Screening is mostly considered a noble and worthwhile effort. Most patients believe screening has no drawbacks and can bring only positive health outcomes.1 As physicians, we know otherwise. This article aims to review and explain some of the challenging myths surrounding screening. The evolution of patient care from acute infectious disease to chronic disease has influenced our approach to screening and contributed to these myths. We assumed we could tackle chronic diseases the same way we had fought infectious diseases; however, not only is the management of chronic diseases not as straightforward, but diagnostic test results for chronic disease are seldom as certain. This uncertainty is amplified for screening test results.
Screening starts with the premise that a test applied to an asymptomatic, eligible person or population, once or at intervals, can identify a treatable precursor to a disease (ie, to prevent illness) or identify a treatable disease at an earlier stage (ie, to prevent more severe morbidity and mortality). For screening to be effective, identification must lead to effective treatments that benefit patients (ie, reduce morbidity and mortality) with acceptable magnitudes of harm. Yet research often fails to provide essential information to quantify both the benefits and the harms of screening.2 Patients and practitioners are then unable to assess the balance and engage in meaningful shared decision making. This fuels assumptions and myths about screening.
Case description
In the morning, while perusing one of your professional magazines online, you are intrigued to learn of a new screening test for dementia that would allow detection years before onset of symptoms. Your initial impression is that this could be a useful screening test in your practice setting. Upon further reflection, you wonder what benefits might occur from earlier detection of dementia when there is no specific therapy that would meaningfully change its course. You also ponder how this diagnosis could affect the life decisions of individual patients who have positive screening results, especially considering the possibility of false positives. You start to question the benefits of earlier detection of other diseases. In fact, you even wonder, “Does screening save lives?”
Myth 1: there are no harms from screening
Some screening tests may have benefits, but the possible harms are seldom discussed. Ideally, when we find more disease, we should have the ability to treat it and improve the patient’s outcome. Unfortunately, this is not true for all diseases detected. Once a diagnosis is made, it is not possible to know if the person has an overdiagnosis (ie, disease that would not have become apparent in the person’s lifetime),3 a disease for which we cannot change the outcome, or a disease for which we can improve the outcome.4 Many think that only the latter occurs.
Overdiagnosis is an inherent consequence of any type of screening. Its occurrence, as well as other potential harms such as false-positive results, should be estimated and discussed with the patient alongside potential benefits to determine whether screening is something to embark on or not. Patient understanding and input into the decision is key. As an example, let us consider a 70-year-old man who feels well. After shared decision making, the patient was screened for abdominal aortic aneurysm (AAA).5 An AAA was discovered and the patient underwent surgery. This patient may have had this surgical intervention for an AAA that would never have caused symptoms in his lifetime. If so, this would be an overdiagnosis. Since the AAA would not have caused symptoms, the patient could not benefit from the intervention and could only potentially be harmed (eg, surgical complications). Even those with small AAAs below the threshold for operation may be harmed by the regular surveillance they undergo. On the other hand, some individuals identified through screening do benefit from earlier surgery. Since physicians cannot predict the future, we cannot know which asymptomatic patients with “disease” will benefit from screening and subsequent interventions. The discussion about the pros and cons of screening needs to happen before the screening decision is made.
Other harms are related to the tests themselves and to further possible investigations or treatments. False positives may be a concern for patients, especially if they are common. In addition to making a healthy person anxious about being ill, further investigations of positive results may require additional diagnostic imaging and biopsies that are not without consequences. Screening has potential benefits, but like any clinical decision, harms should also be discussed and patients’ values and preferences respected. For example, we discuss the benefits and harms of statin initiation in primary prevention with patients before prescribing. The same should be done before making screening decisions.
Myth 2: earlier detection results in better outcomes
One of the most common beliefs is that earlier detection of disease always results in better patient outcomes. Earlier detection is necessary, but not sufficient, for screening to be beneficial. An increase in earlier diagnosis by itself is not directly related to benefit unless a decrease in advanced disease or mortality can be demonstrated.
