Abstract
A new method for the rapid and quantitative fluorometric determination of callose is described. In suspension-cultured cells of Glycine max, synthesis of callose starts within 20 minutes of treatment with chitosan and parallels over hours the accumulation of 1,3-linked glucose in the wall. Poly-l-lysine also elicits callose synthesis. The effect of chitosan is enhanced by Polymyxin B at low concentrations; this antibiotic alone at higher concentrations can also induce callose synthesis. Callose synthesis is immediately stopped when external Ca2+ is bound by ethylene glycolbis-(2-aminoethyl ether)-N,N′-tetraacetate or cation exchange beads, and partly recovers upon restoration of 15 micromolar Ca2+.
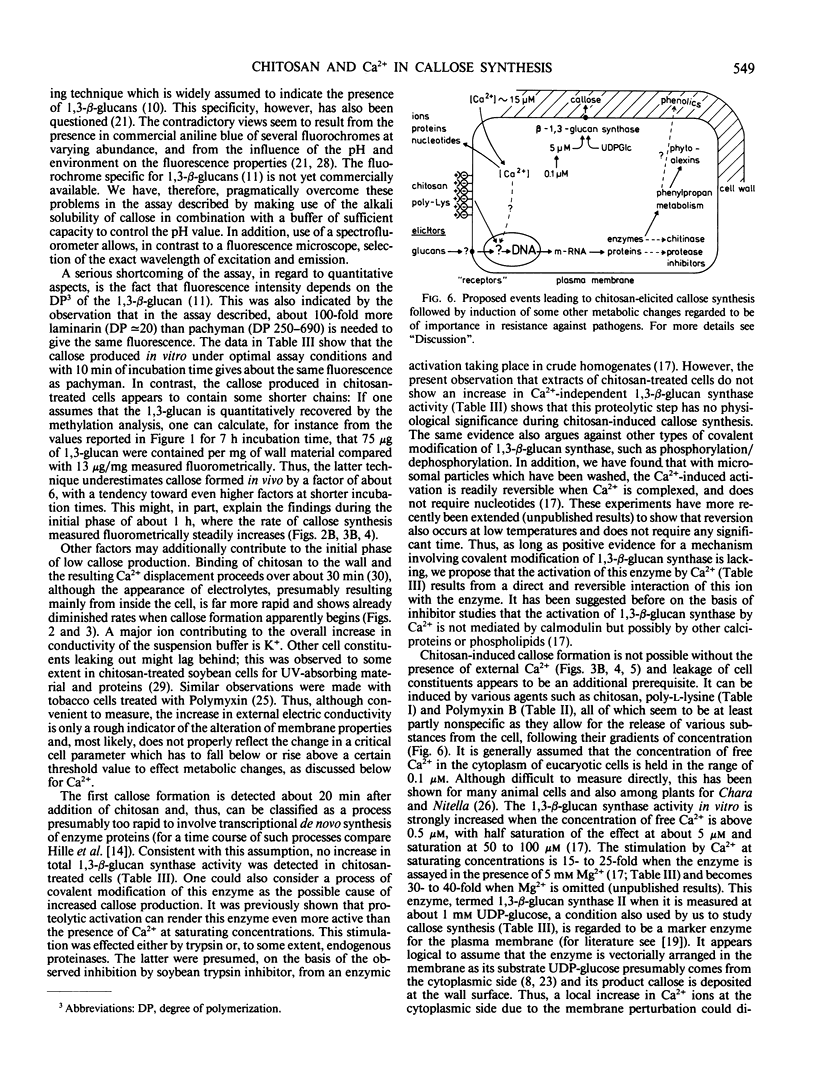
Callose synthesis is observed only when membrane perturbation causing electrolyte leakage from the cells is induced by one of the above treatments. It does not appear to be due to de novo synthesis or proteolytic activation of 1,3-β-d-glucan synthase. It is concluded that this Ca2+-dependent enzyme is directly activated by the influx of Ca2+ occurring concomitantly with the leakage of cell constituents. This suggestion is also discussed in conjunction with the chitosan-induced synthesis of phytoalexin in the same cells.
