Abstract
Tiny flying insects, such as Drosophila melanogaster, fly by flapping their wings at frequencies faster than their brains are able to process. To do so, they rely on self-oscillation: dynamic instability, leading to emergent oscillation, arising from muscle stretch-activation. Many questions concerning this vital natural instability remain open. Does flight motor self-oscillation necessarily lead to resonance—a state optimal in efficiency and/or performance? If so, what state? And is self-oscillation even guaranteed in a motor driven by stretch-activated muscle, or are there limiting conditions? In this work, we use data-driven models of wingbeat and muscle behaviour to answer these questions. Developing and leveraging novel analysis techniques, including symbolic computation, we establish a fundamental condition for motor self-oscillation common to a wide range of motor models. Remarkably, D. melanogaster flight apparently defies this condition: a paradox of motor operation. We explore potential resolutions to this paradox, and, within its confines, establish that the D. melanogaster flight motor is probably not resonant with respect to exoskeletal elasticity: instead, the muscular elasticity plays a dominant role. Contrary to common supposition, the stiffness of stretch-activated muscle is an obstacle to, rather than an enabler of, the operation of the D. melanogaster flight motor.
Keywords: insect flight, self-oscillation, stretch-activated muscle, Drosophila
1. Introduction
The flight motors of dipterans, such as Drosophila spp., are fascinating natural examples of self-oscillation. These motors are complex set of muscles, neural control systems and exoskeletal structures—including wings, halteres and transmission mechanisms—that together generate controlled and powered flight. The primary flight muscles within these motors are remarkable in that they are asynchronous: they are subjected to neural activation as infrequently as every 40th wingbeat [1]. In the intervening periods, the muscles continue to oscillate, and the wings continue to beat, through the phenomenon of stretch-activation: the non-neural delayed activation of asynchronous muscles in response to changing strain [1–3]. The dipteran flight motor, under stretch activation, is a system that converts a form of energy that has no inherent periodicity—chemical energy, in adenosine triphosphate (ATP)—into periodic oscillation. As such, it is a dynamically unstable, self-oscillating system [4,5].
Early on during the study of stretch-activation in insect flight motors, connections were made between motor self-oscillation and motor resonance. The landmark experiments of Machin and Pringle [4], on asynchronous muscles attached to a lightly-damped load system, demonstrated characteristics of the self-oscillatory response that would suggest alignment with the system natural and/or resonant frequency [6]. Subsequently, self-oscillation and resonance have become blended terms in the literature [7–9], and the dipteran flight motor is widely depicted as resonant [10–12]. However, a remarkable feature of the results of Machin and Pringle [4] is now seldom noted: in a general system, there is no particular reason why self-oscillation and resonance should align [13]. Indeed, both older [14] and recent [5] analyses of insect self-oscillation models indicate that convergence to a resonant frequency is not a guaranteed property of the motor system. The identification of multiple resonant frequencies within even simple models of the dipteran flight motor—frequencies split by damping and by the distribution of stiffness/elasticity [6]—adds further colour to this picture. Is self-oscillation still resonant under in vivo heavy damping conditions? [12] If so, resonant in what sense? [6] And what, indeed, is the quantitative mechanism by which motor self-oscillation arises from stretch-activation? [15]
This work seeks to answer these questions. Using literature-reported ex vivo data from D. melanogaster asynchronous flight muscles, data-driven models of the D. melanogaster wingbeat and flight motor, and several novel analysis techniques, we characterize the self-oscillation behaviour of this dipteran's flight motor. In §§2 and 3 we develop data-driven models of the wingbeat and muscular behaviour, respectively, using a new method for characterizing muscular impulse-response data. In §4 we analyse these motor models using symbolic computation, and encounter a fundamental and pervasive paradox: self-oscillation at observed wingbeat amplitudes should not be possible. This paradox holds irrespective of the motor model; motor parameters, including exoskeletal stiffness distribution, exoskeletal damping and quantity of muscle; and the muscular data source. Reported muscular ex vivo behaviour—from both sinusoidal and strain rate impulse response testing—is simply too stiff to match wingbeat requirements. As such, these results challenge the assertion that the high stiffness of asynchronous flight muscles is a feature enabling motor self-oscillation [10,16,17]: in contrast, this stiffness is an obstacle to self-oscillation in heavily-damped insects such as D. melanogaster. They also qualify the relationship between self-oscillation and resonance. In §5 we explore this relationship, and both confirm and extent the conclusions of Machin and Pringle [4]. For lightly-damped models of the dipteran flight motor, self-oscillation is resonant with respect to the thoracic exoskeleton. However, for in-vivo levels of motor damping, resonant behaviour is probably driven by the elasticity of the muscles. Altogether, as we discuss finally in §6, our results provide new insight into the dynamical behaviour of flight muscles: the connection, and distinction, between self-oscillation and resonance in fully integrated models of the dipteran flight motor and musculature. And the new analysis techniques that we develop and apply, including symbolic computation routines, and methods for the analysis of impulse response data, open up avenues for wider meta-analyses of flight motors across multiple species. The prospects for further integrated studies—and for a final answer to the question of whether dipteran flight motors really are resonant—are positive.
2. Modelling flight motor oscillation
2.1. Modelling flight motor dissipation
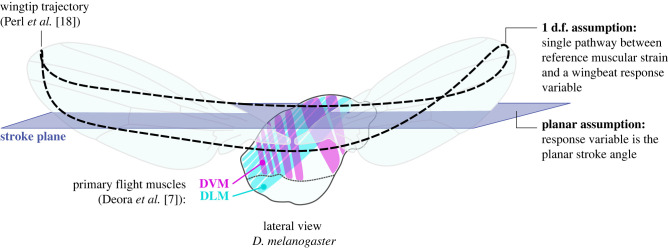
The study of flight motor self-oscillation is predicated on a model of the motor as an oscillator. In constructing such a model several assumptions are typically made (figure 1). First, the single-degree-of-freedom (1 d.f.) assumption—the treatment of a 1 d.f. drivetrain pathway between a reference muscular strain and a wingbeat kinematic variable [12,15]. The presence of series elasticity—between the muscles and each wing—may technically extend the model to 2 d.f. [6,15], but 1 d.f. analyses remain possible. The 1 d.f. models represent reductions of the real continuum mechanics of the exoskeletal structure [19]. Making the 1 d.f. assumption typically involves the assumption of a rigid wing [12], though these assumptions are properly distinct. There is no preclusion of the use of wing deformation for aerodynamic modelling in a 1 d.f. model, cf. [20,21]; and the elastic effect of wing deformation could be included to first order via series elasticity [6,15]. Second, the planar assumption—the analysis of the wingbeat stroke angle as the primary d.f. for muscular forcing [12,15]. The planar assumption also does not preclude the use of non-planar wingbeat kinematics for aerodynamic modelling. It posits only that out-of-plane kinematics do not impact the forcing requirements of the primary flight muscles—either as comparatively insignificant components of muscular power consumption [22,23], or as generated by passive non-muscular effects [24,25].
Figure 1.
Schematic of the flight motor of D. melanogaster, based on [7,18], with 1 d.f. and planar oscillator modelling assumptions illustrated.
Finally, there is the broad question of model fidelity. The simplest conceivable oscillator model is the parallel-elastic linear oscillator [6,11],
| 2.1 |
with canonical parameters ω0, the structural natural frequency; and ζ, the damping ratio. With appropriate definition of force (F, f), the kinematic variable x can be taken either as the wingbeat stroke angle (ϕ), muscular strain (ε), or, a normalized reference (e.g. − 1 ≤ x ≤ 1, for normative hovering flight). We note in passing that the formulation in damping ratio, ζ, implies that dissipation scales with structural stiffness (ω0), which is unrealistic for aerodynamic dissipation. The formulation in damping time constant d is probably more suitable for insect flight motors.
A key deficiency in the linear model (equation (2.1)) is that wingbeat aerodynamic dissipation is certainly nonlinear [26]. A more realistic model is the nonlinear formulation of quadratic damping, i.e. aerodynamic drag [5,26],
| 2.2 |
with quadratic damping parameter β. To improve model fidelity further, exoskeletal structural damping [27,28] can be added. This requires a mixed time-frequency domain model,
| 2.3 |
where γ is the structural damping ratio, or loss tangent [29], and denotes the Fourier transform. A mixed time-frequency formulation is required because FK,γ is non-causal [30,31]: in the time domain, FK,γ(t) depends on both past and future—and, as such, only approximates a real, causal structure. More realistic models typically involve fractional-order terms of the form K(iΩ)γ, preserving causality and roughly constant damping with frequency [3,30].
More widely, frequency-domain analysis is a powerful tool for characterizing motor self-oscillation. Equations (2.1)–(2.3) permit frequency-domain representations in some form. Denoting and , the linear model (equation (2.1)) permits the exact representation
| 2.4 |
The quadratic model (equation (2.2)) requires approximation. If the response, x(t), is simple-harmonic, , approximating observed D. melanogaster wingbeat kinematics [12,32], then an approximate representation is
| 2.5 |
where the quadratic damping ratio c is dependent on amplitude . A derivation is given in the electronic supplementary material (§1), alongside the relations for a peak-damping approximation; for an average-damping approximation. In general, the window is a suitable approximation of by c.
