table 10; Table 3; Table 4; Table 5; Table 6; Table 13; **The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI), ***Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: from 6 weeks up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. BDI‐II: Beck Depression Inventory; CI: confidence interval; D‐Lit: Depression Literacy Questionnaire; FIT: faecal immunochemical test; PHQ‐8: Patient Health Questionnaire; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1One additional RCT could not be included in the narrative synthesis due to missing data in the control group (Thompson 2012).
2Based on reported values from four studies included in the analysis, as one study reported change scores only (Unger 2013).
aDowngraded by ‐1 for imprecision: result was based on a single study with a small sample size.
bDowngraded by ‐2 for imprecision: wide CI and result was based on a single study with a small sample size.
cDowngraded by ‐1 for risk of bias: high risk of bias for blinding and outcome was subjectively measured; unclear risk of bias for allocation concealment.
dDowngraded by ‐1 for inconsistency: there was considerable statistical heterogeneity (> 75%). One study found a large effect whereas the other study found a small effect. However, the direction of effects appeared to be consistent.
eDowngraded by ‐1 for imprecision: small sample size and final SDs for one study were obtained from reported baseline scores, as post‐intervention SDs were not reported.
fDowngraded by ‐1 for risk of bias: high risk of bias for blinding and outcome was subjectively measured.
gDowngraded by ‐1 for imprecision: small sample size and the CI encompassed values indicating both improvement and worsening in this outcome.
hDowngraded by ‐1 for imprecision: result was based on a single study with a small sample size and CI was wide, encompassing a large effect but also little or no effect.
Summary of findings 6. Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic.
| Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic | ||||||
| Patient or population: migrants Setting: all settings Intervention: culturally and literacy adapted audio‐/visual education without personal feedback Comparison: written information on the same topic (standard brochure, or literacy adapted pamphlet) | ||||||
| Outcome category – outcome(s)* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with written information | Risk with audio‐/visual education | |||||
|
Health literacy – Time point a: short‐term (up to 1 month post‐intervention)*** (1) Diabetes health literacy Assessed with:
(2) Appraising health information Measured with:
Lower score is better (3) Applying health information Multiple measures used:
Higher score is better Time point b: medium‐term (3 months post‐intervention) (1) Competencies (inhaler use technique)
Higher score is better (2) Understanding health information Multiple measures used:
Higher scores are better |
Time point a: short‐term (1) Diabetes health literacy |
— | 240 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Audio‐/visual education without personal feedback compared to written information on the same topic probably has little or no effect on diabetes health literacy. | |
| The mean diabetes health literacy in the control group was 53% | The mean diabetes health literacy in the intervention group was 2.00 points higher (0.15 lower to 4.15 higher) | |||||
| (2) Appraising health information | — | 608 (1 RCT) |
⊕⊕⊕⊝ Moderateb | Audio‐/visual education without personal feedback compared to written information on the same topic probably improves the appraisal of health information (decisional conflict) 1 month post‐intervention. | ||
| The mean score in the intervention group was 31.32 | The mean decisional conflict score in the intervention group was 9.88 points lower (12.87 lower to 6.89 lower) than in the control group | |||||
|
(3) Applying health information Made informed decision regarding HPV vaccination |
RR 1.51 (1.29 to 1.77) | 608 (1 RCT)3 |
⊕⊕⊕⊝ Moderateb | Audio‐/visual education without personal feedback compared to written information on the same topic probably improves the application of health information (making an informed decision) 1 month post‐intervention. | ||
| 415 per 1000 | 627 per 1000 (535 to 735) |
|||||
|
Time point b: medium‐term (1) Competencies (inhaler use technique) |
— | 176 (2 RCTs) |
⊕⊕⊝⊝ Lowa,c | Audio‐/visual education without personal feedback compared to written information on the same topic may slightly improve competencies (inhaler use technique) 3 months post‐intervention. | ||
| The mean inhaler use technique in the control groups was 5.2 points4 | The mean inhaler use technique in the intervention group was (0.98 points higher (0.26 higher to 1.70 higher) | |||||
|
(2) Understanding health information One RCT (n = 85) reported that the mean understanding of physician's instruction in the intervention group was 0.04 higher (0.55 lower to 0.63 higher) than in the control group One RCT (n = 43) reported that the mean understanding of pulmonary rehabilitation procedures in the intervention group was 0.30 higher (0.76 lower to 1.36 higher) than in the control group. |
— | 128 (2 RCTs) |
⊕⊕⊝⊝ Lowa,c | Audio‐/visual education without personal feedback compared to written information on the same topic may have little or no effect on understanding of health information 3 months post‐intervention. | ||
| Quality of life – not measured | — | — | — | — | — | The effect of audio‐/visual education without personal feedback on quality of life is unknown, as there was no direct evidence identified. |
|
Health‐related knowledge – Time point a: short‐term (up to 1 month post‐intervention) Any health‐related knowledge Standardised on score 0 (no knowledge) to 100 (perfect knowledge) Time point b: medium‐term (up to 6 months post‐intervention) Any health‐related knowledge Standardised on score 0 (no knowledge) to 100 (perfect knowledge) |
Time point a: short‐term | — | 987 (3 RCTs) |
⊕⊕⊝⊝ Lowd,e | Audio‐/visual education without personal feedback compared to written information on the same topic may slightly improve health‐related knowledge up to 1 month post‐intervention. | |
| The mean health‐related knowledge score ranged from 59.2% to 71.9%5 | The mean knowledge score in the intervention group was 8.35 points higher (0.32 lower to 17.02 higher) | |||||
| Time point b: medium‐term | — | 979 (3 RCTs) |
⊕⊝⊝⊝ Very lowe,f,g | We are uncertain whether audio‐/visual education without personal feedback compared to written information on the same topic improves health‐related knowledge up to 6 months post‐intervention. One study (n = 85) did not report data in a way that could be extracted for meta‐analysis but reported no difference in asthma knowledge; very low‐certaintyc,i |
||
| The mean cancer‐related knowledge score across control groups ranged from 58% to 67% | The mean cancer‐related knowledge score in the intervention groups was 7.30 points higher (3.73 lower to 18.32 higher) | |||||
|
Health outcome – Depression Assessed with:
Lower score is better Time point: long‐term (12 months post‐intervention) |
The mean depression score in the control group was 4.5 | The mean depression score was 0.60 points lower (1.37 lower to 0.17 higher) | — | 445 (1 RCT) |
⊕⊕⊝⊝ Lowh | Audio‐/visual education without personal feedback compared to written information on the same topic may have little or no effect on depression 12 months post‐intervention. |
|
Health behaviour – Time point a: medium‐term (up to 6 months post‐intervention) Any cancer screening uptake Assessed with:
Time point b: long‐term (12 months post‐intervention) Documentation of new advance care planning Assessed with:
|
Time point a: medium‐term Any cancer screening uptake |
RR 1.07 (0.95 to 1.20) | 803 (2 RCTs) | ⊕⊕⊝⊝ Lowe,f | Audio‐/visual education without personal feedback may have little or no effect on any cancer screening uptake up to six months post‐intervention, when compared to written information on the same topic. | |
| 513 per 1000 | 549 per 1000 (487 to 616) | |||||
|
Time point b: long‐term Documentation of new advance care planning |
RR 1.49 (1.13 to 1.97) | 445 (1 RCT) |
⊕⊕⊕⊝ Moderatej | Audio‐/visual education without personal feedback compared to written information on the same topic probably improves documentation of advance care planning 12 months post‐intervention. | ||
| 257 per 1000 | 382 per 1000 (290 to 506) | |||||
|
Self‐efficacy – Time point a: short‐term (immediately post‐intervention) Self‐efficacy in accessing breast cancer‐related advice or information Assessed with:
Any cancer‐related self‐efficacy Multiple measures used (1) Pooled findings:
(2) Unpooled finding:
Higher score is better Time point: medium‐term (up to 6 months post‐intervention) |
Time point a: short‐term One RCT found little or no difference in self‐efficacy in accessing breast cancer‐related advice or information (MD 0.08 higher (0.02 lower to 0.18 higher)) |
— | 240 (1 RCT) |
⊕⊕⊝⊝ Lowj,k | Audio‐/visual education without personal feedback may have little or no effect on cancer‐related self‐efficacy immediately post‐intervention, when compared to written information on the same topic. | |
|
(1) Pooled findings The pooled analysis of 2 RCTs (N = 256) showed that the mean cancer‐related self‐efficacy in the intervention groups was 0.08 standard deviations higher (0.18 lower to 0.33 higher) three months post‐intervention. (2) Unpooled findings One RCT (N = 727) found little or no difference in self‐efficacy regarding Pap testing between the intervention and the control group (RR 1.02, 95% CI 0.98 to 1.06) 6 months post‐intervention. One study (n = 43) that did not report data in a way in which an MD and a spread of scores could be calculated, found that the group receiving audio‐/visual education had a slightly higher mean self‐efficacy for managing COPD but the CIs encompassed both an improvement and a reduction, indicating little or no difference in self‐efficacy 3 months post‐intervention |
— | 1026 (4 RCTs) |
⊕⊕⊝⊝ Lowl,m | Audio‐/visual education without personal feedback may have little or no difference in cancer‐related self‐efficacy 3 months post‐intervention, when compared to written information on the same topic. | ||
| Health service use – not measured | — | — | — | — | — | The effect of audio‐/visual education without personal feedback on health service use is unknown, as there was no direct evidence identified. |
|
Adverse events – Anxiety Assessed with GAD‐7 (score range: 0 to 21) Lower scores are better Time point: long‐term (12 months post‐intervention) |
The mean anxiety score in the control group was 3.76 | The mean anxiety score was 0.70 points lower (1.40 lower to 0.00 higher). | — | 445 (1 RCT) |
⊕⊕⊕⊝ Moderatej | Audio‐/visual education without personal feedback probably has little or no effect on anxiety 12 months post‐intervention. |
| *More detail on scoring and direction for each outcome measure is provided in Table 2; Table 8; Table 5; Table 3; Table 4; Table 6, **The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI), ***Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. CI: confidence interval; DHLS: Diabetes Health Literacy Survey; GAD‐7: Generalised Anxiety Disorder‐7; n.r.: not reported; PHQ‐8: Patient Health Questionnaire; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Cut‐off values for DHLS scores are as follows: inadequate, ≤ 59%; marginal, 60% to 74%; adequate, ≥ 75% (Calderón 2014).
2Scores ≤ 25 are associated with following through on decisions; scores > 37.5 are associated with delay in decision‐making (O'Connor 1993).
3One RCT could not be included in the analysis due to missing effect measures for both the intervention and the control group (Unger 2013).
4Based on results reported in Poureslami 2016b, as there were inconsistencies in the reported final scores of Poureslami 2016a between the publications related to this study. Both studies had four intervention arms. Group 1, 2 and 3 watched different videos and group 4 read a pictorial pamphlet on the same topic. We combined group 1,2 and 3 to create a single pairwise comparison with group 4. The results of each study group are reported narratively in Table 17.
5Based on two out of the three studies included in the analysis, as one study reported change scores only (Unger 2013).
6Scores ranging from 0 to 7 represent no clinically meaningful anxiety or depression (Zigmond 1983).
aDowngraded by ‐1 for imprecision: small sample size and CI encompassed both benefit and harm.
bDowngraded by ‐1 for risk of bias: unclear allocation concealment and high risk of bias for blinding and outcome was either purely subjectively measured (for appraising health information) or a composite variable of self‐reported decision and cut‐off value on a knowledge scale (7 out of 12 correct).
cDowngraded by ‐1 for risk of bias: unclear risk or high risk for multiple domains including random sequence generation and allocation concealment in the included study/studies.
dDowngraded by ‐1 for inconsistency: considerable statistical heterogeneity (> 75%) due to inconsistent direction of effects.
eDowngraded by ‐1 for imprecision: CI was wide and/or encompassed values indicating both improvement and worsening in this outcome.
fDowngraded by ‐1 for risk of bias: unclear or high risk of bias for random sequence generation and allocation concealment in one study.
gDowngraded by ‐1 for inconsistency: considerable statistical heterogeneity (> 75%); two studies were in favour of audio‐/visual education without feedback and one study was in favour of written information on the same topic.
hDowngraded by ‐2 for imprecision: result was based on a single study and the CI encompassed values indicating both an improvement and a worsening in the outcome.
iDowngraded by ‐2 for imprecision: result was based on a single study with a small sample size and four study arms, and the results were not reported in such a way that they could be extracted for meta‐analysis. No composite score for three knowledge items was reported (the authors used a Likert scale but not a true/false questionnaire) and the score range was missing so that the results could not be standardised as scores on a scale ranging from 0 to 100.
jDowngraded ‐1 for imprecision: result was based on a single study and the CI encompassed values indicating a decrease in anxiety but also a null effect.
kDownraded by ‐1 for risk of bias: high risk of bis for blinding and the result was subjectively measured.
lDowngraded by ‐1 for imprecision: the CI of the pooled analysis and the CI of one study that reported a risk ratio were precise but encompassed values indicating both an improvement and a reduction in the outcome. The other study did not report a composite score, but subgroup analyses per study group (four groups) and per item (five items) only; three out of five CIs reported in this study encompassed both an improvement and a reduction. However, the point estimates of all four studies in this synthesis indicated little to no effect on self‐efficacy, so that no further downgrading was conducted.
mDowngraded by ‐1 for risk of bias: unclear risk of bias for blinding in two studies and high risk of bias in one study, and the outcome was subjectively measured. In addition, there was unclear risk of bias for random sequence generation and/or allocation concealment in two studies.
11. Outcome category: health literacy ‐ competencies.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD)* |
Notes |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Poureslami 2016a (4 study arms) | Asthma medication management |
Inhaler use technique; direct observation (2 observers) (Participants demonstrated correct use and had to describe each step, 1 point for appropriate use per step, standard checklist, 0 to 9, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative, peer‐led video): 21 Group 3 (both videos): 20 Group 4 (pamphlet): 22 |
At 3‐month follow‐up | Group 1: 2.71, 95% CI 1.35 to 4.06 Group 2: 1.95, 95% CI 0.99 to 2.91) Group 3: 1.53, 95% CI 0.66 to 2.40 |
Group 4: 1.05 (‐0.10 to 2.20) | Change scores are reported Results adjusted for age, gender, educational level and ethnicity |
| Poureslami 2016b (4 study arms) | COPD medication management | Inhaler use technique; direct observation (2 observers) (Participants demonstrated correct use and had to describe each step, 10‐item‐validated inhaler‐specific checklist, standard checklist, 0 to 9, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative, peer‐led video): 26 Group 3 (both videos): 29 Group 4 (pamphlet): 14 |
At 3‐month follow‐up | Group 1: 6.8
(2.0) Group 2: 5.9 (2.0) Group 3: 5.8 (1.6) |
Group 4: 5.2 (1.4) | — |
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally adapted audio‐/visual education without personal feedback | |||||||
| Poureslami 2016a | Asthma medication management |
Inhaler use technique; direct observation (2 observers) (Participants demonstrated correct use and had to describe each step, 1 point for appropriate use per step, standard checklist, 0 to 9, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative. peer‐led video): 21 |
At 3‐month follow‐up | Group 1: 2.71, 95% CI 1.35 to 4.06 | Group 2: 1.95, 95% CI 0.99 to 2.91) | Change scores are reported Results adjusted for age, gender, educational level and ethnicity |
| Poureslami 2016b | COPD medication management | Inhaler use technique; direct observation (2 observers) (Participants demonstrated correct use and had to describe each step, 10‐item‐validated inhaler‐specific checklist, standard checklist, 0 to 9, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative. peer‐led video): 26 |
At 3‐month follow‐up | Group 1: 6.8 (2.0) | Group 2: 5.9 (2.0) | — |
*Unadjusted mean (SD) if not otherwise reported.
CG: control group; CI: confidence interval; COPD: chronic obstructive pulmonary disease; IG: intervention group; SD: standard deviation
Summary of findings 7. Culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback.
| Culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback | ||||||
| Patient or population: migrants Setting: community Intervention: audio‐/visual education without personal feedback (narrative video) Comparison: another audio‐/visual education without personal feedback (factual knowledge video) | ||||||
| Outcome category – outcome(s)* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with factual knowledge video | Risk with narrative educational video | |||||
|
Health literacy – (1) Competencies (inhaler use technique) Assessed with:
Higher score is better (2) Understanding health information (understanding physician's instruction) Assessed with:
Higher score is better Time point: medium‐term (3 months post‐intervention) (3) Applying health information (intention for cervical cancer screening using Pap test) Assessed with:
Higher score is better Time point: medium‐term (6 months post‐intervention) |
1) Competences (inhaler use technique) | — | 91 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | We are uncertain whether educational videos compared to factual knowledge videos improve competencies (inhaler use technique) 3 months post‐intervention. | |
| The mean inhaler use technique score in the control group was 7 points | The mean inhaler use technique in the group who watched the narrative video was 0.89 lower (1.84 lower to 0.07 higher) than in the group who watched the knowledge video | |||||
|
(2) Understanding health information One RCT (n = 43) reported that the mean understanding of physician's instruction in the group who watched the narrative video was 0.15 lower (0.72 lower to 0.42 higher) than in the group who watched the knowledge video |
— | 43 (1 RCT)1 |
⊕⊝⊝⊝ Very lowa,b | We are uncertain whether educational videos compared to factual knowledge videos improve the understanding of health information 3 months post‐intervention. | ||
| (3) Applying health information | RR 1.97 (0.83 to 4.69) | 109 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | We are uncertain whether narrative educational videos compared to factual knowledge videos improve the application of health information 6 months post‐intervention. | ||
| 125 per 1000 | 246 per 1000 (104 to 586) |
|||||
| Quality of life – not measured | — | — | — | — | — | The effect of a narrative educational video compared to a factual knowledge video on quality of life is unknown as there was no direct evidence identified. |
|
Health‐related knowledge – Any health‐related knowledge
Higher scores are better. Time point: medium‐term (3 to 6 months post‐intervention) |
One RCT (n = 109) found that the mean heath‐related knowledge score in the group who watched the narrative video was 1.12 points higher (4.63 lower to 6.87 higher). The mean cervical cancer knowledge score in the control group was 66%. One RCT (n = 43) found that the mean asthma knowledge score in the group who watched the narrative video was higher than in the group who watched the physician‐led knowledge video (MD 0.85 higher (1.07 lower to 2.76 higher).2 |
— | 152 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | We are uncertain whether narrative educational videos compared to factual knowledge videos improve health‐related knowledge up to 6 months post‐intervention. | |
| Health outcome – not measured | — | — | — | — | — | The effect of narrative educational videos compared to a factual knowledge video on health outcomes is unknown as there was no direct evidence identified. |
|
Health behaviour – Cervical cancer screening Assessed with:
Time point: medium‐term (6 months post‐intervention) |
292 per 1000 | 376 per 1000 (219 to 651) | RR 1.29 (0.75 to 2.23) | 109 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | We are uncertain whether narrative educational videos compared to factual knowledge videos improve cervical cancer screening behaviour 6 months post‐intervention. |
| Self‐efficacy – not measured | — | — | — | — | — | The effect of a narrative educational video compared to a factual knowledge video on self‐efficacy is unknown as there was no direct evidence identified. |
| Health service use – not measured | — | — | — | — | — | The effect of a narrative educational video compared to a factual knowledge video on health service use is unknown as there was no direct evidence identified. |
| Adverse events – not measured | — | — | — | — | — | The effect of a narrative educational video compared to a factual knowledge video on adverse events is unknown as there was no direct evidence identified. |
| *We report on our predefined outcome categories and assigned all outcomes that we considered eligible for this review to one of these categories (see Types of outcome measures). More detail on scoring and direction for each outcome measure is provided in Table 5; Table 11; Table 4); **The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ***Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: from 6 weeks up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1One RCT could not be included in the narrative synthesis as the participants who watched the narrative video and those who watched the knowledge video were not directly compared to each other, but both were compared to a control group who read a pictorial pamphlet (Poureslami 2016b). Details are shown in Table 19.
2No score range was reported, but subgroup analyses adjusted for age, gender, educational level and ethnicity per study group and knowledge item only. Therefore, we could not standardise the reported values on a scale ranging from 0 to 100. However, the three knowledge items were combined to calculate an MD across the items.
aDowngraded by ‐1 for risk of bias: unclear risk of bias for random sequence generation and/or allocation concealment in all studies.
bDowngraded by ‐2 for imprecision: small sample size and/or the results stemmed from a single study. In addition, the CI included values that encompassed both an improvement and a worsening.
12. Outcome category: health literacy ‐ understanding health information.
| Study ID | Health topic | Measure | No. of participants | Time point(s) |
Intervention arm Mean (SD)* |
Control arm Mean (SD)* |
Notes |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Poureslami 2016a (4 study arms) | Asthma | Understanding of and adherence to physician's instructions (5 items, 0 to 5, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative, peer‐led video): 21 Group 3 (both videos): 20 Group 4 (pamphlet): 22 |
3 months post‐intervention | Group 1: 0.53, 95% CI 0.12 to 0.94 Group 2: 0.38, 95% CI ‐0.06 to 0.82 Group 3: 0.24, 95% CI ‐0.19 to 0.66 |
Group 4: 0.35, 95% CI ‐0.22 to 0.92 | Change scores are reported Adjusted for age, gender, educational level and ethnicity |
| Poureslami 2016b (4 study arms) | COPD | Understanding pulmonary rehabilitation procedures Questionnaire; text passage based on Canadian Thoracic Society COPD assessment guidelines, developed by the research team and related questions answered by participants (Correct/incorrect, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative, peer‐led video): 26 Group 3 (both videos): 29 Group 4 (pamphlet): 14 |
3 months post‐intervention | — | Change scores are reported; adjusted for age, gender, educational level and disease severity Group 1 vs group 4: MD 2.14 (95% CI 0.73 to 3.16) Group 2 vs group 4: MD 2.22 (95% CI0.86 to 3.30) Group 3 vs group 4: MD 0.30 (95% CI ‐0.76 to 1.36) |
|
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally and literacy adapted audio‐/visual education without personal feedback | |||||||
| Poureslami 2016a | — | Understanding of and adherence to physician's instructions (5 items, 0 to 5, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative peer‐led video): 21 |
3 months post‐intervention | Group 1: 0.53, 95% CI 0.12 to 0.94 | Group 2: 0.38, 95% CI ‐0.06 to 0.82 | Change scores are reported Adjusted for age, gender, educational level and ethnicity |
| Poureslami 2016b | — | Understanding pulmonary rehabilitation procedures Questionnaire; text passage based on Canadian Thoracic Society COPD assessment guidelines, developed by the research team and related questions answered by participants (Correct/incorrect, higher score is better) |
Group 1 (physician‐led knowledge video): 22 Group 2 (narrative peer‐led video): 26 |
3 months post‐intervention | — | Change scores are reported; adjusted for age, gender, educational level and disease severity Group 2 vs group 4 (pamphlet): 2.22, 95% CI 0.86 to 3.30, P < 0.05 Group 1 vs group 4 (pamphlet): 2.14, 95% CI 0.73 to 3.16 |
|
| 8 Culturally and literacy adapted medical instruction vs no health literacy intervention | |||||||
| Bailey 2012 | Medication understanding | Demonstration by means of correct dosage in dosing tray (5 items, frequency and spacing, 0 to 5, higher score is better) |
IG: 102 CG: 100 |
Immediately post‐intervention | Median: 4.0 (IQR 3.0 to 5.0) | Median: 3.0 (IQR 2.0 to 4.0) | P < 0.0001 |
| Kheir 2014 (3 study arms) | Medication understanding | Interpretation of label contents (11 medicine labels, 1 = no comprehension to 3 = full comprehension, 1 to 3, higher score is better) |
Group 1 (standard text labels + verbal instructions): 40 Group 2 (pictogram‐only): 47 Group 3 (pictogram + verbal instructions): 36 |
Immediately post‐intervention | — | — | For 10 of the 11 medicine instructions, participants in group 3 (pictogram + verbal instructions) consistently scored higher than participants in group 1 (standard text labels + verbal instructions), while group 1 had higher scores than group 2 (pictogram‐only) for 8 of the 11 labels. |
| Mohan 2014 | Medication understanding | MUQ (0 to 100, higher score is better) |
IG: 99 CG: 101 |
At 1‐week follow‐up | 86.4 (12.6) | 76.4 (18.0) | Adjusted difference P < 0.001 |
*Unadjusted mean (SD) if not otherwise reported.
CG: control group; CI: confidence interval; COPD: chronic obstructive pulmonary disease; IG: intervention group; IQR: interquartile range; MD: mean difference; MUQ: Medication Understanding Questionnaire
Summary of findings 8. Culturally and literacy adapted medical instruction versus no health literacy intervention.
| Culturally and literacy adapted medical instruction versus no health literacy intervention | ||||||
| Patient or population: migrants Setting: all settings Intervention: culturally and literacy adapted medical instruction Comparison: no health literacy intervention (usual care, standard written information + verbal instruction) | ||||||
| Outcome category – outcome(s)* | Anticipated absolute effects** (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no health literacy intervention | Risk with literacy adapted written information | |||||
|
Health literacy – Medication understanding Multiple measures used:
Higher scores are better Time point: short‐term (up to 1 week post‐intervention)*** |
Three RCTs reported on 3 health literacy outcomes related to the understanding of medical instructions. One RCT (n = 202) reported that health literacy informed medication instructions improved the correct dosage in the dosing tray immediately post‐intervention (IG: median 4.0, IQR: 3.0 to 5.0; CG: median: 3.0, IQR: 2.0 to 4.0). Another RCT (n = 123) reported that pictograms plus verbal instruction improved the correct interpretation of label contents in 10 out of 11 medical instructions immediately post‐intervention (no composite score reported). One RCT (n = 200) reported that a literacy adapted plain language text in combination with an illustrated medication list improved medication understanding assessed with MUQ at 1 week follow‐up (10 points higher (5.70 higher to 14.30 higher)). |
— | 478 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | Culturally and literacy adapted medical instructions compared to no health literacy intervention may improve medication understanding up to 1 week post‐intervention. | |
| Quality of life – not measured | — | — | — | — | — | The effect of the intervention on quality of life is unknown as there was no direct evidence. |
| Health outcome – not measured | — | — | — | — | — | The effect of the intervention on health outcomes is unknown as there was no direct evidence. |
| Health‐related knowledge – not measured | — | — | — | — | — | The effect of the intervention on health‐related knowledge is unknown as there was no direct evidence. |
|
Health behaviour – Medication adherence Assessed with:
Time point: short‐term (up to 1 week post‐intervention) |
The mean self‐reported medication adherence in the control group was 9.9% | The mean medication adherence score in the intervention group was 0.5 points higher (0.1 lower to 1.1 higher) | — | 200 (1 RCT) | ⊕⊕⊝⊝ Lowc,d | Culturally and literacy adapted medical instructions compared to no health literacy intervention may have little or no effect on health behaviour. |
| Health service use – not measured | — | — | — | — | — | The effect of the intervention on health service use is unknown as there was no direct evidence. |
| Self‐efficacy ‐ not measured | — | — | — | — | — | The effect of the intervention on self‐efficacy is unknown as there was no direct evidence. |
| Adverse events – not measured | — | — | — | — | — | The effect of the intervention on adverse events is unknown as there was no direct evidence. |
| *We report on our predefined outcome categories and assigned all outcomes that we considered eligible for this review to one of these categories (see Types of outcome measures). More detail on scoring and direction for each outcome measure is provided in Table 19; Table 4; **The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ***Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: from 6 weeks up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. ARMS: Adherence to Refills and Medications Scale; CG: control group; CI: confidence interval; IG: intervention group; IQR: interquartile range; MUQ: Medication Understanding Questionnaire; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by ‐1 for risk of bias: one study was at high risk of bias for blinding; unclear allocation concealment in one other study.
bDowngraded by ‐1 for imprecision: data from two studies were not reported in a way that made it possible to calculate an MD.
cDowngraded by ‐1 for risk of bias: high risk of bias for blinding.
dDowngraded by ‐1 for imprecision: results were based on a single study with a small sample size and the CI encompassed both an improvement and a worsening.
Summary of findings 9. Female migrants' benefit of any health literacy intervention versus male migrants' benefit of any health literacy intervention.
| Female migrants' benefit of any health literacy intervention versus male migrants' benefit of any health literacy intervention | ||||||
|
Patient or population: migrants Settings: all settings Intervention: any health literacy intervention Comparison: no health literacy intervention, or written information on the same topic, or unrelated health literacy intervention | ||||||
| Outcome category– outcome(s) | Illustrative comparative risks** (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk for female migrants | Corresponding risk for male migrants | |||||
|
Health literacy – Multiple outcomes and measures used: (1) Generic health literacy
(2) Disease‐specific health literacy
Higher scores are better Time point: short‐term (immediately post‐intervention)*** |
(1) Generic functional health literacy One RCT that compared a health literacy skills building course to no health literacy intervention reported that female migrants scored higher in functional health literacy immediately post‐intervention (2.78 points higher (4.35 lower to 9.91 higher)) |
— | 77 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | We are uncertain whether female migrants' generic functional health literacy improves more than that of male migrants when receiving health literacy skills building courses. | |
|
(2) Disease‐specific health literacy One RCT that compared audio‐/visual education without personal feedback to written information on the same topic found that the intervention may improve diabetes health literacy in women more than in men (MD 5.00 higher (0.62 higher to 9.38 higher)). The mean diabetes health literacy score in men was 56%1 |
— | 118 (1 RCT) |
⊕⊕⊝⊝ Lowc | Female migrants' diabetes‐specific health literacy may improve slightly more than that of male migrants, when receiving audio‐/visual education intervention. | ||
| Quality of life – not measured | — | — | — | — | The effect of any health literacy intervention on female compared to male migrants' quality of life is unknown as there was no direct evidence identified. | |
| Health‐related knowledge – not measured | — | — | — | — | The effect of any health literacy intervention on female compared to male migrants' health‐related knowledge is unknown as there was no direct evidence identified. | |
| Health outcome – not measured | — | — | — | — | The effect of any health literacy intervention on female compared to male migrants' health outcome is unknown as there was no direct evidence identified. | |
|
Health behaviour – Time point a: short‐term (immediately post‐intervention) Cardiovascular health behaviour
Higher score is better Time point b: long‐term (approx. 12 months post‐intervention) New documentation of advance care planning
|
Time point a: short‐term Cardiovascular health behaviour One RCT that compared a health literacy skills building course to no health literacy intervention (standard ESL course) found that women scored higher on the cardiovascular health behaviour questionnaire than men in the intervention group (MD 2.07 (5.04 lower to 9.18 higher)) |
— | 77 (1 RCT) |
⊕⊝⊝⊝ Very lowb,d | We are uncertain whether female migrants' cardiovascular health behaviour improves more than that of male migrants when receiving health literacy skills building courses. | |
|
Time point b: long‐term New documentation of advance care planning One RCT that compared audio‐/visual education without personal feedback to written information on the same topic found that health behaviour improved in both men and women in the intervention group. Female migrants were slightly more likely to have new documentation of advance care planning than male migrants (RR 1.27, 95% CI 0.90 to 1.79) 12 months post‐intervention. |
— | 219 (1 RCT) |
⊕⊕⊝⊝ Lowc | Audio‐/visual education without personal feedback may have little or no effect on new documentation of advance care planning between female and male migrants 12 months post‐intervention. | ||
| Health service use – not measured | — | — | — | — | The effect of any health literacy intervention on female compared to male migrants' health service use is unknown as there was no direct evidence identified. | |
| Self‐efficacy – not measured | — | — | — | — | The effect of any health literacy intervention on female compared to male migrants' self‐efficacy is unknown as there was no direct evidence identified. | |
| Adverse events – not measured | — | — | — | — | The effect of any health literacy intervention on adverse events for female compared to male migrants is unknown as there was no direct evidence identified. | |
| *We report on our predefined outcome categories and assigned all outcomes that we considered eligible for this review to one of these categories (see Types of outcome measures). More detail on scoring and direction for each outcome measure is provided in Table 19 and Table 5; **The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval ) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ***Short‐term: immediately up to 6 weeks after the total intervention programme was completed; medium‐term: from 6 weeks up to and including 6 months after the total intervention programme was completed; long‐term: longer than 6 months after the total intervention programme was completed. CI: confidence interval; CSC: Cardiovascular Health Behaviour Questionnaire; DHLS: Diabetes Health Literacy Survey; ESL: English as a second language; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; TOFHLA: Test of Functional Health Literacy in Adults | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Scoring of diabetes health literacy wasinadequate ≤ 59%, marginal 60% to 70% or adequate ≥ 75%.
aDowngraded by ‐1 for risk of bias: unclear risk of bias for random sequence generation and allocation concealment.
bDowngraded by ‐2 for imprecision: results were based on a single study with a small sample size (fewer than 100) and/or CIs encompassed values favouring either female or male migrants.
cDowngraded by ‐2 for imprecision: results were based on a single study with a small sample size and CIs were wide or encompassed values favouring either female or male migrants.
dDowngraded by ‐1 for risk of bias: unclear risk of bias for random sequence generation and allocation concealment, high risk of bias for blinding and outcome was subjectively measured.
Background
International migration is a complex phenomenon of increasing importance in an era of rising globalisation. More than ever before, international migration touches all countries and affects all areas of daily living (IOM 2017). The growing presence of migrants, and refugees in particular, can have a complex impact on the healthcare systems of respective host countries, which face tremendous pressures in responding fast to new and increasing healthcare needs (Hunter 2016). However, evidence suggests persistent inequalities between migrants and non‐migrants in accessing and using health information and healthcare services (Abbas 2018; Lebano 2020). In addition, the ongoing COVID‐19 pandemic has shown that misinformation may exacerbate health‐related inequalities in the context of migration, and even further highlighted the importance of individual and organisational health literacy (Sentell 2020).
Health literacy, understood as the ability to access, understand, appraise and apply health information (Sørensen 2012), has become a key contributor to effective disease management, improved health outcomes and the overall efficiency of health care. Furthermore, health literacy is an essential concept with regard to health‐related autonomous decisions and health behaviour (Woopen 2015). Evidence suggests that the individual's perceived health literacy is not only associated with healthy lifestyle choices (e.g. physical activity), but also with one's general subjective health status and health‐related quality of life (HLS19 Consortium 2021). In contrast, limitations in health literacy have been shown to be associated with higher rates of chronic diseases, more frequent hospitalisations and emergency treatments, higher healthcare expenditures, the reduced use of preventive measures, lower treatment adherence, and an increased risk of morbidity and mortality (Berkman 2011; Eichler 2009; HLS‐EU Consortium 2012; HLS19 Consortium 2021; Paasche‐Orlow 2007; Rasu 2015).
In studies conducted in Germany, migrants with low language proficiency and older people with a migrant background reported experiencing particular problems in understanding and processing health information, and in translating it into healthy choices (Berens 2022a; Quenzel 2016). These results are in line with studies from Australia, Canada and the USA that report ethnic minority status, limited language proficiency or having a migration experience as a risk factor for health literacy limitations (Beauchamp 2015; Christy 2017; Ng 2014; Sentell 2012). Similar critical evidence was found for the health literacy levels of refugees in Sweden (Wångdahl 2014). Although research on health literacy indicates that having a migrant background is not the sole issue (Berens 2022a; Ganahl 2016; HLS19 Consortium 2021), it seems likely to function as a multiplier in creating health inequalities. Health literacy has shown to be a social determinant of health (Nutbeam 2021; Pelikan 2018). It has a social gradient, including income, social status, education and age (Berkman 2011; HLS‐EU Consortium 2012; HLS19 Consortium 2021), and some of these factors can be even more pronounced in the context of migration. Thus, improving health literacy, both at the individual and population level, is of crucial importance for a sustainable and equitable promotion of public health.
Description of the condition
Health literacy
The notion of health literacy was initially mentioned in the setting of school‐based health education in the 1970s (Simonds 1974). In the medical context, the first definitions referred to health literacy as "the constellation of skills, including the ability to perform basic reading and numerical tasks required to function in the healthcare environment" (AMA 1999). This rather passive understanding of the individual acting as a patient ‐ today referred to as functional health literacy ‐ has rapidly expanded to a more complex concept, including individual competencies and resources to take healthy choices and act on health information as an empowered consumer (Nutbeam 2000). In the European region, research on health literacy gained popularity among researchers and health policy‐makers when the European Health Literacy Consortium presented its work in 2012, providing for the first time population‐based data on citizens' health literacy in eight European countries (HLS‐EU Consortium 2012). Based on a systematic review of existing definitions and conceptual frameworks, the researchers around Sørensen 2012 developed an integrated model of health literacy by systematically considering individual, social and systemic influencing factors, determinants and domains that can affect an individual's health literacy (see Figure 1). Referring to this underlying model, “health literacy is linked to literacy and entails people's knowledge, motivation and competencies to access, understand, appraise, and apply health information in order to make judgements and take decisions in everyday life concerning healthcare, disease prevention and health promotion to maintain or improve quality of life during the life course” (Sørensen 2012). A key component of this definition is the procedural character of health information processing, which is expressed in the following four steps:
1.
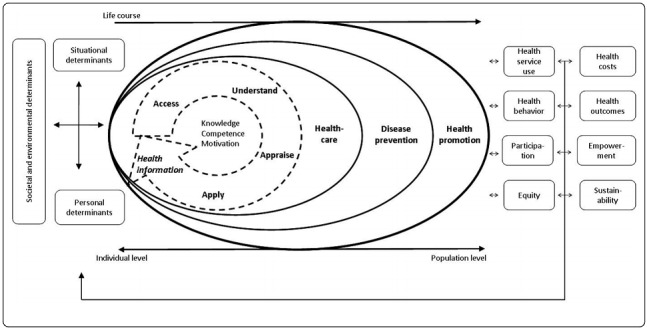
Integrated model of health literacy (Sørensen 2012)
access;
understand;
appraise; and
apply.
Individual prerequisites such as knowledge, motivation and skills or competencies (e.g. reading and writing abilities) are necessary to pass through the four steps of health information processing. Applying these prerequisites, health literacy requires a person to search for and find relevant health information, to understand it sufficiently, to appraise it in the context of one's own value system and finally to apply the information, for example by making healthy choices. Thus, the individual's ability to process health information is closely linked to health‐related behaviour (e.g. medication adherence), which can in turn influence health‐related outcomes (e.g. progression of disease). However, important to note is that causes of limited health literacy are not limited exclusively to an individual. Health literacy is determined by individual abilities and resources on the one hand and structural, situational and political conditions on the other hand (Dodson 2015; Parker 2009). For example, a recent migrant might have sufficient health literacy skills to successfully navigate the healthcare system in the country of origin, but might be challenged by the demands and complexity of the healthcare system in the host country. Thus, the health literacy environment (e.g. clinicians with cultural competence or the type of access to health services and reliable health information) plays a crucial role in determining the specific health literacy‐related challenges that migrants may encounter.
We applied the integrated model of health literacy as an umbrella framework in this review for assessing the effectiveness of health literacy interventions, focusing on the four steps of health information processing (access, understand, appraise and apply), and the involved cognitive, knowledge‐based and motivational aspects that contribute to a person’s health literacy.
Disease‐specific health literacy
A variety of context‐ and disease‐specific definitions and models of health literacy have emerged within many medical disciplines, such as for psychiatry (mental health literacy), oncology (cancer literacy) or endocrinology (diabetes literacy) (Mackert 2015). Health literacy is hereby described with regard to the particular disease‐specific demands concerning an individual, for instance the understanding of and adherence to a certain therapeutic regimen. Such disease‐specific approaches often focus on the acquisition of knowledge about the related disease, implying the causal relationship between knowledge and the respective behaviour. Just to name one, the concept of mental health literacy, for instance, was initially defined as "knowledge and beliefs about mental disorders which aid their recognition, management or prevention" (Jorm 1997). It was later extended with the mental disorder‐related knowledge that is necessary to benefit the mental health of oneself or others, referring thereby to the ability to recognise mental disorders, as well as to having the knowledge about their risk factors and causes, about effective self‐help strategies, and adequate time to seek professional help or to help others (Jorm 2000). To date, several mental disorder‐specific subcategories have emerged (e.g. depression literacy or suicide literacy) and new measurements evolve continuously.
Measurement of health literacy
To date, a broad variety of definitions and models have evolved around the world (Sørensen 2012). However, there is no uniformly applied definition of health literacy to date. Thus, measurements of health literacy are equally diverse, and depend on the underlying definition of health literacy (Altin 2014; Guzys 2015; Haun 2014), and on whether generic or disease‐specific health literacy should be assessed. Generic health literacy can, for example, be assessed using performance‐based or perception‐based assessment tools. Two of the most widely used performance‐based assessment tools are the Rapid Estimate of Adult Literacy in Medicine (REALM) (Davis 1991) and the Test of Functional Health Literacy (TOFHLA) (Parker 1995). These tools measure reading and writing abilities in the medical context (REALM, in this review, is also referred to as print literacy) and text understanding or numeracy skills (TOFHLA, in this review, is also referred to as functional health literacy). Perception‐based assessment tools such as the Health Literacy Questionnaire (HLQ) (Osborne 2013) or the European Health Literacy Questionnaire (HLS‐EU‐Q) (Sørensen 2013) measure self‐reported health literacy, including, for instance, the assessment of self‐perceived difficulties in processing health information with regard to health promotion, disease prevention and disease management (Sørensen 2013).
Disease‐specific assessment tools often address certain aspects of health literacy, which are seen to be important in the respective disease‐specific context (e.g. knowledge or attitudes towards professional help), others are based on established generic health literacy tools such as the TOFHLA or REALM, but use disease‐specific words or phrases (e.g. HIV‐specific terms) rather than general medical terminology. Knowledge is regarded as one of the major components of health literacy (Sørensen 2012), especially when it comes to applying it in certain (disease‐specific) contexts. In health literacy research, knowledge is usually assessed by measures that assess declarative knowledge, which is explicit knowledge that can be verbalised by questionnaires (i.e. knowing facts about a certain skill domain). Procedural knowledge, however, is represented in procedures for performing a certain skill (i.e. knowing how to do things) (Anderson 1982). The latter is closely related to competencies such as reading and writing abilities or numeracy skills. Thus, these skills are often assessed by administering disease‐specific health literacy measures that are based on established performance‐based tools such as TOFHLA.
Migration
We use the term migration as defined by the International Organization for Migration (IOM), which states that migration is “the movement of a person or a group of persons, either across an international border, or within a state. It is a population movement, encompassing any kind of movement of people, whatever its length, composition and causes; it includes migration of refugees, displaced persons, economic migrants, and persons moving for other purposes, including family reunification” (IOM 2018). Voluntary migration is often accompanied by the hope for improved living conditions for oneself or family members, better working opportunities, or study purposes. Forced migration can include coercion or obligation to flee from natural or human‐made disasters, extreme poverty, religious, sexual or political persecution, generalised violence, or armed conflicts such as civil war (IOM 2018; Moore 2004; Nuscheler 2013). However, making a clear‐cut distinction between forced and voluntary migration is not always feasible as the complexity of individual experiences is often on a forced‐voluntary continuum (Erdal 2018). As with health literacy, there is no uniformly applied definition of the term migrant at the international level. According to a recent definition proposed by the IOM, the term migrant can be used as an umbrella term that reflects the "common lay understanding of a person who moves away from his or her place of usual residence, whether within a country or across an international border, temporarily or permanently, and for a variety of reasons" (IOM 2019).
Independent of the reasons for peoples' movement, migration is a life‐changing experience that affects an individual's biography, his or her family development, and shapes several following generations. Migration includes risks and opportunities in social and economic conditions, as well as health (Razum 2008). Poor socio‐economic environments and living conditions, limited access to educational opportunities, and psychological stresses such as chronic work hazards are well examined causal factors leading to health inequalities (Marmot 2005). These factors can have a particularly strong impact on migrants' health because language barriers, racial discrimination or limited health systems knowledge are significant challenges to health improvement and preservation, and recovery from illness (Derose 2007; Harris 2006; Masseria 2010; Timmins 2002). Although migrants are often, at least initially, relatively healthy compared to most people in the host country, international studies indicate that immigrants and refugees tend to be vulnerable to poor mental health, certain communicable diseases such as tuberculosis and HIV/AIDS, and non‐communicable diseases such as diabetes, injuries and maternal and child health problems (Goosen 2014; Kirmayer 2011; Lindert 2009; Rechel 2013; Yun 2012). Certain migration trajectories are linked to specific health adversities before, during and after migration. For example, among refugees escaping from civil war the migration process can be accompanied by violence, exploitation by human traffickers, hunger and infectious diseases (IOM 2013; United Nations 2017). Furthermore, accessing affordable high‐quality health care in the host country can vary among healthcare systems and may depend on the legal status of the migrant (Bozorgmehr 2016; Rechel 2013; WHO 2010).
Gender
Gender is widely considered to describe roles, behaviours, identities and relations, whereas the terms sex typically refers to biological and physiological processes (Hammarström 2012). Given the behavioural and relational nature of the health literacy concept, we refer to differences between men's and women's health literacy as gender differences rather than sex differences (Sandford 1999). Therefore, we used the term gender to denote results concerning female and male migrants (and, had this been applicable, other genders).
Although differing in intensity, gender differences occur in all cultures and can be of critical importance at all stages of the migratory process (Malmusi 2010). Gender may influence both the reasons individuals migrate and the health outcomes they experience before, during and after migration. Thus, the process of migration is inherently gendered, influenced by gender roles, expectations and power dynamics. The intersectionality between gender, migration and their synergistic effects on health have been discussed in the scientific literature (Douki 2007; Malmusi 2010; Wandschneider 2020). Research shows, for example, that certain health risks are more common among women (e.g. sexual violence and abuse, human trafficking, or risks around childbirth and pregnancy), whereas accidents, physical stress or work hazards affect men more commonly (Douki 2007; Llácer 2007; Malmusi 2010; Schouler‐Ocak 2017). Additionally, a systematic review of social epidemiological literature found that stronger adherence to traditional gender norms, higher levels of gender inequality, gender‐based discrimination and gender‐based violence were associated with adverse health outcomes among migrants (Wandschneider 2020). These circumstances can influence why people need health information, and affect how health information is accessed, processed and translated into health‐related action.
Both gender and migration are factors that have received increased attention in relation to their roles as important determinants of health and health literacy (Svensson 2017). Simultaneously, there is a considerable lack of gender aggregated data in international migration research in general (Bircan 2022), and in health literacy research in particular (Aldin 2019; Chakraverty 2022). A recent review of 24 studies that included previously unpublished data from 15 studies found that men with a migrant background, although much less frequently examined, may have slightly lower health literacy than women. However, there was substantial heterogeneity between studies and the difference vanished when excluding studies with a high risk of bias (Chakraverty 2022). Nevertheless, to date it remains unclear how, and in which way, gender affects the health literacy of migrants or if female and male migrants perceive challenges regarding accessing, understanding, appraising and applying health information differently (Aldin 2019; Chakraverty 2020).
Considering equity in health literacy
A lack of evidence on equity has been described as a barrier to the use of systematic reviews by healthcare decision‐makers (Welch 2015). Considering equity in systematic reviews on health literacy is therefore of high importance for the effective implementation of health literacy interventions. Health equity is defined as "the absence of avoidable and unfair inequalities in health" (Welch 2012; Whitehead 1992). The emphasis of this concept is on the avoidance of unfair differences in health and related outcomes among individuals in a population and among different population groups. Differences in health across certain socio‐demographic characteristics, including age, sex and gender, or ethnicity, can be caused by discrimination or inadequate access to healthcare services, which hinders people from preserving and regaining health (Welch 2015).
The integrated model of health literacy developed by Sørensen 2012 (see Description of the condition) draws attention to the importance of equity in health literacy research across individuals and populations. The integrated model served as an equity model for this review because it includes relevant personal determinants such as gender and race, socio‐economic status and education, situational variables (e.g. the current physical environment), and culture as societal and environmental determinants of health literacy. The term race, albeit a scientifically unjustifiable concept (Williams 1997), which is used inconsistently throughout the literature (Kaplan 2003; Williams 1994), is often applied to denote immigrant groups such as so‐called Hispanics/Latinos/Latinas (López 2010). If this term was accompanied by information that the person who was categorised by race is a migrant, we would have used the term race (or the synonymous term 'ethnicity') as a personal determinant of health literacy. Thus, migration can be integrated in the model as a personal (i.e. race or ethnicity), situational (i.e. pre‐, peri‐ and post‐migration status), or societal and environmental factor (i.e. culture) to determine health literacy.
We followed the PRISMA‐Equity (PRISMA‐E) reporting guidelines for systematic reviews to acknowledge equity as an important determinant of health (Welch 2012; Welch 2015). We provided a strong rationale on gender and migration as important factors to be considered in health equity when discussing the improvement of health literacy. We formulated objectives that enabled the exploration of gender differences that may contribute to inequalities in health literacy. We applied an inclusive approach to the study population and ensured inclusion of different groups of migrants. Regarding data collection, we extracted and reported items related to equity using the PROGRESS‐Plus framework. Moreover, we considered issues around equity in our synthesis and discussion of findings (Welch 2015).
Description of the intervention
This review assesses different interventions with the purpose of improving individual health literacy in migrants or one of the four steps of health information processing (access, understand, appraise or apply health information). These interventions may have included community‐based health‐related interventions, such as community education or schooling programmes, and individual‐based health‐related interventions such as online provision of information, personal (face‐to‐face) provision of information, or others. Interventions could have been delivered by any person involved in the health care or social work field and working closely with migrants and their descendants. Furthermore, the outcomes of these interventions should have been measured using either an established assessment tool for health literacy as a construct, or an assessment tool that is capable of measuring the outcomes that are targeted in the intervention and which are related to the respective processing step. Health literacy could have been assessed using remote (e.g. online, telephone) or face‐to‐face questionnaires or surveys. Interventions for improving health literacy that target healthcare providers, services or information materials rather than the consumer, would have been included only if the effects of such interventions were directly measured in female and male migrants (How the intervention might work). We focused on interventions targeting individual health literacy. Broader interventions that address the health literacy environment solely, such as health literacy toolkits for health systems (Dodson 2015), or approaches to creating health literate healthcare organisations, exist (Brach 2012) but were beyond the scope of this review.
How the intervention might work
Specific design features of interventions targeted for low‐health‐literacy populations (e.g. presenting essential information first, presenting information in simple language or formats, or substantiated by video or illustrated narratives) have been shown to be effective in terms of improving comprehension of information. Furthermore, multiple interventions such as intensive self‐ and disease‐management or adherence interventions have shown promise in mitigating the effects of limited health literacy with regard to reduced emergency department visits and hospitalisations, and reduced disease prevalence (Berkman 2011; Sheridan 2011). A meta‐analysis indicated that, on average, health literacy interventions significantly improved participants' health literacy (22%) and treatment adherence (16%) among those who participated in a health literacy intervention compared to those who did not. However, particular methodological and measurement moderators greatly affected the effect sizes of health literacy interventions on participants' level of health literacy. For instance, subjective health literacy measures showed higher effect sizes over objective measures and health literacy improvements were higher when participants self‐assessed their health literacy compared to assessment by a clinician or other members of the clinical team (Miller 2016). Therefore, conclusions have to be drawn carefully, since the effects may be highly variable within the included studies.
Apart from interventions that aimed at improving health literacy in a general sense, we also included interventions that targeted at least one of the four steps of health information processing. Pathways for these interventions may have included empowering people by strengthening their skills in accessing, understanding, appraising or applying health information. For example, a web navigation training intervention (imparting knowledge) has been shown to improve health information search strategies of people living with HIV/AIDS, thereby focusing on the improved ability to search for and find online information (Kalichman 2006). Reproductive health knowledge was strengthened by a health education intervention that aimed to improve understanding of health information (Mbizvo 1997). The appraisal of such information was enhanced by matching content presentation to the health locus of control for recipients (Williams‐Piehota 2004). Individually tailored information on behavioural change increased cholesterol screening rates and physical activity (Kreuter 1996).
A successful interaction with healthcare providers is dependent on the communication skills of the patient on the one hand (e.g. language proficiency) and those of the healthcare professionals on the other hand (e.g. use of plain language and taking time for explanation). Therefore, another pathway for improving migrants' health literacy could have included improving healthcare providers' communication skills, rather than educating the individual migrants themselves. Such interventions could have indirectly improved health literacy skills and, in turn, health‐related outcomes through patient‐provider communication that is respectful and tailored to the patient's health literacy needs. For instance, Tavakoly 2018 found that health provider communication skills training significantly improved patient communication skills, self‐efficacy, adherence to medication and hypertension outcomes.
Beauchamp 2017 developed a three‐step approach that identified health literacy issues of health professionals or consumers; developed appropriate interventions; and implemented, evaluated and improved these interventions by using Plan‐Do‐Study‐Act (PDSA) cycles. Successful interventions involved one of the following four pathways: improvement of clinician skills and resources for health literacy, the active engagement of community volunteers to disseminate health promotion messages, the direct impact on consumers' health literacy and the redesign of existing healthcare services. Such studies indicate that an individual's health literacy can be improved through both direct and indirect means.
Why it is important to do this review
Research on migrants' health is highly relevant to gain a better understanding of migrants’ specific healthcare needs, and how to respond best and most efficiently to these needs. Understanding the effectiveness of available interventions and pathways through which they have their effects is of great interest to decision‐makers in healthcare systems, who face the challenge of rolling out interventions for improving health literacy across populations. Furthermore, it is important to identify effective approaches for improving access, understanding, appraisal and application of health information by migrants, since an appropriate response to healthcare needs entails the proper application of the health information found. However, people with limited health literacy skills face considerable barriers in accessing high‐quality health information, and in understanding, appraising and applying the information for their own healthcare decisions and behaviours (Friis 2016; HLS‐EU Consortium 2012; HLS19 Consortium 2021). These and other challenges should be considered in the research on migrants' health literacy to ensure equitable and humane healthcare systems on the one hand, and empowered individuals on the other hand.
There is no prior Cochrane effectiveness review on migrants' health literacy. There is a published Cochrane effectiveness review on interventions for improving consumers’ online health literacy (Car 2011), and a published Cochrane protocol on interventions improving health literacy in people with kidney disease (Campbell 2016). However, we did not expect overlap between the reviews because health literacy is defined differently in each, and the phenomena and populations under study differ greatly.
Research on health literacy has the overarching aim of establishing a common understanding of health literacy, informing development of appropriate assessment tools, and effective interventions to improve health literacy. Health literacy measurement is evolving, and the majority of international research is targeted at assessing individuals’ ability to function in the healthcare environment, mostly measuring functional aspects of health literacy (i.e. reading and writing abilities in the medical context) and neglecting procedural characteristics of the four health information processing steps in other than clinical settings (Guzys 2015; Haun 2014). In particular, a theory‐driven approach of applying the integrated model of health literacy as an umbrella framework to assess the effectiveness of interventions that address the four health information processing steps has not yet been determined. This review can therefore contribute to a more profound understanding of health literacy as a multidimensional construct by identifying design features of interventions targeted to migrants that address the relevant health information processing steps sufficiently.
Objectives
To assess the effectiveness of interventions for improving health literacy in migrants.
To assess whether female or male migrants may respond differently to the identified interventions.
Such interventions must have addressed health literacy either as a comprehensive construct or at least one of its four health information processing steps (access, understand, appraise, apply). However, we did not aim to equate general health literacy interventions that include a range of activities targeted to all of the four health information processing steps with interventions that aim to improve only one step (e.g. understand). We aimed instead to create a comprehensive picture of the effect of health literacy interventions by applying the integrated model as an umbrella framework for a deductive analysis of the four steps of health information processing.
We did not restrict this review to specific settings or diseases because we aimed to provide an overview of available interventions for improving health literacy that address migrant populations.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐RCTs (trials in which groups of participants were randomised) (see Data collection and analysis). We planned to also include quasi‐RCTs (trials in which randomisation was attempted but subject to potential manipulation, such as allocating participants by day of the week, date of birth or sequence of entry into trial), but no eligible quasi‐RCTs were identified.
Types of participants
We included migrants, referring to immigrants, refugees, asylum seekers, wandering people and other individuals who have migrated (first‐generation migrants). This corresponds with the definition by the International Organisation for Migration (IOM), which states that migration is the “the movement of a person or a group of persons, either across an international border, or within a state. It is a population movement, encompassing any kind of movement of people, whatever its length, composition and causes; it includes migration of refugees, displaced persons, economic migrants, and persons moving for other purposes, including family reunification” (IOM 2018). Thus, movement within a state was considered as migration only if it was embedded within the movement of a population.
We included adults aged 18 years or over. We applied no gender or ethnicity restrictions. We excluded trials if fewer than 80% of participants were adults, and if no subgroup data were available.
We excluded studies that included only extractable data for individuals of established ethnic minority communities (e.g. Latino Americans in the USA), defined as descendants of migrants who have settled in the respective country at least one generation ago. If data for subgroups who were explicitly designated as first‐generation migrants could be extracted then we included the study. We included studies in which at least 80% of participants were migrants according to our definition. If no clear distinction could be made between ethnic minority group and migrant status according to our definition (e.g. when it was not stated which migrant generation was included), we excluded the study.
Types of interventions
We searched for studies that entailed, for instance, interventions that aimed to:
improve health literacy in different settings (e.g. group‐based education programmes for pregnant women on post‐partum care in an immigrant community, or self‐management programmes for improving disease management);
improve health literacy in hard‐to‐reach groups (e.g. telephone interventions to improve patients' engagement in disease management);
improve knowledge or understanding of information about health, disease or treatment (e.g. mitigate effects of limited language proficiency through the provision of information in different languages);
affect the appraisal of health information (e.g. by individually tailoring the information provided); and
improve understanding or use of medical information through culturally and literacy adapted medication labels.
We also searched for studies targeting health professionals' communication skills in consulting patients with low literacy skills (e.g. teach‐back training, if the effect was measured in migrants) or studies that aimed to improve access to health information, e.g. through access to telemedicine in rural areas. However, we did not find any studies assessing the effects of either of these approaches.
We included health literacy interventions that were explicitly named as such, or interventions designed for individuals with low literacy skills without explicitly referring to the concept of health literacy, so long as the intervention's aims and outcomes could be assigned to health literacy as an umbrella concept. Such interventions could have addressed health literacy either as a general concept, or at a minimum, components of health literacy such as knowledge, or one of its four health information processing steps (access, understand, appraise and apply).
We excluded interventions that solely addressed the health literacy environment, i.e. interventions that focused on healthcare organisations or health systems without measuring the effect of these interventions on migrants' health literacy. We also excluded studies that could not be assigned to our umbrella framework of health literacy because the intervention was not designed to improve health literacy or even to mitigate the effects of low literacy. These studies were excluded even if they reported using a health literacy assessment tool.
At the protocol stage, we planned to conduct a main analysis including health literacy interventions that were explicitly named as such and a secondary deductive analysis including health literacy interventions that address at least one of the four health information processing steps (see description above). For example, if a study reported a 'health literacy intervention' as simply providing an information pamphlet on an available health service and reported a health literacy measure, we planned to include the study for the secondary analysis, assigning it to the processing step 'access', since the effect could not be assigned to health literacy as a general concept. We also planned to include such a study in the deductive analysis, if the pamphlet was targeted to individuals with limited language proficiency and the effect measured was the level of understanding that these individuals achieved regarding the information provided. In this case, the intervention was planned to be assigned to the processing step of 'understand' in the deductive analysis.
Due to the diversity of studies found, we were not able to conduct one main analysis, but rather identified several comparisons. We conducted meta‐analyses where possible and deductively categorised the studies' outcomes to our umbrella framework of health literacy (see also Data synthesis). In addition, we decided to exclude studies that solely provided a publicly available pamphlet when the respective pamphlet was not adapted with regard to (health) literacy by the study authors.
Types of outcome measures
Outcome categories referred to empirically indicated associations of health literacy with the respective outcome category (Berkman 2011; HLS‐EU Consortium 2012; Paasche‐Orlow 2007; Paasche‐Orlow 2005). Applied health literacy assessment tools could be either performance‐based or perception‐based (self‐assessment) (see Description of the condition). Within studies, we prioritised validated assessment tools in preference to non‐validated assessment tools. However, we did not exclude studies based on whether the assessment tool used had been validated or not.
If single trials reported more than one outcome that mapped to the same category, we listed all reported outcomes (see Characteristics of included studies), but reported effect measures of the prioritised outcomes only. If an outcome was measured in more than one way in a single trial (e.g. medical record review or self‐report), we reported these outcomes narratively for each included study (see Effects of interventions, and Table 2 to Table 9), but prioritised objective outcome measures (e.g. medical record review) for inclusion in the meta‐analysis in preference to subjective outcome measures (e.g. self‐reported medication taking). If more than one outcome per category was measured in the same way, two review authors made a decision about which was clinically most important or which was the most appropriate measure of the outcome under focus (or both). For example, if a study reported the two objectively assessed outcome measures, 'children's emergency department encounters' and their 'attendance to well visits' for the category 'health service use', we presented the outcome 'emergency department encounters' as this was likely to have a greater clinical impact. We combined outcome data when a single trial measured the same outcome in the same way, but reported the results for subscales separately. For example, Han 2017 assessed breast cancer knowledge and cervical cancer knowledge. In this case, we did not prioritise one outcome over the other, but combined the data, as both knowledge tests reflected the intervention content.
For the category 'health literacy' we built subcategories, referring to them as 'generic health literacy', 'disease‐specific health literacy' or 'components of health literacy'. Again, our aim was to provide an overview of available interventions that addressed health literacy either as a concept or one of its components, such as the four steps of health information processing. In addition, we believed that there are important conceptual distinctions to be made between generic health literacy and disease‐specific health literacy. For example, one study reported five objective measures for assessing health literacy. One of these measures was not an established one, and we had insufficient information about how it was applied; one measure was the numeracy subscale of the TOFHLA (Parker 1995), but three measures were validated, full versions of a performance‐based health literacy assessment tool (Kim 2020). Of these, one measure assessed disease‐specific health literacy (diabetes health literacy; DM‐REALM) (Kim 2020), the other two measures are widely used for assessing generic health literacy. One assesses health numeracy (NVS) (Weiss 2005); the other one is used to assess print literacy (REALM; also referred to as functional health literacy) (Davis 1991). We decided to report the results of the latter three measures as they all are validated tools that measure different aspects of health literacy, which we considered relevant for this review.
We conducted a meta‐analysis when at least two studies, which we judged similar enough in terms of intervention features and comparator, measured the same outcome in the same way (see Data synthesis). If more than one outcome per category per trial was eligible for meta‐analysis, we prioritised objective measures in preference to subjective measures to not double‐count data for the same outcome category for the same population in one analysis. All outcomes reported in the included studies were assigned independently to the review's outcome categories. Any differences in categorisation were resolved by involving a third review author.
Primary outcomes
We aimed to include the following primary outcomes in this review:
health literacy; and
adverse events associated with the intervention (e.g. anxiety).
We also extracted outcomes that we considered as components of health literacy (a) knowledge; b) motivation; c) competencies; d) accessing health information; e) understanding health information; f) appraising health information; g) applying health information).
As prespecified in the protocol for this review, we reported on health‐related knowledge separately in the summary of findings tables and in the results section. We assessed knowledge separately as empirical research strongly indicates that higher levels of (functional) health literacy are associated with higher levels of health‐related knowledge (Berkman 2011; Osborn 2011; Paasche‐Orlow 2005; Paasche‐Orlow 2007; Sheridan 2011). In line with the integrated model, however, we considered knowledge to be one of the major components of health literacy. We planned to examine attitudes and beliefs as an outcome only if a knowledge measure was not applied in the respective study, because as proposed by Berkman 2011, we also believe that attitudes result from knowledge. However, none of the included studies assessed attitudes and beliefs without additionally reporting a separate knowledge measure.
Secondary outcomes
We aimed to include the following secondary outcomes, referring to these as 'outcomes related to health literacy':
quality of life;
health outcome (e.g. subjective health status, depression);
health behaviour (e.g. use of preventive measures, medication adherence);
health‐related knowledge (e.g. disease‐specific knowledge);
health service use (e.g. use of emergency room services, hospitalisation rate);
individual skills (e.g. self‐efficacy, self‐awareness); and
health care costs.
At the protocol stage, we pre‐specified the outcome category 'individual skills (e.g. self‐efficacy, self‐awareness)'. For the sake of clarity, and since self‐efficacy has been shown in several studies to be associated with health literacy (Berens 2021; Berens 2022b; Guntzviller 2016; von Wagner 2009; Xu 2018), we decided to rename this category as 'self‐efficacy', including the different forms of self‐efficacy (e.g. self‐efficacy to manage one's own disease, self‐efficacy to use certain screening measures, or self‐efficacy to identify a disease). We also planned to extract outcomes related to the prespecified category 'health care costs'. Health care costs as a secondary outcome was not assessed, as no data were available from the published main trial reports and due to a lack of resources we were not able to search for separate cost‐effectiveness analyses.
We did not exclude studies based on the outcomes reported, but studies were excluded when it was not apparent that improving health literacy or mitigating the effects of low (health) literacy was an aim of the study.
We included the following main outcomesin the summary of findings tables:
health literacy;
adverse events associated with the intervention (e.g. anxiety);
quality of life;
health outcome (e.g. subjective health status, depression);
health behaviour (e.g. use of preventive measures, exercising rate, medication adherence);
health service use (e.g. use of emergency room services, hospitalisation rate);
health‐related knowledge (e.g. disease‐specific knowledge); and
self‐efficacy.
Timing of outcome assessment
We reported all time points, starting from the earliest time point assessed after the total intervention programme was completed. This included short‐term (up to six weeks from the start of the intervention and immediately after the intervention programme was completed), medium‐term (from six weeks up to and including six months after the intervention programme was completed) and long‐term outcomes (longer than six months after the intervention programme was completed).
Search methods for identification of studies
Electronic searches
We adapted the search strategies as suggested in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2022). The search strategy was developed by an Information Specialist (IM) in close consultation with the review authors. The concept of health literacy has evolved continuously since its first mention in 1974. Thus, we searched for studies that measured health literacy as a comprehensive concept, or one of its processing steps, even if these were not explicitly mentioned as such in the respective study. We included full‐text articles and publications available as abstracts only if sufficient information was available on study design, characteristics of participants and interventions provided.
As a supplement to the protocol, the term 'health literacy' or 'literacy' had to be mentioned at full‐text stage to avoid conceptual fraying. Accordingly, for studies to be included they had to either be designed to improve health literacy, or to mitigate the effects of lower literacy in the context of health.
Searches were run in the following databases from inception until 2 February 2022 (for a full overview, see Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library, all issues up to 2 February 2022);
MEDLINE (OvidSP 1946 to 2 February 2022);
EMBASE (OvidSP1974 up to 2 of February 2022);
PsycINFO (OvidSP 1806 to 2 February 2022); and
CINAHL (EBSCO 1982 to 2 February 2022).
No date, language or geographic restrictions were applied to the search.
Searching other resources
We searched for reference lists of the included studies and relevant systematic reviews. We also searched online trials registers for ongoing and recently completed studies from the inception of each trial register up to 2 February 2022:
ClinicalTrials.gov; and
WHO International Clinical Trials Registry Platform (ICTRP).
At the protocol stage, we planned to additionally handsearch for conference abstracts of certain conferences (e.g. migration conferences). We did not handsearch for conference abstracts due to a lack of resources and because our comprehensive search strategy most likely covered the published conference abstracts. We decided to search ClinicalTrials.gov and ICTRP as the other two clinical trial registries mentioned in the protocol (EU clinical trials register and DRKS) are already included in the ICTRP search portal.
Data collection and analysis
Selection of studies
We applied the following two components of Cochrane's Screen4Me workflow to reduce the number of references retrieved and to assess the search results:
Known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as 'a RCT' or as 'not a RCT'.
The RCT model ‐ a machine learning RCT classifier (Wallace 2017), which is available in the Cochrane Register of Studies (CRS‐Web). The RCT classifier assigns a probability of being a true RCT (from 0 to 100) to each citation. We assumed citations that were assigned a probability score below the cut‐point at a recall of 99% to be non‐RCTs. We manually dual screened those results that scored on or above the cut‐point.
More information about Screen4Me and the evaluations that have been done is available at the Screen4Me website on the Cochrane Information Specialist's portal (see Marshall 2018; McDonald 2017; Noel‐Storr 2018; Thomas 2017).
We did not use the third component, which would have consisted of consulting Cochrane Crowd, Cochrane's citizen science platform where the Crowd help to identify and describe health evidence, due to the relatively small number of references remaining.
Two review authors (AB, AAl) independently screened all titles and abstracts identified from searches to determine which met the inclusion criteria. The full text of any article identified as potentially relevant by at least one review author was retrieved. The same two review authors independently screened full‐text articles for inclusion or exclusion, with discrepancies resolved by discussion and, if necessary, by consultation with a third author (DC) to reach a consensus (Higgins 2022). All potentially relevant articles excluded from the review at this stage are listed as excluded studies, with reasons provided in the Characteristics of excluded studies. The process of study selection is presented in a flow chart (Figure 2), as recommended by the PRISMA statement (Liberati 2009). Citation details and any available information about ongoing studies and of duplicate publications are also provided as each study (rather than each report) was the unit of interest in this review.
2.
Study flow diagram
Data extraction and management
Two review authors (AB, CH) independently extracted data from the included studies. Any discrepancies were resolved by discussion until consensus was reached, or through consultation with a third author (AAl) whenever necessary. We developed and piloted a data extraction form on the basis of the Cochrane Consumers and Communication Data Extraction Template (available at: cccrg.cochrane.org/author-resources) and extended it to serve the specific aims of our review.
We extracted the following information:
general information: author, title, source, publication date, country, language, duplicate publications;
quality assessment (risk of bias): allocation concealment, blinding (participants, personnel, outcome assessors), incomplete outcome data, selective outcome reporting, selective recruitment of cluster participants, other sources of bias (e.g. methods of measurement or baseline imbalances between study groups);
study characteristics: trial design, aim of the intervention, setting and dates, source of participants, inclusion/exclusion criteria, random sequence generation, selective recruitment of cluster participants, treatment, compliance with assigned intervention, length of follow‐up, details of control group characteristics, e.g. recruitment and selection strategy, types of comparisons (e.g. waiting list control);
participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, participants lost to follow‐up, type of intervention;
outcomes: primary outcome categories: health literacy and adverse events; secondary outcome categories: quality of life, health outcome, health behaviour, health‐related knowledge, health service use, individual skills;
data extraction by outcome: use of assessment tool, timing of outcome assessment; and
funding: details of the funding source
Furthermore, because this is an equity‐focused, theory‐driven review, we extended the data extraction form with characteristics we considered relevant regarding health equity and health literacy. This concerned both the included studies and the participants. We used the PROGRESS‐Plus concept (Place of residence, Race/ethnicity/culture, Occupation, Sex, Religion, Education, Socioeconomic status, Social capital, age, disability and sexual orientation) to capture equity‐relevant data, as recommended in the PRISMA‐Equity statement (Welch 2012; Welch 2015). We further extended the data extraction form with intervention features (e.g. language of delivery, cultural adaptation and consumer involvement, and characteristics of the participants (e.g. length of time living in host country) that we considered especially equity‐relevant for migrant populations.
We extracted data on the definition of health literacy that guided the intervention and the assessment tool applied (e.g. a measure for disease‐specific health literacy or generic health literacy). We used the integrated model by Sørensen 2012 to capture components of health literacy that were addressed by the interventions under study. We designated a component as being addressed when the authors explicitly stated that this certain aspect of health literacy was intended to be improved (e.g. through specific design features applied or the use of a certain outcome measure), the methods reported clearly referred to this component, or when the authors referred to an underlying framework or theory of health literacy that contains one of the following:
prerequisites of health literacy (knowledge, motivation and competencies); and
steps of health information processing (access, understand, appraise and apply).
For instance, we judged 'competencies' and 'understand' to be addressed by the intervention when the authors described methods such as learning words and phrases based on medical terminologies as being part of the intervention, or when a performance‐based assessment tool for assessing (functional) health literacy was applied (e.g. TOFHLA) (Parker 1995).
We also extracted information on whether the interventions were developed on the basis of a theoretical framework that explicitly referred to health literacy (e.g. the integrated model of health literacy (Sørensen 2012)) or other established behavioural theories such as the theory of planned behaviour (Ajzen 1991), which might help explain causal pathways of the intervention effectiveness.
We extracted the following information for each health literacy intervention:
theoretical framework underlying the intervention;
procedure (including material provided);
intervention provider (e.g. healthcare professional, trained lay health educators or researchers);
delivery mode (delivered one‐to‐one or in groups, number and frequency of sessions, total duration of programme);
delivery method (face‐to‐face, written, video‐based, web‐based);
language of delivery (host country's language or language concordant/bilingual);
format (individually tailored or standard format);
setting/location (e.g. community setting, clinic, participants' home); and
consumer involvement (e.g. in design and/or evaluation of intervention).
The data extraction form was pilot tested with the first five included studies, and refined throughout the review process. One review author entered all extracted data into RevMan 5 (Review Manager 2014), and a second review author checked for accuracy against the data extraction sheets. We contacted the authors of individual studies to ask for additional information whenever required.
Assessment of risk of bias in included studies
We assessed and reported the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Consumers and Communication guidelines (Ryan 2013), which recommend the explicit reporting of the following individual elements for RCTs: random sequence generation; allocation sequence concealment; blinding (participants and personnel); blinding (outcome assessment); completeness of outcome data, selective outcome reporting; and other sources of bias, such as health literacy measurement (e.g. social desirability in self‐assessment tools). We considered blinding separately for different outcomes where appropriate (for example, blinding may have the potential to affect objective versus subjective outcome measures differently). We judged each item as being at high, low or unclear risk of bias as set out in the criteria provided by Higgins 2011, and provided a quote from the study report and a justification for our judgement for each item in the risk of bias tables.
We deemed studies to be at the highest risk of bias if they scored as high or unclear risk of bias for either the sequence generation or allocation concealment domains, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2022). For cluster‐RCTs, we also assessed and reported the risk of bias associated with an additional domain: selective recruitment of cluster participants. In addition, we judged studies as being at high risk of bias in the domain 'other bias' when the reported data were not adjusted for the cluster design, and we were not able to re‐analyse the data using the appropriate unit of analysis (i.e. when the necessary information such as the intra‐cluster correlation coefficient (ICC), or the number of participants in each cluster, could not be obtained (see Unit of analysis issues)).
Two review authors (AB, AAl) independently assessed the risk of bias of included studies, with any disagreements resolved by discussion or involvement of a third author (DC) to reach a consensus. We contacted study authors for additional information about the included studies, or for clarification of the study methods as required. We incorporated the results of the risk of bias assessment into the review through standard tables, and systematic narrative description and commentary about each of the elements, leading to an overall assessment of the risk of bias of included studies and a judgement about the internal validity of the review’s results.
Measures of treatment effect
For dichotomous outcomes, we analysed data based on the number of events (e.g. emergency room visits) and the number of people assessed in the intervention and comparison groups. We used these data to calculate the risk ratio (RR) and the corresponding 95% confidence interval (CI). Where continuous scales of measurement were used (e.g. health literacy measurement, knowledge scales), we analysed data based on the mean, standard deviation (SD) and number of people assessed in the intervention and comparison groups to calculate the mean difference (MD) and the corresponding 95% CI. If the MD was reported without individual group data, we used this to report the study results.
If more than one study measured the same outcome using different tools, we calculated the standardised mean difference (SMD) and 95% CI using the inverse variance method in RevMan 5 or standardised the scores to range from 0 to 100 points to facilitate pooling of data (e.g. for the outcome knowledge). When change from baseline scores and post‐intervention scores were reported, we prioritised change scores over post‐intervention scores, when repeated outcome measures were used in the studies. If not otherwise possible, we used both change scores and post‐intervention scores to calculate the SMD. We refer to a study of 21 meta‐analyses on osteoarthritis cited in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), which did not find a difference between combined SMDs based on post‐intervention values and combined SMDs based on change scores (da Costa 2013). If results could not be summarised as point estimates with 95% CIs, we presented results narratively in tabular form for each outcome (see Table 2 to Table 9).
Unit of analysis issues
We checked for unit of analysis errors in the included cluster‐RCTs. If errors were found, but sufficient information was available, we re‐analysed the data using the appropriate unit of analysis by considering the intracluster correlation coefficient (ICC). We planned to obtain estimates of the ICC by contacting the authors of included studies, or to impute them using estimates from similar studies. We contacted all authors of studies that lacked information, but we could not obtain any additional information. However, one cluster‐RCT provided sufficient information, including an ICC, to re‐analyse the data in the trial report (Han 2017). One study reported in a secondary reference related to the trial that a cluster‐design was used, but did not account for clustering in any analysis (Kim 2014). Four studies stated that they used generalised estimating equations (GEE) to account for clustering, but at least some of the data we used (e.g. for the outcome knowledge) were either not adjusted for the effective sample size (Han 2017; Taylor 2011), or the information was insufficient as only percentages were reported for our outcomes of interest (Bloom 2014; Tong 2017). For these outcomes, we used the ICC reported by Han 2017 to re‐analyse the data. When we were not able to do so, we reported the unadjusted effect estimates and annotated them as (possible) unit of analysis error.
We used the most conservative ICC reported by Han 2017 for outcomes that have not been assessed by Han 2017, but by other studies to re‐analyse the data. For example, the ICC for health literacy reported by Han 2017 was 0.03, but the ICC for cervical cancer knowledge was 0.02. We used an ICC of 0.03 for health literacy, self‐efficacy and health behaviour, but 0.02 for high blood pressure knowledge to re‐analyse the data reported by Kim 2014.
Dealing with missing data
We contacted study authors to obtain missing data (e.g. for participants, outcomes, effect values stratified by gender or summary data). We contacted the authors of 29 studies at least once, of whom 12 responded. Eight authors provided us with missing information or additional data. When authors responded but were not able to provide us with the missing data, or when we did not receive a response, we categorised these studies as 'Data sought but not used' (see Characteristics of included studies).
Where possible, we conducted all analyses based on the intention‐to‐treat principle. Otherwise, we analysed data as reported. We reported on losses to follow‐up and assessed this as a source of potential bias (see Incomplete outcome data (attrition bias)).
For missing outcome or summary data, we imputed missing data where possible. If estimates for mean and standard deviations were missing, we calculated these statistics from reported data whenever possible, using the approaches described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). When either the baseline or the post‐intervention SD was not reported, we substituted it with the other, so long as we did not expect the intervention to alter the variability of the outcome measure, as recommended in Chapter 6.5.2.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). We aimed to investigate, through sensitivity analyses, the effects of any imputed data on pooled effect estimates. However, due to a lack of studies included in the respective pooled analyses (two studies each), we were not able to conduct any sensitivity analysis for imputed data.
Assessment of heterogeneity
Before we conducted any meta‐analysis, we assessed studies for similarities in terms of setting, intervention, comparison and outcome measures. We then grouped studies according to the characteristics of the interventions (e.g. intervention components, mode and method of delivery), the comparison groups and the outcomes assessed. Where we detected substantial methodological heterogeneity across included studies, we used a narrative approach to data synthesis (see Data synthesis) and reported the results in additional tables where possible (see Table 2 to Table 9). As our aim was to assess the general effectiveness of health literacy interventions in migrants, we did not group studies according to the participants' clinical characteristics for the purposes of our analyses. We reported on the results of our synthesis as recommended by the reporting guideline for Synthesis Without Meta‐Analysis (SWiM) in systematic reviews (Campbell 2020).
Where studies were considered to be similar enough to allow pooling of data in meta‐analyses, we assessed the degree of heterogeneity by visual inspection of forest plots and by examining the Chi² test for heterogeneity. We quantified heterogeneity using the I² statistic. We considered an I² value of 50% or more to represent substantial heterogeneity. However, we interpreted this value in light of the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi² test (Higgins 2022); we considered the direction of effects and the variability in these rather than variability in the size of effects as a basis for our interpretation of heterogeneity. We considered this in our GRADE assessment in that we did not downgrade for inconsistency when the direction of effect was consistent across studies, despite some variability in the size of effects across individual studies (e.g. for the outcome health‐related knowledge). We did, however, downgrade for inconsistency when there was high variability in measurement (e.g. when there was no gold standard measure for assessing a certain outcome) that added further uncertainty to the effects of health literacy interventions for this outcome.
Assessment of reporting biases
We assessed reporting bias qualitatively based on the characteristics of the included studies (e.g. if only small studies that indicated positive findings had been identified), and if information obtained from contacting study authors suggested that there were relevant unpublished studies.
We planned to investigate publication bias by using funnel plots if at least 10 studies were available for inclusion in the review. No meta‐analysis included at least 10 studies, so we did not create funnel plots to assess reporting bias.
Data synthesis
We meta‐analysed data based on whether the interventions in the included trials were similar enough in terms of setting, intervention, comparison and outcome measures to ensure meaningful conclusions from a statistically pooled result. We then pooled results across studies in cases where investigators used similar outcome measures, and we expected the effects to be independent of the type of health topic the participants received information on. We conducted a number of meta‐analyses, as the heterogeneity of the included studies did not allow for pooling all studies that reported a single outcome together. When studies were judged sufficiently similar to be pooled together, but varied in the programme duration, we pooled the results with the most common timing of outcome assessment (e.g. immediately after the programme was completed) and conducted subgroup analyses by length of programme when appropriate (see Subgroup analysis and investigation of heterogeneity).
For inclusion in meta‐analyses, we used the longest time point reported for each study and pooled the data together with studies reporting the same time point for the same outcome. For example, when one study assessed the same outcome two times within the same category (i.e. short‐term, medium‐term or long‐term). However, we made one exception: for Unger 2013, we decided to pool only the shorter time point reported because the authors stated that "the data collectors reported that several students shared their photonovel with students in the text pamphlet group after the posttest." (Unger 2013, p. 405). Thus, intervention fidelity was not assured, which might have introduced a bias concerning the assessment at one‐month follow‐up.
Due to the heterogeneity of included studies we used the random‐effects model for all meta‐analyses. We created forest plots to display individual study results, ordered by weight in ascending order. In addition, we narratively summarised all outcomes that met our inclusion criteria and presented them in additional tables (see Table 2 to Table 9).
We used a three‐step approach to group the included studies and to examine possibilities for meta‐analysis of the results within the prespecified outcome categories. The first author's (AB) grouping was independently reviewed by a second author (AAl or DC). The assessment of whether there was sufficient similarity for subordinating interventions, but also control groups, to one category was made by at least two review authors. All discrepancies were resolved by the involvement of a third review author.
Firstly, studies were grouped in terms of their main components with regard to content‐related and methodological features. The categorisation of main intervention components was piloted with the first five studies and refined throughout the process of the data synthesis.
-
Intense health education with direct provider contact, including:
multiple methods of knowledge transfer, provider delivered (e.g. multimedia presentations, interactive role‐plays, discussions, evaluations).
-
Simple health education without direct provider contact, including:
one or up to two methods of knowledge transfer, media delivered (e.g. written information, interactive online education, educational video, educational messages).
-
Self‐monitoring, including:
provision of take‐home measuring instruments and supervision in order to manage, document and adapt one's own health or course of disease (e.g. blood pressure monitor).
-
Role modelling, including:
information that was substantiated by illustrated narratives or the introduction of role modelling characters using audio‐ and/or visual formats (e.g. photonovel, narrative video).
-
Motivational counselling, including:
provider and/or peer feedback on personal progress (e.g. with the use of motivational interviewing, phone calls, interactive messages).
-
Redesign of written medical instructions, including:
(health) literacy adapted medication labels or written information (e.g. using (culturally adapted) plain language, pictograms).
Secondly, the main intervention components were set in relation to specific design features that we considered relevant for the intervention effect (e.g. interaction with the provider, number and frequency of educational sessions, total duration and intensity of the programme).
The following subcategories resulted from the first two steps of grouping:
culturally and literacy adapted self‐management programme;
culturally adapted health literacy skill building course;
culturally and literacy adapted telephone education;
culturally and literacy adapted audio‐/visual education without personal feedback; and
culturally and literacy adapted medical instruction.
Thirdly, the study groups were ordered according to their comparator.
It was planned to include the following types of comparisons:
health literacy intervention versus no intervention (including usual care); and
health literacy intervention versus another health literacy intervention.
The following comparators were formed according to the trials identified:
no health literacy intervention (i.e. attention placebo intervention, wait‐list control or usual care/no intervention);
unrelated health literacy intervention (i.e. same method or mode of delivery, but information on a different health topic);
written information on the same health topic (i.e. written pamphlet/brochure, written pictogram); and
another health literacy intervention (i.e. information on the same health topic in a different format, e.g. narrative video compared to factual knowledge video).
As the concept of health literacy is related to the processing of health information in different contexts, we referred to comparator interventions that provided information on a different health topic than that in the intervention as 'unrelated health literacy intervention' and reported the results together with comparators categorised as 'no health literacy intervention'. We referred to all comparators that did not fulfil our predefined criteria for health literacy interventions (see Types of interventions) as 'no health literacy intervention'.
For studies with more than two intervention groups, we used the following approaches: we extracted data from two groups, of which at least one applied a health literacy intervention, and provided the strongest contrast. If at least two groups referred to alternative variants of the same intervention, we combined the intervention groups to create a single pair‐wise comparison, as recommended in Chapter 16.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). If the combination of intervention groups was not possible (e.g. due to a lack of information needed or when data were not presented in a way that they could be combined, see Poureslami 2016b), we extracted data from the two groups that provided the strongest contrast as described above.
The following comparisons resulted from the grouping procedure:
culturally and literacy adapted self‐management programme versus no health literacy intervention;
culturally and literacy adapted self‐management programme versus written information on the same topic;
culturally adapted health literacy skill building course versus no or unrelated health literacy intervention;
culturally and literacy adapted telephone education versus unrelated health literacy intervention;
culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention;
culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic;
culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback; and
culturally and literacy adapted medical instruction versus no health literacy intervention.
As our second aim was to assess whether female or male migrants benefit differently from any health literacy intervention, we formed a ninth comparison:
female migrants' versus male migrants' benefit from any health literacy intervention.
Subgroup analysis and investigation of heterogeneity
We intended to conduct subgroup analyses for gender, ethnicity and health literacy assessment (if named as such) (see Objectives). Since health literacy can be defined and measured in different ways, we planned to conduct a subgroup analysis for perception‐based versus performance‐based measurement tools applied in the included studies. However, no self‐assessment tool was used in the included studies. Therefore, it was not possible or meaningful to follow the protocol in terms of conducting subgroup analyses for perception‐based versus performance‐based health literacy assessment.
Due to high heterogeneity of the included interventions, participants and comparators and an insufficient number of studies in any of the meta‐analyses, we were not able to conduct quantitative subgroup analyses for ethnicity or gender either. However, we were able to conduct separate analyses on outcomes for which we could obtain gender‐separate scores from the study authors.
Contrary to the protocol, we conducted post hoc quantitative subgroup analyses for specific design features when we considered studies similar enough to be combined in a meta‐analysis, but nevertheless design‐specific heterogeneity needed to be considered. For example, when there was high variance in the programme duration, we conducted subgroup analyses by the length of the programme (e.g. up to six months versus up to 12 months) to investigate the reasons for heterogeneity.
Sensitivity analysis
We conducted sensitivity analyses for high risk of bias versus low risk of bias studies, when possible. In addition, we conducted sensitivity analyses when heterogeneity was unexplainably high. For example, the results of Kaur 2019 were noticeably better than the results of other studies included in the same meta‐analysis, and we could not explain this with the study design or the participant characteristics.
Summary of findings and assessment of the certainty of the evidence
We presented the results of meta‐analyses and narrative syntheses in summary of findings (SoF) tables for the major comparisons of the review. We provided a source and rationale for each assumed risk in the tables, and used the GRADE approach to assess the certainty in the evidence based on the methods described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022). Furthermore, we used the GRADEpro GDT software for our assessments (GRADEpro GDT). Where meta‐analyses were not possible, we presented results in a narrative format, taking into account the GRADE assessments (Ryan 2016).
We presented all time points for each key outcome in each study in the SoF tables, when the intervention effect on the respective outcome appeared to vary over time (e.g. for knowledge). We made one exception: for Koniak‐Griffin 2015, we report the shorter time point (immediately post‐intervention) because "there was a statistically significant decrease in the control group [at three‐month follow‐up], approaching a 1000‐step decline, whereas intervention participants maintained their activity level." (p.82 f). Moreover, the number of average daily steps in the intervention group fell back to the baseline level (which was 8571 average daily steps (SD 3130)). Thus, the calculated MD does not reflect an actual improvement of the intervention group, so that reporting the results of the three‐month follow‐up assessment in the SoF table would have unintentionally overestimated the intervention effect.
Involvement of consumers
The involvement of consumers is important for obtaining a better understanding of the performance and effectiveness of health literacy interventions, particularly how they reach consumers. This effectiveness review and the linked QES were part of an overarching project on Gender‐specific Health Literacy in Individuals with a Migration background (GLIM) that aimed to examine gender‐specific aspects of health literacy in migrants by applying a mixed‐methods approach. The project was funded by the Federal Ministry of Education and Research in Germany (grant number 01GL1723).
We involved consumers by conducting focus group discussions (FGDs) with female and male healthcare professionals (N = 31) in Germany, of whom more than 50% had a migrant background themselves. Our aim was to examine the perceived health literacy‐related challenges and needs, as well as the applied solutions of healthcare professionals in Germany when engaging with persons with a migrant background (defined as first‐ or second‐generation migrants). We particularly focused on personal factors such as gender, situational conditions such as the current workload, and societal and environmental factors such as system‐related conditions that may impact the flow of information in transcultural treatment settings (Baumeister 2021a; Chakraverty 2020). We used the results of the FGDs to discuss and reflect on the findings of the current review (see Agreements and disagreements with other studies or reviews). Moreover, consumers were involved in the development of the review protocol as consumer referees provided written feedback on it. Consumer referees also read the results of the review and provided written feedback, as part of Cochrane's editorial processes.
At the protocol stage, we had planned to also involve consumers by conducting gender‐separate focus group discussions (FGDs) with female and male migrants, as well as to conduct a final symposium with different stakeholders, such as experts from political and healthcare contexts, to discuss the impact and implications of our primary and secondary findings for healthcare decision‐making at the political level, particularly in Germany. However, due to a lack of financial and human resources, this was not possible.
Results
Description of studies
Results of the search
Our search yielded 17,223 results. After removal of duplicates and application of the RCT classifier, 6941 records were included for title and abstract screening (Figure 2). We assessed 336 possibly eligible references in full text. After reading the full texts, we excluded 223 references that did not fulfil our inclusion criteria.
Included studies
We included 34 studies (94 references) in this review (Figure 2). See the Characteristics of included studies for a full description of the included studies. In addition, eight references that we identified are still awaiting assessment (see Characteristics of studies awaiting classification), and 11 are ongoing (see Ongoing studies).
Study design
Of the 34 included studies, six were cluster‐RCTs (Bloom 2014; Elder 1998; Han 2017; Kim 2014; Taylor 2011; Tong 2017), and 28 were RCTs. All were published in English.
Location
All studies were conducted in high‐income countries, 27 of which were in the United States of America (USA). Four studies were conducted in Canada (Kaur 2019; Poureslami 2016a; Poureslami 2016b; Taylor 2011), two in Asia (Qatar and Singapore) (Kheir 2014; Wong 2020), and one study in Australia (Kiropoulos 2011).
Participants
We used the PROGRESS‐Plus framework to assess equity‐relevant data. A full description of participants is shown in Table 22.
13. PROGRESS‐plus framework.
| PROGRESS | Plus | Health literacy | |||||||
| Study1 | Place of residence; time living in host country | Race/ethnicity/culture/language | Occupation | Gender | Religion | Education | Socioeconomic status, social capital | Age, sexual orientation, disability, migrant status | Assessment tool, range |
|
Bailey 2012 (No. analysed = 202) |
|
|
— |
|
— |
|
|
|
— |
|
Bloom 2014 Total N = 230 |
|
|
— |
|
|
|
— |
|
— |
|
Calderón 2014 (Total N = 240) |
|
|
— |
|
— |
|
|
|
|
|
DeCamp 2020 (Total N = 157) |
|
|
|
— | — |
|
|
|
|
|
Elder 1998 (No. only Latinos = 341) |
|
|
|
|
— |
|
|
|
— |
|
Gwede 2019 (Total N = 76) |
|
|
n = 75
|
|
— |
|
n = 70
|
|
|
|
Han 2017 (Total N = 560) |
|
|
|
|
— |
|
|
|
|
|
Hernandez 2013 (Total N = 146) |
|
|
|
|
— |
|
|
|
|
|
Kaur 2019 (Total N = 140) |
|
|
|
|
— |
|
|
|
|
|
Kheir 2014 (Total N = 123) |
|
|
|
|
— |
|
|
|
— |
|
Kim 2009 (No. analysed = 79) |
|
|
|
|
— |
|
|
|
— |
|
Kim 2014 (No. analysed = 369) |
|
|
— |
|
— |
|
|
|
|
|
Kim 2020 (No. analysed = 209) |
|
|
|
|
— |
|
|
|
|
|
Kiropoulos 2011 (Total N = 202) |
|
|
|
|
— |
|
|
|
|
|
Koniak‐Griffin 2015 (Total N = 223) |
|
|
|
|
— |
|
|
|
— |
| Lepore 2012 (No. analysed = 431 for survey data, N = 490 for medical claims data) |
|
|
— |
|
— | N = 490
|
|
|
— |
|
Mohan 2014 (No. analysed = 200) |
|
|
— |
|
— |
|
— |
|
|
|
Ochoa 2020 (No. analysed = 109) |
|
|
— |
|
— | N = 232
|
N = 232
|
|
— |
|
Otilingam 2015 (Total N = 100) |
|
|
— |
|
— |
|
|
|
n = 73
|
|
Payán 2020 (No. analysed = 193) |
|
|
— |
|
— | N = 240
|
N = 240
|
|
— |
|
Poureslami 2016a (No. analysed = 85) |
|
|
|
|
— |
|
— |
|
— |
|
Poureslami 2016b (Total N = 91) |
|
|
— |
|
— |
|
— |
|
— |
|
Rosal 2005 (No. analysed = 25) |
|
|
|
|
— |
|
|
|
— |
|
Rosal 2011 (Total N = 252) |
|
|
n = 230 11.3% working full or part‐time, 3.5% unemployed/looking for a job, 61.7% disabled, 10.9% retired, 12.6% housewife |
|
— |
|
|
|
— |
|
Soto Mas 2018 (Total N = 181) |
|
|
— |
|
— | n = 155
|
— | n = 155
|
n = 155
|
|
Sudore 2018 (No. of Spanish‐speaking participants = 445) |
|
|
— |
|
49.9% fairly to extremely religious, 59.6% fairly to extremely spiritual |
|
|
|
|
|
Taylor 2011 (Total N = 180) |
|
|
— |
|
— |
|
|
|
— |
|
Thompson 2012 (Total N = 170) |
|
|
— |
|
— | n = 159
|
|
|
— |
|
Tong 2017 (Total N = 329) |
|
|
|
|
— |
|
|
|
— |
|
Unger 2013 (No. analysed = 139) |
|
|
— |
|
— |
|
— |
|
— |
|
Valdez 2015 (Total N = 708) |
|
|
— | n = 707
|
— |
|
n = 707
|
n = 691
|
— |
|
Valdez 2018 (No. analysed = 727) |
|
|
— |
|
— | N = 943
|
|
|
— |
|
van Servellen 2005 (No. analysed = 85) |
|
|
— |
|
— |
|
|
|
|
|
Wong 2020 (No. analysed = 39) |
|
|
|
|
n = 38
|
n = 38
|
n = 38
|
|
n = 37
|
1Not all studies reported numbers on all participants randomised to either the intervention or control arm. Here we report the number of participants randomised, if not otherwise stated.
*Mean (SD), **Median (SD), ***Mean (SE)
Abbreviations:
AHL‐C: Assessment of Health Literacy in Cancer screening; BHLS: Brief Health Literacy Screen; BP: blood pressure; D‐Lit/DLQ: Depression Literacy Questionnaire; DM‐REALM: Diabetes‐specific Rapid Estimate of Adult Literacy in Medicine; GED: general educational development; HBP‐HLS: high blood pressure health literacy scale; HL: health literacy; NVS: newest vital sign; QR: Qatari riyal; REALM: Rapid Estimated of Adult Literacy in Medicine; SD: standard deviation; SILS: Single Item Literacy Screener; TOFHLA: Test of Functional Health Literacy in Adults; TS‐REALD: Two Stage Rapid Estimate of Adult Literacy in Dentistry; y: years
The included studies recruited between 76 (Gwede 2019) and 943 participants (Valdez 2018). In total, 8249 participants were allocated to either an intervention or a control arm. According to the distribution of immigrant groups in the USA, most of the studies focused on participants who were born in Central and South America (19 studies; Calderón 2014; DeCamp 2020; Elder 1998; Gwede 2019; Hernandez 2013; Koniak‐Griffin 2015; Lepore 2012; Mohan 2014; Ochoa 2020; Otilingam 2015; Payán 2020; Rosal 2005; Rosal 2011; Soto Mas 2018; Sudore 2018; Thompson 2012; Unger 2013; Valdez 2018; van Servellen 2005) or East and South Asia (13 studies; Bloom 2014; Bailey 2012; Han 2017; Kaur 2019; Kheir 2014; Kim 2009; Kim 2014; Kim 2020; Poureslami 2016a; Poureslami 2016b; Taylor 2011; Tong 2017; Wong 2020). One study included participants from both Central and South America and Asia (Valdez 2015), and one study included participants who had migrated from Europe (i.e. from Greece or Italy) to Australia (Kiropoulos 2011). The participants' time living in the host country was reported in 25 studies (Bailey 2012; DeCamp 2020; Elder 1998; Gwede 2019; Han 2017; Hernandez 2013; Kheir 2014; Kim 2009; Kim 2014; Kim 2020; Kiropoulos 2011; Koniak‐Griffin 2015; Ochoa 2020; Otilingam 2015; Payán 2020; Poureslami 2016a; Poureslami 2016b; Soto Mas 2018; Sudore 2018; Taylor 2011; Thompson 2012; Tong 2017; Unger 2013; Valdez 2015; Valdez 2018); the average time since immigration ranged from less than one year up to 62 years.
Participants' occupational status was reported in 15 studies (Elder 1998; Gwede 2019; Han 2017; Hernandez 2013; Kaur 2019; Kheir 2014; Kim 2009; Kim 2020; Kiropoulos 2011; Koniak‐Griffin 2015; Poureslami 2016a; Rosal 2005; Rosal 2011; Tong 2017; Wong 2020); two of these provided data on the type of occupation: these were migrant workers in the petrol industry (Kheir 2014), and migrant workers presumably working in Singaporean households (Wong 2020). All studies reported at least some information about the participants' formal education.
Twenty‐one studies reported data related to social capital (e.g. number of children) (DeCamp 2020; Elder 1998; Gwede 2019; Han 2017; Hernandez 2013; Kim 2009; Kim 2020; Kiropoulos 2011; Koniak‐Griffin 2015; Lepore 2012; Ochoa 2020; Otilingam 2015; Payán 2020; Rosal 2011; Sudore 2018; Taylor 2011; Thompson 2012; Tong 2017; Valdez 2015; Valdez 2018; Wong 2020).
In total, 24 studies reported any information related to the participants' socioeconomic status such as income (seven studies; Bailey 2012; Elder 1998; Kheir 2014; Kim 2009; Otilingam 2015; Sudore 2018; van Servellen 2005), or health insurance (two studies; Kim 2014; Lepore 2012), and 15 studies reported information related to both (Calderón 2014; DeCamp 2020; Gwede 2019; Han 2017; Hernandez 2013; Kaur 2019; Kim 2020; Koniak‐Griffin 2015; Ochoa 2020; Payán 2020; Rosal 2005; Rosal 2011; Thompson 2012; Tong 2017; Valdez 2018).
The mean age was reported in 24 studies (Bailey 2012; Calderón 2014; DeCamp 2020; Elder 1998; Gwede 2019; Han 2017; Kheir 2014; Kim 2009; Kim 2014; Kim 2020; Kiropoulos 2011; Koniak‐Griffin 2015; Lepore 2012; Otilingam 2015; Payán 2020; Poureslami 2016a; Rosal 2005; Sudore 2018; Thompson 2012; Tong 2017; Unger 2013; Valdez 2015; Valdez 2018; Wong 2020) ranging from 28.7 years (Elder 1998) to 70.9 years (Kim 2014).
The least described PROGRESS‐Plus domains were religion, sexual orientation and disability. Three studies provided concrete information on the participants' religion (Bloom 2014; Sudore 2018; Wong 2020), whereas one other study assessed how religious beliefs might influence medical‐decision making (Gwede 2019). Four studies recruited their participants from churches (Han 2017; Kim 2009; Kim 2014; Kim 2020). One study reported data on the participants' sexual orientation (van Servellen 2005), whereas no study included participants with any mental or complex disability.
Most participants included in the studies were female (75.4%). Four studies did not provide data on the number of female and male participants randomly assigned to either the intervention or control arm (Elder 1998; Poureslami 2016a; Poureslami 2016b; Unger 2013). Ten studies had an all‐female population (Bloom 2014; DeCamp 2020; Han 2017; Hernandez 2013; Koniak‐Griffin 2015; Ochoa 2020; Otilingam 2015; Payán 2020; Valdez 2018; Wong 2020), and two studies included men only (Kheir 2014; Lepore 2012). Bloom 2014 also educated the husbands of women included in their study, but we had insufficient information to consider these data.
Health literacy
Nineteen studies reported baseline data on health literacy using a validated assessment tool. Of these, 12 additionally reported an outcome measure for health literacy (named as such) to assess the effectiveness of the intervention (Calderón 2014; Han 2017; Hernandez 2013; Kaur 2019; Kim 2014; Kim 2020; Kiropoulos 2011; Otilingam 2015; Soto Mas 2018; Unger 2013; van Servellen 2005; Wong 2020). Ten studies used a disease‐specific assessment tool (Calderón 2014; Han 2017; Hernandez 2013; Kaur 2019; Kim 2014; Kim 2020; Kiropoulos 2011; Unger 2013; van Servellen 2005; Wong 2020). Two of these studies made use of both, a disease‐specific and either one (Hernandez 2013) or more generic health literacy assessment tools (Kim 2020). Two studies reported results on generic functional health literacy (Soto Mas 2018) or health numeracy only (Otilingam 2015). Poureslami 2016a reported that they "assessed patients’ health literacy (as ability to access, understand, and use asthma‐related information)" but the results were not reported. A description of the assessment tools applied as well as the baseline scores of the participants in each study is shown in the Characteristics of included studies section.
Interventions
The identified interventions varied widely with regard to the design features such as methods and modes of delivery, the targeted populations, the health literacy components addressed and the outcomes assessed. An overview of the studies' grouping according to the main intervention components and the comparators is shown in Table 23 and in the Characteristics of included studies section.
14. Grouping of studies according to main intervention components and comparator.
| Study ID | Health topic | Description of intervention arm(s) | Main intervention component | Additional intervention components | Intervention delivery method/mode | Intervention provider | Comparator |
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | |||||||
| Bloom 2014 | Breast cancer | Multimodal educational intervention "Afghan women's breast health program" | Intense health education (multiple methods of knowledge transfer/skills training, personal interaction with provider) | Individual motivational counselling | Weekly face‐to‐face group sessions, followed by individual motivational counselling through health navigators (total programme duration, number and length of group sessions and counselling not reported) | Trained LHE/ health navigators | Wait‐list control (delayed intervention) |
| Koniak‐Griffin 2015 | Cardiovascular disease | Multimodal lifestyle behaviour intervention, "Mujeres Sanas y Precavidas" | Intense health education (multiple methods of knowledge transfer/skills training, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 8 weekly face‐to‐face group sessions lasting 2 hours, followed by 4 months of individual teaching and coaching sessions (4 face‐to‐face sessions and 4 phone calls) | Trained promotoras | Attention placebo control; same quantity, but information on safety and preparedness |
| Rosal 2011 | Type 2 diabetes | Multimodal Diabetes Self‐Management intervention programme “Latinos en Control” | Intense health education (multiple methods of knowledge transfer/skills training, role modelling, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 12 weekly face‐to‐face group sessions lasting 2.5 hours and 8 monthly face‐to‐face group sessions. First session: 1st hour personalised counselling and cooking; remaining time: group protocol and meal | Trained team of 2 leaders and an assistant (either nutritionist or health educator and trained lay individuals or 3 lay individuals supervised by 2 investigators) | Usual care (no additional intervention) |
| van Servellen 2005 | HIV | Multimodal HIV treatment adherence enhancement program “Es por la vida” | Intense health education (multiple methods of knowledge transfer/skills training, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 5 weekly face‐to‐face group sessions (of 3 to 7 participants), followed by 6 months of telephone counselling or face‐to‐face encounters | Nurse practitioner and health educator; trained foreign medical student (only assessment) | Usual care (no additional intervention) |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Han 2017 | Breast/cervical cancer | CHW‐led breast and cervical cancer health literacy skills training | Intense health education (multiple methods of knowledge transfer/skills training, role modelling, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 1 face‐to‐face group session (of 7 to 8 women) lasting 1.5 to 2 hours, followed by 6 months of monthly telephone calls | Trained CHW | Wait‐list control/standard brochure |
| Kaur 2019 | Oral health | “Safeguard Your Smile” oral health literacy intervention | Intense health education (multiple methods of knowledge transfer/skills training, role modelling, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 1 face‐to‐face group session (of 3 to 4 participants) lasting 1 hour; monthly phone calls within a 3‐month follow‐up period | Lead researcher, no further training | Standard brochure |
| Kim 2009 | Type 2 diabetes | Community based, multimodal behavioural Self‐Help Intervention Programme for Diabetes Management (SHIP‐DM, pilot study) | Intense health education (multiple methods of knowledge transfer/skills training, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 6 weekly face‐to‐face group sessions lasting 2 hours followed by 6 months of self‐monitoring and monthly telephone counselling (10 to 25 min) | Trained CHW and research nurses | Wait‐list control/standard brochure |
| Kim 2014 | High blood pressure (HBP) | Multimodal self‐help intervention programme on the control of high blood pressure | Intense health education (multiple methods of knowledge transfer/skills training, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 6 weekly face‐to‐face group sessions (of 6 to 10 participants) lasting 2 hours, followed by 12 months of self‐monitoring (including weekly submission of BP to study website) and monthly telephone counselling |
Trained research staff and research nurses | Wait‐list control/standard brochure |
| Kim 2020 | Type 2 diabetes | Community based, multimodal behavioural Self‐Help Intervention Programme for Diabetes Management (SHIP‐DM) | Intense health education (multiple methods of knowledge transfer/skills training, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 6 weekly face‐to‐face group sessions lasting 2 hours, followed by 12 months of self‐monitoring and monthly telephone counselling | Trained CHW and research nurses | Wait‐list control/standard brochure |
| Rosal 2005 | Type 2 diabetes | Multimodal self‐management intervention programme for metabolic self‐control in individuals with type 2 diabetes | Intense health education (multiple methods of knowledge transfer/skills training, role modelling, personal interaction with provider) | Individual motivational counselling, self‐monitoring | 1 initial face‐to‐face individual session lasting 1 hour, 10 weekly face‐to‐face group sessions lasting 2.5 to 3 hours and 2 individual sessions lasting 15 min (immediately prior to group sessions within 10 weeks period) | Diabetes nurse, nutritionist and research assistant (known to community residents) | Standard brochure |
| 3 Culturally adapted health literacy skills building course vs no/unrelated health literacy intervention | |||||||
| Elder 1998 | Nutrition/cardiovascular health | Health literacy skills training embedded in language course | Intense health education (multiple methods of knowledge transfer/skills training incorporated in existing English as a second language (ESL) course, personal interaction with provider) | — | As many as 5 face‐to‐face group sessions lasting 3 hours | Trained ESL teacher | Same method/mode of delivery, but information on a different health topic |
| Otilingam 2015 | Nutrition/heart and brain health | Group 1: Workshop on nutrition and heart health Group 2: Workshop on nutrition and heart health plus brain health Group 3: Wait‐list control Group 4: Post‐test only wait‐list control |
Group 1, 2 (combined)**: Intense health education (multiple methods of knowledge transfer/skills training, role modelling, personal interaction with provider) | — | 2 face‐to‐face group sessions (of up to 7 participants) lasting 2 hours (1 week apart) | Trained bilingual research assistants | Group 3, 4**: wait‐list control |
| Soto Mas 2018 | Cardiovascular health | Health literacy skills training embedded in language course | Intense health education (multiple methods of knowledge transfer/skills training incorporated in existing ESL course, role modelling, personal interaction with provider) | — | 12 face‐to‐face, group sessions lasting 3.5 hours (total of 42 hours) delivered over a period of 6 weeks | Trained ESL teacher | Usual care (standard ESL course without additional information)1 |
| Taylor 2011 | Hepatitis B | Health literacy skills training embedded in language course | Intense health education (multiple methods of knowledge transfer/skills training incorporate in existing ESL course, role modelling, personal interaction with provider) | — | 1 face‐to‐face, group session lasting 3 hours | Trained ESL teacher | Same method/mode of delivery, but information on a different health topic |
| Tong 2017 | Colorectal cancer (CRC) | LHE‐led CRC group education | Intense health education (multiple methods of knowledge transfer/skills training, personal interaction with provider) | Individual motivational counselling | 2 face‐to‐face group sessions lasting approx. 90 min, separated by 2 months 2 follow‐up phone calls 1 month after each session |
Trained LHE | Same method/mode of delivery, but information on a different health topic |
| Wong 2020 | Mental health (depression) | Cognitive behavioural therapy (CBT)‐based paraprofessional training programme | Intense health education (multiple methods of knowledge transfer/skills training, personal interaction with provider) | — | 4 weekly face‐to‐face, group sessions lasting 3 hours, homework exercises | Master's level clinical psychology trainees | Wait‐list control |
| 4 Culturally adapted telephone education vs unrelated culturally adapted telephone education | |||||||
| Lepore 2012 | Prostate cancer | Tailored telephone education intervention on prostate cancer | Simple health education (2 methods of knowledge transfer: telephone education plus educational pamphlet), personal interaction with provider | Decision support | 2 individual phone calls within a 1‐month period (median = 1 week) plus mailed brochure, 1 health education call lasting approx. 20 min and 1 follow‐up call lasting approx. 5 min | Trained graduate‐level health educator | Same method/mode of delivery, but information on a different health topic |
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| DeCamp 2020 | Child health | "Salud al Día", Spanish‐language interactive text messaging intervention | Simple health education (2 methods of knowledge transfer: factual information, role modelling) | Motivational interactive text/push messages and automated feedback | 1 individual video session lasting 9 min (plus take‐home DVD at 2‐month visit in clinic) and monthly interactive text messages for 10 months, if necessary email contact to clinic nurse | Research staff, clinic staff | Usual care (no additional intervention) |
| Hernandez 2013 | Mental health (depression) | Fotonovela "Secret Feelings" | Simple health education (1 method of knowledge transfer: role modelling), extent of personal interaction with provider unclear | — | 1 face‐to‐face group session (printed fotonovela read out loud by literate participants) | Experienced study site's promotoras | Placebo intervention (group discussion on family communication) |
| Kiropoulos 2011 | Depression | Multicultural Information on Depression Online (MIDonline) website | Simple health education (2 methods of knowledge transfer, role modelling, multiple interactive online modules) | — | 1 individual web‐based session (interactive website) | Not applicable | Placebo intervention (semi‐structured interview about depression) |
| Thompson 2012 | Child nutrition and feeding | Nutrition education via interactive touchscreen | Simple health education (1 method of knowledge transfer: multiple interactive online modules) | Algorithm‐based automated feedback | 1 individual web‐based session (interactive touchscreen computer, 5 modules of 2 to 8 min, total duration approx. 25 min) | Not applicable | Usual care (no additional intervention) |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Calderón 2014 | Type 2 diabetes | Animated bilingual video "¿Que es la Diabetes?/What Is Diabetes?" | Simple health education (1 method of knowledge transfer: role modelling) | — | 1 individual video session lasting 13 min | Not applicable | Easy‐to‐read information on diabetes (language concordant) |
| Gwede 2019 | Colorectal cancer | “LCARES” fotonovela booklet and DVD intervention plus faecal immunochemical test (FIT) | Simple health education (2 methods of knowledge transfer: factual information, role modelling) | Reminder letters | 1 individual video session plus printed fotonovela | Not applicable | Standard brochure |
| Payán 2020 | Breast cancer | Group 1: CUIDARSE ("taking care of oneself") brochure on breast cancer Group 2: CHW‐delivered CUIDARSE ("taking care of oneself") brochure on breast cancer Group 3*: usual care (standard brochure) |
Group 1, 2** (combined): simple health education (1 method of knowledge transfer: role modelling), personal contact, but no additional support or information (oral administration of adapted written information) |
— | 1 face‐to‐face session lasting 15 min (printed brochure verbally administered) (unclear whether delivered in group or individually) | Trained bilingual CHW | Group 3*: usual care (standard brochure) |
| Poureslami 2016a | Asthma | Group 1: physician‐led video Group 2: community video Group 3: both physician‐led and community videos Group 4: literacy adapted pictorial pamphlet (language concordant) |
Group 3*: simple health education (2 methods of knowledge transfer: factual information, role modelling) | — | 1 individual video session (2 videos: 1 factual knowledge video and 1 peer‐led (community) video) | Not applicable | Group 4*: easy‐to‐read pictorial pamphlet on asthma |
| Poureslami 2016b | COPD | Group 1: physician‐led video Group 2: community video Group 3: both physician‐led and community videos Group 4: literacy adapted pictorial pamphlet (language concordant) |
Group 3*: simple health education (2 methods of knowledge transfer: factual information, role modelling) | — | 1 individual video session (2 videos: 1 physician‐led, factual knowledge video and 1 peer‐led (role‐played) video | Not applicable | Group 4*: easy‐to‐read pictorial pamphlet on COPD |
| Sudore 2018 | No specific (advance care planning) | Interactive online advance care planning programme “PREPARE” and AD intervention | Simple health education (2 methods of knowledge transfer: multiple interactive online modules, skills training), personal interaction with provider via telephone | Algorithm‐based automated feedback | 1 web‐based session (interactive website), ongoing access to website, plus literacy adapted printed Advance Directive (AD), reminder phone call 1 to 3 days prior to primary care visit | Trained research staff | Written advance directive |
| Unger 2013 | Mental health (depression) | Fotonovela "Secret Feelings" | Simple health education (1 method of knowledge transfer: role modelling), personal interaction with provider unclear | — | 1 face‐to‐face group session lasting 20 to 30 min (printed fotonovela read by oneself) | One data collector, no further information |
Standard brochure |
| Valdez 2015 | Cervical cancer | Educational DVD on human HPV vaccine | Simple health education (2 methods of knowledge transfer: role modelling, factual information) | — | 1 individual video session (DVD watched at home at individually convenient time) | Not applicable | Usual care (standard brochure) |
| Valdez 2018 | Cervical cancer | Cervical cancer education via interactive touchscreen | Simple health education (1 method of knowledge transfer: multiple interactive online modules) | Algorithm‐based automated feedback | 1 individual web‐based session lasting 20 to 30 min (interactive, multimedia touchscreen kiosk) | Not applicable | Standard brochure |
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally and literacy adapted audio‐/visual education without personal feedback | |||||||
| Ochoa 2020 | Cervical cancer | Tamale Lesson/Conversando entre Tamales", a narrative culturally tailored film on prevention of cervical cancer | Simple health education (1 method of knowledge transfer: role modelling) | — | 1 narrative/story telling video session lasting 11 min | Not applicable | Factual knowledge video |
| Poureslami 2016a | Asthma | Group 1: physician‐led video Group 2: community video Group 3: both physician‐led and community videos Group 4: literacy adapted pictorial pamphlet (language concordant) |
Group 2*: simple health education (1 method of knowledge transfer: role modelling) | — | 1 narrative/story telling video session (peer‐played | Not applicable | Group 1*:(Community) physician‐led, factual knowledge video |
| Poureslami 2016b | COPD | Group 1: physician‐led video Group 2: community video Group 3: both physician‐led and community videos Group 4: literacy adapted pictorial pamphlet (language concordant) |
Group 2*: simple health education (1 method of knowledge transfer: role modelling) | — | 1 narrative video session (peer‐played) | Not applicable | Group 1*: (Community) physician‐led, factual knowledge video |
| 8 Culturally and literacy adapted medical instruction vs no health literacy intervention | |||||||
| Bailey 2012 | No specific (medication understanding) | Health literacy informed Rx bottles | Adapted written medical instructions (health literacy informed medication label) |
— | Written information | Not applicable | Language concordant standard text labels |
| Kheir 2014 | No specific (medication understanding) | Group 1: pictogram‐only label Group 2: pictogram label with verbal instructions Group 3: standard text label with translated verbal instructions |
Group 2*: adapted written medical instructions (pictogram labels) plus translated verbal instructions | — | Written information, face‐to‐face instruction (1 session) | Research staff, interpreter |
Group 3*: standard text label with translated verbal instructions |
| Mohan 2014 | Diabetes (medication understanding) | PictureRx illustrated medication list | Adapted written information (illustrated medication list + plain language bilingual text), personal contact with provider |
— | Written information, face‐to‐face instruction, 2‐min instruction video | Research assistant | Language concordant standard text labels |
AD: advance directive; BP: blood pressure; CHW: community health worker; COPD: chronic obstructive pulmonary disease; CRC: colorectal cancer; ESL: English as a second language; LHE: lay health educator; Rx: prescription; SHIP‐DM: Self‐Help Intervention programme for type 2 Diabetes Management
* Prioritised intervention group to create a single pairwise comparison; ** Groups were combined to create a single pairwise comparison
1Standard ESL curriculum already includes health‐related topics. Therefore, control group assignment might not be accurate.
In the following, the grouped interventions are described with regard to the intervention complexity in descending order.
1 Culturally and literacy adapted self‐management programme
Studies categorised as culturally and literacy adapted self‐management programmes aimed to improve self‐care management in individuals with at least one chronic disease or a certain disease risk and low literacy skills and/or low language proficiency. Interventions were characterised by the following main intervention components: 1) a phase of intense one‐to‐one or group‐based health education and 2) a maintenance phase of self‐monitoring accompanied by 3) at least monthly individual motivational counselling up to a total programme duration of 12 months. The individual counselling sessions during the maintenance phase were usually delivered through telephone or face‐to‐face either by research staff (Kaur 2019; Rosal 2011), registered study nurses and/or trained lay community health workers (e.g. promotoras; lay Hispanic/Latinx community members who are trained to provide health education in the community) (Han 2017; Kim 2009; Kim 2014; Kim 2020; Koniak‐Griffin 2015; Rosal 2005; van Servellen 2005). The counselling sessions were carried out to reinforce the lessons learned, to motivate to maintain self‐care skills, and to provide normative feedback on the participants' progress. Participants included in these interventions were either (predominantly) male HIV‐positive Latino immigrants (van Servellen 2005) or overweight Latinas at risk for developing a cardiovascular disease (Koniak‐Griffin 2015), Korean or (Caribbean) Latinx immigrants with diabetes (Kim 2009; Kim 2020; Rosal 2005; Rosal 2011), Korean immigrants with high blood pressure (Kim 2014), or Korean immigrants at risk for breast or cervical cancer (Han 2017). One study aimed to improve oral health literacy in Punjabi immigrants by teaching correct dental hygiene and raising awareness of oral diseases such as gingivitis and dental plaque (Kaur 2019). All self‐management interventions were individually tailored and facilitated by multidisciplinary teams except for one less complex intervention that was delivered by the lead researcher alone (Kaur 2019).
For one study, we only found an abstract describing a few results of the intervention's evaluation (Bloom 2014) and two publications describing the qualitative formative research to develop the intervention (Shirazi 2013; Shirazi 2015). Thus, the information about the intervention features is limited, but we assume that this intervention most likely fits into this grouping. Briefly, the study was based on extensive community‐based participatory research and addressed Afghan Muslim women's breast health, of whom many have had a family history of breast cancer. It aimed to educate Afghan Muslim women about breast health and to improve mammography screening rates by means of culturally and literacy‐sensitive, faith‐based group education on a weekly basis (total duration is unclear), followed by the support of trained community health navigators to facilitate making and keeping appointments for mammography screening as needed. In addition, the male heads of the family were educated to convince them of the importance of educating their wives about breast health. Further details about the involvement of the participants' husbands, the intensity and total duration of the programme were not reported.
2 Culturally adapted health literacy skills building course
Interventions categorised as culturally adapted health literacy skill building courses were characterised by intense health education delivered in a group format, aiming to improve health literacy skills in the domain of disease prevention. These included multiple strategies of knowledge transfer such as risk communication, interactive role‐plays to practise communication with healthcare providers, culture‐sensitive narratives delivered through diverse multimedia formats (e.g. via video), and several other practices to improve health‐related reading, writing and numeracy skills (e.g. writing short texts or calculating daily doses of calories). Three studies were conducted in the setting of adult language schools embedding face‐to‐face health literacy skills training related to a certain health topic in an existing English as a second language (ESL) course curriculum (Elder 1998; Soto Mas 2018; Taylor 2011). All of these interventions were delivered through trained ESL teachers. The mode of delivery for these courses ranged from one or two face‐to‐face group sessions lasting three hours (Taylor 2011), to more intense courses with 15 hours (Elder 1998), up to 42 hours of intense health literacy training delivered in 12 face‐to‐face group sessions (Soto Mas 2018). Two studies made use of two face‐to‐face group sessions lasting from 90 minutes (Tong 2017) to two hours (Otilingam 2015). In one study, the participants received additional telephone‐based follow‐up sessions that were delivered by trained lay community health workers (Tong 2017). Another study was delivered by trained bilingual research assistants (Otilingam 2015). The interventions were related to cardiovascular health behaviour in Latinx immigrants (Elder 1998; Soto Mas 2018), hepatitis B testing (Taylor 2011), colorectal cancer screening (Tong 2017) or depression (Wong 2020) in South and East Asian immigrants. One study with four arms and two intervention groups provided education about cardiovascular health only (intervention group 1) or cardiovascular health and brain health (intervention group 2) (Otilingam 2015).
3 Culturally and literacy adapted telephone education
One study provided information about prostate cancer through trained graduate‐level health educators who delivered tailored telephone education (lasting 20 minutes) to immigrant men of African descent from the Caribbean (Lepore 2012). In addition, the participants received mailed health brochures on the topic. Participants in the control group received telephone education about healthy nutrition.
4 Culturally and literacy adapted audio‐/visual education without personal feedback
Interventions categorised as culturally and literacy adapted audio‐/visual education without personal feedback made use of simple health education delivered through diverse audio‐ and/or visual formats (e.g. via video, interactive touchscreen computer, websites and/or text messages, or via telephone calls). These studies aimed to improve knowledge and understanding of, and attitudes towards a certain disease or disease prevention service (e.g. screening, vaccines). They were designed to promote a specific health behaviour such as the correct medication dosing or to improve adequate health service use through educational messages embedded in culturally adapted narratives. Two studies aimed to improve the inhaler use in Asian immigrants either with asthma (Poureslami 2016a) or chronic obstructive pulmonary disease (COPD) (Poureslami 2016b). The information was either presented by a physician with the same ethnic background or through video‐recorded role‐plays conducted by peer patients or lay individuals of the community. Four studies made use of printed narratives (Payán 2020) and photonovels (in Spanish "fotonovela"; small comic books that tell a story of a person coping with a certain disorder or a health problem written at a low literacy reading level) (Hernandez 2013; Unger 2013). The included studies were related to depression (Hernandez 2013; Unger 2013), colorectal cancer (Gwede 2019) or breast cancer (Payán 2020). All four studies addressed Latinx immigrants. Payán 2020 and Hernandez 2013 delivered the printed photonovel verbally through a promotora, whereas Gwede 2019 provided an educational DVD in addition to the photonovel. Three other studies also used educational videos including narratives and role modelling elements either relating to diabetes (Calderón 2014), to cervical cancer (Ochoa 2020), or to child vaccinations and infant diseases (DeCamp 2020). Of these, one study additionally provided monthly interactive text messages (for 10 months) (DeCamp 2020). Two studies delivered health information about child nutrition (Thompson 2012) or cervical cancer (Valdez 2018) to Latinx immigrants through interactive touchscreen kiosks. Another two presented the information through interactive websites (Kiropoulos 2011; Sudore 2018), one study embedding case studies of individuals coping with depression in the "MIDonline" website, which was designed to educate Southern European immigrants living in Australia about depression (Kiropoulos 2011). The other study intended to increase engagement in advance care planning among elderly Latinos with chronic illnesses and to mitigate the effects of low literacy (Sudore 2018). The patient‐directed interactive online advance care planning programme (PREPARE for your care) consisted of five modular skill‐building steps including interactive online questions that generated an individual action plan and a summary of participants’ individual wishes. Reminder calls by the research staff were carried out to remind the participants of talking about their wishes with their primary doctor (Sudore 2018).
Narratives in the form of photonovels or embedded in DVDs have also been used in other intervention studies as part of a broader main strategy such as group‐based health education to foster adequate health service use or to model attitudinal change (Han 2017; Kaur 2019; Otilingam 2015; Rosal 2005; Rosal 2011; Soto Mas 2018; Taylor 2011).
5 Culturally and literacy adapted medical instruction
Three studies included a culturally and literacy‐adapted presentation of written medical instructions as a single strategy using either pictograms, which were substantiated by verbal (Kheir 2014) or video instruction (Mohan 2014), or easily understandable, culturally adapted terminology (Bailey 2012). The primary aim of these studies was an improved medication understanding and use of prescribed medication without an additional component of disease‐specific knowledge transfer. All studies were delivered in one session using a written format (Bailey 2012; Kheir 2014). One study additionally included a short video instruction (Mohan 2014). None of these studies were individually tailored.
Comparator
Twenty‐nine studies were two‐arm RCTs and five studies were multiple‐arm RCTs (Kheir 2014; Otilingam 2015; Payán 2020; Poureslami 2016a; Poureslami 2016b). As recommended in Chapter 6.2.9 of the Cochrane Handbook for Systematic Reviews of Interventions, we created single pairwise comparisons for each trial (Higgins 2022a), resulting in two studies that were included in more than one comparison (Poureslami 2016a; Poureslami 2016b). An overview of the comparisons included in this review is shown in Table 23.
Health literacy interventions were compared with 'no health literacy intervention' including usual care and no additional intervention (Bailey 2012; DeCamp 2020; Kheir 2014; Mohan 2014; Rosal 2011; Soto Mas 2018; Thompson 2012; van Servellen 2005), placebo intervention (Hernandez 2013; Koniak‐Griffin 2015; Kiropoulos 2011) and delayed intervention (Bloom 2014; Otilingam 2015; Wong 2020), or with 'unrelated health literacy intervention' (participants received the same intervention but information on a different health topic) (Elder 1998; Lepore 2012; Taylor 2011; Tong 2017). In 14 studies, a health literacy intervention was compared to 'written information on the same health topic' (Calderón 2014; Gwede 2019; Han 2017; Kaur 2019; Kim 2009; Kim 2014; Kim 2020; Han 2017; Payán 2020; Rosal 2005; Sudore 2018; Unger 2013; Valdez 2015; Valdez 2018). In four of these studies, participants in the control group received a brief brochure, but also a delayed intervention after the programme was completed (Han 2017; Kim 2009; Kim 2014; Kim 2020).
One study compared two variants of a health literacy intervention, which were a narrative educational video related to cervical cancer compared to a factual knowledge video on the same topic. We reported the results in comparison 7 'culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback' (Ochoa 2020).
Five studies were multiple‐arm RCTs. Two of these studies, with four arms each, compared a (community) physician‐led factual knowledge video (group 1) to a narrative, peer group role‐played video (group 2), to a group who watched both videos (group 3), or to a control group who read a pictorial pamphlet on the same topic (group 4) (Poureslami 2016a; Poureslami 2016b). As we categorised more than one of these interventions as being a health literacy intervention, we reported these studies in two comparisons. Firstly, we combined groups 1, 2 and 3 to create a single pairwise comparison with group 4 and reported the results in comparison 6. Secondly, we reported the results for group 1 compared to group 2 in comparison 7. One other four‐arm parallel trial compared two variants of the same intervention to two variants of wait‐list control groups (Otilingam 2015). In this study, intervention group 1 consisted of a disease prevention and health literacy skills building course related to cardiovascular health, whereas intervention group 2 consisted of the same course extended by 20 to 30 minutes of education on brain health. The wait‐list control groups differed in the timing of outcome assessments only. Control group 1 was assessed baseline, post‐test and at one‐month follow‐up, whereas control group 2 was assessed post‐test only. We pooled both intervention and control groups to create a single pairwise comparison for the post‐test assessment. We compared the pooled intervention groups to control group 1 for the follow‐up assessment. Another three‐arm parallel trial compared a culturally and literacy adapted printed brochure about breast cancer to read oneself (group 1) to the same brochure, which was delivered by a community health worker (group 2) with a language concordant standard brochure about breast cancer (group 3, 'no health literacy intervention') (Payán 2020). We pooled group 1 and group 2, comparing it to group 3, which we refer to as the control group. Another study had two intervention arms split into three conditions for the analysis (Kheir 2014). Pictogram‐only labels (group 1) were compared with pictogram labels with verbal instructions (group 2) to a standard text label with verbal instructions (group 3, here referred to as the control group). We included group 1 and group 3 only, as they built the greatest contrast.
Theories and frameworks guiding the interventions
Various health‐related theories and frameworks were used to guide intervention development, implementation and/or evaluation. Table 24 presents an overview of the theoretical frameworks named by the study authors.
15. Theoretical frameworks used to guide the intervention development.
| Theoretical framework | Study |
| Behavior Change Wheel (Michie 2011) | Kaur 2019 |
| Behavioral Skills Model (Amico 2011) | DeCamp 2020 |
| Health Behavior Framework1 (Curry 1994) | Taylor 2011 |
| Health Belief Model (Janz 1984) Health Belief Model, Social Learning Theory and self‐efficacy (Rosenstock 1988) Health Belief Model (perceived barriers and benefits, perceived susceptibility, self‐efficacy and cues to action) (Champion 2008) |
Thompson 2012 Otilingam 2015 Payán 2020 |
| Input‐Output Framework (McGuire 2015) | Payán 2020 |
| Adult learning theory (Knowles 1984) Learning theories (Smith 1999; Semple 2000) |
Soto Mas 2018; Rosal 2011 Thompson 2012 |
| Model of culture‐centric narratives (Larkey 2010) | Hernandez 2013 |
| Operant conditioning (Skinner 1953) | Elder 1998 |
| Ottawa Decision Support Framework (Doull 2006) | Lepore 2012 |
| Preventive Health Model (Mc Queen 2008) | Gwede 2019 |
| PRECEDE‐PROCEED model2 (Green 19913) | Kim 2009; Kim 2020; Han 2017 |
| Self‐Help Model (Braden 1990b; Braden 1990a) | Kim 2014 |
| Social‐Cognitive Theory (Bandura 1977; Bandura 2002; Bandura 2004) | Elder 1998; Hernandez 2013; Kim 2009; Rosal 2005; Rosal 2011; Sudore 2018; Soto Mas 2018; Tong 2017 |
| The Interpersonal Communication Competence Model (Spitzberg 1984; Street 2003) | Sudore 2018 |
| Theory of Reasoned Action/Planned Behaviour (Ajzen 1991; Fishbein 19754) | Unger 2013; Valdez 2015 |
| Transtheoretical Model of Health Behavior (Prochaska 1997) | Sudore 2018; Tong 2017; Valdez 2018 |
| Theories about self‐efficacy (Bandura 1994) | Hernandez 2013 |
1Authors mentioned explicitly the Health Belief Model, the Theory of Reasoned Action/Planned Behavior, the PRECEDE model and Social influence theory, which are integrated in the Health Behavior Framework.
2Authors mentioned explicitly premises of the self‐help model (Braden 1990b; Braden 1990a), which is integrated in the PRECEDE‐PROCEED model.
3Green developed PRECEDE in 1974 and Kreuter added PROCEED in 1991.
4The Theory of Reasoned Action was originally developed by Fishbein & Ajzen (1975) (Fishbein 1975); Ajzen complemented it in 1991 (Ajzen 1991).
In summary, 19 established theories were applied in 21 studies, some of which referred to more than one theory guiding the intervention development, implementation and/or evaluation. Established theories and frameworks used referred to both theories of health promotion and health behaviour change, but also to behavioural theories in general. Most studies referred to Bandura's social‐cognitive theory (Bandura 1977; Bandura 2002; Bandura 2004; Elder 1998; Hernandez 2013; Kim 2009; Rosal 2005; Rosal 2011; Soto Mas 2018; Sudore 2018; Tong 2017) or theories of self‐efficacy (Bandura 1994; Bandura 1997; Hernandez 2013). Three studies informed their intervention with the transtheoretical model of health behaviour (Prochaska 1997; Sudore 2018; Tong 2017; Valdez 2018), three studies referred to the health belief model or its variations (Champion 2008; Janz 1984; Otilingam 2015; Payán 2020; Rosenstock 1988; Thompson 2012), and another three studies applied adult learning theory (Knowles 1984) or learning theories in general (Rosal 2011; Semple 2000; Smith 1999; Soto Mas 2018; Thompson 2012). The PRECEDE‐PROCEED model (Green 1991) was used by Han 2017, Kim 2009 and Kim 2020. Unger 2013 and Valdez 2015 referred to the theory of reasoned action/planned behaviour (Ajzen 1991; Fishbein 1975).
Moreover, DeCamp 2020 referred to the behavioural skills model (Amico 2011), Gwede 2019 to the preventive health model (Aguado Loi 2020; Mc Queen 2008), Taylor 2011 used the health behaviour framework, which integrates various health‐ and behaviour‐related theories and concepts including inter alia the social‐cognitive theory or the transtheoretical model (Curry 1994), Sudore 2018 additionally referred to the interpersonal communication competence model (Spitzberg 1984; Street 1995; Street 2003), Kim 2014 used the self‐help model of learned response to chronic illness experiences (Braden 1990b; Braden 1990a), Kaur 2019 informed the intervention with the behaviour change wheel (Michie 2011), Elder 1998 used operant conditioning (Skinner 1953), Payán 2020 additionally referred to the input output framework (McGuire 2015), Lepore 2012 to the Ottawa decision support framework (Doull 2006), and Hernandez 2013 referred to the model of culture‐centric narratives (Larkey 2010). The intervention development of Bloom 2014 was guided by the cultural explanatory models (CEMs) framework (Rajaram 1998) and Chatman's theory of information seeking (Chatman 1996). All studies referenced empirical studies either related to (low) literacy or language proficiency, or health literacy in the context of health to emphasise the relevance and purpose of the intervention study.
Health literacy components addressed in the interventions
A description of the intervention components based on the integrated model of health literacy is shown in Table 25.
16. Health literacy components addressed by the intervention.
| Study ID | Health domain1 | Prerequisites/tools1 | Processing steps1 | |||||
| Knowledge | Motivation | Competencies | Access | Understand | Appraise | Apply | ||
| No./total + |
Health care 13/34 Disease prevention 21/34 Health promotion 0/34 |
31/34 | 25/34 | 15/34 | 22/34 | 34/34 | 23/34 | 33/34 |
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | ||||||||
| Bloom 2014 | Disease prevention | + | u | u | + | + | u | + |
| Koniak‐Griffin 2015 | Disease prevention | + | + | + | + | + | + | + |
| Rosal 2011 | Health care | + | + | + | + | + | + | + |
| van Servellen 2005 | Health care | + | + | + | + | + | + | + |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | ||||||||
| Han 2017 | Disease prevention | + | + | + | + | + | + | + |
| Kaur 2019 | Disease prevention | + | + | + | + | + | + | + |
| Kim 2009 | Health care | + | + | + | + | + | + | + |
| Kim 2014 | Health care | + | + | + | + | + | + | + |
| Kim 2020 | Health care | + | + | + | + | + | + | + |
| Rosal 2005 | Health care | + | + | + | + | + | + | + |
| 3 Culturally adapted health literacy skills building course vs no/unrelated health literacy intervention | ||||||||
| Elder 1998 | Disease prevention | + | + | + | ‐ | + | u | + |
| Otilingam 2015 | Disease prevention | + | + | + | ‐ | + | u | + |
| Soto Mas 2018 | Disease prevention | + | + | + | + | + | + | + |
| Taylor 2011 | Disease prevention | + | + | + | ‐ | + | + | + |
| Tong 2017 | Disease prevention | + | + | + | + | + | + | + |
| Wong 2020 | Disease prevention | + | + | + | + | + | + | + |
| 4 Culturally and literacy adapted telephone education vs unrelated culturally and literacy adapted telephone education | ||||||||
| Lepore 2012 | Disease prevention | + | + | ‐ | + | + | + | + |
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | ||||||||
| DeCamp 2020 | Disease prevention | + | + | ‐ | + | + | u | + |
| Hernandez 2013 | Disease prevention | + | + | ‐ | + | + | + | + |
| Kiropoulos 2011 | Disease prevention | + | u | ‐ | + | + | + | + |
| Thompson 2012 | Disease prevention | + | + | ‐ | ‐ | + | + | + |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | ||||||||
| Calderón 2014 | Health care | + | u | ‐ | + | + | + | + |
| Gwede 2019 | Disease prevention | + | + | ‐ | + | + | u | + |
| Payán 2020 | Disease prevention | + | u | ‐ | ‐ | + | + | + |
| Poureslami 2016a | Health care | + | + | ‐ | ‐ | + | + | + |
| Poureslami 2016b | Health care | + | + | ‐ | ‐ | + | + | + |
| Sudore 2018 | Health care | + | + | ‐ | ‐ | + | + | + |
| Unger 2013 | Disease prevention | + | + | ‐ | + | + | + | + |
| Valdez 2015 | Disease prevention | + | u | ‐ | ‐ | + | u | + |
| Valdez 2018 | Disease prevention | + | u | ‐ | + | + | u | + |
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally and literacy adapted audio‐/visual education without personal feedback | ||||||||
| Ochoa 2020 | Disease prevention | + | + | ‐ | + | + | u | + |
| 8 Culturally and literacy adapted medical instruction vs no health literacy intervention | ||||||||
| Bailey 2012 | Health care | ‐ | ‐ | ‐ | ‐ | + | ‐ | + |
| Kheir 2014 | Health care | ‐ | ‐ | ‐ | ‐ | + | ‐ | ‐ |
| Mohan 2014 | Health care | ‐ | ‐ | ‐ | ‐ | + | ‐ | + |
1 = review authors' assignment; + = addressed (either explicitly stated/measured or implicitly through theory used or methods applied); u = unclear whether health literacy component was addressed; ‐ = health literacy component was not addressed
Most interventions were related to the domain of disease prevention (21/34) (Bloom 2014; DeCamp 2020; Elder 1998; Gwede 2019; Han 2017; Hernandez 2013; Kaur 2019; Kiropoulos 2011; Koniak‐Griffin 2015; Lepore 2012; Ochoa 2020; Otilingam 2015; Payán 2020; Soto Mas 2018; Taylor 2011; Thompson 2012; Tong 2017; Unger 2013; Valdez 2015; Valdez 2018; Wong 2020). These interventions were usually designed to improve the knowledge of, and beliefs and attitudes towards, a certain disease, its treatment or a certain screening measure (e.g. cervical cancer screening). Thirteen interventions were related to the health care domain, aiming to improve participants' disease‐specific self‐management, their medication understanding or skills to navigate the health system. No study addressed the health promotion domain (Bailey 2012; Calderón 2014; Kheir 2014; Kim 2009; Kim 2014; Kim 2020; Mohan 2014; Poureslami 2016a; Poureslami 2016b; Rosal 2005; Rosal 2011; Sudore 2018; van Servellen 2005).
All but three interventions explicitly aimed at improving health‐related knowledge or made use of at least one method of knowledge transfer (31/34) (Bailey 2012; Kheir 2014; Mohan 2014). Motivation was addressed by 23 interventions, including programmes that were, for example, designed to address motivational aspects of behaviour change. For six studies it was unclear if and how motivation was addressed (Bloom 2014; Calderón 2014; Kiropoulos 2011; Payán 2020; Valdez 2015; Valdez 2018) and three interventions did not address aspects of motivation (Bailey 2012; Kheir 2014; Mohan 2014). Seventeen studies aimed at improving competencies such as functional (health) literacy skills. Of these, 15 reported explicit methods for improving literacy or numeracy skills in the context of health (Elder 1998; Han 2017; Kaur 2019; Kim 2009; Kim 2014; Kim 2020; Koniak‐Griffin 2015; Otilingam 2015; Rosal 2005; Rosal 2011; Soto Mas 2018; Taylor 2011; Tong 2017; van Servellen 2005; Wong 2020). Those interventions included, for example, learning medical terminology and health‐related phrases or learning how to calculate nutrition values. Two interventions aimed at improving inhaler use technique for pulmonary diseases (Poureslami 2016a; Poureslami 2016b). For one study, we had insufficient information to permit judgement about whether competencies were addressed (Bloom 2014).
Regarding the four steps of health information processing, accessing health information was addressed by 22 interventions that explicitly or implicitly referred to this step by improving health care navigation skills or knowledge of the healthcare system, or by reducing barriers to accessing health care or health information (Bloom 2014; Calderón 2014; DeCamp 2020; Gwede 2019; Han 2017; Hernandez 2013; Kaur 2019; Kim 2009; Kim 2014; Kim 2020; Kiropoulos 2011; Koniak‐Griffin 2015; Lepore 2012; Ochoa 2020; Rosal 2005; Rosal 2011; Soto Mas 2018; Tong 2017; Unger 2013; Valdez 2018; van Servellen 2005; Wong 2020).
Understanding health information was the most common addressed processing step; all interventions were designed to improve the understanding of health information or applied linguistically or literacy adapted information formats.
Appraising health information was addressed by 23 interventions (Calderón 2014; Han 2017; Hernandez 2013; Kaur 2019; Kim 2009; Kim 2014; Kim 2020; Kiropoulos 2011; Koniak‐Griffin 2015; Lepore 2012; Payán 2020; Poureslami 2016a; Poureslami 2016b; Rosal 2005; Rosal 2011; Soto Mas 2018; Sudore 2018; Taylor 2011; Thompson 2012; Tong 2017; Unger 2013; Wong 2020). These interventions included, for example, components of knowledge transfer to improve trust in professional sources of health information or in healthcare providers. Others aimed at improving informed decision‐making by improving the ability to weigh the pros and cons for a certain screening or treatment option. For eight studies, we do not know if and how the appraisal of health information was addressed (Bloom 2014; DeCamp 2020; Elder 1998; Gwede 2019; Ochoa 2020; Otilingam 2015; Valdez 2015; Valdez 2018). No intervention directly aimed at improving the participants' ability to filter, judge and evaluate whether information is of good quality, how to appraise whether a source of information is reliable (e.g. with regard to online information) or where to find good (online) health information.
All but Kheir 2014 addressed the application of health information. These studies either measured outcomes related to this step of information processing (e.g. behaviour intent or actual health behaviour) or referred to theories related to health literacy that imply a causal relationship between, for example, improved knowledge and a respective health behaviour.
Outcomes
A variety of outcomes, assessed with several measures, were reported in the included studies. We reported effect measures on all of our prespecified outcome categories prioritised as specified in Types of outcome measures. A full description of all outcomes assessed within the included studies is shown in the Characteristics of included studies. An overview of health literacy‐related outcomes considered in this review, including measures applied and timing of outcome assessment, is shown in Table 26 and Table 27.
17. Outcomes considered in this review ‐ components of health literacy.
| Study ID | (Disease‐specific) health literacy* | Prerequisites** | Steps of health information processing | Timing of outcome assessment considered | |||
| Knowledge*** | Competencies | Understand | Appraise | Apply | |||
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | |||||||
| Bloom 2014 | — | Breast health/breast cancer knowledge, not specified, no further details reported | — | — | — | — | After 6 months1 |
| Koniak‐Griffin 2015 | — | Heart disease knowledge; 10‐items adapted from previous survey (true/false), 0 to 10 |
— | — | — | — | Short‐term, immediately post‐intervention, medium‐term (3 months post‐intervention) |
| Rosal 2011 | — | Diabetes knowledge; Audit of Diabetes Knowledge (ADK) (subset of 25 items, true/false), no range of score reported |
— | — | — | — | Short‐term (immediately post‐intervention) |
| van Servellen 2005 | HIV health literacy; modified REALM (24 additional HIV‐relevant medical terms); 0 to 24 (recognition of terms); 0 to 24 (understand (explain) terms) |
HIV knowledge; HIV Illness and Treatment Knowledge and Misconceptions measure (0 to 17) |
— | — | — | — | Short‐term (immediately post‐intervention) |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Han 2017 | Cancer health literacy; AHL‐C; sub‐scales on print literacy and functional health literacy, 0 to 53 | Cervical and breast cancer knowledge; Breast Cancer Knowledge Test (0 to 18); Cervical Cancer Knowledge Test (0 to 20) |
— | — | Decisional balance measure (weighing pros and cons), 5 pros and 9 cons on 5‐point Likert scale | — | Short‐term (immediately post‐intervention) |
| Kaur 2019 | Oral health literacy; TS‐REALD; word recognition test, 27 to 73 | Oral hygiene self‐care knowledge; no range of scores reported | — | — | — | — | Short‐term (immediately post‐intervention) |
| Kim 2009 | — | Diabetes knowledge; Diabetes Knowledge Test (DKT) (0 to 14) |
— | — | — | — | Short‐term (immediately post‐intervention) |
| Kim 2014 | HBP health literacy; HBP Health Literacy Scale, sub‐scales of print/functional literacy and numeracy, 0 to 43 |
HBP knowledge; HBP knowledge questionnaire (0 to 26) | — | — | — | — | Short‐term and medium‐term (immediately post‐intervention and at 6‐month follow‐up) |
| Kim 2020 | (1) Print literacy: REALM, 0 to 66 (2) Diabetes‐specific literacy: DM‐REALM, 0 to 83 (3) Health numeracy: TOFHLA, 7‐item numeracy subscale (NVS), 0 to 6 |
Diabetes knowledge; Diabetes Knowledge Test (DKT) (0 to 14) |
— | — | — | — | Short‐term (immediately post‐intervention) |
| Rosal 2005 | — | Diabetes knowledge; Audit of Diabetes Knowledge Scale (ADKnowl), 23 item‐sets (104 items) on various diabetes‐related topics, true/false/"don't know", no range of score reported | — | — | — | — | Short‐term (2 weeks post‐intervention) and medium‐term (4.5 months after programme completion) |
| 4 Culturally adapted health literacy skills building course vs no/unrelated health literacy intervention | |||||||
| Otilingam 2015 | Health numeracy; NVS, 0 to 6 | Dietary fat knowledge, 9 items (0 to 9) | — | — | — | — | Short‐term (at 1 month post intervention) |
| Soto Mas 2018 | Functional health literacy; TOFHLA, 0 to 100 | — | — | — | — | — | Short‐term (immediately post‐ intervention) |
| Wong 2020 | Depression literacy; D‐Lit, 0 to 22 | Knowledge on cognitive behavioural therapy (CBT); 9 items (multiple choice) | — | — | — | — | Short‐term (immediately post‐intervention and at 2‐month follow‐up) |
| Elder 1998 | — | Nutrition‐related knowledge; nutrition knowledge test, 12 items (0 to 12) | — | — | Intention to change nutritional habits (questionnaire: 3 items (1 to 3)) |
Medium‐term (6‐month follow‐up) | |
| Taylor 2011 | — | Hepatitis B knowledge; questionnaire, 5 items (0 to 5) | — | — | — | — | Medium‐term (at 6‐month follow‐up) |
| Tong 2017 | — | Colorectal cancer knowledge; questionnaire, 5 items (0 to 5) | — | — | — | — | Medium‐term (at 3‐month follow‐up) |
| 5 Culturally and literacy adapted telephone education vs unrelated culturally and literacy adapted telephone education | |||||||
| Lepore 2012 | — | Knowledge on prostate cancer screening; 14 items (true/false), percent correct | — | — | — | Testing intention; decision made to get tested for prostate cancer (yes/no) | Long‐term (8 months after randomisation, approx 7 months post‐intervention) |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| DeCamp 2020 | — | Infant health knowledge; true/false, (0 to 5) | — | — | — | — | Short‐term (immediately up to 3 months post‐intervention)2 |
| Hernandez 2013 | — | Depression knowledge; Depression Knowledge Scale (0 to 17) |
— | — | — | Intention to seek treatment for depression; intention to seek treatment for depression scale (0 to 32) | Short‐term (immediately post‐intervention) |
| Kiropoulos 2011 | Depression literacy; D‐Lit, 0 to 22 | — | — | — | — | — | Short‐term (1 week post‐intervention) |
| Thompson 2012 | — | Parental nutrition and feeding knowledge 12‐item true/false questions and 7 multiple choice questions (4 options), 0 to 19 | — | — | — | Planned changes in behaviour: 3 questions; 1 question related to planned changes in behaviour (yes, perhaps, no), 1 open‐ended question on exactly what behaviours they want to change, and 1 question on plans about talking to the child's doctor, family or friends about the information (yes, probably, no), no score reported | Short‐term (immediately post‐intervention) |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Calderón 2014 | Diabetes Health Literacy; DHLS, 37 items on 4 constructs related to diabetes type; 21 items on knowledge and 16 items on knowledge and cultural perceptions |
— | — | — | — | — | Short‐term (immediately post‐ intervention) |
| Gwede 2019 | — | Awareness of colorectal cancer and screening tests; 6 items (0 to 11) | — | — | — | — | Medium‐term (at 3‐month follow‐up) |
| Payán 2020 | — | Breast cancer risk knowledge; questionnaire, true/false (0 to 16) | — | — | — | — | Short‐term (immediately post‐intervention) and medium‐term (at 3‐month follow‐up) |
| Poureslami 2016a | — | Asthma‐related knowledge, questionnaire, 5‐point Likert scale, range of scores not reported | Inhaler use technique; direct observation (2 observers); participants demonstrated correct use and had to describe each step (0 to 9 standard checklist), higher score is better | Understanding of and adherence to physician's instructions: 5 items, asking participants to explain the instruction in their own words, 0 = incorrect, 1 = correct, higher score is better | — | — | Short‐term (immediately post‐ intervention) and medium‐term (at 3‐month follow‐up)3 |
| Poureslami 2016b | — | — | Inhaler use technique; direct observation (2 observers); participants demonstrated correct use and had to describe each step; 0 to 10, validated checklist, higher score is better | Understanding of pulmonary rehabilitation; text passage based on Canadian Thoracic Society COPD assessment guidelines, developed by the research team and related questions answered by participants. (correct = 1 or incorrect = 0), higher score is better | — | — | Short‐term (at 4 weeks (immediately post‐intervention and medium‐term (at 3‐ month follow‐up) |
| Sudore 2018 | — | — | — | — | — | Engagement in ACP actions; subscale of ACP Engagement survey, 0 to 25, higher score is better |
Long‐term (15 months after enrolment) |
| Unger 2013 | — | Depression knowledge; depression knowledge scale (0 to 17) | — | — | — | Willingness to seek help for depression; modified intention to seek depression care scale (4 to 8) | Short‐term (immediately post‐intervention) |
| Valdez 2015 | — | HPV and cervical cancer knowledge; 12 items on HPV knowledge and awareness, and additional questions related to the intervention content (0 to 12) | — | — | Decisional Conflict Scale, subscales informed decision, values clarity, support, 0 to 100 (each scale), lower score is better | Made informed decision; 3 criteria, composite score: (1) making a vaccination choice, (2) affirming that the decision was an informed choice and (3) having a knowledge score of at least 7 out of 12 knowledge items, higher score is better | Short‐term (at 1‐ month follow‐up) |
| Valdez 2018 | — | Knowledge on cervical cancer, human papillomavirus (HPV) and Pap testing: adapted scale from Pathfinder intervention study, 5 items, yes/no | — | — | — | — | Medium‐term (at 6‐month follow‐up) |
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally and literacy adapted audio‐/visual education without personal feedback | |||||||
| Ochoa 2020 | — | Knowledge regarding Pap test, HPV and cervical cancer; 8 open‐ended questions summed to knowledge score | — | — | — | Cervical cancer screening intention; 2 questions: (1) "When did you have your most recent Pap test" and (2) "Since you saw the film, did you make an appointment for a Pap test?" (yes/no, do not know) | Short‐term and medium‐term (knowledge at 2‐weeks post‐test and at 6‐month follow‐up), behavioural intentions at 22 weeks post‐test and at 6‐month follow‐up |
| 8 Culturally and literacy adapted medical instruction vs no health literacy intervention | |||||||
| Bailey 2012 | — | — | — | Comprehension of medical instruction; demonstration by means of correct dosage in dosing tray (demonstrate correct dose, frequency and spacing; 0 to 5; 0 = incorrect, 1 = correct), numbers of instructions understood, RR, 95% CI | — | — | Short‐term (immediately post‐intervention) |
| Kheir 2014 | — | — | — | Comprehension of medical instructions through interpretation of label contents; level of comprehension (1 to 3; 1 no comprehension to 3 full comprehension) | — | — | Short‐term (immediately post‐intervention) |
| Mohan 2014 | — | — | — | Medication understanding: Medication Understanding Questionnaire (MUQ), 0 to 100 (0 to 3 for each medication), higher score is better | — | — | Short‐term (1 week post‐intervention) |
*Outcomes to be considered in this review; see Characteristics of included studies for an overview of all outcomes assessed within the included studies.
**No study reported a measure for assessing either motivation or the step of accessing health information.
***Results for the outcome category 'health‐related knowledge' were reported separately in the results section as well as in the summary of findings tables.
1Not enough information to categorise into short‐, medium‐ or long‐term assessment.
2Participants were not all assessed at one time point (immediately post intervention up to three months post‐intervention). We report the results as short‐term outcomes.
3Authors only report results of a 3‐month follow‐up assessment.
ACP: advance care planning; ADK: Audit of Diabetes Knowledge; ADKnowl: Audit of Diabetes Knowledge Scale; AHL‐C: Assessment of Health lIteracy in Cancer; CI: confidence interval; COPD: chronic obstructive pulmonary disease; DHLS: Diabetes Health Literacy Survey; DKT: Diabetes Knowledge Test; D‐Lit/DLQ: Depression Literacy Questionnaire; DM‐REALM: Diabetes‐specific Rapid Estimate of Adult Literacy in Medicine; HBP: high blood pressure; HPV: human papillomavirus; MUQ: Medication Understanding Questionnaire; NVS: Newest Vital Sign; REALM: Rapid Estimate of Adult Literacy in Medicine; RR: risk ratio; TOFHLA: Test of Functional Health Literacy in Adults; TS‐REALD: Two Stage Rapid Estimate of Adult Literacy in Dentistry
18. Outcomes considered in this review ‐ additional outcomes related to health literacy.
| Study ID | Quality of* life | Health outcomes | Health behaviour | Self‐efficacy | Health service use | Adverse events | Timing of outcome assessment considered |
| 1 Culturally and literacy adapted self‐management programme vs no health literacy intervention | |||||||
| Bloom 2014 | — | — | Mammography: self‐report, no further details reported | — | — | — | After 6 months1 |
| Koniak‐Griffin 2015 | — | — | Physical activity*: accelerometer data (worn during walking hours for 7 consecutive days) | — | — | — | Short‐term (immediately post‐intervention), medium‐term (3 months post‐intervention) |
| Rosal 2011 | — | — | Blood glucose self‐monitoring*: unannounced phone calls (3 recalls per time point (oral assessment, 3 questions on blood glucose self‐monitoring, higher score is better | Self‐efficacy in diabetes management; self‐efficacy for dietary and physical activity change (Lifestyle Self‐Efficacy Scale for Latinos with Diabetes (LSESLD); 17 items) | — | — | Short‐term (immediately post‐intervention) |
| van Servellen 2005 | — | Self‐reported general health status, 1 item on perceived level of general health in past week* | HIV medication adherence ACTG Adherence behaviours Baseline Questionnaire (self‐report), proportion of those with > 95% adherence within last 4 days |
Medication adherence self‐efficacy Certainty to master medication regimen; 1 item of ACTG Adherence Baseline Questionnaire (3‐point Likert scale), higher score is better | — | — | Short‐term (immediately post‐intervention) |
| 2 Culturally and literacy adapted self‐management programme vs written information on the same topic | |||||||
| Han 2017 | — | — | Adherence to age‐appropriate screening (medical record review) | — | — | — | Short‐term (immediately post‐intervention) |
| Kaur 2019 | — | — | Health behaviour (oral hygiene self‐care behaviour) Questionnaire on oral self‐care knowledge and oral self‐care behaviour, no total score provided |
— | — | — | Short‐term (immediately post‐intervention) |
| Kim 2009 | Quality of life (diabetes‐related QoL) Diabetes Quality of Life Measure (DQOL, 14 items) (0 to 75) | Depression; KDSKA (0 to 21), lower score is better | Adherence to diabetes regimen Diabetes Self‐Care Activities scale, no range reported |
Diabetes self‐efficacy; adapted Stanford Chronic Disease Self‐Efficacy Scale, 8 items, 10‐point Likert scale, 0 to 80, higher score is better | — | — | Short‐term (immediately post‐intervention) |
| Kim 2014 | — | Depression; PHQ‐9 (0 to 27), lower score is better | Self‐reported medication adherence HB‐MAS (8 items, 4‐point Likert scale, 1 (none of the time) to 4 (all of the time), 8 to 32, higher score is better |
Self‐efficacy in managing high blood pressure; 8‐item questionnaire adapted from the HBP belief scale (4‐point Likert scale (1 to 4)) | — | — | Short‐term and medium‐term (immediately post‐intervention and at 6‐month follow‐up) |
| Kim 2020 | Quality of life (diabetes‐related QoL) Diabetes Quality of Life Measure (DQOL, 14 items) (0 to 75) | Depression; Korean Patient Health Questionnaire 9 (PHQ‐9K) (0 to 27), lower score is better | — | Diabetes self‐efficacy; adapted Stanford Chronic Disease Self‐Efficacy Scale, 8 items, 10‐point Likert scale, 0 to 80, higher score is better | — | — | Short‐term (immediately post‐intervention) |
| Rosal 2005 | Diabetes‐related quality of life, adapted ADDQoL, score range not reported | Depression; Center for Epidemiological Studies‐Depression Scale (CES‐D), 0 to 60, lower score is better | Blood‐glucose self‐monitoring*: 24‐hour recall of self‐monitoring blood glucose by asking individuals whether they had checked their blood sugar level in the previous 24 hours, at what time, and what value was obtained, lower score is better | IMDSES, 26‐item, 4‐point Likert‐scale ranging from 1 ("low confidence") to 4 ("high confidence"), 26 to 104, higher score is better | — | — | Short‐term (2 weeks post‐intervention) and medium‐term (4.5 months after programme completion) |
| 2 Culturally adapted health literacy skills building course vs no/unrelated health literacy intervention | |||||||
| Otilingam 2015 | — | — | Fat‐Related Diet Habits Questionnaire, 12 items, mean on 4‐point scale; (1 to 4), higher score is better |
— | — | — | Short‐term (at 1 month follow‐up) |
| Soto Mas 2018 | — | — | Cardiovascular health behaviour; CSC (34 to 136) | — | — | — | Short‐term (immediately post‐intervention) |
| Wong 2020 | — | — | — | — | — | — | Short‐term (immediately post‐intervention and at 2‐month follow‐up) |
| Elder 1998 | — | — | — | Self‐efficacy to change one's diet; questionnaire: 5 items on self‐efficacy: score 1 (low) to 3 (high) | — | — | Medium‐term (at 6‐month follow‐up) |
| Taylor 2011 | — | — | Hepatitis B testing (self‐report and verification through medical records) |
— | — | — | Medium‐term (6‐month follow‐up)2 |
| Tong 2017 | — | — | Up‐to‐date colorectal cancer screening* including faecal occult blood test (FOBT), sigmoidoscopy or colonoscopy (S/C)) (self‐report of test receipt and when the test was obtained) | — | — | — | Medium‐term (at 3‐month follow‐up) |
| 4 Culturally and literacy adapted telephone education vs unrelated culturally and literacy adapted telephone education | |||||||
| Lepore 2012 | — | — | PSA testing; medical claims records (0 = no, 1 = yes) | — | — | State Anxiety; 7‐item sub‐scale of the HADS (0 to 21), lower score is better | Long‐term (8 months after randomisation (anxiety), 2 years after randomisation (PSA testing)) |
| 5 Culturally and literacy adapted audio‐/visual education without personal feedback vs no health literacy intervention | |||||||
| DeCamp 2020 | — | Parent depression; PHQ‐8, 8 items (0 to 24), lower score is better | Up‐to‐date immunisation assessed via EMR | — | ER visits assessed via EMR* | — | Short‐term (immediately up to 3 months post‐intervention)3 |
| Hernandez 2013 | — | — | — | Self‐efficacy to identify need for treatment; Self‐Efficacy to identify the Need for Treatment Scale (0 to 15) | — | — | Short‐term (immediately post‐ intervention) |
| Kiropoulos 2011 | — | Depression; BDI‐II (0 to 63), lower score is better | — | — | — | — | Short‐term (1 week post‐intervention) |
| Thompson 2012 | — | — | — | — | — | — | NA |
| 6 Culturally and literacy adapted audio‐/visual education without personal feedback vs written information on the same topic | |||||||
| Calderón 2014 | — | — | — | — | — | — | NA |
| Gwede 2019 | — | — | Screening for colorectal cancer; return of a completed FIT kit within 90 days of intervention | Self‐efficacy for screening using FIT | — | — | Medium‐term (at 3‐month follow‐up) |
| Payán 2020 | — | — | — | Self‐efficacy in accessing breast cancer‐related advice or information: one item adapted from a cancer confidence question in the 2012 Health Information National Trends Survey; the item asked "Overall, how confident are you that you could get advice or information about breast cancer if you needed it?”, 5‐point scale ranging from "completely confident" to "not confident at all" | — | — | Short‐term (immediately post‐intervention and 3‐month follow‐up) |
| Poureslami 2016a | — | — | — | — | — | — | Medium‐term (at 3‐month follow‐up)4 |
| Poureslami 2016b | — | — | — | COPD self‐efficacy; validated COPD Self‐Efficacy Scale (short version, 5 items), 5‐point Likert scale | — | — | Medium‐term (at 3 month follow‐up) |
| Sudore 2018 | — | Depression*; PHQ‐8, (0 to 24) referred to as adverse events, lower score is better | Documentation of new Advance Care Planning (legal forms and documented discussions with clinicians and/or surrogates) |
— | — | Anxiety (GAD‐7 questionnaire (0 to 21), referred to as adverse events, lower score is better | Long‐term (at 12‐month follow‐up) |
| Unger 2013 | — | — | — | Self‐efficacy to identify depression, 2 items adapted from Lorig et al; 10‐point scale ranging from 1 = "not at all confident" to 10 = "very confident" (mean (SD); range not reported) |
— | — | Short‐term (immediately post‐intervention) |
| Valdez 2015 | — | — | — | — | — | — | NA |
| Valdez 2018 | — | — | Screening behaviour (Pap testing): adapted scale from the Pathfinder intervention study, yes/no (e.g. "Obtained a pap test or made appointment"); further information not reported | Self‐efficacy (Pap testing): adapted scale from the Pathfinder intervention study, binary items (yes/no) (e.g. "Can get a pap smear if needed"); further information not reported | — | — | Medium‐term (at 6‐month follow‐up) |
| 7 Culturally and literacy adapted audio‐/visual education without personal feedback vs another culturally and literacy adapted audio‐/visual education without personal feedback | |||||||
| Ochoa 2020 | — | — | Pap testing behaviour, self‐report, 1 question: "Since you saw the film, have you had a Pap test?" with response options "yes", "no" and "do not know" | — | — | — | Short‐term (at 2 week post‐test) and mid‐term (at 6‐month follow‐up) |
| 8 Culturally and literacy adapted medical instruction vs no health literacy intervention | |||||||
| Bailey 2012 | — | — | — | — | — | — | NA |
| Kheir 2014 | — | — | — | — | — | — | NA |
| Mohan 2014 | — | — | Medication adherence: 8 item sub‐scale of Spanish translation of ARMS, patients' self‐reported adherence under various circumstances (sub‐scale to medication refills), 8 (most adherent to 32 (least adherent), lower score is better |
— | — | — | Short‐term (1 week) |
*Prioritised outcome to be considered in this review; see Characteristics of included studies for a full description of outcomes assessed in the respective study.
1Not enough information to categorise into short‐, medium‐ or long‐term assessment.
2Post‐test assessment only.
3Participants were not all assessed at one time point (immediately post intervention up to three month post intervention). We report the results as short‐term outcomes.
4Authors report that a short telephone‐based outcome assessment was conducted at 6‐month follow‐up, assessing subjective medication adherence, but results are not reported.
ACTG: Adult AIDS Clinical Trials Group; ARMS: Adherence to Refills and Medications Scale; BDI‐II: Beck Depression Inventory‐II; COPD: chronic obstructive pulmonary disease; CSC: Cardiovascular Health Questionnaire; DQOL: Diabetes Quality of Life Measure; EMR: electronic medical record; ER: emergency room; FIT: faecal immunochemical test; FOBT: faecal occult blood test ; GAD‐7: Generalised Anxiety Disorder‐7; HADS: Hospital Anxiety and Depression Scale; HB‐MAS: Hill‐Bone Medication Adherence Scale; HBP: high blood pressure; IMDSES: Insulin Management Self‐Efficacy Scale; KDSKA: Kim Depression Scale for Korean Americans; LSESLD: Lifestyle Self‐Efficacy Scale for Latinos with Diabetes; NA: not applicable; PHQ: Patient Health Questionnaire; PSA: prostate‐specific antigen; QoL: quality of life; SD: standard deviation
The following primary outcomes have been included in this review:
Health literacy: a) generic health literacy (including functional health literacy, print literacy, health numeracy); b) disease‐specific health literacy (including cancer screening health literacy, depression literacy, diabetes health literacy, high blood pressure health literacy, HIV health literacy, oral health literacy).
Adverse events: associated with the intervention: anxiety.
The included secondary outcomes were as follows:
Quality of life: diabetes‐related quality of life.
Health outcome: a) subjective health status (self‐reported general health in past week); b) depression.
Health behaviour: a) blood glucose self‐monitoring; b) cardiovascular health behaviour; c) cancer screening behaviour (including breast cancer screening adherence, cervical cancer screening behaviour, colorectal cancer screening uptake, prostate cancer screening, up‐to‐date colorectal cancer screening); d) diabetes self‐care activities; e) documentation of new advance care planning; f) hepatitis B testing; g) HIV medication adherence; h) oral hygiene self‐care behaviour; i) fat‐related diet habits; j) medication adherence (including adherence to asthma medication, medication adherence (non‐specific), non‐adherence to blood pressure medication); k) physical activity; l) (child's) up‐to‐date immunisation.
Health‐related knowledge: a) asthma knowledge; b) cardiovascular disease (heart) knowledge; c) child health knowledge; d) cervical/breast cancer knowledge; e) colorectal cancer knowledge (including awareness of colorectal cancer and screening test); f) COPD knowledge; g) depression knowledge; h) diabetes knowledge; i) hepatitis B knowledge; j) high blood pressure knowledge; k) HIV knowledge; l) nutrition knowledge (including child nutrition and feeding knowledge); m) oral health knowledge; n) cognitive behaviour therapy knowledge; o) prostate cancer screening knowledge.
Health service use: use of emergency room services.
Self‐efficacy (a) self‐efficacy in managing one's disease (including diabetes and insulin management self‐efficacy, self‐efficacy in managing high blood pressure, medication adherence self‐efficacy, COPD self‐efficacy); b) cancer screening self‐efficacy (including self‐efficacy for colorectal cancer screening using faecal immunochemical test (FIT), self‐efficacy for accessing breast cancer‐related advice or information, self‐efficacy for cervical cancer screening using pap testing); c) self‐confidence in supporting individuals with depression; d) self‐efficacy for identifying depression; e) self‐efficacy to identify need for treatment (related to depression); f) self‐efficacy to change one's diet).
Timing of outcome assessment
Participants were assessed at different time points and over varying follow‐up periods. Many studies assessed participants at multiple time points. Thereby, follow‐up periods with minimal provider contact (e.g. monthly telephone calls) were treated as being part of the intervention programme, since these contacts might have had an effect on our outcomes of interest (e.g. health behaviour). The majority of participants were assessed at short‐term follow‐up (up to six weeks from the start of the intervention and immediately after the intervention programme was completed) (Bailey 2012; Calderón 2014; Han 2017; Hernandez 2013; Kaur 2019; Kheir 2014; Kim 2009; Kim 2020; Kiropoulos 2011; Koniak‐Griffin 2015; Mohan 2014; Ochoa 2020; Otilingam 2015; Payán 2020; Poureslami 2016a; Poureslami 2016b; Rosal 2011; Soto Mas 2018; Thompson 2012; Unger 2013; Valdez 2015; Wong 2020). In 12 studies, participants were assessed at medium‐term follow‐up (up to and including six months after the intervention programme was completed) (DeCamp 2020; Elder 1998; Gwede 2019; Kim 2014; Koniak‐Griffin 2015; Poureslami 2016a; Poureslami 2016b; Rosal 2005; Taylor 2011; Tong 2017; Valdez 2018; van Servellen 2005). Two studies assessed participants longer than six months and up to two years after the intervention programme was completed (Lepore 2012; Sudore 2018).
In one study, the authors stated that participants were assessed six months post‐intervention. However, the information about the design of the intervention and, thus, the total programme length including the supervised follow‐up phase was insufficient to permit judgement about whether the outcomes were assessed short‐term or medium‐term (Bloom 2014).
Health literacy
Twelve studies explicitly stated to have measured either disease‐specific or generic health literacy for assessing the intervention effectiveness. All the included studies assessed outcomes related to at least one of the four health information processing steps (access, understand, appraise and apply) or the prerequisites of health literacy (knowledge, motivation and competencies).
Eight studies reported outcomes on disease‐specific health literacy. Three of these assessed primarily disease‐specific knowledge and attitudes towards a certain disease or disease management. Two of these studies assessed depression literacy using either the original English version (Wong 2020) or an adapted and translated version of the validated Depression‐Literacy questionnaire (D‐Lit) by Griffiths 2004 (Kiropoulos 2011). One study assessed diabetes literacy using the Diabetes Health Literacy Survey (DHLS) (Calderón 2014). The questionnaire was developed and validated in the study and measured diabetes‐related knowledge, knowledge application and cultural perceptions about diabetes management. Five studies made use of disease‐specific health literacy assessment tools that were adapted from established generic measures for assessing functional health literacy, such as the Rapid Estimate of Adult Literacy in Medicine (REALM) (Davis 1991) or the Test of Functional Health Literacy in Adults (TOFHLA) (Parker 1995). One study reported a measure for high blood pressure health literacy using the High Blood Pressure‐Health Literacy Scale (HBP‐HLS) (Kim 2014). The scale was developed and validated by the study authors (Kim 2012). One study measured cancer screening literacy (Han 2017) using the Assessment of Health Literacy in Cancer Screening (AHL‐C), also developed and validated by the study authors (Han 2014). One study reported a measure of HIV health literacy (van Servellen 2005) using an adapted version of the REALM developed by the study authors to assess recognition and understanding of HIV terms and, again, another study reported a measure of oral health literacy (Kaur 2019) using the validated two Stage Rapid Estimate of Adult Literacy in Dentistry (TS‐REALD) (Stucky 2011). One study administered the diabetes‐specific Rapid Estimate of Adult Literacy in Medicine (DM‐REALM), developed and previously validated by the study authors (Kim 2020), referring to the outcome as "health literacy knowledge" (Kim 2020, p. 212). In addition, Kim 2020 administered three other established performance‐based assessment tools for print literacy or health numeracy: the original REALM (Davis 1991), the numeracy subscale of the TOFHLA (Parker 1995) and the health numeracy test newest vital sign (NVS) (Weiss 2005). The NVS was also used by one other study (Otilingam 2015). One study administered the English version of the TOFHLA to assess functional health literacy (Soto Mas 2018). One study reported having assessed health literacy, but did not report the results (Poureslami 2016a).
All the assessment tools applied are performance‐based measures that assess components of health literacy, such as disease‐specific knowledge or functional health literacy, including subscales of print literacy (recognition of medical terms), functional literacy (understanding health‐related phrases and terminology) or numeracy (performing minor mathematical tasks).
Prerequisites of health literacy
Knowledge
See outcome category 'health‐related knowledge'.
Motivation
Two studies measured outcomes related to motivation. However, none of the results were included in our analysis, because the applied scales also addressed theoretical constructs other than motivation. Therefore, the results could not be subordinated to the construct of motivation. One study assessed "Patient activation", which refers to the knowledge, skills and confidence the individuals need to manage their health and health care (DeCamp 2020). The measure captures aspects of motivation and engagement with health and self‐management behaviour. Another study reported a measure that included motivation as a subscale of a broader behaviour change process scale including self‐perceived knowledge, self‐efficacy and readiness for behaviour change related to advance care planning (Sudore 2018).
Competencies (skills acquisition)
Two studies measured skills acquisition, such as correct use of metered dose inhaler by acting out the right steps of inhaler use measured through direct observation. Both studies used validated checklists to tick off the correct steps (Poureslami 2016a; Poureslami 2016b).
Steps of health information processing
Accessing health information
In the guiding health literacy framework (Figure 1), the first step of health information processing is access to health information, which refers to "the ability to seek, find and obtain health information" (Sørensen 2012).
None of the studies reported outcomes that were directly related to accessing health information.
Understanding health information
Understanding health information refers to "the ability to comprehend the health information that is accessed" (Sørensen 2012).
Five studies measured outcomes related to the understanding of health information. One study used the Medication Understanding Questionnaire (MUQ) to measure understanding of adapted medical instructions (Mohan 2014). One study assessed the level of comprehension of medical instructions by asking for the participant's interpretation of the medication label's content (Kheir 2014). Two studies measured outcomes related to the understanding of instructions for inhaler use. Of these, one study reported an outcome measure related to understanding of and adherence to physician's instructions for inhaler use for asthma by asking the participants to explain the instructions in their own words (Poureslami 2016a). The other study reported an outcome measure for the understanding of pulmonary rehabilitation by using a text passage and questions related to COPD, which was developed by the study authors (Poureslami 2016b). One study measured the understanding of medical instructions by means of a dosing tray, which was filled by the participants according to the respective instruction (Bailey 2012).
Appraising health information
Appraising health information is defined as "the ability to interpret, filter, judge and evaluate the health information that has been accessed" (Sørensen 2012). It was assessed in three studies, one reporting a measure on the decisional balance (i.e. the weighing of pros and cons) for the use of cancer screening measures after receiving an educational intervention related to breast and cervical cancer screening (Han 2017). The other two studies measured decisional conflict using the validated decisional conflict scale (O'Connor 1995), of which we report the results of the three subscales informed decision, values clarity and support. We do not report the results for the subscales uncertainty and effective decision as these subscales presume a full decision that reflects the processing step of applying health information rather than the appraisal of health information. One study measured decisional conflict related to human papillomavirus (HPV) vaccination (Valdez 2015) and the other study measured decisional conflict in the realm of prostate cancer screening (Lepore 2012).
Applying health information
Applying health information is defined as the "ability to communicate and use the information" (i.e. patient‐provider interaction) and to make a decision that has a positive impact on one's health or the health of others (i.e. behaviour intent) (Sørensen 2012). Outcome categories such as 'health behaviour' or 'health service use' may not be directly subordinated to this step of health information processing, but can be seen as a consequence of the decisions made based on certain information and therefore are closely related to the processing step of applying health information. Two studies measured participants' behavioural intentions regarding the use of preventive measures, such as for cervical cancer (Ochoa 2020) or prostate cancer (Lepore 2012). One study measured participants' informed decision regarding the vaccination against HPV using the composite variable described above (see appraising health information) (Valdez 2015). Two studies reported an outcome measure that assessed participants' intention to change their diet (Elder 1998) or parents' planned behaviour changes with regard to the nutrition of their children (Thompson 2012). Two studies assessed the intention to seek professional help for a mental health problem (Hernandez 2013; Unger 2013), but Unger 2013 did not provide enough information to calculate a point estimate and a confidence interval.
Secondary outcomes related to health literacy
Quality of life
Two studies reported outcome measures on diabetes‐related quality of life using the Diabetes Quality of Life Measure (DQOL) (Kim 2009; Kim 2020). One study also measured diabetes‐related quality of life using an adapted version of the Audit of Diabetes Dependent Quality of Life (ADDQoL) (Rosal 2005).
Health‐related knowledge
In total, 28 studies assessed health‐related knowledge, including a variety of content‐specific knowledge scales that tested the knowledge derived from the educational content conveyed in the study. Twenty‐two studies measured disease‐specific knowledge (DeCamp 2020; Gwede 2019; Han 2017; Hernandez 2013; Kim 2009; Kim 2014; Kim 2020; Koniak‐Griffin 2015; Lepore 2012; Ochoa 2020; Otilingam 2015; Payán 2020; Poureslami 2016a; Poureslami 2016b; Rosal 2005; Rosal 2011; Taylor 2011; Tong 2017; Unger 2013; Valdez 2015; Valdez 2018; van Servellen 2005). Of these, one study measured parents' knowledge about infant diseases (DeCamp 2020). Seven studies assessed knowledge not directly related to a certain disease, but to another health‐relevant topic. One of them assessed knowledge on cognitive behavioural therapy for depression (Wong 2020). Three studies made use of a nutrition knowledge measure (Elder 1998; Otilingam 2015; Thompson 2012), and, again, another study measured oral self‐care knowledge (Kaur 2019). One study reported to have measured COPD‐related knowledge, but did not report the results (Poureslami 2016b). One study reported data for the intervention group only (Koniak‐Griffin 2015). One study measured knowledge, probably related to breast health or breast cancer as the intervention was related to these topics, but detailed information was not provided in the identified trial reports (Bloom 2014).
Nine studies explicitly referred to knowledge as a considerable component of the health literacy concept (Calderón 2014; Hernandez 2013; Kaur 2019; Kim 2014; Kim 2020; Kiropoulos 2011; Soto Mas 2018; Unger 2013; van Servellen 2005).
Health behaviour
Seventeen studies assessed outcomes that are related to the use of health information. Eight studies measured adherence to medication or therapeutic regimen through participants' self‐report (Kim 2009; Kim 2014; Kim 2020; Otilingam 2015; Mohan 2014; Rosal 2005; Rosal 2011; van Servellen 2005). Of these, two studies reported to have used the Summary of Diabetes Self‐Care Activities Scale (SDSCA) to assess adherence to a diabetes regimen (Kim 2009; Kim 2020), but one did not report the results (Kim 2020). Three studies assessed outcomes related to a healthy lifestyle, such as physical activity, which was measured through the use of objective accelerometer data (Koniak‐Griffin 2015). Others assessed self‐reported cardiovascular health behaviour (Soto Mas 2018) or self‐reported oral hygiene behaviour (Kaur 2019). Four studies measured the use of preventive measures, one assessing the infant's up‐to‐date immunisation via electronic medical records (DeCamp 2020). Three other studies assessed the uptake of screening measures, one using self‐report of colorectal cancer screening (Tong 2017) and one measuring self‐report of breast cancer screening by mammography (Bloom 2014). The third study assessed return of a completed take home faecal immunochemical test kit (FIT kit) within 90 days using pre‐stamped and self‐addressed mailers for objective verification of screening completion (Gwede 2019). One study used medical records to verify cervical and breast cancer screening (Han 2017) and one study used medical records to verify self‐reported hepatitis B screening (Taylor 2011). One study measured the documentation of new advance care planning forms by using a composite variable of legal forms and/or documented discussions about advance care planning with clinicians and/or surrogates (Sudore 2018).
Health outcomes
A total of eight studies assessed health outcomes. One study measured self‐rated general health within the last week (van Servellen 2005). Seven studies reported outcome measures for depression using four different measures (Hernandez 2013; Kim 2009; Kim 2014; Kim 2020; Kiropoulos 2011; Rosal 2005; Sudore 2018). Four used the Patient Health Questionnaire (PHQ) either with eight items (PHQ‐8) (DeCamp 2020; Sudore 2018) or with nine items (PHQ‐9), respectively (Kim 2014; Kim 2020). One study used the Depression Scale for Korean Americans (KDSKA) (Kim 2009). Another study used the Beck Depression Inventory II (BDI‐II) (Kiropoulos 2011), and the other two studies made use of the Center for Epidemiological Studies of Depression Scale (CES‐D Scale) (Hernandez 2013; Rosal 2005). Sudore 2018 referred to depression as an adverse event related to the intervention.
Self‐efficacy
Fourteen studies reported a variety of outcome measures related to self‐efficacy. Seven studies measured self‐efficacy in managing one's own disease or medication (Kim 2009; Kim 2014; Kim 2020; Rosal 2005; Rosal 2011; Poureslami 2016b; van Servellen 2005). Two studies used a measure to assess self‐efficacy either for colorectal cancer screening using a faecal immunochemical test (Gwede 2019) or for cervical cancer using Pap testing (Valdez 2018). One study assessed self‐efficacy in accessing breast cancer‐related advice or information (Payán 2020). Two studies reported outcome measures on self‐efficacy to identify depression or the need for treatment (Hernandez 2013; Unger 2013). One study assessed participants' self‐confidence in supporting individuals with depression (Wong 2020) and another study measured self‐efficacy in changing one's diet (Elder 1998).
Health service use
One study assessed the use of health services with the use of medical records to measure emergency room visits (DeCamp 2020).
Adverse events
Two studies reported adverse events related to the interventions. Both studies reported outcome measures for anxiety, whereas one study used the seven‐item subscale of the Hospital Anxiety and Depression Scale (HADS) (Lepore 2012) and the other study made use of the Generalised Anxiety Disorder‐7 questionnaire (GAD‐7), referring to anxiety as an adverse event related to the intervention (Sudore 2018).
Gender
Ten studies included women only (Bloom 2014; DeCamp 2020; Han 2017; Hernandez 2013; Koniak‐Griffin 2015; Ochoa 2020; Otilingam 2015; Payán 2020; Valdez 2018; Wong 2020); two studies included only men (Kheir 2014; Lepore 2012). Furthermore, some studies, despite having a gender‐mixed study population, had a considerable disproportion of genders: five studies included predominantly women (80% or more, Calderón 2014; Rosal 2005; Soto Mas 2018; Thompson 2012; Valdez 2015), two of which even included more than 90% (Thompson 2012; Valdez 2015). Similarly, two studies included predominantly men (Poureslami 2016b; van Servellen 2005).
Studies awaiting assessment
Eight studies are awaiting assessment due to insufficient information to permit judgement for inclusion or exclusion. For four of these studies, we identified only abstracts indicating that health literacy or literacy in the context of health were addressed in the study design and at least a part of the participants were migrants, but we did not find a trial registry entry, a published protocol or a published final trial report to confirm the assumption (Erwin 2012; Essien 2017; Esquivel 2019; Glaser 2020). For the other studies we found either a study protocol, a trial report or a secondary analysis of the RCT, but the information was still insufficient to permit judgement about inclusion or exclusion (Gonzalez 2020; Joshi 2016; NCT04993326; Pekmezaris 2020).
For most studies, it was unclear if data (from ongoing studies) would be extractable separately for first‐generation migrants or if at least 80% of the participants were first‐generation migrants (Essien 2017; Gonzalez 2020; Joshi 2016; NCT04993326; Pekmezaris 2020). For one study, it was unclear which study design was used (Glaser 2020). We contacted authors of studies for which a final trial report was available asking whether the participants were first‐generation migrants, but did not receive a response. We also contacted authors to clarify the study design used, or to ask when a final trial report would probably be available and whether migrants will be included, but to date none of the final reports have actually been published.
Ongoing studies
We identified 11 ongoing studies from trial registries or during the electronic database searches (see Characteristics of ongoing studies and references to Ongoing studies).
Excluded studies
After screening titles and abstracts, we excluded 6605 references that did not match our inclusion criteria. In addition, we excluded a total of 209 studies (reported in 223 references) after full‐text screening for the following reasons: duplicate, study used the wrong study design (neither a RCT nor a quasi‐RCT or a cluster‐RCT), study included the wrong study population (paediatric population, no separately extractable data on first‐generation migrants, no migrants at all, or primary language/race/ethnicity/minority population only indicating that immigrants were not included), study evaluated the wrong intervention (improving health literacy was not an aim of the study, neither literacy nor health literacy was mentioned in the reference, or no outcome was related to health literacy). The details of relevant excluded trials are provided in the Characteristics of excluded studies.
Risk of bias in included studies
We assessed the risk of bias in the included studies according to the criteria defined at the protocol stage. Not applicable risk of bias domains are empty. Details of the risk of bias assessment for each of the included studies are shown in the risk of bias tables in the Characteristics of included studies, in Figure 3 and in Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eighteen studies described adequate sequence generation and allocation concealment, and we assessed them as being of low risk of selection bias (Calderón 2014; DeCamp 2020; Kheir 2014; Kim 2020; Kiropoulos 2011; Koniak‐Griffin 2015; Lepore 2012; Mohan 2014; Payán 2020; Sudore 2018; Taylor 2011; Thompson 2012; Tong 2017; Unger 2013; Valdez 2015; Valdez 2018; van Servellen 2005; Wong 2020). Eight trials reported adequate sequence generation, but the concealment of allocation was unclear (Bailey 2012; Hernandez 2013; Kaur 2019; Kim 2009; Poureslami 2016a; Poureslami 2016b; Rosal 2005; Rosal 2011). One study reported to have used sealed envelopes to inform participants about their random assignment, but did not provide information about how random assignment was generated (Otilingam 2015). We rated seven studies as being at unclear risk of bias for both random sequence generation and allocation concealment domains, as information was insufficient (Bloom 2014; Elder 1998; Gwede 2019; Han 2017; Kim 2014; Ochoa 2020; Soto Mas 2018).
Blinding
Performance bias
For most of the studies blinding of participants and personnel was not possible, despite best attempts to do so. However, we judged non‐blinded studies to be at high risk of performance bias only when the outcomes assessed were self‐reported or subject to interpretation, assuming that knowledge of participant's group assignment might have affected the results (e.g. for outcomes such as depression or self‐efficacy). In total, we rated 20 studies as being at high risk of bias for this domain (Bloom 2014; DeCamp 2020; Elder 1998; Han 2017; Hernandez 2013; Kaur 2019; Kim 2009; Kim 2014; Kim 2020; Kiropoulos 2011; Mohan 2014; Otilingam 2015; Payán 2020; Poureslami 2016b; Rosal 2005; Rosal 2011; Soto Mas 2018; Tong 2017; Unger 2013; van Servellen 2005). In total, we rated 10 studies as being at low risk of performance bias (Bailey 2012; Calderón 2014; Kheir 2014; Koniak‐Griffin 2015; Lepore 2012; Ochoa 2020; Poureslami 2016a; Sudore 2018; Taylor 2011; Wong 2020). We assessed two of these as being at low risk of performance bias, although some outcomes were subjectively measured (Ochoa 2020; Sudore 2018). One study compared audio‐/visual education without personal feedback via a narrative video to audio‐/visual education without personal feedback via a factual knowledge video. Thus, the intervention only differed in one aspect, so we assumed that this did not lead to substantial risk of bias (Ochoa 2020). In Sudore 2018, the intervention was delivered online and via telephone and the method for enhancing blinding of both the participants and the personnel was described in detail. For example, participants were told that they would review one of two guides on advance care planning but were blinded as to which guide was the active intervention and which was the active control (online programme and additional written advance directive versus written easy‐to‐read advance directive alone). We rated the other eight studies as being at low risk of performance bias as the outcomes considered in this review were objectively measured and not subject to interpretation or the participants were presumably not aware of the intervention received (Calderón 2014; Kheir 2014; Koniak‐Griffin 2015; Lepore 2012; Ochoa 2020; Poureslami 2016a; Taylor 2011; Wong 2020). Therefore, we assumed that even non‐blinding would not have affected the results. Four studies had an unclear risk of performance bias, as participants and personnel might have been blinded, but the information was insufficient to permit judgement. It remained unclear whether potential non‐blinding might have affected the results of subjectively measured outcomes (Gwede 2019; Thompson 2012; Valdez 2015; Valdez 2018).
Detection bias
In concordance with the ratings for performance bias, we distinguished between subjective and objective outcome measures to assess the risk of detection bias, as blinding of group allocation and blinding of outcome assessors might have affected the risk of bias in this domain differently. Almost all studies reported primarily or exclusively subjectively measured outcomes that were dependent on the participants' judgement. Most of these studies made use of self‐report questionnaires that were used repeatedly to assess the participants at different time points during the study period. We rated them as being at high risk of detection bias, when the participants were not, or presumably not, blinded to the intervention they received (Bloom 2014; DeCamp 2020; Elder 1998; Han 2017; Hernandez 2013; Kaur 2019; Kim 2009; Kim 2014; Kim 2020; Kiropoulos 2011; Mohan 2014; Otilingam 2015; Payán 2020; Poureslami 2016b; Rosal 2005; Rosal 2011; Soto Mas 2018; Tong 2017; Unger 2013; van Servellen 2005), and at unclear risk of bias when participants and personnel could have been blinded, but the information was insufficient to permit judgement of 'low risk' or 'high risk'(Gwede 2019; Thompson 2012; Valdez 2015; Valdez 2018). We rated three studies as being at low risk of bias for both subjective and objective outcomes, as the participants were presumably not fully aware of the intervention they received (Sudore 2018), or the interventions differed only very slightly. In one study, a narrative video about cervical cancer was compared to a non‐narrative video on the same topic (Ochoa 2020), and in the other study the participants received telephone education on different health topics (Lepore 2012).
All 34 studies used observer‐reported outcome measures. We rated all but one study, Bloom 2014, as being at low risk of bias, because the outcomes were measured by means of objective criteria without the involvement of the outcome assessors' judgement and/or outcome assessors were blinded.
We assessed Bloom 2014 as being at high risk for the domain 'subjective outcome measures' and at unclear risk of bias for the domain 'objective outcome measures' as participants and personnel were most likely not blinded due to the nature of the study, and health behaviour was measured via self‐report. We do not know if knowledge was subjectively or objectively measured in the study. In the case that knowledge was also subjectively measured, the results for this outcome might also be biased.
Incomplete outcome data
In all studies, participants were analysed according to their original group assignment.
Eight studies reported undertaking intention‐to‐treat analysis and provided details on the methods used, and we assessed them as being at low risk of bias (DeCamp 2020; Han 2017; Kaur 2019; Kheir 2014; Lepore 2012; Otilingam 2015; Sudore 2018; Wong 2020). We also assessed studies as being at low risk for attrition bias when outcome data were available for nearly all participants (Bailey 2012; Bloom 2014; Calderón 2014; Hernandez 2013; Kim 2009; Kiropoulos 2011; Lepore 2012; Mohan 2014; Poureslami 2016a; Poureslami 2016b; Rosal 2005; Rosal 2011; Soto Mas 2018; Thompson 2012), and studies had less than 15% differential loss of follow‐up between intervention and control group and reported the reasons for dropouts per study arm (DeCamp 2020; Gwede 2019; Kim 2014; Kim 2020; Koniak‐Griffin 2015; Payán 2020; Taylor 2011; Unger 2013; van Servellen 2005). We rated Ochoa 2020 as being at low risk of bias although the number of participants who dropped out was not reported separately per study arm, because the study compared two variants of the same intervention (narrative video versus knowledge video), indicating that neither of the interventions particularly led to the relatively high attrition rate of 47 out of 187 participants at six‐month follow‐up.
We rated three studies as being at unclear risk of bias in this domain. Of these, one study neither provided information on the numbers of participants that dropped out nor the reasons for attrition per study arm (Valdez 2018). One study reported considerable differences in the numbers of participants analysed between study groups. In total, 100 participants were not included in the analysis: 74 in the intervention group and 26 in the control group. It was unclear whether the participants did not complete pre‐ and/or post‐test assessment or if they were excluded for other reasons (Valdez 2015). Another study reported attrition rates and results of a statistical attrition analysis, but due to lack of reporting of the total number of participants randomised to each arm as well as those who dropped out per arm, we also rated the risk of attrition bias as being unclear (Elder 1998).
Selective reporting
Fourteen study protocols or registered trial records were available to assess the risk of selective reporting. For the remaining 22 studies, we made decisions regarding the risk of reporting bias based on whether the results for each outcome listed in the methods section were present in the results of each published report. For one study, we found an abstract only. Thus, the information was insufficient to permit a judgement of 'low risk' or 'high risk' (Bloom 2014). We also assessed two other studies as being at unclear risk of bias. In one study, the registered trial record indicated that two additional outcomes, namely 'health care utilisation' and 'problem‐solving and communication skills', should have been assessed additionally at six weeks, and month 6, 12, 18 and 24. The time points of outcome assessment reported in the primary cluster‐RCT ranged up to 18 months, which indicates that another publication might follow (Kim 2014). In one study, the results for communication quality, satisfaction with communication, satisfaction with decision‐making, care consistent with current goals, barriers to advance care planning (ACP) and attitudes about ACP were not reported. However, these measures were not pre‐specified at clinicaltrials.gov, but in one of the two published study protocols (see secondary reference of Sudore 2018). It is unclear whether these measures were used as process variables or whether it was intended to assess these as outcome variables and whether the results are yet to be published (Sudore 2018).
We rated five studies as being at high risk for this domain. One of them indicated having assessed participants' health literacy at different time points (Poureslami 2016a), but results were not reported. Another study reported having assessed participants' knowledge of COPD, but did not report the results (Poureslami 2016b). In one study, all prespecified outcomes reported at clinicaltrials.gov were reported in the published reports, but the results of the control group's knowledge assessment were missing (Koniak‐Griffin 2015). Another study indicated having assessed adherence to a diabetes regimen using the Diabetes Self‐care Activities Scale, but also did not report the results (Kim 2020). Lastly, one study pre‐specified colorectal cancer screening intention as an outcome measure in the trial registry, but the results are missing in the published trial report (Tong 2017).
Selective recruitment of cluster participants
We assessed potential bias resulting from selective recruitment of cluster participants in six cluster‐RCTs. We assessed one study as being at low risk of recruitment bias (Tong 2017). For the other five studies, we did not have enough information to permit judgement of 'low risk' or 'high risk' (Bloom 2014; Elder 1998; Han 2017; Kim 2014; Taylor 2011).
Other potential sources of bias
No study applied a perception‐based tool to measure health literacy. Therefore, in terms of health literacy assessment, social desirability was not a bias of concern in this review.
We rated most studies as being at low risk for other potential sources of bias (i.e. the domain was not applicable for these studies). We rated three cluster‐RCTs as being at low risk of bias as they either properly accounted for the cluster‐design in the analysis (Han 2017), or because we were able to re‐analyse the data using the appropriate unit of analysis (Kim 2014; Taylor 2011). We rated three studies as being at unclear risk of bias in this domain due to insufficient information to permit judgement of 'low risk' or 'high risk' (Bloom 2014; Elder 1998; Tong 2017). We rated Tong 2017 as being at unclear risk of bias because, although the authors reported having accounted for clustering in the analyses, we were not able to verify whether it also accounted for those outcomes that we considered in this review, and due to insufficient information we were not able to re‐analyse the data.
Effects of interventions
See: Table 1; Table 7; Table 12; Table 14; Table 15; Table 16; Table 18; Table 20; Table 21
Comparison 1: Culturally and literacy adapted self‐management programme versus no health literacy intervention
We included four studies in this comparison. Of these, three were RCTs with a total programme length of six (van Servellen 2005) to 12 months (Koniak‐Griffin 2015; Rosal 2011). For one cluster‐RCT, we had limited information regarding the intensity and total length of the programme (Bloom 2014). Table 1 presents the evidence on the effect of culturally and literacy adapted self‐management programmes, when compared to usual care or to no health literacy intervention. In addition, see Data and analyses for pooled data on this comparison and Table 2, Table 19, Table 4, Table 5 and Table 6 for data we did not pool.
Health literacy
One study with 69 participants assessed functional HIV health literacy and reported the results for understanding HIV terms and recognition of HIV terms separately (van Servellen 2005). Self‐management programmes compared to no health literacy intervention may improve understanding of HIV terms (mean difference (MD) 4.25, 95% confidence interval (CI) 1.32 to 7.18; low‐certainty evidence; Analysis 1.1) and recognition of HIV terms (MD 3.32, 95% CI 1.28 to 5.36; low‐certainty evidence; Analysis 1.2) immediately post‐intervention.
1.1. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 1: HIV health literacy: understanding HIV terms (short‐term: immediately post‐intervention)
1.2. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 2: HIV health literacy: recognition of HIV terms (short‐term: immediately post‐intervention)
Quality of life
The effect of self‐management programmes on quality of life is unknown when compared to no health literacy intervention, as there was no direct evidence.
Health‐related knowledge
All four studies in this comparison assessed the effects of self‐management programmes on knowledge immediately after the intervention programme was completed. The studies' knowledge tests were based on the interventions' content (i.e. diabetes mellitus, HIV, breast cancer or heart health). Due to differences in the scales used (Rosal 2011; van Servellen 2005), or missing information to calculate a mean difference and a measure of dispersion for each study group (Bloom 2014; Koniak‐Griffin 2015), we narratively synthesised the results. We transformed the proportion of accurate responses to a percentage scale, ranging from 0% (no correct responses) to 100% (fully correct responses), whenever possible. Results for each outcome at each time point are presented in Table 3. The following results pertain to data that could not be pooled in a meta‐analysis.
The narrative synthesis of two studies indicated that self‐management programmes may make little or no difference to health‐related knowledge immediately post‐intervention, when compared to no health literacy intervention (low‐certainty evidence) (Rosal 2011; van Servellen 2005). One randomised controlled trial (RCT) with 252 participants reported that the mean diabetes knowledge score was slightly higher in the intervention group (MD 5.6; range 2.2 to 9.0, details are shown in Table 3) (Rosal 2011). The mean knowledge score in the control group was 68. The other RCT with 69 participants reported that the mean HIV global disease/treatment knowledge was slightly lower in the intervention group (MD ‐1.18%, 95% CI ‐9.23 to 6.87; Analysis 1.3), but the CI encompassed values that indicate both an improvement and a reduction in knowledge (van Servellen 2005). The same study, however, also reported that the mean knowledge of the risk of getting sicker when stopping taking one's HIV medication was slightly improved in the intervention group (MD 0.33, 95% CI ‐0.01 to 0.67; Analysis 1.4). However, the CI also encompassed values indicating a null effect.
1.3. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 3: Health‐related knowledge: HIV global disease/treatment knowledge, 0 to 100 (short‐term: immediately post‐intervention)
One cluster‐RCT was missing information about the number of participants randomised to each study group, and the intensity and length of the intervention programme. For example, we did not know if participants were assessed in the short term or medium term, as we also did not know for how long and at which intensity they received individual counselling. In addition, data were not reported in a way in which they could be extracted for meta‐analysis (Bloom 2014). Briefly, Bloom 2014 reported that the intervention increased knowledge (MD 0.5, P < 0.0001) six months "post‐test".
One other RCT with 194 participants was missing data for the control group but reported that knowledge about heart health increased in the intervention group three months post‐intervention (Koniak‐Griffin 2015); we did not grade the results due to missing data for the control group.
Self‐management programmes may have little to no short‐term effect on health‐related knowledge. We are uncertain whether self‐management programmes compared to no health literacy interventions improve knowledge in the medium term.
Health outcomes
There is low‐certainty evidence from one RCT with 69 participants that self‐management programmes compared to no health literacy intervention may lead to little or no difference in subjective health status within the past week when assessed immediately post‐intervention (MD 0.38, 95% CI ‐0.13 to 0.89; Analysis 1.5) (van Servellen 2005).
1.5. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 5: Health outcomes: subjective health status (short‐term: immediately post‐intervention)
Health behaviour
Three RCTs with 514 participants measured three health behaviour outcomes including self‐reported blood glucose self‐monitoring, self‐reported adherence to HIV medication and physical activity assessed with an accelerometer. Results for each outcome at each time point assessed are presented in Table 5. The following results pertain to data that could not be pooled in a meta‐analysis.
Rosal 2011 reported greater self‐reported blood glucose‐self‐monitoring in the intervention group immediately post‐intervention (RR 1.30, 95% CI 1.11 to 1.52; 252 participants; Analysis 1.6). van Servellen 2005 reported that the proportion of participants who reported > 95% adherence to HIV medication within the last four days was higher in the intervention group six months after randomisation (change score intervention group: 1.71%, change score control group: ‐4.85%, 69 participants). Koniak‐Griffin 2015 reported that the mean physical activity (average daily steps) was higher in the intervention group immediately post‐intervention (MD 289 daily steps, 95% CI ‐601.41 to 1179.41; 193 participants; Analysis 1.7).
1.6. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 6: Health behaviour: blood glucose self‐monitoring 2 times per day (capped at 2), self‐report (short‐term: immediately post‐intervention)
1.7. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 7: Health behaviour: physical activity, average daily steps (short‐term: immediately post‐intervention)
One cluster‐RCT was missing information about the number of participants randomised to each study group, and the intensity and length of the programme. The study reported that self‐reported mammography screening was higher in the group who received the self‐management programme compared to a wait‐list control group (56% versus 10%; P < 0.0001; very low‐certainty evidence) after six months (Bloom 2014). However, it was unclear whether the participants were supported by health navigators during the total follow‐up time or not. Thus, we do not know whether participants were assessed in the short term or medium term. In addition, the information was insufficient to permit judgement for most risk of bias domains and the authors stated having used generalised estimating equations (GEE) models, but only reported the proportions of participants who self‐reported that they have had a mammogram.
Unpooled findings indicate that self‐management programmes may slightly improve health behaviour immediately post‐intervention, when compared to no health literacy intervention (low‐certainty evidence). However, the outcome measures and effects appear variable.
Koniak‐Griffin 2015 also reported results for physical activity at three‐month follow‐up. The results indicated uncertainty about whether there is a medium‐term effect on physical activity (MD 1336.00, 95% CI 540.86 to 2131.14; 193 participants; very low‐certainty evidence; Analysis 1.8). The certainty of the evidence is very low as the control group had a more than 1000‐step decline from immediately to three months post‐intervention, whereas the number of average daily steps in the intervention group fell back to the baseline level (which was 8577 average daily steps (standard deviation (SD) 2872)). Thus, the calculated MD does not reflect an actual improvement in the intervention group.
1.8. Analysis.

