Abstract
PURPOSE
Hepatocellular carcinoma (HCC), the fourth most common cancer in Africa, has a dismal overall survival of only 3 months like in sub-Saharan Africa. This is affected by the low gross domestic product and human development index, absence of coherent guidelines, and other factors.
METHODS
An open forum for HCC-experienced health care workers from Africa and the rest of the world was held in October 2021. Participants completed a survey to help assess the real-life access to screening, diagnoses, and treatment in the North and Southern Africa (NS), East and West Africa (EW), Central Africa (C), and the rest of the world.
RESULTS
Of 461 participants from all relevant subspecialties, 372 were from Africa. Most African participants provided hepatitis B vaccination and treatment for hepatitis B and C. More than half of the participants use serum alpha-fetoprotein and ultrasound for surveillance. Only 20% reported using image-guided diagnostic liver biopsy. The Barcelona Clinic Liver Cancer is the most used staging system (52%). Liver transplant is available for only 28% of NS and 3% EW. C reported a significantly lower availability of resection. Availability of local therapy ranged from 94% in NS to 62% in C. Sorafenib is the most commonly used systemic therapy (66%). Only 12.9% reported access to other medications including immune checkpoint inhibitors. Besides 42% access to regorafenib in NS, second-line treatments were not provided.
CONCLUSION
Similarities and differences in the care for patients with HCC in Africa are reported. This reconfirms the major gaps in access and availability especially in C and marginally less so in EW. This is a call for concerted multidisciplinary efforts to achieve and sustain a reduction in incidence and mortality from HCC in Africa.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a disease of global significance with high incidence and mortality. HCC is the sixth most common cancer worldwide and the fourth in Africa.1,2 Because of the high prevalence of the causative risk factors of hepatitis C virus (HCV) and hepatitis B virus (HBV), Africa includes 6 of the 15 countries with the highest HCC incidence globally.3 HCC etiology and incidence differ between North and sub-Saharan Africa.4 HBV prevalence is high throughout sub-Saharan Africa, ranging from 1.1 to >10% of the population, with some regional variation.5-8 HCV prevalence is the highest in North Africa, ranging from 2.3% to 7.7%.9,10 Dietary exposure to fungal aflatoxins, alcohol use, obesity, and diabetes-related nonalcoholic fatty liver disease are other drivers and synergistic cofactors in the development of HCC in Africa.5
CONTEXT
Key Objective
Hepatocellular carcinoma (HCC) has one of the highest incidences of the world in Africa and the lowest overall survival rate.
Knowledge Generated
To help build pertinent guidelines for the treatment of HCC in Africa, HCC-experienced health care workers in Africa were engaged. A survey of 372 participants from Africa reassured us of the relative success of providing hepatitis B vaccination and treating hepatitis B and C. Staging system data varied. Liver transplant was only available in North and South Africa. Local therapies were relatively more abundant. Despite the advent of all novel checkpoint inhibitors-based therapies that only 13% reported access to, systemic therapy was limited to sorafenib mostly.
Relevance
This effort reconfirms the major gaps in access and availability of care for HCC in Africa. It is a call for concerted multidisciplinary efforts to achieve and sustain a reduction in incidence and mortality of HCC in Africa.
HCC ranks as the third leading cause of cancer-related deaths worldwide with most HCC-related deaths occurring in Africa and the Asia-Pacific region.4,11 The median overall survival of HCC in sub-Saharan Africa is only 3 months.5
We investigated the HCC care continuum ranging from prevention, surveillance, diagnosis, and treatment across different regions of the African continent using a survey conducted during the inaugural of Africa Guidelines for Hepatocellular Carcinoma meeting. The results of the survey reflect the real-world current care status of prevention, diagnosis, and care for HCC in Africa.
METHODS
Africa Guidelines for Hepatocellular Carcinoma
The committee members of the Africa Guidelines for Hepatocellular Carcinoma met almost monthly over 2020-2021. Most of the committee members or coauthors care for patients with HCC in Africa. The committee elected to categorize the 54 countries of Africa on the basis of geography, gross domestic product (GDP), and human development index (HDI), thereby grouping them into three regions: North and Southern (NS), East and West (EW), and Central (C). An open-access virtual meeting followed. All health care workers who care for patients at risk for or diagnosed with HCC from Africa and the rest of the world (ROW) were invited. Completion of the polling questionnaire was the only prerequisite to attend the meeting.
Survey-Like Polling Questionnaire
The survey was provided in English or French. Survey respondents answered about their professional expertise, clinical practice, and access and use of HCC prevention, surveillance, diagnosis, staging, and treatment options (Data Supplement [Fig S1]).
Statistical Analysis
Participants' responses were summarized by frequency and percentage in the three African and the ROW groups.
A hierarchical logistic regression model was used to examine differences between the four groups in how health care professionals provide and use resources on a set of binary outcomes.
The country was modeled as a random effect to account for the clustered structure of participants nested within the country with respondents from the same country being more likely to have similar outcomes than respondents from other countries. In the primary analyses, ROW was used as the reference group. Secondary analyses were performed with NS as the reference group, for which the results are included in the supplementary material. Odds ratio (OR) and 95% CI were presented.
For outcomes with more than two distinct categories and because of paucity in the data (some outcomes were not observed at all or observed very infrequently for some of the four regions), the conventional method of adaptive quadrature for generalized linear mixed models was not feasible. Clustered multinomial outcomes, like the effect of region within a country, will be assessed using a Bayesian hierarchical model. This work is underway and will be published separately.