The quadratic damping ratio c has several interesting properties. It is dimensionless: the ratio of peak forces arising from dissipative and inertial effects, both scaling with Ω2 [26]. As such, it is the inverse of the Weis-Fogh number (NWF) characterized by Lynch et al. [15]: the relation c = 1/NWF allows a translation from reported Weis-Fogh numbers. Going further, the relationship between quadratic and linear damping ratios, c and ζ, is 2ζω0 = cΩ. If ω0 ≈ Ω—either as a reference frequency or an assumed motor operating point [6]—then c ≈ 2ζ, allowing rough translation from reported motor linear damping ratios [6]. Finally, while equation (2.5) as presented looks like the Fourier transform of a linear time-domain model, we suspect it to be non-causal (cf. equation (2.3)).
2.2. Modelling flight motor elasticity
In addition to differing models of dissipation, differing models of elasticity are also available. For one, both parallel and series elasticities might be present in the flight motor [15,33]. Introducing series elasticity to the quadratic model, equation (2.2), leads to the hybrid system
| 2.6 |
where Kp and Ks are parallel and series stiffnesses, respectively, and u is the muscular d.f., now distinct from x. Equation (2.6) is expressible in the frequency domain. With canonical parameters , , β = D/M, and f = F/M [6]; and the approximation window ,
| 2.7 |
Equation (2.7) reduces to pure series elasticity for ω0,p = 0, and pure parallel elasticity for ω0,s → ∞. To avoid the limit → ∞, the stiffness ω0,s may be reparametrized by a compliance parameter α, for instance, as ω0,s = ω0,p/α; cf. [6].
There is also the general question of elastic nonlinearity: thoracic exoskeletal structures from several insect species have been observed to show strain-hardening nonlinearity [27,34]. An analysis in the electronic supplementary material (§2), illustrates how a cubic-type elasticity can be accounted for in a linear model of the form of equation (2.5), within an appropriate approximation window. As such, equation (2.5) can account for both cubic stiffness and quadratic damping: it is a highly general model, and will be the normative (but not exclusive) model used in this study.
2.3. Identifying motor model properties
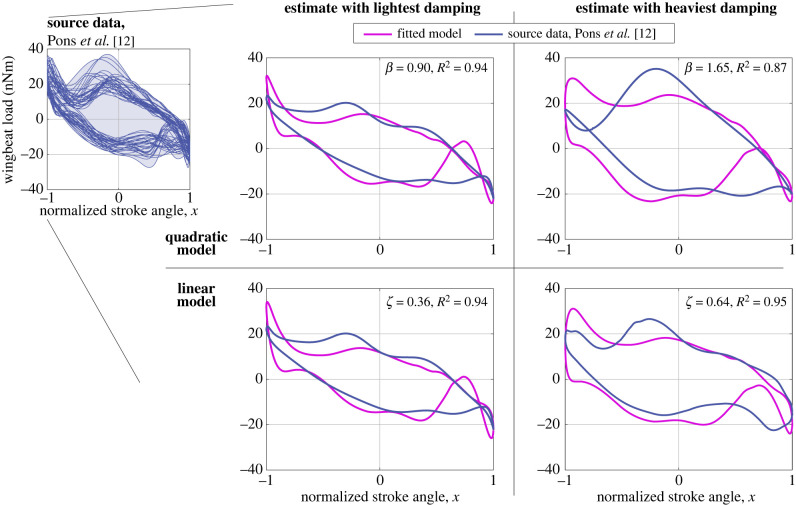
The models of §§2.1 and 2.2 require parameter values: in particular, dissipative parameters c, β and/or d, which we identify from the D. melanogaster meta-dataset of Pons et al. [12]. This dataset contains wingbeat load requirement profiles, meta-analysed from a range of reported experimental and computational data. For parameter identification, the inelastic loads [35] of the linear and quadratic aerodynamic dissipation models, equations (2.1) and (2.2), can be expressed as
| 2.8 |
where Sd and Sβ are scale factors, and − 1 ≤ x ≤ 1 for normative hovering flight. We consider the linear model to enable comparisons with the classical results of Machin and Pringle [4]. Using least-squares fitting we identify {Sd, d} and {Sβ, β} for all load requirement profiles associated with biological wingbeat kinematics. Figure 2 illustrates these fits for the most heavily and most weakly damped profiles in the meta-dataset; in the electronic supplementary material (§4), fit parameters are given for every profile. Across the meta-dataset, d ∈ [0.99, 1.76] · 103 rad s−1, mean d = 1.38 · 103 rad s−1; and β ∈ [0.90, 1.65], mean β = 1.25. Due to differing conditions across the meta-dataset, including in wing geometry and kinematics, the variation in damping values is relatively wide, spanning ±35% of the mean. However, the fit accuracy is good: R2 > 0.9 for all profiles except those using the aerodynamics of Muijres et al. [36], which show R2 down to 0.83 (electronic supplementary material, §4). Using the approximation window , with , we may estimate the quadratic damping ratio as c ∈ [0.70, 1.65], mean c = 1.11, for normative hovering flight. For comparison, this is a Weis-Fogh number ranging over NWF ∈ [0.60, 1.42], or, if ω0 = Ω for the linear model, a linear damping ratio ranging over ζ ∈ [0.36, 0.64], mean ζ = 0.50.
Figure 2.
Motor models fitted to source data for D. melanogaster—quadratic (β) and linear (d, ζ) models, illustrated for the most lightly damped (β = 0.90) and most heavily damped (β = 1.65) load requirement work loops in the source meta-dataset of Pons et al. [12]. The linear model is expressed in ζ rather than d for ease of interpretation.
3. Modelling asynchronous muscle stretch-activation
3.1. Modelling asynchronous muscle dynamics
In the motor models of equations (2.1)–(2.7), the forcing, F or f, is that of the flight muscles. In dipterans, this includes both the primary flight muscles—the sets of asynchronous and antagonistic dorsoventral (DVM) and dorsolongitudinal (DLM) muscles (figure 1)—and an array of steering muscles. Neglecting steering muscles, which account for less than 3% of total muscle mass [37], we may view asynchronous muscle forcing through a viscoelastic lens. At low strain amplitudes, the muscle may be modelled as having a frequency-dependent complex modulus, : the ratio of muscular stress (σ) to strain (ɛ), with real-valued storage (E) and loss (H) moduli components [3,38],
| 3.1 |
The storage modulus represents how elastic the muscle is (or, appears to be): the effective spring stiffness of the forces it generates. The loss modulus represents how much power it produces (or dissipates): its effective damping. When the loss modulus is negative, the muscle produces power, and does work on the motor to move the wings. With this in mind, we may express in terms of the viscoelastic negative loss tangent (r) [29],
| 3.2 |
related also to the phase offset of muscular forcing, δ, as r = −tan δ [29].
Equations (3.1) and (3.2) are the basis for a model of muscular forcing, f(t). First, if the DVM and DLM operate as an exactly antagonistic pair (180° phase offset), then this pair resolves to a single equivalent . Then, in the frequency domain, , where the constant of proportionality accounts for muscular cross-sectional area and an assumed linear kinematic relationship between muscular strain (ɛ) and the wingbeat kinematic variable (x or u). As such,
| 3.3 |
where NE and NM(Ω) are undetermined constants of proportionality. These constants will be identified during the analysis; however, as detailed in the electronic supplementary material (§3), we may also estimate NE for D. melanogaster as
| 3.4 |
given reported muscle length (Lmuscle), total cross-sectional area (Amuscle) and strain amplitude (); and wing inertia (Iwing) and stroke amplitude (, ±, in rad).
Specific forms of for D. melanogaster are well-established. Two key forms are those of Kawai and Brandt [39], applied to insect flight muscle by Sicilia & Smith [2]; and the modified form, specific to insect flight muscle, used by Maughan et al. [3] and others [38,40]. Both these forms model three muscular processes, denoted A, B and C, as:
| 3.5 |
where our b and c correspond to 2πb and 2πc in [38,40]. Process A represents the viscoelasticity of passive structures in the muscle [3,41]. The fractional-order term A(iΩ)k, k ∈ [0, 1] preserves both causality and roughly constant damping with frequency; cf. [30,31]. Processes B and C arise from muscular activation. The conversion of chemical energy (ATP) to mechanical work during activation occurs through the cross-bridge cycle [41]—the cyclic interaction between myosin- and actin-containing filaments—and this cycle is associated with changes in complex moduli. Process B is a work emitting process, and Process C a work absorbing process [3,38,39]. For both model types, process parameters can be identified in ex vivo empirical results for both steady and transient response testing. Indeed, muscle behaviour as per equation ((3.5) can be seen as velocity feedback: a transfer function, , between stress and strain rate, identifiable from strain-rate impulse response data [42,43]. In addition, we note that, while nonlinearity in stretch-activated muscles is known to occur [44,45], connecting nonlinear model formulations [2,14] with observed behaviour is difficult.