Comparison 1: Culturally and literacy adapted self‐management programme versus no HL intervention, Outcome 8: Health behaviour: physical activity, average daily steps (short‐term: three months post‐intervention)
Self‐efficacy
Two RCTs measured self‐efficacy to manage one's disease (Rosal 2011; van Servellen 2005). The pooled analysis with 333 participants indicated that self‐management programmes compared to no health literacy interventions probably improve self‐efficacy slightly immediately post‐intervention (standardised mean difference (SMD) 0.28, 95% CI 0.06 to 0.50; Analysis 1.9).
Health service use
The effect of self‐management programmes on health service use is unknown when compared to no health literacy intervention, as there was no direct evidence.
Adverse events
The effect of self‐management programmes on health service use is unknown when compared to no health literacy intervention, as there was no direct evidence.
Comparison 2: Culturally and literacy adapted self‐management programme versus written information on the same topic
We included six studies in this comparison with a total programme length of up to three (Rosal 2005; Kaur 2019), six (Han 2017; Kim 2009) and 12 months (Kim 2014; Kim 2020). The following results pertain to the short‐term assessments (immediately after the programme was completed) unless otherwise described. One cluster‐RCT reported additional results for six months after the programme was completed (Kim 2014). Table 7 presents the evidence relating to the effect of culturally and literacy adapted self‐management programmes compared to written information on the same topic. In addition, see Data and analyses for pooled data on this comparison and Table 2, Table 11, Table 8, Table 3, Table 4, Table 5 and Table 6 for the data that we did not pool.
Health literacy
Four RCTs reported either measures for generic health literacy, including health numeracy (assessed with NVS) and print literacy (assessed with REALM) (Kim 2020), or for disease‐specific health literacy, including cancer screening health literacy (assessed with AHL‐C) (Han 2017), oral health literacy (assessed with TS‐REALD) (Kaur 2019), high blood pressure health literacy (assessed with HBP Health Literacy Scale) (Kim 2014), or diabetes health literacy assessed with DM‐REALM (Kim 2020).
Generic health literacy
There is moderate‐certainty evidence from one RCT with 209 participants that self‐management programmes compared to written information on the same topic probably improve health numeracy slightly (MD 0.7, 95% CI 0.15 to 1.25; Analysis 2.1) and that they probably improve print literacy immediately post‐intervention (MD 9.00, 95% CI 2.90 to 15.10; Analysis 2.2) (Kim 2020).
2.1. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 1: Generic health literacy: health numeracy, NVS (short‐term: immediately post‐intervention)
2.2. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 2: Generic health literacy: print literacy, REALM (short‐term: immediately post‐intervention)
Disease‐specific health literacy
The pooled analysis of two RCTs (Kaur 2019; Kim 2020) and two cluster‐RCTs (Han 2017; Kim 2014) with 955 participants indicated that self‐management programmes compared to written information may improve disease‐specific health literacy (SMD 0.67, 95% CI 0.27 to 1.07; I² = 89%; low‐certainty evidence; Analysis 2.3). The test for subgroup differences by programme length was significant (Chi² = 4.89, df = 1, P = 0.03, I² = 79.2%; Analysis 2.4), revealing that participants who participated in shorter programmes (three to six months) and who were, thus, assessed after shorter follow‐up periods (that were accompanied by at least monthly motivating telephone calls) had higher scores in disease‐specific health literacy than those who participated in longer programmes of up to 12 months. Sensitivity analysis including only studies without high risk of bias (n = 2) showed a greater effect of self‐management programmes compared to written information on the same topic, but the lower limit of the pooled CI included a value favouring written information on the same topic (SMD 0.87, 95% CI ‐0.05 to 1.78, I² = 94%; Analysis 2.5). Since the results of Kaur 2019 were noticeably better than the results of other studies, we conducted an additional sensitivity analysis for this outcome. Excluding Kaur 2019 from the analysis, however, did not considerably alter the interpretation of the results. The calculated standardised mean difference still indicated an important effect, but the statistical heterogeneity was reduced (SMD 0.47, 95% CI 0.19 to 0.76, I² = 76%; Analysis 2.6).
2.5. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 5: Any disease‐specific health literacy (short‐term: immediately post‐intervention) ‐ studies without high risk of bias
One cluster‐RCT with 242 participants additionally reported on high blood pressure health literacy six months post‐intervention. The self‐management programme may improve high blood pressure health literacy slightly six months after the programme was completed (MD 4.10, 95% CI 0.97 to 7.23; low‐certainty; Analysis 2.7) (Kim 2014).
2.7. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 7: High blood pressure health literacy, HBP health literacy scale (medium‐term: 6 months post‐intervention)
Self‐management programmes may improve any disease‐specific health literacy immediately post‐intervention, and they may improve high blood pressure health literacy slightly at six‐month follow‐up.
Steps of health information processing (appraising health information)
One cluster‐RCT with 329 participants assessed decisional balance (i.e. weighing pros and cons) for using mammography or Pap testing for breast cancer screening or cervical cancer screening, respectively (Han 2017). The results indicated that self‐management programmes compared to written information on the same topic may lead to little or no difference in decisional balance, when assessed immediately after the six‐month programme was completed (MD 1.15, 95% CI ‐0.23 to 2.53; low‐certainty evidence; Analysis 2.8).
Quality of life
The pooled analysis of two RCTs with 288 participants indicated uncertainty about whether self‐management programmes improved diabetes‐related quality of life immediately post‐intervention (MD 9.06, 95% CI 2.85 to 15.27; I² = 60%; very low‐certainty evidence; Analysis 2.9) (Kim 2020; Kim 2009).
2.9. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 9: Diabetes‐related quality of life, DQOL (short‐term: immediately post‐intervention) ‐ all studies
One study with 25 participants reported on diabetes‐related quality of life, but due to incomplete reporting, both the direction and the size of the effect was unclear (Rosal 2005). However, the reported CI encompassed both benefit and harm, indicating that the intervention makes little to no difference to quality of life. The certainty of the evidence was very low.
We are uncertain whether self‐management programmes improve quality of life immediately post‐intervention.
Health‐related knowledge
Six studies assessed the effects of self‐management programmes on knowledge (Han 2017; Kaur 2019; Kim 2009; Kim 2014; Kim 2020; Rosal 2005). The studies' knowledge tests were based on the interventions' content (i.e. heart health, diabetes mellitus and HIV). We transformed the proportion of accurate responses to a percentage scale ranging from 0% (no correct responses) to 100% (fully correct responses).
The pooled analysis of six studies indicated that self‐management programmes may improve health‐related knowledge (MD 11.45, 95% CI 4.75 to 18.15; I² = 92%; low‐certainty evidence; Analysis 2.10). Due to the substantial statistical heterogeneity in this analysis, we conducted a subgroup analysis by programme length. It revealed that participants who participated in shorter programmes (three to six months), thus being assessed after shorter follow‐up periods (supported by the study team), had slightly more correct answers than those who participated in longer programmes of up to 12 months with a longer maintenance phase. However, each subgroup's pooled CI remained wide and the test for subgroup differences was non‐significant (Chi² = 0.02, df = 1, P = 0.89, I² = 0%; Analysis 2.11). Sensitivity analysis excluding studies with high risk of bias indicated that the effect of self‐management programmes on health‐related knowledge was even higher than indicated by the main analysis (MD 17.58, 95% CI 11.05 to 24.11, I2 = 79%; 3 RCTs, 428 participants; Analysis 2.12). Since the results of Kaur 2019 were noticeably better than the results of other studies, we conducted an additional sensitivity analysis for this outcome. Excluding Kaur 2019 from the analysis, however, did not considerably alter the interpretation of the results. The calculated mean difference still indicated an important, but smaller, effect on knowledge (MD 8.76, 95% CI 3.57 to 13.96, I² = 82%; Analysis 2.13).
2.12. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 12: Any health‐related knowledge, 0 to 100 (short‐term: immediately post‐intervention) ‐ studies without high risk of bias
The pooled analysis of two studies with 298 participants indicated that self‐management programmes may lead to little or no difference in health‐related knowledge up to six months post‐intervention (MD 3.87, 95% CI ‐0.46 to 8.19, I² = 30%; low‐certainty evidence; Analysis 2.14) (Kim 2014; Rosal 2005).
Self‐management programmes compared to written information on the same topic may improve health‐related knowledge immediately post‐intervention. However, the medium‐term analysis indicated that they may lead to little or no difference in health‐related knowledge up to six months post‐intervention.
Health outcomes
The pooled analysis of four RCTs with 555 participants indicated uncertainty about whether self‐management programmes have an effect on depression immediately post‐intervention (SMD ‐0.19, 95% CI ‐0.62 to 0.23, I² = 79%; very low‐certainty evidence; Analysis 2.15) (Kim 2009; Kim 2014; Kim 2020; Rosal 2005).
2.15. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 15: Health outcome: any depression (short‐term: immediately post‐intervention)
The pooled analysis of two studies with 267 participants indicated that self‐management programmes compared to written information may lead to little or no difference in depression up to six months after the programme was completed (MD ‐0.32, 95% CI ‐0.90 to 0.27, I² = 53%; low‐certainty evidence; Analysis 2.16) (Kim 2014; Rosal 2005).
2.16. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 16: Health outcome: any depression (medium‐term: up to 6 months post‐intervention)
We are uncertain whether self‐management programmes improve depression either immediately or six months post‐intervention.
Health behaviour
Five studies reported on five different health behaviour outcomes. In four studies, participants were assessed in the short term (immediately after the programme was completed) (Han 2017; Kaur 2019; Kim 2009; Kim 2014). In two studies, participants were assessed in the medium term (up to six months post‐intervention) (Kim 2014; Rosal 2005). Outcome measures included diabetes self‐care activities (Kim 2009), oral self‐care behaviour (Kaur 2019), cervical/breast cancer screening adherence (Han 2017), non‐adherence to blood pressure medication (Kim 2014), and blood glucose self‐monitoring (Rosal 2005). The following results pertain to data that could not be pooled in a meta‐analysis.
Kim 2009 reported that the self‐management programme improved diabetes self‐care activities post‐intervention, when compared to written information on the same topic (MD 15, 95% CI 7.87 to 22.13; 79 participants; Analysis 2.17). Kaur 2019 found that the intervention improved self‐reported oral self‐care behaviour immediately post‐intervention, when compared to written information on the same topic (MD 3.1, 95% CI 2.5 to 3.7; 140 participants; Analysis 2.18). One cluster‐RCT with 336 participants reported that the intervention improved cervical and breast cancer screening adherence (risk ratio (RR) 7.17, 95% CI 3.96 to 12.99; Analysis 2.19) (Han 2017). Kim 2014 found little or no difference in non‐adherence to blood pressure medication immediately post‐intervention (MD ‐0.4, 95% CI ‐0.87 to 0.07; 1 cluster‐RCT, 242 participants; Analysis 2.20), when compared to written information on the same topic.
2.17. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 17: Health behaviour: diabetes self‐care activities (short‐term: immediately post‐intervention)
2.18. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 18: Health behaviour: oral hygiene self‐care behaviour (short‐term: immediately post‐intervention)
2.19. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 19: Health behaviour: screening adherence (mammogram and Pap test), medical record review (short‐term: immediately post‐intervention)
2.20. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 20: Health behaviour: non‐adherence to blood pressure medication (short‐term: immediately post‐intervention)
Kim 2014 additionally reported results for non‐adherence to blood pressure medication at six months after the programme was completed, indicating lower non‐adherence scores in the intervention group (MD ‐0.40, 95%‐CI ‐0.78 to ‐0.02; Analysis 2.21). Rosal 2005 reported greater self‐reported blood glucose‐self‐monitoring in the intervention group four and a half months post‐intervention, but the CI encompassed both a large improvement and a reduction in this outcome (RR 1.96, 95% CI 0.76 to 5.03; 23 participants; Analysis 2.22)
2.21. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 21: Health behaviour: non‐adherence to blood pressure medication (medium‐term: 6 months post‐intervention)
2.22. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 22: Health behaviour: blood glucose self‐monitoring 2 times per day (capped at 2), self‐report (medium‐term: 4 1/2 months post‐intervention)
Kim 2020 stated having measured diabetes self‐care activities but did not report the results.
The unpooled findings indicated that self‐management programmes may improve some health behaviours immediately post‐intervention (low‐certainty evidence) and they may slightly improve some health behaviours up to six months post‐intervention (low‐certainty evidence). However, measures and effect sizes for both the short‐term and the medium‐term assessments appeared to be variable.
Self‐efficacy
The pooled analysis of four studies with 552 participants showed that the mean score for self‐efficacy to manage one's own disease was higher across the intervention groups (SMD 0.47, 95% CI 0.30 to 0.64; I² = 0%; moderate‐certainty evidence; Analysis 2.23) (Kim 2009; Kim 2014; Kim 2020; Rosal 2005). The sensitivity analysis excluding studies at high risk of bias indicated a larger, but still moderate, effect on self‐efficacy (SMD 0.58, 95% CI 0.34 to 0.81; I² = 0%; low‐certainty evidence; Analysis 2.24).
2.24. Analysis.