Bell and Nijsten6 have commented on how screening for melanoma increased early disease detection without having any impact on later-stage disease. Similarly, Japan’s story of screening for neuroblastoma (starting in 1985) is cautionary. This cancer has a better prognosis when it is diagnosed before age 1, so the goal of the program was to identify neuroblastoma earlier, when the prognosis is better. Screening increased the incidence of neuroblastoma but did not change the number of children diagnosed later (after age 1) and the mortality remained similar to that of other countries without screening programs.7 As in the melanoma screening example, screening discovered cases earlier but had no overall benefits. Japan’s program was discontinued in 2004.8
Another example of detecting disease earlier was the “epidemic” of thyroid cancer in South Korea that followed the increased use of thyroid ultrasound scans. In the face of an increasing rate of thyroid cancers, many South Koreans thought that screening would be helpful. Based on the extolled virtues of early detection, screening for thyroid cancer through ultrasound scans resulted in tens of thousands of patients being overdiagnosed, with no change in mortality, even though nearly all patients were treated, many with sequelae.9 With no impact on patient-important benefits and a large increase in harms (eg, medicalization, treatment harms), screening more often and detecting more disease was not beneficial. While thyroid screening was not promoted in Canada, noticeable increases in the use of imaging through the 1990s and early 2000s, mainly in middle-aged women, were observed, again with no change in mortality.10
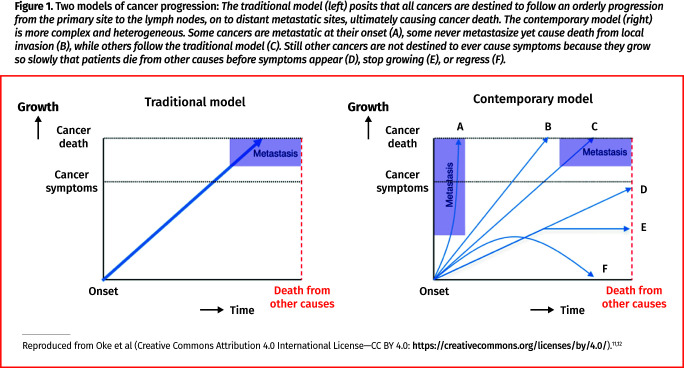
We often think that diseases behave consistently and are predictable, as depicted in the traditional model of cancer progression in Figure 1,11,12 but the reality is more variable, as shown in the contemporary model. Even cancer is not always a linear and progressive disease, and as such the question “Is earlier better?” must be answered before suggesting any screening. We need evidence that earlier is in fact better, at least for some individuals, and a sense of the magnitude of benefits and harms.
Figure 1.
Two models of cancer progression: The traditional model (left) posits that all cancers are destined to follow an orderly progression from the primary site to the lymph nodes, on to distant metastatic sites, ultimately causing cancer death. The contemporary model (right) is more complex and heterogeneous. Some cancers are metastatic at their onset (A), some never metastasize yet cause death from local invasion (B), while others follow the traditional model (C). Still other cancers are not destined to ever cause symptoms because they grow so slowly that patients die from other causes before symptoms appear (D), stop growing (E), or regress (F).
Myth 3: newer technology produces more benefit
When new technologies allow more disease to be detected, we need to ensure that their use results in an overall positive balance in terms of patient-important outcomes.13 As examples, we can think of improved imaging or the addition of more tests to tests that already exist, such as higher-resolution computed tomography. Since the arrival of this “improved” test, there has been a steep increase in pulmonary embolism diagnoses, resulting in increased incidence without a substantial decrease in mortality.14 This is a clear example of overdiagnosis (through incidental findings, not screening). In screening, digital mammography combined with breast tomosynthesis may detect more breast cancers than mammography alone,15 but this should not be seen as assurance of better health. It might be beneficial, but information on the magnitude of the potential benefits and harms is needed to inform our patients.
Table 1 describes the various ways screening may expand disease recognition.16 Persistent issues are the lack of recognition of potential harms and unproven benefits from the increased incidence of cases.
Table 1.
Common approaches used to promote earlier or increased detection of disease and what to consider
| APPROACH TO EXPAND DISEASE DETECTION | REFLECTION |
|---|---|
| Expand the age range of screening to start earlier or end later | Incidence of the disease may differ or competing morbidity and mortality may worsen the benefit-harm balance. Examples include the controversy over the age to start screening for breast cancer with mammography. Transparent information about the magnitude of benefits and harms is key to shared decision making |
| Increase the frequency of screening | Belief in the benefit of more frequent screening to not “miss” cases while not considering the potential harms (eg, annual Papanicolaou tests were once thought necessary) |
| Use more sensitive screening tests | Use of more sensitive imaging may identify smaller lesions without evidence of benefit from clinical trials. An example would be if magnetic resonance imaging were recommended instead of mammography for breast cancer screening in women at average risk |
| Expand disease definitions | Lowering the threshold for abnormality will increase the proportion of the population diagnosed with a given condition. Examples include changes in the criteria for hypertension, diabetes, and autism spectrum disorder16 |
Myth 4: screening saves lives
For many screening programs, especially cancer screening, we are told that screening saves lives. Unfortunately, this has rarely been shown, even if some studies have found reductions in disease-specific mortality. For example, systematic reviews of breast cancer screening have shown a small reduction in deaths attributed to breast cancer17 but not in overall mortality. This is important since key messages often get whittled down to “breast cancer screening saves lives,” whereas the message should be that for every 1000 women screened repeatedly over time, “breast cancer screening can reduce deaths from breast cancer.” The number varies by age, but it is around 1 in 1000 women screened in their 50s or 60s.18
Showing a reduction in all-cause mortality is a challenge for any test, particularly for screening tests where most patients are at very low risk of death. Randomized controlled trials would need to be very large or the effect size would need to be substantial.19 A strategy is to combine multiple trials to increase statistical power. By so doing, the only cancer screening test to show a statistically significant reduction in all-cause mortality is flexible sigmoidoscopy for colorectal cancer (relative risk=0.97; 95% CI 0.959 to 0.992, P=.004) with an absolute risk reduction of 3.0 deaths per 1000 screened (95% CI 1.0 to 4.0) over 11.5 years of follow-up.20 Since cervical cancer screening decreases incidence of disease, it is likely this screening also reduces mortality.21
Since screening focuses on asymptomatic people and the results of screening can lead to medicalization, it is important for our patients (and for ourselves) that we understand what the benefit is (eg, disease-specific mortality) and its magnitude. We should avoid the more general idea of saving lives. Confronting screening myths with evidence is an important step in recognizing how screening can be used more sensibly (Table 2).