All analyses were done in SAS 9.4 (SAS Institute Inc, Cary, NC) or R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). All P values were two-sided, and P value of <.05 was considered to be of statistical significance.
RESULTS
Survey Participants
All 461 participants completed the survey between August and October 2021. Eighty-one percent (372) of the participants were from Africa, of whom the largest representation was from NS (202, 44%), followed by EW (133, 29%) and C (37, 8%) Africa. Of the other 89 (19%) participants from the ROW, 61 (68%) were from the United States.
For all participants, professional expertise included gastroenterology and hepatology (42%), medical oncology/radiation oncology (27%), general internal medicine (11%), hepatobiliary/liver transplant surgery (5%), and other health care professionals (16%).
HCC Prevention and Surveillance
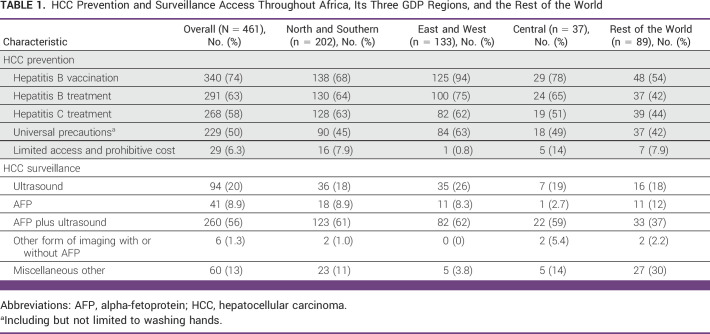
Most participants provided primary prevention including HBV vaccination (74%) and HBV (63%) and HCV treatment (58%) plus universal precautions (50%). Compared with the ROW, EW were more likely to endorse HBV vaccination (OR, 9.78 [95% CI, 3.5 to 27.5]), HBV treatment (OR, 4.46 [95% CI, 1.60 to 12.49]), and universal precautions (OR, 2.28 [95% CI, 1.00 to 5.21]). The EW group was also less likely to report limited access and prohibitive costs of preventative measures (OR, 0.08 [95% CI, 0.01 to 0.77]; Fig 1). Compared with NS, respondents in the EW were significantly more likely to provide HBV vaccination (OR, 7.35 [95% CI, 2.80 to 19.33]) and significantly less likely to report limited access and prohibitive costs of preventive measures (OR, 0.09 [95% CI, 0.01 to 0.87]; Data Supplement [Table S1]).
FIG 1.
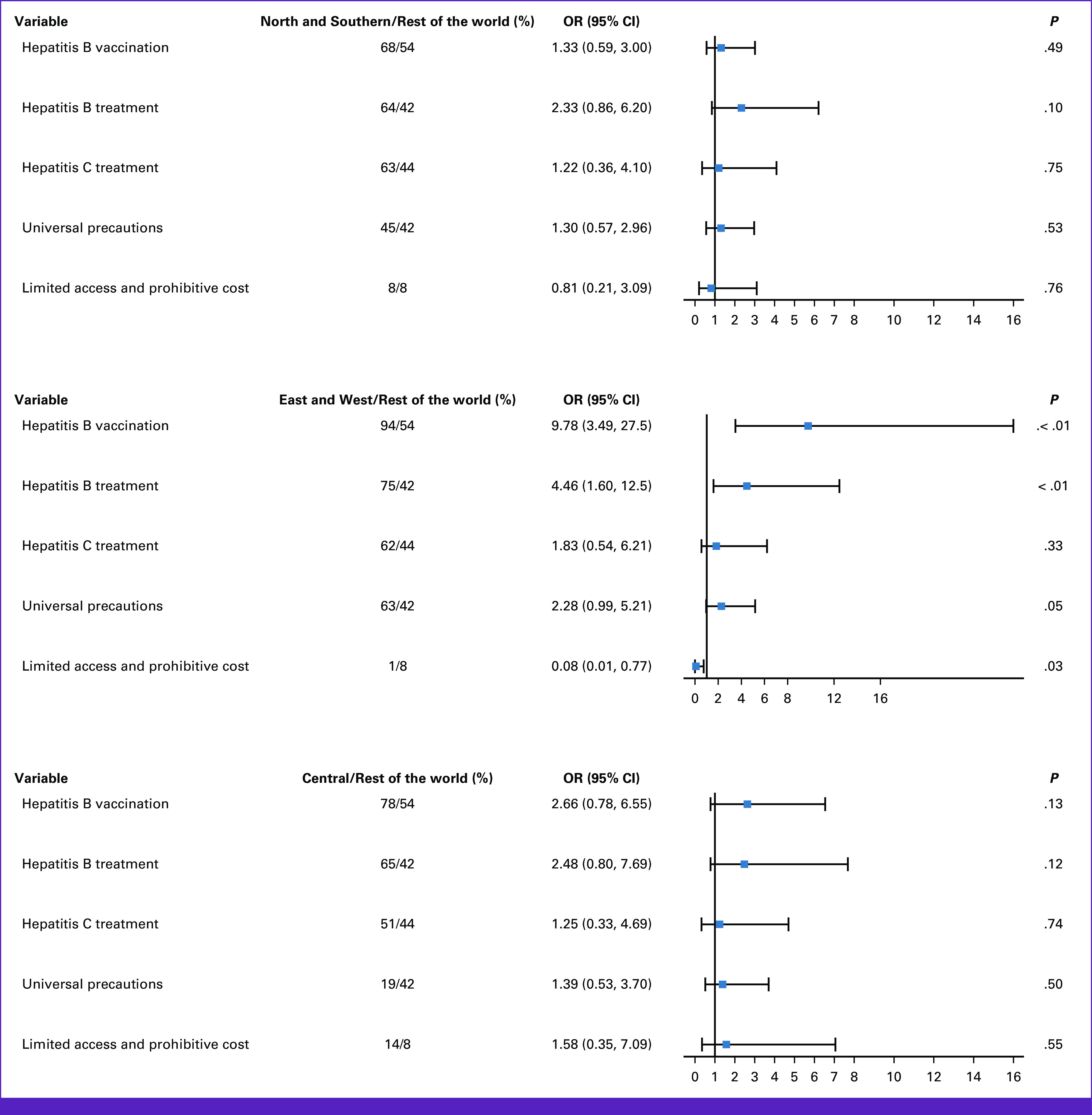
Methods of HCC prevention forest plot. HCC, hepatocellular carcinoma; OR, odds ratio.