Combining the muscle models of equations (3.1)–(3.5) with the motor models of §2.2 leads to frequency-domain characteristic equations for the motor—equations describing a free (unforced) system, in which self-oscillation may emerge. For instance, the quadratic model (equation (2.8)) under general complex modulus excitation (equation (3.3)), has the characteristic equation,
| 3.6 |
a combination which we will use as a normative model. Analysing characteristic equations, such as equation (3.6), can proceed in a forward or an inverse direction: analysing the response (Ω) for given structural properties (c, ω0, NE, …), or the structural properties required to effect a given response, respectively. We will pursue both analysis directions over §4.
3.2. Identifying muscle model properties
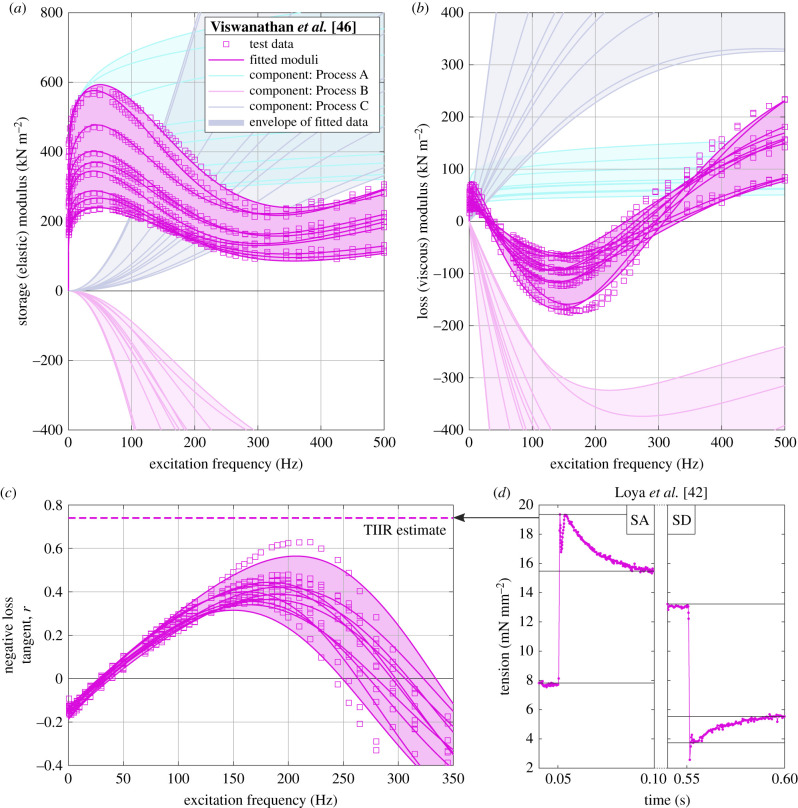
Parameters in equation (3.5) can be fitted to ex vivo muscular testing data [42–44]. In this study, we use data from two independent sources: firstly, and principally, the complete wild-type D. melanogaster sinusoidal testing dataset reported by Viswanathan et al. [46]. This dataset is composed of test data and fitted parameters for 10 muscle fibre sinusoidal tests, carried out in solutions of suitably high-concentration ATP (the muscular energy source); and concentrations of calcium (the ion triggering activation) at which power output is maximized [38,46]. Figure 3 illustrates this dataset, alongside the reported fitting to the Maughan-type model, in moduli and negative loss tangent. Reported fit parameter values range over A ∈ [132, 373], k ∈ [0.088, 0.112], B ∈ [647, 4061], b ∈ [1393, 2032], C ∈ [651, 3921] and c ∈ [2382, 3091]. As can be seen in figure 3, moduli estimates are reasonably widely spread, varying by a factor of 2×. Estimates of the peak negative loss tangent range over 0.3 to 0.6, consistent with other studies of D. melanogaster indirect flight muscle (table 1). The calculations behind the estimates of table 1 are given in the electronic supplementary material (§5).
Figure 3.
Ex vivo muscular data, leading to estimates of muscular negative loss tangent, r. Sinusoidal testing dataset from Viswanathan et al. [46], with associated fit and process-wise composition (Processes A, B, C): (a) storage modulus, (b) loss modulus, (c) negative loss tangent. (d) Strain rate impulse response data for stretch-activation (SA) and shortening-deactivation (SD) from Loya et al. [42], with associated time-independent impulse-response (TIIR) estimate of r overlaid on (c). This TIIR estimate is the largest among the impulse-response data for D. melanogaster surveyed in table 1.
Table 1.
Estimates of peak r for D. melanogaster indirect flight muscle.
| dataset | estimated peak r |
|---|---|
| sinusoidal testing: | |
| Trujillo et al. [47] | 0.48 |
| Yang et al. [41] | 0.32 |
| Barton et al. [48] | 0.16 |
| Henkin et al. [49] | 0.31 |
| Dickinson et al. [40] | 0.38 |
| Warmke et al. [50] | 0.55 |
| strain rate impulse response (TIIR): | |
| Loya et al. [42] | 0.74 |
| Glasheen et al. [51] | |
| Swank solution | 0.46 |
| Reedy solution | 0.26 |
| Wang et al. [52] | |
| 1% strain step change | 0.46 |
| 2.5% strain step change | 0.39 |
Secondly, we use the impulse-response characterization of the dual phenomenon of stretch-activation (SA) and shortening-deactivation (SD, i.e. SA upon contraction) in wild-type D. melanogaster flight muscle reported by Loya et al. [42]. It is possible to fit equation (3.5) directly to this impulse-response data; cf. [5], but raw data and the full set of fitted rate constants (b, c, …) are rarely reported [42]; and amplitude constants (B, C, …) are typically unreported. For this reason, we develop our own time-independent impulse-response (TIIR) estimates of the peak r, based on only a few reported SA/SD measurements—accounting for the directionally nonlinear distinction between SA and SD. Details of these estimates are given in the electronic supplementary material (§6). The TIIR estimate for the single pair of SA/SD profiles reported by Loya et al. [42], is peak r ≈ 0.74 (figure 3c, table 1). The TIIR process is also applicable to other impulse-response datasets in the literature: the electronic supplementary material (§6) presents TIIR calculations for the SA-only data of Glasheen et al. [51], under two different muscle bathing solutions, and of Wang et al. [52], under two different strain step changes. Estimated peak r values for these datasets are shown in table 1. There is broad consistency between estimates of r across sinusoidal testing and impulse response datasets, with no estimate exceeding r ≈ 0.74 [42]. We note, however, that these estimates are coarse: in particular, TIIR estimates are likely to represent overestimates of the peak r for their respective datasets; but also, do not account for nonlinearities that may make muscular oscillation differ from step response behaviour [53]. The value of the TIIR process is that it allows conservative estimates of maximum r—a crucial parameter for our analysis over §§4 and 5—to be made using only low-precision graphs of strain rate impulse response, which are unsuitable for detailed fitting to viscoelastic models such as equation (3.5).
In general, these peak r estimates are for muscular strain amplitudes () lower than those anticipated in vivo. In D. virilis tethered flight, these amplitudes average ±1.8% [54], though these estimates are coarse, and apparently inconsistent with the observation that D. melanogaster flight muscle generates maximum power at amplitudes as low as ±0.4% [52]. Nevertheless, Viswanathan et al. [46] prescribe an amplitude of only ±0.0625%; Loya et al. [42], an effective ±0.5%. These low strain amplitudes are a key limitation of this study: both because a complex-modulus model may break down at high amplitudes [44,45], and because the parameters of any model may change between low and high amplitudes. Model breakdown can be overcome, to a degree, via direct analysis of work loop data (§4.2); but parameter variation remains unclear. Only a TIIR estimate derived from Wang et al. [52] reaches clearly biological levels of ±1.3%, and while this estimate is consistent (peak r ≈ 0.39), nonlinearities with respect to strain amplitude could still lead to qualitatively different behaviour at high strain amplitudes.
4. Conditions for motor self-oscillation
4.1. Self-oscillation conditions for parallel-elastic systems
We begin by considering a forward-problem approach to the normative D. melanogaster motor model, equation (3.6). In this initial analysis, we take the stiffness parameter (NM) and negative loss tangent (r) to be given constants—in theory, their values at the motor self-oscillation frequency, which is as yet undetermined. In practice, r is confined to a narrow window: between 0 and 0.6 for D. melanogaster motor operation at any frequency (figure 3). NM we take as unknown. As such, the motor characteristic equation can be expressed
| 4.1 |
and solved for the complex-valued operating characteristic, Ω. In general, Ω has two solutions: , only one of which is physical. The real part, , is the response frequency—only the solution branch with positive real part, denoted Ω+, is physical. The imaginary part, , is the response dissipation factor. represents divergent oscillation; convergent oscillation; and critical self-oscillation: the steady operation of an insect flight motor.