Table 2.
Screening myths
| MYTH | REALITY |
|---|---|
| Earlier is better | While early detection is key for a successful screening test, we need more information. Evidence from trials should show the intervention improves health if the disease is found earlier and that resultant harms are acceptable |
| More is better | Detecting more disease is not a synonym of benefit. We need information on the balance between benefits and harms before proceeding. The most unbiased indicator of benefit is all-cause mortality, but this outcome is rarely achieved. False-positive results and overdiagnosis are important indicators of harm |
| Newer is better | Newer tests tend to be seen through a positive lens. They may detect more disease, but the use of these new methods should be subjected to clinical trials to demonstrate the magnitude of benefits and harms |
| Screening saves lives | This is the most enduring myth of all, but the reality is more nuanced. Patients should know the ultimate impact on their lives from screening based on mortality from all causes. Disease-specific mortality can result in a more favourable perception of benefit. Absolute estimates of benefit (and harm) should be transparently provided for meaningful shared decision making |
Case resolution
At lunch, you return to the online article about the screening test for dementia. It states that the test detected the disease earlier compared with usual care, but there is no information on patient-important outcomes (eg, need for long-term care, quality of life, mortality) and harms were not reported. You realize your reflections had been spot on; more research is needed to assess this intervention. You decide to write a comment below the article. In reading previous comments you realize you are not the only one with doubts about the clinical importance of this discovery.
Conclusion
While there should be a sufficient burden of disease for screening to potentially be appropriate, an increase in incidence should not be the only reason to suggest more screening. Some screening is aimed at very rare diseases with catastrophic outcomes because effective approaches are available to avert these consequences (eg, metabolic diseases in newborns), but, in general, population-based screening for something exceedingly rare (eg, cervical cancer in women younger than 25) would cause many harms (eg, false positives) with very few, if any, benefits. Diseases may increase in incidence because of changing epidemiology (eg, tobacco smoking, obesity, diabetes). If this is suspected, efforts should be made to determine whether systematically addressing these risk factors would be more effective than screening.
In 1968 Wilson and Jungner brought attention to screening by identifying 10 principles to guide its use.22 With the knowledge we have gained since then, we realize that what we had thought was relatively straightforward is much more complicated.23
Key points
▸ Earlier diagnosis by itself is not directly related to benefit unless a decrease in advanced disease or mortality can be demonstrated.
▸ Screening has potential benefits but is not without potential harms such as overdiagnosis. Understanding potential benefits and harms should precede and inform shared decision making with patients about screening.
▸ Since screening focuses on asymptomatic people and the results of screening can lead to medicalization, it is important for patients and practitioners to understand what the benefit is (eg, disease-specific mortality) and its magnitude.
Suggested reading
Welch HG. Should I be tested for cancer? Maybe not and here’s why. Oakland, CA: University of California Press; 2006.
Welch HG, Schwartz LM, Woloshin S. Overdiagnosed. Making people sick in the pursuit of health. Boston, MA: Beacon Press; 2012.
Dickinson JA, Thériault G, Grad R, Bell NR, Szafran O. Assessing new screening tests. Panacea or profligate? Can Fam Physician 2022;68:815-22 (Eng), e310-7 (Fr).
Footnotes
Competing interests
None declared
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to https://www.cfp.ca and click on the Mainpro+ link.
La traduction en français de cet article se trouve à https://www.cfp.ca dans la table des matières du numéro de novembre 2023 à la page e216.