Comparison 2: Culturally and literacy adapted self‐management programme versus written information, Outcome 24: Self‐efficacy to manage one's disease (short‐term: immediately post‐intervention) ‐ studies without high risk of bias
One cluster‐RCT with 242 participants also reported data for the six‐month assessment, indicating that self‐management programmes compared to written information may lead to little or no difference in high blood pressure self‐efficacy six months post‐intervention (MD ‐0.20, 95% CI ‐1.16 to 0.76; low‐certainty evidence; Analysis 2.25) (Kim 2014).
Self‐management programmes compared to written information on the same topic probably improve self‐efficacy immediately post‐intervention, but they may result in little or no effect on self‐efficacy six months post‐intervention.
Health service use
The effect of self‐management programmes on health service use is unknown as there was no direct evidence.
Adverse events
The effect of self‐management programmes on adverse events is unknown as there was no direct evidence.
Comparison 3: Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention
We included three RCTs (Otilingam 2015; Soto Mas 2018; Wong 2020) and three cluster‐RCTs (Elder 1998; Taylor 2011; Tong 2017) in this comparison. Participants were assessed in the short term (immediately post‐intervention) and medium term (three to six months post‐intervention). The following results pertain to the short‐term assessments (immediately after the programme was completed) unless otherwise described. Table 12 presents the evidence relating to the effect of culturally adapted health literacy skills building courses compared to either no health literacy intervention or an unrelated health literacy intervention. In addition, see Data and analyses for pooled data on this comparison and Table 2, Table 10, Table 3, Table 5 and Table 6 for data that we did not pool.
Health literacy
Generic health literacy
Two RCTs measured generic functional health literacy using either the full version of the Test of Functional Health Literacy in Adults (TOFHLA) (Soto Mas 2018) or newest vital sign (NVS) (Otilingam 2015).
The pooled analysis of these two RCTs with 229 participants found that health literacy skills building courses may improve any generic functional health literacy up to one month post‐intervention, when compared to no or unrelated health literacy intervention (SMD 0.48, 95% CI 0.20 to 0.75; I² = 0%; low‐certainty evidence; Analysis 3.1).
Disease‐specific health literacy
One RCT with 37 participants indicated that health literacy skills building courses may lead to little or no difference in depression literacy immediately post‐intervention, when compared to no or unrelated health literacy intervention (MD 0.17, 95% CI ‐1.28 to 1.62; low‐certainty evidence; Analysis 3.2) (Wong 2020).
3.2. Analysis.

Comparison 3: Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention, Outcome 2: Depression literacy, D‐Lit (short‐term: outcome assessment immediately post‐intervention)
Steps of health information processing (applying health information)
One cluster‐RCT with 287 participants indicated uncertainty about whether health literacy skills building courses improve the intention to change nutritional habits, when compared to no or unrelated health literacy intervention (MD 0.05; P > 0.05; very low‐certainty evidence; see Table 10) (Elder 1998).
Quality of life
The effect of the intervention on quality of life is unknown as there was no direct evidence identified.
Health‐related knowledge
The pooled analysis of two RCTs with 111 participants indicated that health literacy skills building courses may improve health‐related knowledge immediately post‐intervention, when compared to no or unrelated health literacy intervention (MD 10.87, 95% CI 5.69 to 16.06; I² = 0%; low‐certainty evidence; Analysis 3.3) (Otilingam 2015; Wong 2020). The knowledge score across control groups ranged from 48.1% to 61.8%. In absolute terms, this means that the group receiving no or unrelated health literacy intervention had, on average, 57 out of 100 answers correct whereas those in the self‐management group had 68 answers correct on average (from 63 to 73 correct).
Three cluster‐RCTs, which could not be pooled because most studies did not report the results in an extractable way for meta‐analysis, measured health‐related knowledge six months post‐intervention (Elder 1998; Taylor 2011; Tong 2017). One cluster‐RCT with 168 participants reported that the health literacy skills building course slightly improved hepatitis B knowledge six months post‐intervention (MD 0.81, 95% CI 0.43 to 1.19; Analysis 3.4) (Taylor 2011). One cluster‐RCT with 291 participants reported that the intervention slightly improved nutrition knowledge six months post‐intervention (MD 0.79; P ≤ 0.001) (Elder 1998). One cluster‐RCT with 329 participants that did not report a composite knowledge score, but proportions of correct answers for five knowledge questions, found that the proportion of participants with correct answers was higher in the intervention group for all five knowledge domains with an MD ranging from 15.1% to 36.8% and P values ranging from < 0.0001 to 0.012 (Tong 2017). For more details on this outcome, see Table 3.
3.4. Analysis.

Comparison 3: Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention, Outcome 4: Hepatitis B knowledge (medium‐term: 6 months post‐intervention)
Health literacy skills building courses may slightly improve health‐related knowledge six months post‐intervention, when compared to no or unrelated health literacy intervention (low‐certainty evidence).
Health outcomes
The effect of the intervention on health outcomes is unknown as there was no direct evidence identified.
Health behaviour
Two RCTs (Otilingam 2015; Soto Mas 2018) and two cluster‐RCTs (Taylor 2011; Tong 2017) reported on three health behaviour outcomes. The following results pertain to data that could not be pooled in a meta‐analysis.
Two RCTs reported on two health behaviour measures immediately post‐intervention and indicated uncertainty about whether health literacy skills building courses improve health behaviour at this time point. One RCT with 74 participants found little or no difference in self‐reported fat‐related dietary habits one month post‐intervention (MD 0.25, 95% CI 0.00 to 0.50; Analysis 3.5) (Otilingam 2015). One RCT with 155 participants also found little or no difference in self‐reported cardiovascular health behaviour immediately post‐intervention (MD 1.2; P value = 0.067, see Table 5) (Soto Mas 2018).
Two cluster‐RCTs with 440 participants measured screening adherence six months post‐intervention (Taylor 2011; Tong 2017). The pooled analysis indicated that health literacy skills building courses may improve or reduce screening adherence six months post‐intervention, when compared to no or unrelated health literacy intervention (RR 2.68, 95% CI 0.33 to 21.83; low‐certainty evidence; Analysis 3.6). The effect sizes appear to vary considerably, indicating an inconclusive result.
Health literacy skills building courses compared to no or unrelated health literacy intervention may lead to little or no difference in any health behaviour immediately post‐intervention. When assessed at six‐month follow‐up, they may improve or reduce health behaviour (cancer screening adherence), but the importance of the effect is unclear as the effect sizes appeared to be variable.
Self‐efficacy
One cluster‐RCT with 290 participants indicated uncertainty about whether health literacy skills building courses improve self‐efficacy to change one's diet six months post‐intervention (MD 0.03; P = 0.64; very low‐certainty evidence) (Elder 1998). For more details, see Table 6.
Health service use
The effect of the intervention on health service use is unknown as there was no direct evidence.
Adverse events
The effect of the intervention on adverse events is unknown as there was no direct evidence.
Comparison 4: Culturally and literacy adapted telephone education versus unrelated health literacy intervention
We included one RCT in this comparison. Lepore 2012 compared telephone education about prostate cancer to an unrelated health literacy intervention that came in the form of telephone education about nutrition. Participants were assessed in the long term (approximately seven months post‐intervention for the outcomes decisional conflict (related to appraising health information), knowledge, prostate cancer screening intention and anxiety, and two years post‐intervention for the outcome actual prostate‐specific antigen (PSA) testing). Table 14 presents the evidence relating to the effect of culturally and literacy adapted telephone education compared to unrelated health literacy intervention. In addition, data related to this study are shown in Table 11, Table 10, Table 3, Table 5 and Table 9.
Steps of health information processing
Culturally and literacy adapted telephone education compared to unrelated health literacy intervention probably improves the appraisal of health information by reducing decisional conflict (‐5.70, 95% CI ‐10.24 to ‐1.16; 431 participants; moderate‐certainty evidence; Analysis 4.1), but probably leads to little or no difference in applying health information (prostate cancer screening intention) (RR 1.00, 95% CI 0.92 to 1.10; 431 participants; moderate‐certainty evidence; Analysis 4.2), when assessed approximately seven months post‐intervention.
4.1. Analysis.

Comparison 4: Culturally and literacy adapted telephone education versus unrelated health literacy intervention, Outcome 1: Health literacy ‐ appraise: decisional conflict (long‐term: approx. 7 months post‐intervention)
4.2. Analysis.

Comparison 4: Culturally and literacy adapted telephone education versus unrelated health literacy intervention, Outcome 2: Health literacy ‐ apply: prostate cancer screening intention (long‐term: approx. 7 months post‐intervention)
Quality of life
The effect of telephone education on quality of life is unknown as there was no direct evidence.
Health‐related knowledge
Culturally and literacy adapted telephone education compared to unrelated health literacy intervention probably improves prostate cancer knowledge slightly approximately seven months post‐intervention (MD 6.9, 95% CI 6.88 to 6.92; 431 participants; moderate‐certainty evidence; Analysis 4.3). In absolute terms, the group receiving unrelated telephone education had, on average, 55 out of 100 answers correct whereas those in the self‐management group had 62 answers correct on average (from 62 to 62 correct).
4.3. Analysis.

Comparison 4: Culturally and literacy adapted telephone education versus unrelated health literacy intervention, Outcome 3: Prostate cancer knowledge, 0 to 100 (long‐term: approx. 7 months post‐intervention)
Health outcomes
The effect of telephone education on health outcomes is unknown as there was no direct evidence.
Health behaviour
The data reported by Lepore 2012 indicated that telephone education compared to unrelated telephone education probably results in little or no difference in prostate cancer testing two years post‐intervention (RR 0.93, 95% CI 0.82 to 1.07; 490 participants; moderate‐certainty evidence; Analysis 4.4).
4.4. Analysis.

Comparison 4: Culturally and literacy adapted telephone education versus unrelated health literacy intervention, Outcome 4: Health behaviour: prostate cancer testing (long‐term: 2 years post‐intervention)
Self‐efficacy
The effect of telephone education on self‐efficacy is unknown as there was no direct evidence.
Health service use
The effect of telephone education on health service use is unknown as there was no direct evidence.
Adverse events
The data reported by Lepore 2012 indicated that telephone education compared to unrelated telephone education probably leads to little or no difference in anxiety (assessed with the seven‐item subscale of the Hospital Anxiety and Depression Scale, HADS) approximately seven months post‐intervention (MD ‐0.14, 95% CI ‐0.55 to 0.27; 431 participants; moderate‐certainty evidence; Analysis 4.5).
4.5. Analysis.

Comparison 4: Culturally and literacy adapted telephone education versus unrelated health literacy intervention, Outcome 5: Adverse events: anxiety (long‐term: approx. 7 months post‐intervention)
Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention
We included four RCTs in this comparison (DeCamp 2020; Hernandez 2013; Kiropoulos 2011; Thompson 2012). Table 15 presents the evidence relating to the effect of culturally and literacy adapted audio‐/visual education compared to usual care, no health literacy intervention or unrelated health literacy intervention. In addition, see Data and analyses for pooled data on this comparison and Table 2, Table 10, Table 3, Table 4, Table 5, Table 6 and Table 13 for data we did not pool.
Health literacy
Disease‐specific health literacy
One RCT with 202 participants reported results for depression literacy assessed with the Depression Literacy Questionnaire (D‐Lit) (Kiropoulos 2011). Audio‐/visual education without personal feedback compared to no health literacy intervention probably improves depression literacy one week post‐intervention (MD 8.62, 95% CI 7.51 to 9.73; moderate‐certainty evidence; Analysis 5.1).
5.1. Analysis.

Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention, Outcome 1: Health literacy: depression literacy, D‐Lit (short‐term: at 1‐week post‐intervention)
Steps of health information processing (applying health information)
One RCT with 120 participants indicated that audio‐/visual education without personal feedback may slightly improve the intention to seek treatment for depression immediately post‐intervention (MD 1.8, 95% CI 0.43 to 3.17; low‐certainty evidence; Analysis 5.2), when compared to no health literacy intervention (Hernandez 2013).
5.2. Analysis.

Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention, Outcome 2: Health literacy: apply ‐ intent to seek treatment (short‐term: immediately post‐intervention)
Quality of life
The effect of audio‐/visual education without personal feedback on quality of life is unknown, as there was no direct evidence.
Health‐related knowledge
Two studies assessed the effect of audio‐/visual education compared to no health literacy intervention on health‐related knowledge (DeCamp 2020; Hernandez 2013). The knowledge tests in the studies were based on the content of the interventions (i.e. child health and depression). We transformed the proportion of accurate responses to a percentage scale ranging from 0% (no correct responses) to 100% (fully correct responses).
The pooled analysis with 293 participants indicated that audio‐/visual education without personal feedback compared to no health literacy intervention may slightly improve health‐related knowledge up to one month post‐intervention, but the effect sizes appear to vary considerably (MD 8.44, 95% CI ‐2.56 to 19.44; I² = 97%; low‐certainty evidence; Analysis 5.3).
5.3. Analysis.

Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention, Outcome 3: Any health‐related knowledge, 0 to 100 (short‐term: immediately up to 3 months post‐intervention)
Health outcome
The pooled analysis of two RCTs with 337 participants indicated that audio‐/visual education without personal feedback may lead to little or no difference in any depression immediately up to three months post‐intervention (SMD ‐0.15, 95% CI ‐0.40 to 0.10; low‐certainty evidence; Analysis 5.4), when compared to no health literacy intervention.
5.4. Analysis.

Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention, Outcome 4: Health outcome: any depression (short‐term: up to 1 week post‐intervention)
Health behaviour
One RCT with 135 participants assessed children's up‐to‐date immunisation immediately and up to three months post‐intervention (participants were not assessed at the same time) (DeCamp 2020). The results of DeCamp 2020 (RR 1.07, 95% CI 0.91 to 1.25; moderate‐certainty evidence; Analysis 5.5) indicated that audio‐/visual education without personal feedback probably results in little or no difference in children's up‐to‐date immunisation immediately and up to three months post‐intervention, when compared to no health literacy intervention.
5.5. Analysis.

Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention, Outcome 5: Health behaviour: child's up‐to‐date immunisation (short‐term: immediately up to 3 months post‐intervention)
Self‐efficacy
The results of one RCT with 133 participants indicated that audio‐/visual education without personal feedback may improve self‐efficacy to identify the need for treatment of depression immediately post‐intervention (MD 3.51, 95% CI 2.53 to 4.49; low‐certainty evidence; Analysis 5.6), when compared to no health literacy intervention (Hernandez 2013).
5.6. Analysis.

Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention, Outcome 6: Self‐efficacy to identify need for treatment (short‐term: immediately post‐intervention)
Health service use
One RCT with 157 participants assessed children's emergency room visits immediately and up to three months post‐intervention, indicating that audio‐/visual education without personal feedback compared to no health literacy intervention probably reduces children's emergency room visits up to three months post‐intervention (MD ‐0.59, 95% CI ‐1.11 to ‐0.07; moderate‐certainty evidence; Analysis 5.7) (DeCamp 2020).
5.7. Analysis.

Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention, Outcome 7: Health service use: emergency room visits, medical record review (short‐term: immediately up to 3 months post‐intervention)
Adverse events
The effect of audio‐/visual education without personal feedback on adverse events is unknown, as there was no direct evidence identified.
Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic
We included nine RCTs in this comparison (Calderón 2014; Gwede 2019; Payán 2020; Poureslami 2016a; Poureslami 2016b; Sudore 2018; Unger 2013; Valdez 2015; Valdez 2018). Participants were assessed in the short term immediately post‐intervention up to 15 months after study enrolment. Table 16 presents the evidence relating to the effect of culturally and literacy adapted media interventions compared to another culturally and literacy adapted media intervention. In addition, see Data and analyses for pooled data on this comparison and Table 2, Table 17, Table 19, Table 11, Table 10, Table 3, Table 4, Table 5, Table 6 and Table 9 for data we did not pool.
Health literacy
Disease‐specific health literacy
One RCT with 240 participants measured diabetes health literacy immediately post‐intervention, indicating that audio‐/visual education without personal feedback compared to written information on the same topic probably leads to little or no difference in diabetes health literacy (MD 2.00, 95% CI ‐0.15 to 4.15; moderate‐certainty evidence; Analysis 6.1) (Calderón 2014).
6.1. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 1: Diabetes health literacy, DHLS (short‐term: immediately post‐intervention )
Prerequisites and tools
The pooled analysis of two RCTs with 176 participants indicated that audio‐/visual education without personal feedback compared to written information on the same topic may slightly improve competencies (inhaler use technique) three months post‐intervention (MD 0.98, 95% CI 0.26 to 1.70; low‐certainty evidence; Analysis 6.2) (Poureslami 2016a; Poureslami 2016b).
6.2. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 2: Health literacy ‐ competencies: inhaler use technique, checklist 0 to 10 (medium‐term: 3 months post‐intervention)
Steps of health information processing
Two RCTs with 128 participants reported results either for understanding physician's instruction (MD 0.04, 95% CI ‐0.55 to 0.63; 85 participants; Analysis 6.3) (Poureslami 2016a), or for understanding pulmonary rehabilitation procedures (MD 0.30, 95% CI ‐0.76 to 1.36; 43 participants) (Poureslami 2016b), both indicating that audio‐/visual education without personal feedback compared to written information on the same topic may lead to little or no difference in understanding of health information three months post‐intervention (low‐certainty evidence). We found moderate‐certainty evidence from one RCT with 608 participants, which reported results for appraising and applying health information (Valdez 2015). The study found that audio‐/visual education without personal feedback compared to written information probably improves appraising health information by reducing decisional conflict, assessed with the three subscales 'informed decision', 'values clarity' and 'support' at one month post‐intervention (MD ‐9.88, 95% CI ‐12.87 to ‐6.89; Analysis 6.4). This was also found for applying health information (making an informed decision regarding HPV vaccination) one month post‐intervention (RR 1.51, 95% CI 1.29 to 1.77; Analysis 6.5).
6.3. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 3: Health literacy ‐ understanding physician's instructions (medium‐term: 3 months post‐intervention)
6.4. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 4: Health literacy ‐ appraise: decisional conflict (short‐term: 1 month post‐intervention)
6.5. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 5: Health literacy ‐ apply: informed decision against HPV vaccination (short‐term: 1 month post‐intervention)
Quality of life
The effect of audio‐/visual education without personal feedback on quality of life is unknown, as there was no direct evidence.
Health‐related knowledge
Six studies measured any health‐related knowledge either immediately and up to one month post‐intervention (Payán 2020; Unger 2013; Valdez 2015), or up to six months after the intervention was completed (Gwede 2019; Payán 2020; Poureslami 2016a; Valdez 2018). Poureslami 2016b stated having assessed COPD‐related knowledge, but did not report the results. The knowledge tests in the studies were based on the content of the interventions (i.e. heart health, diabetes mellitus and HIV). We transformed the proportion of accurate responses to a percentage scale ranging from 0% (no correct responses) to 100% (fully correct responses).
The pooled analysis of three RCTs with 987 participants indicated that audio‐/visual education without personal feedback compared to written information on the same topic may slightly improve health‐related knowledge up to one‐month post‐intervention (MD 8.35, 95% CI ‐0.32 to 17.02; I² = 93%; low‐certainty evidence; Analysis 6.6) (Payán 2020; Unger 2013; Valdez 2015). Subgroup analysis revealed that the use of an audiovisual (multimedia) format (here an educational DVD) was more effective in improving health‐related knowledge (MD 15.00, 95% CI 12.61 to 17.39; 1 study, 608 participants) than a printed visual format (here photonovels delivered either by community health workers or in a group session delivered by lay health workers) (MD 4.75, 95% CI ‐3.33 to 12.84; 2 studies, 379 participants). The test for subgroup differences was significant (Chi² = 5.68, df = 1, P = 0.02, I² = 82.4%; Analysis 6.7).
The pooled analysis of three RCTs with 979 participants indicated uncertainty about whether audio‐/visual education without personal feedback compared to written information on the same topic improves cancer‐related knowledge up to six months post‐intervention (MD 7.30, 95% CI ‐3.73 to 18.32, I² = 90%; very low‐certainty evidence; Analysis 6.8) (Gwede 2019; Payán 2020; Valdez 2018). The subgroup analysis showed that audiovisual (multimedia) formats (MD 12.27, 95% CI 8.28 to 16.26) were superior to printed visual formats (MD ‐2.80, 95% CI ‐8.00 to 2.40). The test for subgroup differences was significant (Chi² = 20.32, df = 1, P < 0.00001, I² = 95.1%; Analysis 6.9).
One study with 85 participants and four study arms could not be included in the pooled analysis as no composite score was reported (Poureslami 2016a). Only change scores and CIs per group and per item were reported. In addition, we had insufficient information about the score range, so combining the results of the knowledge items and pooling them with other data by calculating a standardised mean difference would have led to information loss. Briefly, the study found that audio‐/visual education may make little or no difference to asthma knowledge three months post‐intervention as almost all CIs were wide and included both benefit and harm (very low‐certainty evidence). Results for all study groups are shown in Table 3.
Culturally and literacy adapted audio‐/visual education without personal feedback may improve health‐related knowledge in the short term, when compared with written information on the same topic. We do not know whether it has an effect on health‐related knowledge in the medium term as the certainty of the evidence is very low.
Health outcome
One RCT with 445 participants measured depression 12 months post‐intervention using the Patient Health Questionnaire (PHQ‐8) (Sudore 2018). The results indicated that audio‐/visual education without personal feedback compared to written information on the same topic may result in little or no difference in depression 12 months post‐intervention (MD ‐0.60, 95% CI ‐1.37 to 0.17; low‐certainty evidence; Analysis 6.10).
6.10. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 10: Health outcome: depression, PHQ‐8 (long‐term: 12 months post‐intervention)
Health behaviour
Two RCTs measured cancer screening uptake either related to colorectal cancer (assessed via return of faecal immunochemical test) (Gwede 2019) or cervical cancer (assessed via self‐reported Pap testing) (Valdez 2018). The pooled analysis with 803 participants indicated that audio‐/visual education without personal feedback may lead to little or no difference in any cancer screening uptake up to six months post‐intervention, when compared to written information on the same topic (RR 1.07, 95% CI 0.95 to 1.20, I² = 0%; low‐certainty evidence; Analysis 6.11).
6.11. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 11: Health behaviour: any cancer screening uptake (medium‐term: up to 6‐month follow‐up)
One RCT with 445 participants measured new documentation of advance care planning assessed via medical record (Sudore 2018). The results indicated that audio‐/visual education without personal feedback compared to written information on the same topic probably improves documentation of advance care planning 12 months post‐intervention (RR 1.49, 95% CI 1.13 to 1.97; moderate‐certainty evidence; Analysis 6.12).
6.12. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 12: Health behaviour: new documentation of advance care planning (long‐term: 12 months post‐intervention)
Self‐efficacy
One RCT with 240 participants reported on self‐efficacy in accessing breast cancer‐related advice or information immediately post‐intervention (Payán 2020) and indicated that audio‐/visual education compared to written information on the same topic may result in little or no difference in self‐efficacy in accessing breast cancer‐related advice or information (MD 0.08, 95% CI ‐0.02 to 0.18; low‐certainty evidence; Analysis 6.13).
6.13. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 13: Breast cancer self‐efficacy (short‐term: immediately post‐intervention)
Four studies measured self‐efficacy three to six months post‐intervention (Gwede 2019; Payán 2020; Poureslami 2016b; Valdez 2018). The results of two studies could be pooled. The following results pertain to the synthesis of the pooled analysis and the unpooled findings of the two other studies.
The pooled analysis of two RCTs with 256 participants found little or no effect of audio‐/visual education without personal feedback on any cancer‐related self‐efficacy three months post‐intervention (SMD 0.08, 95% CI ‐0.18 to 0.33, I² = 0%; Analysis 6.14) (Gwede 2019; Payán 2020). One study with 727 participants that could not be incorporated in the pooled analysis due to variance in the reported outcome data, also found that audio‐/visual education made little or no difference to self‐efficacy regarding Pap testing between the intervention groups (RR 1.02, 95% CI 0.98 to 1.06; Analysis 6.15) (Valdez 2018). One study with 43 participants and four study arms could not be incorporated in the pooled analysis as the data were not reported in a way that could be extracted for meta‐analysis (Poureslami 2016b). The study found little or no effect on self‐efficacy three months post‐intervention. In this study no composite score was reported, but only subgroup analyses per intervention group compared to a control group and per item (five items). In addition, three out of the five CIs encompassed both an improvement and a reduction in self‐efficacy. More details are shown in Table 6.
6.14. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 14: Cancer‐related self‐efficacy (medium‐term: at 3‐month follow‐up)
6.15. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 15: Self‐efficacy regarding Pap testing (medium‐term: at 6‐month follow‐up)
Audio‐/visual education without personal feedback compared to written information on the same topic may have little or no effect on self‐efficacy when assessed in the medium term.
Health service use
The effect of audio‐/visual education without personal feedback on health service use is unknown as there was no direct evidence.
Adverse events
One RCT with 445 participants measured anxiety using the Generalised Anxiety Disorder Scale (GAD‐7) (Sudore 2018). The results demonstrated that audio‐/visual education without personal feedback probably leads to little or no difference in anxiety 12 months post‐intervention (MD ‐0.70, 95% CI ‐1.40 to 0.00; moderate‐certainty evidence; Analysis 6.16).
6.16. Analysis.

Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic, Outcome 16: Adverse event: anxiety, GAD‐7 (long‐term: 12 months post‐intervention)
Comparison 7: Culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback
We included three RCTs comparing a narrative video (here referred to as intervention) to a factual knowledge video (here referred to as control). One study aimed to improve knowledge about cervical cancer and cervical cancer screening behaviour in Spanish‐speaking immigrants (Ochoa 2020). The other studies aimed to improve knowledge about asthma (Poureslami 2016a) or COPD (Poureslami 2016b) and its medication management in Asian immigrants. Participants were all assessed in the medium term, either three months (Poureslami 2016a; Poureslami 2016b) or six months post‐intervention (Ochoa 2020). Poureslami 2016a and Poureslami 2016b stated that participants were also assessed six months post‐intervention, but results were not reported. Table 18 presents the evidence relating to the effect of culturally and literacy adapted audio‐/visual education without personal feedback (narrative video) versus another culturally and literacy adapted audio‐/visual education without personal feedback (factual knowledge video). In addition, see Data and analyses for pooled data on this comparison and Table 17, Table 19, Table 10, Table 3 and Table 5 for data we did not pool.
Health literacy
Prerequisites and tools
The pooled analysis of two RCTs with 91 participants indicated uncertainty about whether educational (narrative) videos compared to factual knowledge videos improve competencies (inhaler use technique) three months post‐intervention (MD ‐0.89, 95% CI ‐1.84 to 0.07; very low‐certainty evidence; Analysis 7.1) (Poureslami 2016a; Poureslami 2016b).
7.1. Analysis.

Comparison 7: Culturally and literacy adapted audio‐/visual education without personal feedback versus another audio‐/visual education without personal feedback, Outcome 1: Health literacy ‐ competencies: inhaler use technique, checklist 0 to 10 (medium‐term: 3 months post‐intervention)
Steps of health information processing
The results of one RCT with 43 participants indicated uncertainty about whether narrative videos compared to factual knowledge videos have an effect on understanding of physician's instruction three months post‐intervention (MD ‐0.15, 95% CI ‐0.72 to 0.42; very low‐certainty evidence; Analysis 7.2) (Poureslami 2016a). One study could not be included in the narrative synthesis as the participants who watched the narrative video and those who watched the knowledge video were not directly compared to each other, but both were compared to a control group who read a pictorial pamphlet (Poureslami 2016b). Details are shown in Table 19.
7.2. Analysis.

Comparison 7: Culturally and literacy adapted audio‐/visual education without personal feedback versus another audio‐/visual education without personal feedback, Outcome 2: Health literacy ‐ understanding physician's instruction (medium‐term: 3 months post‐intervention)
Ochoa 2020 reported results for intention to have cervical cancer screening (Pap testing) that indicated uncertainty about whether educational (narrative) videos compared to factual knowledge videos improve the application of health information (intention to have cervical cancer screening) six months post‐intervention (RR 1.97, 95% CI 0.83 to 4.69; 109 participants; very low‐certainty evidence; Analysis 7.3).
7.3. Analysis.