Equation (4.1)'s solution in Ω is
| 4.2 |
which can be reformulated as
with
| 4.3 |
These expressions are derived with symbolic computation in MATLAB-MuPAD [55]—see the Data accessibility statement.
Equation (4.3) gives the conditions for self-oscillation to manifest. Locating , we compute a condition for critical self-oscillation in terms of a critical quadratic damping factor, c = ccrit
| 4.4 |
We may confirm with symbolic computation that, under any NM, r and ω0 > 0, if c < ccrit the system will diverge, and if c > ccrit, it will converge. If then, we have a motor with given peak r (a muscle of fixed properties) but with NM and ω0 that we could hypothetically control, then the maximum possible c that we could self-excite would be
| 4.5 |
That is to say, it is not possible to self-excite a system with c > r under any conditions—no matter the exoskeletal stiffness/elasticity (ω0) or the quantity of muscle (NM). This is a remarkable limit on the kinds of flight motor for which self-oscillation is possible. And it is a limit that forms the beginnings of a paradox—given that reported r values (0–0.6, §3.2) and c-values (0.9–1.65, §2.3) apparently do not obey this limit.
4.2. The self-oscillation paradox in Drosophila melanogaster
To generalize the analysis of §4.1 to frequency-dependent moduli, we transition to an inverse-problem analysis. If the motor is specified to self-oscillate steadily at a given frequency Ω = Ω*, then the characteristic equation for operation of our normative motor model at this state is
| 4.6 |
where E(Ω*) and H(Ω*) are given by the moduli models in §3.2. The unknown variables are the level of dissipation (c) and exoskeletal elasticity (ω0) in the motor, and the muscle factor (NE) required for steady self-oscillation. If we are interested in the maximum dissipation (cmax) at which the motor is capable of self-oscillation, then, we may solve this inverse problem for the critical muscular factor (NE,crit) and dissipation (ccrit) consistent with self-oscillation,
| 4.7 |
and thus
| 4.8 |
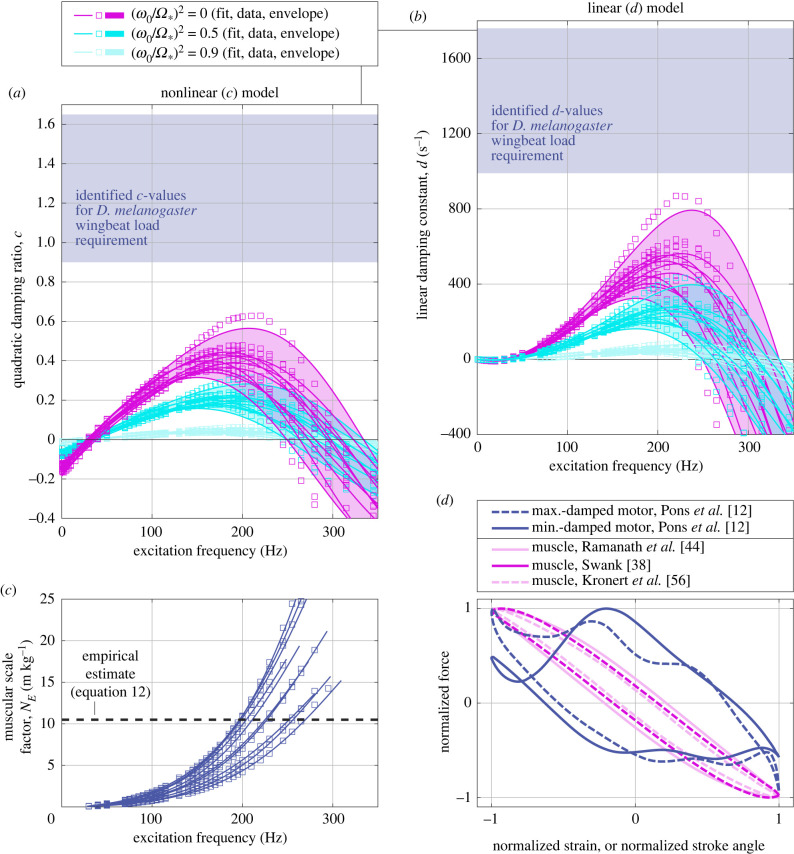
Equation (4.8) has three implications. First, the motor never oscillates at below the exoskeletal natural frequency, ω0: the presence of muscular elasticity, NE,critE(Ω*), necessitates that Ω* > ω0. Second, exoskeletal elasticity does not reduce the burden on the muscles: the quantity of muscle required to self-oscillate a given level of damping, measured as NE,crit/ccrit, is constant w.r.t. ω0. Third, consistent with §4.1, ccrit decreases with exoskeletal elasticity ω0, and reaches maximum cmax = r(Ω*) at ω0 = 0. As such, cmax = r(Ω*) is a hard limit on the motor's capability for self-oscillation. If we track ccrit over Ω* for the muscular data of Viswanathan et al. [46], we observe that it is impossible for D. melanogaster flight muscle to excite a motor with dissipation greater than c ≈ 0.6 (figure 4a); or, in the best-case TIIR estimate, c ≈ 0.74.
Figure 4.
The self-oscillation paradox in the flight motor of D. melanogaster. (a) Critical levels of damping for the nonlinear model, ccrit, as a function of the exoskeletal stiffness, (ω0/Ω*)2, and compared with estimates of c for the flight motor (figure 2). (b) Critical levels of damping for the linear model, dcrit, as a function of the exoskeletal stiffness, compared with estimates of motor d (figure 2). (c) The critical muscular scale factor, NE,crit, required for self-oscillation under ω0/Ω* = 0, compared with the estimate of NE from motor properties (equation (3.4)). Note that the ratios NE,crit/ccrit and NE,crit/dcrit are independent of ω0/Ω*. (d) The self-oscillation paradox illustrated in empirical nonlinear data: work loops for the motor [12] and muscle [38,44,56], illustrating the difference in relative damping.
This limit presents a paradox: by several estimates, the level of dissipation in D. melanogaster during normative hovering flight exceeds this limit significantly. Figure 4 illustrates this paradox in three different ways: in the inconsistency between the maximum quadratic damping that the muscle and exoskeletal transmission can cause to self-oscillate, and the actual quadratic damping model for the wingbeat (figure 4a); in the analogous inconsistency for linear damping (figure 4b); and in a direct inconsistency of work loops for muscular forcing and wingbeat load requirements (figure 4d). The level of elasticity, i.e. level of tilt, in these loops is inconsistent and cannot be made consistent by any scale factor (NE, NH—these do not alter the ellipse tilt); nor by any addition of exoskeletal elasticity (ω0—this exacerbates the inconsistency). Forcing and loading are fundamentally inconsistent.
4.3. The self-oscillation paradox across motor models
One question of immediate relevance is whether this paradox holds across different motor models—representing different physical configurations and effects that may be present within the motor. Is it simply a feature of our selected model? We show that it is not, by applying the same symbolic computation-based forward and/or inverse analyses as in §§4.1 and 4.2 to the differing models of motor dissipation and elasticity described in §§2.1 and 2.2. The details of this process are given in the electronic supplementary material (§7)—to summarize:
First, the addition of exoskeletal structural damping (equation (2.3)) reduces the level of aerodynamic damping that is permissible while maintaining self-oscillation. Exoskeletal damping, certainly present at some level within the D. melanogaster flight motor, makes self-oscillation even more difficult.
Second, the scaling of aerodynamic damping in the motor may not be exactly quadratic, given the low Reynolds number of operation (approx. 100) [12]. As an example of lower-degree scaling, if damping is taken to be linear (d, equation (2.1)), then, under equivalent forward and inverse formulations,
| 4.9 |
In contrast with cmax, dmax does scale with muscle mass NM—though this entails an increase in self-oscillation frequency, or Ω*. In theory, if r > 0 can be maintained, then it is always possible to out-scale linear damping with muscular forcing. However, apart from the relatively unrealistic scaling of the linear model, D. melanogaster flight muscle shows r > 0 only up to roughly 300 Hz (figure 3). Over this window, estimates of d for D. melanogaster again exceed dmax significantly (figure 4b).
Third, the distribution of elasticity across the flight motor may not be exactly parallel. Series elasticity (equations (2.6) and (2.7)) can arise from the flexibility of the wing hinge [15,33] and local modes of deformation within the exoskeleton [6]; and could act as a first-order approximation of the elastic effects of distributed wing flexibility—though the deformation observed in D. melanogaster wings during flight is relatively minimal [32,57]. We can account for these effects via a hybrid parallel-series model of the flight motor (equation (2.7)). Such a model has critical quadratic damping,
| 4.10 |
under a forward analysis. For reference, a plot of this function is given in the electronic supplementary material (§7). From equation (4.10), positive α and ν can only reduce ccrit, and cmax = r. By the same token, linearized strain-hardening effects in any elastic term do not increase cmax, as cmax occurs when exoskeletal elasticity is absent (ν = α = 0). Wing hinge flexibility and other series-elastic effects cannot alter the cmax = r self-oscillation limit—a limit which now appears fundamental to a range of linearized oscillators.