More than half (56%) of the participants reported using serum alpha-fetoprotein (AFP) and ultrasound in combination for surveillance while smaller proportions reported using ultrasound alone (20%) or AFP alone (9%; Table 1). The odds of using other forms of imaging with or without AFP for HCC surveillance versus ultrasound were significantly lower for the EW compared with the ROW (OR, 0.06 [95% CR, 0.002 to 0.844]; Data Supplement [Fig S2]). No significant association of HCC surveillance was found when comparing EW or C with NS (Data Supplement [Table S2]). Among the 60 respondents in the miscellaneous other category, 35 (17% from C, 57% from NS, and 9% from EW) reported limited HCC surveillance because of cost (n = 3), not using any method (n = 30), or do not believe data (n = 2).
TABLE 1.
HCC Prevention and Surveillance Access Throughout Africa, Its Three GDP Regions, and the Rest of the World
HCC Diagnosis, Staging Classification, and Guideline Utilization
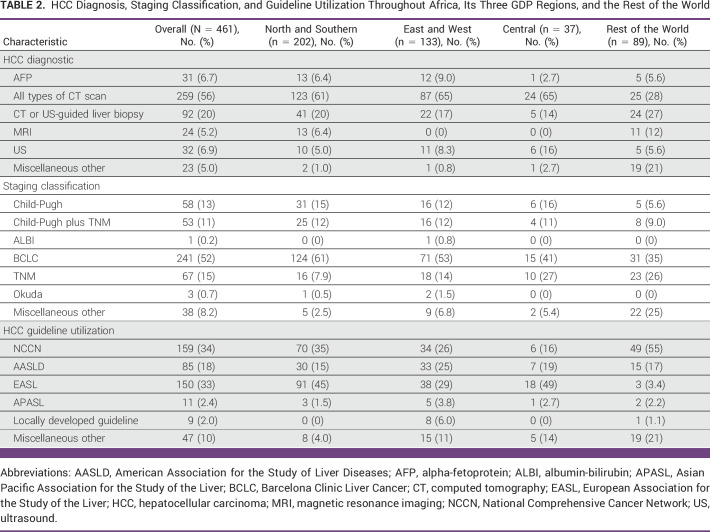
More than half of total number of respondents (56%) reported using computed tomography (CT) scan for diagnosis (Table 2). Magnetic resonance imaging (MRI) was used by only 5.2% solely from NS. Only 20% reported using image-guided liver biopsy. Compared with the ROW, the odds of using MRI as a diagnostic tool versus CT or ultrasound-guided liver biopsy was less in EW (OR, 0.047 [95% CR, 0.002 to 0.819]; Data Supplement [Fig S3]). Compared with NS, the odds of using ultrasound alone versus using CT- or ultrasound-guided liver biopsy was lower for EW (OR, 0.057 [95% CR, 0.002 to 0.970]).
TABLE 2.
HCC Diagnosis, Staging Classification, and Guideline Utilization Throughout Africa, Its Three GDP Regions, and the Rest of the World
Overall, the Barcelona Clinic Liver Cancer (BCLC) classification was the most reported used staging system (52%): 61% in NS, 53% in EW, 41% in C, and 35% in the ROW (Table 2). Compared with the ROW, the odds of using the TNM staging classification as opposed to using BCLC staging was significantly lower in Africa (OR, 0.23 [95% CR, 0.057 to 0.869]; Data Supplement [Fig S4]). Compared with NS, there was no significant association of region with use of HCC staging (Data Supplement [Table S4]). About a third of respondents reported using National Comprehensive Cancer Network (NCCN; 34%) or European Association for the Study of the Liver (EASL) guidelines (33%) for the management of HCC. However, in C, only 16% reported using the NCCN guidelines while 49% used EASL guidelines. There was also no significant association of region with use of different HCC guidelines within Africa (Data Supplement [Table S5]).