Comparison 7: Culturally and literacy adapted audio‐/visual education without personal feedback versus another audio‐/visual education without personal feedback, Outcome 3: Health literacy ‐ apply: Pap testing intention, self‐report (medium‐term: 6 months post‐intervention)
Quality of life
The effect of narrative videos compared to factual knowledge videos on quality of life is unknown, as there was no direct evidence identified.
Health‐related knowledge
Two RCTs in this comparison reported results for health‐related knowledge (Ochoa 2020; Poureslami 2016a). The knowledge tests in the studies were based on the content of the interventions (i.e. cervical cancer and asthma). We transformed the proportion of accurate responses to a percentage scale ranging from 0% (no correct responses) to 100% (fully correct responses) for the results of Ochoa 2020 only, as in Poureslami 2016a no score range was reported, but only subgroup analyses per study group and knowledge item. Therefore, we could not standardise the reported values on a scale ranging from 0 to 100. Nevertheless, the three knowledge items were combined to calculate an MD across the items.
The findings of Ochoa 2020 indicated uncertainty about whether watching a narrative video about cervical cancer has an effect on health‐related knowledge, when compared to a factual knowledge video on the same topic (MD 1.12, 95% CI ‐4.63 to 6.87; 109 participants; Analysis 7.4) six months post‐intervention. The mean cervical cancer knowledge score in the control group was 66%. However, there was an unclear risk of bias for random sequence generation and allocation concealment and the CI encompassed both an improvement and a worsening. The results of Poureslami 2016a also indicated uncertainty about the effect of watching a narrative video about asthma management on health‐related knowledge when compared to a factual knowledge video on the same topic three months post‐intervention (MD 0.85, 95% CI ‐1.07 to 2.76; 43 participants; Analysis 7.5).
7.4. Analysis.

Comparison 7: Culturally and literacy adapted audio‐/visual education without personal feedback versus another audio‐/visual education without personal feedback, Outcome 4: Cervical cancer knowledge, 0 to 100 (medium‐term: 6 months post‐intervention)
7.5. Analysis.

Comparison 7: Culturally and literacy adapted audio‐/visual education without personal feedback versus another audio‐/visual education without personal feedback, Outcome 5: Asthma knowledge (medium‐term: 3 months post‐intervention)
We are uncertain whether narrative educational videos compared to factual knowledge videos improve health‐related knowledge up to six months post intervention.
Health outcome
The effect of narrative educational videos compared to factual knowledge videos on health outcomes is unknown, as there was no direct evidence.
Health behaviour
The results of Ochoa 2020 indicated uncertainty about whether narrative educational videos compared to factual knowledge videos improve cervical cancer screening behaviour six months post‐intervention (RR 1.29, 95% 0.75 to 2.23; 109 participants; very low‐certainty evidence; Analysis 7.6).
7.6. Analysis.

Comparison 7: Culturally and literacy adapted audio‐/visual education without personal feedback versus another audio‐/visual education without personal feedback, Outcome 6: Health behaviour: cervical cancer screening (medium‐term: at 6‐month follow‐up)
Self‐efficacy
The effect of narrative videos compared to factual knowledge videos on self‐efficacy is unknown, as there was no direct evidence.
Adverse events
The effect of narrative videos compared to factual knowledge videos on adverse events is unknown, as there was no direct evidence.
Comparison 8: Culturally and literacy adapted medical instruction versus no health literacy intervention
We included three RCTs with 478 participants in this comparison (Bailey 2012; Kheir 2014; Mohan 2014). Participants were assessed up to one week post‐intervention. Table 20 presents the evidence relating to the effect of culturally and literacy adapted medical instruction compared to another culturally and literacy adapted media intervention. In addition, see Data and analyses for data presented in forest plots and Table 19, and Table 5 for all data in this comparison.
Health literacy
Steps of health information processing (understanding health information)
One RCT with 202 participants reported that health literacy informed medication instructions improved the correct dosage in the dosing tray immediately post‐intervention (intervention group: median 4.0, interquartile range (IQR) 3.0 to 5.0; control group: median 3.0, IQR 2.0 to 4.0) (Bailey 2012). Another RCT with 123 participants reported that pictograms plus verbal instruction improved the correct interpretation of label contents in 10 out of 11 medical instructions immediately post‐intervention, when compared with standard text labels and verbal instruction (no composite score reported) (Kheir 2014). One RCT with 200 participants reported that a literacy adapted plain language text in combination with an illustrated medication list improved medication understanding assessed with the Medication Understanding Questionnaire (MUQ), with a score range of 0 (no knowledge) to 100 (perfect knowledge) at one‐week follow‐up (MD 10, 95% CI 5.70 to 14.30; Analysis 8.1) (Mohan 2014).
8.1. Analysis.

Comparison 8: Culturally and literacy adapted medical instruction versus no health literacy intervention, Outcome 1: Understand: medication understanding (short‐term: immediately post‐intervention)
Culturally and literacy adapted medical instructions compared to no health literacy intervention may improve medication understanding up to one week post‐intervention.
Quality of life
The effect of the intervention on quality of life is unknown as there was no direct evidence.
Health‐related knowledge
The effect of the intervention on health‐related knowledge is unknown as there was no direct evidence.
Health outcome
The effect of the intervention on health outcomes is unknown as there was no direct evidence.
Health behaviour
One RCT with 200 participants measured self‐reported medication adherence at one week post‐intervention (Mohan 2014), indicating that culturally and literacy adapted medical instructions compared to no health literacy intervention may result in little or no difference in health behaviour one week post‐intervention (MD 0.5, 95% CI ‐0.1 to 1.1; low‐certainty evidence).
Self‐efficacy
The effect of the intervention on self‐efficacy is unknown as there was no direct evidence.
Health service use
The effect of the intervention on health service use is unknown as there was no direct evidence.
Adverse events
The effect of the intervention on adverse events is unknown as there was no direct evidence.
Comparison 9: Female migrants' benefit of any health literacy intervention versus male migrants' benefit of any health literacy intervention
The study authors of three intervention studies provided gender‐separate data upon request (Calderón 2014; Soto Mas 2018; Sudore 2018). Only Soto Mas 2018 reported gendered scores for functional health literacy in the published trial report. Nevertheless, the gendered scores for health behaviour were obligingly provided at our request. Table 21 presents the evidence relating to female and male migrants' benefits of any health literacy intervention.
Health literacy
Generic functional health literacy
One RCT with 77 participants in the intervention group that compared a health literacy skills building course to no health literacy intervention indicated uncertainty about whether female compared to male migrants' generic functional health literacy improves more immediately post‐intervention (MD 2.78, 95% CI ‐4.35 to 9.91; very low‐certainty evidence; Analysis 9.1) (Soto Mas 2018). Additional information on the findings related to this study are described in Comparison 3 (see also Table 12).
9.1. Analysis.

Comparison 9: Female migrants' benefit of any health literacy intervention versus male migrants' benefit of any health literacy intervention, Outcome 1: Generic health literacy, TOFHLA (short‐term: immediately post‐intervention)
Disease‐specific health literacy
The results of one RCT with 118 participants in the intervention group that compared audio‐/visual education without personal feedback to written information on the same topic indicated that female migrants' diabetes health literacy may improve slightly more than that of male migrants (MD 5.00, 95% CI 0.62 to 9.38; low‐certainty evidence; Analysis 9.2) (Calderón 2014).
9.2. Analysis.

Comparison 9: Female migrants' benefit of any health literacy intervention versus male migrants' benefit of any health literacy intervention, Outcome 2: Diabetes health literacy, DHLS (short‐term: immediately post‐intervention)
Quality of life
The effect of any health literacy intervention on female compared to male migrants' quality of life is unknown as there was no direct evidence.
Health‐related knowledge
The effect of any health literacy intervention on female compared to male migrants' health‐related knowledge is unknown as there was no direct evidence.
Health outcome
The effect of any health literacy intervention on female compared to male migrants' health outcome is unknown as there was no direct evidence.
Health behaviour
The results of one RCT with 77 participants in the intervention group that compared a health literacy skills building course to no health literacy intervention (standard English as a second language (ESL) course) indicated uncertainty about whether female compared to male migrants' cardiovascular health behaviour improves more immediately post‐intervention (MD 2.07, 95% CI ‐5.04 to 9.18; very low‐certainty evidence; Analysis 9.3) (Soto Mas 2018). Additional information on the findings related to this study is described in Comparison 3 (see also Table 12).
9.3. Analysis.

Comparison 9: Female migrants' benefit of any health literacy intervention versus male migrants' benefit of any health literacy intervention, Outcome 3: Cardiovascular health behaviour (short‐term: immediately post‐intervention)
The results of one other RCT with 219 participants in the intervention group indicated that audio‐/visual education without personal feedback may lead to little or no difference in new documentation of advance care planning between female and male migrants 12 months post‐intervention (RR 1.27, 95% CI 0.90 to 1.79; low‐certainty evidence; Analysis 9.4) (Sudore 2018). Additional information on the findings related to this study is described in Comparison 6 (see also Table 16).
9.4. Analysis.