Fourth, there is only one form of linearized elasticity that could resolve the self-oscillation paradox. Unstable elasticity, , as could arise within the well of a bistable mechanism within the thoracic transmission, could enable ccrit > r. Estimates of the factor by which observed D. melanogaster damping (cobs) exceeds peak muscular capability (robs, from Viswanathan et al. [46]) range from cobs/robs = 1.4 in the best case, to 4.7 in the worst case (figure 4a). The levels of parallel instability (in equation (4.4) and (4.8)) generating these factors are
| 4.11 |
a measure which varies from 0.30 to 0.79 across best-worst cases. Exoskeletal instability would have to be at least 30% as strong as effective elasticity of the muscles themselves to resolve the paradox. The only way to resolve the paradox simply by choice of model is to invoke instability, which is problematic in morphological terms, as we discuss further in §6.2.
5. Is motor self-oscillation resonant?
5.1. Excitation to effective energy resonance
In §4 we focused on the conditions for motor self-oscillation to emerge. A consequent topic concerns the frequency to which this self-oscillation converges, and how this frequency maps on to the resonant landscape of the motor [6]: its classical and/or energy resonant frequencies. Within the response frequencies, , of the motor models in §4, one central principle can be identified. The storage modulus, E(Ω), functions in a dynamical sense like elasticity—storing and releasing energy, or appearing to do so. If we treat E(Ω) as elasticity, we can divide any motor-muscle system into an effective system composed of the motor dynamics plus E(Ω); and an effective forcing, H(Ω). For any linearized motor dynamics
| 5.1 |
Then, if self-oscillation in the motor is simple-harmonic (§2), then it necessarily converges to the energy resonant frequency [6] of the effective dynamics, denoted ωe,eff. This is because the effective forcing is incapable of generating negative work and therefore can only generate effective energy resonant states
| 5.2 |
If the effective dynamics are parallel-elastic, with linear, quadratic or viscoelastic damping, the effective energy resonant frequency is the effective natural frequency—the natural frequency associated with the elasticity of the exoskeleton plus activated muscle; cf. [6]. In the hybrid systems, provided self-oscillation is simple-harmonic, this frequency will be the effective energy resonant frequency, computable from [6].1
5.2. Recovering and extending the results of Machin and Pringle [4]
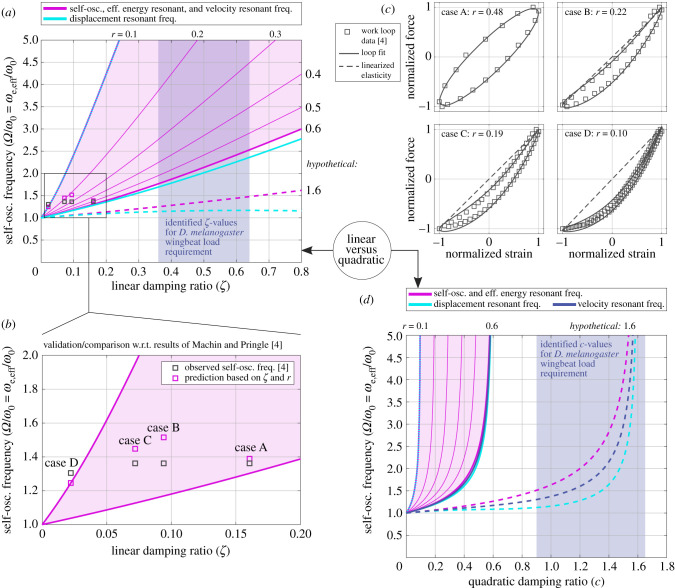
Excitation to effective energy resonance provides theoretical context to the classical ex vivo experimental results of Machin and Pringle [4]: observations of self-oscillation in an asynchronous basalar muscle from the Indian rhinoceros beetle Oryctes rhinoceros connected to an artificial parallel-elastic linear oscillator. We compare the observations in Cases A–D of fig. 8 in Machin and Pringle [4], with predictions in our modelling context—both as model validation, and to investigate the ways in which these ex vivo observations may break down in vivo. For each case, we extract reported muscular work loop data and estimate r, as detailed in the electronic supplementary material (§8). Computing also the artificial oscillator damping ratio, , from reported mass (M), stiffness (K) and damping coefficients (D), we predict the self-oscillation frequency Ω from the principle of excitation to effective energy resonance. By substituting (equation (2.4)) and the NM(Ω) forcing formulation (equation (3.3)) into equation (5.1), we may solve for Ω = ωe,eff, with ωe,eff being the natural frequency of combined exoskeletal () and muscular (NM) effects. That is
| 5.3 |
using the estimates of r from the recorded muscular work loops. The predictions of equation (5.3) match well the self-oscillation frequency actually observed by Machin and Pringle [4] (figure 5a,b). The errors observed (less than 15%) are explicable by factors such as uncertainty and non-ellipticity in the muscular work loops (figure 5c); and unmeasured damping phenomena (e.g. aerodynamic damping). The comparison in figure 5b validates our self-oscillation analysis against experimental data for linear oscillators excited by insect flight muscle.
Figure 5.
Relationship between self-oscillation frequency and effective resonant frequencies in linear and quadratic motor models, alongside the ex vivo experimental results of Machin and Pringle [4]. (a) Predictions of self-oscillation frequency for the linear model, alongside motor effective resonant frequencies; identified ζ for D. melanogaster; and Machin and Pringle's results. (b) Close-up of the comparison with Machin and Pringle's results, with Cases A–D indicated. (c) Fit results for the work loops recorded by Machin and Pringle, used to identify r for model prediction. (d) Predictions of self-oscillation frequency for the quadratic model, alongside motor effective resonant frequencies.
However, Machin and Pringle's [4] ex vivo data do not extend to the levels of damping associated with D. melanogaster hovering flight (figure 5a). We can perform this extension with the model: to ζ ≈ 0.6; a hypothetical r = 1.6 resolving the self-oscillation paradox; and separately, a quadratic damping model. The extension reveals ways in which these ex vivo results might not generalize to D. melanogaster in vivo. Two factors cause a breakdown: the level of damping, and its scaling with frequency. As damping increases, self-oscillation occurs at ascending frequencies—at hovering flight conditions, substantially higher than the exoskeletal natural frequency, ω0 (figure 5a). The nature of this frequency ascent is, however, dependent on how damping scales with frequency (figure 5d). This illustrates, following Ellington [26], the importance of precise models of frequency-based scaling of flight motor aerodynamic power consumption. The first key implication of figure 5a,d is that muscular self-oscillation may drive the system far from the exoskeletal natural frequency, ω0. In conditions representative of D. melanogaster, the frequency of self-oscillation is determined largely by muscular, not exoskeletal, effects.
Alongside this extension to in vivo conditions, we may also map the behaviour of other resonant frequencies within the motor effective dynamics. Following [6], we focus on displacement (ωdisp,eff) and velocity (ωvel,eff) resonant frequencies,
| 5.4 |
For the linear and quadratic model, these frequencies are
| 5.5 |
with
| 5.6 |
As illustrated in figure 5, under Machin & Pringle's [4] conditions, all these frequencies are closely aligned, and motor self-oscillation can be considered resonant in multiple senses. However, under the heavy quadratic damping more representative of D. melanogaster in vivo, resonant frequencies can be widely separated, and motor self-oscillation can show substantial (greater than 50%) deviation from classical resonance. The second key implication of figure 5a,d is that the conclusions of Machin and Pringle [4] break down in vivo: the D. melanogaster is not necessarily resonant in all senses of the term. It is probably energy resonant in the sense of effective energy resonance (§5.1) but is probably not resonant in the classical sense of peak transfer function response. We should note, however, that this result has two key limitations, discussed further in §6. First, the effective dynamics framework, equation (5.1), is an optimistic characterization of the muscle's elastic behaviour: its real elastic energy storage may differ [12], and effective energy resonance may not translate to real energy savings. Second, following Machin and Pringle [4], we assume that stable exoskeletal elasticity (ω0 > 0) is present, and that the self-oscillation paradox is resolved via a muscle with high negative loss tangent (r ≈ 1.6)—that is, a muscle with high power output and low stiffness. The alternative case of near-zero or unstable exoskeletal elasticity (ω0 ≤ 0) is not covered by this treatment, and may lead to different conclusions regarding the nature of resonance in the motor. As per §6, further data-driven analysis is needed to resolve the self-oscillation paradox in the dipteran flight motor before the nature of resonance in this motor can be precisely characterized.