HCC Early-Stage Disease Treatment
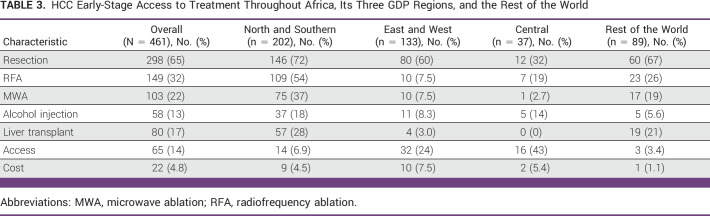
Overall, curative treatments are more widely available in NS and the ROW, less so in EW with limited availability in C (Table 3). Use of surgical resection, radiofrequency ablation (RFA), and microwave ablation (MWA) by respondents were 65%, 32%, and 22%, respectively. Compared with the ROW, there was a significant decrease in the odds of using surgical resection (OR, 0.15 [95% CI, 0.05 to 0.48]; P < .01) for participants from C (Data Supplement [Fig S5]). A total of 17% of respondents reported the availability of liver transplant, with 28% and 21% in NS and ROW, respectively, and only 3% from EW and none from C. Compared with the ROW, the odds of providing liver transplant were not significantly different in NS (OR, 0.93 [95% CI, 0.19 to 4.52]) but significantly lower in EW (OR, 0.14 [95% CI, 0.02 to 0.91]; Data Supplement [Fig S5]). With NS as a reference, there was a significantly lower odds of liver transplant in EW (OR, 0.15 [95% CI, 0.02 to 0.95]; P = .04; Data Supplement [Table S6]). Overall, 19% of respondents reported that no curative treatment is provided because of lack of access or cost. This proportion was higher in C (48%) and EW (32%) than NS Africa (11%) or the ROW (4.5%). Compared with the ROW, the odds of no curative treatment modality because of limited access and prohibitive cost were significantly higher for C (OR, 19.3 [95% CI, 4.7 to 79.6]) and EW (OR, 8.1 [95% CI, 2.1 to 30.3]; Data Supplement [Fig S5]). For NS, there was a substantial but nonsignificant increase in the odds of no curative treatment compared with the ROW (OR, 3.8 [95% CI, 0.98 to 14.8]; P = .05; Data Supplement [Fig S5]). In comparison with NS, there was a significantly increased odds of no curative treatment because of limited access and prohibitive cost in C (OR, 5.1 [95% CI, 1.9 to 13.8]; P < .01) but a nonsignificant increase in EW (OR, 2.1 [95% CI, 0.89 to 5.0]; P = .10; Data Supplement [Table S6]).
TABLE 3.
HCC Early-Stage Access to Treatment Throughout Africa, Its Three GDP Regions, and the Rest of the World
Intermediate-Stage Disease
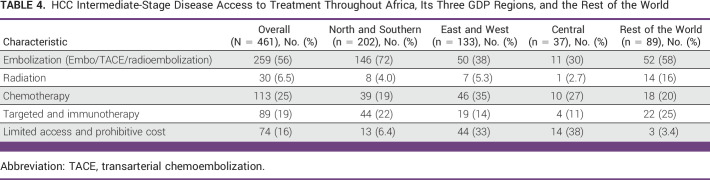
Intra-arterial locoregional treatment (eg, transarterial bland embolization, chemoembolization, or radioembolization) was widely available in NS (72%) and the ROW (58%) while only about a third of respondents answered that these therapeutic modalities are available in the rest of Africa (Table 4). Compared with the ROW, locoregional treatments had a nonsignificant decreased odds of being available in EW (OR, 0.34 [95% CI, 0.07 to 1.73]; P = .19) and C (OR, 0.19 [95% CI, 0.03 to 1.14]; P = .07; Data Supplement [Fig S6]). Compared with NS, locoregional treatments also had a nonsignificant decreased odds of availability in EW (OR, 0.48 [95% CI, 0.09 to 2.51]; P = .386) and C (OR, 0.28 [95% CI, 0.05 to 1.68]; P = .162; Data Supplement [Table S7]). Overall, 16% reported that no treatment is provided for intermediate-stage HCC because of lack of access or prohibitive cost. This proportion was higher in C (38%) and EW (33%) than NS (6%) and the ROW (3%). The odds of no access to locoregional treatment was significantly higher in C (OR, 17.7 [95% CI, 2.79 to 113.7]; P < .01) and EW (OR, 10.97 [95% CI, 1.86 to 64.61]; P = .01) compared with the ROW (Data Supplement [Fig S6]). Compared with NS, the findings were similar, with the odds of no treatment because of limited access and prohibitive cost significantly higher in C (OR, 7.95 [95% CI, 1.95 to 32.49]; P < .01) and EW (OR, 4.94 [95% CI, 1.33 to 18.37]; P = .017; Data Supplement [Table S7]).
TABLE 4.