Comparison 9: Female migrants' benefit of any health literacy intervention versus male migrants' benefit of any health literacy intervention, Outcome 4: Health behaviour: new documentation of advance care planning (long‐term: approx. 12 months post‐intervention)
Self‐efficacy
The effect of any health literacy intervention on female compared to male migrants' self‐efficacy is unknown as there was no direct evidence.
Health service use
The effect of any health literacy intervention on female compared to male migrants' health service use is unknown as there was no direct evidence.
Adverse events
The effect of any health literacy intervention on adverse events for female compared to male migrants is unknown as there was no direct evidence.
Discussion
Summary of main results
The primary objective of this review was to assess the effectiveness of interventions for improving health literacy in migrants. We included 34 studies in this review. Given our broad inclusion criteria regarding the interventions, participants and control groups, we expected heterogeneity between the identified studies. Additionally, there was great variation in the outcome measures and time points of assessment across studies. To address these factors appropriately, we grouped the included studies according to the main intervention components, the complexity of the intervention and the comparator, resulting in eight 'main comparisons'. In addition, we built a ninth comparison to address our second objective, which was to assess whether female and male migrants respond differently to any health literacy intervention.
Comparison 1: Culturally and literacy adapted self‐management programme versus no health literacy intervention
See Table 1.
When compared to no health literacy intervention, self‐management programmes may improve disease‐specific HIV health literacy (understanding of HIV terms and recognition of HIV terms) in the short term. We found low‐certainty evidence that self‐management programmes may slightly improve any health behaviour, but the effects vary in size. Self‐management programmes may lead to little or no difference in health‐related knowledge or subjective health status immediately post‐intervention, when compared to no health literacy intervention. We found moderate‐certainty evidence that self‐management programmes probably improve self‐efficacy slightly immediately post‐intervention.
We do not know whether self‐management programmes have an effect on quality of life, or health service use, as the certainty of the evidence was either very low or we did not identify direct evidence for these outcomes. Adverse events related to the intervention were not reported in any of the included trials in this comparison.
Comparison 2: Culturally and literacy adapted self‐management programme versus written information on the same topic
See Table 7.
When assessed in the short term, self‐management programmes compared to written information on the same topic probably slightly improve health numeracy and probably improve generic print literacy. We found low‐certainty evidence that self‐management programmes may improve any disease‐specific health literacy, when compared to written information on the same topic. The pooled analysis of six studies indicated that self‐management programmes may improve health‐related knowledge immediately post‐intervention. We also found low‐certainty evidence that they may improve any health behaviour immediately post‐intervention, with variable effects. Moderate‐certainty evidence indicated that self‐management programmes compared to written information probably have a short‐term effect on self‐efficacy.
When assessed in the medium term, self‐management programmes may slightly improve high blood pressure health literacy. With regard to the steps of health information processing, we found low‐certainty evidence that self‐management programmes may lead to little or no difference in the appraisal of health information (decisional balance for using mammography or Pap testing) in the medium term. The pooled analysis of two studies indicated that there may be little or no effect on health‐related knowledge when assessed in the medium term. Self‐management programmes may slightly improve some health behaviours, but both the outcome measures and size of effects appeared to be variable. Low‐certainty evidence also indicated that there may be little or no medium‐term effect on depression. Self‐management programmes compared to written information on the same topic may result in little or no effect on high blood pressure self‐efficacy six months post‐intervention.
We do not know if self‐management programmes improve quality of life, depression or health service use immediately post‐intervention as our certainty in the evidence is either very low (quality of life, depression), or we did not find direct evidence for these outcomes (health service use). No study in this comparison reported adverse events (e.g. anxiety). We also do not know whether there are any long‐term effects of self‐management programmes compared to written information due to a lack of evidence.
Comparison 3: Culturally adapted health literacy skills building course versus no or unrelated health literacy intervention
See Table 12.
We found that health literacy skills building courses may improve any generic functional health literacy in the short term (up to one month post‐intervention), when compared to no or an unrelated health literacy intervention. However, health literacy skills building courses may result in little or no difference in disease‐specific health literacy (depression literacy) immediately post‐intervention. We do not know if the intervention improves the intention to change nutritional habits (here referred to as applying health information) as the certainty of the evidence is very low. Health literacy skills building courses may improve health‐related knowledge, but may have little or no effect on any health behaviour immediately post‐intervention.
When assessed in the medium term (six months post‐intervention), they may slightly improve knowledge, and they may improve or reduce health behaviour (cancer screening adherence); the measures and effect sizes appeared to vary considerably.
We are uncertain whether health literacy skills building courses improve quality of life, health outcomes or self‐efficacy, due to a lack of evidence or a very low certainty of evidence. No study in this comparison reported adverse events (e.g. anxiety). We also do not know whether there are any long‐term effects of health literacy courses due to a lack of evidence.
Comparison 4: Culturally and literacy adapted telephone education versus unrelated health literacy intervention
See Table 14.
We included only one study in this comparison. All participants were assessed in the long term (approximately seven months post‐intervention up to two years follow‐up (for health behaviour outcomes)). Culturally and literacy adapted telephone education compared to unrelated health literacy intervention probably has an important long‐term effect on the appraisal of health information by decreasing decisional conflict, but probably results in little or no difference in prostate cancer screening intention or in actual prostate cancer testing (at two‐year follow‐up). The results of one study further suggest that telephone education probably slightly improves health‐related knowledge approximately seven months post‐intervention. Based on the results of this study, telephone education compared to unrelated telephone education probably does not cause harm as little or no long‐term effect on anxiety has been found.
We do not know whether telephone education improves quality of life, health outcomes, self‐efficacy or health service use, as we did not identify direct evidence for these outcomes. We also do not know whether there is any short‐ or medium‐term effect of telephone education on health literacy outcomes due to a lack of evidence.
Comparison 5: Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention
See Table 15.
We found moderate‐certainty evidence that audio‐/visual education without personal feedback compared to no health literacy intervention probably improves depression literacy in the short term. We found low‐certainty evidence indicating that it slightly improves the intention to seek treatment for depression (here referred to as applying health information), health‐related knowledge and self‐efficacy, but there may be little or no effect on any depression immediately in the short term.
We found moderate‐certainty evidence indicating that audio‐/visual education without personal feedback probably has little or no effect on health behaviour (children's up‐to‐date immunisation), but probably improves health service use (by reducing emergency room visits), both assessed immediately and up to three months post‐intervention (short‐ to medium‐term).
We do not know whether audio‐/visual education without personal feedback has any effect on the participants' quality of life, or whether there are any adverse events related to this intervention, as we did not identify direct evidence for these outcomes.
Comparison 6: Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic
See Table 16.
Audio‐/visual education without personal feedback compared to written information on the same topic probably has little or no short‐term effect on diabetes health literacy. However, we found moderate‐certainty evidence indicating that audio‐/visual education without personal feedback compared to written information probably has a short‐term effect on appraising health information (by reducing decisional conflict) and on applying health information (making an informed decision regarding HPV vaccination). Audio‐/visual education may slightly improve health‐related knowledge in the short term, but we do not know whether this also improves at longer time points (six months) as our certainty in the evidence is very low.
We found low‐certainty evidence that audio‐/visual education may result in little or no difference in self‐efficacy, when assessed either in the short term or medium term. When assessed in the medium term (three months post‐intervention), audio‐/visual education may slightly improve competencies (inhaler use technique). We found low‐certainty evidence indicating that it may lead to little or no difference in understanding health information (understanding physician's instruction/pulmonary rehabilitation procedure) in the medium term.
When assessed in the long term, audio‐/visual education without personal feedback compared to written information on the same topic may result in little or no difference in depression or any cancer screening uptake, but moderate‐certainty evidence indicates that it probably improves new documentation of advance care planning in the long term.
We did not identify any direct evidence for quality of life or health service use. Therefore, the effect of the intervention on these outcomes is unknown. We found no evidence that audio‐/visual education causes harm, but the results of one study indicated that there is probably little or no difference in anxiety 12 months post‐intervention.
Comparison 7: Culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback
See Table 18.
We do not know whether narrative educational videos have an effect on either health literacy, quality of life, knowledge, health outcomes, self‐efficacy, health service use or adverse events, as there was either no direct evidence (for the outcomes quality of life, health outcomes, self‐efficacy, health service use and adverse events) or the certainty of the evidence is very low (for the outcomes health literacy, knowledge and health behaviour).
Comparison 8: Culturally and literacy adapted medical instruction versus no health literacy intervention
See Table 20.
We found low‐certainty evidence indicating that culturally and literacy adapted medical instructions compared to no health literacy intervention may improve medication understanding and may lead to little or no difference in medication adherence up to one week post‐intervention.
We do not know whether culturally and literacy adapted medical instructions have an effect on quality of life, health‐related knowledge, health outcomes, health service use or self‐efficacy. We also do not know if there are any adverse events related to the intervention due to a lack of evidence.
Comparison 9: Female migrants' versus male migrants' benefit of any health literacy intervention
See Table 21.
We found low‐certainty evidence indicating that female migrants' diabetes health literacy may improve slightly more than that of male migrants when receiving audio‐/visual education. However, one other study found that female migrants' health behaviour (new documentation of advance care planning) may be little or no different to that of male migrants 12 months post‐intervention, when receiving audio‐/visual education without personal feedback.
We do not know whether female or male migrants benefit differently from any health literacy intervention with regard to generic health literacy, quality of life, health‐related knowledge, health outcomes, individual skills or health service use as there was no direct evidence or the certainty of the evidence is very low (health literacy, health behaviour). In addition, we do not know if there are any adverse events related to the interventions that may affect female migrants more or less than male migrants as none of the studies reported adverse events separately for female or male migrants.
Overview of intervention effects
The following Table 1 provides an overview of the review findings at the outcome level, presenting the results on intervention effects based on high‐, moderate‐ or low‐certainty evidence.
Table 1. Summary of intervention effects
| Outcome category and outcomes | Interventions that have an effect on the outcome (high‐certainty evidence) | Interventions that probably have an effect on the outcome (moderate‐certainty evidence) | Interventions that may have an effect on the outcome (low‐certainty evidence) | Female versus male migrants' benefits from any health literacy intervention |
|
Health literacy 1) Generic health literacy 2) Disease‐specific health literacy 3) Components of health literacy |
— |
(1) Generic health literacy Time point a: short‐term* Comp 2: SMP vs written information
2) Disease‐specific health literacy Comp 5: AVE w/o personal feedback vs no health literacy intervention
Comp 6: AVE w/o personal feedback vs written information
3) Components of health literacy Time point a: short‐term Comp 6: AVE w/o personal feedback vs written information
Time point c: long‐term Comp 4: Telephone education vs unrelated health literacy intervention
|
1) Generic health literacy Time point a: short‐term Comp 1: SMP vs no health literacy intervention
Comp 3: HL‐SBC vs no/unrelated HL‐SBC
2) Disease‐specific health literacy Time point a: short‐term Comp 2: SMP vs written information
Comp 3: HL‐SBC vs no/unrelated HL‐SBC
Time point b: medium‐term Comp 2: SMP vs written information
3) Components of health literacy Time point a: short‐term Comp 8: AMI vs no health literacy intervention
Comp 2: SMP vs written information
Comp 5: AVE w/o personal feedback vs no health literacy intervention
Time point b: medium‐term Comp 6: AVE w/o personal feedback vs written information
|
2) Disease‐specific health literacy Time point a: short‐term Intervention: AVE w/o personal feedback
|
| Quality of life | — | — | — | — |
| Health‐related knowledge | — |
Time point c: long‐term Comp 4: Telephone education vs unrelated health literacy intervention
|
Time point a: short‐term Comp 1: SMP vs no health literacy intervention
Comp 2: SMP vs written information
Comp 5: AVE w/o personal feedback vs no health literacy intervention
Comp 3: HL‐SBC vs no/unrelated HL‐SBC
Comp 6: AVE w/o personal feedback vs written information
Time point b: medium‐term Comp 2: SMP vs written information
Comp 3: HL‐SBC vs no/unrelated HL‐SBC
|
— |
| Any health outcome | — | — |
Time point a: short‐term Comp 1: SMP vs no health literacy intervention
Time point b: medium‐term Comp 2: SMP vs written information
Comp 5: AVE without personal feedback vs no health literacy intervention
Comp 6: AVE without personal feedback vs written information
|
— |
| Any health behaviour | — |
Time point a: short‐term Comp 5: AVE w/o personal feedback vs no health literacy intervention
Time point c: long‐term Comp 4: Telephone education vs unrelated health literacy intervention
Comp 6: AVE w/o personal feedback vs written information
|
Time point a: short‐term Comp 1: SMP vs no health literacy intervention
Comp 2 SMP vs written information
Comp 3: HL‐SBC vs no/unrelated HL‐SBC
Comp 8: AMI vs no health literacy intervention
Time point b: medium‐term Comp 2 SMP vs written information
Comp 3: HL‐SBC vs no/unrelated HL‐SBC
|
Time point c: long‐term Intervention: AVE w/o personal feedback
|
| Self‐efficacy | — |
Time point a: short‐term Comp 1: SMP vs no health literacy intervention
Comp 2: SMP vs written information
|
Time point a: short‐term Comp 5: AVE w/o personal feedback vs no health literacy intervention
Comp 6: AVE w/o personal feedback vs written information
Time point b: medium‐term Comp 2: SMP vs written information
Comp 6: AVE w/o personal feedback vs written information
|
— |
|
Health service use ‐ Child's emergency room visits |
— |
Time point b: medium‐term Comp 5: AVE w/o personal feedback vs no health literacy intervention
|
— | — |
|
Adverse events ‐ Anxiety |
— |
Time point c: long‐term Comp 4: Telephone education vs unrelated health literacy intervention
Comp 6: AVE w/o personal feedback vs written information
|
— | — |
| *Short‐term: immediately up to six weeks after the total intervention programme was completed; medium‐term: up to and including six months after the total intervention programme was completed; long‐term: longer than six months after the total intervention programme was completed. ACP: advance care planning; AMI: adapted medical instruction; AVE: audio‐/visual education; Comp: comparison; HBP: high blood pressure; HL‐SBC: health literacy skills building course; SMP: self‐management programme; w/o: without | ||||
Overall completeness and applicability of evidence
Due to the high degree of heterogeneity between the included studies in terms of the type and delivery of the interventions, the characteristics of the participants, the measured outcomes and the control groups, it was neither possible nor appropriate to pool all results and conduct meta‐analyses with all studies for all outcomes. However, we were able to pool some results and conducted meta‐analyses of studies we judged similar enough to be synthesised together (i.e. when at least two studies in one comparison measured the same outcome comparably). Nevertheless, despite strict grouping, there was considerable statistical heterogeneity in some analyses, reducing the extent to which we can draw firm conclusions from this review.
We investigated heterogeneity through post hoc subgroup analysis by specific design features such as programme length, and through sensitivity analysis excluding studies at high risk of bias. For example, we pooled data from interventions using multimedia formats such as educational DVDs or interactive touchscreen computers with those using print formats such as photonovel only; both were categorised as 'audio‐/visual education without personal feedback'. Although we conducted subgroup analyses by such design features to investigate the reasons for heterogeneity, this should be taken into account when interpreting the results.
In addition, we did not restrict our inclusion criteria to a certain health context and included first‐generation migrants with a range of different conditions, or those being at risk of developing certain conditions (e.g. certain types of cancer). Thus, the statistical heterogeneity may have reflected either differences across the clinically diverse studies and/or the heterogeneity of migrant groups, or variations in the interventions evaluated. Therefore, the pooled effect sizes and confidence intervals should be interpreted as a range across migrant groups and across conditions, which may not be applicable to a specific migrant group or a certain health condition in particular.
We planned to conduct quantitative subgroup analyses by ethnicity, gender and health literacy assessment tool (performance‐based versus perception‐based tool). However, no study made use of a perception‐based tool to measure health literacy. Due to the studies' heterogeneity described above and an insufficient number of studies in any of the meta‐analyses, we were not able to conduct quantitative subgroup analyses for ethnicity or gender either. In addition, many of the included studies only had small samples, and few also contained unclear reports or missing data that we had to impute, impeding the interpretation of the quantitative and qualitative synthesis. Moreover, the described heterogeneity also led us to pooling outcomes that did not assess exactly the same constructs or conditions. For example, the outcome self‐efficacy for managing one's own disease was related to either diabetes, HIV, blood pressure or other conditions. In addition, and in the absence of a standardised measure that would have been applicable to all the studies, we did not restrict our synthesis to validated outcome measures, which may also lower the comparability and generalisability of our results.
Interpretation of the results was affected by heterogeneity in so far as decisions about whether there was an important effect or not were, at least for some outcomes, based on our subjective interpretation of the results. In some cases, we calculated standardised mean differences (SMD) to enable pooling and used rules of thumb for standardised effect measures as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). However, that was not possible for all outcome measures. Particularly when the measure was used by one study only, so that we could not calculate an SMD, or when we could not obtain a 'minimally important difference' for the respective outcome measure from the literature.
The studies included in this review were primarily of short‐ or medium‐term duration; only a few outcome assessments were available at longer time points (i.e. longer than six months after completion of the intervention programme). Thus, for the majority of intervention types included, we do not know whether there are important long‐term effects on health literacy or on health literacy‐related outcomes. In addition, only two trials reported measuring unintended consequences or adverse events. Both audio‐/visual education and telephone education probably have little or no long‐term effect on anxiety. However, we do not know whether there are any adverse events or unintended consequences in the other interventions identified. Many studies included in this review were small and thus have likely been underpowered to detect adverse events. In addition, we found no evidence for an effect of any health literacy intervention on quality of life as we either did not identify direct evidence for this outcome (only three studies measured quality of life) or our certainty in the evidence is very low.
The majority of studies were based on established social‐cognitive theories or models of health behaviour change. None of the included studies were guided by the integrated model of health literacy (Sørensen 2012). Other established health literacy models such as the three‐level health literacy framework proposed by Nutbeam 2000 were also rather neglected. Only Kim 2020 developed a health literacy framework based on the definition of Ratzan 2000. Other studies that explicitly referred to the concept of health literacy primarily referenced empirical research that showed associations between limited health literacy or low literacy and the respective health problem under study without applying a certain health literacy framework or model for developing, implementing or evaluating the intervention.
We used the integrated model by Sørensen 2012 to guide the whole review process including data extraction, grouping of studies, data synthesis and interpretation of the results. To our knowledge, this is the first systematic review that uses such a comprehensive approach to synthesise evidence related to health literacy in the context of migration. Grouping health literacy intervention studies according to a set of cautiously developed criteria might help decision makers, future reviewers and other researchers to derive meaning from health literacy interventions. However, this review shows that applying the integrated model of health literacy and taking into account its components (i.e. knowledge, motivation, competencies and the four steps of health information processing) as a framework for assessing the effectiveness of health literacy interventions is, at least to date, limited.
We assume the following reasons for this finding: the interventions identified were primarily conducted in North America. None of the studies were conducted in Europe, where the integrated model of health literacy has its origin and is widely known. In addition, the more comprehensive approach of taking into account not only aspects of functional literacy or numeracy in the context of health, but also the procedural characteristics of health information processing, is quite young. Thus, the majority of the studies addressed literacy aspects and aimed to improve understanding or model health behaviour through mitigating the effects of low literacy and low language proficiency in the respective health context. However, implicitly, the studies' aims were often to improve either the accessing, understanding, appraising and/or applying of health information, even though the investigators did not use the concept of 'health literacy' to describe these aims. Hence, all studies implicitly (e.g. through methods used or theories applied) or explicitly (e.g. by mentioning this aspect in one of the published reports) addressed at least components of health literacy in the design or evaluation of the intervention.
Furthermore, it might not have been expedient on our side to subordinate the outcomes to the components of health literacy as this approach leaves space for interpretation. However, all decisions regarding the categorisation and priorisation of outcomes were made by at least two review authors. Furthermore, again, our aim was not to assess the effects of one specific intervention on migrants' health literacy assessed with established, validated tools only. We rather aimed to draw a comprehensive picture of those health literacy interventions available for migrants and assess at least components of the concept of health literacy (e.g. the four steps of health information processing). Therefore, it was not surprising that only 12 out of the 34 included studies reported an outcome measure for either generic or disease‐specific health literacy to assess the intervention effectiveness.
The vast majority of studies reported a measure for health‐related knowledge that was based on the intervention's content (27 studies). Empirical research strongly indicates that higher levels of (functional) health literacy are associated with higher levels of health‐related knowledge (Berkman 2011; Osborn 2011; Paasche‐Orlow 2007). In line with that, we considered knowledge to be one of the major components of health literacy. We found that health literacy interventions may have a short‐term effect on health‐related knowledge, ranging from less important to important effects. Some findings, however, seemed, at first sight, paradoxical. For example, we found that self‐management programmes may lead to little or no difference in knowledge, when compared to no health literacy intervention (comparison 1), but they may have an important short‐term effect on knowledge, when compared to written information on the same topic (comparison 2). This may be for the reason that there were only two studies included in the narrative synthesis of comparison 1, with one very small study (N = 69) reporting inconclusive results for knowledge and the other study (N = 252) reporting a mean difference of 5.6% in favour of the intervention. For both comparisons, however, our certainty in the evidence was low (i.e. the true effect may be substantially different from the estimate of the effect).
None of the included studies directly assessed the effects of health literacy interventions on motivation, but the majority of intervention studies made use of methods that targeted improved motivation and/or the interventions were guided by established behaviour change theories. Two studies reported on outcomes related to motivation. However, none of the results were reported in this review, as the applied scales also address theoretical constructs other than motivation (e.g. subjective knowledge or self‐efficacy) and no subscale data were reported.
Outcome measures for competencies (e.g. reading and writing abilities or skills acquisition) were assessed in two studies, although it should be noted that all studies that reported an outcome measure for health literacy made use of established performance‐based assessment tools such as REALM (Davis 1991) or TOFHLA (Parker 1995). These measures assess either reading and writing abilities (REALM), or understanding of text phrases and numeracy skills (TOFHLA) in the context of health. The disease‐specific health literacy measures used were either also REALM‐ or TOFHLA‐based, or they assessed disease‐specific knowledge and/or beliefs (e.g. depression literacy assessed with the D‐Lit by Griffiths 2004 or diabetes health literacy assessed with the DHLS by Calderón 2014). None of the studies used a self‐assessment health literacy tool measuring self‐perceived difficulties in accessing, understanding, appraising or applying health information in different health domains.
Regarding the four steps of health information processing, accessing health information was the only step not measured by any study to assess the intervention effectiveness. However, whether participants accessed health information was often implicitly addressed through outcome measures related to health behaviour or health service use (e.g. the use of preventive measures or rates of emergency department encounters). Five studies measured understanding of health information, which is closely related to functional health literacy, or how the construct is often assessed (see Description of the condition).
Only three studies assessed the appraisal of health information (i.e. the ability to filter, judge and evaluate the information received). This is noticeable, as in our understanding of health literacy, the ability to evaluate the information found not only in terms of its quality and trustworthiness, but also in light of one's own value system, is crucial for autonomous decision‐making. Particularly regarding difficult health decisions (e.g. the use of certain, more or less invasive, screening measures or treatment options), it is important to recognise whether information is of high quality on the one hand and to thoughtfully outweigh the pros and cons (e.g. of a health service) on the other hand. According to European population studies, both migrants (Berens 2022a) and the majority population (HLS19 Consortium 2021) reported the greatest difficulties in appraising health information. In particular, judging different treatment options or judging the reliability of online information were perceived as challenging. The evidence we found regarding an effect of health literacy interventions on this processing step was either moderate‐ (two studies) or low‐certainty (one study), but nevertheless based on only three studies. None of these studies measured the ability to judge whether an informational source or particular health information is trustworthy or reliable. However, all three studies measured decisional processes such as weighing pros and cons regarding cancer screening measures, indicating that health literacy interventions can have a positive impact on migrants' ability to make informed decisions that are congruent with one's value system.
Six studies measured behaviour intent, which is related to applying health information as it reflects a decision made. However, most studies measured health behaviour, which is widely regarded as an outcome of the health literacy process, as fully informed, autonomous decisions that are based on high‐quality information may ultimately turn information into value congruent action.
We assessed the characteristics of study populations using the PROGRESS‐Plus framework, thereby acknowledging equity as an important determinant of health. All studies were conducted in high‐income countries, predominantly in North American, urban areas. Accordingly, we found a predominance of migrants who were born in Central and South America or East and South Asia in the studies, aged between 28.7 years to 70.9 years, and a 75% proportion of females. The average time since immigration ranged from less than one year up to 62 years, many of whom immigrated at least five years ago. All studies reported at least some information about the participants' education, whereas most studies included so‐called "disadvantaged populations" of low (health) literacy and/or low socioeconomic status. The least described PROGRESS‐Plus domains were religion, sexual orientation, disability and migrant status. However, three studies provided concrete information about the participant's religion, one study explored how participants' religious beliefs affected decision‐making and four studies (including Korean Americans) recruited participants from religious communities. One study included Afghan Muslim women and described the intervention as being "faith‐based". In total, 19 (56%) studies reported baseline data on health literacy using a validated assessment tool. Twelve studies additionally assessed health literacy (named as such) as an outcome. Most studies included primarily, or at least to a considerable part, participants with limited generic (functional) health literacy or disease‐specific health literacy.
As this review aimed to assess the effectiveness of interventions for improving health literacy in migrants, and to assess whether female or male migrants benefit differently from these interventions, we included only studies that, at least implicitly, took into account health equity. Interestingly, a considerable proportion of the included studies neither defined health literacy or even literacy in the context of health, nor assessed health literacy (named as such). However, all studies shared the aim of either improving health literacy, or mitigating the effects of low literacy in migrants who were either low literate (partly even in their own language) or did not speak the host country's language well. In addition, all interventions were culturally tailored and linguistically or literacy adapted.
Migrants who are more comfortable and fluent in their native language may have better comprehension of health‐related information when it is presented in their mother tongue. By using migrants' native language, health literacy interventions may better capture the nuances of the migrants' culture, beliefs and health practices and transfer these idiosyncrasies into the respective cultural context of the host country. This may be particularly important for the successful implementation of health literacy interventions designed for migrants, as health literacy is not only about understanding health information but also about appraising it against one’s set of values and applying it in the appropriate cultural context (Sørensen 2012). Thus, adapting a health literacy intervention culturally and linguistically may lead to an improved intervention experience, increased learning outcomes and more accurate assessments of the participants' health literacy levels. However, this review could not show which intervention components exactly increase the effectiveness of health literacy interventions, which in particular was due to the heterogeneity of the included studies. It is important to note, however, that a variety of intervention formats, besides classic written or oral approaches, have the potential to improve information transfer in migrants (see Effects of interventions). For example, short educational videos, group education or interactive online programmes may help to increase health literacy by considering the needs of people with low literacy skills, while carefully integrating cultural aspects identified as barriers for accessing, understanding, appraising or applying information on a certain health topic. A thorough investigation of which intervention components are most effective and appropriate for which migrant community may enhance the significance of future reviews and, thus, the design and implementation of future health literacy interventions.
The research strand on mental health literacy emerged from health literacy research, but has largely developed separately from it. What they have in common is that dealing successfully with one's own illness, navigating the health system and interacting with health professionals are essential concerns (Baumeister 2021b). Audio‐/visual education such as web‐based interventions including (inter‐)active elements have shown to be a promising approach with regard to increasing mental health literacy and awareness for mental health problems such as depression (Brijnath 2016). Research has also shown that there are considerable cultural differences in beliefs about mental illness, particularly in relation to help‐seeking beliefs (Altweck 2015; Jorm 2000; Jorm 2005). In addition, some migrant groups are particularly vulnerable to psychological distress compared to the majority population (Brijnath 2020), and can be confronted with additional stressors such as fear of deportation and discriminatory events (Valentín‐Cortés 2020). In this review, only four studies aimed to improve mental health literacy (or knowledge about certain mental disorders, e.g. depression) in migrants, revealing that there is currently a substantial lack of intervention studies in this context and a need for developing and evaluating targeted, culture‐sensitive interventions that aim to improve mental health literacy among migrants.
We were able to obtain gendered scores related to the intervention effects of only three studies and there was a disproportionate share of studies that included only, or predominantly, women. Twelve studies included either female (10 studies) or male migrants (two studies) only, another five studies included predominantly women (> 80%) and two studies included predominantly men. We contacted all authors with mixed‐gender study populations asking for subgroup data, but received information from only three authors (Calderón 2014; Soto Mas 2018; Sudore 2018). As we intended to assess whether female or male migrants respond differently to either of the interventions, we included only those studies that reported gender‐separate scores for the participants randomised to the intervention group in our gender‐focused analyses. Thus, we ended up with results that were all based on single studies with very small sample sizes, impeding the degree to which we can draw conclusions from the evidence found for any gender differences.
We found low‐certainty evidence from one study indicating that female migrants may benefit more from audio‐/visual education without personal feedback with regard to diabetes‐specific health literacy, when receiving audio‐/visual education without personal feedback. One other study, evaluating a similar intervention type, found that there may be little or no difference in health behaviour between female and male migrants when receiving audio‐/visual education. For the other predefined outcome categories, however, we either did not identify evidence assessing gender differences or our certainty in the evidence is very low. Thus, we cannot certainly tell whether female or male migrants benefit differently from the identified interventions or whether the needs regarding future health literacy interventions differ substantially between the genders.
Quality of the evidence
We conducted a GRADE assessment for each outcome included in this review. The certainty of the evidence for outcomes was predominantly rated as being low or very low, but we also found moderate‐certainty evidence for some outcomes in different comparisons (e.g. for disease‐specific health literacy or knowledge; see Effects of interventions). Across all comparisons, the most common reasons for downgrading were risk of bias for random sequence generation and/or allocation concealment or blinding, or the imprecision of effect estimates. These were often imprecise due to small sample sizes or wide confidence intervals with values indicating both an improvement or a worsening in the respective outcome. In addition, some studies did not report the results in such a way that they could be extracted for meta‐analysis. For one cluster‐RCT (Elder 1998), we were not able to re‐calculate the data by using the appropriate unit of analysis. For two cluster‐RCTs (Bloom 2014; Tong 2017), both of which reported having used GEE models to account for clustering, we were not sure if the appropriate unit of analysis was used as the data were reported as proportions only (e.g. proportion of participants who correctly answered questions regarding colorectal cancer).
Regarding the blinding of outcome assessors, most studies were rated at high risk of bias. This was due to the fact that we judged non‐blinding to influence particularly the results of subjectively measured outcomes (e.g. depression, self‐efficacy), meaning that participants also acted as their own outcome assessors. The nature of most studies, however, made blinding unfeasible, so we did not judge this to affect objectively measured outcomes such as knowledge. In addition, for 13 studies, we had insufficient information to permit judgement about low or high risk regarding random sequence generation and/or allocation concealment.
Potential biases in the review process
Health literacy is a multidimensional construct (Figure 1), which is defined and measured inconsistently (Mackert 2015), and so is migration. Thus, we used a correspondingly broad search strategy. However, although our searches were comprehensive, it is possible that not all potentially relevant studies were identified and screened for this review (this may be especially the case because health literacy is so variably described and the research is cross‐disciplinary). We included first‐generation migrants aged 18 years or over and did not restrict our search by health context, gender or participants' ethnicity. Nevertheless, it is possible that we have excluded studies in the abstract screening or at full‐text stage that would have actually fitted into this review's objective. For example, to limit the amount of (heterogeneous) studies in this review, we decided during the screening process that either 'health literacy' or 'literacy' had to be mentioned in the published trial report. In addition, the intention to consider at least literacy‐related aspects such as the use of literacy‐adapted materials in the development, design and delivery of the intervention had to be evident. These studies did not have to describe themselves as 'health literacy intervention', but at least 'literacy' had to be mentioned as a concept and the outcomes had to be assignable to the integrated model of health literacy as an umbrella framework. This approach has its limitations in so far as it is possible that our understanding of health literacy influenced our view of potentially eligible studies. We might have excluded studies at full‐text stage that actually evaluated interventions quite similar to those included in this review, but that missed explicitly stating that aspects of 'health literacy' or even 'literacy' were considered in the study. Thus, there may be other health literacy‐relevant studies (according to our understanding based on Sørensen 2012), which could have contributed to the evidence base in this review.
For the reasons described above (see Summary of main results; Overall completeness and applicability of evidence), we anticipated the inclusion of a variety of studies that address certain aspects of health literacy in different settings, which have to be grouped according to their study features, thereby accepting at least some loss of information. We made efforts to group studies that fit together best according to the main intervention components, the intervention complexity and the comparators. However, this approach is limited as judgements of similarity between interventions and comparators depended on several aspects. Firstly, our subjective interpretation of what the concept of health literacy constitutes. Secondly, our judgement about to what extent certain intervention features (e.g. intense group education with active components or passive education through audio‐/visual formats) affect the results of our predefined outcome categories. Thirdly, it depended on the quality of information that was reported in each trial, considering that some interventions were poorly described. In addition, the assignment of the interventions to one of the eight main comparisons was not always a clear‐cut decision. For example, two interventions did not fit perfectly into the category 'culturally and literacy adapted self‐management programme' as they had less intense phases of group education and/or less intense follow‐up phases. In addition, both interventions were developed for individuals at risk of developing a certain disease, but not for individuals already affected. However, both programmes included self‐management components such as breast self‐examination (Han 2017) or practising good oral hygiene (Kaur 2019). These were compared to written information on the same topic.
Furthermore, we took these specific design features into account by conducting post hoc subgroup analyses for the length of the programme. We differentiated between studies that evaluated a less intense intervention programme with a shorter follow‐up phase and studies that evaluated longer programmes. Thus, our grouping procedure may be somewhat biased. In addition, the interpretation of results could have been facilitated by combining control groups (e.g. written information and no health literacy intervention). In this way, more studies would have contributed to the evidence synthesis in each comparison. Thus, more general conclusions about whether a certain type of health literacy intervention (e.g. self‐management programme) is effective when compared to a control group receiving no or minimal (written) information could have been made. However, again, we wanted to assess whether the processing of the respective health information delivered can be facilitated through the interventions identified. Thus, we think it is important to distinguish between control groups receiving information on a different health topic (than that of the intervention) or those receiving information on the same health topic, but to a minimal extent.
Trials with positive findings are more likely to be published, which might have influenced the selection of included studies in this review. In addition, the small number of studies for most outcomes did not allow for a quantitative analysis of publication bias and six out of the 34 studies were at unclear or high risk of selective outcome reporting, indicating that there may have been a bias arising from a failure to report all negative findings. However, efforts were made to overcome a potential publication bias through searching clinical trial registries for prospectively registered trials.
Agreements and disagreements with other studies or reviews
We found a prior review evaluating the effectiveness of health literacy interventions in immigrants, focusing on the role of nurses in the development and implementation of these interventions (Fernández‐Gutiérrez 2018). The review included nine studies, two of which we also included in this review (Soto Mas 2018; van Servellen 2005), and found that the interventions were effective in improving functional health literacy and knowledge. However, only two studies were RCTs, the studies were not grouped according to intervention components and comparators, and no meta‐analysis, only a narrative synthesis, was conducted. Thus, the comparability of results is limited.
We found one other review that aimed to evaluate the characteristics and the effectiveness of health literacy curricula incorporated in English as second language (ESL) courses (Chen 2015). The review concluded that these curricula are effective in terms of improving (functional) health literacy and knowledge. Three out of seven curricula evaluated in the review were also included in this current review, referring to these studies as 'health literacy skills building courses' (see Table 12). The findings do not differ considerably from ours, although we described our findings with more uncertainty. Chen 2015, however, did not conduct a systematic risk of bias assessment and four out of the seven curricula included in the review were evaluated using other than randomised controlled designs in the primary studies. We found low‐certainty evidence indicating that health literacy skills building courses may improve generic (functional) health literacy and also knowledge slightly.
Stormacq 2020 assessed the effectiveness of health literacy interventions on health‐related outcomes in socially disadvantaged adults living in a community, thereby including migrants in at least some studies. In this review, any health literacy interventions were compared to 1) standard care, no intervention or delayed intervention, or 2) minimal/alternative interventions. Three of the included studies were also included in this review (Kim 2009; Koniak‐Griffin 2015; Mohan 2014). Stormacq 2020 found that 13 out of 22 studies were effective in improving a variety of health‐related outcomes (mainly clinical outcomes), in preventive health practices and behaviours, and in health‐promoting behaviours. In addition, the authors concluded that multi‐faceted interventions appeared to be superior to single‐modality interventions and identified some intervention components including cultural appropriateness, tailoring, skills building, goal setting and active discussions that contributed to the interventions' effectiveness. However, the authors' GRADE assessment judged the effects of health literacy interventions on all but one outcome, namely quality of life (low‐certainty), to be of very low certainty. We found only very low‐certainty evidence for an effect on quality of life that stemmed from three studies.
The review Fox 2022 aimed to characterise the research evaluating the effectiveness of health literacy interventions for refugees and migrants in high‐income countries without systematically synthesising the results of each study in terms of health literacy‐related outcomes. The review included 23 studies, 10 of which were also included in this review. The authors concluded that there was high heterogeneity between the intervention studies, the outcomes, as well as the outcome measures, impeding the comparison of the intervention effectiveness. These characteristics are similar to the findings of the current review.
We found no other systematic review that assessed whether women and men benefit differently from health literacy interventions, whether they are migrants or not. This is unsurprising considering that gender, or even sex differences, are highly neglected aspects in primary studies on health literacy of migrants. There is only one other systematic review on gender differences in the health literacy of migrants, which was also conducted by our review group (Chakraverty 2022). The results indicate that there are only marginal differences between female and male migrants' health literacy, when assessed with validated assessment tools. In addition, we found that studies on male migrants' health literacy in particular are sparse. However, as health literacy is a relational construct, which is dynamic and context‐sensitive, we think that there are gender‐specific aspects of health literacy that should be taken into account when designing, implementing and evaluating health literacy interventions.
In preparation for this review, and as part of an overarching project on gender‐specific aspects of health literacy in individuals with a migrant background, we conducted focus group discussions (FGDs) with healthcare professionals in Germany. Of these, more than 50% were either first‐ or second‐generation migrants themselves. The findings from the FGDs were analysed with a focus on organisational health literacy in the context of transcultural treatment settings (Baumeister 2021a), and in terms of the healthcare professionals' views on how gender as a personal determinant of health literacy may affect the interaction with their migrant patients (Chakraverty 2020). We found that there are certain gender‐specific aspects of health literacy that affect how female and male migrants access, understand, appraise and apply health information. For example, we found that cultural and gender norms played a significant role for migrant women of Turkish or Arab origin with regard to accessing and understanding health information. This was expressed, for example, in a preference for access to female doctors (e.g. for personal reasons such as feelings of shame or humiliation when having to undress for a physical examination). Other findings were related to gender‐specific aspects of language barriers, as some healthcare professionals stated that immigrant women of Turkish origin had limited language proficiency (i.e. German), more so than their male counterparts (Chakraverty 2020). Furthermore, gender may also be relevant in the realm of mental health literacy, as the participants of the FGDs reported a higher awareness of mental health issues in female migrants as compared to male migrants. The women's growing acceptance of psychotherapy was described as slowly spreading to the migrant men as well.
It was not always clear, however, whether issues of understanding each other were foremost or solely grounded in a lack of language proficiency or due to low literacy skills. In addition, an omnipresent systemic lack of time and economic pressure was described by many healthcare professionals as one of the major barriers to an effective and satisfactory flow of information in transcultural treatment situations (Baumeister 2021a). In particular, time restrictions were perceived as hindering factors in adequately addressing female and male migrants' health literacy needs, including the healthcare professionals' response to potential gender‐related issues. There are few, but some, other studies indicating that traditional gender roles, cultural norms and religious aspects do play a role in how female and male migrants access and process health information (e.g. Cherrington 2011; Shirazi 2013; Shirazi 2015). All these studies use qualitative study methods, indicating that exploring gender differences in the health literacy of migrants is, at least to date, more promising with the means of qualitative participatory research, than with quantitative measures only.
To sum up, the circumstance of our only finding very marginal differences in female and male migrants' benefit from health literacy interventions does not mean that there are not gender‐specific aspects that need to be taken into account in the design, delivery and evaluation of health literacy interventions.
Authors' conclusions
Implications for practice.
The degree of heterogeneity between the included studies was considerable and in some comparisons only a limited number of studies, partly with small sample sizes, were included. Therefore, the pooled effect sizes and confidence intervals should be interpreted as a range across migrant groups and across conditions, which may not be applicable to a specific migrant group or a certain health condition in particular.
We found moderate‐ to low‐certainty evidence that some health literacy interventions can have small to moderate positive effects on health literacy. We also found moderate‐certainty evidence for a short‐term effect of self‐management programmes on self‐efficacy and moderate‐ to low‐certainty evidence for a moderate (short‐term) to small (medium‐term) effect of self‐management programmes and audio‐/visual education without personal feedback on knowledge. We also found a small long‐term effect of telephone education on knowledge (moderate‐certainty). Results regarding the effects of health literacy interventions on health behaviour are mixed, as the measures and the effect sizes appear to vary considerably. Audio‐/visual education without personal feedback probably has a positive effect on health service use but, nevertheless, the evidence stemmed from only one study. We do not know whether any health literacy intervention improves health‐related quality of life in migrants, as we only identified very low‐certainty evidence, or the outcome was not directly measured.
We found no evidence that health literacy interventions cause harm, but it is important to note that only two studies reported on adverse events such as anxiety. Both studies indicated that there are probably few or no negative long‐term effects of audio‐/visual or telephone‐based education on anxiety.
We found only three studies reporting gender differences. Low‐certainty evidence indicated that female migrants' diabetes health literacy may improve slightly more than that of male migrants when receiving audio‐/visual education (AVE) without personal feedback, but there may be little or no difference between genders in health behaviour with AVE. For other intervention types and outcomes, the certainty of the evidence was either very low or no evidence was found. Thus, we cannot tell with any certainty whether the needs regarding future health literacy interventions differ substantially between female and male migrants.
Implications for research.
There is a need for more high‐quality studies, and adequately powered randomised controlled trials (RCTs) that explicitly aim to improve health literacy in migrants. There is a particular need for high‐quality, long‐term studies that measure comprehensive health literacy, for example, but not exclusively, based on the integrated model of health literacy (Sørensen 2012). This review shows that most intervention studies conducted in this area aimed to improve individuals’ ability to function in the healthcare environment, mostly measuring functional health literacy (i.e. reading and writing abilities in the medical context) and neglecting the procedural characteristics of the four health information processing steps. Also, most studies were conducted in North America or other high‐income countries, indicating a need to conduct studies worldwide, representing various countries and healthcare systems. In addition, comprehensive evaluations of health literacy interventions using robust and well‐validated tools will improve this field.
There is a lack of studies that examine whether female and male migrants respond differently to health literacy interventions. In addition, there is a lack of intervention studies in this field that include male migrants only. In order to assess which components of health literacy should be addressed in future interventions, and to better understand which gender aspects should be considered in the development, implementation and evaluation of health literacy interventions, it is essential to take into account the perspectives and needs of female and male migrants, at best with the use of community‐based participatory research methods. Future research should also provide thorough theoretical foundations for examining and improving health literacy in female and male migrants. This is necessary to explore the influence of migration, gender and its interactions with other factors such as education, social status and age in relation to health literacy, so that future interventions can consider aspects of health‐related equity that are important for health information processing and, thus, for autonomous decisions regarding one's own health and the health of others.
History
Protocol first published: Issue 4, 2019
Notes
This review is based on guidance provided by Cochrane Consumers and Communication (CCCG 2016).
This review was developed in parallel with the linked Cochrane qualitative evidence synthesis (QES) (Aldin 2019), through continuous exchange between Annika Baumeister (first author of this review) and Angela Aldin (first author of the linked QES).
Acknowledgements
We thank the editors and staff of Cochrane Consumers and Communication, particularly the Managing Editor Louisa Walsh, who guided us through the submission process. We deeply thank the Co‐ordinating Editor, Rebecca Ryan, for her detailed feedback during the whole peer review process and her very helpful input on methodological issues regarding this review in general, and the summary of findings tables in particular. We also thank Anne Parkhill, Information Specialist of Cochrane Consumers and Communication, for reviewing the search methods.
We thank Julia Beideck for her support in data extraction. We thank Tina Jakob, Ümran Sema Seven and Görkem Anapa for contributing methodological and content expertise as co‐authors on the protocol for this review.
We also thank Dr. Pinika Patel, Faculty of Medicine and Health, The University of Sydney, as well as Gauri Kapoor who peer reviewed this review and provided us with helpful thoughts and suggestions.
Finally, we thank Jenny Bellorini, Cochrane Central Production Service, for copy editing this review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (via Cochrane Library)
ID Search
#1 MeSH descriptor: [Multilingualism] explode all trees
#2 multilingualism*:ti,ab,kw
#3 "as a second language":ti,ab,kw
#4 bilingual*:ti,ab,kw
#5 (second language):ti,ab,kw
#6 (foreign language):ti,ab,kw
#7 (proficiency and language):ti,ab,kw
#8 MeSH descriptor: [Communication Barriers] explode all trees
#9 (barrier near/7 language):ti,ab,kw
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 MeSH descriptor: [Transients and Migrants] explode all trees
#12 migrant*:ti,ab,kw
#13 (migration* near/3 (background* or human*)):ti,ab,kw
#14 MeSH descriptor: [Emigrants and Immigrants] explode all trees
#15 MeSH descriptor: [Undocumented Immigrants] explode all trees
#16 MeSH descriptor: [Emigration and Immigration] explode all trees
#17 (immigrant* or immigrat*):ti,ab,kw
#18 (emigrant* or emigrat*):ti,ab,kw
#19 (minorit* near/3 (population* or group*)):ti,ab,kw
#20 (ethnic* near/3 (population* or group* or patient* or background* or specific* or minorit* or identit*)):ti,ab,kw
#21 (displaced and (people or person*)):ti,ab,kw
#22 MeSH descriptor: [Vulnerable Populations] explode all trees
#23 MeSH descriptor: [Refugees] explode all trees
#24 (foreigner* or asylum* or refugee* or undocumented or non‐native or nonnative or foreign‐born or foreignborn):ti,ab,kw
#25 (cultur* near/5 (differences* or cross* or background*)):ti,ab,kw
#26 (linguisticall* near/5 (differences* or cross* or background*)):ti,ab,kw
#27 (border* and crossing):ti,ab,kw
#28 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 MeSH descriptor: [Access to Information] explode all trees
#30 ((access or gain access or obtain or seek out or find or indentify) near/5 (information* or health*)):ti,ab,kw
#31 MeSH descriptor: [Comprehension] explode all trees
#32 (understand or comprehend or comprehension):ti,ab,kw
#33 (appraise or evaluate or process or interpret or assess):ti,ab,kw
#34 "assessment of information":ti,ab,kw
#35 (apply or decide):ti,ab,kw
#36 (use* near/3 (information* or health)):ti,ab,kw
#37 MeSH descriptor: [Decision Making] explode all trees
#38 ((make or making or made or take) near/4 decision*):ti,ab,kw
#39 (acting or act or action):ti,ab,kw
#40 judge*:ti,ab,kw
#41 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40
#42 MeSH descriptor: [Consumer Health Information] explode all trees
#43 MeSH descriptor: [Information Literacy] explode all trees
#44 MeSH descriptor: [Health Literacy] explode all trees
#45 (information* near/3 health*):ti,ab,kw
#46 (health* near/3 (literac* or servic* or decision* or concept* or competenc* or system* or knowledg* or status or level* or needs or insurance or status or behaviour*)):ti,ab,kw
#47 #42 or #43 or #44 or #45 or #46
#48 MeSH descriptor: [Health Education] explode all trees
#49 MeSH descriptor: [Educational Status] explode all trees
#50 (health* near/3 education*):ti,ab,kw
#51 MeSH descriptor: [Health Services Accessibility] explode all trees
#52 #48 or #49 or #50 or #51
#53 #41 and (#47 or #52)
#54 health litera*:ti,ab,kw
#55 medical literacy:ti,ab,kw
#56 (health and literacy):ti
#57 (functional and health and literacy):ti,ab,kw
#58 low‐litera*:ti,ab,kw
#59 (litera* or illitera*):ti,ab,kw
#60 (read or comprehen*):ti,ab,kw
#61 MeSH descriptor: [Reading] explode all trees
#62 MeSH descriptor: [Comprehension] explode all trees
#63 MeSH descriptor: [Health Promotion] explode all trees
#64 MeSH descriptor: [Health Education] explode all trees
#65 MeSH descriptor: [Patient Education as Topic] explode all trees
#66 MeSH descriptor: [Communication Barriers] explode all trees
#67 MeSH descriptor: [Communication] explode all trees
#68 MeSH descriptor: [Attitude to Health] explode all trees
#69 MeSH descriptor: [Comprehension] explode all trees
#70 MeSH descriptor: [Educational Status] explode all trees
#71 #69 and #70
#72 (family and literacy):ti,ab,kw
#73 drug labeling:ti,ab,kw
#74 MeSH descriptor: [Drug Prescriptions] explode all trees
#75 comprehension:ti,ab,kw
#76 ((cancer or diabetes or genetics) and (literacy or comprehension))
#77 (adult and (educational status or (educational and status) or literacy))
#78 (limited and (educational status or (educational and status) or literacy))
#79 (patient* and (educational status or (educational and status) or literacy))
#80 (patient* and (comprehension or understanding))
#81 #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62
#82 #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81
#83 #81 and #82
MEDLINE (via Ovid)
# Searches
1 "Transients and Migrants"/
2 migrant*.tw,kf,ot.
3 (migration* adj3 (background* or human*)).tw,kf,ot.
4 exp "Emigrants and Immigrants"/
5 Undocumented immigrants/
6 "Emigration and Immigration"/
7 (immigrant* or immigrat*).tw,kf,ot.
8 (emigrant* or emigrat*).tw,kf,ot.
9 (minorit* adj3 (population* or group*)).tw,kf,ot.
10 (ethnic* adj3 (population* or group* or patient* or background* or specific* or minorit* or identit*)).tw,kf,ot.
11 (displaced and (people or person$1)).tw.
12 Vulnerable populations/
13 Refugees/
14 (foreigner* or asylum* or refugee* or undocumented or non‐native or nonnative or foreign‐born or foreignborn).tw,kf,ot.
15 (cultur* adj5 (differences* or cross* or background*)).tw,kf,ot.
16 (border* and crossing).tw.
17 ((culturall* or linguisticall*) adj3 (diverse* or patient* or parent* or communit* or background* or student* or wom?n or famil*)).tw,kf,ot.
18 or/1‐17
19 multilingualism/
20 multilingualism*.tw,kf,ot.
21 "as a second language".tw,kf,ot.
22 bilingual.tw,kf,ot.
23 second language.tw.
24 foreign language.tw.
25 (proficiency and language).tw.
26 communication barriers/
27 (barrier adj3 language).tw,kf,ot.
28 or/19‐27
29 18 or 28
30 Access to Information/
31 ((access or gain access or obtain or seek out or find or identify) adj5 (information* or health*)).tw.
32 Comprehension/
33 (understand or comprehend or comprehension).tw.
34 (appraise or evaluate or process or interpret or assess).tw.
35 assessment of information.tw.
36 (apply or decide).tw.
37 (use* adj3 (information* or health)).tw.
38 (capacit* adj4 health).tw.
39 accept*.tw,kf,ot.
40 Decision Making/
41 ((make or making or made or take) adj4 decision*).tw.
42 ("behavior change" or "behaviour change").tw,kf,ot.
43 (acting or act or action).tw.
44 judge*.tw.
45 or/30‐44
46 exp Consumer Health Information/ or Information literacy/
47 Health Literacy/
48 (information* adj3 health*).tw.
49 (health* adj3 (literac* or servic* or decision* or concept* or competenc* or system* or knowledg* or status or level* or needs or insurance or status or behaviour*)).tw.
50 or/46‐49
51 Health Education/ or Educational Status/
52 (health* adj3 education*).tw.
53 Health Services Accessibility/sn [Statistics & Numerical Data]
54 or/51‐53
55 45 and (50 or 54)
56 health litera$2.af.
57 medical literacy.af.
58 (health and literacy).ti.
59 (functional and health and literacy).tw.
60 low‐litera$2.ti.
61 litera$2.ti.
62 illitera$2.ti.
63 reading/ or comprehension/
64 (read* or comprehen*).tw,kf.
65 health promotion/
66 health education/
67 patient education/
68 communication barriers/
69 communication/
70 health knowledge,attitudes,practice/
71 attitude to health/
72 comprehension/ and *educational status/
73 (family and literacy).ti.
74 (drug labeling.af. or Drug Prescriptions/) and comprehension.af.
75 ((cancer or diabetes or genetics) and (literacy or comprehension)).ti.
76 (adult and (educational status or (educational and status) or literacy)).af.
77 (limited and (educational status or (educational and status) or literacy)).af.
78 (patient$1 and (educational status or (educational and status) or literacy)).af.
79 (patient$1 and (comprehension or understanding)).ti.
80 or/56‐64
81 or/65‐79
82 80 and 81
83 randomized controlled trial.pt.
84 controlled clinical trial.pt.
85 randomi?ed.ab.
86 placebo.ab.
87 drug therapy.fs.
88 randomly.ab.
89 trial.ab.
90 groups.ab.
91 or/83‐90
92 exp animals/ not humans/
93 91 not 92
94 29 and (55 or 82) and 93
Embase (via Ovid) # Searches
1 exp migrant/
2 migrant*.tw,kw.
3 (migration* adj3 (background* or human*)).tw,kw.
4 (emigrant* or immigrant*).tw,kw.
5 (undocumented* adj3 immigrant*).tw,kw.
6 (ethnic* adj3 (population* or group* or patient* or background* or specific* or minorit* or identit*)).tw,kw.
7 (displaced and (people or person$1)).tw.
8 (low* adj3 income*).ti,ab.
9 (minorit* adj3 (population* or group*)).tw,kw.
10 exp refugee/
11 Vulnerable population/
12 (foreigner* or asylum* or refugee* or undocumented or non‐native or nonnative or foreign‐born or foreignborn).tw,kw.
13 (cultur* adj5 (differences* or cross* or background*)).tw,kw.
14 (border* and crossing).tw.
15 ((culturall* or linguisticall*) adj3 (tailor* or diverse* or patient* or parent* or communit* or background* or student* or wom?n or famil*)).tw,kw.
16 "cultural factor"/
17 or/1‐16
18 multilingualism/
19 multilingualism*.tw,kw.
20 "as a second language".tw,kw.
21 bilingual.tw,kw.
22 second language.tw.
23 foreign language.tw.
24 (proficiency and language).tw.
25 communication barriers/
26 (barrier adj3 language).tw,kw.
27 or/18‐26
28 17 or 27
29 access to information/
30 ((access or gain access or obtain or seek out or find or identify) adj5 (information* or health*)).tw.
31 comprehension/
32 (understand or comprehend or comprehension).tw.
33 (appraise or evaluate or process or interpret or assess).tw.
34 judge*.tw.
35 assessment of information.tw.
36 (apply or decide).tw.
37 (use* adj3 (information* or health)).tw.
38 (capacit* adj4 health).tw.
39 accept*.tw.
40 decision making/
41 ((make or making or made or take) adj4 decision*).tw.
42 ("behavior change" or "behaviour change").tw.
43 (acting or act or action).tw.
44 or/29‐43
45 consumer health information/
46 information literacy/
47 health literacy/
48 (information* adj3 health*).tw.
49 (health* adj3 (literac* or servic* or decision* or concept* or competenc* or system* or knowledg* or status or level* or needs or insurance or status or behaviour*)).tw.
50 health education/
51 educational status/
52 (health* adj3 education*).tw.
53 exp health care delivery/
54 or/45‐53
55 44 and 54
56 health litera$2.mp.
57 medical literacy.mp.
58 (health and literacy).ti.
59 (functional and health and literacy).tw.
60 low‐litera$2.ti.
61 litera$2.ti.
62 illitera$2.ti.
63 reading/ or comprehension/
64 (read* or comprehen*).tw,kw.
65 or/56‐64
66 *health promotion/
67 *health education/
68 *patient education/
69 *communication barriers/
70 *communication/
71 *health knowledge, attitudes, practice/
72 *attitude to health/
73 *comprehension/ and *educational status/
74 (family and literacy).ti.
75 (drug labeling.mp. or Prescription/) and comprehension.mp.
76 ((cancer or diabetes or genetics) and (literacy or comprehension)).ti.
77 (adult and (educational status or (educational and status) or literacy)).mp.
78 (limited and (educational status or (educational and status) or literacy)).mp.
79 (patient$1 and (educational status or (educational and status) or literacy)).mp.
80 (patient$1 and (comprehension or understanding)).ti.
81 or/66‐80
82 65 and 81
83 55 or 82
84 randomized controlled trial/
85 controlled clinical trial/
86 single blind procedure/ or double blind procedure/
87 crossover procedure/
88 random*.tw.
89 placebo*.tw.
90 ((singl* or doubl*) adj (blind* or mask*)).tw.
91 (crossover or cross over or factorial* or latin square).tw.
92 (assign* or allocat* or volunteer*).tw.
93 or/84‐92
94 28 and 83 and 93
CINAHL (via EBSCO)
# Query
S84 S82 AND S83
S83 (DE "Placebo" OR ((random* OR controlled) AND trial*) OR randomly OR randomized OR placebo* OR double‐blind)
S82 (S10 or S28) and (S54 or S81)
S81 S79 and S80
S80 S64 or S65 or S66 or S67 or S68 or S69 or S70 or S71 or S72 or S73 or S74 or S75 or S76 or S77 or S78
S79 S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63
S78 TI (patient* and (comprehension or understanding))
S77 SU (patient* and (educational status or (educational and status) or literacy))
S76 SU (limited and (educational status or (educational and status) or literacy))
S75 SU (adult and (educational status or (educational and status) or literacy))
S74 TI (cancer or diabetes or genetics) and (literacy or comprehension)
S73 SU (drug labeling or prescriptions, drugs) and comprehension
S72 TX family and literacy
S71 MA COMPREHENSION AND MA EDUCATIONAL STATUS
S70 MA "Health Personnel Attitudes"
S69 DE "Health Attitudes"
S68 DE "Health Knowledge" OR DE "Health Behavior"
S67 DE COMMUNICATION
S66 DE COMMUNICATION BARRIERS
S65 DE HEALTH EDUCATION
S64 DE HEALTH PROMOTION
S63 DE COMPREHENSION
S62 DE READING
S61 TX illitera* OR TX literac*
S60 TX read* OR TX comprehen*
S59 TX low‐litera*
S58 TX functional and health and literacy
S57 TX health and literacy
S56 TX medical literacy
S55 TX health litera*
S54 S44 and (S49 or S53)
S53 S50 or S51 or S52
S52 MA HEALTH SERVICES ACCESSIBILITY
S51 TX health* N3 education*
S50 DE HEALTH EDUCATION OR (DE EDUCATION AND DE STATUS)
S49 S45 or S46 or S47 or S48
S48 TX health* N3 (literac* or servic* or decision* or concept* or competenc* or system* or knowledg* or status or level* or needs or insurance or status or behaviour*)
S47 TX information* N3 health*
S46 DE HEALTH LITERACY
S45 MA CONSUMER HEALTH INFORMATION OR DE INFORMATION LITERACY
S44 S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43
S43 TX judge*
S42 TX acting or act or action
S41 TX "behavior change" or "behaviour change"
S40 TX ((make or making or made or take) N4 decision*)
S39 DE DECISION MAKING
S38 TX accept*
S37 TX capacit* N4 health
S36 TX use* N3 (information* or health)
S35 TX apply or decide
S34 TX assessment of information
S33 TX appraise or evaluate or process or interpret or assess
S32 TX (understand or comprehend or comprehension)
S31 DE COMPREHENSION
S30 TX (access or gain access or obtain or seek out or find or indentify) N5 (information* or health*)
S29 MA "ACCESS TO INFORMATION"
S28 S11 or S12 or S13 Or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27
S27 TX (culturall* or linguisticall*) N3 (diverse* or patient* or parent* or communit* or background* or student* or woman or women or famil*)
S26 TX border* and crossing
S25 TX cultur* N3 (differences* or cross* or background*)
S24 TX (foreigner* or asylum* or refugee* or undocumented or non‐native or nonnative or foreign‐born or foreignborn)
S23 (DE REFUGEES OR DE ASYLUM SEEKING OR DE POLITICAL ASYLUM)
S22 MA VULNERABLE POPULATIONS
S21 TX (displaced and (people or person*))
S20 TX ethnic* N2 (population* or group* or patient* or background* or specific* or minorit* or identit*)
S19 TX minorit* N2 (population* or group*)
S18 TX emigrant* OR TX emigrat*
S17 TX immigrant* OR TX immigrat*
S16 DE IMMIGRATION
S15 DE HUMAN MIGRATION
S14 MA "EMIGRANTS AND IMMIGRANTS"
S13 TX migration* N3 (background* or human*)
S12 TX migrant*
S11 MA "TRANSIENTS AND MIGRANTS"
S10 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9)
S9 TX (barrier N3 language)
S8 (DE "Communication Barriers")
S7 TX (proficiency and language)
S6 TX foreign language
S5 TX second language
S4 TX "as a second language"
S3 TX multilingualism*
S2 TX bilingual
S1 (DE "Multilingualism" OR DE "Bilingualism" OR DE "Bilingual Education" OR DE "English as Second Language") PsycINFO (via EBSCO)
PsycINFO (via OVID)
# Searches
1 Multilingualism/ or Bilingualism/ or "Bilingual Education"/ or "English as Second Language"/
2 (bilingual* or multilingual* or "second language" or "foreign language").tw.
3 (proficiency and language).tw.
4 "Communication Barriers"/
5 (barrier adj3 language).tw.
6 IMMIGRATION/ or exp HUMAN MIGRATION/
7 (migrant* or immigrant* or immigrat* or emigrant* or emigrat*).tw.
8 (migration* adj3 (background* or human*)).tw.
9 (minorit* adj2 (population* or group*)).tw.
10 (ethnic* adj2 (population* or group* or patient* or background* or specific* or minorit* or identit*)).tw.
11 (displaced and (people or person*)).tw.
12 exp At Risk Populations/
13 asylum seeking/ or political asylum/ or refugees/
14 (foreigner* or asylum* or refugee* or undocumented or non‐native or nonnative or foreign‐born or foreignborn).tw.
15 (cultur* adj3 (difference* or cross* or background*)).tw.
16 (border* and crossing).tw.
17 ((culturall* or linguisticall*) adj3 (diverse* or patient* or parent* or communit* or background* or student* or woman or women or famil*)).tw.
18 or/1‐17
19 information specialists/
20 ((access or gain access or obtain or seek out or find or indentify) adj5 (information* or health*)).tw.
21 exp Comprehension/
22 (understand or comprehend or comprehension or appraise or evaluate or process or interpret or assess or "assessment of information" or apply or decide or accept*).tw.
23 (use* adj3 (information* or health)).tw.
24 (capacit* adj4 health).tw.
25 exp Decision Making/
26 ((make or making or made or take) adj4 decision*).tw.
27 ("behavior change" or "behaviour change" or acting or act or action or judge*).tw.
28 or/19‐27
29 health information/ or information literacy/ or exp health literacy/
30 (information* adj3 health*).tw.
31 (health* adj3 (literac* or servic* or decision* or concept* or competenc* or system* or knowledg* or status or level* or needs or insurance or status or behaviour*)).tw.
32 or/29‐31
33 exp Health Education/
34 EDUCATION/ and STATUS/
35 (health* adj3 education*).tw.
36 exp Health Care Access/
37 or/33‐36
38 28 and (32 or 37)
39 exp Health Literacy/
40 (health litera* or medical literacy or read* or comprehen* or literac* or low‐litera* or illitera*).tw.
41 (health and literacy).tw.
42 exp Reading/
43 exp Comprehension/
44 or/39‐43
45 Health Promotion/ or Health Education/ or Communication Barriers/ or Health Knowledge/ or Health Behavior/ or Health Attitudes/ or Health Personnel Attitudes/
46 exp Educational Attainment Level/
47 Comprehension/ and exp Educational Attainment Level/
48 (family and literacy).tw.
49 exp Prescription Drugs/
50 Comprehension/ and exp Prescription Drugs/
51 ((cancer or diabetes or genetics) and (literacy or comprehension)).ti.
52 (adult and (educational status or (educational and status) or literacy)).tw.
53 (limited and (educational status or (educational and status) or literacy)).tw.
54 (patient* and (educational status or (educational and status) or literacy)).tw.
55 (patient* and (comprehension or understanding)).ti.
56 or/45‐55
57 44 and 56
58 18 and (38 or 57)
59 (control: or random:).tw. or exp treatment/
60 clinical trials/ or "treatment outcome clinical trial".md. or ((randomi?ed adj7 trial*) or ((single or doubl* or tripl* or treb*) and (blind* or mask*)) or (controlled adj3 trial*) or (clinical adj2 trial*)).ti,ab,id.
61 59 or 60
62 58 and 61
Data and analyses
Comparison 1. Culturally and literacy adapted self‐management programme versus no HL intervention.
Comparison 2. Culturally and literacy adapted self‐management programme versus written information.
Comparison 3. Culturally adapted health literacy skills building course versus no/unrelated health literacy intervention.
Comparison 4. Culturally and literacy adapted telephone education versus unrelated health literacy intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Health literacy ‐ appraise: decisional conflict (long‐term: approx. 7 months post‐intervention) | 1 | 431 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐10.24, ‐1.16] |
| 4.2 Health literacy ‐ apply: prostate cancer screening intention (long‐term: approx. 7 months post‐intervention) | 1 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.92, 1.10] |
| 4.3 Prostate cancer knowledge, 0 to 100 (long‐term: approx. 7 months post‐intervention) | 1 | 431 | Mean Difference (IV, Random, 95% CI) | 6.90 [6.88, 6.92] |
| 4.4 Health behaviour: prostate cancer testing (long‐term: 2 years post‐intervention) | 1 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.82, 1.07] |
| 4.5 Adverse events: anxiety (long‐term: approx. 7 months post‐intervention) | 1 | 431 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.55, 0.27] |
Comparison 5. Culturally and literacy adapted audio‐/visual education without personal feedback versus no health literacy intervention.
Comparison 6. Culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic.
Comparison 7. Culturally and literacy adapted audio‐/visual education without personal feedback versus another audio‐/visual education without personal feedback.
Comparison 8. Culturally and literacy adapted medical instruction versus no health literacy intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Understand: medication understanding (short‐term: immediately post‐intervention) | 1 | 200 | Mean Difference (IV, Random, 95% CI) | 10.00 [5.70, 14.30] |
Comparison 9. Female migrants' benefit of any health literacy intervention versus male migrants' benefit of any health literacy intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Generic health literacy, TOFHLA (short‐term: immediately post‐intervention) | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 2.78 [‐4.35, 9.91] |
| 9.2 Diabetes health literacy, DHLS (short‐term: immediately post‐intervention) | 1 | 118 | Mean Difference (IV, Random, 95% CI) | 5.00 [0.62, 9.38] |
| 9.3 Cardiovascular health behaviour (short‐term: immediately post‐intervention) | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 2.07 [‐5.04, 9.18] |
| 9.4 Health behaviour: new documentation of advance care planning (long‐term: approx. 12 months post‐intervention) | 1 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.90, 1.79] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bailey 2012.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: 2 cities, San Francisco and Chicago, USA Ethical approval: yes Recruitment setting: 6 clinics and 3 community‐based organisations (urban area) Method of recruitment: 1) approaching patients in waiting rooms, 2) having healthcare professionals direct patients to a research assistant of the study, 3) announcing the study or distributing flyers during group classes or clinic visits Length of follow‐up: no follow‐up Dropouts: 1 person did not complete the whole interview A priori calculation of effect size/power?: yes |
|
| Participants |
Description: low English proficient Chinese (Cantonese or Mandarin), Korean, Russian, Spanish or Vietnamese‐speaking adults Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 1 was excluded after randomisation, did not complete the entire interview PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean (SD): 17.0 (0.7) Race/ethnicity: Chinese, Korean, Russian, Spanish, Vietnamese Gender
Education (years): 1% < 9 y, 14.4% 9 to 11 y, 29.2% 12 y or GED, 14.9% some college, 21.8% ≥ college graduate Socioeconomic status/income: 44.7% USD 10,000, 36.7% USD 10,000 to 19,999, 18.6% ≥ USD 20,000 Age (years), mean (SD), range: 63.6 (0.91), 18 to 85 Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: health literacy informed RX instructions Theoretical framework: health literacy "best practices" Description: concordant prescription instructions using health literacy ‘best practices’. The medication‐taking was parted into 4 distinct time periods: morning, noon, evening and bedtime. Simple terms, lowercase and uppercase letters and numeric characters were used to facilitate patients’ understanding.
Comparator Type: no health literacy intervention Description: standard instructions with typical terminology based upon those generated by a national chain pharmacy offering language assistance services. |
|
| Outcomes | Outcomes assessed in the study: medication understanding, regimen dosing, regimen consolidation Outcomes considered in this review
Methods of assessing outcomes
Note: a research assistant handed the participant the dosing tray and a Rx bottle and stated, “Using this tray, please show me when you would take this medicine over the course of one full day.” Research assistants recorded the number of pills the participant placed in each of the 24 compartments. Participants could refer to the Rx label throughout the exercise. The process was repeated for 5 individual medication labels. Language of assessment: Spanish Translation procedure: not applicable; bilingual research assistant Timing of outcome assessment: short‐term (immediately post‐intervention) |
|
| Health literacy |
Definition: “capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.” (IOM, 2004) Health literacy components addressed by the intervention Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: not reported Funding: funding was provided by the California Endowment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Research assistants used a random number list, created by the study team, to assign participants to receive either standard or ConcordantRx instructions." There were more male participants in intervention arm 44.6% vs 31.0%, P < 0.05. However, the type of randomisation indicates that imbalances occurred by chance. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation list was created by study team, but further description of allocation is not provided. This indicates an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No information on whether participants were aware of which group they were assigned to and whether personnel were aware of the assignment. However, the intervention consisted of a single exposure of two different medication labels and participants were assessed immediately with the use of objective criteria. Therefore, we assume that even non‐blinding would not have affected the results. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | "1) Rx understanding, 2) regimen dosing and 3) regimen consolidation. Each was measured with a dosing tray, which consisted of 24 compartments, each labeled with one hour of the day. As some cultures use a 24 hour clock (i.e. 1400 vs. 2:00 pm) two different versions of trays were created. Participants were shown both and allowed to choose their preferred format. RAs demonstrated how to use the tray, then verified participant understanding of the tool." "Participants had to demonstrate the correct dose, frequency and spacing inferred by each instruction to be coded as ‘correct.’ Spacing criteria was developed by the research team with the assistance of two general internal medicine physicians." The outcome assessment is performance‐based and was conducted immediately post‐intervention. No statement was made on whether outcome assessors were blinded. However, even if the outcome assessors judged whether medication dosing was correct, it was objectively assessed and not dependent on a subjective judgement of either the interviewer or the participant. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "203 were randomised and initiated the study interview. 202 completed the entire interview and were included in analyses." One person dropped out: reason is provided, but not reported to which intervention the person was initially randomised to and no intention‐to‐treat analysis. However, the attrition rate indicates low risk of bias, since outcome data are available for nearly all participants randomised and the intervention only differed in type of Rx instruction provided. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported in the methods section are reported in the results of the paper. |
Bloom 2014.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT, 2 arms Geographic location: California, USA Ethical approval: not reported Recruitment setting: 17 Korean American churches and 3 senior centres Method of recruitment: not reported Length of follow‐up: probably 6 months (unclear when programme ended) Dropouts: 2 women in the control group were lost to follow‐up A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: female Afghan Muslim refugees with low English proficiency Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 230 women were included in the study. Total numbers were not reported separately for each study group. Authors state that general linear models using generalised estimating equations (GEE) methods were used to account for clustering (sample and analysis), to adjust for baseline knowledge levels. PROGRESS‐Plus Baseline imbalances: women in the intervention group had higher levels of knowledge Place of residence: urban, USA Race/ethnicity: Afghan refugees Gender: 100% female Note: the women's husbands received education too, but details not reported. Education: limited English proficiency and low literacy; no further details reported Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: 'The Afghan Women’s Breast Health Program' Theoretical framework: Cultural Explanatory Models (CEMs) framework (Rajaram 1998) and Chatman's Theory of Information Seeking (Chatman 1996) Description: following community‐based participatory research methods (CBPR) a community advisory boards was formed and involved to design the study. Lay health educators (female and male) facilitated culturally and literacy sensitive faith‐based group education for Afghan Muslim women about breast health using multiple methods of knowledge transfer (e.g. storytelling) and trained community health navigators/health advisors supported the women afterwards to facilitate making and keeping appointments as needed.
Note: most of this information stems from the related formative research (Shirazi 2013; Shirazi 2015) and from a publicly available video (www.youtube.com/watch?v=v7YbebbMYi8). For example, the authors state that it was planned to use interactive methods and storytelling as a result of the interviews with 53 Afghan women that were conducted previously. In addition, an education programme for the male heads of the household was implemented "to turn potential gatekeepers into family health advocates"(Bloom 2014) through trustful relationships and education, but we could not find detailed information about this additional study component. Comparator Type: no health literacy intervention (wait‐list control) Description: the control group received a delayed intervention. |
|
| Outcomes | Outcomes assessed in the study: breast cancer knowledge, mammography Outcomes considered in this review
Methods of assessing outcomes
Language of assessment: not reported Timing of outcome assessment: baseline, at 6‐month follow‐up (insufficient information to categorise into short‐term or medium‐term assessment as it is unclear for how long and at what intensity women were supported by the community health navigators after receiving group education). |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: National Institutes of Health, National Cancer Institute, USA. The Alameda County Program to Reduce Cancer Disparities (ANCP), U54 CA 153506 to the University of California, Berkeley, CA 94720‐7360 and the Afghan Coalition of Fremont, California. Additional notes: we only found a conference abstract for the RCT; authors were contacted and asked for additional information but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomised design was used, but the information is insufficient to permit judgement about "low risk" or "high risk". |
| Allocation concealment (selection bias) | Unclear risk | The information is insufficient to permit judgement about "low risk" or "high risk". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were most likely not blinded due to the nature of the study. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | "Women in the intervention group were more likely to report getting a mammogram between pre‐ and post‐test" Participants and personnel were most likely not blinded due to the nature of the study and health behaviour was measured via self‐report. In addition, we do not whether knowledge was subjectively or objectively measured in the study. If knowledge was subjectively measured, too. The results for knowledge might be biased as well. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Unclear risk | We do not whether knowledge was subjectively or objectively measured in the study. Thus, the information is insufficient to permit judgement about "low risk" or "high risk". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Retention from pre‐ to post‐test was 99% (two women in the control group were lost to follow‐up)." Low attrition rate and reasons provided. |
| Selective reporting (reporting bias) | Unclear risk | The information is insufficient to permit judgement about "low risk" or "high risk". |
| Selective recruitment of cluster participants | Unclear risk | The information is insufficient to permit judgement about "low risk" or "high risk". |
| Other bias | Unclear risk | Insufficient information to permit judgement of "low risk" or "high risk". |
Calderón 2014.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: California, Los Angeles, USA Ethical approval: yes Recruitment setting: South Central Family Health Center (SCFHC), South Los Angeles Method of recruitment: a SCFHC’s certified diabetes nurse screened information for new type 2 diabetes patients for study inclusion criteria; health navigator ("promotora") met with patients referred by the diabetes nurse and provided more information about the study. Flyers were distributed at the clinic and posted on billboards in waiting areas. Length of follow‐up: no follow‐up Dropouts: no dropouts A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: economically disadvantaged Spanish‐speaking Latino/Hispanics with type 2 diabetes Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, USA Race/ethnicity: Latino Gender
Education: 86.7% < high school, 13.3% ≥ high school Socioeconomic status/ income: 75.6% < USD 10,000, 24.4% ≥ USD 10,000 Health insurance: 31.3% insured Age (years), range; distribution: 18 to > 60 y; 20.7% 18 to 39 y, 88.6% 40 to 60 y, 20.7% > 60 y Health literacy (baseline) Assessment tool, range (score): STOFHLA, 0 to 36, higher score is better (validated tool)
|
|
| Interventions |
Intervention: animated video about diabetes ¿Que es la Diabetes?; What Is Diabetes? Theoretical framework: not reported; reference to various programmes with animation‐based teaching elements and to Doak 1996 Description: animated video whose icon "Corazon Quelate" (Heart that beats; Spanish version)/"Lotta Hart" (English version) describes typical characteristics of middle‐aged Latinx/Hispanic/African American who are inclined to be overweight. One character is diagnosed with diabetes. The video covers 3 main topics about diabetes: (1) general information, (2) clinical management and (3) self‐management. To explain more complex consequences of diabetes, the video resorts to animated illustrations.
Comparator Type: written information on the same topic Description: 5 pages of easy‐to‐read diabetes information (5th grade reading level) available from the National Diabetes Information Clearinghouse of the National Institute of Diabetes and Digestive and Kidney diseases (NIDDK). In addition, information about diabetes definition, cause and risk factors, clinical management and self‐management (accessed from the Spanish version of 'Your Guide to Diabetes: Type 1 and Type 2'). |
|
| Outcomes | Outcomes assessed in the study: diabetes health literacy Outcomes considered in this review
Methods of assessing outcomes Interviewer administered questionnaire; show cards were used to display response options as the interviewer read survey questions.
Language of assessment: Spanish and English Translation procedure: back‐translation procedure Reliability/validity: validated in the study, coefficient α = 0.79 Timing of outcome assessment: baseline, short‐term (immediately post‐ intervention) |
|
| Health literacy |
Definition: “the degree to which individuals have the capacity to obtain, process and understand basic health information needed to make appropriate health decisions.” (AMA 1999, Nielson‐Bohlman 2004) Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: not reported Funding: Agency for Healthcare Research and Quality (1R24‐HS014022‐01A1), the National Institute of Minority Health and Health Disparities (P20MD000182, P20MD000516, U54MD008149, MD000103), National Institute of Ageing (P30‐AG021684), and National Center for Research Resources (UL1TR000124). Additional notes: unadjusted data and gender‐separate scores for the outcome 'diabetes health literacy' were obtained from the study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment was done via numbers concealed in sealed envelopes that were generated by the study statistician through randomization software." |
| Allocation concealment (selection bias) | Low risk | "Neither the SCFHC diabetes nurse educator who recruited patients nor Drew’s health navigator/promotora who tested participants knew the content of the envelopes (allocation concealment). Therefore, neither knew the group (animation or text) to which participants would be assigned (allocation status)." It can be strongly assumed that participants could not foresee assignment either. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were most likely aware of the intervention they received due to the nature of the study. It is not clear whether the personnel who assessed the participants was blinded. However, outcomes measured were not subject to interpretation |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Diabetes health literacy was assessed with a questionnaire that predominantly measures factual knowledge. It was administered by an interviewer. It is not clear whether the interviewer was blinded, participants could not be blinded anyway. However, the outcome was assessed objectively and immediately post‐intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are available for all participants, indicating a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section are reported in the results of the study. |
DeCamp 2020.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Maryland, USA Ethical approval: yes Recruitment setting: large urban, academic general paediatrics clinic Method of recruitment: review of the clinic schedule for completed initial newborn visits; potentially eligible parents were sent an informational letter about the study. A bilingual research assistant recruited potential participants either by follow‐up phone call or during a subsequent newborn visit. Length of follow‐up: length of programme: 10 months; follow‐up survey at child age: 12 to 15 months, which was 1 to 3 months after the programme was completed Dropouts: 22 participants lost to follow‐up (7 in the intervention group (5 moved or switched clinics, 2 were unable to be contacted) and 15 in the control group (4 moved or switched clinic, 5 were unable to be contacted and 6 declined) A priori calculation of effect size/power?: yes |
|
| Participants |
Description: immigrant parents or legal guardians of Latin descent with US‐born infants < 2 months of age Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: an intention‐to‐treat analysis was performed for primary outcomes (analysed via electronic medical record (EMR)); secondary outcomes that were not abstracted from the EMR included only individuals who finished follow‐up survey. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean (SD): 7.3 (5.3) Race/ethnicity: Latinos Occupation: 79.0% spouse or partner employed Gender: 100% female Education: 40.8% ≤ 8th grade, 26.1% some high school, 33.1% some high school or greater Socioeconomic status/income (annual): 42.7% < USD 20,000, 24.2% USD 20,000 to 30,000, 7.6% > USD 30.000, 19.1% did not report or unknown Health insurance: all children publicly insured Social capital: 20.3% single, 79.6% spouse or partner Age (years), mean (SD): 29.3 (6.2) Health literacy (baseline) Assessment tool, range (score): Spanish‐language version of the Newest Vital Sign (NVS), 6 items, 0 to 6, higher score is better (validated in English and Spanish)
English proficiency was assessed using the US Census Bureau question, “How well do you speak English?”
|
|
| Interventions |
Intervention: Salud al Día, an interactive text messaging intervention to reduce ED use and increase vaccine adherence Theoretical framework: situated Information, Motivation, Behavioral Skills (sMIB) model (Amico 2011) Description: parents received interactive personalised text messages, push messages and watched an animated Spanish‐language educational video. Sequences included appointment reminders, support for obtaining medicines, support for completing referrals, and illness care monitoring and education. Interactive text messages included reminders of flu vaccine or information on parent support programmes and public benefit programmes. Certain response records generated an email to a clinic nurse who contacted participants and offered further support.
Comparator Type: no health literacy intervention (usual care/no additional intervention) Description: usual care for infants in the 1st year of life |
|
| Outcomes | Outcomes assessed in the study: infant health knowledge, up‐to‐date immunisations*, well visits, parent depression, emergency department use, parent experience of care rating, change in mean parent engagement, receipt of 2 doses of the influenza vaccine, well visit no‐shows and cancellations, clinic visit provider continuity, number of sick care visits, speciality care referral completion, participant‐generated telephone encounters, electronic medical record (EMR) patient portal (MyChart) status, Supplemental Nutrition Assistance programme (SNAP)/food stamp participation Outcomes considered in this review
*Prioritised outcome in the category 'health behaviour' based on consensus opinion of the authors Methods of assessing outcomes Surveys were orally administered by bilingual research assistants, either in‐person (enrolment and follow‐up) or via telephone (midpoint). Responses were captured using a touchscreen tablet computer and Research Electronic Database Capture software.
Language of assessment: English, Spanish Timing of outcome assessment: baseline, short‐term (at 11 to 14 months after randomisation, which was 1 to 3 months after the programme was completed) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: NCT02647814 Funding: funding was provided by the Gordon and Betty Moore Foundation. Additional notes: authors provided additional information on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment was performed by computer random number generation in blocks of 10, with a 1:1 allocation ratio." |
| Allocation concealment (selection bias) | Low risk | Low risk of bias due to randomisation method used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Participants and research staff were not blinded to which intervention participants were allocated to. Clinical staff and providers were not aware of group assignment unless revealed by the participant." Personnel and participants were not blinded and some outcomes of interest were subjectively measured. Therefore, results of subjective outcomes might be bias |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | "All surveys were orally administered by bilingual research assistants. Survey responses were captured simultaneously with administration using a Touchscreen tablet computer and Research Electronic Database Capture software" Participants were aware of group assignment and depression was measured via self‐reported questionnaire, which might have introduced a bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants and research staff were aware of group assignment. However, knowledge, health behaviour (child's up‐to‐date immunisation) and health service use (emergency department use) were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Analyses of primary outcomes were conducted per the intention‐to‐treat principle. Analyses of secondary and process outcomes that were not abstracted from the EMR included only those individuals with corresponding follow‐up survey data." Authors report numbers and reasons of dropouts separately for each study arm using a CONSORT diagram. In total, 22 participants were lost to follow‐up, n=7 (8.86%) in the intervention group and n=15 (19.23%) in the control group. The dropout rates are unbalanced. However, the differential loss between intervention and control arm is less than 15% (10.37%) and the reasons are reported transparently. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported in the results. |
Elder 1998.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT, 2 arms Geographic location: California, USA Ethical approval: unclear Recruitment setting: recruited from 3 community college sites, which took place during a 1‐week period at each site Method of recruitment: recruitment presentations Length of follow‐up: 6 months Dropouts: 72% of those completing baseline surveys also completed 6‐month follow‐up surveys (294) Note: exact numbers of dropouts are not reported. A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: adult students attending English as a Second Language (ESL) classes in the San Diego area Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 408 participants took part in the study. Numbers randomised are not reported separately for each study arm, but total numbers of participants who were assessed at all 3 time points (baseline, post‐intervention, 6‐month follow‐up, see 'additional tables'). PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years): 45.0% < 3 y Race/ethnicity: Latino, European, Asian, Others; Latino: 86.7% Gender:
Note: not reported per arm Education (years), mean (SD); distribution: 9.8 (3.7); 48.0% ≥ 9 y Socioeconomic status: "(...) two‐thirds of the group had monthly income less than $1099" (Elder 1998, p. 569). Social capital: "approximately one‐third was married" (Elder 1998, p. 569) Age (years), mean (SD): 28.7 (9.8) Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: 'Language for Health' Theoretical framework: Social‐cognitive Theory (Bandura 1977; Bandura 2002; Bandura 2004), Operant Conditioning (Skinner 1953) Description: educational intervention, which is incorporated in existing ESL course; classes about heart health/nutrition education. The classes included topics such as (1) understanding dietary fat and cholesterol, (2) classification of foods, (3) modifying eating habits, (4) reading food labels, (5) understanding blood pressure and its relationship to salt intake, (6) shopping for low fat and low‐cholesterol foods, and (7) modifying recipes. Curricula conformed to statewide ESL guidelines, including several methods of knowledge transfer.
Comparator Type: same method/mode of delivery, but information on a different health topic Description: same quantity of health‐related information on stress management topics incorporated into the same standardised ESL course format. |
|
| Outcomes | Outcomes assessed in the study: nutrition‐related knowledge, belief that change in diet leads to better health, intention to change one's diet, self‐efficacy to change diet, blood pressure, cholesterol, waist and hip circumference/weight, fat avoidance score, stress knowledge (to test salience of attention‐placebo manipulation) Outcomes considered in this review
Methods of assessing outcomes Paper‐pencil questionnaires for patient‐reported outcomes
Language of assessment: bilingual (Spanish and English) Timing of outcome assessment: baseline, 3 months after randomisation (short‐term) and at 6‐month follow‐up (medium‐term) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was provided by the National Heart, Lung, and Blood Institute (no. 5R01 HL46776‐02); no clinicaltrial.gov registration. Additional notes: authors were contacted and asked for additional information but provision was not possible (no longer access to data set). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Instructors were randomly assigned to teach one of the two educational programmes". It was stated that intervention and control groups "did not differ significantly on any baseline physiological, psychosocial, or demographic variable with one exception: Women constituted slightly more of the intervention group than the control group, c2 = 4.0, df=1, p < .05". Insufficient information to permit a judgement of "low risk" or "high risk"; no serious baseline differences reported. |
| Allocation concealment (selection bias) | Unclear risk | No statement on blinding of allocation concealment. Therefore, the information does not allow to permit judgement of "low risk" or "high risk" of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants were most likely not blinded due to the natue of the study. This might have affected the results of subjectively measured outcomes. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | "Physiological assessments were usually conducted during class time in a designated room on campus. A comprehensive paper‐pencil survey, available in English and Spanish, was administered in the classroom (...) Male and female research staff were available at physiological assessments and paper‐pencil survey assessments." Participants were not blinded and subjective outcomes were measured using repeated questionnaires. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants and research staff were aware of group assignment. However, knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "For the most part, participants completing surveys also provided physiological measures at baseline (90t three month post‐test (93%) and at the 6 month follow‐up (86%). Seventy‐two percent of those completing baseline surveys also completed 6 month follow‐up surveys and 69% of those providing baseline physiological measures also provided these at the 6 month follow‐up assessment. A thorough attrition analysis was conducted using procedures suggested by Biglan et al. (1991). No evidence was found for differences in the rate of attrition by condition (X2=0.06, d.f.=1, p=0.8). More importantly, ANOVAs showed that there was no differential attrition by condition with regard to demographic characteristics or any nutrition‐related physiological or psychosocial measure." Attrition rates were reported and the statistical attrition analysis revealed no significant differences with regard to demographic characteristics. However, exact numbers of participants included in each study arm as well as numbers of dropouts per arm are not reported. Therefore, information is insufficient to permit judgement of "low risk" or "high risk". |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section are reported in the results of the paper. |
| Selective recruitment of cluster participants | Unclear risk | "Participants were adult students, over 18 years of age, attending ESL classes in the San Diego area. Participants were recruited from three community college sites. Recruitment at each site took place during a 1 week period. Because of the high percentage of native Spanish‐speaking students in the targeted classes, classroom‐recruitment presentations were conducted in English and in Spanish when necessary." Timing and sequence of cluster randomisation is unclear. Therefore, information is insufficient to permit judgement of "high risk" or "low risk". |
| Other bias | Unclear risk | "Results showed the intraclass correlations were negligible and so mixed model analysis of variance (ANOVA) procedures were conducted to test intervention effects." Results were not adjusted for the cluster‐design. It is unclear how this affected the results, as the intracluster correlation coefficient is not reported and we had insufficient information to re‐analyse the data. |
Gwede 2019.
| Study characteristics | ||
| Methods |
Study design: RCT (pilot), 2 arms Geographic location: Southwest Florida, USA Ethical approval: yes Recruitment setting: 2 community clinics Method of recruitment: potential participants were selected from a community clinic, eligible participants were provided with further study information and written consent was obtained. Length of follow‐up: 3 months Dropouts: 6 participants were lost to follow‐up in the intervention group (reasons: more than 5 attempts, called second contact and contacted clinic for updated info but was unsuccessful); 2 participants discontinued intervention (reason: declined to further participate in study); 7 participants were lost to follow‐up in the control group (reasons: more than 5 attempts, called second contact and contacted clinic for updated info but was unsuccessful); 2 participants discontinued intervention (reason: declined to further participate in study) A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: patients of Latin/Hispanic descent, not up‐to‐date with colorectal cancer (CRC) screening guidelines at average risk of CRC Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean (based on n = 71 participants who were not born in the US): 23.4 Race/ethnicity: Hispanics/Latino/as Occupation (n = 75): 52.6% employed, 40.8% not employed, 4.0% retired, 1.0% student Gender:
Education: 43.4% elementary or less, 18.4% some high school, 17.1% high school graduate, 21.0% > high school Socioeconomic status/income (annual) (n = 70): 44.3% < USD 10,000, 55.1% ≥ USD 10,000 Health insurance: 25.5% insured Social capital: 69.7% married/living together, 13.1% divorced/separated, 7.9% widowed, 9.2% never married/single Age (years), mean (SD), range: 57.2 (6.0), 50 to 74 Health literacy (baseline) Assessment tool, range (score): validated (Spanish) Single Item Literacy Screener (SILS), 2 single items assessing difficulties in reading written materials (1st question) and confidence in completing health forms by oneself (2nd question) 1st question: 0 to 5; 0 for ‘very confident’ to 3 for ‘almost always ask for help’, lower score is better 2nd question: 0 to 3; 0 for 'never' to 2 for 'always'
|
|
| Interventions |
Intervention: Latinos Colorectal Cancer Awareness, Research, Education and Screening (LCARES) Theoretical framework: Preventive Health Model (PHM) (Aguado Loi 2020; Mc Queen 2008) Description: the participants received a culture‐sensitive photonovel booklet (here referred as fotonovela) and an educational DVD. The fotonovela contained stories with characters that represented a test‐specific behaviour of the FIT screening while the DVD‐storyline depicted characters that modelled the test‐specific behaviour of a FIT screening. The participants watched the DVD in the clinic receiving a copy of it and the fotonovela to take home. In addition, participants received a FIT kit, written and oral user instructions, and a self‐addressed stamped envelope to return the FIT kit. Email reminders were sent after 2 weeks.
Comparator Type: written information on the same topic Description: standard Spanish‐language booklet plus FIT, written and oral instructions to use FIT kit; reminder letters 2 weeks after study entry for participants who did not return FIT kit (like the intervention group) |
|
| Outcomes | Outcomes assessed in the study: awareness of CRC and screening tests, CRC screening uptake (return of a completed FIT kit within 90 days of intervention delivery), time to FIT kit return, Preventive Health Model (PHM) variables (i.a. self‐efficacy for screening using FIT) Outcomes considered in this review
Methods of assessing outcomes Bilingual study co‐ordinators assessed measures at baseline (in‐person) and by phone at 3‐month follow‐up. All questions were read aloud for all participants.
Note: items of the HINTS survey reflect subjective knowledge ("Have you heard about..."); other items not further described.
Language of assessment: Spanish Reliability/validity: not reported for awareness; validated Spanish version for self‐efficacy Timing of outcome assessment: baseline, after 3 months (medium‐term) |
|
| Health literacy |
Definition: “Thus, an important feature in promoting screening behaviors is the provision of culturally, and linguistically salient information that is mindful of audiences at‐risk of low‐literacy (e.g. those who may have difficulty in obtaining, processing and understanding health information)” (Gwede 2019, p. 311). Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: the study was supported by the Florida Department of Health’s Biomedical Research Branch, Bankhead Coley [grant number: 4BB09]; no clinicaltrials.gov registration. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After completion of baseline assessments, participants were randomized (1:1) to receive either the LCARES or comparison condition." Intervention group had a higher percentage identifying as ‘other’ race and an annual income less than $10.000”, n= 21 (75%) versus n=10 (30%). The sample size is small, therefore imbalances might have occurred by chance. However, information is insufficient to permit judgement of "high risk" or "low risk", as the randomisation procedure is not clearly described. |