References
- 1.Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med 2015;175(2):274-86. [DOI] [PubMed] [Google Scholar]
- 2.Johansson M, Borys F, Peterson H, Bilamour G, Bruschettini M, Jørgensen KJ. Addressing harms of screening—a review of outcomes in Cochrane reviews and suggestions for next steps. J Clin Epidemiol 2021;129:68-73. Epub 2020 Oct 1. [DOI] [PubMed] [Google Scholar]
- 3.InformedHealth.org . What is overdiagnosis? Cologne, Germany: Institute for Quality and Efficiency in Health Care; 2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430655. Accessed 2023 Aug 23. [Google Scholar]
- 4.Harris RP, Wilt TJ, Qaseem A; High Value Care Task Force of the American College of Physicians . A value framework for cancer screening: advice for high-value care from the American College of Physicians. Ann Intern Med 2015;162(10):712-7. [DOI] [PubMed] [Google Scholar]
- 5.Canadian Task Force on Preventive Health Care . Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ 2017;189(36):E1137-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell KJL, Nijsten T. Melanoma overdiagnosis: why it matters and what can be done about it [comment]. Br J Dermatol 2022;187(4):459-60. Epub 2022 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawada T, Sugimoto T, Kawakatsu H, Matsumura T, Matsuda Y. Mass screening for neuroblastoma in Japan. Pediatr Hematol Oncol 1991;8(2):93-109. [DOI] [PubMed] [Google Scholar]
- 8.Ioka A, Inoue M, Yoneda A, Nakamura T, Hara J, Hashii Y, et al. Effects of the cessation of mass screening for neuroblastoma at 6 months of age: a population-based study in Osaka, Japan. J Epidemiol 2016;26(4):179-84. Epub 2015 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med 2014;371(19):1765-7. [DOI] [PubMed] [Google Scholar]
- 10.Topstad D, Dickinson JA. Thyroid cancer incidence in Canada: a national cancer registry analysis. CMAJ Open 2017;5(3):E612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oke JL, Brown SJ, Senger C, Welch HG. Deceptive measures of progress in the NHS long-term plan for cancer: case-based vs. population-based measures. Br J Cancer 2023;129(1):3-7. Epub 2023 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attribution 4.0 international. Mountain View, CA: Creative Commons. Available from: https://creativecommons.org/licenses/by/4.0/#. Accessed 2023 Oct 12. [Google Scholar]
- 13.Dickinson JA, Thériault G, Grad R, Bell NR, Szafran O. Assessing new screening tests. Panacea or profligate? Can Fam Physician 2022;68:815-22 (Eng), e310-7 (Fr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ 2013;347:f3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pattacini P, Nitrosi A, Giorgi Rossi P, Iotti V, Ginocchi V, Ravaioli S, et al. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the Reggio Emilia tomosynthesis randomized trial. Radiology 2018;288(2):375-85. Epub 2018 Jun 5. [DOI] [PubMed] [Google Scholar]
- 16.Thériault G, Grad R, Dickinson JA, Singh H, Antao V, Bell NR, et al. Beware of overdiagnosis harms from screening, lower diagnostic thresholds, and incidentalomas. Can Fam Physician 2023;69:97-100 (Eng), e33-7 (Fr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Little J, Barbeau P, Stevens A, Beck A, Skidmore B, et al. Breast cancer screening: part A. An evidence report to inform an update of the Canadian Task Force on Preventive Health Care 2011 guideline. Ottawa, ON: Public Health Agency of Canada; 2017. Available from: https://canadiantaskforce.ca/wp-content/uploads/2019/02/Systematic-Review-Evidence-Report_v2_FINAL.pdf. Accessed 2023 Aug 23. [Google Scholar]
- 18.Klarenbach S, Sims-Jones N, Lewin G, Singh H, Thériault G, Tonelli M, et al. Recommendations on screening for breast cancer in women aged 40-74 years who are not at increased risk for breast cancer. CMAJ 2018;190(49):E1441-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad V, Lenzer J, Newman DH. Why cancer screening has never been shown to “save lives”—and what we can do about it. BMJ 2016;352:h6080. [DOI] [PubMed] [Google Scholar]
- 20.Swartz AW, Eberth JM, Josey MJ, Strayer SM. Reanalysis of all-cause mortality in the U.S. Preventive Services Task Force 2016 evidence report on colorectal cancer screening. Ann Intern Med 2017;167(8):602-3. Epub 2017 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caird H, Simkin J, Smith L, Van Niekerk D, Ogilvie G. The path to eliminating cervical cancer in Canada: past, present and future directions. Curr Oncol 2022;29(2):1117-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva, Switz: World Health Organization; 1968. [Google Scholar]
- 23.Dobrow MJ, Hagens V, Chafe R, Sullivan T, Rabeneck L. Consolidated principles for screening based on a systematic review and consensus process. CMAJ 2018;190(14):E422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]