HCC Intermediate-Stage Disease Access to Treatment Throughout Africa, Its Three GDP Regions, and the Rest of the World
Advanced-Stage Disease
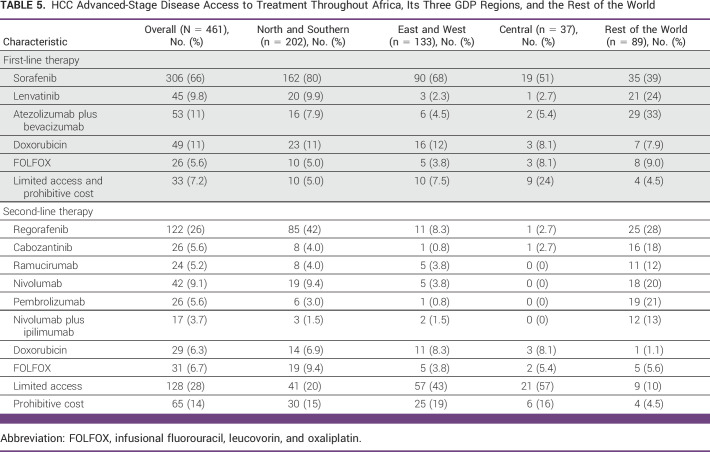
Within the confines of the available and/or approved medications worldwide at the time of this study, for first-line treatment, 66% of participants reported using sorafenib,12 and it was the most commonly available therapy across Africa (80% in NS, 68% in EW, and 51% in C) while its proportion of first-line use was much lower in the ROW (39%; Table 5). Compared with the ROW, there was a nonsignificant two-fold increase in the use of sorafenib treatments in NS (OR, 2.13 [95% CI, 0.68 to 6.73]) and EW (OR, 2.14 [95% CI, 0.68 to 6.80]; P = .195), respectively (Data Supplement [Fig S7]). Only 12.9% of respondents in Africa answered that they use more recently approved medications including lenvatinib13 and atezolizumab and bevacizumab combination14 treatment for advanced HCC. In contrast, a quarter and a third reported using lenvatinib and atezolizumab and bevacizumab combination treatment for advanced HCC in the ROW (Table 5). Compared with the ROW, the odds of using lenvatinib were significantly reduced among participants from NS (OR, 0.22 [95% CI, 0.05 to 0.93]; P = .04), EW (OR, 0.07 [95% CI, 0.01 to 0.40]; P < .01), and C (OR, 0.09 [95% CI, 0.01 to 0.91]; P = .04; Data Supplement [Fig S7]). Similarly, atezolizumab plus bevacizumab was less likely to be used in first-line treatment among participants from NS (OR, 0.18 [95% CI, 0.08 to 0.39]; P < .01), EW (OR, 0.10 [95% CI, 0.04 to 0.27]; P < .01), and C (OR, 0.12 [95% CI, 0.03 to 0.56]; P < .01; Data Supplement [Fig S7]).
TABLE 5.
HCC Advanced-Stage Disease Access to Treatment Throughout Africa, Its Three GDP Regions, and the Rest of the World
A quarter of respondents from C reported that they do not have access to any treatment for advanced HCC. The odds of having no access to first-line systemic treatment were nearly six-fold in C compared with NS (OR, 5.60 [95% CI, 1.22 to 25.69]; P = .02) and 7.2 times higher in C when compared with the ROW (OR, 7.21 [95% CI, 1.17 to 44.27]; P = .03; Data Supplement [Fig S7]).
When compared with NS, a significantly limited access to second-line systemic treatment was reported in EW (OR, 4.3 [95% CI, 1.1 to 16.9]; P = .035) and C (OR, 7.0 [95% CI, 1.6 to 30.5]; P = .01; Data Supplement [Table S8]). Regorafenib15 was most used in NS (42%) and the ROW (28%) while treatment was not provided in most cases in EW and C because of high cost, lack of access, clinical deterioration, and short overall survival (Table 5). Compared with the ROW, the odds of limited access to second-line systemic treatment were nonsignificantly increased for EW (OR, 3.9 [95% CI, 0.96 to 15.4]; P = .057) but significantly increased for C (OR, 6.2 [95% CI, 1.4 to 27.8]; P = .018; Data Supplement [Fig S7]). No significant difference in lack of treatment because of limited access between NS versus the ROW was observed (Data Supplement [Fig S8]). When compared with NS, significantly limited access to second-line systemic treatment was noted in EW (OR, 4.3 [95% CI, 1.1 to 16.9]; P = .035) and C (OR, 7.0 [95% CI, 1.6 to 30.5]; P = .01) was noted (Data Supplement [Table S9]).
DISCUSSION
With the continued advances in screening, diagnosis, and particularly therapeutic interventions for HCC, the lack of any recent comprehensive multidisciplinary assessment of the status of HCC control efforts in Africa is a serious concern. The large data set from 461 participants of the inaugural Africa Guidelines for Hepatocellular Carcinoma exposed a number of potential unanticipated surprises and misperceptions of the health care community. The extent of the dearth of access to adequate care in Africa, a region with one of the highest incidences of liver cancer in the world, is a major global health concern.
Limited resources for the management of viral hepatitis and HCC in Africa are well recognized.16 Our survey findings are reassuring of health care workers motivation to achieve high levels of vaccination and treatment like previously reported data.17,18 HBV vaccination (74%) and HBV (63%) and HCV (58%) treatments are all available in many countries, and additional efforts toward the application of universal precautions reflect the success of efforts to enhance awareness of these strategies. However, the discrepancy in availability and endorsement of preventive measures between EW compared with NS and C raises concern in view of the anticipated better availability in NS. On another note, the success of the program for universal treatment for HCV in Egypt is a highlight, catalyzed by a strong governmental support and a collaborative pharmaceutical industry effort.19
The advanced or terminal stage HCC presentation in up to 95% of patients in most sub-Saharan African countries20 is a key consequence of the absence of surveillance programs. Although the respondents in our study reported a strong preference for use of the current global standard of surveillance with the combination of ultrasound and serum AFP measurement, our findings underscore a potential discordance between the practices of community health care workers and primary care providers in Africa, who are the primary point of contact for persons at risk for HCC and the opinions of hospital-based liver disease and liver cancer specialists.