| Allocation concealment (selection bias) | Unclear risk | No statement on concealment of allocation. Therefore, information is insufficient to permit judgement of "low risk" or "high risk" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No statement about whether participants and personnel were blinded and the effect on subjectively measured outcomes is unclear. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | Unclear risk | Subjective outcomes were measured with the use of repeated questionnaires and participants were probably not blinded to group allocation. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | "Screening uptake was evaluated by return of a completed FIT kit to the study team at the cancer center using pre‐stamped and self‐addressed mailers. This provided an objective verification of screening completion. The primary outcome was return of a completed FIT kit within 90 days of intervention delivery (coded as yes or no). Time to FIT kit return was a secondary outcome." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 76 enrolled, 40 were randomized to the LCARES intervention and 36 were randomized to the comparison condition. Accrual required 7 months. Fifty‐nine participants completed the 3‐month follow‐up interview (32 in LCARES condition and 27 in the comparison condition). A total of 13 participants were considered lost to follow‐up." Thirteen participants were excluded from analysis due to lost‐to follow up (n=9 in intervention group and n=8 in control group, respectively). No intention‐to‐treat analysis was performed for subjective outcomes. However, authors transparently report on attrition rates per study arm including the reasons for dropouts (illustrated by a CONSORT diagram). Differential loss between intervention and control arm is less than 15%. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods are reported in the results. |
Han 2017.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT, 2 arms Geographic location: Baltimore, Maryland–Washington, DC, USA Ethical approval: yes Recruitment setting: 23 Korean American churches Method of recruitment: 29 trained female community health workers (CHWs) from 23 ethnic churches recruited Korean American women from their respective churches. Trained bilingual research assistants visited the church, obtained written informed consent and collected data. Length of follow‐up: 6 months (total programme duration) Dropouts: lost to follow‐up at 3 months: 10 participants (reasons: 4 change of mind; 3 lack of time; 1 car accident; 1 moving out of state; 1 death); at 6 months: 7 participants (reasons: 4 no longer available; 2 change of mind; 1 out of contact) A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Korean American women, who had not had either a mammogram or a Pap test within the past 24 months Health topic: breast/cervical cancer; 5.4% had family history of breast cancer Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: intention‐to‐treat analysis was performed to account for missing data; methods reported. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean (SD), range: 15.4 (9.7), 1 to 62 Race/ethnicity: Korean Americans Occupation: 57.9% working full or part‐time, 42.1% unemployed, retired or other Gender:
Education: 35.2% high school graduate or less, 64.8% some college or more Socioeconomic status/income: 26.4% very comfortable or comfortable, 34.5% just OK, 39.5% uncomfortable or very uncomfortable Health insurance: 37.9% insured Social capital: 85.5% married or partnered, 11.1% separated, widowed or divorced, 3.4% never married Age (years), mean (SD): 46.1 (8.5) Health literacy (baseline) Assessment tool, range (score): Assessment of Health Literacy in Cancer screening (AHL‐C), 0 to 53, higher score is better
|
|
| Interventions |
Intervention: CHW–led intervention to improve breast and cervical cancer screening literacy Theoretical framework: Predisposing, Reinforcing, and Enabling Constructs in Education/environmental Diagnosis and Evaluation (PRECEDE)–Policy, Regulatory, and Organizational Constructs in Educational and Environmental Development (PROCEED) model Description: trained CHWs delivered health literacy skills training in group meetings. The components addressed participants' understanding of key medical terminology with regard to breast and cervical cancer screening, screening of relevant medical instructions, and knowledge of healthcare system navigation for obtaining screening. A DVD and a picture guidebook produced by the researchers were handed out, too. In group meetings, key medical phrases in English and role‐play scenarios presented in the DVD and guidebook were practised. In follow‐up calls new skills and knowledge was reinforced.
Comparator Type: written information on the same topic Description: the wait‐list control group received publicly available educational brochures related to breast and cervical cancer and a delayed intervention. |
|
| Outcomes | Outcomes assessed in the study: cancer screening health literacy, cancer knowledge (breast/cervical cancer), perceptions about cancer (decisional balance), adherence to age‐appropriate screening guidelines Outcomes considered in this review
Methods of assessing outcomes Self‐administered questionnaires for patient‐reported outcomes, medical records for health service use.
Note: The AHL‐C is a performance‐based measure that assesses print literacy, numeracy, and familiarity with and comprehension of cancer‐specific words.
Note: "The Cronbach a for the original scale ranged from 0.83 to 0.90, and α coefficients were 0.80 for mammogram and 0.84 for Pap testing in this sample."
Language of assessment: Korean (applies to knowledge and decisional balance) Translation procedure (if necessary): validated tool (applies to knowledge and decisional balance) Timing of outcome assessment: baseline, short‐term (at 3 months and at 6 months after randomisation) |
|
| Health literacy |
Definition: "Health literacy ‐ the degree to which individuals have the capacity to obtain, process, and understand basic health information and services to make appropriate health decisions" (Ratzan 2000) Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID:NCT00857636 Funding: funding was provided by the National Cancer Institute (no. R01 CA129060). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "We randomized the participating churches (intervention = 11; wait list control = 12) on the basis of their size and location. Insufficient information about the randomisation procedure and some minor baseline imbalances reported (subjective income (p=0.046) and English proficiency (p=0.046)). |
| Allocation concealment (selection bias) | Unclear risk | No statement on concealment of allocation. Therefore, the information is insufficient to permit judgement of "low risk" or "high risk". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants were not blinded to intervention allocation due to the nature of the study. Therefore, the results of subjectively measured outcomes might be biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Participants were not blinded and 'decisional balance' was measured by repeated questionnaire. This might have introduced a bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants were not blinded but health literacy and knowledge were measured objectively and not subject to interpretation. Pap‐Test use and mammography were assessed by self‐report but additionally by medical record review, indicating a low risk of bias for this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some incomplete data but not substantial. Reasons provided and sufficiently accounted for in the analysis; see consort diagram in appendix. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section are reported in the results of the paper. |
| Selective recruitment of cluster participants | Unclear risk | Timing and sequence of cluster randomisation is unclear. Therefore, information is insufficient to permit judgement of "high risk" or "low risk". |
| Other bias | Low risk | Authors sufficiently accounted for cluster‐design in the analysis. |
Hernandez 2013.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: California, USA Ethical approval: yes Recruitment setting: large multiservice community clinic Method of recruitment: through regularly offered health educational classes, at community events and other local services, snowball sampling Length of follow‐up: no follow‐up Dropouts: no dropouts; 3 in the intervention group were excluded from analysis (reasons: 2 participants had invalid measures due to missing responses and 1 due to wrong assignment) and 1 in the control group (reason: had invalid measures due to missing responses) A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: Latinas at risk for depression Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 4 were excluded after randomisation, 3 in intervention group (2 had invalid measures due to missing responses, and 1 due to wrong assignment); 1 in control group (invalid measures due to missing responses). PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), distribution: 7.7% < 5 y, 34.0% 6 to 10 y, 57.7% > 10 y Race/ethnicity: Latinas (78.8% Mexico, 21.1% other) Occupation: 33.8% employed Gender:
Education: 36.6% grade school, 25.3% middle school, 14.0% some high school, 10.5% high school or General Educational Development (GED), 10.5% some college or beyond Socioeconomic status/income (annual): 69.7% < USD 19,000, 19.0% USD 20,000 to 30,000, 11.2% > USD 30,000 Health insurance: 45.0% insured Social capital: 58.4% married, 24.6% living with partner, 7.7% never married, 9.1% divorced or widowed Age (years), range: 18 to 55 Health literacy (baseline) Assessment tool, range, level: Spanish version of Short Test of Functional Health Literacy in Adults (S‐TOFHLA), 0 to 36; 23% inadequate HL (0 to 16); 16% marginal HL (17 to 22 ); 62.6% adequate HL (23 to 36)
|
|
| Interventions |
Intervention: fotonovela "Secret Feelings", entertainment‐education for populations with low health literacy Theoretical framework: social‐cognitive theory (Bandura 1977; Bandura 2002; Bandura 2004); culture‐centric narrative (Larkey 2010) Description: the intervention consisted of 1 session including 30 to 45 minutes pretest questionnaires, 20 to 30 minutes exposure to a photonovel (here referred as fotonovela) presenting a story of a depressed middle‐aged Latina mother, 30 to 40 minutes post‐test questionnaires. The storyline addressed adaptive illness perceptions, help‐seeking behaviours, depression symptoms and treatment options, as well as common fears and misconceptions associated with treatment. The fotonovela was written at 4th grade reading level and read out loud with each literate participant taking turns.
Comparator Type: no health literacy intervention Description: discussion on family communication and intergenerational relationships developed by the study site's clinicians; first author delivered intervention and received training |
|
| Outcomes | Outcomes assessed in the study: depression knowledge, intent to seek treatment, depression, self‐efficacy to identify the need for treatment, stigma about mental health care, antidepressant stigma Outcomes considered in this review
Methods of assessing outcomes Self‐administered questionnaires (supported by verbal instructions of interviewer); verbal administration to 11 participants who were illiterate or had difficulty completing the forms
Language of assessment: Spanish Translation procedure (if necessary): scales for intent to seek treatment and self‐efficacy were translated into Spanish by a bilingual native speaker of Spanish and reviewed by 2 additional bilingual native speakers of Spanish. Feedback and edits were discussed until consensus was achieved. Timing of outcome assessment: baseline, short‐term (immediately after intervention) |
|
| Health literacy |
Definition: "Health literacy refers to health knowledge and health management skills influenced by reading fluency, prior health knowledge and experiences, as well as conceptual knowledge of health care" (Baker 2006). Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention (depression) |
|
| Notes |
Trial ID: not reported Funding: funding was provided by a grant from the Health Initiative of the Americas’ programme de Investigación de Migración y Salud (PIMSA). Additional notes: the intervention builds on the results of Unger 2013, exploring the fotonovela's compatibility with the promotora model of health education. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using STATA 11 software, those eligible for participation were randomly assigned to either the control or experimental group." Baseline differences in previous depression treatment reported. As the method of randomisation was appropriate imbalances probably occurred by chance. |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of measures to conceal the allocation of participants to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded due to the nature of the study; subjectively measured outcomes might be biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | "Each group received verbal instructions for completion of the pretest and posttest that were verbally administered to 11 illiterate participants or to those with difficulty completing the forms." Outcome assessors were not blinded and subjective outcomes were measured by verbally administered questionnaires to participants who were not blinded to group allocation. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants and personnel were not blinded but depression knowledge was measured objectively and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “One hundred forty‐six women were recruited for this study. Three participants, one from the control group and two from the experimental group, had invalid measures due to several missing responses. One participant assigned to the experimental group reported being enrolled in counselling at the time of pretest and posttest administration, so her data were not used. Thus, a total of 142 participants were included: 67 in the control group and 75 in the experimental group.” Slightly imbalanced attrition rate (n = 3 vs n = 1). Reasons for exclusion of participants post randomisation are reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section were reported in the results of the paper. |
Kaur 2019.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Montreal metropolitan areas, Canada Ethical approval: yes Recruitment setting: a community partner organisation, Punjabi community temples, community centres and grocery stores Method of recruitment: referrals from members of a community partner organisation, word of mouth, visits to Punjabi community temples, community centres and grocery stores Length of follow‐up: 3 months (total duration of the programme) Dropouts: 21 (reasons: work schedules, lack of interest or unavailability) A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Punjabi immigrants with good general health Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, Canada Race/ethnicity: Punjabis Occupation: 63.6% full‐time workers (including 14.3% self‐employed), 5.0% part‐time workers, 1.4% occasional workers, 22.1% homemakers, 2.9% unemployed Gender:
Education: 37.7% college/technical education, 26.8% university education, 35.5% high school or less Socioeconomic status/income (annual): 52.1% CAD 0 to 49,999, 19.3% CAD 50,000 to 89,999, 6.4% CAD ≥ 90,000, 20.7% unknown Health insurance: 72.9% insured Age (years), range; distribution: 18 to 60; 26.4% 18 to 31 y, 46.4% 32 to 45 y, 27.1% 46 to 60 y Health literacy (baseline) Assessment tool, range, score: Two Stage Rapid Estimate of Adult Literacy in Dentistry (TS‐REALD), 27 to 73, higher score is better
|
|
| Interventions |
Intervention: “Safeguard Your Smile” (SYS) oral health literacy intervention Theoretical framework: Behavior Change Wheel (Michie 2011) Description: participants received a 1‐hour group intervention including 5 components: (1) reviewing a photonovel showing risk factors of dental plaque and gingivitis and benefits and risks of action/inaction, (2) a demonstration of tools and skills of oral hygiene and a teach‐back of learned techniques (3) encouragement of participants to plan their dental hygiene and register a concrete plan and to track progress of a routine, and (4) a follow‐up call to reinforce learned skills and motivate to maintain self‐care behaviour.
Comparator Type: written information on the same topic Description: conventional English language oral hygiene self‐care pamphlet |
|
| Outcomes | Outcomes assessed in the study: oral health literacy, oral hygiene self‐care knowledge, oral hygiene self‐care behaviour, plaque index, gingival index Outcomes considered in this review
Methods of assessing outcomes Self‐administered questionnaires
Note: validated word recognition routing test; participants are asked to read a list of 5 dental words aloud, 1 point per correct pronunciation. Participants are categorised depending on their scores into 3 groups for further testing: (1) low literacy stage‐2 (4‐word test), (2) average literacy stage‐2 (6‐word test), (3) high literacy stage‐2 (3‐word test); score from routing test is added to the stage‐2 score to produce a raw score, that is translated into a scaled score.
Note: the questionnaires were translated into Punjabi language and "provided to the participants who could not read or write in English". Language of assessment: English for health literacy; Punjabi or English (applies to knowledge and behaviour) Translation procedure: translated into the Punjabi language (applies to knowledge and behaviour) Reliability/validity: validated tool (applies to health literacy) Timing of outcome assessment: baseline and 3 months after randomisation (immediately post‐intervention) |
|
| Health literacy |
Definition: "Oral health literacy refers to the "degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make oral health related decisions" (National Center for Health Statistics 2012). Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: NCT02521155 Funding: related to PhD thesis of first author Universté de Montréal; no additional funding declared Additional notes: authors were contacted and asked for additional information but without success; qualitative data related to the formative research are reported in the linked QES (Aldin 2019) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After recruitment and obtaining free and informed consent, 140 participants were randomly assigned to the experimental or control group using a computer‐generated random sequence provided by a statistician at the Université de Montréal, Canada." "Participants randomized into intervention and control groups differed as a function of age since females in the age group 32 to 45 years were over‐represented in the intervention group compared to the control group." There was a baseline imbalance reported. However, the radnomisation method used indicates that they may have occured by chance. In addition, the samle size was small which can result in chance‐based imbalances, too. |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of measures to conceal the allocation of participants to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants were not blinded to intervention allocation. It was explicitly stated that this was a non‐blinded RCT. Therefore, results of subjectively measured outcomes might be biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Outcome assessors were not blinded and health behaviour was measured with repeated questionnaires. This might have introduced a bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Outcome assessors were not blinded but health literacy and knowledge were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Initially 140 participants were recruited and consented to participate in the study. However, 21 people (15%) dropped out between pre‐test and post‐test primarily due to reasons such as work schedules, lack of interest or unavailability." "A sensitivity analysis was performed using the Worst Outcome Carried Forward (WOCF) to handle study dropouts and unanswered questionnaire items.The WOCF in this study consisted of using the pre‐intervention values measured as observed data in the post‐intervention. This strategy ensures that, even if the data is not missing at random, our results are robust to the worst‐case scenario." Authors report reasons for dropouts, but not the numbers of dropouts per group. However, the attrition rate is moderate, the methods used to account for missing data are appropriate. Therefore, a low risk of bias is present. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported at clinicaltrials.gov are reported in the published reports. |
Kheir 2014.
| Study characteristics | ||
| Methods |
Study design: RCT, 3 arms Geographic location: Doha, Qatar Ethical approval: yes Recruitment setting: at their workplace Method of recruitment: major contracting companies representing the main suppliers of workers to Qatar Petroleum (QP) were contacted; mid‐level supervisors informed the workers and extended invitation Length of follow‐up: no follow‐up Dropouts: no dropouts A priori calculation of effect size/power?: yes |
|
| Participants |
Description: foreign workers with low literacy skills Health topic
Inclusion criteria
Exclusion criteria
Group 1
Group 2
Group 3
Note: in this study all study arms were compared to each other. We created a single‐pairwise comparison referring to group 2 as intervention group and to group 3 as control group as they built the greatest contrast. PROGRESS‐Plus Place of residence: urban Race/ethnicity: Asians Time in Arab‐speaking country (years), mean range: 4.6 to 6.1 y Occupation: workers at QP company Gender: 100% male Education (years), mean (SD): 6.1 (3.4) Socioeconomic status: each participant was compensated with QAR 50 (equivalent to about USD 14), which translates to about 2 to 3 days average wage Age (years), mean (SD): 32.1 (8.5) Health literacy (baseline) Not measured Note: all participants had low literacy skills. Inclusion criteria were less than 8 years of formal education and low English and Arabic language skills (self‐assessed). The majority of the study population self‐assessed themselves as poor in English (70.0%) and Arabic literacy (94.0%). |
|
| Interventions |
Intervention: pictogram label with verbal instructions (group 2) Theoretical framework: not reported Description: the interviewer handed the pictogram‐only labelled medication box to the participant and asked each participant to offer their interpretation of the label contents. This was repeated for all 11 of the medicine instructions (group 1 and 2). Current practice verbal instructions were given to participants. All verbal communication between the interviewers and the participants was conducted through an interpreter (group 2).
Comparator (group 3) Type: no health literacy intervention Description: standard text label with verbal instructions (interpreted by interviewer fluent in respective language) |
|
| Outcomes | Outcomes assessed in the study: comprehension of medical instructions Outcomes considered in this review
Methods of assessing outcomes
Note: an appropriately labelled medication box was handed to participant by interviewer; participant was then asked to offer their interpretation of the label contents. The process was repeated for all 11 of the medicine instructions. Current practice verbal instructions (in English and Arabic) were given to participants in intervention group 1 and 2 only. Verbal communication between interviewer and participant was conducted through an interpreter. Each level of comprehension was pre‐defined using guidelines for categorising the results to maximise consistency between the 2 interviewers. Language of assessment: English Translation procedure: the verbatim transcript of the entire discussions that were not in English were later translated Timing of outcome assessment: short‐term (immediately post‐intervention) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: not reported Funding: funding was provided by a grant from Qatar National Research Fund under its Undergraduate Research Experience programme (no. UREP 10‐111‐3‐026). Additional information: authors were contacted and asked for additional information but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned into three study arms using computer‐generated random numbers" The randomisation procedure indicates a low risk of bias. |
| Allocation concealment (selection bias) | Low risk | "The interviewer handed the appropriately labelled medication box to the participant and asked each participant to offer their interpretation of the label contents." There is no statement whether the allocation was concealed. However, the randomisation was computer‐generated and the participants were asked to interpret a labelled medication box directly afterwards. Even if the participants had known the group they would be allocated to in advance, we do not think that it would have introduced a bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel and participants were not blinded to intervention allocation but outcomes were objectively measured immediately post‐intervention |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | "The interviewer handed the appropriately labeled medication box to the participant and asked each participant to offer their interpretation of the label contents. This was repeated for all 11 of the medicine instructions. Current practice verbal instructions (in English and Arabic) were given to participants in Groups A and C only. All verbal communication between the interviewers and the participants was conducted through an interpreter.The level of comprehension was determined as either 1 (no comprehension), 2 (partial comprehension) or 3 (full comprehension). To maximize consistency between the two interviewers, each level of comprehension was clearly defined and guidelines for categorizing the results were agreed upon as follows: full comprehension – complete understanding of the label leading to correct and safe use of the medicine; nil comprehension – total misunderstanding of the label leading to high risk for incorrect medicine usage; partial comprehension – indication of some comprehension with possible risk when taking the medicine." Outcome assessors were not blinded. However, as the participants were assessed immediately after the participant received the medication label and by means of predefined criteria including two interviewers, we assume a low risk for detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants were assessed immediately; hence, incomplete data due to lost to follow‐up were not possible. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section were reported in the results section of the paper. |
Kim 2009.
| Study characteristics | ||
| Methods |
Study design: RCT (pilot), 2 arms Geographic location: Baltimore‐Washington area, USA Ethical approval: yes Recruitment setting: Korean Resource Center (KRC), a community‐based site in partnership with the research team Method of recruitment: multiple sources (list of participants in the authors' previous studies, ethnic media (e.g. newspapers, radio stations), ethnic Korean churches, Korean grocery stores) Length of follow‐up: 30 weeks after randomisation (immediately after programme was completed) Dropouts: 4 lost to follow‐up at 6 months after baseline, 1 in the intervention group and 3 in the control group (reason: lack of time) A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Korean American immigrants with type 2 diabetes Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years): 53.2% > 20 y Race/ethnicity: Korean Americans Occupation: 70.3% employed Gender:
Education: 48.1% higher level of education Socioeconomic status/income (annual family income): 59.2% > USD 40,000 Social capital: 87.3% married Age (years), mean (SD): 56.4 (7.9) Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: SHIP‐DM Theoretical framework: theories of health literacy; PRECEDE–PROCEED model (Green 1991) Description: community‐based, multimodal behavioural SHIP‐DM that consisted of 3 main intervention modes: (1) 6 weeks of behavioural group education programmes related to diabetes mellitus, (2) home glucose monitoring with tele transmission (HGMT) and (3) individual counselling. The weekly educational group sessions included features to increase knowledge about diabetes, psychological education and health literacy education. Participants were provided with a glucose monitor, an electronic BP monitor and an HGMT‐system. Measurement data were transmitted and made accessible for nurse counsellors. Participants received monthly measurement reports through nurse counsellors. Monthly telephone counselling included data reviewing, reinforcement of lessons learned, discussion of issues related to diabetes self‐management, assistance and emotional support.
Comparator Type: written information on the same topic Description: control group participants received a standard brochure about diabetes and a delayed intervention. |
|
| Outcomes | Outcomes assessed in the study: diabetes knowledge, self‐efficacy, self‐care activities, depression, diabetes‐related quality of life, A1C level, fasting glucose, lipid batteries, blood pressure, height, weight (BMI), attitudes towards diabetes Outcomes considered in this review
Methods of assessing outcomes All outcomes considered in this review were assessed with the use of structured questionnaires.
Note: psychometric properties were obtained from a review of 5 randomised interventions and 2 observational studies (combined sample of 1988 people with diabetes) (Toobert 2000).
Language of assessment: Korean Translation procedure: back‐translation procedure and panel consensus approach (applies to knowledge and self‐efficacy) Timing of outcome assessment: baseline, at 18 weeks and at 30 weeks after randomisation (short‐term). We report on the 30‐week assessment only as this is the earliest time point after the intervention programme was completed. |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: NCT00505960 Funding: funding was provided by the National Institutes of Health (NIDDK R34 DK071957), LifeScan, Inc (HCC002154), and the Johns Hopkins University School of Medicine General Clinical Research Center (M01‐RR00052), from the National Center for Research Resources/National Institutes of Health. Additional notes: authors were contacted and asked for additional information (e.g. gender‐separate scores) but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 83 participants with confirmed eligibility were then randomly assigned to either the SHIP‐DM intervention group (n = 41) or the control (delayed intervention) group (n = 42) by computer‐automated random assignment." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because of the nature of this intervention and the design of the study, blinding of subjects to random assignment was not feasible." Non‐blinding might have affected the results of subjectively measured outcomes. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Personnel and participants were not blinded to study condition. Subjective outcomes were measured with repeated questionnaires. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Outcome assessors were not blinded but knowledge was objectively measured and not subjective to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One participant from the intervention group and 3 from the control group withdrew because of a lack of time (retention rate = 95.2%). Outcome data are available for almost all participants indicating a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods are reported in the results section. |
Kim 2014.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT, 2 arms Geographic location: Baltimore, Washington, USA Ethical approval: yes Recruitment setting: 17 Korean American churches and 3 senior centres Method of recruitment: 22 Korean American churches and senior centres were selected as intervention and control group sites; potential participants were screened, enroled and tested at each site Length of follow‐up: 18 months (6 months after completion of the 1‐year programme) Dropouts: 41 in the intervention group, thereof 34 after 6 months (15 refused classroom education, 16 with incomplete education, 3 did not conduct home blood pressure transmission, 3 did not receive telephone counselling), 4 after 12 months (1 Parkinson’s disease, 1 lost contact, 1 visited Korea, 1 refused) and 3 after 18 months (1 deceased with fire, 1 lung cancer, 1 refused). 30 dropped out in the control group, thereof 23 after 6 months (3 returned to Korea, 18 refused, 2 lost contact) and 7 after 12 months (2 deceased, 2 refused, 1 moved out, 2 got sick) Note: reporting discrepancies with regard to attrition rates shown in the CONSORT diagram and in the text (38 vs 37 vs 34 in the intervention group after 6 months) A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: Korean American seniors with high blood pressure (HBP) Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: only participants who completed the study were included in the analysis. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean (SD): 25.0 (11.0) Race/ethnicity: Korean Americans Gender:
Education: 37.4% ≤ middle school graduate, 28.2% high school graduate, 34.4% ≥ some college Socioeconomic status, health insurance: 82.7% insured Age (years), mean (SD), distribution: 70.9 (5.3), 42.0% ≤ 69 y, 51.5% 70 to 79 y, 6.5% ≥ 80 y Health literacy (baseline) Assessment tool, range, score: HBP health literacy scale, 0 to 43, higher score is better
|
|
| Interventions |
Intervention: multimodal SHIP on the control of HBP Theoretical framework: Self‐Help Model of Learned Response to Chronic Illness Experiences Description: SHIP to control HBP; intervention consisted of (1) education and training, (2) blood pressure home monitoring and (3) telephone counselling. Weekly educational sessions over 6 weeks were delivered by trained registered nurses and nutritionists. Health literacy training included learning medical terminologies and practising communication with healthcare providers. Sessions also covered (1) HBP management, (2) complications of uncontrolled blood pressure, (3) diet and nutrition, (4) food labels and exercise, (5) medications and food‐drug interactions and (6) problem‐solving skills. For blood pressure home monitoring participants were equipped with a blood pressure monitor with tele‐transmission. Participants were instructed to measure their blood pressure at home 2x/day with 3 readings at each measure and to transmit blood pressure data once a week to a contractor. The contractor set up a monthly report, which was used by counsellors and participants for goal setting. Trained bilingual CHWs undertook telephone counselling once a month for 12 months to strengthen healthy behaviours of the participants, deal with barriers and support.
Comparator Type: written information on the same topic Description: participants received a brief educational brochure that also listed available resources in the community at baseline and an abbreviated educational session after all data were collected at 18 months. |
|
| Outcomes | Outcomes assessed in the study: HBP health literacy, HBP knowledge, self‐efficacy in managing high blood pressure, medication adherence, depression, blood pressure Outcomes considered in this review
Methods of assessing outcomes
Note: the HBP health literacy scale covers 2 domains ‐ print literacy and functional health literacy for HBP management. Items are scored as correct or incorrect and then summed.
Note: combined measure of the 12‐item Check Your HBPIQ instrument and 14 items based on literature review of study authors. It is unclear whether the scale underwent a translation process. Secondary publications indicate a back‐to‐back translation procedure (Han 2011).
Note: it is unclear whether the scale underwent a translation process. Secondary publications indicate a back‐to‐back translation procedure (Kim 2006).
Note: researchers used a total score of ≥ 5 as cut‐point for presence of depressive symptoms. It is unclear if the Korean version of the PHQ‐9 was applied. Secondary publications indicate a back‐to‐back translation procedure (Kim 2015). Language of assessment: unclear for knowledge, self‐efficacy, adherence and depression; PHQ‐9 is validated in English and Korean Timing of outcome assessment: baseline, and at 6, 12 (short‐term) and 18 months (long‐term) after randomisation |
|
| Health literacy |
Definition: “(...) 'The degree to which individuals have the capacity to obtain, process, and understand basic health information and services to make appropriate health decisions' (...) (Nielson‐Bohlman 2004)" (Kim 2012, p. 2). Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: NCT00406614 Funding: funding was provided by a grant from the National Heart, Lung, and Blood Institute (no. R01 HL085567). Additional notes: information on test instruments was extracted from multiple publications related to this study. For an overview of all publications, see Kim 2014. Authors were contacted for additional information but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we used a randomized clinical control trial with the intervention delayed for the control group. Using adaptive stratified randomization, we selected 22 Korean American churches and senior centers as intervention and control group sites, depending on size or location." "We used a cluster randomization using ethnic churches as the unit of random assignment in order to reduce the potential risk of treatment diffusion between participants." (Kim 2012, p.4) Insufficient information to permit judgement of "high risk" or "low risk". |
| Allocation concealment (selection bias) | Unclear risk | No statement on concealment of allocation. Therefore, information is insufficient to permit judgement of "low risk" or "high risk". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, personnel and participants were not blinded to intervention allocation, results of subjectively measured outcomes might be biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | "After participants provided written informed consent, bilingual registered nurses (RNs) obtained 3 BP measurements, and trained bilingual research staff conducted face‐to‐face interviews for initial data collection. For both the intervention and control groups, data collection was repeated at 6, 12, and 18 months. Participants and personnel were not blinded and subjective outcomes were assessed by repeated questionnaires. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants and personnel were not blinded but health literacy and knowledge were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "At 6, 12, and 18 months, the numbers of participants who stayed in the study were 379 (86.1%), 372 (84.5%), and 369 (83.9%); at 18 months, the distribution was nearly even (184 in the intervention group; 185 in the control group). Over the 18 months, 71 (16.1%) participants dropped out for reasons such as cessation of contact (phone disconnection, residence change), schedule conflict, personal problems, or physical conditions. Some dropped out because they thought their BP was not high enough to require rigorous management. There were no differences in sociodemographic characteristics between those who remained in the study and those who dropped out. Analysis included only those who completed the study." Authors transparently report on attrition rates per study arm including the reasons for dropouts (illustrated by a CONSORT diagram). Differential loss between intervention and control arm is less than 15%. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the methods were reported in the results of the papers. However, study registration in clinicatrials.gov. indicates that 'health care utilization' and 'problem solving and communication skills' should have been assessed additionally at 6 weeks, month 6, 12, 18 and 24. Timepoints reported in the primary RCT range up to 18 month, which indicates the another publication might follow. Therefore, reporting bias is unclear. |
| Selective recruitment of cluster participants | Unclear risk | Information is insufficient to permit judgement of "high risk" or "low risk". |
| Other bias | Low risk | Data have been re‐analysed using the appropriate unit of analysis (with the use of the ICC reported by Han 2017). |
Kim 2020.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Baltimore, Maryland, USA Ethical approval: yes Recruitment setting: natural community setting; 32 churches, 86 outreach to a supermarket, outreach to trade association meetings Method of recruitment: media campaigns, outreach to places populated or frequented by Korean Americans (e.g. ethnic churches, supermarkets, festivals), referrals by Korean healthcare providers Length of follow‐up: 12 months (total duration of the programme) Dropouts: 15 in the intervention group, thereof 4 after 3 months (3 were too busy, 1 got enough), 4 after 6 months (1 was too busy, 2 due to cancer, 1 was out of contact), 2 after 9 months (1 due to family, 1 moved) and 5 after 12 months (2 were too tired, 1 was too busy, 1 stayed in Korea, 1 due to bankruptcy); 26 in the control group, thereof 17 after 3 months (2 visited Korea, 4 were too busy, 2 due to no ride, 1 due to language issue, 1 due to family, 1 due to cancer, 6 refused), 5 after 6 months (1 due to lymphoma, 2 were too busy, 1 refused, 1 due to mental issue), 2 after 9 months (1 due to cancer, 1 refused) and 2 after 12 months (1 was too busy, 1 refused) A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Korean Americans with type 2 diabetes Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: only participants who completed the programme were included in the analysis. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean (SD): 23.8 (11.0) Race/ethnicity: Korean Americans Occupation: 59.3% full/part‐time Gender:
Education (years), mean (SD): 13.4 (3.0) Socioeconomic status/income (monthly), mean: USD 3780; 63.2% housing own, 67.7% comfortable living Health insurance: 50.2% insured Social capital: 89.5% married; family size (persons), mean (SD): 3.0 (1.2) Age (years), mean (SD): 58.7 (8.4) Health literacy (baseline) Print literacy (referred to as "health literacy knowledge"): assessment tool, range, score Rapid Estimated of Adult Literacy in Medicine (REALM), 66 medical terms, 0 to 66, higher score is better
Diabetes mellitus‐specific Rapid Estimate of Adult Literacy in Medicine (DM‐REALM), 82 diabetes‐specific words, 0 to 88, higher score is better
Comprehension scale, 0 to 28, higher score is better
Functional health literacy (health numeracy): Test of Functional Health Literacy in Adults (S‐TOFHLA), numeracy subscale, 0 to 7, higher score is better
Newest Vital Sign (NVS), 0 to 6, higher score is better
Note: HL measures were correlated with each other: REALM and DM‐REALM (r = 0.91, P value < 0.001), TOFHLA (r = 0.68, P value < 0.001) and NVS (r = 0.47, P value < 0.001) |
|
| Interventions |
Intervention: SHIP‐DM Theoretical framework: theories of health literacy, PRECEDE–PROCEED model (Green 1991) Description: the community‐based, multimodal behavioural SHIP‐DM that consisted of 3 main intervention modes: (1) 6 weeks behavioural education programmes, (2) self‐monitoring and (3) individual counselling. (1) Weekly educational group sessions included features to enhance participants' knowledge of diabetes mellitus, psychological and health literacy education. (2) Participants were provided with a glucose monitor, strips and lancet(s) with instructions on how to use the equipment and registering measurements. Participants were requested to log their daily blood glucose levels twice a day for 12 months. (3) Telephone counselling was conducted once a month using motivational interviewing to counsel participants in disease‐specific demands and to encourage them to maintain self‐care skills and a healthy lifestyle.
Comparator Type: written information on the same topic Description: participants received a brief educational brochure at baseline that highlighted the critical self‐management principles of SHIP‐DM; the brochure also contained available care and educational resources in the community. An abbreviated educational session was offered to control group members at 12 months. |
|
| Outcomes | Outcomes assessed in the study: functional health literacy, health numeracy, diabetes‐specific health literacy, diabetes‐specific knowledge, diabetes‐specific self‐efficacy, adherence to diabetes regimen*, depression, diabetes‐related quality of life, comprehension**, social support*, dietary intake (using the 24‐hour recall method)*, HbA1c, blood pressure, weight, cholesterol Outcomes considered in this review
Notes: *results not reported in the identified publications; **comprehension was assessed via "comprehension scale" (it is not clear whether the comprehension scale was part of one of the health literacy assessment tools or whether it was used additionally; no additional explanations in the publications) Methods of assessing outcomes Health literacy was assessed with the use of 3 validated assessment tools on functional health literacy and health numeracy, respectively.
Language of assessment: language of assessment is not reported for functional health literacy; other measures were assessed in Korean Translation procedure: not reported for functional health literacy, health numeracy and quality of life Timing of outcome assessment: baseline and at 3, 6, 9 and 12 months after randomisation (short‐term, immediately after programme was completed) |
|
| Health literacy |
Definition: "(...) HL is 'the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions' (Ratzan 2000, p. vi)". Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID:NCT01264796 Funding: funding was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (no. R18 DK083936) with material support from LifeScan, including devices (OneTouch glucometer, OneTouch UltraSoft test strips, and OneTouch UltraSoft lancets) for study participants. In addition, the Johns Hopkins Institute for Clinical and Translational Research supported the cost of blood serum lab tests. Additional notes: the outcomes considered in this review are reported in two references. We have chosen the publication of the results on our primary outcome health literacy as the primary report, but we extracted data from all available reports related to this study. For an overview of all identified reports linked to this study, see Kim 2020. Authors were contacted and asked for additional information (e.g. gender‐separate scores) but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A total of 250 KA immigrants with uncontrolled T2DM were enrolled in our programme and randomized into either the intervention (n = 120) or the control (n = 130) group, with computer software ensuring equivalence between groups on key factors that might influence the primary outcome of A1C (e.g.., disease severity, age, body mass index, and gender)" |
| Allocation concealment (selection bias) | Low risk | Randomisation method indicated low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants were not blinded due to the nature of the intervention; results of subjectively measured outcomes might be biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Participants were not blinded to study condition and subjective outcomes were measured with repeated questionnaires. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants were not blinded but health literacy and knowledge were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Analyses of changes in this study included only participants with complete follow‐up data.” No intention‐to‐treat analysis, but completers only analysis was performed. Many dropouts in both arms (from 120 to 105 in intervention group (12.5%) and 130 to 104 in control group (20%)). However, reasons are provided and similar across groups. Attrition rate does not exceed the recommended 20% for short‐term follow‐up according to Cochrane RoB guidance. Differential loss between intervention and control group is less than 15%. |
| Selective reporting (reporting bias) | High risk | Results on adherence to diabetes regimen assessed with the diabetes Selfcare Activities Scale, social support (no information on the tool used) and dietary intake (using a 24‐hour recall) are not reported. |
Kiropoulos 2011.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Melbourne, Australia Ethical approval: yes Recruitment setting: Greek and Italian social welfare clubs, print and radio media directed at Greek‐ and Italian‐speaking residents in Melbourne Method of recruitment: advertising in Greek and Italian social welfare clubs, print and radio media, participants who opted to take part in the study contacted researchers listed in advertisements Length of follow‐up: 1 week after intervention Dropouts: no dropouts A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Greek‐born and Italian‐born immigrants living in Australia Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, Australia Time living in host country (years), mean (SD): 43.8 (9.0) Race/ethnicity: Greeks and Italians Occupation: 5.0% never worked, 57.9% unskilled, 31.2% tradesperson/clerical, 4% manager/professional, 28.2% working now, 70.8% are not working now Gender:
Education: 15.3% no/incomplete primary, 42.1% completed primary, 24.3% some secondary school, 9.9% all secondary school, 8.4% some/completed tertiary Social capital: 28.2% married, 71.8% not married, 14.9% living with spouse, 52.0% living with children, 24.8% living with other relatives, 14.4% currently living alone, 85.6% not currently living alone Age (years), mean (SD): 65.4 (8.57) Health literacy (baseline) Assessment tool, range, score: D‐Lit scale, 22 items, 0 to 22, higher score is better
|
|
| Interventions |
Intervention: Multicultural Information on Depression Online (MIDonline) website Theoretical framework: not reported Description: for the MIDonline website the interviewer and participant sat together in front of the computer. In the first 10 minutes the interviewer explained the purpose of the website and instructed participants on how to use it. Participants were then given 1 hour to read through the online material by themselves. The MID online website provides culturally tailored multilingual information about depression designed for middle‐ to older‐aged consumers who are not English‐native speakers. The website incorporates (1) information about symptoms and case studies of depression, (2) how depression is diagnosed, (3) related disorders, (4) causes, (5) treatment options, (6) how to find a bilingual mental health professional and professional psychological care, (7) stigma related to mental illness and multilingual translated resources.
Comparator Type: placebo intervention; semi‐structured interview about depression Description: semi‐structured interview with a bilingual interviewer who asked open‐ended questions relating to the participant’s beliefs about depression including the causes, symptoms, course and development, treatments and outcomes of depression; no additional material |
|
| Outcomes | Outcomes assessed in the study: depression literacy (depression knowledge), depression severity, depression stigma Outcomes considered in this review
Methods of assessing outcomes Face‐to‐face questionnaires administered by bilingual psychologists
Note: 4 items of the original questionnaire were replaced to reflect the content of the MIDonline website.
Language of assessment: language concordant Translation procedure (if necessary): all self‐report scales were translated from English into Greek and Italian by the first author and other bilingual psychologists; all item translations were reconsidered by a second bilingual psychologist and researcher; more difficult or ambiguous items were examined for meaning with lay members of the Greek and Italian communities. Validity was checked by examining the psychometric properties of the scales after data were collected, preceding further analysis. Timing of outcome assessment: prior and immediately after intervention, 1‐week follow‐up (short term) |
|
| Health literacy |
Definition: "depression literacy (also called depression knowledge)" (Kiropoulos 2011, p. 2), not further defined Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was provided by a major research grant from Beyondblue, the National Depression Initiative. Additional notes: authors were contacted and asked for additional information (e.g. gender‐separate scores) but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned by the first author following a simple randomization procedure using a computerized list of random numbers to one of two intervention groups (either the MIDonline intervention (n = 110) or the control group (n = 92) using a 1:1 allocation with stratification at level of country). The sequence of numbers was concealed until the intervention was assigned." |
| Allocation concealment (selection bias) | Low risk | "The sequence of numbers was concealed until the intervention was assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Interviewers and participants were not blinded to condition assignment" Non‐blinding might have affected the results of subjectively measured outcomes. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Outcome assessors were not blinded and depression was measured using a repeated questionnaire. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants and personnel were not blinded but depression literacy was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0% attrition rate. Therefore, a risk of attrition bias is not indicated. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods are reported in the results of the paper. |
Koniak‐Griffin 2015.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Los Angeles, California, USA Ethical approval: yes Recruitment setting: parent education centres, churches, laundromats, organisations providing basic services to children and families (e.g. ESL classes, job training, social services) Method of recruitment: recruitment was conducted in 4 consecutive intervention cycles. Trained recruiters gave small group and individual presentations providing an overview of study and programme announcements. Length of follow‐up: 9 months (3 months after programme completion) Dropouts: 59 participants were lost to follow‐up; 13 in the intervention group and 17 in the control group after 6 months and 11 in the intervention group and 18 in the control group after 9 months. A priori calculation of effect size/power?: yes |
|
| Participants |
Description: low‐income Latina immigrants that are overweight Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: authors report having conducted a modified intention‐to‐treat analysis using mixed‐effects models for repeated measures over time; 13 participants were excluded from physical activity analysis because they did not meet the accelerometer recording criteria. PROGRESS‐Plus Place of residence: urban Time living in host country (years), mean (SD), range (n = 204): 18.6 (8.3), 1 to 40 Race/ethnicity: Latinas Occupation: 74.6% unemployed Gender: female only Education (grade) (n = 220): 52.5% ≤ 8th grade, 33.6% 9th to 12th grade, 12.6% ≥ 13 years Socioeconomic status/income (annual): ≤ USD 20,000 54.7%, USD 20,001 to 40,000 28.7%, USD 40,001 to 75,000 16.6% Health insurance: 31.8% insured Social capital: 72.2% married/living with a partner, 27.8% divorced/widowed/single Age (years), mean (SD), range: 44.6 (7.9), 35 to 64 Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: lifestyle behaviour intervention "Mujeres Sanas y Precavidas" Theoretical framework: community‐based participatory research conceptual framework Description: the culturally targeted promotora‐led programme included group education plus individual teaching and coaching units about healthy lifestyle behaviours to reduce cardiovascular disease risks. Promotoras presented standardised content in pairs and showed an instructor‐led stretching and exercising DVD, produced by an official public health department. In coaching sessions, food and physical activity diaries of participants were discussed with promotors (inter alia). The intervention promoted four key messages: (1) healthy food choices, (2) portion control, (3) managing emotional eating and (4) increasing physical activity. Participants received a pedometer, a copy of the exercise video presented in the classes and culturally‐appropriate recipes.
Comparator Type: no health literacy intervention (attention placebo control) Description: 6‐month educational programme on safety and preparedness topics (e.g. in case of earthquakes) followed by the possibility of 8 individual teaching and coaching contacts where class content was reviewed in in‐depth discussions. After completion of the study, participants were offered 2 classes on key information about a promotora‐led health intervention ("Su Corazón, Su Vida"). |
|
| Outcomes | Outcomes assessed in the study: knowledge of heart disease, physical activity*, dietary habits, body weight, height and waist circumference, blood pressure, blood lipids and glucose Outcomes considered in this review
Note: *prioritised outcome, category 'health behaviour' Methods of assessing outcomes
Note: "Items also assessed prevention behaviours and awareness that early treatment exists."
Note: "The Lifecorder activity counts were converted into METS (1 MET = 3.5 mL/kg min), thus enabling classification of intensity according to accepted standards as well as measurement of steps". Participants received verbal and written instructions with illustrations on the devices. Note: a bilingual research assistant, blinded to participant’s group assignment, administered the questionnaires via face‐to‐face interviews. Language of assessment: Spanish Timing of outcome assessment: baseline, 6 months after randomisation (short‐term, immediately after programme was completed) and 9 months after randomisation (medium‐term, 3 months after programme was completed) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: prevention |
|
| Notes |
Trial ID: NCT01333241 Funding: funding was obtained by the National Heart, Lung, and Blood Institute (R01 HL086931) and was part of a registered clinical trial. Additional notes: authors were contacted and asked for additional information (e.g. control groups' post‐intervention knowledge scores) but provision of data was not possible. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a web‐based programme custom‐developed for this study. Participants were assigned to the Lifestyle Behavior Intervention or the control group in a 1:1 ratio using a block randomization procedure." |
| Allocation concealment (selection bias) | Low risk | The randomisation method indicates a low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel and participants were not blinded due to the nature of the study. However, outcomes considered in this review were objectively measured. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Both physical activity and knowledge were objectively measured. No subjective judgement of personnel required. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Retention was 86.5% and 87.0% or the 6‐ and 9‐month evaluations, respectively. (...) The retention rates across groups were not statistically different” The attrition rate is lower than 20% and the differential loss between study groups is not significant. A modified intention‐to‐treat‐analysis was conducted for physical activity; a completers only analysis was performed for participant‐reported outcomes. |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes reported at clinicaltrials.gov are reported in the published reports. However, results of the control group's knowledge assessment were not reported. |
Lepore 2012.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: New York, USA Ethical approval: yes Recruitment setting: the sampling frame was constructed from the health insurance beneficiaries (~355,000) list of a large healthcare workers union in the New York City metropolitan area. Method of recruitment: participants were drawn from the sampling frame and recruited via advance letters and reply cards. Length of follow‐up: 2 years for prostate‐specific antigen (PSA) claims, self‐report data were collected 8 months after randomisation (programme duration approx. 1 month). Dropouts: 29 were lost to follow‐up in the intervention group (reasons: 25 could not be reached for follow‐up, 4 refused to complete the study); 30 were lost to follow‐up in the control group (reasons: 25 could not be reached for follow‐up, 4 refused to complete the study, 1 pulled from study); in the allocation process 15 did not receive allocated intervention (reasons: 11 could not be reached, 4 refused to complete, 0 pulled from study); 16 in the control group did not receive allocated intervention (reasons: 11 could not be reached, 4 refused to complete, 1 pulled from study) A priori calculation of effect size/power?: yes |
|
| Participants |
Description: black immigrant men from the Caribbean Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: a partial intention‐to‐treat‐analysis was performed; participants were included in analyses even if they did not receive the allocated intervention. PROGRESS‐Plus Place of residence: urban, USA Race/ethnicity: black men of African descent Gender: 100% male Education: 31.3% less than high school, 31.8% high school degree, 36.9% college education or degree Socioeconomic status: Health insurance: all had access to health insurance that covered prostate cancer tests Social capital: 83.7% married Age (years), mean (SD): 55.04 (6.29) Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: Tailored telephone education on prostate cancer Theoretical framework: Ottawa Decision Support Framework (Doull 2006) Description: tailored telephone education about prostate cancer testing that included print education material, tailored and balanced information about prostate cancer risk and tests, and a values' clarification exercise. The intervention addressed participants' knowledge, values and decision conflict for prostate cancer screening, and aimed to increase their ability and motivation to talk with a physician about testing. Calls were audio‐recorded and checked for fidelity.
Comparator Type: unrelated health literacy intervention (same methods but information on a different health topic) Description: print brochure on fruit and vegetable consumption and tailored telephone education including information about the recommended amounts of fruits and vegetables, appropriate serving size, and the importance of eating a colourful variety of fruits and vegetables. |
|
| Outcomes | Outcomes assessed in the study: knowledge on prostate cancer screening, testing intention, benefits‐to‐risk ratio of testing, and verified PSA testing, state of anxiety, decisional conflict, verified physician visit to discuss testing, congruence between intention and actual behaviour Outcomes considered in this review
Note: We would have reported on the results of the following subscales: informed decision, values clarity and support. The subscales uncertainty and effective decision presume a completed decision, thus rather reflecting the processing step of applying health information. However, the authors report on the full subscales informed decision, values clarity and 1 item of the support subscale only justifying that with many participants (N = 81) having been still undecided after the intervention and reasons of reliability. These items "were dropped along with items 6 and 8 [subscale support] in order to bring reliability up to an acceptable level (Cronbach’s alpha = .62)." Methods of assessing outcomes Questionnaires were telephone‐administered by data collector blinded to group assignment.
Language of assessment: English Reliability/validity: only reported for state of anxiety, α = 0.66 pretest, 0.70 posttest Timing of outcome assessment: baseline (knowledge only), long‐term (approx. 7 months follow‐up for self‐reported outcomes and at 1‐ and 2‐year follow‐up for PSA testing) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: NCT01415375 Funding: funding was provided by the National Cancer Institute of the National Institutes of Health (grant R01 CA104223). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was conducted within three age strata (45–49, 50–54, and 55–70 years old) using the PLAN procedure of SAS (Cary, NC)." "The Principal Investigator used a computer generated randomization schedule to randomize the participant and emailed the randomization assignment to the interventionist." |
| Allocation concealment (selection bias) | Low risk | The randomisation procedure used indicates a low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Data collectors were blind to condition but the interventionists were not" Data collectors were blinded, but intervention providers were not. However, we assume that participants were unaware of the allocated intervention, as both the intervention and control group received telephone education. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | Low risk | "Data collectors were blind to condition but the interventionists were not" Participants were presumably not aware of the intervention received |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | "Data collectors were blind to condition but the interventionists were not" Knowledge and PSA testing were measured objectively and were not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Attrition was low (12%) and did not vary by condition. Most (93.6%) participants received their allocated intervention, but a few could not be reached by telephone. Medical claims data on prostate cancer testing and physician visits were 100% complete." Dropout rates are low and the differential loss between intervention and control group is 0.3%. Participants excluded from the analysis already had incomplete data at baseline stage. Questions were orally administered indicating that incomplete data did not result from participants' low literacy. An intention‐to‐treat analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified at clinicaltrials.gov are reported in the results. |
Mohan 2014.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Tennessee, USA Ethical approval: yes Recruitment setting: safety net clinic, Nashville Method of recruitment: research assistants screened patient charts and received referrals from clinic staff to identify patients with reported diabetes; patients were directly approached by research assistants in the clinic waiting room and other clinic areas. Length of follow‐up: 1 week after intervention Dropouts: 2 in the intervention group were lost to follow‐up, 1 in the control group were lost to follow‐up A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: Latinos with diabetes prescribed for at least 1 chronic medication Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 4 participants were subsequently excluded from each arm for not meeting eligibility criteria. PROGRESS‐Plus Place of residence: urban, USA Race/ethnicity: Latinos Gender:
Education (years), mean: 8; 29.0% had at least high school education Age (years), mean: 50 Health literacy (baseline) Assessment tool, range, score: Brief Health Literacy Screen (BHLS), validated in Englisch and Spanish, 3 to 15, higher score is better
59% had limited health literacy |
|
| Interventions |
Intervention: PictureRx illustrated medication list Theoretical framework: not reported Description: the participant's prescribed medication regimen was entered into a secure website by a research assistant to prepare and print a colour PictureRx illustrated medication schedule. It showed the full medication regimen, dosing of medication and included a picture of each medication to show its purpose. Medication instructions were printed in plain language (English and Spanish). The research assistant explained the PictureRx to the participant and showed a 2‐minute video about it. Patients received a 1‐page sheet with tips on how to use the PictureRx.
Comparator Type: no health literacy intervention Description: usual care; the treating provider reviewed medication instructions with the patient and the patient received a handwritten list of medications in their preferred language, with instructions for use and the drug indications, but no illustrations. |
|
| Outcomes | Outcomes assessed in the study: medication understanding, medication adherence Outcomes considered in this review
Methods of assessing outcomes Baseline questionnaire after enrolment in the study administered by research assistant, telephone administered follow‐up interview (also by research assistant)
Language of assessment: Spanish Timing of outcome assessment: short‐term (at 1‐week follow‐up) |
|
| Health literacy |
Definition: "(…) evidence suggests that health literacy – or the constellation of skills needed to effectively function in the health care environment – plays an important role." (Mohan 2012, p. 2) Timing of assessment: baseline Health literacy components addressed by the intervention Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: not reported Funding: funding was provided by Small Business Innovation Research award (no. R43 MD004048) (Riley/Boyington), from the HHS National Institute on Minority Health and Health Disparities (NIH) of the National Institutes of Health. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization codes were prepared in advance using a computer random number generator, in permuted blocks of varying size, and sealed individually in opaque envelopes to maintain concealment of treatment allocation." Participants in the intervention arm were more likely to be male (38% vs 23%; P = 0.017) and more likely to be white (98% vs 92%; P = 0.05). However, the type of randomisation indicates that imbalances occurred by chance. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed, indicating a low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Research staff and patients were not blinded. Investigators and the biostatistician were blinded." Personnel and participants were not blinded to group allocation and medication adherence was measured subjectively. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | "Research staff and patients were not blinded. Investigators and the biostatistician were blinded." Outcome assessors were not blinded and medication adherence was measured via self‐report, indicating a high risk of bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | No blinding of participants and personnel but medication understanding was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "208 patients were randomized, 105 to usual care and 103 to the intervention. Upon further assessment, 4 patients were subsequently excluded from each arm for not meeting eligibility criteria, leaving 101 patients in the usual care arm and 99 in the intervention arm. Of those 200 patients, 197 (98.5%) completed the follow‐up outcome assessment, including the medication understanding measure." "The primary analysis was an intention‐to‐treat comparison of medication understanding among patients randomized to receive the intervention versus patients randomized to usual care alone." Attrition rates are low and numbers and reasons for dropouts are reported in figure 2. An intention‐to‐treat‐analysis was performed. Therefore, the risk of attrition bias is low. |
| Selective reporting (reporting bias) | Low risk | Both outcomes reported in the methods section are reported in the results of the paper. |
Ochoa 2020.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Los Angeles County, USA Ethical approval: not reported Recruitment setting: at participant's home via telephone Method of recruitment: participants were recruited via random digit dialling (RDD) procedures Length of follow‐up: 6 months post‐intervention Dropouts: in total, 191 dropped out; 113 did not complete post‐test survey, 48 did not complete the survey at all (3) time points, another 31 were not included in the analysis, as they were born in the USA A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: monolingual Spanish‐speaking woman of Mexican origin Health topic
Inclusion criteria
Exclusion criteria
Note: participants born in the USA were excluded for analysis; authors indicate that "foreign‐born and US‐born Hispanics show differences of opinion in some key issues." Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean: 25.12 Race/ethnicity: Hispanic, Mexican Gender: 100% female Education: 49.8% < high school, 31.25% high school, 19.0% some college degree Socioeconomic status/income (annual): 41.6% < USD 20,000, 35.4% USD 20,000 to < 40,000, 16.05% USD 40,000 to < 60,000, 6.9% ≥ USD 60,000 Health insurance: 73.45% insured Social capital: 78.95% married/living with partner, 10.7% separated/divorced/widowed, 10.35% never married (single) Age (years), range: 25 to 45 Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: narrative culturally tailored film about cervical cancer Theoretical framework: not reported Description: participants were exposed to a linguistically and culturally tailored narrative/story‐telling film showing a Mexican‐American family that prepares for the daughter's birthday party. One of the daughters tells her sister that she had an abnormal Pap test and has been diagnosed with the human papillomavirus infection (HPV). In the course of the film the daughter provides information about HPV, cervical cancer and the importance of Pap tests to detect cervical cancer while the older woman presented in the film recognise the benefits of testing for cervical cancer. At the end of the film the 3 main characters are going to the local clinic for the conducting of a Pap test.
Comparator Type: factual knowledge video on the same topic Description: Latina women featured film similar in length providing information via charts and figures. It also showed doctors and patients talking about cervical cancer, risk factors and their importance as well as the Pap testing procedure. |
|
| Outcomes | Outcomes assessed in the study: knowledge, attitudes towards Papanicolauou test (Pap test), behavioural intentions regarding cervical cancer, testing behaviour Outcomes considered in this review
Methods of assessing outcomes Outcomes were assessed via questionnaires; no further information
Note: as only monolingual Spanish‐speaking Latinas were included, one can assume that the questionnaires were conducted in Spanish. Timing of outcome assessment: short‐term and medium‐term (knowledge was assessed baseline, post‐intervention at 2 weeks and at 6‐month follow‐up, question (1) behavioural intention was assessed at baseline, question (2) was assessed at post‐test and at 6‐month follow‐up, health behaviour was assessed at post‐test and at 6‐month follow‐up) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was provided by the National Cancer Institute (NCI) (grant no. RO1CA144052), the SC Clinical and Translation Science Institute at USC (CTSI) (award number UL1TR000130), and the Norris Comprehensive Cancer Center (NCCC) (NCI ‐ P30CA014089). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... participants were randomly assigned to one of two experimental conditions: half of the participants were assigned to view the narrative film (Tamale Lesson/ Conversando entre Tamales), and the other half were assigned to view the nonnarrative film (It’s Time/Es Tiempo)." "On average, women who were assigned to watch the narrative film reported longer length in the USA (26.6 vs 23.3; p = 0.005) compared with women who were assigned to the nonnarrative film." Insufficient information regarding the randomisation procedure to permit judgement of "high risk" or "low risk"; small sample size so that baseline imbalances might have occurred by chance. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high risk or low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not clear whether participants and personnel were blinded. However, interventions only differed in one aspect (narrative versus non‐narrative video). We assume that this did not lead to bias. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | Low risk | Subjective outcomes were measured by repeated questionnaires and participants were probably not blinded to group allocation. However, interventions only differed in one aspect (narrative versus non‐narrative video). We assume that this did not lead to bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Unclear blinding but knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Three hundred women were randomized, a total of 187 women completed the post‐test survey, and 140 women completed the surveys at three points in time, of which 109 were included in this study (see Fig. 1). For analysis, we excluded participants who were born in the USA because it has been found that foreign‐born and US‐born Hispanics show differences of opinion on some key issues." A completers only analysis was conducted. Reasons for excluding US‐born Latinas are provided, but numbers of dropouts and reasons for dropouts are not reported per study arm. However, the study compared a variant of the same intervention. Thus, we do not assume that one of the interventions led to a higher attrition rate to any particular degree than the other one. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section are reported in the results. |
Otilingam 2015.
| Study characteristics | ||
| Methods |
Study design: RCT, 4 arms Geographic location: California, Los Angeles, USA Ethical approval: yes Recruitment setting: predominantly Mexican American community in Los Angeles County Method of recruitment: potentially eligible participants were invited via telephone to meet individually with a research assistant at the clinic. Invitations to participate in the nutrition study were issued to a series of random samples drawn from the parent study until a sufficient number of women agreed to participate. Length of follow‐up: 1 month post‐intervention Dropouts: 8 (2 in the heart plus brain condition and 3 in the heart only condition received only partial intervention and did not complete post‐test); 3 (1 in each intervention group and 1 in the wait‐list control group) were lost to follow‐up. A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: healthy Latinas Health topic
Inclusion criteria
Exclusion criteria
Intervention group 1
Intervention group 2
Control group 1
Control group 2
Note: an intention‐to‐treat‐analysis was performed including all participants randomised; we used completers‐only analysis for meta‐analysis as final scores were reported for completers only. Results for both completers‐only analysis and intention‐to‐treat‐analysis (repeated measures analysis of variance for testing the difference between intervention and control groups) are reported in Table 2, Table 3 and Table 5. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean: 34.3 Race/ethnicity: Latinas Gender
Education (highest level): 41.0% none or elementary, 35.0% high school, 10.0% community/technical college, 14.0% college Socioeconomic status: 39.0% family income ≤ USD 20,000 Social capital (number of children living at home age < 17): mean 2.1 Age (years), mean, range: 58.95, 48 to 84 Health literacy (baseline) Assessment tool, range, score: NVS, 6 items, 0 to 6, higher score is better
|
|
| Interventions |
Interventions Nutrition and heart health plus brain health workshop (group 1) and Nutrition and heart health workshop (group 2)* Theoretical framework: Social Learning Theory and health belief model (Rosenstock 1988); theories/empirical evidence related to literacy in the context of health and limited language proficiency Description: two workshops with the first one conducted one week after pretest. The workshops included culturally tailored nutrition education techniques. Photographs and other visual aids were featured to circumvent potential concerns of low reading literacy. Both intervention groups received the nutrition education. The additional “Brain Connection” module content was delivered to intervention group 2 only during the first workshop (20 to 30 min). It incorporated research findings about the relationship between metabolic syndrome and increased risk for dementia, a visual representation in which a non‐pathological brain was compared with the brain of someone with Alzheimer’s disease, research findings about the relationship between saturated fat consumption and increased risk of cardiovascular as well as cerebrovascular diseases, and knowledge about dementia.
Comparator Type (group 3, 4): no health literacy intervention Description: participants in control group 1 and in control group 2 were offered an invitation to participate in two 2‐hour workshops based on materials given to participants in the heart plus brain health condition after the intervention was completed. *Intervention groups were combined to create a single‐pairwise comparison with group 3 for the 1‐month follow‐up assessment (results for control group 4 were reported post‐test only and we used the 1‐month assessment for meta‐analysis). |
|
| Outcomes | Outcomes assessed in the study: health numeracy, dietary fat knowledge, behaviours to reduce dietary fat Outcomes considered in this review
Methods of assessing outcomes Participants were administered materials orally in Spanish or English per preference; no further information.
Language of assessment: per preference (Spanish or English) Timing of outcome assessment: baseline, immediately after intervention and at 1‐month follow‐up (short‐term) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: the authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by NIH Grants P50 AG005142 (principal investigator [PI]: Helena Chui), R25 MH071544 (PIs: Barry Lebowitz, Jilip Jeste), U10EY11753 (PI: Rohit Varma), a Wallis Annenberg Fellowship (Poorni Otilingam), and an unrestricted grant from Research to Prevent Blindness, New York (Rohit Varma). Additional notes: leader manuals and all handouts and posters on the brain condition are available at dornsife.usc.edu/labs/scrap/usc-alzheimers-disease/. Authors provided additional information (e.g. score range for Dietary Fat Habits Questionnaire) on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study comprised a randomized controlled trial" "All potentially eligible participants were invited by telephone to meet individually with a research assistant at the clinic to complete the informed consent and to be given a sealed envelope with their random assignment to a study condition (so that research assistants were blind to condition until the envelope was opened)." There is only a statement that the participants were randomised, but no information on the randomisation procedure used. Therefore, information is insufficient to permit judgement of high risk or low risk of bias. |
| Allocation concealment (selection bias) | Low risk | "All potentially eligible participants were invited by telephone to meet individually with a research assistant at the clinic to complete the informed consent and to be given a sealed envelope with their random assignment to a study condition (so that research assistants were blind to condition until the envelope was opened)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to group allocation due to the nature of the study and health behaviour was subjectively measured. This might have introduced bias. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Health behaviour was measured via self‐report and participants were not blinded to group allocation. This might have introduced bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants were not blinded but health numeracy and knowledge were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 100 individuals were randomized to the four conditions, with 92% completing all times of measurement for their condition. Two members of the heart plus brain condition and three members of the heart only condition received only a partial intervention and did not complete the posttest, and another one participant from each intervention condition was lost at follow‐up. One member of the wait list control group was lost at follow‐up." "PROC MIXED allowed for including all participants, even if they discontinued after providing 1 or 2 times of measurement, or if they were in the posttest only wait list control group." The attrition rate is low and reasons for loss to follow‐up are transparently reported, indicating a low risk of bias. An intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section are reported in the results. |
Payán 2020.
| Study characteristics | ||
| Methods |
Study design: RCT, 3 arms Geographic location: California, USA Ethical approval: yes Recruitment setting: outpatient clinic waiting rooms in a large public hospital providing care for underserved populations Method of recruitment: bilingual, bicultural and trained Latina research staff, approached woman for recruitment and to assess eligibility Length of follow‐up: 3 months Dropouts: completion rate was 100% for the first 2 time points of outcome assessment (baseline and post‐intervention); 80.4% completed the 3‐month follow‐up assessment. In total, 47 did not complete the 3‐month follow‐up (12 in group 1, 18 in group 2 and 17 in the control group). A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: low‐income Latinas Health topic
Inclusion criteria
Exclusion criteria
Group 1
Group 2
Group 3
Note: in this study all study arms are compared to each other. We created a single pair‐wise comparison by combining group 1 and 2 and referring to them as the intervention group. We refer to group 3 as the control group. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years) (n = 240): 69.9% ≥ 15 y Race/ethnicity: Latinas Gender: 100% female Education: 64.2% ≥ 6th grade level of education Socioeconomic status/ income (annual household income): 93.4% < USD 30,000 Health insurance: 79.6% Social capital: 46.8% married, 30.5% separated, 22.7% single Age (years), mean (SD), range: 52.3 (8.8), 35 to 72 Health literacy (baseline) Not measured |
|
| Interventions |
Interventions:CUIDARSE brochure (group 1), CHW‐delivered CUIDARSE brochure (group 2)* Theoretical framework: input‐output framework (McGuire 2015), Health Belief Model (Champion 2008) Description: the brochure CUIDARSE contained four fictional narratives describing Latinas with different risk levels for developing breast cancer. The content incorporated information on basic prevention, the risks, advantages and disadvantages of preventive actions and modifiables well as non‐modifiable risk factors for developing breast cancer (group 1, 2). The brochure was orally administered by trained CHWs without additional support (group 2).
Comparator Type: no health literacy intervention (standard brochure) Description: participants in group 3 received a Spanish‐language consumer guide on reducing breast cancer risk from the Agency for Healthcare Research and Quality (AHRQ). *Groups were combined to create a single pair‐wise comparison. |
|
| Outcomes | Outcomes assessed in the study: breast cancer risk knowledge, self‐efficacy to access breast cancer‐related advice or information, perceived breast cancer susceptibility Outcomes considered in this review
Methods of assessing outcomes Outcomes were assessed via questionnaires, 3‐month follow‐up assessments were telephone‐administered by trained bilingual, bicultural research staff.
Language of assessment: English or Spanish Translation procedure: back‐to‐back translation, translation discrepancies were resolved by a bilingual committee (principal investigator, project coordinator, and other bilingual and bicultural staff) Reliability/validity: adapted from validated tools, no further information reported Timing of outcome assessment: baseline, short‐term (immediately post‐intervention) and medium‐term (at 3‐month follow‐up) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was provided by the AHRQ, Grant No. R18HS019264. Additional notes: authors provided additional information (related to intervention delivery and language of assessments) and data (unadjusted mean (SD) for knowledge and self‐efficacy) upon request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by recruitment clinic and individual level of education (≥6 or <6 years of education) to prevent imbalanced group assignment due to possible confounders." "The control group had fewer participants born in El Salvador compared to Groups 1 and 2 (13.4% vs. 25.3% vs. 29.1%). The control group also had fewer participants with higher acculturation levels (≥15 years in the United States) compared to Groups 1 and 2 (58.5% vs. 74.7% vs. 75.9%)" Baseline differences were reported for two variables. However, the sample size was small and there is no evidence that there was a problem in the randomisation process. |
| Allocation concealment (selection bias) | Low risk | "All participants completed a baseline survey before being randomized to one of three study arms using sealed randomization envelopes. Data collectors were blind to the study condition up until this point." Concealment of allocation was ensured through the use of "sealed randomization envelopes", indicating a low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were most likely not blinded to group allocation due to the nature of the study and self‐efficacy was subjectively measured. This might have introduced bias. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Self‐efficacy was measured via questionnaire and participants were not blinded to group allocation. This might have introduced bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The response rate was 100% at baseline and postintervention (n = 240) and decreased to 80.4% (n = 193) after 3 months." No intention‐to‐treat analysis was performed. In total, 47 participants did not complete the 3‐month follow‐up (n = 12 in group 1, n = 18 in group 2 and n = 17 in the control groups) and no reasons are given for the loss to follow‐up. However, the differential loss between intervention and control groups is less than 15%, indicating that the reasons for dropouts were not caused by the nature of the intervention. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section are reported in the results. |
Poureslami 2016a.
| Study characteristics | ||
| Methods |
Study design: RCT, 4 arms Geographic location: Vancouver, Canada Ethical approval: yes Recruitment setting: collaborating physicians’ clinics Method of recruitment: convenience sampling method: physicians identified participants for the qualitative and quantitative study from the community Length of follow‐up: 6 months*, outcomes reported were assessed at 3‐month follow‐up Dropouts: 2 (1 Punjabi, 1 Chinese) did not complete 3‐month follow‐up and were excluded from analysis A priori calculation of effect size/power? yes *Inconsistencies in length of intervention in 2 study reports (9‐month vs 10‐month). However, the intervention was a single exposure to 1 of 2 educational videos or both videos, respectively, or a brief pamphlet (control group). Follow‐up tests were conducted immediately post‐intervention (1 month after baseline assessment) and at 3‐month follow‐up. In addition, authors report that a short telephone‐based follow‐up was conducted at 6‐month follow‐up, but did not report the results. Figure 1 also indicates a 9‐month follow‐up assessment that is not reported in the text either. |
|
| Participants |
Description: Chinese or Punjabi immigrants with physician‐diagnosed asthma using asthma medications daily Health topic
Inclusion criteria
Exclusion criteria
Intervention groups
Note: according to the flow diagrams shown in the published trial reports (Poureslami 2016a), 21 participants watched the physician‐led video (vs 22 according to texts and tables). We used the numbers displayed in texts and tables, assuming that the numbers displayed in the flow diagrams might be wrong. PROGRESS‐Plus Place of residence: urban, Canada Time living in host country: participants had immigrated to Canada within the past 5 years Race/ethnicity: Chinese and Punjabi Occupation: 21.2% employed, 29.4% unemployed, 43.5% retired, 5.9% volunteer job
Education: 17.6% never attended formal school, 24.7% completed elementary school, 34.1% completed high school, 23.5% post‐high‐school education Age (years), mean (SD), range: 62.9 (15.3), 21 to 87 Health literacy (baseline) Not reported |
|
| Interventions | Theoretical framework: theories of health literacy; formative research to inform intervention development Comparison 1: audio‐/visual education without personal feedback versus written information on the same topic Intervention: clinical, knowledge video, narrative community video or both (groups 1,2, and 3)* Description: participants watched either one or two educational videos at the clinic or at home. The knowledge video provided clinical information about asthma symptoms, medication techniques and self‐management strategies. The correct method of inhaler use was demonstrated by a well‐known physician from the same ethnic background as the participants. In the community video, participants and caregivers role‐played a scenario, offering opinions and narratives about asthma and its management in short videos. The contents of both videos were similar, showing cultural beliefs and practices from 3 target ethnic communities. The correct way of using inhalers was performed by respiratory educators from the target communities at the end of both the physician‐led and community videos.
Comparator Type: (written information on the same topic Description: culturally and literacy adapted pictorial pamphlets containing the same information in written format; developed by the research team using a community‐based participatory approach. Comparison 2: culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback Intervention: narrative, community video (group 2) Description: participants watched the narrative, community video (see description above) Comparator: physician‐led, knowledge video (group 1) Description: participants watched the physician‐led, knowledge video (see description above) *From this study, we have formed two comparisons: firstly, we combined group 1, 2 and 3 to create a single‐pairwise comparison with group 4 reporting the results in the comparison 'culturally and literacy adapted audio‐/visual education without personal feedback versus written information om the same topic'. Secondly, we compared the results of group 1 with those of group 2, reporting them in the comparison 'culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback'. |
|
| Outcomes | Outcomes assessed in the study: asthma‐related knowledge, inhaler use technique, understanding physician's instructions, asthma‐related knowledge (knowledge of symptoms, triggers and factors that make asthma worse), qualitative open‐ended questions on patients' overall beliefs and concerns about asthma and its management In addition, authors state that they "added some questions to assess patients’ health literacy" but the results are not reported. Outcome measures considered in this review:
Methods of assessing outcomes: Outcomes were assessed face‐to‐face (at 3 months) and via telephone by trained bilingual facilitators
Note: checklist for inhaler use technique included the following steps: (1) shake device (metered‐dose inhaler); (2) load the inhaler; (3) breathe out away from inhaler; (4) put the inhaler in mouth behind teeth; (5) breathe in deeply; (6) hold breath for 5 to 10 seconds; (7) breathe out from nose; (8) wait for 60 seconds before taking the second puff, if needed; and (9) recap and rinse mouth, if needed.
Translation procedure: professional translators translated the written materials to the 3 target languages and provided back‐translation Timing of outcome assessment: baseline, medium‐term (at 3‐month follow‐up), results of 6‐month assessment are not reported |
|
| Health literacy |
Definition: health literacy as "ability to access, understand, and use asthma‐related information" (Poureslami 2012, p. 544) Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: NCT01474928 Funding: funding was provided by the Canadian Institutes of Health Research (CIHR) and partly by the Centre for Lung Health at the University of British Columbia. Additional notes: authors were contacted and asked for additional information (e.g. with regard to the health literacy assessment) but without success. Data have been extracted from multiple trial reports (see all references related to Poureslami 2016a). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eighty‐seven subjects were randomized into the intervention, and 85 completed the study" Insufficient information to permit judgement of low risk or high risk because there is no information on the method used for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No statement on concealment of allocation and whether investigators or participants could foresee assignment. Therefore, the information is insufficient to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study team was not blind to the subject group assignment. We also involved a family member who normally took care of the subject at home (the immediate caregiver at the home) in the interviews and learning process across the study groups." According to the study register (clinicaltrials.gov) this was a single‐blind study in which only the participants were masked to the group they were assigned to. However, due to the nature of the study, it is unclear whether blinding of the participants was effective. Personnel could have been blinded, but the authors state that they were not. However, the outcomes considered in this review were objectively measured. Thus, we do not assume that non‐blinding affected the results. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | "We interviewed each patient alone in their native language to ensure confidentiality. The interviews were facilitated by bilingual and bicultural experienced moderators from the same community who were not aware of the study hypothesis. The facilitators signed an agreement to keep the information confidential." Although it is unclear whether blinding to study hypothesis also includes blinding to the intervention allocation, knowledge, understanding of physician's instruction and inhaler technique acquisition were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Eighty‐seven subjects were randomized into the intervention, and 85 completed the study (42 Chinese and 43 Punjabi, age 21–87 y [mean SD 62.9 15.3 y], 42 males and 43 females) (Table 1)." The attrition rate is presented in a CONSORT diagram; the number of dropouts per arm is not explicitly reported in the text. When comparing all numbers across the publications, one could assume that the participants dropped out from the control group. Only 2 participants dropped out in total and reasons are provided, indicating a low risk of bias. |
| Selective reporting (reporting bias) | High risk | "We assessed patients’ functional knowledge, health literacy, and health practices (as explained in the section “Measurement”) related to asthma at the baseline interview (pretest). We then conducted our intervention 1 month immediately after the pretest, and then had a further follow up 3 months post‐intervention. Furthermore, 6 months after the post‐intervention, the patients were invited to participate in a telephone follow‐up survey to assess their self‐reported use of the peak flow meter, whether they followed their action plans, and whether they used their prescribed medications regularly." An outcome measure for health literacy is reported in the methods section but not in the result section of the paper. In addition, in the report of time point a (Poureslami 2012), an additional telephone follow‐up was conducted to assess medication adherence, but results are not reported in any of the publications. |
Poureslami 2016b.
| Study characteristics | ||
| Methods |
Study design: RCT, 4 arms Geographic location: Vancouver, Canada Ethical approval: yes Recruitment setting: outpatient respiratory clinics Method of recruitment: collaborating physicians identified and referred potential candidates, bilingual facilitators contacted candidates Length of follow‐up: 3 months* Dropouts: no dropouts A priori calculation of effect size/power?: yes *Inconsistencies between text and figure 1; according to figure 1 follow‐ups should have been conducted at 3, 6 and 9 months after intervention. Quote: "All outcomes were measured at baseline, then at 4 weeks and 3 months after intervention (...) Data were collected over a 4‐month period through 3 in‐person assessments. The baseline assessment preceded the intervention; the post‐intervention assessment occurred immediately following the intervention (4 weeks after baseline); a follow‐up assessment occurred 3 months following intervention." |
|
| Participants |
Description: Chinese immigrants with chronic obstructive pulmonary disease (COPD) Health topic
Inclusion criteria
Exclusion criteria
Intervention groups
Note: according to figure 1, 29 participants watched the clinical video (vs 22 according to the text and to table 1) and 22 participants watched both videos (vs 29 according to text and to table 1). We used the numbers displayed in the text and in table 1, assuming that the numbers displayed in figure 1 might be wrong. PROGRESS‐Plus Place of residence: urban, Canada Time living in host country: participants had immigrated to Canada within the past 12 years Race/ethnicity: Chinese Gender:
Note: not reported per arm Education: 46.2% low education, 53.8% high education Age (years), median; distribution: 75; 40.7% ≤ 75, 59.3% > 75 Health literacy (baseline) Not measured |
|
| Interventions | Theoretical framework: theories of health literacy Comparison 1: audio‐/visual education without personal feedback versus written information on the same topic Intervention: clinical, knowledge video, narrative community video or both (groups 1, 2 and 3)* Description: participants watched either a physician‐led, knowledge video (group 1), a narrative, community video (group 2) related to COPD management. The researchers used the same content to develop the lay videos and the clinical videos in the 2 languages. In the last scene of both videos, an experienced respiratory educator from the same language group as the participants demonstrated the correct use of different inhalers. The “clinician video” was a 20‐minute physician‐led video, providing clinical information about COPD symptoms and self‐management strategies. In the “lay video,” peer patients role‐played a scenario offering opinions and narratives about COPD self‐management in a 12‐minute video clip. 2 lay videos with similar content in Mandarin and Cantonese languages were developed.
Comparator Description: easy‐to‐understand pictorial self‐management pamphlet at grade 5 literacy level using the same content from the active intervention in a printed format, translated and back‐translated in Cantonese and Mandarin. Comparison 2: culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback Intervention: narrative, community video (group 2) Description: participants watched the narrative, community video (see description above) Comparator: physician‐led, knowledge video (group 1) Description: participants watched the physician‐led, knowledge video (see description above) *From this study, we have formed two comparisons: firstly, we combined group 1, 2 and 3 to create a single‐pairwise comparison with group 4 reporting the results in comparison 6 'culturally and literacy adapted audio‐/visual education without personal feedback versus written information on the same topic'. Secondly, we compared the results of group 1 with those of group 2, reporting them in comparison 7 'culturally and literacy adapted audio‐/visual education without personal feedback versus another culturally and literacy adapted audio‐/visual education without personal feedback'. |
|
| Outcomes | Outcomes assessed in the study: COPD knowledge**, inhaler technique, understanding of pulmonary rehabilitation procedure*, understanding of steps to manage COPD, self‐efficacy for COPD self‐management Outcomes considered in this review
*Prioritised outcome in category 'health literacy ‐ understand', as it was unclear how 'understanding of steps to manage COPD was assessed' **Authors state that "some questions of BRISTOL COPD Knowledge Questionnaire [BCKQ]" (knowledge and actions needed to prevent or treat COPD exacerbation) were used, but the results are not reported. Methods of assessing outcomes Trained bilingual facilitators assessed outcomes face‐to‐face.
Language of assessment: Cantonese, Mandarin Translation procedure: professional translators translated the written materials and provided back‐translation. In addition, translations were reviewed and commented by COPD patients during initial focus groups. Reliability/validity: for self‐efficacy, a validated tool was used. Timing of outcome assessment: baseline, short‐term (at 4 weeks after randomisation; results not reported) and medium‐term (at 3‐month follow‐up) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: NCT01474707 Funding: funding was provided by an operating grant from CIHR. Additional notes: data were extracted from study report and from information collected at clinicaltrials.gov. Authors were contacted and asked for additional information (e.g. with regard to the knowledge assessments) but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization was applied to assign patients into the study groups, including three experimental groups and one control group. Because of our previous knowledge regarding the re‐effectiveness of educational pamphlets on disease management, we applied an unequal randomization approach to deliberately assign more participants in intervention groups. Our aim was to ensure enrolling adequate numbers of participants in the intervention groups to detect the effect of educational interventions on attainment of self‐management skills. It is a helpful approach, particularly when a 2:1 ratio is employed, and we managed our random allocation close to a 2:1 ratio for each intervention/control pairing." |
| Allocation concealment (selection bias) | Unclear risk | No statement on concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Data collection was conducted by trained bilingual facilitators, blinded throughout the study, as was the data analyst." Personnel were blinded throughout the study. However, due to the nature of the study, participants were most likely aware of the intervention to which they were allocated. This might have affected the results of subjectively measured outcomes. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | "Data collection was conducted by trained bilingual facilitators, blinded throughout the study, as was the data analyst. An identical questionnaire was used in the three different assessments." Outcome assessors were blinded. However, self‐efficacy was measured subjectively with the use of repeated questionnaires. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | No blinding of participants but understanding of pulmonary rehabilitation procedures was objectively measured and inhaler technique acquisition was assessed objectively by two blinded outcome assessors by means of a checklist indicating a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts, therefore the risk of bias is low. |
| Selective reporting (reporting bias) | High risk | "Given the lack of an existing COPD self‐management questionnaire in Chinese language, the study assessment tool also included some questions developed by the research team using the Bristol COPD Knowledge Questionnaire regarding disease‐related knowledge and actions needed to prevent or treat a COPD exacerbation." The results on knowledge were not reported. |
Rosal 2005.
| Study characteristics | ||
| Methods |
Study design: RCT (pilot), 2 arms Geographic location: Massachusetts, USA Ethical approval: yes Recruitment setting: community health centre (CHC), elder health service (affiliated to the CHC) and online (community‐wide database) Method of recruitment: participants were randomly recruited by each recruitment site; the director of each site chose 1 of every 5 individuals from a list ordered by a record number. Length of follow‐up: 6 months after randomisation Dropouts: "Assessment completion rates were 100% at baseline (95% CI = 86%, 100%) and 92% (95% CI = 74%, 99%) at the 3‐ and the 6‐month assessments." No further details reported. A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: low‐income Spanish‐speaking individuals with type 2 diabetes Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, USA Race/ethnicity: Hispanic, Puerto Rican Occupation: 24.0% housewife, 20.0% disabled, 4.0% unemployed, 4.0% never worked, 48.0% pension Gender:
Education: 50.0% ≤ 5th grade, 24.0% 6th to 8th grade, 24.0% 9th to 12th grade Socioeconomic status/income (annual): 84.0% ≤ USD 10,000, 16.0% USD 10,001 to 20,000 Health insurance: 40.0% Medicaid only, 60.0% Medicaid and supplemental Age (years), mean (SD), range: 62.6 (8.6), 45 to 82 Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: self‐management intervention for metabolic self‐control in individuals with type 2 diabetes Theoretical framework: Social Cognitive Theory, intervention delivery was guided by the patient‐centred counselling model Description: the intervention consisted of an initial 1‐hour individual session, followed by 10 weekly 2.5‐ to 3‐hour group sessions and 2 15‐minute individual sessions during the 10‐week period immediately prior to the group sessions. The programme was designed to improve diabetes knowledge, attitudes and self‐management skills. For the intervention purpose, a soap opera was read aloud in the group session, which conveyed diabetes‐related cues in the context of a love story, as well as self‐management and successful coping strategies regarding barriers to diabetes self‐management. To enhance the intervention effect, pauses were made during the reading to discuss and emphasise certain aspects. In addition, the intervention used a traffic light system developed with the participants to visually depict educational messages.
Comparator Type: written information (simple booklet) Description: control group participants and intervention group participants received a simple booklet describing the importance of lifestyle factors regarding diabetes management and providing recommendations for diet, physical activity and self‐monitoring of blood glucose (SMBG). Note: the control condition was included to provide data on the feasibility of conducting a future RCT with the target population. |
|
| Outcomes | Outcomes assessed in the study: psychosocial variables (diabetes knowledge, self‐efficacy for diet, exercise, self‐monitoring, oral glycaemic agents, insulin, depression, diabetes‐related quality of life), physiological variables (HbA1c, percentage in HbA1c, total cholesterol, high‐density/low‐density lipoprotein, triglycerides, Log (triglycerides), BMI, waist circumference, systolic/diastolic blood pressure), behavioural variables (physical activity, blood glucose self‐monitoring*, dietary intake in total kcal, total fat, saturated fat, total carbohydrates, fibre (no composite score reported)) Outcomes considered in this review
*Prioritised outcome in the category 'health behaviour' based on consensus opinion of the authors Methods of assessing outcomes Assessments were telephone administered by a trained, native‐Spanish‐speaking dietitian (only health behaviour) or interviewer, respectively.
Note: details of the tools were taken from various publications, cited by the study authors (ADKnowl: Speight 2001, IMDSES: Bernal 2000, CES‐D: Sawyer‐Radloff 1977). It is unclear whether the information also applies to the adapted versions. Psychometric properties originate, according to study authors, from "preliminary psychometric data of the adapted scales". Adaption of the tools included the (1) modification for telephone administration by an interviewer and (2) qualitative analysis utilising cognitive interviewing to assess clarity, understanding of instructions and wording of the items for the target population. Language of assessment: Spanish Timing of outcome assessment: short‐term (3 months after randomisation, which was 2 weeks after the programme was completed), and medium‐term (6 months after randomisation) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: not reported Funding: the study was supported by an American Diabetes Association Innovation Awards supported in part by Novo Nordisk Pharmaceuticals. Additional notes: authors were contacted and asked for additional information (e.g. gendered scores) but provision was not possible (no longer access to data set). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A list of individuals with type 2 diabetes was randomly generated by each recruitment site (all individuals with a diagnosis of type 2 diabetes at each site had an equal chance of being selected to be in the list), with the director of each site choosing one of every five individuals from a list ordered by record number (...) Upon recruitment and attainment of baseline information, individuals were randomized into either an intervention or a control condition (...) Participants were grouped as closely as possible by age, gender, and insulin status (whether or not they used insulin) and randomized to intervention or control in a 3:2 ratio." Some minor baseline differences for some variables are reported. However, the sample size is very small and the randomisation procedure indicates that these imbalances probably occurred by chance. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, personnel and participants were not blinded; results of subjectively measured outcomes might be biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | "In addition, psychosocial measures were previously adapted for use with this population, and assessments were conducted by interviewers who were blind to treatment condition." Interviewers were blinded to study condition, but participants were not. Subjective outcomes were measured with repeated questionnaires. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Interviewers were blinded to study condition, but participants were not. However, knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Assessment completion rates were 100% at baseline (95% CI = 86%, 100%) and 92% (95% CI = 74%, 99%) at the 3‐ and the 6‐month assessments." It is unclear if there were any imbalances in the dropout rates between intervention and control group. However, the overall attrition rate is low, indicating a low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section are reported in the results. |
Rosal 2011.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Massachusetts, USA Ethical approval: yes Recruitment setting: 5 CHCs Method of recruitment: research co‐ordinators screened participants and obtained primary care providers' (PCP) approval for participation of screened patients; the co‐ordinators sent letters signed by PCPs informing patients about the study and then contacted the patients; eligible and interested individuals were scheduled for a recruitment visit where consent procedures were implemented. Length of follow‐up: 12 months (total programme duration) Dropouts: no dropouts A priori calculation of effect size/power?: yes |
|
| Participants |
Description: low‐income Latinos with type 2 diabetes Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, USA Race/ethnicity: (Caribbean) Latinos Occupation (n = 230): 11.3% working full or part‐time, 3.5% unemployed/looking for a job, 61.7% disabled, 10.9% retired, 12.6% housewife Gender:
Education (n = 250): 28.0% ≤ 4th grade, 28.0% 5th to 8th grade, 19.2% 9th to 12th grade (not high school graduate), 24.8% ≥ high school Socioeconomic status/income (annual) (n = 217): 55.3% < USD 10,000 Health insurance: 89.3% public insurance, 6.0% commercial insurance, 2.8% free care, 2.0% no insurance Social capital: 25.8% married or living with partner, 39.0% divorced/widowed/separated, 25.2% never married Age (years): 16.3% 18 to 44 y, 29.8% 45 to 54 y, 32.9% 55 to 64 y, 21.0% ≥ 65 y Health literacy (baseline) Not measured Note: literacy was assessed by self‐reported education (56% of participants had a formal education ≤ 8th grade). |
|
| Interventions |
Intervention: diabetes self‐management intervention “Latinos en Control” Theoretical framework: Social‐cognitive Theory, Adult Learning Theory Description: 1‐year diabetes self‐management programme consisting of an intense phase and a follow‐up phase of face‐to‐face group sessions. In the first session, participants received a 1‐hour personalised counselling and cooking. In addition, participants were provided with a pedometer to self‐monitor health‐related behaviour and physical indicators. The intervention sessions concerned healthy nutrition and food preparation. During group sessions, each participant spent about 10 min in a one‐on‐one discussion with research staff to talk about behavioural goals, assess progress, feedback and facilitating improvements. Each session, participant’s received feedback on their blood glucose variability and their self‐management behaviour.
Comparator Type: usual care (no additional intervention) Description: usual care |
|
| Outcomes | Outcomes assessed in the study: diabetes knowledge, self‐efficacy in diabetes management, physical activity, blood glucose self‐monitoring, HbA1c, dietary intake, diet Note: no composite score for dietary intake and diet reported. Outcome measures considered in this review
*Prioritised outcome in the category 'health behaviour' based on consensus opinion of the authors. Methods of assessing outcomes
Note: details of the tool have been taken from publications cited by the study authors (Rosal 2003; Speight 2001). It is unclear whether the information also applies to the adapted version and whether the 104‐item subset was used. Psychometric properties originate according to study authors from "preliminary psychometric data of the adapted scales". Adaption of the tools included the (1) modification for telephone administration by an interviewer and (2) qualitative analysis utilising cognitive interviewing to assess clarity, understanding of instructions and wording of the items for the target population. The ADKnowl was translated and cross‐checked in several stages by several professional English‐ and Spanish‐native translators.
Note: the tool has been previously developed and validated by study authors; to be found in Wang 2013.
Language of assessment: bilingual (English or Spanish) Translation procedure: translated, validated versions Reliability/validity: self‐efficacy: Cronbach's α = 0.85; not reported for knowledge Timing of outcome assessment: short‐term (12 months after randomisation, immediately after completion of the intervention programme) Results stratified according to gender: no |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: not reported Funding: funding was provided by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant (no. R18‐DK‐65985) and a grant from the Robert Wood Johnson Foundation and Novo Nordisk Pharmaceutical (to Milagros C. Rosal). Additional notes: authors were contacted and asked for additional information (e.g. gendered scores) but provision was not possible. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was at the individual level and stratified by site, sex, HbA1c level, and insurance status. Within each strata, subjects were randomized in randomly allocated blocks.” |