6. Discussion and conclusion
6.1. Resolution of the self-oscillation paradox via muscular nonlinearity
The self-oscillation paradox is based on the inconsistency between observed muscular negative loss tangents, r, and reported wingbeat damping—quadratic damping ratio c, or other damping metrics. One resolution to the paradox is that either of these parameters are not representative of D. melanogaster in vivo. It is difficult to make a case that wing inertial forces are substantially greater, or wing drag forces smaller, than existing estimates [6,12,15]—the only realistic contributing factor in this direction is the possibility that distributed wing flexibility, which is not accounted for in the source aerodynamic models, reduces the drag load on the wing, and thus decreases c. To resolve the self-oscillation paradox, D. melanogaster wing flexibility would have to reduce drag forces (cΩ2) to below half of current estimates, maintaining roughly equivalent lift. The flexibility observed in D. melanogaster wings during flight is not large [32,57]; and existing studies of insectoid flexible flapping wings indicate reductions in drag-per-lift of up to approximately 20% in the very best cases [58–60]. As such, wing flexibility could contribute slightly to resolving the self-oscillation paradox in D. melanogaster, but does not offer a complete resolution.
However, there is a stronger case that estimates of r may be unrepresentative. It is possible that there are biochemical factors that cause the muscle to generate substantially more power in vivo, at the same stiffness; or the same power at lower stiffness due to differences in activation conditions—e.g. changes in role of elastic proteins [16] and/or the behaviour of the cross-bridge cycle [41]. It is also possible that active muscle is subject to strain-softening nonlinearity—decreasing stiffness with increasing strain—as observed in relaxed flight muscle [17]. Further data is needed on the nonlinear behaviour of activated muscle with respect to strain, as well as the actual strain amplitude of D. melanogaster in vivo. However, it should be noted that, while strain-based nonlinearities in general are known to be present [2,14,44,45], the very limited data available at high strain amplitude—a single TIIR estimate (table 1)—would suggest that significant increases in r are not occurring.
6.2. Resolution of the self-oscillation paradox via motor static instability
A second potential resolution concerns the fact that the condition c ≤ r for self-oscillation does not hold for exoskeletal unstable elasticity, or static instability. Contemplating exoskeletal instability recalls the outdated hypothesis of the dipteran click mechanism [61,62]; but an instability to resolve the self-oscillation paradox could be qualitatively different. It could be relatively weak: at minimum only 30% as strong as muscular effective elasticity (§5.2). As such, it might not generate observable static instability in the motor even when the muscles are inactive. It would also be required only at small scales: not necessarily in larger dipterans, and perhaps in small insects from other orders, such as ptilids [63]. Indeed, there is a general question of the scaling and generality of the self-oscillation paradox. If, as expected, the paradox resolves naturally (c ≤ r) at larger scales of insect, what is the threshold? And is the same paradox present in other insects at similar or smaller scales than D. melanogaster? Ptilids are a particularly relevant test case, as the bristled wings of these tiny beetles generate very low inertial forces—as low as 1% of aerodynamic forces [64]. This leads to an extremely dissipation-dominated wingbeat [63], i.e. c → ∞, which could only emerge under a flight muscle with no effective stiffness (r → ∞), or under exoskeletal static instability. Differences between ptilid and dipteran muscle architecture could be a confounding factor [65], but it appears insufficient to postulate a mechanism that only reduces the elasticity/stiffness of the motor system: this elasticity must be brought to zero.
There is also a further point here, alluding to §7.1. A different static instability is already present in the motor: the muscle cross-bridge dynamics. A notable feature of the A-B-C decomposition of muscular processes (§3.1) is that the cross-bridge cycle, Processes B and C, acts as static instability: reducing the effective elasticity of the muscle (figure 3). In reported data, the cross-bridge dynamics are not strong enough to drive the muscle into static neutral stability or instability, but if this effect was more powerful in vivo, or muscular structural elasticity (Process A) was weaker, this might occur. Cross-bridge dynamics are a further potentially nonlinear phenomenon to track with increasing muscle strain amplitude (§7.1)
6.3. Resonance as an emergent property of the flight motor
In §6, we leveraged self-oscillation as a tool to understand motor resonance, following the classical results of Machin & Pringle [4]. The self-oscillation paradox restricts this analysis: either to low wingbeat amplitudes, or to conjectural solutions to the paradox (e.g. r ≥ c). Solving the paradox and eliminating this restriction is an avenue for future research, but even within the current framework, we are able to make one conclusion with a high degree of confidence: that D. melanogaster is not resonant with respect to exoskeletal elasticity (§5.2). It might be resonant, in some sense, with respect to muscular elasticity, but details are unclear. The muscular storage modulus (E) is elastic-like, but it is optimistic to assume that it represents solely passive energy absorption [12,66]. Feedback-driven forcing functions can have a storage modulus, but store little or no energy: e.g. a delayed viscous force, , or the electronic implementation of muscular forcing devised by Lynch et al. [5]. In such cases, the appearance of a storage modulus is an aliasing effect [12]. Given the possibility of aliasing, the effective resonant frequencies studied in §5 may not actually be optimal: there is a need for a more pessimistic analysis of flight muscle behaviour.
As can be seen, there are several lines of analysis for future characterizations of flight motor resonance. These lines of analysis complement existing approaches that focus on alignment of experimentally observed frequencies [34]; or other features attributed to resonance [67]. Following [5,28], we study a third aspect of flight motor resonance: resonance as an emergent property of the dipteran flight motor, which can be characterized by modelling individual components of the flight motor (wing, exoskeleton, muscle, etc.); and studying the behaviour of these components as they interact. Such a characterization involves the development both of new component-specific data-driven modelling techniques, such as the TIIR process (§3.2); and of techniques for interactive or integrative analysis, such as symbolic computation (§4). Together, these techniques can drive classical experimental results in new directions: allowing us, for instance, to extend the classical results of Machin & Pringle [4] into conditions more representative of D. melanogaster in vivo.
There is also scope for further extensions of this approach, e.g. into forward [68], take-off [69] or manoeuvring [70] flight conditions. These conditions are likely to reflect interesting variations of the paradox: they show broadly similar levels of damping [69,70], or possibly decreased in the case of forward flight [68], but differences in inertial and dissipative loading raise the question of how the motor adapts to ensure load consistency and continued self-oscillation. Is this adaption entirely reflected in wingbeat kinematic changes, or do the structural properties of the motor adapt too? In this direction, the question of flight motor self-oscillation is connected to wider questions regarding the mechanisms of insect flight control. Remarkably, despite progress in the question of flight control [71], the decades-old question of whether the dipteran flight motor is actually resonant still remains unresolved. However, by integrating data across the flight motor, and leveraging new modes of analysis, answers are beginning to emerge.
Endnotes
There remain open questions in such systems—e.g. does the non-existence of an energy-resonant frequency in certain hybrid systems [6] imply the impossibility of self-oscillation?
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Code containing MATLAB-MuPAD symbolic computation proofs and processed results from source datasets are available on the Swedish National Data Service from: https://snd.gu.se/sv/catalogue/dataset/2023-205/1 [72]. Supporting analyses are provided in the electronic supplementary material [73].
Declaration of AI use
I have not used AI-assisted technologies in creating this article.
Author's contributions
A.P.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, writing—original draft, writing—review and editing.