The lack of Africa guidelines for HCC is one of the consequences of the current state of health infrastructure to care for HCC in Africa. Global guidelines adapted for Africa have been proposed and implemented in a few settings. Currently available guidelines include the NCCN Harmonized Guidelines for sub-Saharan Africa21 and the NCCN Middle East North Africa (MENA) region initiative.22 Our data show that awareness and utilization of the NCCN guidelines is limited. Beside the use of international guidelines, some respondents reported the use of local guidelines, such as those of the Society for Gastroenterology and Hepatology in (SOGHIN)23 and the Tanzania guidelines.24 Of note, neither were independently validated.
The development and validation of African-derived guidelines for HCC is a core and critical component of our efforts. Our results confirm the need for innovation and implementation of transformative, low cost, regionally and culturally appropriate cancer control strategies.
The significant regional variation in the provision of different treatments in Africa is noted. This is best illustrated by the limited availability and access to curative treatments in EW and C. For systemic treatment, the fact that sorafenib remains the most commonly available first-line therapy for advanced HCC across all regions of Africa is of serious concern, as several other options are shown to be superior in first line. This, in addition to the quarter of respondents from C reporting no access to any treatments for advanced HCC, let alone second-line treatments is an awakening call. While clinical and translational scientists are proud to have contributed to the development and use the novel therapies including the immune checkpoint inhibitors mainly in the Western Hemisphere,12-14, 25-28 Africa with one the two highest incidences of HCC worldwide still lacks access to these therapies. Another level of disparity is noted within Africa where the substantially smaller proportion of patients receiving any HCC treatment in most of Africa compared with Egypt (3% v 76%; P < .0001) and the shorter median survival of 2.5 versus 10.9 months (P < .000) is a direct proof right from Africa that improved access to care and contributes to substantial improvement in survival.19
A major strength is the readiness of African health care workers to respond and provide direct answers. The categorization of the countries into three groups is an informed collective opinion of the committee members. We are cognizant of other groupings and the limited or no access to colleagues in other countries. This confirms the lack of absolute regional divisions and recognizes that many more variables may determine the optimal grouping of countries. We acknowledge that the ROW is mainly describing current practice in the United States. We were unable to further separate Europe or North America from the ROW because of the small sample size. We anticipate in future efforts to invite and engage more experts with diverse backgrounds from other parts of the world including Asia-Pacific and Europe. Grouping by special patient groups need also to be studied. This is especially pertinent to patients living with human immunodeficiency virus in Africa, the epicenter of the AIDS epidemic.29 We acknowledge that the gathered data were not formally validated. It is likely though the large size of the cohorts helped reduce any impact of inconsistent or outlier responses. Except for a few responses, the scope of this effort failed to generate comprehensive information on palliative and supportive care. This remains a major gap in Africa especially in the setting of late-stage presentation and the minimal or no access to effective therapies.
In conclusion, the results of this study highlight the high level of knowledge and understanding of the current best practices among health care workers across Africa while also revealing major gaps in access and availability. The effort helped build the nucleus for a pan-African community bringing together local and global experts in HCC. We hope such efforts will lead to concerted, multilevel efforts by governments, health care institutions, and advocacy groups to achieve and sustain the long-term commitment to reduce the incidence and mortality of HCC in Africa.
ACKNOWLEDGMENT
We thank and congratulate all respondents for their input. Special thanks to the Memorial Sloan Kettering Certified Medical Education team members: Susan Wasserman, Amy Edouard, Jamie Silverstein, Katie O'Connell, Peggy Tully, Michelle Chen, and Jessica Bauer. We appreciate the help and support of the Information Technology team at Memorial Sloan Kettering led by Sam Palmucci. We also gratefully acknowledge the following organizations for providing educational grants for this live activity: AstraZeneca, Autem Medical, BeiGene, Berry Oncology Co., LTD, and Yiviva, Inc.
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Eisai, Ipsen, Merck Serono, AstraZeneca, Yiviva, Roche/Genentech, Autem Medical, Incyte, Exelixis, QED Therapeutics, Servier, Helio Health, Boehringer Ingelheim, Newbridge Pharmaceuticals, Novartis, Astellas Pharma, Berry Genomics, BioNtech, Bristol Myers Squibb/Medarex, Fibrinogen, Merus NV, Neogene Therapeutics, Tempus, Thetis Pharma, Vector Health
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Puma Biotechnology (Inst), QED Therapeutics (Inst), Arcus Ventures (Inst), BioNtech (Inst), Genentech/Roche (Inst), Helsinn Healthcare (Inst), Yiviva (Inst), Elicio Therapeutics (Inst), Agenus (Inst), Parker Institute for Cancer Immunotherapy (Inst), Pertzye (Inst)