| Allocation concealment (selection bias) | Unclear risk | “Given the nature of the study, we could not blind participants’ PCPs; however, providers were not informed of their patients’ study assignments.” Not clearly stated whether blinding refers to concealed allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, personnel and participants could not be blinded, indicating a high risk of bias for subjectively measured outcomes. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Subjective outcomes were measured with the use of repeated questionnaires and participants were not blinded to group allocation. This might have introduced bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 252 patients were enrolled and participated in the study, with 128 randomized to the control condition and 124 randomized to the intervention condition." Follow‐up data are reported for 252 participants, so it can be concluded that the outcome data are complete, indicating a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section are reported in the results of the paper. |
Soto Mas 2018.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Texas, USA Ethical approval: yes Recruitment setting: general population Method of recruitment: local Spanish radio and television stations announced study Length of follow‐up: no follow‐up Dropouts: 18 in the intervention group and 8 in the control group were excluded from analysis (completed less than 75.0% of sessions) A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: Spanish‐speaking adults with low to intermediate English proficiency Health topic:
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: only participants who completed more than 75% of the sessions were included in the final analysis. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years) (n = 145): 2.2% < 1 y, 12.7% 1 to 3 y, 8.3% 4 to 7 y, 70.2% 8 y or more, 6.6% missing Race/ethnicity: Latinos Gender:
Education (n = 154): 5.2% elementary school, 11.7% middle school, 40.9% high school, 18.8% associate/technical degree, 20.1% bachelor's degree, 1.9% master's degree, 1.3% doctoral degree Age (years): 9.0% 20 to 30 y, 38.7% 31 45 y, 52.3% ≥ 46 y Note: complete data provided only for n = 155 analysed participants. Health literacy (baseline) Assessment tool, range, level: English TOFHLA (full version) 0 to 100, ≤ 59 inadequate, 60 to 74 marginal, 75 ≤ adequate
|
|
| Interventions |
Intervention: Health literacy and ESL curriculum Theoretical framework: theories of health literacy and health behaviour, sociocultural approaches to literacy and communication, Adult Learning Theory Description: the intervention consisted of a conventional ESL course, which was extended by health literacy‐related content and skills development. It focused on improving English proficiency in listening, speaking, reading and writing while developing health literacy and cardiovascular disease prevention knowledge skills. The health literacy curriculum consisted of 12 separate units that opened with a vignette in Spanish language describing the experiences with health and the healthcare system of a recently arrived immigrant family. The content addressed the development of skills related to prose, documents, numeracy, clinical practices, preventive practices and navigation of the health care system.
Comparator Type: usual care (standard ESL course without additional information) Description: a second teacher delivered conventional curriculum to all control groups, the conventional ESL programme is not specific to health literacy but, it includes content related to civic and life skills (e.g. make an appointment, use community resources, communicate schedule information) and maths (e.g. complete a bar graph, calculate net pay), in addition, 2 units are related to health “ailments and injuries,” and “food and nutrition.” Note: standard ESL curriculum already includes health related topics. Therefore, control group assignment might not be accurate. |
|
| Outcomes | Outcomes assessed in the study: functional health literacy, cardiovascular health behaviour Outcomes considered in this review
Methods of assessing outcomes Self‐administered questionnaires, health literacy assessment, but in group setting; general completion instructions were read out loud to the group.
Language of assessment: English (health literacy) and Spanish (health behaviour) Translation procedure: the CRC was a translated version; not reported for health literacy Reliability/validity: validated tools Timing of outcome assessment: baseline and short‐term (immediately after intervention at 6 weeks after first session) |
|
| Health literacy |
Definition: “The degree to which individuals can obtain, process, and understand the basic health information and services they need to make appropriate health decisions.” (Ratzan 2000, pp. v‐vi) Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was provided by the National Heart, Lung, and Blood Institute, National Institutes of Health (Title: Health Literacy and ESL: Integrating Community‐Based Models for the U.S.‐Mexico Border Region. No. 1R21 HL091820‐01A2. PI: Francisco Soto Mas). Additional notes: the study was reported in multiple publications. For an overview of the included reports linked to this study, see (Soto Mas 2018). Gendered scores for health behaviour were provided by the study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Those who met all requirements were randomly assigned to either the intervention or control group. When more than one family member or relative qualified, only one person per household was selected for the study." "Years in the US (P=0.024) and level of education (P=0.022) were the only demographic variable unbalanced between intervention and control at baseline with controls more likely to have lived in the US longer and more likely to have less than high school education. The intervention group had higher TOFHLA and higher numeracy scores at baseline compared to controls." Insufficient information to permit judgement of low risk or high risk, as the method of randomisation is not reported. |
| Allocation concealment (selection bias) | Unclear risk | No statement on allocation concealment. Therefore, information is insufficient to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants and personnel was not possible and cardiovascular health behaviour was subjectively measured. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Cardiovascular health behaviour was measured via self‐report and participants were not blinded to group allocation. This might have introduced bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants and personnel were not blinded but health literacy was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All participants who attended the last session completed the posttest. Only participants who completed more than 75% of the sessions were included in the final analysis." The dropout rate was higher for the intervention group compared to the control group (N = 18 vs N = 10); no intention‐to‐treat analysis was performed, but a completers only analysis was done. However, reasons for dropouts were transparently given, and intervention and control only differed in their content, so that the imbalanced dropout rate was presumably not caused by the intervention. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods were reported in the results of the publications. |
Sudore 2018.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: California, USA Ethical approval: yes Recruitment setting: 4 primary care clinics within the San Francisco Health Network Method of recruitment: a Health Insurance Portability and Accountability Act (HIPAA) waiver was obtained to identify individuals who met inclusion criteria and exclusion criteria and had upcoming primary care appointments. After receiving clinician approval, recruitment letters were sent, written at a 5th‐grade reading level in English or Spanish. If patients did not opt out, staff called them to assess interest and eligibility. Length of follow‐up: 15 months after randomisation (12 months post‐intervention) Dropouts: 29 withdrew from intervention group (7 lost interest, 1 was too sick, 9 took study too long, 4 found study upsetting, 3 were too busy, 5 other reasons, not further described); 21 withdrew from control group (5 lost interest, 2 were too sick, 3 took study too long, 1 found study upsetting, 2 were too busy, 8 other reasons, not further described) Note: dropouts are reported for both English and Spanish‐speaking participants separately in a supplement file (eTable1). A priori calculation of effect size/power?: yes |
|
| Participants |
Description: chronically or seriously ill elderly Latinos Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: intention‐to‐treat analysis was performed to account for missing data. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean: 26 Race/ethnicity: White Latino or Hispanic (98.9%), White non‐Latino or Hispanic, Multiethnic or other Gender:
Religion: 49.9% fairly to extremely religious, 59.6% fairly to extremely spiritual Education: 83.6% ≤ high school Socioeconomic status/income: 27.4% not enough to make ends meet Social capital (measure of total support score): 36.7; 37.5% in a marriage or long‐term relationship, 88.8% have adult children, 98.0% have a potential surrogate Age (years), mean (SD): intervention group: 64 (6.8); control group: 64 (7.2) Health literacy (baseline) Assessment tool, range, level: S‐TOFHLA, 0 to 36, 0 to 22 limited, 23 to 36 adequate
Note: BHLS in Spanish and English was used for block randomisation (inadequate vs adequate); C‐index = 0.82, (0.77 to 0.87) for inadequate health literacy |
|
| Interventions |
Intervention: advance care planning programme “PREPARE” and AD intervention Theoretical framework: Social‐cognitive Theory (Bandura 1977; Bandura 2002; Bandura 2004), Transtheoretical Model (Prochaska 1997), interpersonal communication competence model (Spitzberg 1984; Street 1995; Street 2003) Description: the intervention consisted of a patient‐directed, online‐advance care planning programme written at 5th grade reading level that participants read in English or Spanish; voice‐overs of texts and closed‐captioning of videos were provided (www.prepareforyourcare.org). The website consisted of 5 modular skill‐building steps and personal values questions about the participant's medical care, the creation of an action plan and participants’ individual wishes. Additionally, participants received an easy‐to‐read written Advance Directive (AD) to take home alongside the summary of wishes, PREPARE information in pamphlet, booklet and DVD format and the website login. Before the doctor’s visit, participants were reminded to talk to their physician about the PREPARE materials.
Comparator Type: written information on the same topic Description: easy‐to‐read AD in English or Spanish to read in research offices and to take home. |
|
| Outcomes | Outcomes assessed in the study: documentation of new advance care planning (ACP), depression, anxiety, ACP behaviour change and action processes, ease of use and satisfaction with PREPARE, communication quality*, satisfaction with communication*, satisfaction with decision‐making*, care consistent with current goals*, barriers to ACP*, attitudes about ACP* Outcomes considered in this review
* results are not reported. Methods of assessing outcomes Face‐to‐face or phone‐based assessment by blinded interviewer.
Notes: all notes in the medical record were handsearched; forms and discussions were assessed separately; 2 independent, blinded reviewers double‐coded primary outcomes.
Note: authors refer to depression and anxiety as adverse events. According to our pre‐defined outcome categories, we report only anxiety as a potential adverse event related to the intervention.
Language of assessment: Spanish Reliability/validity: validated tools Timing of outcome assessment: long‐term (15 months after randomisation, which was at 12‐month follow‐up) Adverse events: adjusted mean depression and anxiety scores did not differ between study arms. |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: NCT01990235 Funding: funding was provided by grant from the National Institutes of Health (NIH) National Institute on Aging (NIA) (no. R01 AG045043) and a Patient‐Centered Outcomes Research Institute (PCORI) Award (CDR‐1306‐01500). Funding was obtained by Rebecca L. Sudore. Additional notes: the trial is reported in multiple publications including results of qualitative formative research. We have chosen the publication in which the results of the primary outcomes are reported. For a full overview of included publications related to this study, see Sudore 2018 [https://revman.cochrane.org/#/296117111501030413/dashboard/htmlView/1.203.173?revertEnabled=false&versionWithProductionChanges=false#STD‐Sudore‐2018]. Baseline characteristics and results for both Spanish‐speaking and English‐speaking participants were reported separately. We only used the data available for Spanish‐speaking participants and calculated relative numbers, when necessary, based on the reported information. Gendered scores for the outcome documentation of ACP planning were obtained from the study authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A statistician not involved in recruitment or data collection uses a computer‐based random number generator to create a randomisation scheme using block randomisation by health literacy (adequate health literacy vs limited health literacy, as determined by a validated question concerning confidence with medical forms). Random block sizes of 4, 6 and 8 are used to ensure an equal number of patients with limited health literacy in each group. Randomisation information is associated with a unique patient identification number and is kept separate from other patient data." Higher rate of prior documentation of ACP among Spanish speakers in the AD‐only arm compared with Spanish speakers in the PREPARE arm. However, the type of randomisation indicates random imbalances. |
| Allocation concealment (selection bias) | Low risk | "Clinicians were blinded. Participants could not be blinded but were told during consent there was a “50‐50 chance” of getting 1 of 2 ACP interventions, and the nonassigned intervention was not described." This method of randomisation reduces foreknowledge of group allocation, indicating a low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants are told that each research participant will review one of two guides, but study participants are blinded as to which guide is the active intervention and which is the active control. Since each group obtains ACP materials, such as the easy‐to‐read advance directive, blinding is enhanced." Besides best attempts to blind the participants, the nature of these interventions does not allow for complete blinding of the participants. However, since participants only knew that they would review one of two ACP materials, the risk of bias is reduced to a low to moderate level. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | Low risk | "Participants are told that each research participant will review one of two guides, but study participants are blinded as to which guide is the active intervention and which is the active control. Since each group obtains ACP materials, such as the easy‐to‐read advance directive, blinding is enhanced. To ensure blinding of all outcome assessments, research staff who conduct follow‐up interviews are never the same staff member who completed the baseline interview and randomisation for that participant. At the start of all follow‐up interviews, participants are reminded not to discuss the study materials they reviewed. If, however, during the follow‐up interview, the research assistant becomes unblinded (eg, the participant mentions the PREPARE website), this information is noted in our database, and the participant is assigned to a new blinded research assistant for all subsequent interviews." See comment above. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | "All primary outcome data were double‐coded by 2 independent, blinded reviewers as described in the trial protocol in Supplement 1". Personnel were blinded for outcome assessment. ACP documentation is an objective outcome, as it does not require subjective judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors report the numbers of participants lost to follow‐up in a CONSORT diagram and provide reasons for dropouts. An intention‐to‐treat‐analysis was performed, indicating a low risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Results for the outcomes communication quality, satisfaction with communication, satisfaction with decision‐making, care consistent with current goals, barriers to ACP and attitudes about ACP are not reported. However, these measures were not pre‐specified at clinicaltrials.gov, but in one of the two published study protocols (see secondary reference, Sudore 2016). It is unclear whether these measures were used as process variables or whether it was intended to assess these as outcome variables, and whether the results for these outcomes are yet to be published. |
Taylor 2011.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT, 2 arms Geographic location: British Columbia, Canada Ethical approval: yes Recruitment setting: 6 community‐based organisations that provide ELSA education Method of recruitment: a regular ESL‐class teacher and a project teacher collaborated for recruitment; a regular teacher explained the purpose and eligibility criteria for the study, but all students could attend the health education class. Project staff then distributed Chinese language recruitment flyers (which provided detailed information about the project) and answered questions. Length of follow‐up: 6 months Dropouts: 38 refused to complete a follow‐up survey, could not be contacted after multiple attempts or had disconnected phones and/or email addresses. Thereof, 15 in the intervention group and 23 in the control group. Note: dropout rates are not displayed per study arm. A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: Asian immigrants visiting ESL class Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 40 classes were randomised to hepatitis B curriculum and 40 classes were randomised to physical activity curriculum; 218 fulfilled inclusion criteria. Analysis included only the participants who provided follow‐up data (180). Generalised estimating equations were used to account for cluster‐randomisation. PROGRESS‐Plus Place of residence: urban, Canada Time living in host country (years): 45.0% < 2 y, 55.0% ≥ 2 y Race/ethnicity: Asian Gender:
Education (years): 65.0% < 16 y, 35.0% ≥ 16 y Social capital: 86.0% currently married, 14.0% not currently married Age (years): 46.0% < 40 y, 54.0% ≥ 40 y Note: data are provided only for analysed participants. Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: ESL Curriculum addressing Hepatitis B Theoretical framework: Health Behavior Framework (Curry 1994) Description: the ESL curriculum consisted of partner exercises and group exercises related to hepatitis B including information about the high rate of HBV infection in Chinese‐Canadian communities, the ways in which hepatitis B can be transmitted from one person to another and potential consequences of hepatitis B infection. At the end of the ESL classes, students received a pamphlet (with Chinese and English text) entailing key learning points.
Comparator Type: unrelated health literacy intervention Description: 3‐hour ESL curriculum about physical activity |
|
| Outcomes | Outcomes assessed in the study: hepatitis‐B‐related knowledge, hepatitis B testing Outcomes considered in this review
Methods of assessing outcomes An interviewer conducted a telephone interview at 6‐month follow‐up.
Language of assessment: Chinese, Farsi, Korean, Punjabi Translation procedure: study material (e.g. consent form and questionnaires) was translated into Chinese, Farsi, Korean and Punjabi using forward‐translation, back‐translation and reconciliation. Reliability/validity: not reported Timing of outcome assessment: only post‐intervention assessment, medium‐term (at 6‐month follow‐up) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was provided by grant (no. R01‐CA‐113663) from the US National Cancer Institute. One of the authors (Dr. C. Bajdik) is the recipient of a Scholar Award from the Michael Smith Foundation for Health Research. Additional notes: authors were contacted and asked for additional information (e.g. knowledge scores) but without success (study too old, authors no longer have access to the data). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A blocked randomization scheme was used whereby classes from each of the six participating community organizations formed a stratum and were randomized within the stratum. Students who had never received serologic testing for HBV were identified from a self‐administered baseline survey. Each student who attended a project class and indicated he/she had never been tested for HBV was asked to complete an interviewer‐administered follow‐up survey six months after attending his/her project class." |
| Allocation concealment (selection bias) | Low risk | "Certified ESL teachers with experience in teaching ELSA level three classes were hired and trained (in either the HBV or physical activity curriculum). Different teachers delivered education to the experimental and control group classes." Project staff collaborated with the regular teacher and project teacher for each class to schedule recruitment and associated project classes. Project classes were generally scheduled within one week of recruitment classes. At each recruitment class, the regular teacher explained that the study would see if health education in English classes can improve immigrants' health; a guest speaker would be coming to the class to provide instruction about a health topic; and only students who spoke Cantonese, Farsi, Korean, Mandarin, and Punjabi were being invited to be part of the study (but all students could attend the health education class). Project staff then distributed recruitment flyers in the study languages (that provided detailed information about the project) and answered questions." The intervention was delivered by externally hired teachers, whereas the project staff and regular teachers informed the participants about the study without mentioning the content of the intervention. Therefore, foreknowledge of group allocation is unlikely for both intervention provider and participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel and participants were not blinded to group allocation due to the nature of the study, but outcomes were objectively measured and not subject to interpretation. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants and personnel were not blinded. However, knowledge was objectively measured by a true/false questionnaire and HBV testing was objectively assessed by verifying self‐reported HBV testing through medical record review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Follow‐up surveys were completed by 180 (83%) of the 218 students who had no history of hepatitis B testing. (The other 38 students refused to complete a follow‐up survey, could not be contacted after multiple attempts or had disconnected phones and/or email addresses). Therefore, our analysis included 180 students." N = 38 refused to complete a follow‐up survey (n = 15 in the intervention group and n = 23 in the control group). The authors report attrition rates per group and provide reasons for loss to follow‐up. Differential loss between the intervention and control group is less than 15%. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported in the methods are reported in the results of the paper. |
| Selective recruitment of cluster participants | Unclear risk | No information about the time point when participants were recruited and enrolled. |
| Other bias | Unclear risk | "Because the study randomization was by group rather than by individual, Generalized Estimating Equations (GEE) were also used for the evaluation. Our multivariable GEE analyses adjusted for the following variables: ESL organization, class time (day versus evening), country of origin (China, India, Iran, or other Asian country), years since immigration (<2 versus ≥2), gender, age in years (<40 versus ≥40), years of education (<16 versus ≥16), and marital status (currently married versus not currently married)." The authors state that they accounted for clustering in the analysis. This does not relate to the data we considered in the meta‐analysis, but we re‐analysed the data with the use of the ICC reported by Han 2017. Therefore, we assume a low risk of bias in this domain. |
Thompson 2012.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Maryland, USA Ethical approval: yes Recruitment setting: urban hospital‐based academic paediatric clinic Method of recruitment: 2 trained bilingual, bicultural research assistants recruited parents in the clinic waiting room; interested parents were consented by the use of an oral consent process. Length of follow‐up: no follow‐up Dropouts: no dropouts A priori calculation of effect size/power?: yes |
|
| Participants |
Description: low‐income Spanish‐speaking parents of infants and toddlers Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 2 participants in the control group were excluded from the analysis because they were missing any responses to the knowledge questionnaire. However, these participants were included in the analysis for the secondary outcome 'planned changes in behaviour'. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean (n = 158): 6.02 Race/ethnicity: Latinos/Latinas Gender (n = 148):
Education (years) (n = 159): 41.0% 6 y or less, 51.0% 7 to 12 y, 8.0% some or all of university degree Socioeconomic status/income: "low‐income" population (Thompson 2012) Health insurance: "More than 95% of clinic patients are publicly insured" (Thompson 2012, p. 413) Social capital (number of children), mean: 2.3 Age (years), mean: 27.55 Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: nutrition education via touchscreen Theoretical framework: behavioural, cognitive and humanistic learning theories, Health Belief Model, cultural targeting strategies Description: the intervention group members viewed 5 culturally and linguistically adapted modules on nutrition and feeding presented on an interactive platform using a touchscreen computer. The modules contained a series of short educational messages and included text, pictures and audio material that accounted for the educational levels and health literacy of the participants. The modules were interactive, meaning questions requiring participants' responses with feedback given. Content was partly tailored based upon these responses.
Comparator Type: usual care (no additional intervention) Description: participants in the control group did not receive any intervention. |
|
| Outcomes | Outcomes assessed in the study: parental nutrition and feeding knowledge, planned changes in behaviour Outcomes considered in this review
Methods of assessing outcomes Face‐to‐face orally administered questionnaires by trained bilingual research assistants
Language of assessment: language concordant Translation procedure (if necessary): back‐translation technique Reliability/validity: developed for the study, no psychometric properties reported Timing of outcome assessment: baseline, short‐term (immediately after intervention) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention (prevent childhood diseases through nutritional failure) |
|
| Notes |
Trial ID: NCT01272492 Funding: funding was provided by Johns Hopkins University. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized by the use of a block randomization process. We used block randomization, 10‐per‐block, to prevent sample size imbalances which could affect the study’s power. At the start of the trial, an opaque container was filled with 10 envelopes with equal representation of intervention and control assignments. The research assistant removed an envelope from this container to determine each participant’s group assignment. After ten participants, she repeated the process." |
| Allocation concealment (selection bias) | Low risk | The randomisation procedure used indicates a low risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not reported and behaviour intent was subjectively measured. It is unclear whether the results were affected. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | Unclear risk | Participants were probably not blinded to group allocation and behaviour intent was assessed using a verbally administered questionnaire. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Unclear blinding but knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Only 2 participants were missing any responses to the knowledge questions.These individuals were not included in the analyses for the total summed knowledge score and the breastfeeding domain‐specific summed knowledge score." No participant was lost to follow‐up and only 2 participants were excluded from the analysis due to missing responses. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported at clinicaltrials.gov are reported in the published reports. |
Tong 2017.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT, 2 arms Geographic location: California, USA Ethical approval: yes Recruitment setting: community, community‐based organisations (e.g. Hmong Women's Heritage Association (HWHA)) Method of recruitment: lay health educators (LHE) were recruited through Hmong radio and HWHA clients. After receiving training on participant recruitment, LHEs recruited participants through their own social networks. Participants were recruited through radio announcements and HWHA clients, each LHE recruited 12 to 15 participants. Length of follow‐up: 6 months after first session (3 months after intervention programme was completed) Dropouts: 1 in the intervention group (could not be contacted), 4 in the control group (could not be contacted) A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Hmong Americans without personal history of CRC Health topic
Inclusion criteria
Note: randomisation was conducted on the level of LHE. The intervention was implemented in 3 time periods (waves). Each LHE participated only in 1 wave. 29 Hmong LHEs (aged 21 to 55, 82.7% women, 14 in the intervention group) were recruited. One LHE in the control group dropped out before study activities began, and that LHE’s 2 participants were assigned to another control group LHE. Exclusion criteria
Intervention group
Control group
PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean (SD), range; distribution: 15.4 (9.7), 1 to 62; 83.6% > 10 y, 16.4% ≤ 10 y Race/ethnicity: Hmong Americans (born in Laos) Occupation: 90.9% not employed Gender:
Education: 88.8% no formal education Socioeconomic status/income (annual): 53.8% < USD 20,000, 4.0% USD 20,000 or more, 42.2% don't know/missing Health insurance: 95.1% insured Social capital: 65.3% married or living with a partner Age (years), mean; distribution: 60.4, 73.3% 50 to 64 y, 26.7% 65 to 75 y Health literacy (baseline) 88.8% of participants had no formal education, indicating low literacy even in their native language. |
|
| Interventions |
Intervention: CRC education Theoretical framework: Social‐cognitive Theory (Bandura 1977; Bandura 2002; Bandura 2004), Transtheoretical Model (Prochaska 1997) Description: LHEs were trained to deliver CRC prevention information. The intervention addressed (1) knowledge of CRC risk and prevention, (2) expectations about CRC screening, (3) self‐efficacy and (4) intention (motivation and readiness to obtain screening). A CRC flip chart was supposed to encourage CRC screening by describing needs and benefits of screening, screening frequency and barriers to screening. For the flip chart, cultural images and translation were adapted.
Comparator Type: unrelated health literacy intervention Description: 2 lectures on healthy nutrition for cardiovascular health and diabetes prevention delivered by health educators. The follow‐up telephone calls for the control group were conducted by NPA LHEs who asked participants about their diet. |
|
| Outcomes | Outcomes assessed in the study: CRC awareness, CRC knowledge**, CRC ever screening, up‐to‐date CRC screening* Outcomes considered in this review
*Prioritised outcome measure based on consensus decision of the authors; **We only report the results of CRC knowledge as awareness reflects subjective rather than objective knowledge of colorectal screening measures. Methods of assessing outcomes
Language of assessment: bilingual (Hmong and English) Note: translation procedure and reliability/validity were not reported. Timing of outcome assessment: medium‐term (6 months after first session) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: NCT01904890 Funding: funding was provided by the National Cancer Institute (no. U54 CA153499). Tung T. Nyguyen, Susan Stewart and Moon S. Chen, Jr. contributed funding acquisition. Additional notes: We would have included CRC screening intention (reported as an outcome measure at clinicalTrials.gov) in our analysis as an outcome measure for "apply" health information, but results are not reported. Authors were contacted and asked for additional information (e.g. gendered scores) but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used a two‐arm cluster randomized controlled trial (RCT), with clustering at the level of the LHEs, who were recruited through Hmong radio and HWHA clients. After receiving training on participant recruitment, LHEs recruited participants through their own social networks. Some participants were recruited through radio announcements and HWHA clients. LHEs were randomized by a computer programme to either the intervention or control arm after completing recruitment." Randomisation was conducted at the level of the LHE. The LHE recruited the participants on their own. However, since the LHE educators were randomised after completing the recruitment, the risk of selective recruitment of cluster participants is low. |
| Allocation concealment (selection bias) | Low risk | "The LHEs were trained on protection of human subjects in recruitment and participation but did not administer consent. Following the training, each LHE recruited 12–15 participants using a script describing the purpose of the project and scope of participant involvement. After completing recruitment and being randomized, the intervention LHEs received a second training session to conduct small group sessions and deliver CRC information. The control LHEs did not receive a second training session as the HWHA staff delivered the NPA information." "Third, it is possible that LHEs may choose participants who may be more likely to get screening, but we attempted to deal with this selection bias by blinding LHEs and participants to study arm assignment until after recruitment was completed.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to the nature of the study and CRC screening was assessed via self‐report. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | CRC screening was assessed via self‐report and participants were not blinded to their allocated group, which might have introduced bias. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | No blinding but knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The retention rate at 6‐month follow‐up was 98%, with 5 participants who could not be contacted." "All participants were included in analyses regardless of prior CRC screening history. Generalized estimating equations (GEE) were used in all models to account for clustering by LHE. Analyses were conducted on an intention‐to‐treat basis, with baseline values carried forward for dropouts. All analyses were conducted with SAS software, version 9.3 (SAS Institute, Inc., Cary, NC); statistical significance was assessed at the 0.05 level (2‐sided)." N = 1 in the intervention group and N = 4 in the control group dropped out, with reasons provided. The attrition rate indicates a low risk of bias, as outcome data are available for nearly all participants randomised. |
| Selective reporting (reporting bias) | High risk | CRC screening intention was pre‐specified as an outcome measure at clinicaltrials.gov, but the results are not reported. |
| Selective recruitment of cluster participants | Low risk | Participants were recruited prior to randomisation of the LHE, indicating a low risk of recruitment bias. |
| Other bias | Unclear risk | "Generalized estimating equations (GEE) were used in all models to account for clustering by LHE. Analyses were conducted on an intention‐to‐treat basis, with baseline values carried forward for dropouts. All analyses were conducted with SAS software, version 9.3 (SAS Institute, Inc., Cary, NC); statistical significance was assessed at the 0.05 level (2‐sided)." The authors accounted for clustering by LHE. We re‐analysed the data for the outcome 'up‐to‐date colorectal cancer screening', but the results for the outcome 'knowledge' were not reported in a way in which we could verify if adjusted values were reported (proportions of correct answers were reported only) and the data could not be re‐analysed. Thus, we do not know if a unit of analysis error is present for the outcome 'knowledge'. |
Unger 2013.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: California, USA Ethical approval: not reported Recruitment setting: 3 community adult schools Method of recruitment: students enrolled in all classes were invited, except for classes related to medical education (e.g. medical assistant) Length of follow‐up: 1 month Dropouts: no dropouts A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: healthy immigrant Latinos currently enrolled in community adult schools Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 185 participants were randomised either to intervention or control group, 135 were analysed. 18 were excluded from the analysis because they did not self‐identify as Hispanic/Latino (3 were White, 3 were African American, 1 was "Other" and 11 did not answer the question). Authors provided numbers of participants randomised and analysed on request. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years): 43.2% 11 y or more, 18.7% 6 to 10 y, 13.7% 1 to 5 y, 5.8% less than 1 y, 2.9% missing Race/ethnicity: Hispanics/Latinos Gender:
Note: not reported per arm Education: 62.6% less than high school, 37.4% high school or more Age (years), mean (SD), range; distribution: 35.8 (12.9), 18 to 90; 34.5% 18 to 29, 25.2% 30 to 39, 20.9% 40 to 49, 13.7% 50 to 59, 2.9% 60 to 90, 2.9% missing Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: fotonovela "Secret Feelings" Theoretical framework: Theory of Planned Behavior, Theory of Reasoned Action (Ajzen 1991; Fishbein 1975) Description: participants read the fotonovela "Secret Feelings", a 30‐page comic book‐sized fotonovela, printed in Spanish and English at 4th grade reading level. The fotonovela was about a Latino family coping with depression. The main educational messages embedded in the narrative are that (1) depression is a real and serious medical condition that affects a person's functioning, (2) people with depression should seek professional help and (3) treatment for depression is available and effective.
Comparator Type: written information on the same topic Description: participants received an evidence‐based text pamphlet "Depression" by the National Institute of Mental Health (NIH publication 08 3561), which conveys similar information in a non‐narrative format, 26 pages, targeted to low literacy audience, publicly available in Spanish and English, language according to preference. |
|
| Outcomes | Outcomes assessed in the study: depression knowledge, willingness to seek help for depression, self‐efficacy to identify depression, stigma about mental health care, antidepressant stigma, dissemination of fotonovela through social networks Outcomes considered in this review
Methods of assessing outcomes Self‐administered questionnaires
Language of assessment: Spanish and English according to preference (each question was shown in both languages) Translation procedure: back‐translation technique (applies to literacy and self‐efficacy) Timing of outcome assessment: immediately before and after intervention, and at 1‐month follow‐up |
|
| Health literacy |
Definition(s): "Health literacy is the degree to which people have the capacity to obtain, process, and understand health information to make appropriate health decisions" (Kutner 2006). "Mental health literacy (knowledge about mental health disorders and treatments); stigmatization of depression; attribution of depression to non‐medical causes including nervios (nerves), fallo mental (mental deficiency or failure), and locura (craziness); reluctance to discuss emotional problems with strangers, and reluctance to take antidepressant medication" (Unger 2013, p. 399). Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: not reported Additional notes: we only report on the results of time point 1, which was immediately after the intervention, as "several students shared their fotonovelas with students in the text pamphlet group after the posttest." (Unger 2013, p. 405). Therefore, results of the 1‐month follow‐up might be biased. Authors provided information on numbers randomised to each study arm on request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The data collector gave each participant an envelope containing a pretest survey, a Fotonovela or text pamphlet, and a posttest survey. The envelopes were shuffled randomly prior to the data collection so that assignment of students to experimental condition would be random." This randomisation method introduces a low risk of bias. Baseline imbalances were not reported. |
| Allocation concealment (selection bias) | Low risk | "Participants were instructed to open their envelopes and fill out the pretest survey." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, blinding of participants and personnel was most likely not possible. Therefore, the results of subjective outcomes are possibly biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Participants were not blinded to group allocation and subjective outcomes were assessed with repeated questionnaires. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Knowledge was measured objectively and was not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Pretest and posttest data were collected from 185 students. Of those, 157 (85 %) completed the 1‐month follow‐up. Of those, 18 were excluded from the analysis because they did not self‐identify as Hispanic/Latino (3 were White, 3 were African American, 1 was ‘‘Other’’, and 11 did not answer the question). The remaining 139 students were included in the analytic sample." The authors provided additional information on the total numbers randomised on request; differential loss between the intervention and control group is less than 15%. No intention‐to‐treat analysis was performed, but a completers only analysis was done. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported int the methods were reported in the results of the paper. |
Valdez 2015.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Santa Clara County, CA, USA; Los Angeles, CA, USA Ethical approval: yes Recruitment setting: Latino study population was recruited in Santa Clara County, CA, USA, Korean study population was recruited in Los Angeles, CA, USA; no further information reported Method of recruitment: participants were recruited by a trained, bicultural, research assistant in their respective region. Length of follow‐up: 4 weeks after intervention Dropouts: in total, 100 participants were not included in the analysis, 74 in the intervention group and 26 in the control group. It is unclear, whether the participants did not complete pre‐ and/or post‐intervention assessment or if they were excluded for other reasons. A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Latino and Korean American parents Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: from the intervention group 167 participants were located in Los Angeles and 197 were located in San Jose. From the control group 153 were located in Los Angeles and 191 were located in San Jose. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years), mean; distribution: 14.3; 93.6% foreign‐born, (n = 700) 14.9% < 5 y, 18.9% 6 to 10 y, 28.4% 11 to 15 y, 37.9% 16+ y Race/ethnicity: Latino and Korean American Gender (n = 707):
Education (years of formal education): 19.6% < 6, 16.7% 7 to 11 y, 18.5% 12 y, 9.9% 13 to 15 y, 35.3% 16+ y Social capital (number of children (mean; distribution); marital status): 2.8; 52.3% 1 to 2, 39.4% 3 to 4, 8.3% 5+; 72.7% married/living together Age (years), mean; distribution (n = 691): 41.7; 12.3% < 35 y, 22.3% 35 to 39 y, 34.6% 40 to 44 y, 17.2% y, 11.2% 50+ y Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: educational intervention for HPV vaccine Theoretical framework: Theory of Planned Behaviour (Ajzen 1991) Description: the intervention consisted of an educational DVD that delivered evidence‐based information about cervical cancer. DVD content addressed 3 main topics: (1) HPV, (2) the association between HPV infection and cervical cancer, and (3) key aspects of HPV vaccine. Participants watched the DVD in privacy in their homes at an individually convenient time.
Comparator Type: written information on the same topic Description: participants in the control arm received a language‐appropriate CDC flyer on HPV vaccine. |
|
| Outcomes | Outcomes assessed in the study: HPV and cervical cancer knowledge, decisional conflict, made informed decision regarding HPV vaccination Outcomes considered in this review
*We report on the results of the following subscales: informed decision, values clarity and support. The subscales uncertainty and effective decision presume a completed decision, thus rather reflecting the processing step of applying health information; **Prioritised outcome for the category 'health literacy ‐ applying health information' based on consensus decision of the authors. Methods of assessing outcomes Outcomes were assessed via telephone interview.
Language of assessment: per preference Translation procedure: HINTS was available in English and Spanish, CHIS was also available in Korean; content‐specific questions were developed for the study. Timing of outcome assessment: baseline, at 1‐month follow‐up |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was provided by the National Institute on Minority Health and Health Disparities Grant No. 2R44MD005198‐03A1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were then randomized, stratified by study site (Los Angeles or San Jose), using a programmed algorithm on the laptop computer and assigned to an intervention or control study arm." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No statement about whether participants or personnel were blinded. Participants in the control group received a CDC flyer, which was most likely publicly available. It is unclear whether the results of subjectively measured outcomes are biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | Unclear risk | Subjective outcome was measured with the use of repeated questionnaires administered via telephone interview. It is unclear whether the interviewer and participants were blinded. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are considerable differences in the numbers of participants analysed between study groups. In total, N = 100 participants were not included in the analysis, n = 74 in the intervention group and n = 26 in the control group. It is unclear whether the participants did not complete pre‐ and/or post‐test assessment or if they were excluded for other reasons. Therefore, the information is insufficient to permit judgement. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section are reported in the results. |
Valdez 2018.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Los Angeles, San Jose and Fresno, USA Ethical approval: yes Recruitment setting: community clinics at 3 sites in California Method of recruitment: participants who visited the community clinics were recruited by bilingual, bicultural female research assistants; a verbally administered screening questionnaire determined eligibility. Length of follow‐up: 6 months post‐intervention Dropouts: attrition rate was 12.8% in Fresno, 18.9% in San Jose and 35.4% in Los Angeles; overall attrition rate was 22.9% (216) Note: 29 participants reported at baseline that they had received a Pap test within the past 2 years (they did not meet the inclusion criteria). The authors included these women in the analysis as being in the contemplation stage ("plans to have a pap test in the next 12 months" (Valdez 2018, p. 223). A priori calculation of effect size/power?: not reported |
|
| Participants |
Description: low‐income Latinas Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: participants were analysed as randomised, but complete cases only. PROGRESS‐Plus Place of residence: urban, USA Time living in host country (years); distribution: 80.0% foreign born, 26.0% 1 to 5 y, 18.0% 6 to 10 y, 20.0% 11 to 15 y, 36.0% 16+ y Race/ethnicity: Latina Gender: 100% female Education (years of formal education), mean (SD); distribution: 8.2 (3.8); 39.0% 1 to 6 y, 34.0% 7 to 11 y, 21.0% 12 y, 6.0% 13+ y Socioeconomic status/ income: criteria for inclusion was annual household income of ≤ USD 24,680 Health insurance: 51.0% insured Social capital (martial status; number of children (mean (SD); distribution): 21.0% single, 43.0% married, 15.0% living together, 15.0% divorced/separated, 5.0% widowed; 3.0 (2.2) children; 10.0% no children, 14.0% 1 child, 21.0% 2 children, 22.0% 3 children, 15.0% 4 children, 18.0% 5+ children Age (years), mean (SD), range: 39.1 (11.8), 21 to 69 Health literacy (baseline) Not measured |
|
| Interventions |
Intervention: Cervical Cancer Education Programme Theoretical framework: transtheoretical model (Prochaska 1997) Description: the intervention included a one‐time education programme delivered through interactive, multimedia touchscreen kiosks. Participants received on‐screen prompts, individualised according to language and age group. The age‐tailored features included behavioural models and multimedia elements to create cultural, linguistic and literacy‐adapted features. The programme incorporated 8 interactive modules. Module content comprised various information on cervical cancer, HPV and Pap testing and how health resources in a treatment setting can be claimed.
Comparator Type: written information on the same topic Description: participants in the control arm received an 8‐panel, 2 colour brochure developed by the Office of Woman's Health of the California Department of Health Services on gynaecological cancers provided in English and Spanish. The procedure corresponds to usual care. |
|
| Outcomes | Outcomes assessed in the study: cervical cancer knowledge, attitudes towards cervical cancer and Pap testing, self‐reported screening behaviour, self‐efficacy regarding Pap testing Outcomes considered in this review
*Self‐efficacy was assessed with three statements. We only report on the results of the statement "Can get a pap smear if needed" as "Every woman should get pap smear" and "Pap smears can save our lives" reflect attitudes and beliefs rather than self‐efficacy; **Health behaviour was assessed with three items: We included one question to assess screening behaviour reported in the study, as"Kiosk main reason for getting a pap test" and "Kiosk information especially influenced decision to get a pap test" do not directly ask for participants' screening behaviour. Methods of assessing outcomes Baseline assessments were delivered through touchscreen kiosk deployed in waiting rooms at the collaborating clinics. Post‐intervention assessments were conducted via structured, language concordant, telephone interviews by bilingual, bicultural, female interviewers. Study used adapted scales from the Pathfinders intervention study conducted by the Northern California Cancer Center (Zapka 2004).
Language of assessment: language concordant (knowledge), Spanish/English per preference (behaviour) Translation procedure: back‐to‐back translation Reliability/validity: added questions were examined for face validity by subject‐matter experts and assessed for clarity and comprehension through individual cognitive interviewing with 10 Latinas. Timing of outcome assessment: baseline, medium‐term (at 6‐month follow‐up) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was provided by the National Cancer Institute, Grant No. 5R44CA093110‐3. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The kiosks were programmemed with an algorithm that used a random number generator to randomly assign participants to study arms. Upon completion of a pretest survey conducted on the kiosks, participants were randomly assigned to either an intervention or control condition with equal probability, stratified by study site and kiosk." |
| Allocation concealment (selection bias) | Low risk | The method used in the randomisation process indicates a low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel were blinded; there is no information on whether participants were blinded. It is unclear whether subjectively measured outcomes were affected. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | Unclear risk | "Participants in both conditions were reassessed at 6 months from baseline through a structured, language concordant, telephone interview by bilingual‐bicultural, female interviewers who were blinded to participants’ group assignment." Health behaviour was measured with the use of questionnaires that were administered via telephone and participants were most likely aware of the intervention they received. It is unclear whether this might have affected the results. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | "Participants in both conditions were reassessed at 6 months from baseline through a structured, language concordant, telephone interview by bilingual‐bicultural, female interviewers who were blinded to participants’ group assignment." Participants were most likely not blinded, but knowledge was objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Attrition rates at post‐test were 12.8 % in Fresno, 18.9 % in San Jose, and 35.4 % in Los Angeles, with an overall attrition rate of 22.9 %." Distribution of dropouts between study groups is not reported and reasons for attrition are not provided. The authors state having performed an intention‐to‐treat analysis, but present results for completers only. It is unclear whether the risk of attrition bias is high or low. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
van Servellen 2005.
| Study characteristics | ||
| Methods |
Study design: RCT (pilot), 2 arms Geographic location: California, USA Ethical approval: yes Recruitment setting: 2 administratively linked HIV community‐based not‐for‐profit clinics Method of recruitment: clinical trial staff screened medical records of the clinic and approached eligible patients by phone and/or letter. Length of follow‐up: 6 months (total programme duration) Dropouts: 9 in the intervention group, thereof 2 after 6 weeks (reason: unable to be reached initially after the instructional component of the programme) and 7 after 6 months; 7 in the control group, thereof 2 after 6 weeks (reason: unable to be reached initially after the instructional component of the programme) and 5 after 6 months A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Latinos with HIV‐infection Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: 93 participants were randomised either to intervention or control group. Authors did not provide numbers on participants randomised to different treatment groups. PROGRESS‐Plus Place of residence: urban, USA Race/ethnicity: Latinos Gender:
Education (years): 81.0% < 12 y Socioeconomic status/income (per month): 41.0% ≤ USD 500 Age (years), mean, range: 40.7, 21 to 78 Health literacy (baseline) Assessment tool, range, level: modified REALM (24 additional HIV‐relevant medical terms), higher score is better
|
|
| Interventions |
Intervention: HIV treatment adherence enhancement programme “Es por la vida” Theoretical framework: no specific Description: the intervention consisted of modular group sessions including (1) basic HIV/AIDS information, (2) barriers and facilitators of adherence management, (3) maintaining quality of life and controlling illness‐related stress, (4) reducing risks related to transmitting HIV and management of substance use (5) and communication skills with healthcare providers and maintaining effective family and community support systems. All materials were read and discussed. There were additional follow‐up phone calls and face‐to‐face conversations with a nurse practitioner focussing on barriers to HIV treatment adherence and strategies to reduce those barriers. Problem‐solving and motivational interviewing strategies were used by reviewing content that has not been fully understood and identifying ways to lower barriers of adherence management, or to identify support systems.
Comparator Type: no health literacy intervention Description: standard clinic care, no additional intervention |
|
| Outcomes | Outcomes assessed in the study: functional HIV health literacy, HIV‐related knowledge, adherence self‐efficacy, medication adherence (self‐report), general health status (self‐report), viral load, relationship and communication with healthcare provider Outcomes considered in this review
Methods of assessing outcomes Questionnaires administered by a bilingual foreign medical
Note: health literacy measures and questions were designed by clinic staff in collaboration with the study team. 24 HIV terms were added to the original set of medical terms of the REALM by keeping with the original format. For example, terms ranged from HIV, virus and symptoms (lower level of difficulty) to terms such as viral replication, protease inhibitors, HIV‐resistant strains (higher level difficulty). Participants were asked first if they had heard these terms (global recognition) and second, whether they could explain them (global understanding).
Note: we prioritised the variable '95% adherence to antiretroviral medication regimen in the past 4 days' over '90.0% adherence in the past 4 days'.
Note: "Most measures were already translated into Spanish but were reviewed again by the bilingual research assistant to ensure proper translation of ideas and concepts. Questions not previously translated were submitted for translation by an independent linguistic and cultural consultant who used the standard multi‐step forward/backward translation with additional evaluation by our bilingual research staff." (van Servellen 2003, p. 288) Language of assessment: Spanish Translation procedure (if necessary): not reported Reliability/validity: no psychometric properties reported (applies to adherence self‐efficacy, medication adherence and health status) Timing of outcome assessment: baseline, at 6 weeks (after group sessions) and at 6 months (short‐term) after randomisation |
|
| Health literacy |
Definition: "According to various reports, the accepted distinguishing characteristics of health‐literate individuals include the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions (IOM, 2004) Furthermore, individuals’ health literacy skills and capacities are influenced by their education, culture, and language (Adams, 2003). It follows that HIV‐related health literacy would include those skills and knowledge to obtain, process, and understand HIV‐related information, and that these skills and knowledge are influenced by the particular educational level, culture, and language of the group in question.” (van Servellen 2005, p. 747) Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: health care |
|
| Notes |
Trial ID: not reported Funding: funding was provided by a grant from the University‐wide AIDS Research programme and State Office of AIDS (no. R00‐LA‐112). Additional notes: we tried to contact the authors to ask for additional information but without success. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Upon enrollment, all participants received a code number from a published table of random numbers and assigned to either the pilot intervention group or comparison group.” |
| Allocation concealment (selection bias) | Low risk | The method of randomisation indicates a low risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study, participants and personnel were not blinded; results of subjectively measured outcomes might be biased. |
| Blinding of outcome assessment (detection bias) subjective outcome measures | High risk | Subjective outcomes were measured with repeated questionnaires and participants were not blinded to group allocation. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants and personnel were not blinded but health literacy and knowledge were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Baseline and 6 weeks (immediately after instructional modular programme) data were available for 41 intervention and 40 comparison group patients. From 6 weeks to 6 months, an additional 5 participants in the comparison group and 7 participants in the pilot group were lost to follow‐up, for an attrition rate of 21% for the intervention group and 17% for the comparison group. Analysis of the characteristics of these 16 patients revealed that they had a poorer understanding of HIV terms (11.00 versus 13.38) [F(1,82) 3.96, p 0.05] and a statistically significant higher viral load than those who remained (99,328 versus 36,973) [F(1,83) 4.34, p 0.04]. They were also less apt to take part in decisions about their care (1.88 versus 2.41) [F(1,82) 4.62, p 0.03]. The numbers of and reasons for participants lost to follow‐up are reported and equal for both the control and intervention group. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported in the methods section are reported in the results of the paper. |
Wong 2020.
| Study characteristics | ||
| Methods |
Study design: RCT, 2 arms Geographic location: Singapore, Southeast Asia Ethical approval: yes Recruitment setting: office of the Humanitarian Organization for Migration Economics (HOME), a non‐governmental organisation located in Singapore Method of recruitment: through social media and HOME Length of follow‐up: 2‐month follow‐up Dropouts: 2 in the intervention group, thereof 1 post‐intervention (reason: repatriated back to the Philippines) and 1 at 2‐month follow‐up (reason: lost to follow‐up), 5 in the control group, thereof 1 post wait‐list measurement (reason: work schedule problem) and 4 at 2‐month follow‐up (reason: lost to follow‐up, not in town, repatriated back to the Philippines) A priori calculation of effect size/power?: yes |
|
| Participants |
Description: Filipino domestic workers Health topic
Inclusion criteria
Exclusion criteria
Intervention group
Control group
Note: the control group attended the programme following completion of the programme by the intervention group. PROGRESS‐Plus Place of residence: urban, Southeast Asia Time living in host country (years) (time working in Singapore), mean, range: 9.45, 1 to 24 Race/ethnicity: Filipino Occupation: working in Singaporean households; number of days off in current job: 58.95% 1/week and public holidays, 66.8% 1/week, 2.5% 2/month, 5.15% 3/month Gender: 100% female Religion: 71.85% Roman Catholic, 28.15% other Christian faith Education (n = 38): 72.0% completed high school (secondary) 4 years, 28.0% completed university Note: 9 ≤ years of formal education was an inclusion criterion. Social capital (n = 38): 48.4% were single or never married, 25.8% were married, 25.8% were separated, divorced or widowed Age (years), mean (SD): 38.6 (6.3) Health literacy (baseline) Assessment tool, range (score): Depression Literacy Questionnaire (D‐Lit, here referred to as "DLQ"), 22 items, true/false questions, 0 to 22, higher score is better (validated tool)
|
|
| Interventions |
Intervention: CBT‐based paraprofessional training programme Theoretical framework: formative research to inform intervention development Description: participants received a CBT‐based paraprofessional group training following a manual from another CBT‐based training that has been previously developed for refugees from Burma in North Carolina (USA). The manual was a version adapted to the needs of foreign domestic workers (FDWs) in Singapore. Participants in the training group attended in HOMEs office 4 weekly English language sessions, held by 2 masters' level clinical psychology trainees. Participants received session handouts and homework practices. The training sessions aimed to support skills regarding depression via didactics, discussions and role‐plays. Training addressed (1) recognition of signs and symptoms of depression, (2) improving attitudes towards treatment‐seeking for depression and (3) provision of basic CBT skills to be able to support peers and to increase awareness of available resources in the community.
Comparator Type: no health literacy intervention (delayed intervention) Description: the wait‐list control group received a delayed intervention immediately after the training group's programme completion. |
|
| Outcomes | Outcomes assessed in the study: depression literacy, CBT knowledge, attitudes towards seeking professional help, self‐confidence in supporting individuals with depression, depression‐related stigma Outcomes considered in this review
Methods of assessing outcomes Outcomes were assessed via questionnaires
Language of assessment: English Timing of outcome assessment: baseline, short‐term (immediately after intervention) and at 2‐month follow‐up (both groups combined) Note: intervention and control group were both assessed at 2‐month follow‐up. The waiting list control group received the training programme immediately after the intervention group's completion (between post‐intervention assessment and follow‐up assessment) and were also assessed at 2‐month follow‐up. The results for the follow‐up assessment are reported for the combined groups only. Therefore, these results could not be incorporated in the analysis (see Table 2 and Table 3) Adverse events: "No participants reported any unintended effects or harms resulting from attending the training program." (Wong 2020, p. 577) |
|
| Health literacy |
Definition: not reported Health literacy components addressed by the intervention Prerequisites and tools
Steps of information processing
Health domain: disease prevention |
|
| Notes |
Trial ID: not reported Funding: funding was obtained by a start‐up Grant awarded to Dr. Shian‐Ling Keng by the Faculty of Arts and Social Sciences in National University of Singapore (NUS) and to Marian Wong as a master's thesis grant by the Department of Psychology at NUS (R‐581‐000‐153‐133). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Forty FDWs were randomized in blocks to either the training group or the WL group based on computer‐generated random numbers (www.randomizer.org)." |
| Allocation concealment (selection bias) | Low risk | "The generation of random numbers and allocation were conducted by an independent research assistant (who was not involved in the recruitment or data collection procedure of the study) based on the sequence of participants’ enrolment into the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Due to the nature of the study, personnel and participants were not blinded, but outcomes were objectively measured. |
| Blinding of outcome assessment (detection bias) objective outcome measures | Low risk | Participants were not blinded to study condition, but depression literacy and CBT knowledge were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In total, n = 2 in the intervention group dropped out, of which n = 1 post‐intervention (repatriated back to the Philippines) and n = 1 at 2‐month follow‐up (lost to follow‐up); n = 5 in the control group, of which n = 1 post wait‐list measurement (work schedule problem) and n = 4 at 2‐month follow‐up (lost to follow‐up, not in town, repatriated back to the Philippines). Dropout rates differed only slightly between the intervention and control group, indicating a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section are reported in the results. |
Abbreviations used:
ACP: advance care planning; ACTG: Adult AIDS Clinical Trials Group; AD: advance directive; ADKnowl: Audit of Diabetes Knowledge Scale; AHL‐C: Assessment of Health Literacy in Cancer screening; AHRQ: Agency for Healthcare Research and Quality; ARMS: Adherence to Refills and Medications Scale; ATSPH‐SF: Attitudes Towards Seeking Professional Psychological Help‐Short Form; BCKQ: Bristol COPD Knowledge Questionnaire; BDI‐II: Beck Depression Inventory‐II; BHLS: Brief Health Literacy Screen; BMI: body mass index; CBT: Cognitive Behavioural Therapy; CBPR: community based participatory research; CDC: Centers for Disease Control and Prevention; CES‐D: Center for Epidemiological Studies‐Depression Scale; CHC: community health centre; CHIS: California Health Information Survey; CHW: trained community health workers; CI: confidence interval; CIHR: Canadian Institutes of Health Research; COPD: chronic obstructive pulmonary disease; CRC: colorectal cancer; CSC: Cardiovascular Health Questionnaire; D‐Lit/DLQ: Depression Literacy Questionnaire; DHLS: Diabetes Health Literacy Survey; DKT: Diabetes Knowledge Test; DM‐REALM: Diabetes‐specific Rapid Estimate of Adult Literacy in Medicine; DQOL: Diabetes Quality of Life Measure; DSM: Diagnostic and Statistical Manual of Mental Disorders; ED: emergency department; EMR: Electronic Medical Record; ESL: English as a second language; FDW: foreign domestic workers; FGD: focus group discussion; FIT: faecal immunochemical test; FOBT: faecal occult blood test; GED: general educational development; GEE: generalized estimating equations; HADS: Hospital Anxiety and Depression Scale; HB‐MAS: Hill‐Bone Medication Adherence Scale; HbA1c: haemoglobin A1c; HBP: high blood pressure; HBV: hepatitis B Virus; HGMT: home glucose monitoring with teletransmission; HINTS: Health Information National Trends Survey; HIPAA: Health Insurance Portability and Accountability Act; HL: health literacy; HLS: health literacy scale; HOME: Humanitarian Organization for Migration Economics; HPV: human papilloma virus; HWHA: Hmong Women's Heritage Association; ICC: intra‐cluster correlation coefficient; ICER: Incremental Cost‐Effectiveness Ratio; IMDSES: Insulin Management Self‐Efficacy Scale; IOM: Institute of Medicine; KDSKA: Kim Depression Scale for Korean Americans; Knowledge CBT‐Q: Knowledge of CBT questionnaire; KRC: Korean Resource Center; LHE: lay health educators; LSESLD: Lifestyle Self‐Efficacy Scale for Latinos with Diabetes; MET: metabolic equivalents; MIDonline: Multicultural Information on Depression online; MUQ: Medication Understanding Questionnaire; NCI: National Cancer Institute; NIA: National Institute on Aging; NIDDK: National Institute of Diabetes and Digestive and Kidney diseases; NIH: National Institutes of Health; NIMHD: National Institute on Minority Health and Health Disparities; NPA: nutrition and physical activity; NVS: newest vital sign; Pap test: Papanicolaou test; PCORI: Patient‐Centered Outcomes Research Institute; PCP: primary care providers; PHM: Preventive Health Model; PHQ‐9K: Korean version of PHQ‐9; PHQ: Patient Health Questionnaire; PRECEDE: Predisposing, Reinforcing, and Enabling Constructs in Education/environmental Diagnosis and Evaluation; PROCEED: Policy, Regulatory, and Organizational Constructs in Educational and Environmental Development; PSA: prostate‐specific antigen; QES: qualitative evidence synthesis; QoL: quality of life; QP: Qatar Petroleum; RCT: randomised controlled trial; RDD: random digit dialling; REALM: Rapid Estimated of Adult Literacy in Medicine; RoB: risk of bias; RR: risk ratio; Rx: prescription; S‐TOFHLA: Spanish version of Short Test of Functional Health Literacy in Adults; SCFHC: South Central Family Health Center; SD: standard deviation; SDSCA: Summary of Diabetes Self‐Care Activities; SE: standard error; SHIP‐DM: Self‐Help Intervention programme for type 2 Diabetes Management; SHIP: Self‐Help Intervention Programme; SILS: Single Item Literacy Screener; SMBG: self‐monitoring of blood glucose; sMIB: Behavioral Skills model; SNAP: Supplemental Nutrition Assistance programme; SYS: Safeguard your Smile; TOFHLA: Test of Functional Health Literacy in Adults; TS‐REALD: Two Stage Rapid Estimate of Adult Literacy in Dentistry; y: years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmad 2012 | Wrong intervention |
| Albright 2005 | Wrong population |
| Alcala 2016 | Wrong study design |
| Alegria 2014 | Wrong population |
| Alegria 2019 | Wrong intervention |
| Apter 2015 | Wrong patient population |
| Aragones 2010 | Wrong intervention |
| Arnold 2019 | Wrong population |
| Athavale 2016 | Wrong population |
| Bahromov 2011 | Wrong intervention |
| Baker 2013 | Wrong study design |
| Banna 2011 | Wrong study design, wrong intervention, wrong patient population |
| Bastani 2010 | Wrong intervention |
| Bastani 2015 | Wong intervention |
| Beauchamp 2020 | Wrong intervention |
| Bermejo 2013 | Wrong patient population, wrong intervention |
| Brenner 2015 | Wrong patient population |
| Brenner 2016 | Wrong patient population |
| Calderón‐Mora 2020 | Wrong intervention |
| Carrasquillo 2012 | Wrong intervention |
| Carrasquillo 2014 | Wrong population |
| Carrasquillo 2015 | Wrong population |
| Carrasquillo 2017 | Wrong intervention |
| Carrasquillo 2018 | Wrong population |
| Castejon 2013 | Wrong intervention |
| Chai 2018 | Wrong population |
| Chakkalakal 2017 | Wrong population |
| Chalela 2015 | Wrong population |
| Chan 2014 | Wrong population |
| Chan 2015 | Wrong population |
| Christy 2019 | Wrong population |
| Cohan 2009 | Wrong population |
| Collado 2014 | Wrong intervention |
| Dancel 2013 | Wrong population |
| Davis 2017 | Wrong population |
| Del 2017 | Wrong population |
| DeStephano 2010 | Wrong study design |
| Dietrich 2006 | Wrong population |
| Diez 2018 | Wrong population, wrong intervention |
| Drieling 2011 | Wrong patient population, wrong intervention |
| Drks 2019 | Wrong intervention |
| Dueweke 2017 | Wrong intervention |
| Duggan 2012 | Wrong intervention, wrong population |
| Dwight‐Johnson 2010 | Wrong intervention |
| Elder 2016 | Wrong publication type, wrong intervention |
| Ell 2007 | Wrong intervention |
| Ell 2017 | Wrong intervention |
| Erenoğlu 2020 | Wrong intervention |
| Esquivel 2014 | Wrong population |
| Eylem 2015 | Wrong intervention |
| Fang 2019 | Wrong intervention |
| Fehniger 2014 | Wrong population |
| Felicitas‐Perkins 2017 | Wrong intervention |
| Field 2009 | Wrong population |
| Field 2010 | Wrong intervention |
| Fischer 2013 | Wrong intervention |
| Fischer 2015 | Wrong population |
| Fortmann 2015 | Wrong intervention |
| Frosch 2011 | Wrong patient population, wrong intervention |
| Gademan 2012 | Wrong population |
| Gany 2007 | Wrong intervention |
| Garbers 2012 | Wrong population |
| Garland 2007 | Wrong intervention |
| Gelberg 2019 | Wrong intervention |
| Goel 2016 | Wrong population, wrong intervention |
| Golchert 2019 | Wrong intervention |
| Gold 2014 | Wrong intervention |
| Gonzales 2014 | Wrong intervention |
| Gonzales 2016 | Wrong study design |
| Gonzales 2020 | Wrong patient population |
| Goodyer 2006 | Wrong population |
| Gordon 2014 | Wrong population |
| Gordon 2016 | Wrong population |
| Greenhalgh 2005 | Wrong patient population |
| Greenhalgh 2011 | Wrong population |
| Gustafsson 2015 | Wrong intervention |
| Gwadz 2017 | Wrong patient population, wrong intervention |
| Gwynn 2016 | Wrong population |
| Hahn 2015 | Wrong study design |
| Han 2010 | Wrong population, wrong intervention |
| Handley 2008 | Wrong population |
| Harmsen 2005 | Wrong population |
| Helland‐Kigen 2013 | Wrong population |
| Helland‐Kigen 2013a | Duplicate |
| Hernandez 2015 | Duplicate |
| Hijazi 2013 | Wrong intervention |
| Hijazi 2014 | Wrong intervention |
| Holzel 2014 | Wrong population |
| Holzel 2016 | Wrong intervention |
| Horowitz 2011 | Wrong population |
| Howell 2011 | Wrong population |
| Howie‐Esquivel 2014 | Wrong population |
| Howie‐Esquivel 2014a | Duplicate |
| Interian 2013 | Wrong study design |
| Jacobson 2016 | Wrong study design |
| Jang 2018 | Wrong intervention |
| Jerant 2014 | Wrong population |
| Jerant 2014a | Duplicate |
| Jervelund 2018 | Wrong study design |
| Jih 2016 | Wrong population |
| Jihyun 2018 | Wrong intervention |
| Jimenez 2015 | Wrong intervention |
| Jimenez 2017 | Wrong intervention |
| Juarez 2013 | Wrong population, wrong intervention |
| Juarez‐Carrillo 2012 | Wrong intervention |
| Juon 2016 | Wrong intervention |
| Kandula 2014 | Wrong intervention |
| Kandula 2020 | Wrong intervention |
| Kendall 2017 | Wrong population, wrong intervention |
| Kepka 2011 | Wrong intervention |
| Kieffer 2013 | Wrong intervention |
| Kieffer 2013a | Duplicate |
| Kim 2010 | Wrong intervention |
| Kim 2014a | Wrong intervention |
| Kim 2019 | Wrong population |
| Kiropoulos 2011a | Duplicate |
| Ko 2017 | Wrong publication type |
| Kocken 2008 | Wrong intervention |
| Kohlstadt 2016 | Wrong population |
| Koniak‐Griffin 2011 | Wrong population |
| Kurth 2016 | Wrong population |
| Kurtovich 2010 | Duplicate |
| Kwon 2015 | Wrong intervention |
| Kwong 2013 | Wrong intervention |
| Ladley 2018 | Wrong population |
| Lam 2003 | Wrong intervention |
| Lasser 2010 | Duplicate |
| Lee 2014 | Wrong population |
| Lee 2014a | Wrong intervention |
| Lee 2017 | Wrong intervention |
| Lee‐Lin 2016 | Wrong intervention |
| Li 2014 | Wrong population |
| Lindberg 2020 | Wrong intervention |
| Lood 2015 | Wrong intervention |
| Ma 2017 | Wrong intervention |
| Ma 2018 | Wrong intervention/wrong patient population |
| Ma 2019 | Wrong intervention |
| Macabasco‐O'Connell 2011 | Wrong population |
| Macabasco‐O'Connell 2011a | Duplicate |
| Makoul 2009 | Wrong study design |
| Makoul 2011 | Wrong population |
| Marcus 2015 | Wrong intervention |
| Medina‐Ramirez 2019 | Wrong intervention |
| Meredith 2014 | Wrong population |
| Millan‐Ferro 2017 | Wrong population |
| Miranda 2019 | Wrong study design |
| Mitchell 2015 | Wrong intervention |
| Moore 2016 | Wrong intervention |
| Myers 2018 | Wrong intervention |
| Møen 2020 | Wrong population |
| Navarro 1995 | Wrong intervention |
| NCT00857636 | Duplicate |
| NCT03980808 | Wrong patient population, wrong intervention |
| NCT04831463 | Wrong intervention |
| Nedjat‐Haiem 2012 | Wrong study design, wrong intervention |
| Nguyen 2009 | Wrong population, wrong intervention |
| Nickell 2019 | Wrong intervention |
| O'Connor 2014 | Wrong population |
| O'Connor 2020 | Wrong intervention |
| Oh 2017 | Wrong intervention, wrong study design |
| Patel 2019 | Wrong population |
| Pekmezi 2009 | Wrong intervention |
| Pekmezi 2012 | Wrong intervention |
| Peragallo 2005 | Wrong intervention |
| Percac‐Lima 2016 | Wrong population |
| Poureslami 2011a | Duplicate |
| Poureslami 2011b | Wrong study design, wrong population |
| Qi 2011 | Wrong intervention |
| Radlick 2020 | Wrong intervention |
| Reddy 2014 | Wrong intervention |
| Reijneveld 2003 | Wrong intervention |
| Reuland 2017 | Wrong population |
| Rhodes 2011 | Wrong intervention |
| Ridgeway 2021 | Wrong intervention |
| Rosas 2015 | Wrong intervention |
| Saha 2013 | Wrong intervention |
| Saha 2018 | Wrong intervention |
| Salazar 2012 | Wrong population |
| Schensul 2009 | Wrong population, wrong intervention |
| Schillinger 2008 | Wrong patient population |
| Schlumbrecht 2016 | Wrong study design |
| Siddiqui 2017 | Wrong intervention |
| Silvani 2015 | Wrong intervention |
| Spalluto 2019 | Wrong intervention |
| Sundquist 2010 | Wrong intervention |
| Sußkind 2019 | Wrong intervention |
| Swerissen 2006 | Wrong intervention |
| Taylor 2002 | Wrong intervention |
| Taylor 2009b | Wrong population |
| Thom 2018 | Wrong population |
| Tsai 2018 | Wrong study design |
| Tu 2006 | Wrong population, wrong intervention |
| Tuot 2015 | Wrong population |
| Turner 2018 | Wrong population |
| Unlu 2010 | Wrong intervention |
| Uygun 2020 | Wrong intervention |
| Vargas 2010 | Wrong population |
| Vincent 2014 | Wrong population |
| Vlaar 2017 | Wrong intervention |
| Walker 2007 | Wrong study design, wrong intervention |
| Walker 2012 | Wrong population |
| Wang 2015 | Wrong intervention |
| Wells 2011 | Wrong population |
| Wieland 2018 | Wrong population |
| Wong 2008 | Wrong intervention |
| Wong 2021 | Wrong intervention |
| Wu 2015 | Wrong intervention |
| Yun 2016 | Wrong study design |
| Zhang 2013 | Wrong intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Erwin 2012.
| Methods | RCT |
| Participants | Latinx |
| Interventions | Cancer education versus diabetes education |
| Outcomes | — |
| Notes | Abstract only, insufficient information to permit judgement |
Esquivel 2019.
| Methods | Pilot RCT |
| Participants | US Latinos with heart failure |
| Interventions | Educational intervention versus usual care |
| Outcomes | Acceptability and appropriateness of a culturally tailored educational intervention |
| Notes | Abstract of feasibility study only, no trial ID |
Essien 2017.
| Methods | RCT |
| Participants | Spanish‐speaking participants |
| Interventions | Peer mentorship in diabetes versus unknown |
| Outcomes | Unknown |
| Notes | Conference abstract only, no trial ID, unclear if data are extractable for first‐generation migrants |
Glaser 2020.
| Methods | Unclear, probably cluster‐RCT |
| Participants | Non‐English speaking population |
| Interventions | Culturally tailored education about colorectal cancer |
| Outcomes | Colorectal cancer screening |
| Notes | Conference abstract only, unclear study design |
Gonzalez 2020.
| Methods | RCT |
| Participants | Ethnically diverse and socio‐economically disadvantaged patients |
| Interventions | Telephone education about diabetes mellitus versus enhanced usual care |
| Outcomes | Depression, medication adherence, self‐efficacy |
| Notes | Study protocol, unclear if data on first‐generation migrants are extractable |
Joshi 2016.
| Methods | Quasi‐RCT |
| Participants | Hispanic women |
| Interventions | Computer‐based bilingual breastfeeding educational programme |
| Outcomes | Knowledge, self‐efficacy and intent to breastfeed |
| Notes | Unclear if participants are first‐generation migrants (at least 80%); additional information requested from author but not provided |
NCT04993326.
| Methods | RCT |
| Participants | African Americans |
| Interventions | Online diabetes self‐management education and support along with COVID‐19 prevention and protection (vaccination) education and resource information versus usual care |
| Outcomes | Understanding of diabetes self‐management, understanding of COVID‐19 risks |
| Notes | Unclear if data are extractable for first‐generation migrants; clinicaltrials.gov identifier: NCT04993326 |
Pekmezaris 2020.
| Methods | Diabetes management programme for Hispanic/Latino |
| Participants | |
| Interventions | Diabetes telemonitoring versus comprehensive outpatient management |
| Outcomes | |
| Notes | Ongoing study; unclear if data are extractable for first‐generation migrants; clinicaltrials.gov identifier: NCT03960424 |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619001019190.
| Study name | The Strong Families Trial: Randomised controlled trial of a family strengthening program to prevent unhealthy weight gain among 5‐ to 11‐year old children from at risk families |
| Methods | RCT |
| Participants | Parents |
| Interventions | Face‐to‐face behavioural parenting and lifestyle (BPL) intervention |
| Outcomes | Usual care |
| Starting date | 23 February 2023 (recruitment) |
| Contact information | andre.renzaho@westernsydney.edu.au |
| Notes |
Blashill 2021.
| Study name | Pilot randomised controlled trial of a patient navigation intervention to enhance engagement in the PrEP continuum among young Latino MSM |
| Methods | Pilot RCT |
| Participants | Latino men |
| Interventions | Patient navigation intervention versus usual care plus written information |
| Outcomes | Knowledge, self‐efficacy, attitudes and beliefs, adherence |
| Starting date | 2019 |
| Contact information | kwells@mail.sdsu.edu |
| Notes | Clinicaltrials.gov identifier: NCT04048382 |
Castro 2013.
| Study name | Design of a randomized controlled trial for multiple cancer risk behaviors among Spanish‐speaking Mexican‐origin smokers |
| Methods | RCT |
| Participants | High‐risk Mexican‐origin smokers who are overweight/obese |
| Interventions | Health education (HE) versus motivation and problem‐solving (MAPS) intervention |
| Outcomes | Smoking status, servings of fruits and vegetables, and both self‐reported and objectively measured physical activity |
| Starting date | — |
| Contact information | — |
| Notes | Study protocol only; NCT01504919 |
NCT03726619.
| Study name | e‐CHEC‐uP: Scaling up an Efficacious Cancer Screening Intervention for Women With Limited English |
| Methods | RCT |
| Participants | Korean American Women |
| Interventions | One‐time online‐based education followed by phone counselling over 6 months versus one‐time face‐to‐face education followed by phone counselling over 6 months |
| Outcomes | Breast and cervical cancer screening measures, health literacy, breast and cervical cancer knowledge, cancer screening‐related self‐efficacy |
| Starting date | 14 July 2019 |
| Contact information | |
| Notes |
NCT03909347.
| Study name | PLAN: dementia Literacy Education and Navigation for Korean Elders With Probable Dementia and Their Caregivers |
| Methods | RCT |
| Participants | 288 self‐identified first‐generation Korean Americans |
| Interventions | Dementia literacy education and navigation versus usual care |
| Outcomes | Dementia literacy |
| Starting date | July 2020 |
| Contact information | hhan3@jhu.edu |
| Notes | Clinicaltrials.gov identifier: NCT03909347 |
NCT04125680.
| Study name | English as Second Language Health Literacy programme |
| Methods | RCT, 2 arms |
| Participants | Hispanic adult learners |
| Interventions | ESL curriculum that focuses on using pedagogies for health literacy as a practice |
| Outcomes | Prevention behaviours, prevention knowledge, health literacy, health service use |
| Starting date | February 2020 |
| Contact information | feuerher@umich.edu |
| Notes | — |
NCT04319458.
| Study name | Testing Mediators and Moderators of a Fotonovela for Depression to Promote Help‐seeking Behavior |
| Methods | RCT |
| Participants | Latinx/Hispanics |
| Interventions | Secret feelings fotonovela versus NIH Brochure: Depression: What You Need to Know |
| Outcomes | Help‐seeking behaviour |
| Starting date | — |
| Contact information | — |
| Notes | clinicaltrials.gov identifier: NCT04319458 |
NCT04564209.
| Study name | Information Visualizations to Facilitate Clinician‐patient Communication in HIV Care (Info Viz: HIV) |
| Methods | RCT |
| Participants | Latinx |
| Interventions | Infographic intervention |
| Outcomes | Standard care |
| Starting date | 18 August 2021 |
| Contact information | — |
| Notes | — |
NCT04905030.
| Study name | Education, Immigration and HPV Vaccination: an Informational Randomized Trial |
| Methods | Informational RCT |
| Participants | Immigrant women in Sweden |
| Interventions | Three types of HPV vaccination information |
| Outcomes | Decision to vaccinate child against HPV, posterior beliefs about false risks of the HPV vaccine |
| Starting date | 2021 |
| Contact information | — |
| Notes | ClinicalTrials.gov identifier: NCT04905030 |
Waterman 2019.
| Study name | Working Within an Integrated Learning Healthcare System to Improve Living Kidney Donation Knowledge Across the CKD Continuum for All Racial Groups |
| Methods | RCT |
| Participants | English and Spanish‐speaking adults |
| Interventions | ET@Home education versus usual care |
| Outcomes | Knowledge, ability to make an informed decision about transplant, self‐efficacy |
| Starting date | 2017 |
| Contact information | — |
| Notes | Clinicaltrials.gov identifier: NCT03389932 |
Weise 2021.
| Study name | Low‐threshold, culturally‐sensitive group psychoeducation programme for asylum seekers (LoPe) |
| Methods | RCT |
| Participants | Asylum seekers |
| Interventions | Culturally sensitive, low‐threshold psychoeducation versus wait‐list control |
| Outcomes | Knowledge, changes in mental distress, openness towards psychotherapy and resilience |
| Starting date | 2020 |
| Contact information | — |
| Notes | Trial registration identifier: DRKS00020564 |
ESL: English as a second language; HPV: human papillomavirus; NIH: National Institutes of Health; RCT: randomised controlled trial
Differences between protocol and review
Extending this review with a qualitative evidence synthesis
The author team of this effectiveness review aimed to conduct a qualitative evidence synthesis (QES) in parallel: Gender differences in health literacy of migrants: a synthesis of qualitative evidence (protocol) (Aldin 2019). The QES aimed to add to this effectiveness review by exploring whether gender differences in the health literacy of migrants exist, and which factors underlie these differences in the four health information processing steps. Additionally, it attempted to identify factors associated with gender and migration that may play a role in the design, delivery and effectiveness of health literacy interventions for female and male migrants. The QES has not yet been completed. At the time of publication, the possibility of the companion QES being completed to complement the current review is being explored.
Criteria for considering studies for this review
Types of interventions
At the protocol stage (Baumeister 2019), we planned to conduct a main analysis including health literacy interventions that were explicitly named as such and a secondary deductive analysis including health literacy interventions that address at least one of the four health information processing steps (see "description of the condition" section). For example, if a study reported a 'health literacy intervention' as simply providing an information pamphlet on an available health service and reported a health literacy measure, we planned to include the study for the secondary analysis, assigning it to the processing step 'access', since the effect cannot be assigned to health literacy as a general concept. We also planned to include such a study in the deductive analysis, if the pamphlet was targeted to individuals with limited language proficiency and the effect measured was the level of understanding that these individuals achieve regarding the information provided. In this case, the intervention was planned to be assigned to the processing step of 'understand' in the deductive analysis.
Due to the diversity of studies found, we were not able to conduct one main analysis, but rather conducted meta‐analyses where possible and deductively categorised the studies' outcomes to our umbrella framework of health literacy (see also Data synthesis). In addition, we decided to exclude studies that solely provided a publicly available pamphlet when the respective pamphlet was not adapted with regard to (health) literacy by the study authors.
Types of outcome measures
Secondary outcomes
At the protocol stage, we pre‐specified the outcome category 'individual skills (e.g. self‐efficacy, self‐awareness)'. For the sake of clarity, and since self‐efficacy has been shown in several studies to be associated with health literacy (Berens 2021; Berens 2022b; Guntzviller 2016; von Wagner 2009; Xu 2018), we decided to rename this category of outcomes as 'self‐efficacy', including the different forms of self‐efficacy (e.g. self‐efficacy to manage one's own disease, self‐efficacy to use certain screening measures or self‐efficacy to identify a disease). We also planned to extract outcomes related to the prespecified category 'Healthcare costs'. Healthcare costs as a secondary outcome was not assessed as no data were available from the published main trial reports and due to a lack of resources we were not able to search for separate cost‐effectiveness analyses.
Search methods for identification of studies
Searching other resources
At the protocol stage, we planned to additionally handsearch for conference abstracts of certain conferences (e.g. migration conferences). We did not handsearch for conference abstracts due to a lack of resources and because our comprehensive search strategy most likely covered the published conference abstracts. We decided to search ClinicalTrials.gov and ICTRP as the other two clinical trial registries mentioned in the protocol (the EU clinical trials register and DRKS) are already included in the ICTRP search portal.
Data collection and analysis
Subgroup analysis
We intended to conduct subgroup analyses for gender, ethnicity and health literacy assessment (if named as such) (Objectives). Since health literacy can be defined and measured in different ways, we planned to conduct a subgroup analysis for different measurement tools applied in the included studies (performance‐based versus self‐assessment tools).
No self‐assessment health literacy tool was applied in the included studies, therefore it was not possible or meaningful to follow the protocol in terms of conducting subgroup analyses for self‐reported versus performance‐based health literacy assessment. Due to the high heterogeneity of studies in terms of interventions, participants and comparators, and an insufficient number of studies in any of the meta‐analyses, we were not able to conduct a quantitative subgroup analysis for gender or ethnicity either. However, we conducted separate analysis by gender, where possible.
Contrary to the protocol, we conducted post hoc quantitative subgroup analyses for specific design features when we considered studies similar enough to be combined in a meta‐analysis, but nevertheless design‐specific heterogeneity needed to be considered. For example, when there was high variance in the programme duration, we conducted subgroup analyses by length of the programme (e.g. up to six months versus up to 12 months) to investigate the reasons for heterogeneity.
Involvement of consumers
At the protocol stage, we had planned to also involve consumers by conducting gender‐separate focus group discussions (FGDs) with female and male migrants, as well as to conduct a final symposium with different stakeholders, such as experts from political and healthcare contexts, to discuss the impact and implications of our primary and secondary findings for healthcare decision‐making at the political level, particularly in Germany. However, due to a lack of financial and human resources, this was not possible.
Contributions of authors
Annika Baumeister (AB) developed the protocol and wrote the review (study screening and selection, data extraction, study quality assessment, data synthesis, interpretation of findings, GRADE assessment, creation of summary of findings (SoF) tables and evidence profiles, writing the text of the review).
Angela Aldin (AAl) assisted in the development of this review (study screening and selection, data synthesis and interpretation of findings, study quality assessment, GRADE assessment), proofread and commented on the draft. She was in constant exchange with Annika Bameister due to the parallel development of the qualitative evidence synthesis linked to this effectiveness review.
Digo Chakraverty (DC) assisted in the study selection and data synthesis (grouping of studies), proofread and commented on the draft.
Constanze Hübner (CH) assisted in study screening and data extraction, proofread and commented on the draft.
Anne Adams (AAd) provided statistical expertise, proofread and commented on the review draft.
Ina Monsef (IM) developed the search strategies and conducted electronic searches, proofread and commented on the review draft.
Nicole Skoetz (NS) provided methodological advice, proofread and commented on the review draft.
Elke Kalbe (EK) provided content expertise, proofread and commented on the review draft.
Christiane Woopen (CW) provided content expertise, proofread and commented on the review draft.
Sources of support
Internal sources
-
Research Unit Ethics, Institute for the History and Ethics of Medicine, University of Cologne, Faculty of Medicine and University Hospital Cologne, Germany
Provision of the offices, including technical equipment.
External sources
-
German Federal Ministry of Education and Research, Germany
This review is funded by the German Federal Ministry of Education and Research, grant number 01GL1723.
Declarations of interest
Annika Baumeister (AB): award of the grant from the Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Angela Aldin (AAl): award of the grant from the Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Digo Chakraverty (DC): award of the grant from the Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Constanze Huebner (CH): none known.
Anne Adams (AAd): none known. She is an editor with Cochrane, but was not involved in the editorial process for this review.
Ina Monsef (IM): none known. She is the Information Specialist for Cochrane Haematology, but was not involved in the editorial process for this review.
Nicole Skoetz (NS): award of the grant from the Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest; she is Co‐ordinating Editor of Cochrane Haematology, but was not involved in the editorial process for this review.
Elke Kalbe (EK): award of the grant from the Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
Christiane Woopen (CW): award of the grant from the Federal Ministry of Education and Research for the University Hospital of Cologne to perform this systematic review does not lead to a conflict of interest.
New
References
References to studies included in this review
Bailey 2012 {published data only (unpublished sought but not used)}
- Bailey SC, Hasnain-Wynia R, Chen AH, Sarkar U, Schoua-Glusberg A, Lindquist LA. Developing multilingual prescription instructions for patients with limited English proficiency. Journal of Health Care for the Poor and Underserved 2012;23(1):81-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Bailey SC, Sarkar U, Chen AH, Schillinger D, Wolf MS. Evaluation of language concordant, patient-centered drug label instructions. Journal of General Internal Medicine 2012;27(12):1707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 2014 {published data only (unpublished sought but not used)}
- Bloom J, Shirazi M, Shirazi A, Stewart S, Popal, R. Breast cancer screening in an immigrant population: the Afghan women’s breast health program. Psycho-Oncology 2014;23(3):53.
- Shirazi M, Bloom J, Shirazi A, Popal R. Afghan immigrant women's knowledge and behaviors around breast cancer screening. Psycho-Oncology 2013;22(8):1705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi M, Shirazi A, Bloom J. Developing a culturally competent faith-based framework to promote breast cancer screening among Afghan immigrant women. Journal of Religion and Health 2015;54(1):153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calderón 2014 {published and unpublished data}
- Calderón J, Shaheen M, Hays RD, Fleming ES, Norris KC, Baker RS. Improving diabetes health literacy by animation. The Diabetes Educator 2014;40(3):361-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeCamp 2020 {published data only}
- DeCamp LR, Godage SK, Araujo DV, Cortez JD, Wu L, Psoter KJ, et al. A texting intervention in Latino families to reduce ed use: a randomized trial. Paediatrics 2020;145(1):e20191405. [DOI] [PubMed]
- Valenzuela-Araujo D, Godage SK, Quintanilla K, Dominguez CJ, Polk S, DeCamp LR. Leaving paper behind: improving healthcare navigation by Latino immigrant parents through video-based education. Journal of Immigrant and Minority Health 2020;23(2):329-36. [DOI] [PubMed] [Google Scholar]
Elder 1998 {published data only (unpublished sought but not used)}
- Elder JP, Candelaria JI, Woodruff SI, Criqui MH, Talavera GA, Rupp JW et al. Initial results of language for health: cardiovascular disease nutrition education for Latino English-as-a-second-language students. Health Education Research 1998;13(4):567-75. [DOI] [PubMed] [Google Scholar]
Gwede 2019 {published data only}
- Aguado Loi CX, Tyson DM, Chavarria EA, Gutierrez L, Klasko L, Davis S, et al. ‘Simple and easy:’ providers’ and Latinos’ perceptions of the fecal immunochemical test (FIT) for colorectal cancer screening. Ethnicity & Health 2020;25(2):206-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy SM, Cousin LA, Sutton SK, Chavarria EA, Abdulla R, Gutierrez L, et al. Characterizing health literacy among Spanish language-preferring Latinos ages 50-75. Nursing Research 2021;70(5):344-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwede CK, Sutton SK, Chavarria EA, Gutierez L, Abdulla R, Christy S, et al. A culturally and linguistically salient pilot intervention to promote colorectal cancer screening among Latinos receiving care in a federally qualified health center. Health Education Research 2019;34(3):310-20. [DOI] [PMC free article] [PubMed]
Han 2017 {published data only (unpublished sought but not used)}
- Choi E, Heo GJ, Song Y, Han H-R. Community health worker perspectives on recruitment and retention of recent immigrant women in a randomized clinical trial. Family and Community Health 2016;39(1):53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H-R, Kim K, Cudjoe J, Kim MT. Familiarity, navigation, and comprehension: key dimensions of health literacy in pap test use among Korean American women. Journal of Health Communication 2019;24(6):585-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H-R, Song Y, Kim M, Hedlin HK, Kim K, Lee BH, et al. Breast and cervical cancer screening literacy among Korean American women: a community health worker-led intervention. American Journal of Public Health 2017;107(1):159-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim B, Choi E, Song Y, Han HR. Knowledge, perceptions, and decision making about human papillomavirus vaccination among Korean American women: a focus group study. Women's Health Issues 2015;25(2):112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim S, Gallo JJ, Nolan MT, Han HR. Decision making about pap test use among Korean immigrant women: a qualitative study. Health Expectations 2017;20(4):685-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Xue Q, Walton-Moss B, Nolan MT, Han H. Decisional balance and self-efficacy mediate the association among provider advice, health literacy and cervical cancer screening. European Journal of Oncology Nursing 2018;32:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Xue Q-L, Walton-Moss B, Nolan MT, Han H-R. Decisional balance and self-efficacy mediate the association among provider advice, health literacy and cervical cancer screening. European Journal of Oncology Nursing 2018;32:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster AL, Frick KD, Huh B-Y, Kim KB, Kim M, Han H-R. Economic evaluation of a community health worker-led health literacy intervention to promote cancer screening among Korean American women. Journal of Health Care for the Poor and Underserved 2015;26(2):431-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez 2013 {published data only}
- Hernandez MY, Organista KC. Entertainment-education? A fotonovela? A new strategy to improve depression literacy and help-seeking behaviors in at-risk immigrant Latinas. American Journal of Community Psychology 2013;52(3-4):224-35. [DOI] [PubMed] [Google Scholar]
- Hernandez MY, Organista KC. Qualitative exploration of an effective depression literacy fotonovela with at risk Latina immigrants. American Journal of Community Psychology 2015;56(1-2):79-88. [DOI] [PubMed] [Google Scholar]
Kaur 2019 {published data only (unpublished sought but not used)}
- Kaur N, Kandelman D, Potvin L. Development and pilot testing of an oral hygiene self-care photonovel for Punjabi immigrants: a qualitative study. Canadian Journal of Dental Hygiene 2021;55(1):30-8. [PMC free article] [PubMed] [Google Scholar]
- Kaur N, Kandelman D, Potvin L. Effectiveness of “safeguard your smile,” an oral health literacy intervention, on oral hygiene self-care behaviour among Punjabi immigrants: a randomized controlled trial. Canadian Journal of Dental Hygiene 2019;53(1):18-27. [PMC free article] [PubMed] [Google Scholar]
- Kaur N. Development and randomized controlled trial evaluation of “safeguard your smile” an oral health literacy intervention promoting oral hygiene self-care behavior among Punjabi immigrants. Montréal (Canada): Université de Montréal, 2017. [PMC free article] [PubMed] [Google Scholar]
Kheir 2014 {published data only (unpublished sought but not used)}
- Kheir N, Awaisu A, Radoui A, El BA, Jean L, Dowse R. Development and evaluation of pictograms on medication labels for patients with limited literacy skills in a culturally diverse multiethnic population. Research in Social and Administrative Pharmacy 2014;10(5):720-30. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only (unpublished sought but not used)}
- Kim MT, Han H-R, Song H-J, Lee J-E, Kim J, Ryu JP, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educator 2009;35(6):986-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only (unpublished sought but not used)}
- Han H, Chan K, Song H, Nguyen T, Lee J, Kim MT. Development and evaluation of a hypertension knowledge test for Korean hypertensive patients. Journal of Clinical Hypertension 2011;13(10):750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H-R, Chan K, Song H, Nguyen T Lee J-E, Kim MT. Development and evaluation of a hypertension knowledge test for Korean hypertensive patients. Journal of Clinical Hypertension 2011;13(10):750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H-R, Kim KB, Kim MT. Evaluation of the training of Korean community health workers for chronic disease management. Health Education Research 2007;22(4):513-21. [DOI] [PubMed] [Google Scholar]
- Han HR, Song Y, Song HJ, Kim MT. Influence of living arrangements on the management and control of hypertension: a mixed-methods study of Korean American elderly. Journal of Immigrant and Minority Health 2013;15(5):944-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KB, Han H-R, Huh B, Nguyen T, Lee H, Kim MT. The effect of a community-based self-help multimodal behavioral intervention in Korean American seniors with high blood pressure. American Journal of Hypertension 2014;27(9):1199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M T, Han H-R, Park H J, Lee H, Kim KB. Constructing and testing a self-help intervention program for high blood pressure control in Korean American seniors—a pilot study. Journal of Cardiovascular Nursing 2006;21(2):77-84. [DOI] [PubMed] [Google Scholar]
- Kim MT, Song H-J, Han H-R, Song Y, Nam S, Nguyen TH, et al. Development and validation of the high blood pressure-focused health literacy scale. Patient Education and Counseling 2012;87(2):165-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2020 {published data only (unpublished sought but not used)}
- Kim MT, Han H-R, Song H-J, Lee J-E, Kim J, Ryu JP, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educator 2009;35(6):986-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MT, Kim KB, Huh B, Nguyen T, Han H-R, Bone LR, et al. The effect of a community-based self-help intervention: Korean Americans with type 2 diabetes. American Journal of Preventive Medicine 2015;49(5):726-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MT, Kim KB, Ko J, Murry N, Xie B, Radhakrishnan K, et al. Health literacy and outcomes of a community-based self-help intervention: a case of Korean Americans with type 2 diabetes. Nursing Research 2020;69(3):210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MT, Zhushan L, Nguyen TH, Murry N, Ko J, Kim KB, et al. Development of a diabetes-focused print health literacy scale using the rapid estimate of adult literacy in medicine model. Health literacy Research and Practice 2020;4(4):e237-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Han HR, Lee JE, Kim J, Kim KB, Nguyen T, et al. Translating current dietary guidelines into a culturally tailored nutrition education program for Korean American immigrants with type 2 diabetes. Diabetes Education 2010;36(5):752-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiropoulos 2011 {published data only}
- Kiropoulos LA, Griffiths KM, Blashki G. Effects of a multilingual information website intervention on the levels of depression literacy and depression-related stigma in Greek-born and Italian-born immigrants living in Australia: a randomized controlled trial. Journal of Medical Internet Research 2011;13(2):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koniak‐Griffin 2015 {published data only (unpublished sought but not used)}
- Albarran CR, Heilemann MV, Koniak-Griffin D. Promotoras as facilitators of change: Latinas' perspectives after participating in a lifestyle behaviour intervention program. Journal of Advanced Nursing 2014;17(10):2303-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koniak-Griffin D, Brecht M-L, Takayanagi S, Villegas J, Melendrez M. Physical activity and cardiometabolic characteristics overweight Latina women. Journal of Immigrant and Minority Health 2014;16:856-64. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koniak-Griffin D, Brecht ML, Takayanagi S, Villegas J, Melendrez M, Balcazar H. A community health worker-led lifestyle behavior intervention for Latina (Hispanic) women: feasibility and outcomes of a randomized controlled trial. International Journal of Nursing Studies 2015;52(1):75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Your heart, your life. A community health worker's manual for the Hispanic community. National Institutes of Health, 2008. [NIH PUBLICATION: No. 08-3674] [Google Scholar]
Lepore 2012 {published data only}
- Lepore SJ, Wolf RL, Basch CE, Godfrey M, McGinty E, Shmukler C, et al. Informed decision making about prostate cancer testing in predominantly immigrant black men: a randomized controlled trial. Annals of Behavioral Medicine 2012;44(3):320-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RL, Lepore SJ, Vandergrift JL, Basch CE, Yaroch AL. Tailored telephone education to promote awareness and adoption of fruit and vegetable recommendations among urban and mostly immigrant black men: a randomized controlled trial. Preventive Medicine 2009;48(1):32-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RL, Lepore SJ, Vandergrift JL, Wetmore-Arkader L, McGinty E, Pietrzak G, et al. Knowledge, barriers, and stage of change as correlates of fruit and vegetable consumption among urban and mostly immigrant black men. Journal of the American Dietetic Association 2008;108(8):1315-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohan 2014 {published data only}
- Blake SC, McMorris K, Jacobson KL, Gazmararian JA, Kripalani S. A qualitative evaluation of a health literacy intervention to improve medication adherence for underserved pharmacy patients. Journal of Health Care for the Poor and Underserved 2010;21(2):559-67. [DOI] [PubMed] [Google Scholar]
- Mohan A, Brian R M, Schmotzer B, Boyington. Improving medication understanding among Latinos through illustrated medication lists. American Journal of Managed Care 2014;20(12):547-55. [PubMed] [Google Scholar]
- Mohan A, Riley MB, Boyington D, Johnston P, Trochez K, Jennings C, et al. Development of a patient-centered bilingual prescription drug label. Journal of Health Communication 2013;18 Suppl 1:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Riley MB, Boyington D, Kripalani S. PictureRx: illustrated medication instructions for patients with limited health literacy. Journal of the American Pharmacists Association 2012;52(5):122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan AV, Riley MB, Boyington DR, Kripalani S. Illustrated medication instructions as a strategy to improve medication management among Latinos: a qualitative analysis. Journal of Health Psychology 2013;18(2):187-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ochoa 2020 {published data only}
- Baezconde-Garbanati L, Murphy ST, Moran MB, Cortessis VK. Reducing the excess burden of cervical cancer among Latinas: translating science into health promotion initiatives. Californian Journal Health Promotion 2013;11(1)(1):45-57. [PMC free article] [PubMed] [Google Scholar]
- Ochoa CY, Murphy ST, Frank LB, Baezconde-Garbanati LA. Using a culturally tailored narrative to increase cervical cancer detection among spanish-speaking Mexican-American women. Journal of Cancer Education 2020;35(4):736-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otilingam 2015 {published data only}
- Otilingam P, Gatz M. Development of a culturally-tailored heart + brain health-focused nutritional educational intervention: buenos habitos alimenticios para una buena salud [Abstract]. In: Journal of Nutrition, Health, & Ageing. Vol. 12. 2008:425.
- Otilingam PG, Gatz M, Tello E, Escobar AJ, Goldstein A, Torres M, et al. Buenos hábitos alimenticios para una buena salud: evaluation of a nutrition education program to improve heart health and brain health in Latinas. Journal of Aging and Health 2015;27(1):177-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Payán 2020 {published and unpublished data}
- Payán DD, Maggard-Gibbons M, Flórez KR, Mejía N, Hemmelgarn M, Kanouse D, et al. Taking care of yourself and your risk for breast cancer (CUIDARSE): a randomized controlled trial of a health communication intervention for Latinas. Health Education & Behavior 2020;47(4):569-80. [DOI: 10.1177/1090198120920529] [DOI] [PubMed] [Google Scholar]
Poureslami 2016a {published data only (unpublished sought but not used)}
- Poureslami I, Nimmon L, Doyle-Waters M, Rootman I, Schulzer M, Kuramoto L, et al. Effectiveness of educational interventions on asthma self-management in Punjabi and Chinese asthma patients: a randomized controlled trial. Journal of Asthma 2012;49(5):542-51. [DOI] [PubMed] [Google Scholar]
- Poureslami I, Rootman I, Doyle-Waters MM, Nimmon L, Fitzgerald JM. Health literacy, language, and ethnicity-related factors in newcomer asthma patients to Canada: a qualitative study. Journal of Immigrant and Minority Health 2011;13(2):315-22. [DOI] [PubMed] [Google Scholar]
- Poureslami I, Shum J, Nimmon L, FitzGerald JM. Culturally specific evaluation of inhaler techniques in asthma. Respiratory Care 2016;61(12):1588-96. [DOI] [PubMed] [Google Scholar]
- Shum M, Poureslami I, Liu J, FitzGerald JM. Perceived barriers to asthma therapy in ethno-cultural communities: the role of culture, beliefs and social support. Health 2017;9(7):1029-46. [Google Scholar]
Poureslami 2016b {published data only (unpublished sought but not used)}
- Poureslami I, Kwan S, Lam S, Khan NA, FitzGerald JM. Assessing the effect of culturally specific audiovisual educational interventions on attaining self-management skills for chronic obstructive pulmonary disease in Mandarin- and Cantonese-speaking patients: a randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:1811-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poureslami IM, Shum J, Cheng N, FitzGerald JM. Does culture or illness change a smoker's perspective on cessation? American Journal of Health Behavior 2014;38(5):657-67. [DOI] [PubMed] [Google Scholar]
Rosal 2005 {published data only (unpublished sought but not used)}
- Rosal MC, Goins KV, Carbone ET, Cortes DE. Views and preferences of low-literate Hispanics regarding diabetes education: results of formative research. Health Education & Behavior 2004;31(3):388-405. [DOI] [PubMed] [Google Scholar]
- Rosal MC, Olendzki RD, Reed GW, Gumieniak O, Scavron J, Ockene I. Diabetes self-management among low-income Spanish-speaking patients: a pilot study. Annals of Behavioral Medicine 2005;29(3):225-35. [DOI] [PubMed] [Google Scholar]
Rosal 2011 {published data only}
- Carbone ET, Rosal MC, Torres MI, Goins KV, Bermudez OI. Diabetes self-management: perspectives of Latino patients and their health care providers. Patient Education and Counseling 2007;66(2):202-10. [DOI] [PubMed] [Google Scholar]
- Rosal MC, Carbone ET, Goins KV. Use of cognitive interviewing to adapt measurement instruments for low-literate Hispanics. Diabetes Educator 2003;29(6):1006-17. [DOI] [PubMed] [Google Scholar]
- Rosal MC, Ockene IS, Restrepo A, White MJo, Borg A, Olendzki B, et al. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income Latinos: Latinos en control. Diabetes Care 2011;34(4):838-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosal MC, White MJ, Borg A, Scavron J, Candib L, Ockene I, et al. Translational research at community health centers: challenges and successes in recruiting and retaining low-income Latino patients with type 2 diabetes into a randomized clinical trial. Diabetes Educator 2010;36(5):733-49. [DOI] [PubMed] [Google Scholar]
- Rosal MC, White MJ, Restrepo A, Olendzki B, Scavron J, Sinagra E, et al. Design and methods for a randomized clinical trial of a diabetes self-management intervention for low-income Latinos: Latinos en Control. BMC Medical Research Methodology 2009;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Lemon SC, Welch G, Rosal MC. Development and validation of the lifestyle self-efficacy scale for Latinos with diabetes (LSESLD). Ethnicity & Disease 2013;23(4):428-35. [PMC free article] [PubMed] [Google Scholar]
Soto Mas 2018 {published and unpublished data}
- Mein E, Fuentes B, Soto Mas F, Muro A. Incorporating digital health literacy into adult ESL education on the US-Mexico border. Rhetoric, Professional Communication, and Globalization 2012;3(1):162-74. [PMC free article] [PubMed] [Google Scholar]
- Soto Mas F, Ji M, Fuentes BO, Tinajero J. The health literacy and ESL study: a community-based intervention for Spanish-speaking adults. Journal of Health Communication 2015;20(4):369-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto Mas F, Mein E, Fuentes B, Thatcher B, Balcazar H. Integrating health literacy and ESL: an interdisciplinary curriculum for Hispanic immigrants. Health Promotion Practice 2013;14(2):263-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto Mas F, Schmitt CL, Jacobson HE, Myers OB. A cardiovascular health Intervention for Spanish speakers: the health literacy and ESL curriculum. Journal of Community Health 2018;43(4):717-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudore 2018 {published and unpublished data}
- Freytag J, Street RL, Barnes DE, Shi Y, Volow AM, Shim JK, et al. Empowering older adults to discuss advance care planning during clinical visits: The PREPARE Randomized Trial. Journal of the American Geriatrics Society 2020;68(6):1210-7. [DOI: 10.1111/jgs.16405] [DOI] [PMC free article] [PubMed]