Conflict of interest declaration
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Gordon S, Dickinson MH. 2006. Role of calcium in the regulation of mechanical power in insect flight. Proc. Natl Acad. Sci. USA 103, 4311-4315. ( 10.1073/pnas.0510109103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicilia S, Smith DA. 1991. Theory of asynchronous oscillations in loaded insect flight muscle. Math. Biosci. 106, 159-201. ( 10.1016/0025-5564(91)90076-U) [DOI] [PubMed] [Google Scholar]
- 3.Maughan D, Moore J, Vigoreaux J, Barnes B, Mulieri LA. 1998. Work production and work absorption in muscle strips from vertebrate cardiac and insect flight muscle fibers. In Mechanisms of work production and work absorption in muscle (eds Sugi H, Pollack GH), pp. 471-480. Boston, MA: Berlin, Germany: Springer US. ( 10.1007/978-1-4684-6039-1_52) [DOI] [PubMed] [Google Scholar]
- 4.Machin KE, Pringle JWS. 1959. The physiology of insect fibrillar muscle – II Mechanical properties of a beetle flight muscle. Proc. R. Soc. Lond. B 151, 204-225. ( 10.1098/rspb.1959.0060) [DOI] [PubMed] [Google Scholar]
- 5.Lynch J, Gau J, Sponberg S, Gravish N. 2022. Autonomous actuation of flapping wing robots inspired by asynchronous insect muscle. In 2022 Int. Conf. on Robotics and Automation (ICRA), pp. 2076-2083. Philadelphia, PA: IEEE. ( 10.1109/ICRA46639.2022.9812028) [DOI] [Google Scholar]
- 6.Pons A, Beatus T. 2022. Distinct forms of resonant optimality within insect indirect flight motors. J. R. Soc. Interface 19, 20220080. ( 10.1098/rsif.2022.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deora T, Gundiah N, Sane SP. 2017. Mechanics of the thorax in flies. J. Exp. Biol. 220, 1382-1395. ( 10.1242/jeb.128363) [DOI] [PubMed] [Google Scholar]
- 8.Deora T, Sane SS, Sane SP. 2021. Wings and halteres act as coupled dual oscillators in flies. Elife 10, e53824. ( 10.7554/eLife.53824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josephson RK, Malamud JG, Stokes DR. 2000. Asynchronous muscle: a primer. J. Exp. Biol. 203, 2713-2722. ( 10.1242/jeb.203.18.2713) [DOI] [PubMed] [Google Scholar]
- 10.Hao Y, Miller MS, Swank DM, Liu H, Bernstein SI, Maughan DW, Pollack GH. 2006. Passive stiffness in Drosophila indirect flight muscle reduced by disrupting paramyosin phosphorylation, but not by embryonic myosin S2 hinge substitution. Biophys. J. 91, 4500-4506. ( 10.1529/biophysj.106.088492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pons A, Beatus T. 2023. Band-type resonance: non-discrete energetically optimal resonant states. Nonlinear Dyn. 111, 1161-1192. ( 10.1007/s11071-022-07888-4) [DOI] [Google Scholar]
- 12.Pons A, Perl I, Ben-Dov O, Maya R, Beatus T. 2023. Solving the thoracic inverse problem in the fruit fly. Bioinspir. Biomim. 18, 046002. ( 10.1088/1748-3190/accc23) [DOI] [PubMed] [Google Scholar]
- 13.Groszkowski J. 1964. Frequency of self-oscillations. Oxford, UK: Pergamon. [Google Scholar]
- 14.Vilfan A, Duke T. 2003. Synchronization of active mechanical oscillators by an inertial load. Phys. Rev. Lett. 91, 114101. ( 10.1103/PhysRevLett.91.114101) [DOI] [PubMed] [Google Scholar]
- 15.Lynch J, Gau J, Sponberg S, Gravish N. 2021. Dimensional analysis of spring-wing systems reveals performance metrics for resonant flapping-wing flight. J. R. Soc. Interface 18, 20200888. ( 10.1098/rsif.2020.0888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bullard B, Burkart C, Labeit S, Leonard K. 2006. The function of elastic proteins in the oscillatory contraction of insect flight muscle. J. Muscle Res. Cell Motil. 26, 479-485. ( 10.1007/s10974-005-9032-7) [DOI] [PubMed] [Google Scholar]
- 17.White DC. 1983. The elasticity of relaxed insect fibrillar flight muscle. J. Physiol. 343, 31-57. ( 10.1113/jphysiol.1983.sp014880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perl I, Maya R, Sabag O, Beatus T. 2023. Lateral instability in fruit flies is determined by wing–wing interaction and wing elevation kinematics. Phys. Fluids 35, 041904. ( 10.1063/5.0138255) [DOI] [Google Scholar]
- 19.Ando N, Kono T, Ogihara N, Nakamura S, Yokota H, Kanzaki R. 2022. Modeling the musculoskeletal system of an insect thorax for flapping flight. Bioinspir. Biomim. 17, 066010. ( 10.1088/1748-3190/ac8e40) [DOI] [PubMed] [Google Scholar]
- 20.Kang C, Shyy W. 2014. Analytical model for instantaneous lift and shape deformation of an insect-scale flapping wing in hover. J. R. Soc. Interface 11, 20140933. ( 10.1098/rsif.2014.0933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker SM, Thomas ALR, Taylor GK. 2010. Deformable wing kinematics in free-flying hoverflies. J. R. Soc. Interface 7, 131-142. ( 10.1098/rsif.2009.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman GJ, Wang ZJ. 2007. Energy-minimizing kinematics in hovering insect flight. J. Fluid Mech. 582, 153-168. ( 10.1017/S0022112007006209) [DOI] [Google Scholar]
- 23.Mou XL, Liu YP, Sun M. 2011. Wing motion measurement and aerodynamics of hovering true hoverflies. J. Exp. Biol. 214, 2832-2844. ( 10.1242/jeb.054874) [DOI] [PubMed] [Google Scholar]
- 24.Bergou AJ, Ristroph L, Guckenheimer J, Cohen I, Wang ZJ. 2010. Fruit flies modulate passive wing pitching to generate in-flight turns. Phys. Rev. Lett. 104, 148101. ( 10.1103/PhysRevLett.104.148101) [DOI] [PubMed] [Google Scholar]
- 25.Beatus T, Cohen I. 2015. Wing-pitch modulation in maneuvering fruit flies is explained by an interplay between aerodynamics and a torsional spring. Phys. Rev. E 92, 022712. ( 10.1103/PhysRevE.92.022712) [DOI] [PubMed] [Google Scholar]
- 26.Ellington CP. 1984. The aerodynamics of insect flight. IV. Aerodynamic mechanisms. Phil. Trans. R. Soc. Lond. B 305, 79-113. ( 10.1098/rstb.1984.0054) [DOI] [Google Scholar]
- 27.Gau J, Gravish N, Sponberg S. 2019. Indirect actuation reduces flight power requirements in Manduca sexta via elastic energy exchange. J. R. Soc. Interface 16, 20190543. ( 10.1098/rsif.2019.0543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gau J, Wold ES, Lynch J, Gravish N, Sponberg S. 2022. The hawkmoth wingbeat is not at resonance. Biol. Lett. 18, 20220063. ( 10.1098/rsbl.2022.0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuss FK. 2015. The loss tangent of visco-elastic models. In Nonlinear approaches in engineering applications (eds Dai L, Jazar RN), pp. 137-157. Cham, Switzerland: Springer International. ( 10.1007/978-3-319-09462-5_6) [DOI] [Google Scholar]
- 30.Gaul L, Klein P, Kemple S. 1991. Damping description involving fractional operators. Mech. Syst. Signal Process. 5, 81-88. ( 10.1016/0888-3270(91)90016-X) [DOI] [Google Scholar]
- 31.Gaul L, Bohlen S, Kempfle S. 1985. Transient and forced oscillations of systems with constant hysteretic damping. Mech. Res. Commun. 12, 187-201. ( 10.1016/0093-6413(85)90057-6) [DOI] [Google Scholar]
- 32.Ben-Dov O, Beatus T. 2022. Model-based tracking of fruit flies in free flight. Insects 13, 1018. ( 10.3390/insects13111018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casey C, Heveran C, Jankauski M. 2023. Experimental studies suggest differences in the distribution of thorax elasticity between insects with synchronous and asynchronous musculature. J. R. Soc. Interface 20, 20230029. ( 10.1098/rsif.2023.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jankauski MA. 2020. Measuring the frequency response of the honeybee thorax. Bioinspir. Biomim. 15, 046002. ( 10.1088/1748-3190/ab835b) [DOI] [PubMed] [Google Scholar]
- 35.Pons A, Beatus T. 2022. Elastic-bound conditions for energetically optimal elasticity and their implications for biomimetic propulsion systems. Nonlinear Dyn. 108, 2045-2074. ( 10.1007/s11071-022-07325-6) [DOI] [Google Scholar]
- 36.Muijres FT, Elzinga MJ, Melis JM, Dickinson MH. 2014. Flies evade looming targets by executing rapid visually directed banked turns. Science 344, 172-177. ( 10.1126/science.1248955) [DOI] [PubMed] [Google Scholar]
- 37.Walker SM, Schwyn DA, Mokso R, Wicklein M, Müller T, Doube M, Stampanoni M, Krapp HG, Taylor GK. 2014. In vivo time-resolved microtomography reveals the mechanics of the blowfly flight motor. PLoS Biol. 12, e1001823. ( 10.1371/journal.pbio.1001823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swank DM. 2012. Mechanical analysis of Drosophila indirect flight and jump muscles. Methods 56, 69-77. ( 10.1016/j.ymeth.2011.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai M, Brandt PW. 1980. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J. Muscle Res. Cell Motil. 1, 279-303. ( 10.1007/BF00711932) [DOI] [PubMed] [Google Scholar]