Joanne F. Chou
Stock and Other Ownership Interests: Paige.AI (Inst)
Patents, Royalties, Other Intellectual Property: Company Paige.AI MSK has licensed intellectual property related to digital pathology slides and algorithm development to Paige. MSK also holds equity in Paige.AI (Inst)
Tiago Biachi de Castria
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Bayer
Speakers' Bureau: Roche, MSD, Servier, AstraZeneca, Bristol Myers Squibb Foundation, Lilly
Travel, Accommodations, Expenses: A2 Biotherapeutics
Fiyinfolu Balogun
Honoraria: Mediflix, Varian Medical Systems
Patents, Royalties, Other Intellectual Property: Mission Driven Tech. Brachytherapy
Blaise Irénée Atipo Ibara
Employment: CHU DE BRAZZAVILLE
C. Wendy Spearman
Speakers' Bureau: Gilead Sciences, Abbott Diagnostics
Travel, Accommodations, Expenses: Gilead Sciences
Ju Dong Yang
Consulting or Advisory Role: Eisai, Exact Sciences, Exelixis, AstraZeneca, Fujifilm
Lewis R. Roberts
Honoraria: Focal Medical Communications, Science First Communications Private Limited, Mumbai, India, Roche Kenya Limited, Nairobi, Kenya, Kolkata Liver Meeting, Kolkata, India - Liver Foundation, West Bengal, India
Consulting or Advisory Role: Bayer (Inst), GRAIL (Inst), RedHill Biopharma (Inst), TAVEC (Inst), Exact Sciences (Inst), Hepion Pharmaceuticals (Inst), Novartis Venture Fund (Inst), Medscape (Inst), Roche (Inst), Global Life Sciences (Inst), Clinical Care Options (Inst), Lynx Group (Inst), AstraZeneca (Inst), Genentech (Inst), Roche (Inst), Pontifax (Inst), MedEd Design LLC (Inst), Roche Africa (Inst)
Speakers' Bureau: Bayer
Research Funding: ARIAD (Inst), BTG (Inst), Exact Sciences (Inst), Gilead Sciences (Inst), Wako Diagnostics (Inst), Glycotest (Inst), RedHill Biopharma (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No. 9,469,877: Materials and Methods for Diagnosis, Prognosis, Monitoring of Recurrence and Assessment of Therapeutic/Prophylactic Treatment of Pancreaticobiliary Cancer (Inst), Five Prime Therapeutics. Royalties, Five Prime Therapeutics. Royalties (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Ghassan K. Abou-Alfa, Mary Afihene, Akwi Asombang, Olusegun Isaac Alatise, Adda Bounedjar, Lina Cunha, Hailemichael Desalegn Mekonnen, Ntokozo Ndlovu, Jerry Ndumbalo, Precious Takondwa Makondi, Christian Tzeuton, Adwoa Afrakoma Agyei-Nkansah, Fiyinfolu Balogun, Eduard Jonas, Stephen Kimani, Peter Kingham, Reshad Kurrimbukus, Nazik Hammad, Vikash Sewram, Ju Dong Yang, Lewis R. Roberts, Ashraf O. Abdelaziz
Financial support: Ghassan K. Abou-Alfa, Reshad Kurrimbukus
Administrative support: Ghassan K. Abou-Alfa, Jerry Ndumbalo, Peter Kingham, Reshad Kurrimbukus
Provision of study materials or patients: Ghassan K. Abou-Alfa, Mary Afihene, Papa Saloum Diop, Ntokozo Ndlovu, Jerry Ndumbalo, Christian Tzeuton, Adwoa Afrakoma Agyei-Nkansah, Alain Bougouma, Blaise Irénée Atipo Ibara
Collection and assembly of data: Ghassan K. Abou-Alfa, Mary Afihene, Olusegun Isaac Alatise, Adda Bounedjar, Lina Cunha, Hailemichael Desalegn Mekonnen, Papa Saloum Diop, Mahamat Moussa Ali, Ntokozo Ndlovu, Jerry Ndumbalo, Precious Takondwa Makondi, Tiago Biachi de Castria, Adwoa Afrakoma Agyei-Nkansah, Alain Bougouma, Blaise Irénée Atipo Ibara, Eloumou Bagnaka Servais Albert Fiacre, Vikash Sewram, Ju Dong Yang, Ashraf O. Abdelaziz
Data analysis and interpretation: Ghassan K. Abou-Alfa, Mary Afihene, Marinela Capanu, Yuelin Li, Joanne F. Chou, Akwi Asombang, Olusegun Isaac Alatise, Adda Bounedjar, Reda Elwakil, Ntokozo Ndlovu, Jerry Ndumbalo, Precious Takondwa Makondi, Christian Tzeuton, Tiago Biachi de Castria, Fiyinfolu Balogun, Eduard Jonas, Stephen Kimani, Peter Kingham, Nazik Hammad, Mona Fouad, Noha El Baghdady, Vikash Sewram, C. Wendy Spearman, Ju Dong Yang, Lewis R. Roberts
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Eisai, Ipsen, Merck Serono, AstraZeneca, Yiviva, Roche/Genentech, Autem Medical, Incyte, Exelixis, QED Therapeutics, Servier, Helio Health, Boehringer Ingelheim, Newbridge Pharmaceuticals, Novartis, Astellas Pharma, Berry Genomics, BioNtech, Bristol Myers Squibb/Medarex, Fibrinogen, Merus NV, Neogene Therapeutics, Tempus, Thetis Pharma, Vector Health
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Puma Biotechnology (Inst), QED Therapeutics (Inst), Arcus Ventures (Inst), BioNtech (Inst), Genentech/Roche (Inst), Helsinn Healthcare (Inst), Yiviva (Inst), Elicio Therapeutics (Inst), Agenus (Inst), Parker Institute for Cancer Immunotherapy (Inst), Pertzye (Inst)
Joanne F. Chou
Stock and Other Ownership Interests: Paige.AI (Inst)
Patents, Royalties, Other Intellectual Property: Company Paige.AI MSK has licensed intellectual property related to digital pathology slides and algorithm development to Paige. MSK also holds equity in Paige.AI (Inst)
Tiago Biachi de Castria
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Bayer
Speakers' Bureau: Roche, MSD, Servier, AstraZeneca, Bristol Myers Squibb Foundation, Lilly
Travel, Accommodations, Expenses: A2 Biotherapeutics
Fiyinfolu Balogun
Honoraria: Mediflix, Varian Medical Systems
Patents, Royalties, Other Intellectual Property: Mission Driven Tech. Brachytherapy
Blaise Irénée Atipo Ibara
Employment: CHU DE BRAZZAVILLE
C. Wendy Spearman
Speakers' Bureau: Gilead Sciences, Abbott Diagnostics
Travel, Accommodations, Expenses: Gilead Sciences
Ju Dong Yang
Consulting or Advisory Role: Eisai, Exact Sciences, Exelixis, AstraZeneca, Fujifilm
Lewis R. Roberts