- McMahan RD, Knight SJ, Fried TR, Sudore RL. Advance care planning beyond advance directives: perspectives from patients and surrogates. Journal of Pain and Symptom Management 2013;46(3):355-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudore RL, Barnes DE, Le GM, Ramos R, Osua SJ, Richardson SA, et al. Improving advance care planning for English-speaking and Spanish-speaking older adults: study protocol for the prepare randomised controlled trial. BMJ Open 2016;6(7):e011705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudore RL, Knight SJ, McMahan RD, Feuz M, Farrell D, Miao Y, et al. A novel website to prepare diverse older adults for decision making and advance care planning: a pilot study. Journal of Pain and Symptom Management 2014;47(4):674-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudore RL, Schillinger D, Katen MT, Shi Y, Boscardin WJ, Osua S, et al. Engaging diverse English- and Spanish-speaking older adults in advance care planning: the PREPARE randomized clinical trial. JAMA Internal Medicine 2018;178(12):1616-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2011 {published data only (unpublished sought but not used)}
- Coronado GD, Taylor V, Acorda E, Hoai DH, Thompson B. Development of an english as a second language curriculum for hepatitis B virus testing in Chinese Americans. Cancer 2005;104(12 Suppl):2948-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VM, Coronado G, Acorda E, Teh C, Tu S-P, Yasui Y, et al. Development of an ESL curriculum to educate Chinese immigrants about hepatitis B. Journal of Community Health 2008;33(4):217-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VM, Gregory HT, Bajdik C, Teh C, Lam W, Acorda E, et al. Hepatitis B ESL education for Asian immigrants. Journal of Community Health 2011;36(1):35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VM, Teh C, Lam W, Acorda W, Li L, Coronado G, Y, et al. Evaluation of a hepatitis b educational ESL curriculum for Chinese immigrants. Canadian Journal of Public Health 2009;100(6):463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2012 {published data only}
- Thompson DA, Joshi A, Hernandez RG, Bair-Merritt MH, Arora M, Luna R, et al. Nutrition education via a touchscreen: a randomized controlled trial in Latino immigrant parents of infants and toddlers. Academic Pediatrics 2012;12(5):412-9. [DOI] [PubMed] [Google Scholar]
Tong 2017 {published data only (unpublished sought but not used)}
- Tong EK, Nguyen TT, Lo P, Stewart SL, Gildengorin GL, Tsoh JY, et al. Lay health educators increase colorectal cancer screening among Hmong Americans: a cluster randomized controlled trial. Cancer 2017;123(1):98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unger 2013 {published and unpublished data}
- Cabassa LJ, Contreras S, Aragón R, Molina GB, Baron M. Focus group evaluation of "secret feelings": a depression fotonovela for Latinos with limited English proficiency. Health Promotion Practice 2011;12(6):840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabassa LJ, Oh H, Humensky JL, Unger JB, Molina GB, Baron M. Comparing the impact on Latinos of a depression brochure and an entertainment-education depression fotonovela. Psychiatric Services (Washington, D.C.) 2015;66(3):313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JB, Cabassa LJ, Molina GB, Contreras S, Baron M. Evaluation of a fotonovela to increase depression knowledge and reduce stigma among Hispanic adults. Journal of Immigrant and Minority Health 2013;15(2):398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valdez 2015 {published data only}
- Valdez A, Stewart SL, Tanjasiri SP, Levy V, Garza A. Design and efficacy of a multilingual, multicultural HPV vaccine education intervention. Journal of Communication in Healthcare 2015;8(2):106-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valdez 2018 {published data only}
- Valdez A, Napoles AM, Stewart SL, Garza A. A randomized controlled trial of a cervical cancer education intervention for Latinas delivered through interactive, multimedia kiosks. Journal of Cancer Education 2018;33(1):222-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Servellen 2005 {published data only (unpublished sought but not used)}
- Servellen G, Carpio F, Lopez M, Garcia-Teague L, Herrera G, Monterrosa F, et al. Program to enhance health literacy and treatment adherence in low-income HIV-infected Latino men and women. AIDS Patient Care and STDs 2003;17(11):581-94. [DOI] [PubMed] [Google Scholar]
- Servellen G, Nyamathi A, Carpio F, Pearce D, Garcia-Teague L, Herrera G, et al. Effects of a treatment adherence enhancement program on health literacy, patient–provider relationships, and adherence to haart among low-Income hiv-positive spanish-speaking Latinos. AIDS Patient Care and STDs 2005;19(11):745-59. [DOI] [PubMed] [Google Scholar]
Wong 2020 {published data only}
- Wong MHM, Keng S-L, Buck PJ, Suthendran S, Wessels A, Østbye T. Effects of mental health paraprofessional training for Filipina foreign domestic workers in Singapore. Journal of Immigrant and Minority Health 2020;22(3):571-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmad 2012 {published data only}
- Ahmad F, Shakya Y, Li J, Khoaja K, Norman CD, Lou W, et al. A pilot with computer-assisted psychosocial risk-assessment for refugees. BMC Medical Informatics and Decision Making 2012;12:71. [DOI: 10.1186/1472-6947-12-71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Albright 2005 {published data only}
- Albright CL, Pruitt L, Castro C, Gonzalez A, Woo S, King AC. Modifying physical activity in a multiethnic sample of low-income women: one-year results from the IMPACT (Increasing Motivation for Physical ACTivity) project. Annals of Behavioral Medicine 2005;30(3):191-200. [DOI: 10.1207/s15324796abm3003_3] [DOI] [PubMed] [Google Scholar]
Alcala 2016 {published data only}
- Alcala HE, Albert SL, Trabanino SK, Garcia RE, Glik DC, Prelip ML, et al. Access to and use of health care services among Latinos in East Los Angeles and Boyle Heights. Journal of Health Promotion and Maintenance 2016;39(1):62-71. [DOI: 10.1097/FCH.0000000000000090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alegria 2014 {published data only}
- Alegria M, Ludman E, Kafali EN, Lapatin S, Vila D, Shrout PE, et al. Effectiveness of the engagement and counseling for Latinos (ECLA) intervention in low-income Latinos. Medical Care 2014;52(11):989-97. [DOI: 10.1097/MLR.0000000000000232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alegria 2019 {published data only}
- Alegría M, Falgas-Bague I, Collazos F, Camacho RC, Markle SL, Wang Y, et al. Evaluation of the integrated intervention for dual problems and early action among Latino immigrants with co-occurring mental health and substance misuse symptoms: a randomized clinical trial. JAMA Network Open 2019;2(1):e186927. [DOI: 10.1001/jamanetworkopen.2018.6927] [DOI] [PMC free article] [PubMed] [Google Scholar]
Apter 2015 {published data only}
- Apter AJ, Bryant-Stephens T, Morales KH, Wan F, Hardy S, Reed-Wells S, et al. Using IT to improve access, communication, and asthma in African American and Hispanic/Latino adults: rationale, design, and methods of a randomized controlled trial. Contemporary Clinical Trials 2015;44:119-28. [DOI: 10.1016/j.cct.2015.08.001] [DOI] [PubMed] [Google Scholar]
Aragones 2010 {published data only}
- Aragones A, Schwartz MD, Shah NR, Gany FM. A randomized controlled trial of a multilevel intervention to increase colorectal cancer screening among Latino immigrants in a primary care facility. Journal of General Internal Medicine 2010;25(6):564-7. [DOI: 10.1007/s11606-010-1266-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnold 2019 {published data only}
- Arnold CL, Rademaker AW, Morris JD, Ferguson LA, Wiltz G, Davis TC. Follow-up approaches to a health literacy intervention to increase colorectal cancer screening in rural community clinics: a randomized controlled trial. Cancer 2019;125(20):3615-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Athavale 2016 {published data only}
- Athavale P, Thomas M, Delgadillo-Duenas AT, Leong K, Najmabadi A, Harleman E, et al. Linking high risk postpartum women with a technology enabled health coaching program to reduce diabetes risk and improve wellbeing: program description, case studies, and recommendations for community health coaching programs. Journal of Diabetes Research 2016:4353956. [DOI: 10.1155/2016/4353956] [DOI] [PMC free article] [PubMed]
- Handley MA, Harleman E, Gonzalez-Mendez E, Stotland NE, Althavale P, Fisher L, et al. Applying the COM-B model to creation of an IT-enabled health coaching and resource linkage program for low-income Latina moms with recent gestational diabetes: the STAR MAMA program. Implementation Science 2016;11(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahromov 2011 {published data only}
- Bahromov M, Weine S. HIV prevention for migrants in transit: developing and testing TRAIN. AIDS Education and Prevention 2011;23(3):267-80. [DOI: 10.1521/aeap.2011.23.3.267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2013 {published data only}
- Baker DW, Brown T, Buchanan DR, Weil J, Cameron KA, Ranalli L, et al. Improving rates of annual colorectal cancer screening among Latino patients. Journal of General Internal Medicine 2013;28(10):106. [Google Scholar]
Banna 2011 {published data only}
- Banna JC, Townsend MS. Assessing factorial and convergent validity and reliability of a food behaviour checklist for spanish-speaking participants in US department of agriculture nutrition education programmes. Public Health Nutrition 2011;14(7):1165-76. [DOI: 10.1017/S1368980010003058] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bastani 2010 {published data only}
- Bastani R, Glenn BA, Herrmann, AK, Crespi CM, Wong WK, Jo AM, et al. Community-based intervention to reduce liver cancer disparities in Asian Americans: a cluster randomized trial. Cancer Prevention Research 2010;3(12 Suppl 2). [DOI: 10.1158/1940-6207] [DOI]
Bastani 2015 {published data only}
- Bastani R, Glenn BA, Maxwell AE, Jo AM, Herrmann AK, Crespi CM, et al. Cluster-randomized trial to increase hepatitis B testing among Koreans in Los Angeles. Cancer Epidemiology, Biomarkers & Prevention 2015;24(9):1341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beauchamp 2020 {published data only}
- Beauchamp A, Mohebbi M, Cooper A, Pridmore V, Livingston P, Scanlon M, et al. The impact of translated reminder letters and phone calls on mammography screening booking rates: two randomised controlled trials. PLOS One 2020;15(1):e0226610. [DOI] [PMC free article] [PubMed]
Bermejo 2013 {published data only}
- Bermejo I, Frank F, Komarahadi F, Albicker J, Ries Z, Kriston L, et al. Transcultural prevention of alcohol dependency: effects of a culturally and migration-sensitive approach [Transkulturelle Prävention von Alkoholerkrankungen—Effekte eines kultur- und migrationssensitiven Ansatzes]. Verhaltenstherapie & Psychosoziale Praxis [Behavioral Therapy & Psychosocial Paxis] 2013;45(1):91-100. [Google Scholar]
Brenner 2015 {published data only}
- Brenner AT, Callan D, Espaillat M, Harbi K, Hoffman R, McWilliams A, et al. Effect of a colorectal cancer screening decision aid on decision making outcomes in a low-income, majority Latino patient population. Journal of General Internal Medicine 2015;30:146. [Google Scholar]
Brenner 2016 {published data only}
- Brenner AT, Hoffman R, McWilliams A, Pignone MP, Rhyne RL, Tapp H, et al. Colorectal cancer screening in vulnerable patients: promoting informed and shared decisions. American Journal of Preventive Medicine 2016;51(4):454-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calderón‐Mora 2020 {published data only}
- Calderón-Mora J, Byrd TL, Alomari A, Salaiz R, Dwivedi A, Mallawaarachchi I, et al. Group versus individual culturally tailored and theory-based education to promote cervical cancer screening among the underserved Hispanics: a cluster randomized trial. American Journal of Health Promotion 2020;34(1):15-24. [DOI] [PubMed] [Google Scholar]
Carrasquillo 2012 {published data only}
- Carrasquillo O, Patberg E, Kenya S, Alonzo Y. A profile of poorly controlled diabetics in South Florida: the Miami healthy heart initiative. Journal of General Internal Medicine 2012;27:109. [Google Scholar]
Carrasquillo 2014 {published data only}
- Carrasquillo O, McCann S, Amofah A, Pierre L, Rodriguez B, Alonzo Y, et al. Rationale and design of the research project of the South Florida center for the reduction of cancer health disparities (SUCCESS): study protocol for a randomized controlled trial. Trials 2014;15:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrasquillo 2015 {published data only}
- Carrasquillo O, Kobetz-Kerman EN, Alonzo Y. A randomized trial of self-sampling for human papilloma virus among minority immigrant women in need of cervical cancer screening: findings from the South Florida center for reducing cancer disparities. Journal of General Internal Medicine 2015;30:90. [Google Scholar]
Carrasquillo 2017 {published data only}
- Carrasquillo O, Lebron C, Alonzo Y, Li H, Chang A, Kenya S. Effect of a community health worker intervention among Latinos with poorly controlled type 2 diabetes: the Miami healthy heart initiative randomized clinical trial. JAMA Internal Medicine 2017;177(7):948-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo O, Patberg E, Alonzo Y, Li H, Kenya S. Rationale and design of the Miami healthy heart Initiative: a randomized controlled study of a community health worker intervention among Latino patients with poorly controlled diabetes. International Journal of General Medicine 2014;7:115-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Patberg E, Cueto V, Li H, Singh B, Kenya S, et al. Community health workers, access to care, and service utilization among Florida Latinos: a randomized controlled trial. American Journal of Public Health 2018;108(9):1249-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenya S, Lebron CN, Chang AY, Li H, Alonzo YA, Carrasquillo O. A profile of Latinos with poorly controlled diabetes in South Florida. Journal of Community Hospital Internal Medicine Perspectives 2015;5(2):26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrasquillo 2018 {published data only}
- Carrasquillo O, McCann S, Amofah A, Pierre LA, McCann S, et al. HPV self-sampling for cervical cancer screening among ethnic minority women in South Florida: a randomized trial. Journal of General Internal Medicine 2018;33(7):1077-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castejon 2013 {published data only}
- Castejon AM, Calderon JL, Perez A, Millar C, McLaughlin-Middlekauff J, Sangasubana N, et al. A community-based pilot study of a diabetes pharmacist intervention in Latinos: impact on weight and hemoglobin A1c. Journal of Health Care for the Poor and Underserved 2013;24(4):48-60. [DOI] [PubMed] [Google Scholar]
Chai 2018 {published data only}
- Chai W, Zou G, Shi J, Chen W, Gong X, Wei X, et al. Evaluation of the effectiveness of a WHO-5A model based comprehensive tobacco control program among migrant workers in Guangdong, China: a pilot study. BMC Public Health 2018;18(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chakkalakal 2017 {published data only}
- Chakkalakal RJ, White RO, Schildcrout J, Stanley B, Wallston K, Rothman RL. Evaluating glycemic control in low-income patients with type 2 diabetes. Journal of General Internal Medicine 2017;32(2):182. [Google Scholar]
Chalela 2015 {published data only}
- Chalela P, Munoz E, Gallion K J, Karnad A, Ramirez AG. Empowering Latina breast cancer patients to make informed decisions about clinical trials: a multicommunication approach. Cancer Epidemiology, Biomarkers & Prevention 2015;24(10 Suppl 6):439-49. [Google Scholar]
Chan 2014 {published data only}
- Chan B, Goldman LE, Sarkar U, Schneidermann M, Critchfield J, Kushel M. Can an in-hospital care transition intervention improve patient experience in the safety net? A randomized controlled trial. Journal of General Internal Medicine 2014;29:44-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2015 {published data only}
- Chan B, Goldman LE, Sarkar U, Schneidermann M, Kessell E, Guzman D, et al. The effect of a care transition intervention on the patient experience of older multi-lingual adults in the safety net: results of a randomized controlled trial. Journal of General Internal Medicine 2015;30(12):1788-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christy 2019 {published data only}
- Christy SM, Sutton SK, Gwede CK, Chavarria EA, Davis SN, Abdulla R, et al. Examining the durability of colorectal cancer screening awareness and health beliefs among medically underserved patients: baseline to 12 months post-intervention. Journal of Cancer Education 2019;34(2):297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohan 2009 {published data only}
- Cohan D, Gomez E, Greenberg M, Washington S, Charlebois ED. Patient perspectives with abbreviated versus standard pre-test HIV counseling in the prenatal setting: a randomized-controlled, non-inferiority trial. PLOS One 2009;4(4):e5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collado 2014 {published data only}
- Collado A, Long KE, MacPherson L, Lejuez CW. The efficacy of a behavioral activation intervention among depressed US Latinos with limited English language proficiency: study protocol for a randomized controlled trial. Trials 2014;15:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dancel 2013 {published data only}
- Dancel L, Perrin EM, Yin HS, Sanders LM, Delamater A, Perreira K, et al. Acculturation and infant feeding styles in a Latino population: results from an ongoing randomized controlled trial of obesity prevention. Gastroenterology 2013;144(5):397. [Google Scholar]
Davis 2017 {published data only}
- Davis SN, Christy SM, Chavarria EA, Abdulla R, Sutton SK, Schmidt AR, et al. A randomized controlled trial of a multicomponent, targeted, low-literacy educational intervention compared with a nontargeted intervention to boost colorectal cancer screening with fecal immunochemical testing in community clinics. Cancer 2017;123(8):1390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del 2017 {published data only}
- Del Salto G, Patel D, Curtis LM, Parker R, Rachman F, Rittner SS, et al. An electronic health record-enabled (EHR) universal medication schedule to promote adherence: a pragmatic trial. Journal of General Internal Medicine 2017;32(2):113. [Google Scholar]
DeStephano 2010 {published data only}
- DeStephano CC, Flynn PM, Brost BC. Somali prenatal education video use in a United States obstetric clinic: a formative evaluation of acceptability. Patient Education and Counseling 2010;81(1):137-41. [DOI] [PubMed] [Google Scholar]
Dietrich 2006 {published data only}
- Dietrich AJ, Tobin JN, Cassells A, Robinson CM, Greene MA, Sox CH, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Annals of Internal Medicine 2006;144(8):563-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diez 2018 {published data only}
- Diez E, Lopez MJ, Mari-Dell'Olmo M, Nebot L, Perez G, Villalbi JR, et al. Effects of a counselling intervention to improve contraception in deprived neighbourhoods: a randomized controlled trial. European Journal of Public Health 2018;28(1):10-5. [DOI] [PubMed] [Google Scholar]
Drieling 2011 {published data only}
- Drieling RL, Ma J, Stafford RS. Evaluating clinic and community-based lifestyle interventions for obesity reduction in a low-income Latino neighborhood: Vivamos Activos Fair Oaks Program. BMC Public Health 2011;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drks 2019 {published data only}
- Feasibility, acceptance and effectiveness of step-by-step, a smartphone-based self-help program for Syrian refugees: a pilot study. http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00017838 2019.
Dueweke 2017 {published data only}
- Dueweke AR, Bridges AJ. The effects of brief, passive psychoeducation on suicide literacy, stigma, and attitudes toward help-seeking among Latino immigrants living in the United States. Stigma and Health 2017;2(1):28-42. [Google Scholar]
Duggan 2012 {published data only}
- Duggan C, Coronado G, Martinez J, Byrd TL, Carosso E, Lopez C, et al. Cervical cancer screening and adherence to follow-up among Hispanic women study protocol: a randomized controlled trial to increase the uptake of cervical cancer screening in Hispanic women. BMC Cancer 2012;12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwight‐Johnson 2010 {published data only}
- Dwight-Johnson M, Lagomasino IT, Hay J, Zhang L, Tang L, Green JM, et al. Effectiveness of collaborative care in addressing depression treatment preferences among low-income Latinos. Psychiatric Services 2010;61(11):1112-8. [DOI] [PubMed] [Google Scholar]
Elder 2016 {published data only}
- Elder JP, Arredondo EM, Haughton J, Perez LG, Martinez ME. Fe en accion/faith in action: promotion of cancer screening among churchgoing Latinas. Cancer Epidemiology, Biomarkers & Prevention 2016;25(Suppl 3):A23. [Google Scholar]
Ell 2007 {published data only}
- Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Preventive Medicine 2007;44(1):26-33. [DOI] [PubMed] [Google Scholar]
Ell 2017 {published data only}
- Ell K, Aranda MP, Wu S, Oh H, Lee P-J, Guterman J. Promotora assisted depression care among predominately Hispanic patients with concurrent chronic illness: public care system clinical trial design. Contemporary Clinical Trials 2016;46:39-47. [DOI] [PubMed] [Google Scholar]
- Ell K, Aranda MP, Wu S, Oh H, Lee PJ, Guterman J. Promotora assisted depression and self-care management among predominantly Latinos with concurrent chronic illness: safety net care system clinical trial results. Contemporary Clinical Trials 2017;61:1-9. [DOI] [PubMed] [Google Scholar]
Erenoğlu 2020 {published data only}
- Erenoğlu R, Sözbir ŞY. The effect of health education given to Syrian refugee women in their own language on awareness of breast and cervical cancer, in Turkey: a randomized controlled trial. Journal of Cancer Education 2020;35(2):241-7. [DOI] [PubMed] [Google Scholar]
Esquivel 2014 {published data only}
- Esquivel JH, Roman Z, Clark RA, Fredericks B, Dracup K. Culturally-appropriate education can improve self-care in Hispanic patients with heart failure: a pilot study. Journal of Cardiac Failure 2014;20(8):110. [Google Scholar]
Eylem 2015 {published data only}
- Eylem O, Van Straten A, Bhui K, Kerkhof A. Protocol: reducing suicidal ideation among Turkish migrants in the Netherlands and in the UK: effectiveness of an online intervention. International Review of Psychiatry 2015;27(1):72-81. [DOI] [PubMed] [Google Scholar]
Fang 2019 {published data only}
- Fang CY, Lee M, Feng Z, Tan Y, Levine F, Nguyen C, et al. Community-based cervical cancer education: changes in knowledge and beliefs among Vietnamese American women. Journal of Community Health 2019;44(3):525-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fehniger 2014 {published data only}
- Fehniger J, Livaudais-Toman J, Karliner L, Kerlikowske K, Tice JA, Quinn J, et al. Perceived versus objective breast cancer, breast cancer risk in diverse women. Journal of Women's Health 2014;23(5):420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felicitas‐Perkins 2017 {published data only}
- Felicitas-Perkins JQ, Palalay MP, Cuaresma C, Ho RC, Chen MS, Dang J, et al. A pilot study to determine the effect of an educational DVD in Philippine languages on cancer clinical trial participation among Filipinos in Hawai'i. Hawai'i Journal of Medicine & Public Health 2017;76(7):171-7. [PMC free article] [PubMed] [Google Scholar]
Field 2009 {published data only}
- Field C, Caetano R, Harris TR, Roudsari B. Understanding ethnic differences in drinking outcomes following a brief alcohol intervention: the role of ethnic matching between patient and provider. Alcoholism: Clinical and Experimental Research 2009;33:269. [Google Scholar]
Field 2010 {published data only}
- Field C, Caetano R. The role of ethnic matching between patient and provider on the effectiveness of brief alcohol interventions with Hispanics. Alcoholism: Clinical and Experimental Research 2010;34(2):262-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 2013 {published data only}
- Fischer S, Kutner J, Cervantes L. Apoyo con carino: RCT of patient navigation to improve palliative care outcomes. Journal of Pain and Symptom Management 2013;45(2):376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 2015 {published data only}
- Fischer IP, Fischer HH, Pereira RI, Rozwadowski JM, Gutierrez-Raghunath S, Furniss AL, et al. Text messaging versus usual care for weight loss in patients with pre-diabetes. Journal of General Internal Medicine 2015;30:265-6. [Google Scholar]
Fortmann 2015 {published data only}
- Fortmann AL, Garcia MI, Ruiz M, Gallo LC, Schultz J, Carlson A, et al. Acceptability and feasibility of an mHealth self-management intervention in underserved Hispanics with poorly controlled type 2 diabetes. Endocrine Reviews 2015;36(2).
Frosch 2011 {published data only}
- Frosch DL, Uy V, Ochoa S, Mangione CM. Evaluation of a behavior support intervention for patients with poorly controlled diabetes. Archieves of Internal Medicine 2011;171(22):2011-7. [DOI] [PubMed] [Google Scholar]
Gademan 2012 {published data only}
- Gademan MG, Deutekom M, Hosper K, Stronks K. The effect of exercise on prescription on physical activity and wellbeing in a multi-ethnic female population: a controlled trial. BMC Public Health 2012;12:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gany 2007 {published data only}
- Gany F, Leng J, Shapiro E, Abramson D, Motola I, Shield DC, et al. Patient satisfaction with different interpreting methods: a randomized controlled trial. Journal of General Internal Medicine 2007;22(2):312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garbers 2012 {published data only}
- Garbers S, Meserve A, Hatcher R, Kottke M, Chiasson MA. Efficacy of a self-administered computerized counseling module in improving contraceptive method choice and continuation. Contraception 2012;85(3):321. [Google Scholar]
Garland 2007 {published data only}
- Garland WH, Wohl AR, Valencia R, Witt MD, Squires K, Kovacs A, et al. The acceptability of a directly-administered antiretroviral therapy (DAART) intervention among patients in public HIV clinics in Los Angeles, California. AIDS Care 2007;19(2):159-67. [DOI] [PubMed] [Google Scholar]
Gelberg 2019 {published data only}
- Gelberg L, Rico MW, Herman DR, Belin TR, Chandler M, Ramirez E, et al. Comparative effectiveness trial comparing MyPlate to calorie counting for mostly low-income Latino primary care patients of a federally qualified community health center: study design, baseline characteristics. BMC Public Health 2019;19(1):990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goel 2016 {published data only}
- Goel MS, O'Conor R. Increasing screening mammography among predominantly Spanish speakers at a federally qualified health center using a brief previsit video. Patient Education and Counseling 2016;99(3):408-13. [DOI] [PubMed] [Google Scholar]
Golchert 2019 {published data only}
- Golchert J, Roehr S, Berg F, Grochtdreis T, Hoffmann R, Jung F, et al. HELP@APP: development and evaluation of a self-help app for traumatized Syrian refugees in Germany—a study protocol of a randomized controlled trial. BMC Psychiatry 2019;19(131). [DOI: ] [DOI] [PMC free article] [PubMed]
Gold 2014 {published data only}
- Gold A, Yu N, Buro B, Garden-Robinson J. Discussion map and cooking classes: testing the effectiveness of teaching food safety to immigrants and refugees. Journal of Nutrition Education and Behavior 2014;46(6):547-53. [DOI] [PubMed] [Google Scholar]
Gonzales 2014 {published data only}
- Gonzales FA, Le HN, Perry DF. Using an optimality index to understand perinatal health disparities: a pilot study with Latina immigrants. Journal of Transcultural Nursing 2014;25(3):265-72. [DOI] [PubMed] [Google Scholar]
Gonzales 2016 {published data only}
- Gonzales FA, Hurtado de Mendoza A, Santoyo-Olsson J, Napoles AM. Do coping strategies mediate the effects of emotional support on emotional well-being among Spanish-speaking Latina breast cancer survivors? Psycho-Oncology 2016;25(11):1286-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzales 2020 {published data only}
- Gonzales FR. ¡Yo no estoy loca! A behavioral health telenovela style entertainment education video to reduce stigma and increase mental health literacy among Latina adults with limited English proficiency [Dissertation]. Reno (USA): University of Nevada, Reno, August 2020. [Google Scholar]
Goodyer 2006 {published data only}
- Goodyer L, Savage I, Dikmen Z. Inhaler technique in Turkish people with poor English: a case of information discrimination? Pharmacy World & Science 2006;28(2):107-14. [DOI] [PubMed] [Google Scholar]
Gordon 2014 {published data only}
- Gordon E, Feinglass J, Martinez S, Vera K, Carney P, O'Connor K, et al. A website for Hispanics/Latinos about living donation and transplantation: a randomized controlled trial of increased knowledge. Transplantation 2014;98(1):479. [Google Scholar]
Gordon 2016 {published data only}
- Gordon EJ, Feinglass J, Carney P, Vera K, Olivero M, Black A, et al. A culturally targeted website for Hispanics/Latinos about living kidney donation and transplantation: a randomized controlled trial of increased knowledge. Transplantation 2016;100(5):1149-60. [DOI] [PubMed] [Google Scholar]
Greenhalgh 2005 {published data only}
- Greenhalgh T, Collard A, Begum N. Sharing stories: complex intervention for diabetes education in minority ethnic groups who do not speak English. BMJ 2005;330(7492):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenhalgh 2011 {published data only}
- Greenhalgh T, Campbell-Richards D, Vijayaraghavan S, Collard A, Malik F, Griffin M, et al. New models of self-management education for minority ethnic groups: pilot randomized trial of a story-sharing intervention. Journal of Health Services Research & Policy 2011;16(1):28-36. [DOI] [PubMed] [Google Scholar]
Gustafsson 2015 {published data only}
- Gustafsson S, Lood Q, Wilhelmson K, Häggblom-Kronlöf G, Landahl S, Dahlin-Ivanoff S. A person-centred approach to health promotion for persons 70+ who have migrated to Sweden: promoting aging migrants' capabilities implementation and RCT study protocol. BMC Geriatrics 2015;15:10. [DOI: doi: 10.1186/s12877-015-0005-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gwadz 2017 {published data only}
- Gwadz MV, Collins LM, Cleland CM, Leonard NR, Wilton L, Gandhi M, et al. Using the multiphase optimization strategy (MOST) to optimize an HIV care continuum intervention for vulnerable populations: a study protocol. BMC Public Health 2017;17(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gwynn 2016 {published data only}
- Gwynn KB, Winter MR, Cabral HJ, Wolf MS, Hanchate AD, Henault L, et al. Racial disparities in patient activation: evaluating the mediating role of health literacy with path analyses. Patient Education and Counseling 2016;99(6):1033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hahn 2015 {published data only}
- Hahn EA, Burns JL, Jacobs EA, Ganschow PS, Garcia SF, Rutsohn JP, et al. Health literacy and patient-reported outcomes: a cross-sectional study of underserved English- and Spanish-speaking patients with type 2 diabetes. Journal of Health Communication 2015;20(Suppl 2):4-15. [DOI] [PubMed] [Google Scholar]
Han 2010 {published data only}
- Han HR, Kim J, Kim KB, Jeong S, Levine D, Li C, et al. Implementation and success of nurse telephone counseling in linguistically isolated Korean American patients with high blood pressure. Patient Education and Counseling 2010;80(1):130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handley 2008 {published data only}
- Handley MA, Shumway M, Schillinger D. Cost-effectiveness of automated telephone self-management support with nurse care management among patients with diabetes. Annals of Family Medicine 2008;6(6):512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harmsen 2005 {published data only}
- Harmsen H, Bernsen R, Meeuwesen L, Thomas S, Dorrenboom G, Pinto D, et al. The effect of educational intervention on intercultural communication: results of a randomised controlled trial. British Journal of General Practice 2005;55(514):343-50. [PMC free article] [PubMed] [Google Scholar]
Helland‐Kigen 2013 {published data only}
- Helland-Kigen KM, Råberg Kjøllesdal MK, Hjellset VT, Bjørge B, Holmboe-Ottesen G, Wandel M. Maintenance of changes in food intake and motivation for healthy eating among Norwegian-Pakistani women participating in a culturally adapted intervention. Public Health Nutrition 2013;16(1):113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Helland‐Kigen 2013a {published data only}
- Helland-Kigen KM, Råberg Kjøllesdal MK, Hjellset VT, Bjørge B, Holmboe-Ottesen G, Wandel M. Maintenance of changes in food intake and motivation for healthy eating among Norwegian-Pakistani women participating in a culturally adapted intervention. Public Health Nutrition 2013;16(1):113-22. [DOI] [PMC free article] [PubMed]
Hernandez 2015 {published data only}
- Hernandez MY, Organista KC. Qualitative exploration of an effective depression literacy fotonovela with at risk Latina immigrants. American Journal of Community Psychology 2015;1-2(56):79-88. [DOI] [PubMed] [Google Scholar]
Hijazi 2013 {published data only}
- Hijazi A, Ziadni M, Arnetz BB, Haddad L, Lumley M. Narrative exposure therapy to treat traumatic stress in middle eastern refugees: a clinical trial. Psychosomatic Medicine 2013;75(3):A118. [Google Scholar]
Hijazi 2014 {published data only}
- Hijazi AM, Lumley MA, Ziadni MS, Haddad L, Rapport LJ, Arnetz BB. Brief narrative exposure therapy for posttraumatic stress in Iraqi refugees: a preliminary randomized clinical trial. Journal of Traumatic Stress 2014;27(3):314-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holzel 2014 {published data only}
- Holzel LP, Ries Z, Zill JM, Kriston L, Dirmaier J, Harter M, et al. Development and testing of culturally sensitive patient information material for Turkish, Polish, Russian and Italian migrants with depression or chronic low back pain (KULTINFO): study protocol for a double-blind randomized controlled trial. Trials 2014;15(1):265-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holzel 2016 {published data only}
- Holzel LP, Ries Z, Kriston L, Dirmaier J, Zill JM, Rummel-Kluge C, et al. Effects of culture-sensitive adaptation of patient information material on usefulness in migrants: a multicentre, blinded randomised controlled trial. BMJ Open 2016;6(11):e012008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horowitz 2011 {published data only}
- Horowitz C, Vasquez C. Relevance and rigor: community-based diabetes prevention. Journal of Diabetes 2011;3:31. [Google Scholar]
Howell 2011 {published data only}
- Howell EA, Balbierz A, Jason W, Howard L. Mothers avoiding depression through empowerment intervention trial (made it). Journal of General Internal Medicine 2011;26:222-3. [Google Scholar]
Howie‐Esquivel 2014 {published data only}
- Howie-Esquivel J, Bibbins-Domingo K, Clark R, Evangelista L, Dracup K. A culturally appropriate educational intervention can improve self-care in Hispanic patients with heart failure: a pilot randomized controlled trial. Cardiology Research 2014;5(3-4):91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howie‐Esquivel 2014a {published data only}
- Howie-Esquivel J, Bibbins-Domingo K, Clark R, et al. A culturally appropriate educational intervention can improve self-care in Hispanic patients with heart failure: a pilot randomized controlled trial. Cardiology Research 2014;5(3-4):91-100. [DOI] [PMC free article] [PubMed]
Interian 2013 {published data only}
- Interian A, Lewis-Fernandez R, Dixon LB. Improving treatment engagement of underserved U.S. racial-ethnic groups: a review of recent interventions. Psychiatric Services 2013;64(3):212-22. [DOI] [PubMed] [Google Scholar]
Jacobson 2016 {published data only}
- Jacobson HE, Hund L, Soto Mas F. Predictors of English health literacy among U.S. Hispanic immigrants: the importance of language, bilingualism and sociolinguistic environment. Literacy and Numeracy Studies 2016;24(1):43-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2018 {published data only}
- Jang M, Plocienniczak MJ, Mehrazarin K, Bala W, Wong K, Levi JR. Evaluating the impact of translated written discharge instructions for patients with limited English language proficiency. International Journal of Pediatric Otorhinolaryngology 2018;111:75-9. [DOI] [PubMed] [Google Scholar]
Jerant 2014 {published data only}
- Jerant A, Kravitz RL, Sohler N, Fiscella K, Romero RL, Parnes B, et al. Sociopsychological tailoring to address colorectal cancer screening disparities: a randomized controlled trial. Annals of Family Medicine 2014;12(3):204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jerant 2014a {published data only}
- Jerant A, Kravitz RL, Sohler N, Fiscella K, Romero RL, Parnes B, et al. Sociopsychological tailoring to address colorectal cancer screening disparities: a randomized controlled trial. Annals of Family Medicine 2014;12(3):204-14. [DOI] [PMC free article] [PubMed]
Jervelund 2018 {published data only}
- Jervelund SS, Maltesen T, Lawaetz Wimmelmann C, Petersen JH, Krasnik A. Ignorance is not bliss: the effect of systematic information on immigrants’ knowledge of and satisfaction with the Danish healthcare system. Scandinavian Journal of Public Health 2017;45(2):161-74. [DOI] [PubMed] [Google Scholar]
- Jervelund SS, Maltesen T, Wimmelmann CL, Petersen JH, Krasnik A. Know where to go: evidence from a controlled trial of a healthcare system information intervention among immigrants. BMC Public Health 2018;18(1):863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jih 2016 {published data only}
- Jih J, Le G, Woo K, Tsoh JY, Stewart S, Gildengorin G, et al. Educational interventions to promote healthy nutrition and physical activity among older Chinese Americans: a cluster-randomized trial. American Journal of Public Health 2016;106(6):1092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jihyun 2018 {published data only}
- Jihyun K, Nam CK. Effects of birth control empowerment program for married immigrant Vietnamese women in South Korea. Korean Journal of Women Health Nursing 2017;23(1):1-10. [DOI] [PubMed] [Google Scholar]
Jimenez 2015 {published data only}
- Jimenez DE, Reynolds CF, Alegria M, Harvey P, Bartels SJ. The Happy Older Latinos are Active (HOLA) health promotion and prevention study: study protocol for a pilot randomized controlled trial. Trials 2015;16:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jimenez 2017 {published data only}
- Jimenez D, Reynolds CF, Alegria M, Harvey PS, Bartels SJ. Preventing anxiety in depression in older Latinos: the Happy Older Latinos are Active (HOLA) health promotion study. American Journal of Geriatric Psychiatry 2017;25(3):124. [Google Scholar]
Juarez 2013 {published data only}
- Juarez G, Hurria A, Uman G, Ferrell B. Impact of a bilingual education intervention on the quality of life of Latina breast cancer survivors. Oncology Nursing Forum 2013;3:331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juarez‐Carrillo 2012 {published data only}
- Juarez-Carrillo PM. Comparison of two environmental health education methods to reduce exposure to residential pesticides in Hispanic households in the U.S.-Mexico borderland. Open Access Theses & Dissertations 2011:2513.
Juon 2016 {published data only}
- Juon HS, Strong C, Kim F, Park E, Lee S. Lay health worker intervention improved compliance with hepatitis b vaccination in Asian Americans: randomized controlled trial. PLOS One 2016;11(9):e0162683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kandula 2014 {published data only}
- Kandula NR, Dave SS, Patel Y, Seguil PE, De Chavez PJ, Kumar S, et al. Translating a heart disease lifestyle intervention for use in south Asian immigrant communities: preliminary results of a pilot randomized controlled trial. Journal of General Internal Medicine 2014;29(1):239. [Google Scholar]
Kandula 2020 {published data only}
- Kandula NR, Bernard V, Swapna D, Ehrlich-Jones L, Counard C, Shah N, et al. The South Asian HEalthy Lifestyle Intervention (SAHELI) trial: protocol for a mixed-methods, hybrid effectiveness implementation trial for reducing cardiovascular risk in South Asians in the United States. Contemporary Clinical Trials 2020;92:105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendall 2017 {published data only}
- Kendall P, Scharff R, Baker S, LeJeune J, Sofos J, Medeiros L. Food safety instruction improves knowledge and behavior risk and protection factors for foodborne illnesses in pregnant populations. Maternal and Child Health Journal 2017;21(8):1686-98. [DOI] [PubMed] [Google Scholar]
Kepka 2011 {published data only}
- Kepka D, Coronado GD, Rodriguez HP, Thompson B. Evaluation of a radionovela to promote HPV vaccine awareness and knowledge among Hispanic parents. Journal of Community Health 2011;36(6):957-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kieffer 2013 {published data only}
- Kieffer EC, Caldwell CH, Welmerink DB, Welch KB, Sinco BR, Guzman JR. Effect of the healthy MOMs lifestyle intervention on reducing depressive symptoms among pregnant Latinas. American Journal of Community Psychology 2013;51(1-2):76-89. [DOI] [PubMed] [Google Scholar]
Kieffer 2013a {published data only}
Kim 2010 {published data only}
- Kim JH, Menon U, Wang E, Szalacha L. Assess the effects of culturally relevant intervention on breast cancer knowledge, beliefs, and mammography use among Korean American women. Journal of Immigrant and Minority Health 2010;12(4):586-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014a {published data only}
- Kim S, Ruiz-Barros V, Tang A, Kuo C, Quan J, Horton C, et al. Provider-augmented automated telephone self-management (ATSM) lowers A1C in a high-risk, safety net population. Diabetes 2014;63:A84. [Google Scholar]
Kim 2019 {published data only}
- Kim M, Lee H, Kiang P, Allison J. Development and acceptability of a peer-paired, cross-cultural and cross-generational storytelling HPV intervention for Korean American college women. Health Education Research 2019;34(5):483-94. [DOI] [PMC free article] [PubMed]
Kiropoulos 2011a {published data only}
- Kiropoulos LA, Griffiths KM, Blashki G. Effects of a multilingual information website intervention on the levels of depression literacy and depression-related stigma in Greek-born and Italian-born immigrants living in Australia: randomized controlled trial. Journal of Medical Internet Research 2011;13(2):19-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2017 {published data only}
- Ko NY, Gunn C, Bak S, Wang N, Nelson K, Flacks JH. Predictors of social support among newly diagnosed breast cancer patients seeking care at an urban safety net academic medical center. Journal of General Internal Medicine 2017;32(2 Suppl 1):283. [Google Scholar]
Kocken 2008 {published data only}
- Kocken PL, Zwanenburg EJ, Hoop T. Effects of health education for migrant females with psychosomatic complaints treated by general practitioners. A randomised controlled evaluation study. Patient Education and Counseling 2008;70(1):25-30. [DOI] [PubMed] [Google Scholar]
Kohlstadt 2016 {published data only}
- Kohlstadt I, Gittelsohn J, Fang Y. NutriBee intervention improves diet and psychosocial outcomes by engaging early adolescents from diverse and disadvantaged communities. Journal of the American College of Nutrition 2016;35(5):443-51. [DOI] [PubMed] [Google Scholar]
Koniak‐Griffin 2011 {published data only}
- Koniak-Griffin D, Lesser J, Takayanagi S, Cumberland WG. Couple-focused human immunodeficiency virus prevention for young Latino parents: randomized clinical trial of efficacy and sustainability. Archives of Pediatrics and Adolescent Medicine 2011;165(4):306-12. [DOI] [PubMed] [Google Scholar]
Kurth 2016 {published data only}
- Kurth AE, Chhun N, Cleland CM, Crespo-Fierro M, Pares-Avila JA, Lizcano JA, et al. Linguistic and cultural adaptation of a computer-based counseling program (CARE+ Spanish) to support HIV treatment adherence and risk reduction for people living with HIV/AIDS: a randomized controlled trial. Journal of Medical Internet Research 2016;18(7):e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurtovich 2010 {published data only}
- Kurtovich E, Ivey SL, Neuhauser L, Graham C, Constantine W, Barkan H. A multilingual mass communication intervention for seniors and people with disabilities on Medicaid: a randomized controlled trial. Health Services Research 2010;45(2):397-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwon 2015 {published data only}
- Kwon I, Choi S, Mittman B, Bharmal N, Liu H, Vickrey B, et al. Study protocol of 'Worth the Walk': a randomized controlled trial of a stroke risk reduction walking intervention among racial/ethnic minority older adults with hypertension in community senior centers. BMC Neurology 2015;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwong 2013 {published data only}
- Kwong K, Chung H, Cheal K, Chou JC, Chen T. Depression care management for Chinese Americans in primary care: a feasibility pilot study. Community Mental Health Journal 2013;49(2):157-65. [DOI] [PubMed] [Google Scholar]
Ladley 2018 {published data only}
- Ladley A, Hieger AW, Arthur J, Broom M. Educational text messages decreased emergency department utilization among infant caregivers: a randomized trial. Academic Pediatrics 2018;18(6):636-41. [DOI] [PubMed] [Google Scholar]
Lam 2003 {published data only}
- Lam TK, McPhee SJ, Mock J, Wong C, Doan HT, Nguyen T, et al. Encouraging Vietnamese-American women to obtain pap tests through lay health worker outreach and media education. Journal of General Internal Medicine 2003;18(7):516-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lasser 2010 {published data only}
- Lasser K, Valley-Shah L, Lisboa S, Casimir N, Murillo J, Emmons K, et al. A randomized controlled trial of patient navigation to promote colorectal cancer screening in community health centers. Journal of General Internal Medicine 2010;25:213. [Google Scholar]
Lee 2014 {published data only}
- Lee E, Menon U, Nandy K, Szalacha L, Kviz F, Cho Y, et al. The effect of a couples intervention to increase breast cancer screening among Korean Americans. Oncology Nursing Forum 2014;41(3):185-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014a {published data only}
- Lee HY, Tran M, Jin SW, Bliss R, Yeazel M. Motivating underserved Vietnamese Americans to obtain colorectal cancer screening: evaluation of a culturally tailored DVD intervention. Asian Pacific Journal of Cancer Prevention 2014;15(4):1791-6. [DOI] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee H, Ghebre R, Le C, Jang YJ, Sharratt M, Yee D. Mobile phone multilevel and multimedia messaging intervention for breast cancer screening: pilot randomized controlled trial. JMIR mHealth and uHealth 2017;5(11):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee‐Lin 2016 {published data only}
- Lee-Lin F, Nguyen T, Pedhiwala N, Dieckmann NF, Menon U. A longitudinal examination of stages of change model applied to mammography screening. Western Journal of Nursing Research 2016;38(4):441-58. [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li X, Lin D, Wang B, Du H, Tam Cheuk C, Stanton B. Efficacy of theory-based HIV behavioral prevention among rural-to-urban migrants in China: a randomized controlled trial. AIDS Education and Prevention 2014;26(4):296-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindberg 2020 {published data only}
- Lindberg NM, Vega-López S, LeBlanc ES, Leo MC, et al. Lessons learned from a program to reduce diabetes risk among low-income Hispanic women in a community health clinic. Frontiers in Endocrinology;11:489882. [DOI] [PMC free article] [PubMed]
Lood 2015 {published data only}
- Lood Q, Gustafsson S, Dahlin Ivanoff S. Bridging barriers to health promotion: a feasibility pilot study of the 'promoting aging migrants' capabilities study'. Journal of Evaluation in Clinical Practice 2015;21(4):604-13. [DOI] [PubMed] [Google Scholar]
Ma 2017 {published data only}
- Ma GX, Fang CY, Seals B, Feng Z, Tan Y, Siu P, et al. A community-based randomized trial of hepatitis b screening among high-risk Vietnamese Americans. American Journal of Public Health 2017;107(3):433-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2018 {published data only}
- Ma GX, Lee MM, Tan Y, Hanlon AL, Feng Z, Shireman TI, et al. Efficacy of a community-based participatory and multilevel intervention to enhance hepatitis b virus screening and vaccination in underserved Korean Americans. Cancer 2018;124(5):973-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2019 {published data only}
- Ma GX, Lee M, Beeber M, Das R, Feng Z, Wang MQ, et al. Community-clinical linkage intervention to improve colorectal cancer screening among underserved Korean Americans. Cancer Health Disparities 2019;3:e1-e15. [PMC free article] [PubMed] [Google Scholar]
Macabasco‐O'Connell 2011 {published data only}
- Macabasco-O'Connell A, Mendez A, Pignone M. Impact of a heart failure "teach to goal" intervention in low literacy patients. Journal of Cardiac Failure 2011;17(8 Suppl 1):88. [Google Scholar]
Macabasco‐O'Connell 2011a {published data only}
- Macabasco-O'Connell A, Mendez A, Pignone M. Impact of a heart failure "teach to goal" intervention in low literacy patients. Journal of Cardiac Failure 2011;17(8 Suppl 1):88.
Makoul 2009 {published data only}
- Makoul G, Cameron KA, Baker DW, Francis L, Scholtens D, Wolf MS. A multimedia patient education program on colorectal cancer screening increases knowledge and willingness to consider screening among Hispanic/Latino patients. Patient Education and Counseling 2009;76(2):220-6. [DOI] [PubMed] [Google Scholar]
Makoul 2011 {published data only}
- Makoul G, Scholtens D, Negrini A, Negrinie A, Cameron KA, Thompson J, et al. Educating underserved patients about colorectal cancer screening: multimedia vs print. Journal of General Internal Medicine 2011;26:10.
Marcus 2015 {published data only}
- Hartman SJ, Dunsiger SI, Bock BC, Larsen BA, Linke S, Pekmezi D, et al. Physical activity maintenance among Spanish-speaking Latinas in a randomized controlled trial of an internet-based intervention. Journal of Behavioral Medicine 2017;40(3):392-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B, Marcus B, Pekmezi D, Hartman S, Gilmer T. A web-based physical activity intervention for Spanish-speaking Latinas: a costs and cost-effectiveness analysis. Journal of Medical Internet Research 2017;19(2):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke SE, Dunsiger SI, Gans KM, Hartman SJ, Pekmezi D, Larsen BA, et al. Association between physical activity intervention website use and physical activity levels among Spanish-speaking Latinas: randomized controlled trial. Journal of Medical Internet Research 2019;21(7):e13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Hartman SJ, Larsen BA, Pekmezi D, Dunsiger SI, Linke S, et al. Pasos Hacia La Salud: a randomized controlled trial of an internet-delivered physical activity intervention for Latinas. International Journal of Behavioral Nutrition and Physical Activity 2016;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Hartman SJ, Pekmezi D, Dunsiger SI, Linke SE, Marquez B, et al. Using interactive internet technology to promote physical activity in Latinas: rationale, design, and baseline findings of pasos hacia la salud. Contemporary Clinical Trials 2015b;44:149-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Medina‐Ramirez 2019 {published data only}
- Medina-Ramirez P, Sutton SK, Martinez U, Meade CD, Byrne MM, Brandon K O, et al. A randomized controlled trial of a smoking cessation self-help intervention for Spanish-speaking Hispanic/Latinx smokers: study design and baseline characteristics. Contemporary Clinical Trials 2019;85:105836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meredith 2014 {published data only}
- Meredith LS, Eisenman DP, Green BL, Kaltman S, Wong EC, Han B, et al. Design of the violence and stress assessment (ViStA) study: a randomized controlled trial of care management for PTSD among predominantly Latino patients in safety net health centers. Contemporary Clinical Trials 2014;38(2):163-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Millan‐Ferro 2017 {published data only}
- Millan-Ferro A, Twahirwa M, Yamba L, Garces MA, Leal ML, Herrera M, et al. Participation of Latinos with type 2 diabetes in a culturally and linguistically oriented virtual diabetes self-care and education program. Diabetes 2017;66 Suppl 1:A605. [Google Scholar]
Miranda 2019 {published data only}
- Miranda R, Meeks KA, Snijder MB, den Born BJ, Fransen MP, Peters RJ, et al. Health literacy and hypertension outcomes in a multi-ethnic population: the HELIUS study. European Journal of Public Health 2019;30(3):545-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2015 {published data only}
- Mitchell DC, Andrews T, Schenker MB. Pasos saludables: a pilot randomized intervention study to reduce obesity in an immigrant farmworker population. Journal of Occupational and Environmental Medicine 2015;57(10):1039-46. [DOI] [PubMed] [Google Scholar]
Moore 2016 {published data only}
- Moore AA, Karno MP, Ray L, Ramirez K, Barenstein V, Portillo M J, et al. Development and preliminary testing of a promotora-delivered, Spanish language, counseling intervention for heavy drinking among male, Latino day laborers. Journal of Substance Abuse Treatment 2016;62:96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2018 {published data only}
- Myers RE, Stello B, Daskalakis C, Sifri R, González ET, DiCarlo M, et al. Decision support and navigation to increase colorectal cancer screening among Hispanic patients. Cancer Epidemiology, Biomarkers & Prevention 2018;28(2):384-91. [DOI] [PubMed] [Google Scholar]
Møen 2020 {published data only}
- Møen KA, Kumar B, Igland J, Diaz E. Effect of an intervention in general practice to increase the participation of immigrants in cervical cancer screening: a cluster randomized clinical trial. JAMA Network Open 2020;3(4):e201903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Navarro 1995 {published data only}
- Navarro AM, Senn KL, Kaplan RM, McNicholas L, Campo MC, Roppe B. Por la vida intervention model for cancer prevention in Latinas. Journal of the National Cancer Institute 1995;18:137-45. [PubMed] [Google Scholar]
NCT00857636 {published data only}
- NCT00857636. Behavioral intervention study for better breast and cervical cancer control for Korean American women. https://clinicaltrials.gov/show/NCT00857636 (first received 6 March 2009).
NCT03980808 {published data only}
- NCT03980808. American sign language-accessible diabetes education. https://clinicaltrials.gov/show/NCT03980808 (first received 10 June 2019).
NCT04831463 {published data only}
- NCT04831463. The effect of the program on the health perceptions and responsibilities of immigrant men on utiling healthcare services (IHAP). https://clinicaltrials.gov/ct2/show/NCT04831463 (first received 5 April 2021).
Nedjat‐Haiem 2012 {published data only}
- Nedjat-Haiem FR, Lorenz KA, Ell K, Hamilton A, Palinkas L. Experiences with advanced cancer among Latinas in a public health care system. Journal of Pain and Symptom Management 2012;43(6):1013-24. [DOI] [PubMed] [Google Scholar]
Nguyen 2009 {published data only}
- Nguyen TT, Le G, Nguyen T, Le K, Lai K, Gildengorin G, et al. Breast cancer screening among Vietnamese Americans: a randomized controlled trial of lay health worker outreach. American Journal of Preventive Medicine 2009;37(4):306-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nickell 2019 {published data only}
- Nickell A, Stewart SL, Burke NJ, Guerra C, Cohen E, Lawlor C, et al. Engaging limited English proficient and ethnically diverse low-income women in health research: a randomized trial of a patient navigator intervention. Patient Education and Counseling 2019;102(7):1313-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2014 {published data only}
- O'Connor K, Fisher S, Platt V. A single community-wide, low-literacy and culturally appropriate education resource-is it possible? Asia-Pacific Journal of Clinical Oncology 2014;9:85. [Google Scholar]
O'Connor 2020 {published data only}
- O'Connor TM, Perez O, Beltran A, Colon GI, Arredondo E, Parra CR, et al. Cultural adaptation of 'healthy dads, healthy kids' for Hispanic families: applying the ecological validity model. International Journal of Behavioral Nutrition and Physical Activity 2020;17(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 2017 {published data only}
- Oh H, Ell K, Palinkas LA. Self-care behavior change and depression among low-income predominantly Hispanic patients in safety-net clinics. Social Work in Health Care 2017;56(8):714-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2019 {published data only}
Pekmezi 2009 {published data only}
- Dominick GM, Dunsiger SI, Pekmezi DW, Marcus BH. Health literacy predicts change in physical activity self-efficacy among sedentary Latinas. Journal of Immigrant and Minority Health 2013;15(3):533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekmezi DW, Neighbors CJ, Lee CS, Gans KM, Bock BC, Morrow KM, et al. A culturally adapted physical activity intervention for Latinas: a randomized controlled trial. American Journal of Preventive Medicine 2009;37(6):495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pekmezi 2012 {published data only}
- Dominick GM, Dunsiger SI, Pekmezi DW, Larsen B, Marquez B, Nodora J, et al. Moderating effects of health literacy on change in physical activity among Latinas in a randomized trial. Journal of Racial and Ethnic Health Disparities 2015;2(3):351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Dunsiger SI, Pekmezi D, Larsen BA, Marquez B, Bock BC, et al. Twelve-month physical activity outcomes in Latinas in the seamos saludables trial. American Journal of Preventive Medicine 2015;48(2):179-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Dunsiger SI, Pekmezi DW, Larsen BA, Bock BC, Gans KM, et al. The seamos saludables study: a randomized controlled physical activity trial of Latinas. American Journal of Preventive Medicine 2013;45(5):598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekmezi Di, Dunsiger S, Gans K, Bock B, Gaskins Ra, Marquez B, et al. Rationale, design, and baseline findings from Seamos Saludables: a randomized controlled trial testing the efficacy of a culturally and linguistically adapted, computer-tailored physical activity intervention for Latinas. Contemporary Clinical Trials 2012;33(6):1261-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peragallo 2005 {published data only}
- Peragallo N, DeForge B, O'Campo P, Lee SM, Young JK, Cianelli R, et al. A randomized clinical trial of an HIV-risk-reduction intervention among low-income Latina women. Nursing Research 2005;54(2):108-18. [DOI] [PubMed] [Google Scholar]
Percac‐Lima 2016 {published data only}
- Percac-Lima S, Ashburner JM, Zai A, Chang Y, Oo S, Guimaraes E, et al. Patient navigation for comprehensive cancer screening in high-risk patients using a population-based health information technology system: a randomized clinical trial. Journal of General Internal Medicine 2016;176(7):930-7. [DOI] [PubMed] [Google Scholar]
Poureslami 2011a {published data only}
- Poureslami I, Rootman I, Doyle-Waters MM, Nimmon L, Fitzgerald JM. Health literacy, language, and ethnicity-related factors in newcomer asthma patients to Canada: a qualitative study. Journal of Immigrant and Minority Health 2011;13(2):315-22. [DOI] [PubMed] [Google Scholar]
Poureslami 2011b {published data only}
- Poureslami I, FitzGerald JM, Doyle-Waters MR. "Using Community-Based Participatory Research (CBPR) to develop culturally and linguistically appropriate educational materials to assess asthma patients" knowledge and health literacy in ethno-cultural groups. American Journal of Respiratory and Critical Care Medicine 2011;183:A1429. [Google Scholar]
Qi 2011 {published data only}
- Qi B, Resnick B, Smeltzer SC, Bausell B. Self-efficacy program to prevent osteoporosis among Chinese immigrants. Nursing Research 2011;60(6):393-404. [DOI] [PubMed] [Google Scholar]
Radlick 2020 {published data only}
- Radlick RL, Svedberg P, Nygren JM, Przedpelska S, Gammon D. Digitally enhanced mentoring for immigrant youth social capital: protocol for a mixed methods pilot study and a randomized controlled trial. JMIR Research Protocols 2020;9(3):e16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2014 {published data only}
- Reddy SM, Quintiliani L, Bowen DJ. Information and communication media use by female residents of public housing. Journal of General Internal Medicine 2014;29:127. [Google Scholar]
Reijneveld 2003 {published data only}
- Reijneveld SA, Westhoff MH, Hopman-Rock M. Promotion of health and physical activity improves the mental health of elderly immigrants: results of a group randomised controlled trial among Turkish immigrants in the Netherlands aged 45 and over. Journal of Epidemiology & Community Health 2003;57(6):405-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reuland 2017 {published data only}
- Reuland DS, Brenner AT, Hoffman R, McWilliams A, Rhyne RL, Getrich C, et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: a randomized clinical trial. JAMA Internal Medicine 2017;177(7):967-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhodes 2011 {published data only}
- Rhodes SD, McCoy TP, Vissman AT, DiClemente RJ, Duck S, Hergenrather KC, et al. A randomized controlled trial of a culturally congruent intervention to increase condom use and HIV testing among heterosexually active immigrant Latino men. AIDS and Behavior 2011;15(8):1764-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ridgeway 2021 {published data only}
- Ridgeway JL, Jenkins SM, Borah BJ, Suman VJ, Patel BK, Ghosh K et al. Evaluating educational interventions to increase breast density awareness among Latinas: a randomized trial in a Federally Qualified Health Center. Cancer 2021;128(5):1038-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosas 2015 {published data only}
- Rosas LG, Thiyagarajan S, Goldstein BA, Drieling RL, Romero PP, Ma J, et al. The effectiveness of two community-based weight loss strategies among obese, low-income US Latinos. Journal of the Academy of Nutrition and Dietetics 2015;115(4):537-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saha 2013 {published data only}
- Saha S, Leijon M, Gerdtham U, Sundquist K, Sundquist J, Arvidsson D, et al. A culturally adapted lifestyle intervention addressing a Middle Eastern immigrant population at risk of diabetes, the MEDIM (impact of migration and ethnicity on diabetes in Malmö): study protocol for a randomized controlled trial. Trials 2013;14:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saha 2018 {published data only}
- Saha S, Gerdtham UG, Siddiqui F, Bennet L. Valuing a lifestyle intervention for middle eastern immigrants at risk of diabetes. International Journal of Environmental Research and Public Health 2018;15(3):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salazar 2012 {published data only}
- Salazar R, Lopez M, Hong J, Perez-Stable E. Prevenir es mejor que lamentar: a culturally tailored colorectal cancer screening intervention for Latinos. Journal of General Internal Medicine 2012;27:283-4. [Google Scholar]
Schensul 2009 {published data only}
- Schensul JJ, Radda K, Coman E, Vazquez E. Multi-level intervention to prevent influenza infections in older low income and minority adults. American Journal of Community Psychology 2009;43(3-4):313-29. [DOI] [PubMed] [Google Scholar]
Schillinger 2008 {published data only}
- Schillinger D, Hammer H, Wang F, Palacios J, McLean I, Tang A, et al. Seeing in 3-D: examining the reach of diabetes self-management support strategies in a public health care system. Health Education and Behavior 2008;35(5):664-82. [DOI] [PubMed] [Google Scholar]
Schlumbrecht 2016 {published data only}
- Schlumbrecht M, Yarian R, Salmon K, Niven C, Singh D. Targeted ovarian cancer education for Hispanic women: a pilot program in Arizona. Journal of Community Health 2016;41(3):619-25. [DOI] [PubMed] [Google Scholar]
Siddiqui 2017 {published data only}
- Siddiqui F, Koivula R, Kurbasic A, Lindblad U, Nilsson PM, Bennet L. Changes in objective physical activity in a randomised lifestyle intervention among diabetes prone Middle-Eastern immigrants and its association with insulin sensitivity. Diabetologia 2017;60(Suppl 1):165. [Google Scholar]
Silvani 2015 {published data only}
- Silvani G, Albonetti A, Acquati S, Zanasi P, Inostroza Velozo HN. How to improve worse pregnancy outcomes in immigrant pregnant women such as in comparable Italian ones by insulin treatment and/or education. Diabetologia 2015;1:485-6. [Google Scholar]
Spalluto 2019 {published data only}
- Spalluto LB, Audet CM, McBride MV, Barajas CP, Beard K, Campbell T, et al. Group versus individual educational sessions with a promotora and Hispanic/Latina women's satisfaction with care in the screening mammography setting: a randomized controlled trial. American Journal of Roentgenology 2019;213(5):1029-36. [DOI] [PMC free article] [PubMed]
Sundquist 2010 {published data only}
- Sundquist J, Hagstromer M, Johansson SE, Sundquist K. Effect of a primary health-care-based controlled trial for cardiorespiratory fitness in refugee women. BMC Family Practice 2010;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sußkind 2019 {published data only}
- Suskind DL, Leung CL, Hundertmark AC, Ladner PH, Leffel KR, Miller K. Impacting low-SES caregiver knowledge, language behaviors, and infant vocabulary skills through a web-based pediatric primary care intervention: a randomized controlled trial. Pediatrics 2019;144:71. [Google Scholar]
Swerissen 2006 {published data only}
- Swerissen H, Belfrage J, Weeks A, Jordan L, Walker C, Furler J, et al. A randomised control trial of a self-management program for people with a chronic illness from Vietnamese, Chinese, Italian and Greek backgrounds. Patient Education and Counseling 2006;64(1-3):360-8. [DOI] [PubMed] [Google Scholar]
Taylor 2002 {published data only}
- Taylor VM, Hislop TG, Jackson JC, Tu SP, Yasui Y, Schwartz SM, et al. A randomized controlled trial of interventions to promote cervical cancer screening among Chinese women in North America. Journal of the National Cancer Institute 2002;94(9):670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2009b {published data only}
- Taylor VM, Hislop TG, Tu S, Teh C, Acorda E, Yip M, et al. Evaluation of a hepatitis B lay health worker intervention for Chinese Americans and Canadians. Journal of Community Health 2009;34(3):165-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thom 2018 {published data only}
- Thom DH, Willard-Grace R, Tsao S, Hessler D, Huang B, DeVore D, et al. Randomized controlled trial of health coaching for vulnerable patients with chronic obstructive pulmonary disease. Annals of the American Thoracic Society 2018;15(10):1159-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2018 {published data only}
Tu 2006 {published data only}
- Tu SP, Taylor V, Yasui Y, Chun A, Yip MP, Acorda E, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: a randomized controlled trial. Cancer 2006;107(5):959-66. [DOI] [PubMed] [Google Scholar]
Tuot 2015 {published data only}
- Tuot DS, Velasquez A, McCulloch CE, et al. The kidney awareness registry and education (KARE) study: protocol of a randomized controlled trial to enhance provider and patient engagement with chronic kidney disease. BMC Nephrology 2015;16:166. [DOI] [PMC free article] [PubMed]
Turner 2018 {published data only}
- Turner BJ, Liang Y, Rodriguez N, Bobadilla R, Simmonds MJ, Yin Z. Randomized trial of a low-literacy chronic pain self-management program: analysis of secondary pain and psychological outcome measures. Journal of Pain 2018;19(12):1471-9. [DOI] [PubMed] [Google Scholar]
Unlu 2010 {published data only}
- Ünlü B, Riper H, Straten A, Cuijpers P. Guided self-help on the internet for Turkish migrants with depression: the design of a randomized controlled trial. Trials 2010;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uygun 2020 {published data only}
- Uygun E, Ilkkursun Z, Sijbrandij M, Aker AT, Bryant R, Cuijpers P, et al. Protocol for a randomized controlled trial: peer-to-peer group Problem Management Plus (PM+) for adult Syrian refugees in Turkey. Trials 2020;21(1):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vargas 2010 {published data only}
- Vargas PA, Robles E, Harris J, Radford P. Using information technology to reduce asthma disparities in underserved populations: a pilot study. Journal of Asthma 2010;47(8):889-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vincent 2014 {published data only}
- Vincent D, McEwen MM, Hepworth JT, Stump CS. The effects of a community-based, culturally tailored diabetes prevention intervention for high-risk adults of Mexican descent. Diabetes Educator 2014;40(2):202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vlaar 2017 {published data only}
- Vlaar EM, Nierkens V, Nicolaou M, Middelkoop BJ, Busschers WB, Stronks K, et al. Effectiveness of a targeted lifestyle intervention in primary care on diet and physical activity among South Asians at risk for diabetes: 2-year results of a randomised controlled trial in the Netherlands. BMJ Open 2017;7(6):e012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2007 {published data only}
- Walker EA, Caban A, Schechter CB, Basch CE, Blanco E, DeWitt T, et al. Measuring comparative risk perceptions in an urban minority population: the risk perception survey for diabetes. Diabetes Educator 2007;33(1):103-10. [DOI] [PubMed] [Google Scholar]
Walker 2012 {published data only}
- Walker EA, Chamany S, Schechter CB, Davis N, Gonzalez J, Difrancesca G, et al. Psychosocial, behavioral and demographic characteristics in an a1c registry telephonic intervention study. Diabetes 2012;61:A204. [Google Scholar]
Wang 2015 {published data only}
- Wang JH, Ma GX, Liang W, Gehan E, Tan Y, MaKambi K, et al. Effects of a culturally targeted physician communication intervention on colorectal cancer screening among older Chinese American patients. Cancer Epidemiology Biomarkers and Prevention 2015;24(10 Suppl 1):B74. [Google Scholar]
Wells 2011 {published data only}
- Wells KJ, Meade CD, Calcano E, Lee J, Rivers D, Roetzheim RG. Innovative approaches to reducing cancer health disparities: the Moffitt cancer center patient navigator research program. Journal of Cancer Education 2011;26(4):649-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wieland 2018 {published data only}
- Wieland ML, Hanza MM, Weis JA, Meiers SJ, Patten CA, Clark MM, et al. Healthy Immigrant families: randomized controlled trial of a family-based nutrition and physical activity intervention. American Journal of Health Promotion 2018;32(2):473-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2008 {published data only}
- Wong CC, Tsoh JY, Tong EK, Hom FB, Cooper B, Chow EA. The Chinese community smoking cessation project: a community sensitive intervention trial. Journal of Community Health 2008;33(6):363-73. [DOI] [PubMed] [Google Scholar]
Wong 2021 {published data only}
- Wong CL, Choi KC, Chen J, Law BMH, Chan DNS, So WKW. A community health worker-led multicomponent program to promote cervical cancer screening in South Asian women: a cluster RCT. American Journal of Preventive Medicine 2021;61(1):136-45. [DOI] [PubMed] [Google Scholar]
Wu 2015 {published data only}
- Wu TY, Lin C. Developing and evaluating an individually tailored intervention to increase mammography adherence among Chinese American Women. Cancer Nursing 2015;38(1):40-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2016 {published data only}
- Yun K, Paul P, Subedi P, Kuikel L, Nguyen GT, Barg FK. Help-seeking behavior and health care navigation by Bhutanese refugees. Journal of Community Health 2016;41(3):526-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang T, Tian X, Ma F, Yang Y, Yu F, Zhao Y, et al. Community based promotion on VCT acceptance among rural migrants in Shanghai, China. PLOS One 2013;8(4):e60106. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Erwin 2012 {published data only}
- Erwin D, Thelemaque L, Saad-Harfouche F, Jandorf L. Esperanza y Vida intervention to increase breast and cervical cancer screening by Latinas: what are the crucial factors in outreach that influence behavior change? Psycho-Oncology 2012;21:22-3. [Google Scholar]
Esquivel 2019 {published data only}
- Esquivel JH, Ottoboni LK, Selman L. Spanish-speaking heart failure patients perceive increased confidence and health communication skills following Spanish-language self-care intervention. Circulation 2019;140(Suppl 1):A10715. [Google Scholar]
Essien 2017 {published data only}
- Essien UR, Seiglie JA, Chiou C A, Cohen MJ. Peer mentorship in diabetes: implementation in a Spanish-speaking population. Journal of General Internal Medicine 2017;32(2 Suppl 1):783-84. [Google Scholar]
Glaser 2020 {published data only}
- Glaser KM, Flores T, Lynch M, Mossop J, Abrams A, Johnson C, et al. Providing colorectal cancer screening interventions at federally qualified health centers (FQHCs): addressing the issues of language, culture, and health literacy through culturally tailored education and navigation [abstract]. Cancer Epidemiology Biomarkers and Prevention 2020;29(6 Suppl 1):B105.
Gonzalez 2020 {published data only}
- Gonzalez JS, Hoogendoorn CJ, Linnell J, Fishman S, Jonas V, Pham-Singer H, et al. Design and methods of NYC care calls: an effectiveness trial of telephone-delivered type 2 diabetes self-management support. Contemporary Clinical Trials 2020;98:106166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joshi 2016 {published data only}
- Joshi A, Amadi C, Meza J, Aguire T, Wilhelm S. Evaluation of a computer-based bilingual breastfeeding educational program on breastfeeding knowledge, self-efficacy and intent to breastfeed among rural Hispanic women. International Journal of Medical Informatics 2016;91:10-9. [DOI] [PubMed] [Google Scholar]
NCT04993326 {published data only}
- Advancing DSME/S and COVID-19 prevention and protection through "emPOWERed to Change" program. https://classic.clinicaltrials.gov/ct2/history/NCT04993326?V_2=View#StudyPageTop (first received 6 August 2021).
Pekmezaris 2020 {published data only}
- Pekmezaris R, Williams MS, Pascarelli B, Finuf KD, Harris YT, Myers AK, et al. Adapting a home telemonitoring intervention for underserved Hispanic/Latino patients with type 2 diabetes: an acceptability and feasibility study. BMC Medical Informatics and Decision Making 2020;20(1):324. [DOI] [PMC free article] [PubMed]
References to ongoing studies
ACTRN12619001019190 {published data only}
- ACTRN12619001019190. The Strong Families Trial: Randomised controlled trial of a family strengthening program to prevent unhealthy weight gain among 5- to 11-year old children from at risk families. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=377560 (first received 16 July 2019). [DOI] [PMC free article] [PubMed]
Blashill 2021 {published data only}
- Blashill AJ, Gordon JR, Rojas SA, Ramers CB, Lin C, Carrizosa CM, et al. Pilot randomised controlled trial of a patient navigation intervention to enhance engagement in the PrEP continuum among young Latino MSM: a protocol paper. BMJ Open 2021;11(5):e040955. [DOI: 10.1136/bmjopen-2020-040955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castro 2013 {published data only}
- Castro Y, Basen-Engquist K, Fernandez ME, Strong LL, Eakin EG, Resnicow K, et al. Design of a randomized controlled trial for multiple cancer risk behaviors among Spanish-speaking Mexican-origin smokers. BMC Public Health 2013;18(13):237. [DOI: 10.1186/1471-2458-13-237] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03726619 {published data only}
- NCT03726619. e-CHEC-uP: Scaling up an efficacious cancer screening intervention for women with limited English. https://classic.clinicaltrials.gov/show/NCT03726619 (first received 31 October 2018). [HTTPS://CLASSIC.CLINICALTRIALS.GOV/CT2/SHOW/NCT03726619: (first posted October 31, 2018)]
NCT03909347 {published data only}
- NCT03909347. PLAN: Dementia literacy education and navigation for Korean elders with probable dementia and their caregivers (PLAN). clinicaltrials.gov/ct2/show/NCT03909347 (first received 10 April 2019).
NCT04125680 {unpublished data only}
- NCT04125680. Community-based participatory English as a second language health literacy program to prevent lead exposure in flint. clinicaltrials.gov/ct2/show/NCT04125680 (first received 14 October 2019).
NCT04319458 {published data only}
- NCT04319458. Testing mediators and moderators of a fotonovela for depression to promote help-seeking behavior. clinicaltrials.gov (first received 24 March 2020).
NCT04564209 {published data only}
- NCT04564209. Information visualizations to facilitate clinician-patient communication in HIV care (Info Viz: HIV). https://classic.clinicaltrials.gov/ct2/show/NCT04564209 (first received 25 September 2020).
NCT04905030 {published data only}
- NCT04905030. Education, immigration and HPV vaccination: an informational randomized trial. https://clinicaltrials.gov/study/NCT04905030 (first received 19 May 2021).
Waterman 2019 {published data only}
- Waterman AD, Anderson C, Alem A, Peipert JD, Beaumont JL, Henry SL, et al. A randomized controlled trial of explore transplant at home to improve transplant knowledge and decision-making for CKD 3–5 patients at Kaiser Permanente Southern California. BMC Nephrology 2019;20(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weise 2021 {published data only}
- Weise C, Grupp F, Reese J-P, Schade-Brittinger C, Ehring T, Morina N, et al. Efficacy of a low-threshold, culturally-sensitive group psychoeducation programme for asylum seekers (LoPe): study protocol for a multicentre randomised controlled trial. BMJ Open 2021;11(10):e047385. [DOI: 10.1136/ bmjopen-2020-047385] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abbas 2018
- Abbas M, Aloudat T, Bartolomei J, Carballo M, Durieux-Paillard S, Gabus L et al. Migrant and refugee populations: a public health and policy perspective on a continuing global crisis. Antimicrobial Resistance and Infection Control 2018;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aguado Loi 2020
- Aguado Loi CX, Martinez TD, Chavarria EA, Gutierrez L, Klasko L, Davis S, et al. ‘Simple and easy:’ providers’ and latinos’ perceptions of the fecal immunochemical test (FIT) for colorectal cancer screening. Ethnicity & Health 2020;25(2):206-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ajzen 1991
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes 1991;50(2):179-211. [Google Scholar]
Aldin 2019
- Aldin A, Chakraverty D, Baumeister A, Monsef I, Noyes J, Jakob T, et al. Gender differences in health literacy of migrants: a synthesis of qualitative evidence. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD013302. [DOI: 10.1002/14651858.CD013302] [DOI] [Google Scholar]
Altin 2014
- Altin SV, Finke I, Kautz-Freimuth S, Stock S. The evolution of health literacy assessment tools: a systematic review. BMC Public Health 2014;14(1):1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altweck 2015
- Altweck L, Marshall T, Ferenczi N, Lefringhausen K. Mental health literacy: a cross-cultural approach to knowledge and beliefs about depression, schizophrenia and generalized anxiety disorder. Frontiers in Psychology 2015;6:Article 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
AMA 1999
- Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. Health Literacy: Report of the Council on Scientific Affairs. Journal of the American Medical Association 1999;281(6):552-7. [PubMed] [Google Scholar]
Amico 2011
- Amico KR. A situated-information motivation behavioral skills model of care Initiation and maintenance (sIMBCIM): an IMB model based approach to understanding and intervening inengagement in care for chronic medical conditions. Journal of Health Psychology 2011;16(7):1071-81. [DOI] [PubMed] [Google Scholar]
Anderson 1982
- Anderson JR. Acquisition of cognitive skill. Psychological Review 1982;89(4):369-406. [Google Scholar]
Baker 2006
- Baker DW. The meaning and the measure of health literacy. Journal of General Internal Medicine 2006;21(8):878-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bandura 1977
- Bandura A. Social learning theory. Englewood Cliffs: Prentice Hall, Inc., 1977. [Google Scholar]
Bandura 1994
- Bandura A. Self-efficacy. In: Ramachandran VS, editors(s). Encyclopedia of Human Behavior. Vol. 4. New York (NY): Academic Press, 1994:71-81. [Google Scholar]
Bandura 1997
- Bandura A. Self-efficacy: the exercise of control. New York (NY): Freeman WH, 1997. [Google Scholar]
Bandura 2002
- Bandura A. Social cognitive theory of mass communication. In: Bryant J, Zillmann D, editors(s). Media Effects: Advances in Theory and Research. Lawrence Erlbaum Associates Publishers, 2002:121-53. [Google Scholar]
Bandura 2004
- Bandura A. Health promotion by social cognitive means. Health Education & Behavior 2004;31(2):143-64. [DOI] [PubMed] [Google Scholar]
Baumeister 2021a
- Baumeister A, Chakraverty C, Aldin A, Seven ÜS, Skoetz N, Kalbe E et al. “The system has to be health literate, too” -perspectives among healthcare professionals on health literacy in transcultural treatment settings. BMC Health Services Research 2021;21:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumeister 2021b
- Baumeister A, Mantell PK, Woopen C. Health Literacy in the Context of Mental Illness: Concept Analysis, State of Research, Intervention Approaches [Gesundheitskompetenz im kontext psychischer erkrankungen: konzeptanalyse, forschungsstand, interventionsansätze]. In: Rathmann K, Dadaczynski K, Orkan O, Messer M, editors(s). Living Reference Work. Berlin, Heidelberg: Springer Berlin Heidelberg, 2020:1-11. [DOI: 10.1007/978-3-662-62800-3_38-1] [DOI] [Google Scholar]
Beauchamp 2015
- Beauchamp A, Buchbinder R, Dodson S, Batterham RW, Elsworth GR, McPhee C et al. Distribution of health literacy strengths and weaknesses across socio-demographic groups: a cross-sectional survey using the health literacy questionnaire (HLQ). BMC Public Health 2015;15:678. [DOI: 10.1186/s12889-015-2056-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beauchamp 2017
- Beauchamp A, Batterham RW, Dodson S, Astbury B, Elsworth GR, McPhee C, et al. Systematic development and implementation of interventions to optimise health literacy and access (OPHELIA). BMC Public Health 2017;17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berens 2021
- Berens E-M, Pelikan JM, Schaeffer D. The effect of self-efficacy on health literacy in the German population. Health Promotion International 2021;37:1. [DOI: 10.1093/heapro/daab085] [DOI] [PubMed] [Google Scholar]
Berens 2022a
- Berens E-M, Klinger J, Mensing M, Carol S, Schaeffer D. Health Literacy of people with migration background in Germany. Results of the HLS-MIG. Short summary. Bielefeld University 2022. [DOI: 10.4119/unibi/2960263] [DOI]
Berens 2022b
- Berens EM, Klinger J, Carol S, Schaeffer D. Differences in health literacy domains among migrants and their descendants in Germany. Frontiers in Public Health 2022;23(10):988782. [DOI: 10.3389/fpubh.2022.988782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkman 2011
- Berkman ND, Sheridan SL, Donahue KE, Halper DJ, Viera A, Crotty K, et al. Health literacy interventions and outcomes: an updated systematic review. Evidence Report/Technology Assessment, No.199. Prepared by RTI International-University of North Carolina Evidence-based Practice Center under contract No. 290-2007-10056-I. AHRQ Publication No.11-E006 2011.
Bernal 2000
- Bernal H, Woolley S, Schensul JJ, Dickinson JK. Correlates of self-efficacy in diabetes self-care among Hispanic adults with diabetes. Diabetes Educator 2000;26(4):673-80. [DOI] [PubMed] [Google Scholar]
Bircan 2022
- Bircan T, Yilmaz S. A critique of gender‐blind migration theories and data sources. International Migration 2022;00:1-16. [Google Scholar]
Bozorgmehr 2016
- Bozorgmehr K, Mohsenpour A, Saure D, Stock C, Loerbroks A, Joos S, et al. Systematic review and evidence mapping of empirical studies on health status and medical care among refugees and asylum seekers in Germany (1990-2014) [Systematische Übersicht und „Mapping“ empirischer Studien des Gesundheitszustands und der medizinischen Versorgung von Flüchtlingen und Asylsuchenden in Deutschland (1990–2014)]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2016;59(5):599-620. [DOI] [PubMed] [Google Scholar]
Brach 2012
- Brach C, Keller D, Hernandez LM, Baur C, Parker R, Dreyer B, et al. Ten attributes of health literate health care organizations. National Academy of Sciences 2012.
Braden 1990a
- Braden J. A test of the self-help model: learned response to chronic illness experience. Nursing Research 1990;39(1):42-7. [PubMed] [Google Scholar]
Braden 1990b
- Braden CJ. Learned self-help response to chronic illness experience: a test of three alternative learning theories. Scholarly Inquiry for Nursing Practice 1990;4(2):23-41; discussion 43-5. [PubMed] [Google Scholar]
Brijnath 2016
- Brijnath B, Protheroe J, Mahtani KR, Antoniades J. Do web-based mental health literacy interventions improve the mental health literacy of adult consumers? Results from a systematic review. Journal of Medical Internet Research 2016;18(6):e165. [DOI: 10.2196/jmir.5463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brijnath 2020
- Brijnath B, Antoniades J, Temple J. Psychological distress among migrant groups in Australia: results from the 2015 national health survey. Social Psychiatry and Psychiatric Epidemiology 2020;55(4):467-75. [DOI: 10.1007/s00127-019-01782-y] [DOI] [PubMed] [Google Scholar]
Campbell 2016
- Campbell ZC, Stevenson JK, McCaffery KJ, Jansen J, Campbell KL, Lee VW, et al. Interventions for improving health literacy in people with chronic kidney disease. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD012026. [DOI: 10.1002/14651858.CD012026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ Open 2020;368:16890. [DOI] [PMC free article] [PubMed]
Car 2011
- Car J, Lang B, Colledge A, Ung C, Majeed A. Interventions for enhancing consumers' online health literacy. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No: CD007092. [DOI: 10.1002/14651858.CD007092.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
CCCG 2016
- Cochrane Consumers and Communication Group. Standard protocol text and additional guidance for review authors. cccrg.cochrane.org/author-resources (accessed 6 November 2018).
Chakraverty 2020
- Chakraverty D, Baumeister A, Aldin A, Jakob T, Seven ÜS, Woopen C, et al. Gender-specific aspects of health literacy: perceptions of interactions with migrants among health care providers in Germany. International Journal of Environmental Research and Public Health 2020;17(7):2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chakraverty 2022
- Chakraverty D, Baumeister A, Aldin A, Seven ÜS, Monsef I, Skoetz N et al. Gender differences of health literacy in persons with a migration background: a systematic review and meta-analysis. BMJ Open 2022;12:e056090. [DOI: 10.1136/bmjopen-2021-056090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Champion 2008
- Champion VL, Skinner CS. The health belief model. In: Glanz K, Rimer BK, Viswanath K, editors(s). Health Behavior and Health Education: Theory, Research, and Practice. 4th edition. San Francisco: Jossey-Bass, 2008:189-93. [Google Scholar]
Chatman 1996
- Chatman EA. The impoverished life-world of outsiders. Journal of the American Society for Information Science and Technology 1996;47(3):193-206. [Google Scholar]
Chen 2015
- Chen X, Goodson P, Acosta S. Blending health literacy with an English as a second language curriculum: a systematic literature review. Journal of Health Communication 2015;20(Suppl 2):101-11. [DOI: 10.1080/10810730.2015.1066467] [DOI] [PubMed] [Google Scholar]
Cherrington 2011
- Cherrington A, Ayala GX, Scarinci I, Corbie-Smith G. Developing a family-based diabetes program for Latino immigrants: do men and women face the same barriers? Family & Community Health: The Journal of Health Promotion & Maintenance 2011;34(4):280-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christy 2017
- Christy SM, Gwede CK, Sutton SK, Chavarria E, Davis SN, Abdulla R, et al. Health literacy among medically underserved: the role of demographic factors, social influence, and religious beliefs. Journal of Health Communication 2017;22(11):923-31. [DOI: 10.1080/10810730.2017.1377322] [DOI] [PMC free article] [PubMed] [Google Scholar]
Curry 1994
- Curry SJ, Emmons KM. Theoretical models for predicting and improving compliance with breast cancer screening. Annals of Behavioral Medicine 1994;16(4):302-16. [Google Scholar]
da Costa 2013
- da Costa BR, Nuesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, et al. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. Journal of Clinical Epidemiology 2013;66:847-55. [DOI] [PubMed] [Google Scholar]
Davis 1991
- Davis TC, Crouch MA, Long SW, Jackson RH, Bates P, George RB, et al. Rapid assessment of literacy levels of adult primary care patients. Family Medicine 1991;23(6):433-5. [PubMed] [Google Scholar]
Derose 2007
- Derose KP, Escarce JJ, Lurie N. Immigrants and health care: sources of vulnerability. Health Affairs 2007;26(5):1258-68. [DOI: 10.1377/hlthaff.26.5.1258] [DOI] [PubMed] [Google Scholar]
Doak 1996
- Doak C, Doak L, Root J. Teaching Patients With Low Literacy Skills. 2nd edition. Philadelphia (PA): JB Lippincott, 1996. [Google Scholar]
Dodson 2015
- Dodson S, Good S, Osborne RH. Health literacy toolkit for low- and middle-income countries: a series of information sheets to empower communities and strengthen health systems. New Delhi: World Health Organization, Regional Office for South-East Asia, 2015. [ISBN: 978-92-9022-475-4] [Google Scholar]
Douki 2007
- Douki S, Zineb SB, Nacef F, Halbreich U. Women's mental health in the Muslim world: cultural, religious, and social issues. Journal of Affective Disorders 2007;102(1-3):177-89. [DOI] [PubMed] [Google Scholar]
Doull 2006
- Doull M, O’Connor A, Jacobsen MJ, Robinson V, Cook L, Nyamai-Kisia C, et al. Investigating the decision-making needs of HIV-positive women in Africa using the Ottawa decision-support framework: knowledge gaps and opportunities for intervention. Patient Education and Counseling 2006;63(3):279-91. [DOI] [PubMed] [Google Scholar]
Eichler 2009
- Eichler K, Wieser S, Brügger U. The costs of limited health literacy: a systematic review. International Journal of Public Health 2009;54(5):313-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elder 2000
- Elder JP, Candelaria JI, Woodruff SI, Criqui MH, Talavera GA, Rupp JW. Results of language for health: cardiovascular disease nutrition education for Latino English-as-a-second-language students. Health Education & Behavior 2000;27(1):50-63. [DOI] [PubMed] [Google Scholar]
Erdal 2018
- Erdal MB, Oeppen C. Forced to leave? The discursive and analytical significance of describing migration as forced and voluntary. Journal of Ethnic and Migration Studies 2018;44(6):981-98. [Google Scholar]
Fernández‐Gutiérrez 2018
- Fernández‐Gutiérrez M, Bas‐Sarmiento P, Albar‐Marín MJ, Paloma‐Castro O, Romero‐Sánchez JM. Health literacy interventions for immigrant populations: a systematic review. International Nursing Review 2018;65(1):54-64. [DOI] [PubMed] [Google Scholar]
Fishbein 1975
- Fishbein M, Ajzen I. Belief, attitude, intention, and behavior: An introduction to theory and research. Reading, MA: Addison-Wesley 1975.
Fox 2022
- Fox S, Kramer E, Agrawal P, Aniyizhai A. Refugee and migrant health literacy interventions in high-income countries: a systematic review. Journal of Immigrant and Minority Health 2022;24(1):207-36. [DOI] [PubMed] [Google Scholar]
Friis 2016
- Friis K, Lasgaard M, Osborne RH, Maindal HT. Gaps in understanding health and engagement with healthcare providers across common long-term conditions: a population survey of health literacy in 29,473 Danish citizens. BMJ Open 2016;6(1):e009627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganahl 2016
- Ganahl K, Dahlvik J, Röthlin F, Alpagu F, Sikic-Fleischhacker A, Peer, S et al. Health literacy in persons with a migration background from Turkey and former Yugoslavia in Austria. Results of a quantitative and qualitative study [Gesundheitskompetenz bei Personen mit Migrationshintergrund aus der Türkei und Ex - Jugoslawien in Österreich. Ergebnisse einer quantitativen und qualitativen Studie]. Vienna. Ludwig Bolzmann Institut for Health Promotion Research 2016.
Goosen 2014
- Goosen ESM. A safe and healthy future? Epidemiological studies on the health of asylum seekers and refugees in the Netherlands [PhD thesis]. Amsterdam (NL): University of Amsterdam, 2014. [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Green 1991
- Green LW, Kreuter MW. Health promotion planning: an educational and environmental approach. 2nd edition. Mountain View, CA: Mayfield Pub. Co., 1991. [Google Scholar]
Griffiths 2004
- Griffiths KM, Christensen H, Jorm AF, Evans K, Groves C. Effect of web-based depression literacy and cognitive-behavioural therapy interventions on stigmatising attitudes to depression: randomised controlled trial. British Journal of Psychiatry 2004;185(4):342-9. [DOI] [PubMed] [Google Scholar]
Guntzviller 2016
- Guntzviller, LM, King, AJ, Jensen, JD, Davis LA. Self-efficacy, health literacy, and nutrition and exercise behaviors in a low-Income, Hispanic population. Journal of Immigrant and Minority Health 2016;19:489-93. [DOI: 10.1007/s10903-016-0384-4] [DOI] [PubMed] [Google Scholar]
Guzys 2015
- Guzys D, Kenny A, Dickson-Swift V, Threlkeld G. A critical review of population health literacy assessment. BMC Public Health 2015;15(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammarström 2012
- Hammarström A, Annandale E. A conceptual muddle: an empirical analysis of the use of ‘sex’ and ‘gender’ in ‘gender-specific medicine’ journals. PloS One 2012;7(4):e34193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2014
- Han HR, Huh B, Kim MT, Kim J, Nguyen T. Development and validation of the assessment of health literacy in breast and cervical cancer screening. Journal of Health Communication 2014;19 Suppl 2(0 2):267-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2006
- Harris R, Tobias M, Jeffreys M, Waldegrave K, Karlsen S, Nazroo J. Effects of self-reported racial discrimination and deprivation on Māori health and inequalities in New Zealand. Lancet 2006;367(9527):2005-9. [DOI] [PubMed] [Google Scholar]
Haun 2014
- Haun JN, Valerio MA, McCormack L, Sørensen K, Paasche-Orlow MK. Health literacy measurement: an inventory and descriptive summary of 51 instruments. Journal of Health Communication 2014;19(2):302-33. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Higgins 2022a
- Higgins JPT, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook .
HLS‐EU Consortium 2012
- HLS-EU Consortium. Comparative report of health literacy in eight EU member states. The European health literacy survey HLS-EU. ec.europa.eu/chafea/documents/news/Comparative_report_on_health_literacy_in_eight_EU_member_states.pdf 2012.
HLS19 Consortium 2021
- The HLS19 Consortium of the WHO Action Network M-POHL. Executive summary of the international report on the methodology, results, and recommendations of the European health literacy population survey 2019-2021 (HLS19) of M-POHL. Vienna: Austrian National Public Health Institute, 2021. [Google Scholar]
Hunter 2016
- Hunter P. The refugee crisis challenges national health care systems: countries accepting large numbers of refugees are struggling to meet their health care needs, which range from infectious to chronic diseases to mental illnesses. European Molecular Biology Organization Reports 2016;17(4):492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
IOM 2013
- International Organization for Migration. International migration, health and human rights. www.ohchr.org/Documents/Issues/Migration/WHO_IOM_UNOHCHRPublication.pdf 2013.
IOM 2017
- International Organization for Migration. World migration report 2018. www.iom.int/wmr/world-migration-report-2018 2017.
IOM 2018
- International Organization for Migration. Key migration terms. www.iom.int/key-migration-terms#Migrant (accessed 1 April 2018).
IOM 2019
- International Organization for Migration. Glossary on migration. IML Series No. 34, 2019.
Janz 1984
- Janz NK, Becker MH. The health belief model: a decade later. Health Education Quarterly 1984;11(1):1-47. [DOI] [PubMed] [Google Scholar]
Jorm 1997
- Jorm AF, Korten AE, Jacomb PA, Christensen H, Rodgers B, Politt P. Public beliefs about causes and risk factors for depression and schizophrenia. Social Psychiatry and Psychiatry Epidemiology 1997;32(3):143-8. [DOI] [PubMed] [Google Scholar]
Jorm 2000
- Jorm AF. Mental health literacy. Public knowledge and beliefs about mental disorders. British Journal of Psychiatry 2000;177:396-401. [DOI] [PubMed] [Google Scholar]
Jorm 2005
- Jorm AF, Nakane Y, Christensen H, Yoshioka K, Griffiths KM, Wata Y. Public beliefs about treatment and outcome of mental disorders: a comparison of Australia and Japan. BMC Medicine 2005;3(1):12. [DOI: 10.1186/1741-7015-3-12] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalichman 2006
- Kalichman SC, Cherry C, Cain D, Pope H, Kalichman M, Eaton L, et al. Internet-based health information consumer skills intervention for people living with HIV/AIDS. Journal of Consulting and Clinical Psychology 2006;74(3):545-54. [DOI] [PubMed] [Google Scholar]
Kaplan 2003
- Kaplan JB, Bennett T. Use of race and ethnicity in biomedical publication. JAMA 2003;289(20):2709-16. [DOI] [PubMed] [Google Scholar]
Kim 2006
- Kim MT, Han H, Park HJ, Lee H, Kim KB. Constructing and testing a self-help intervention pogram for high blood pressure control in Korean American seniors - a pilot study. Journal of Cardiovascular Nursing 2006;21(2):77-84. [DOI] [PubMed] [Google Scholar]
Kim 2012
- Kim MT, Song HJ, Han HR, Song Y, Nam S, Nguyen TH, et al. Development and validation of the high blood pressure-focused health literacy scale. Patient Education and Counseling 2012;87(2):165-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015
- Kim K, Kim B, Choi E, Song Y, Han HR. Knowledge, perceptions, and decision making about human papillomavirus vaccination among Korean American women: a focus group study. Women's Health Issues 2015;25(2):112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirmayer 2011
- Kirmayer LJ, Narasiah L, Munoz M, Rashid M, Ryder AG, Guzder J, et al. Common mental health problems in immigrants and refugees: general approach in primary care. Canadian Medical Association Journal 2011;183(12):E959-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knowles 1984
- Knowles MS. The Adult Learner: A Neglected Species. 3rd edition. Houston, TX: Gulf Publishing Company, 1984. [Google Scholar]
Kreuter 1996
- Kreuter MW, Strecher VJ. Do tailored behavior change messages enhance the effectiveness of health risk appraisal? Results from a randomized trial. Health Education Research 1996;11(1):97-105. [DOI] [PubMed] [Google Scholar]
Kutner 2006
- Kutner M, Greenberg E, Jin Y, Paulsen C. The health literacy of America’s adults. Results from the 2003 national assessment of adult literacy. National Center for Education Statistics 2006.
Larkey 2010
- Larkey LK, Hecht M. A model of effects of narrative as culture-centric health promotion. Journal of Health Communication 2010;15(2):114-35. [DOI] [PubMed] [Google Scholar]
Lebano 2020
- Lebano A, Hamed S, Bradby H, Gil-Salmerón A, Durá-Ferrandis E, Garcés-Ferrer J, et al. Migrants' and refugees' health status and healthcare in Europe: a scoping literature review. BMC Public Health 2020;20(1):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, Paynter R, Rader T, Thomas J, Wieland LS. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook .
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindert 2009
- Lindert J, Ehrenstein OS, Priebe S, Mielck A, Brähler E. Depression and anxiety in labor migrants and refugees - a systematic review and meta-analysis. Social Science and Medicine 2009;69(2):246-57. [DOI] [PubMed] [Google Scholar]
Llácer 2007
- Llácer A, Zunzunegui MV, Amo J, Mazarrasa L, Bolumar F. The contribution of a gender perspective to the understanding of migrants' health. Journal of Epidemiology and Community Health 2007;61(Suppl 2):ii4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorig 1996
- Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Thousand Oaks (CA): Sage Publications, 1996. [Google Scholar]
López 2010
- López E, Morales G, Saucedo C, Aguirre-Girón L, Mack S, Goodkin K. A proposition against using the terms "Hispanic" and "Latino" in research on HIV - associated neurocognitive disorders. Ethnicity & Disease 2010;20(4):479-84. [PubMed] [Google Scholar]
Mackert 2015
- Mackert M, Champlin S, Su Z, Guadagno M. The many health literacies: advancing research or fragmentation? Health Communication 2015;30(12):1161-5. [DOI] [PubMed] [Google Scholar]
Malmusi 2010
- Malmusi D, Borrell C, Benach J. Migration-related health inequalities: showing the complex interactions between gender, social class and place of origin. Social Science & Medicine 2010;71(9):1610-9. [DOI] [PubMed] [Google Scholar]
Marmot 2005
- Marmot M. Social determinants of health inequalities. Lancet 2005;365(9464):1099-104. [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masseria 2010
- Masseria C, Mladovsky P, Hernández-Quevedo C. The socio-economic determinants of the health status of Roma in comparison with non-Roma in Bulgaria, Hungary and Romania. European Journal of Public Health 2010;20(5):549-54. [DOI] [PubMed] [Google Scholar]
Mbizvo 1997
- Mbizvo MT, Kasule J, Gupta V, Rusakaniko S, Kinoti SN, Mpanju-Shumbushu W, et al. Effects of a randomized health education intervention on aspects of reproductive health knowledge and reported behaviour among adolescents in Zimbabwe. Social Science & Medicine 1997;44(5):573-7. [DOI] [PubMed] [Google Scholar]
Mc Queen 2008
- McQueen A, Tiro JA, Vernon SW. Construct validity and invariance of four factors associated with colorectal cancer screening across gender, race, and prior screening. Cancer Epidemiology Biomarkers & Prevention 2008;17(9):2231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2017
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit, 2017 Sep 13-17. Cape Town, South Africa, 2017.
McGuire 2015
- McGuire WJ. McGuire’s classic input-output framework for constructing persuasive messages. In: Rice RE, Atkin CK, editors(s). Public Communication Campaigns. 4th edition. Thousand Oaks (CA): Sage Publications, 2015:133-46. [Google Scholar]
Michie 2011
- Michie S, Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. BMC Implementation Science 2011;6(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2016
- Miller TA. Health literacy and adherence to medical treatment in chronic and acute illness: a meta-analysis. Patient Education and Counseling 2016;99(7):1079-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohan 2012
- Mohan A, Riley B, Boyington D, Kripalani S. PictureRx: illustrated medication instructions for patients with limited health literacy. Journal of the American Pharmacists Association 2012;52(5):e122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2004
- Moore WH, Shellman SM. Fear of persecution. Journal of Conflict Resolution 2004;48(5):723-45. [Google Scholar]
National Center for Health Statistics 2012
- Centers for Disease Control and Prevention Health, Resources and Services Administration, Indian Health Service, National Institutes of Health. Oral health. In: Cunningham R, Dumas R, Hinkle S, Lipphardt A, Park K, Reid J, et al, editors(s). Healthy People 2010 Final Review. 2nd edition. Hyattsville (MD): US Government Printing Office, 2012. [Google Scholar]
Ng 2014
- Ng E, Omariba DWR. Immigration, generational status and health literacy in Canada. Health Education Journal 2014;73:668-82. [DOI: 10.1177/0017896913511809] [DOI] [Google Scholar]
Nielson‐Bohlman 2004
- Nielson-Bohlman L, Panzer AM, Hamlin B, Kindig DA. Health literacy: a prescription to end confusion. Commitee on Health Literacy; Institute of Medicine (US) 2004.
Noel‐Storr 2018
- Noel-Storr AH and the Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live. Oxford, UK, 2018. [Google Scholar]
Nuscheler 2013
- Nuscheler F. Internationale Migration. Flucht und Asyl. 2nd edition. Vol. 14. Berlin: Springer-Verlag, 2013. [Google Scholar]
Nutbeam 2000
- Nutbeam D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promotion International 2000;15(3):259-67. [Google Scholar]
Nutbeam 2021
- Nutbeam D, Lloyd JE. Understanding and responding to health literacy as a social determinant of health. Annual Review of Public Health 2021;42(1):159-73. [DOI] [PubMed] [Google Scholar]
O'Connor 1993
- O'Connor AM. User manual - decisional conflict scale 1993 [updated 2010]. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf (accessed 14 June 2023).
O'Connor 1995
- O’Conner AM. Validation of a decisional conflict scale. Medical Decision Making 1995;15(25):25-30. [DOI] [PubMed] [Google Scholar]
Osborn 2011
- Osborn CY, Paasche-Orlow MK, Bailey SC, Wolf MS. The mechanisms linking health literacy to behavior and health status. American Journal of Health Behavior 2011;35(1):118-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborne 2013
- Osborne RH, Batterham RW, Elsworth GR, Hawkins M, Buchbinder R. The grounded psychometric development and initial validation of the health literacy questionnaire (HLQ). BMC Public Health 2013;13(1):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paasche‐Orlow 2005
- Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. Journal of General Internal Medicine 2005;20(2):175-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paasche‐Orlow 2007
- Paasche-Orlow MK, Wolf MS. The causal pathways linking health literacy to health outcomes. American Journal of Health Behavior 2007;31(1):19-26. [DOI] [PubMed] [Google Scholar]
Parker 1995
- Parker RM, Baker DW, Williams MV, Nurss JR. The test of functional health literacy in adults: a new instrument for measuring patients' literacy skills. Journal of General Internal Medicine 1995;10(10):537-41. [DOI] [PubMed] [Google Scholar]
Parker 2009
- Parker R. Measuring health literacy: Why? So what? Now what? Roundtable on health literacy. In: Measures of health literacy. Workshop summary. Washington (DC): IOM (Institute of Medicine), National Academy Press, 2009:91-4. [Google Scholar]
Pelikan 2018
- Pelikan JM, Ganahl K, Roethlin F. Health literacy as a determinant, mediator and/or moderator of health: empirical models using the European health literacy survey dataset. Global Health Promotion 2018;25(4):57-66. [DOI: ] [DOI] [PubMed] [Google Scholar]
Prochaska 1997
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. American Journal of Health Promotion 1997;12(1):38-48. [DOI] [PubMed] [Google Scholar]
Quenzel 2016
- Quenzel G, Vogt D, Schaeffer D. Differences in health literacy of adolescents with lower educational attainment, older people and migrants. Gesundheitswesen 2016;78(11):708-10. [DOI] [PubMed] [Google Scholar]
Rajaram 1998
- Rajaram S, Rashidi A. Minority women and breast cancer screening: the role of cultural explanatory models. Journal of Preventive Medicine 1998;27:757-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rasu 2015
- Rasu RS, Bawa WA, Suminski R, Snella K, Warady B. Health literacy impact on national healthcare utilization and expenditure. International Journal of Health Policy and Management 2015;4(11):747-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratzan 2000
- Ratzan SC, Parker RM. Introduction. In: Ratzan SC, Parker RM, Selden R, Zorn M, editors(s). National Library of Medicine current bibliographies in medicine: Health Literacy. Bethesda (MD): National Institutes of Health, U.S. Department of Health and Human Services, 2000. [Google Scholar]
Razum 2008
- Razum O, Zeeb H, Meesmann U, Schenk L, Bredehorst M, Brzoska P, et al. Migration and health [Migration und Gesundheit]. Schwerpunktbericht der Gesundheitsberichterstattung des Bundes 2008;4:138.
Rechel 2013
- Rechel B, Mladovsky P, Ingleby D, Mackenbach JP, McKee M. Migration and health in an increasingly diverse Europe. Lancet 2013;381(9873):1235-45. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosal 2003
- Rosal MC, Carbone ET, Goins KV. Use of cognitive interviewing to adapt measurement instruments for low-literate Hispanics. Diabetes Education 2003;29:1006-17. [DOI] [PubMed] [Google Scholar]
Rosenstock 1988
- Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Education Quarterly 1988;15(2):175-83. [DOI] [PubMed] [Google Scholar]
Ryan 2013
- Ryan R, Hill S, Prictor M, McKenzie J, Cochrane Consumers and Communication Group. Study quality guide. Available from cccrg.cochrane.org/authorresources (accessed 12 April 2018).
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence. Version 3.0. Approved (S. Hill) December 1st 2016. http://cccrg.cochrane.org/author-resources 2016 (accessed 24 April 2022).
Sandford 1999
- Sandford S. Contingent ontologies - sex, gender and 'woman' in Simone de Beauvoir and Judith Butler. Radical Philosophy 1999;97:18-29. [Google Scholar]
Sawyer‐Radloff 1977
- Sawyer-Radloff L. The CES–D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385-401. [Google Scholar]
Schouler‐Ocak 2017
- Schouler-Ocak M, Kurmeyer C. Study on Female Refugees [Repräsentative Untersuchung von geflüchteten Frauen in unterschiedlichen Bundesländern in Deutschland]. Berlin: Federal Ministry for Migration, Refugees and Integration; 2017 February.
Schünemann 2022
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Semple 2000
- Semple A. Learning theories and their influence on the development and use of educational technologies. Australian Science Teachers Journal 2000;46(3):21-8. [Google Scholar]
Sentell 2012
- Sentell T, Braun KL. Low health literacy, limited english proficiency, and health status in Asians, Latinos, and other racial/ethnic groups in California. Journal of Health Communication 2012;17:82-99. [DOI: 10.1080/10810730.2012.712621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sentell 2020
- Sentell T, Vamos S, Okan O. Interdisciplinary perspectives on health literacy research around the world: More important than ever in a time of COVID-19. International Journal of Environmental Research and Public Health 2020;17(9):3010. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2011
- Sheridan SL, Halpern DJ, Viera AJ, Berkman ND, Donahue KE, Crotty K. Interventions for individuals with low health literacy: a systematic review. Journal of Health Communication 2011;16(3):30-54. [DOI] [PubMed] [Google Scholar]
Shirazi 2013
- Shirazi M, Bloom J, Shirazi A, Popal R. Afghan immigrant women's knowledge and behaviors around breast cancer screening. Psycho-Oncology 2013;22(8):1705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shirazi 2015
- Shirazi M, Shirazi A, Bloom J. Developing a culturally competent faith-based framework to promote breast cancer screening among Afghan immigrant women. Journal of Religion and Health 2015;54(1):153-9. [DOI: doi: 10.1007/s10943-013-9793-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simonds 1974
- Simonds SK. Health education as social policy. Health Education Monographs 1974;2(1):1-10. [Google Scholar]
Skinner 1953
- Skinner BF. Science and Human Behavior. New York (NY): Macmillan, 1953. [Google Scholar]
Smith 1999
- Smith M. Learning theory. https://infed.org/learning-theory-models-product-and-process/ 1999.
Speight 2001
- Speight J, Bradley C. The ADKnowl: Identifying knowledge deficits in diabetes care. Diabetic Medicine 2001;18(8):626-33. [DOI] [PubMed] [Google Scholar]
Spitzberg 1984
- Spitzberg BH, Cupach WR. Interpersonal Communication Competence. Beverly Hills: Sage, 1984. [Google Scholar]
Stormacq 2020
- Stormacq C, Wosinski J, Boillat E, Van den Broucke S. Effects of health literacy interventions on health-related outcomes in socioeconomically disadvantaged adults living in the community: a systematic review. Joanna Briggs Institute evidence synthesis 2020;18(7):1389-469. [DOI: 10.11124/JBISRIR-D-18-00023] [DOI] [PubMed] [Google Scholar]
Street 1995
- Street BV. Social literacies: Critical approaches to literacy in development, ethnography and education. London: Routledge, 1995. [Google Scholar]
Street 2003
- Street RL. Interpersonal communication skills in health care contexts. In: Greene JO, Burleson BR, editors(s). Handbook of communication and Social Interaction Skills. Mawah: Lawrence Erlbaum Associates, 2003:909-33. [Google Scholar]
Stucky 2011
- Stucky BD, Lee JY, Lee S-YD, Rozier RG. Development of the two-stage rapid estimate of adult literacy in dentistry. Community Dentistry and Oral Epidemiology 2011;39(5):474-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Svensson 2017
- Svensson P, Carlzen K, Agardh A. Exposure to culturally sensitive sexual health information and impact on health literacy: a qualitative study among newly arrived refugee women in Sweden. Culture, Health and Sexuality 2017;19(7):752-66. [DOI: 10.1080/13691058.2016.1259503] [DOI] [PubMed] [Google Scholar]
Sørensen 2012
- Sørensen K, den Broucke S, Fullam J, Doyle G, Pelikan J, Slonska Z, et al. Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health 2012;12(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sørensen 2013
- Sørensen K, den Broucke S, Pelikan J, Fullam J, Doyle G, Slonska Z, et al. Measuring health literacy in populations: illuminating the design and development process of the European health literacy survey questionnaire (HLS-EU-Q). BMC Public Health 2013;13(1):948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tavakoly 2018
- Tavakoly SS, Peyman N, Behzhad F, Esmaeily H, Taghipoor A, Ferns G. Health providers' communication skills training affects hypertension outcomes. Medical Teacher 2018;40(2):154-63. [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas J, Noel-Storr A, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. [DOI] [PubMed] [Google Scholar]
Timmins 2002
- Timmins C. The impact of language barriers on the health care of Latinos in the United States: a review of the literature and guidelines for practice. Journal of Midwifery and Women's Health 2002;47(2):80-96. [DOI] [PubMed] [Google Scholar]
Toobert 2000
- Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23(7):943-50. [DOI] [PubMed] [Google Scholar]
United Nations 2017
- United Nations Department of Economic and Social Affairs Population Division. International migration report 2017: highlights (ST/ESA/SER.A/404). www.un.org/en/development/desa/population/migration/publications/migrationreport/docs/MigrationReport2017_Highlights.pdf (accessed 2 April 2018).
Valentín‐Cortés 2020
- Valentín-Cortés M, Benavides Q, Bryce R, Rabinowitz E, Rion R, Lopez WD, Fleming PJ. Application of the minority stress theory: understanding the mental health of undocumented Latinx immigrants. American Journal of Community Psychology 2020;66(3-4):325-36. [DOI: 10.1002/ajcp.12455] [DOI] [PubMed] [Google Scholar]
van Servellen 2003
- Servellen G, Brown JS, Lombardi E, Herrera G. Health literacy in low-income Latino men and women receiving antiretroviral therapy in community-based treatment centers. AIDS Patient Care and STDs 2003;17(6):283-98. [DOI] [PubMed] [Google Scholar]
von Wagner 2009
- Wagner C, Semmler C, Good A, Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: the role of information processing. Patient Education and Counseling 2009;75(3):352-7. [DOI] [PubMed] [Google Scholar]
Wallace 2017
- Wallace BC, Noel-Storr AH, Marshall IJ, Cohen AM, Smalheiser NR, et al. Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. Journal of the American Medical Informatics Association 2017;24(6):1165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wandschneider 2020
- Wandschneider L, Batram-Zantvoort S, Razum O, Miani C. Representation of gender in migrant health studies – a systematic review of the social epidemiological literature. International Journal for Equity in Health 2020;19:181. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013
- Wang ML, Lemon SC, Welch G, Rosal MC. Development and validation of the lifestyle self-efficacy scale for Latinos with diabetes (LSESLD). Ethnicity & Disease 2013;23(4):428-35. [PMC free article] [PubMed] [Google Scholar]
Weiss 2005
- Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, et al. Quick assessment of literacy in primary care: the newest vital sign. Annals of Family Medicine 2005;3(6):514-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2012
- Welch V, Petticrew M, Tugwell P, Moher D, O'Neill J, Waters E, et al. PRISMA-Equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLOS Medicine 2012;9(10):e1001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2015
- Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. International Journal for Equity in Health 2015;14:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitehead 1992
- Whitehaead M. The concepts and principles of equity and health. International Journal of Health Services 1992;22(3):429-45. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization Regional Office for Europe. How health systems can address health inequities linked to migration and ethnicity 2010. www.euro.who.int/_data/assets/pdf_file/0005/127526/e94497.pdf (accessed 3 March 2018).
Williams 1994
- Williams DR, Lavizzo-Mourey R, Warren RC. The concept of race and health status in America. Public Health Reports 1994;109(1):26-41. [PMC free article] [PubMed] [Google Scholar]
Williams 1997
- Williams DR. Race and health: basic questions, emerging directions. Annals of Epidemiology 1997;7(5):322-33. [DOI] [PubMed] [Google Scholar]
Williams‐Piehota 2004
- Williams-Piehota P, Schneider TR, Pizarro J, Mowad L, Salovey P. Matching health messages to health locus of control beliefs for promoting mammography utilization. Psychology and Health 2004;19(4):407-23. [Google Scholar]
Woopen 2015
- Woopen C. Health literacy [Gesundheitskompetenz]. In: Sturma D, Heinrichs B, editors(s). Handbuch Bioethik. Stuttgart: JB Metzler, 2015:280-6. [Google Scholar]
Wångdahl 2014
- Wångdahl J, Lytsy P, Mårtensson L, Westerling R. Health literacy among refugees in Sweden - a cross-sectional study. BMC Public Health 2014;14(1):1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2018
- Xu XY, Leung, AYM, Chau PH. Health literacy, self-efficacy, and associated factors among patients with diabetes. HLRP: Health Literacy Research and Practice 2018;2(2):e67-e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2012
- Yun K, Hebrank K, Graber LK, Sullivan MC, Chen I, Gupta J. High prevalence of chronic non-communicable conditions among adult refugees: implications for practice and policy. Journal of Community Health 2012;37(5):1110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zapka 2004
- Zapka JG, Puleo E, Taplin SH, Goins KV, Ulcickas Yood M, Mouchawar J, et al. Processes of care in cervical and breast cancer screening and follow-up – the importance of communication. Preventive Medicine 2004;39(1):81-90. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavia 1983;67(6):361-70. [DOI: 10.1111/j.1600-0447.1983.tb09716.x] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Baumeister 2019
- Baumeister A, Aldin A, Chakraverty D, Monsef I, Jakob T, Seven ÜS, et al. Interventions for improving health literacy in migrants. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD013303. [DOI: 10.1002/14651858.CD013303] [DOI] [PMC free article] [PubMed] [Google Scholar]