- 40.Dickinson MH, et al. 1997. Phosphorylation-dependent power output of transgenic flies: an integrated study. Biophys. J. 73, 3122-3134. ( 10.1016/S0006-3495(97)78338-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang C, Kaplan CN, Thatcher ML, Swank DM. 2010. The influence of myosin converter and relay domains on cross-bridge kinetics of Drosophila indirect flight muscle. Biophys. J. 99, 1546-1555. ( 10.1016/j.bpj.2010.06.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loya AK, Van Houten SK, Glasheen BM, Swank DM. 2022. Shortening deactivation: quantifying a critical component of cyclical muscle contraction. Am. J. Physiol.-Cell Physiol. 322, C653-C665. ( 10.1152/ajpcell.00281.2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molloy JE, Kyrtatas V, Sparrow JC, White DCS. 1987. Kinetics of flight muscles from insects with different wingbeat frequencies. Nature 328, 449-451. ( 10.1038/328449a0) [DOI] [Google Scholar]
- 44.Ramanath S, Wang Q, Bernstein SI, Swank DM. 2011. Disrupting the myosin converter-relay interface impairs Drosophila indirect flight muscle performance. Biophys. J. 101, 1114-1122. ( 10.1016/j.bpj.2011.07.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuminetti R, Rossmanith G. 1980. Small amplitude non-linearities in the mechanical response of an asynchronous flight muscle. J. Muscle Res. Cell Motil. 1, 345-356. ( 10.1007/BF00711935) [DOI] [PubMed] [Google Scholar]
- 46.Viswanathan MC, et al. 2020. A role for actin flexibility in thin filament-mediated contractile regulation and myopathy. Nat. Commun. 11, 2417. ( 10.1038/s41467-020-15922-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trujillo AS, Hsu KH, Puthawala J, Viswanathan MC, Loya A, Irving TC, Cammarato A, Swank DM, Bernstein SI. 2021. Myosin dilated cardiomyopathy mutation S532P disrupts actomyosin interactions, leading to altered muscle kinetics, reduced locomotion, and cardiac dilation in Drosophila. Mol. Biol. Cell 32, 1690-1706. ( 10.1091/mbc.E21-02-0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barton B, Ayer G, Heymann N, Maughan DW, Lehmann F-O, Vigoreaux JO. 2005. Flight muscle properties and aerodynamic performance of Drosophila expressing a flightin transgene. J. Exp. Biol. 208, 549-560. ( 10.1242/jeb.01425) [DOI] [PubMed] [Google Scholar]
- 49.Henkin JA, Maughan DW, Vigoreaux JO. 2004. Mutations that affect flightin expression in Drosophila alter the viscoelastic properties of flight muscle fibers. Am. J. Physiol.-Cell Physiol. 286, C65-C72. ( 10.1152/ajpcell.00257.2003) [DOI] [PubMed] [Google Scholar]
- 50.Warmke J, Yamakawa M, Molloy J, Falkenthal S, Maughan D. 1992. Myosin light chain-2 mutation affects flight, wing beat frequency, and indirect flight muscle contraction kinetics in Drosophila. J. Cell Biol. 119, 1523-1539. ( 10.1083/jcb.119.6.1523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glasheen BM, Eldred CC, Sullivan LC, Zhao C, Reedy MK, Edwards RJ, Swank DM. 2017. Stretch activation properties of Drosophila and Lethocerus indirect flight muscle suggest similar calcium-dependent mechanisms. Am. J. Physiol.-Cell Physiol. 313, C621-C631. ( 10.1152/ajpcell.00110.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Q, Zhao C, Swank DM. 2011. Calcium and stretch activation modulate power generation in Drosophila flight muscle. Biophys. J. 101, 2207-2213. ( 10.1016/j.bpj.2011.09.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harischandra N, Clare AJ, Zakotnik J, Blackburn LML, Matheson T, Dürr V. 2019. Evaluation of linear and non-linear activation dynamics models for insect muscle. PLoS Comput. Biol. 15, e1007437. ( 10.1371/journal.pcbi.1007437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan WP, Dickinson MH. 1996. In vivo length oscillations of indirect flight muscles in the fruit fly Drosophila virilis. J. Exp. Biol. 199, 2767. ( 10.1242/jeb.199.12.2767) [DOI] [PubMed] [Google Scholar]
- 55.Sorgatz A, Wehmeier S. 1999. Towards high-performance symbolic computing: using MuPAD as a problem solving environment. Math. Comput. Simul. 49, 235-246. ( 10.1016/S0378-4754(99)00053-1) [DOI] [Google Scholar]
- 56.Kronert WA, et al. 2018. Prolonged cross-bridge binding triggers muscle dysfunction in a Drosophila model of myosin-based hypertrophic cardiomyopathy. eLife 7, e38064. ( 10.7554/eLife.38064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maya R, Lerner N, Ben-Dov O, Pons A, Beatus T. 2023. A hull reconstruction-reprojection algorithm for pose estimation of free-flying fruit flies. J. Exp. Biol. 226, jeb245853. ( 10.1242/jeb.245853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao L, Huang Q, Deng X, Sane SP. 2010. Aerodynamic effects of flexibility in flapping wings. J. R. Soc. Interface 7, 485-497. ( 10.1098/rsif.2009.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Truong H, Engels T, Kolomenskiy D, Schneider K. 2020. Influence of wing flexibility on the aerodynamic performance of a tethered flapping bumblebee. Theor. Appl. Mech. Lett. 10, 382-389. ( 10.1016/j.taml.2020.01.056) [DOI] [Google Scholar]
- 60.Eldredge JD, Toomey J, Medina A. 2010. On the roles of chord-wise flexibility in a flapping wing with hovering kinematics. J. Fluid Mech. 659, 94-115. ( 10.1017/S0022112010002363) [DOI] [Google Scholar]
- 61.Miyan JA, Ewing AW. 1985. Is the ‘click’ mechanism of dipteran flight an artefact of CC14 anaesthesia? J. Exp. Biol. 116, 313-322. ( 10.1242/jeb.116.1.313) [DOI] [Google Scholar]
- 62.Harne RL, Wang KW. 2015. Dipteran wing motor-inspired flapping flight versatility and effectiveness enhancement. J. R. Soc. Interface 12, 20141367. ( 10.1098/rsif.2014.1367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farisenkov SE, Kolomenskiy D, Petrov PN, Engels T, Lapina NA, Lehmann F-O, Onishi R, Liu H, Polilov AA. 2022. Novel flight style and light wings boost flight performance of tiny beetles. Nature 602, 96-100. ( 10.1038/s41586-021-04303-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Callaghan F, Sarig A, Ribak G, Lehmann F-O. 2022. Efficiency and aerodynamic performance of bristled insect wings depending on Reynolds number in flapping flight. Fluids 7, 75. ( 10.3390/fluids7020075) [DOI] [Google Scholar]
- 65.Polilov AA, Beutel RG. 2009. Miniaturisation effects in larvae and adults of Mikado sp. (Coleoptera: Ptiliidae), one of the smallest free-living insects. Arthropod Struct. Dev. 38, 247-270. ( 10.1016/j.asd.2008.11.003) [DOI] [PubMed] [Google Scholar]
- 66.Alexander R, Bennet-Clark HC. 1977. Storage of elastic strain energy in muscle and other tissues. Nature 265, 114-117. ( 10.1038/265114a0) [DOI] [PubMed] [Google Scholar]
- 67.Hrncir M, Gravel A-I, Schorkopf DLP, Schmidt VM, Zucchi R, Barth FG. 2008. Thoracic vibrations in stingless bees (Melipona seminigra): resonances of the thorax influence vibrations associated with flight but not those associated with sound production. J. Exp. Biol. 211, 678-685. ( 10.1242/jeb.013920) [DOI] [PubMed] [Google Scholar]
- 68.Nagai H, Isogai K, Fujimoto T, Hayase T. 2009. Experimental and numerical study of forward flight aerodynamics of insect flapping wing. AIAA J. 47, 730-742. ( 10.2514/1.39462) [DOI] [Google Scholar]
- 69.Kolomenskiy D, Maeda M, Engels T, Liu H, Schneider K, Nave J-C. 2016. Aerodynamic ground effect in fruitfly sized insect takeoff. PLoS ONE 11, e0152072. ( 10.1371/journal.pone.0152072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker SM, Taylor GK. 2021. A semi-empirical model of the aerodynamics of manoeuvring insect flight. J. R. Soc. Interface 18, 20210103. ( 10.1098/rsif.2021.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beatus T, Guckenheimer J, Cohen I. 2015. Controlling roll perturbations in fruit flies. J. R. Soc. Interface 12, 20150075. ( 10.1098/rsif.2015.0075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pons A. 2023. Data from: The self-oscillation paradox in the flight motor of Drosophila melanogaster. Swedish National Data Service. (https://snd.gu.se/sv/catalogue/dataset/2023-205/1) [DOI] [PubMed]
- 73.Pons A. 2023. The self-oscillation paradox in the flight motor of Drosophila melanogaster. Figshare. ( 10.6084/m9.figshare.c.6916126) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pons A. 2023. Data from: The self-oscillation paradox in the flight motor of Drosophila melanogaster. Swedish National Data Service. (https://snd.gu.se/sv/catalogue/dataset/2023-205/1) [DOI] [PubMed]
- Pons A. 2023. The self-oscillation paradox in the flight motor of Drosophila melanogaster. Figshare. ( 10.6084/m9.figshare.c.6916126) [DOI] [PubMed]
Data Availability Statement
Code containing MATLAB-MuPAD symbolic computation proofs and processed results from source datasets are available on the Swedish National Data Service from: https://snd.gu.se/sv/catalogue/dataset/2023-205/1 [72]. Supporting analyses are provided in the electronic supplementary material [73].