Honoraria: Focal Medical Communications, Science First Communications Private Limited, Mumbai, India, Roche Kenya Limited, Nairobi, Kenya, Kolkata Liver Meeting, Kolkata, India - Liver Foundation, West Bengal, India
Consulting or Advisory Role: Bayer (Inst), GRAIL (Inst), RedHill Biopharma (Inst), TAVEC (Inst), Exact Sciences (Inst), Hepion Pharmaceuticals (Inst), Novartis Venture Fund (Inst), Medscape (Inst), Roche (Inst), Global Life Sciences (Inst), Clinical Care Options (Inst), Lynx Group (Inst), AstraZeneca (Inst), Genentech (Inst), Roche (Inst), Pontifax (Inst), MedEd Design LLC (Inst), Roche Africa (Inst)
Speakers' Bureau: Bayer
Research Funding: ARIAD (Inst), BTG (Inst), Exact Sciences (Inst), Gilead Sciences (Inst), Wako Diagnostics (Inst), Glycotest (Inst), RedHill Biopharma (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No. 9,469,877: Materials and Methods for Diagnosis, Prognosis, Monitoring of Recurrence and Assessment of Therapeutic/Prophylactic Treatment of Pancreaticobiliary Cancer (Inst), Five Prime Therapeutics. Royalties, Five Prime Therapeutics. Royalties (Inst)
Travel, Accommodations, Expenses: Gilead Sciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zakharia K, Luther CA, Alsabbak H, et al. : Hepatocellular carcinoma: Epidemiology, pathogenesis, and surveillance - implications for sub-Saharan Africa. S Afr Med J 108:35-40, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Okeke E, Davwar PM, Roberts L, et al. : Epidemiology of liver cancer in Africa: Current and future trends. Semin Liver Dis 40:111-123, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Polaris Observatory Collaborators : Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol Hepatol 3:383-403, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Ervik M, Lam F, et al. : Global Cancer Observatory: Cancer Today. Lyon, France, International Agency for Research on Cancer, 2020. https://gco.iarc.fr/today [Google Scholar]
- 5.Yang JD, Hainaut P, Gores GJ, et al. : A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16:589-604, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baecker A, Liu X, La Vecchia C, et al. : Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev 27:205-212, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JD, Roberts LR: Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol 7:448-458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemoine M, Thursz MR: Battlefield against hepatitis B infection and HCC in Africa. J Hepatol 66:645-654, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Polaris Observatory HCV Collaborators : Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol Hepatol 7:396-415, 2022 [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB: Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142:1264-1273.1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, et al. : Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378-390, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Finn RS, Qin S, et al. : Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391:1163-1173, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Qin S, Ikeda M, et al. : Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894-1905, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Qin S, Merle P, et al. : Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56-66, 2017 [DOI] [PubMed] [Google Scholar]
- 16. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management—PMC (nih.gov) [DOI] [PMC free article] [PubMed]
- 17.World Health Organization : WHO-UNICEF Estimates of HepB3 Coverage. WHO, 2019. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragehepb3.html [Google Scholar]
- 18.Thomas DL: Global elimination of chronic hepatitis. N Engl J Med 380:2041-2050, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Teaima MH, Al-Nuseirat A, Abouhussein D, et al. : Pharmaceutical policies and regulations of oral antiviral drugs for treatment of hepatitis C in Egypt-case study. J Pharm Policy Pract 14:106, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JD, Mohamed EA, Aziz AO, et al. : Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: A multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol 2:103-111, 2017 [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network : NCCN Harmonized Guidelines. http://NCCN.org [Google Scholar]
- 22.National Comprehensive Cancer Network : Middle East & North Africa (MENA) Editions of NCCN Guidelines. http://NCCN.org [Google Scholar]
- 23.Ndububa DA, Abdulkareem FB, Mustapha SK, et al. : Soghin clinical practice guidelines for hepatocellular carcinoma. Niger J Gastroenterol Hepatol 15:1-15, 2023 [Google Scholar]
- 24.Ministry of Health endorsed National Cancer Treatment : Guidelines. Tanzania. Available in print format through the Tanzania Ministry of Health, 2023 [Google Scholar]
- 25.Abou-Alfa GK, Lau G, Kudo M, et al. : Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 1, 2022 [DOI] [PubMed] [Google Scholar]
- 26.Abou-Alfa GK, Meyer T, Cheng AL, et al. : Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379:54-63, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu AX, Kang YK, Yen CJ, et al. : Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20:282-296, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Finn RS, Ryoo BY, Merle P, et al. : Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol 38:193-202, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Kedar Mukthinuthalapati VVP, Sewram V, Ndlovu N, et al. : Hepatocellular carcinoma in sub-Saharan Africa. JCO Glob Oncol 10.1200/GO.20.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]