Full text
PDF

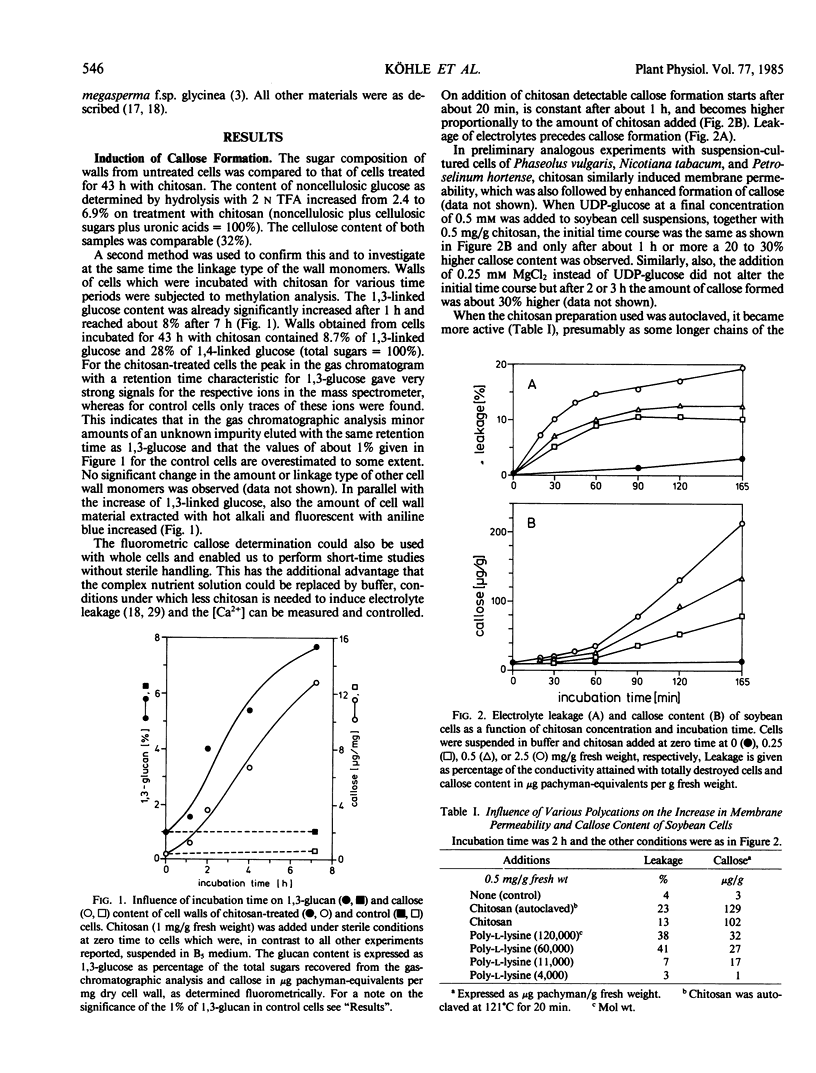
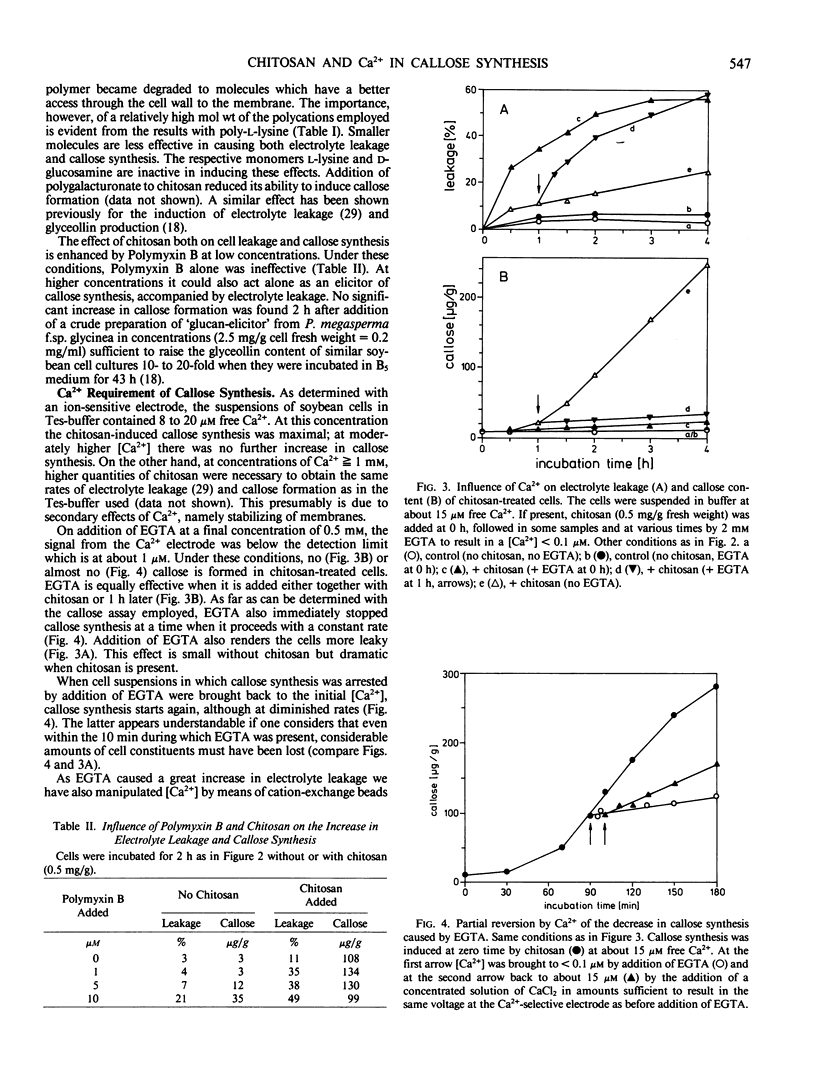
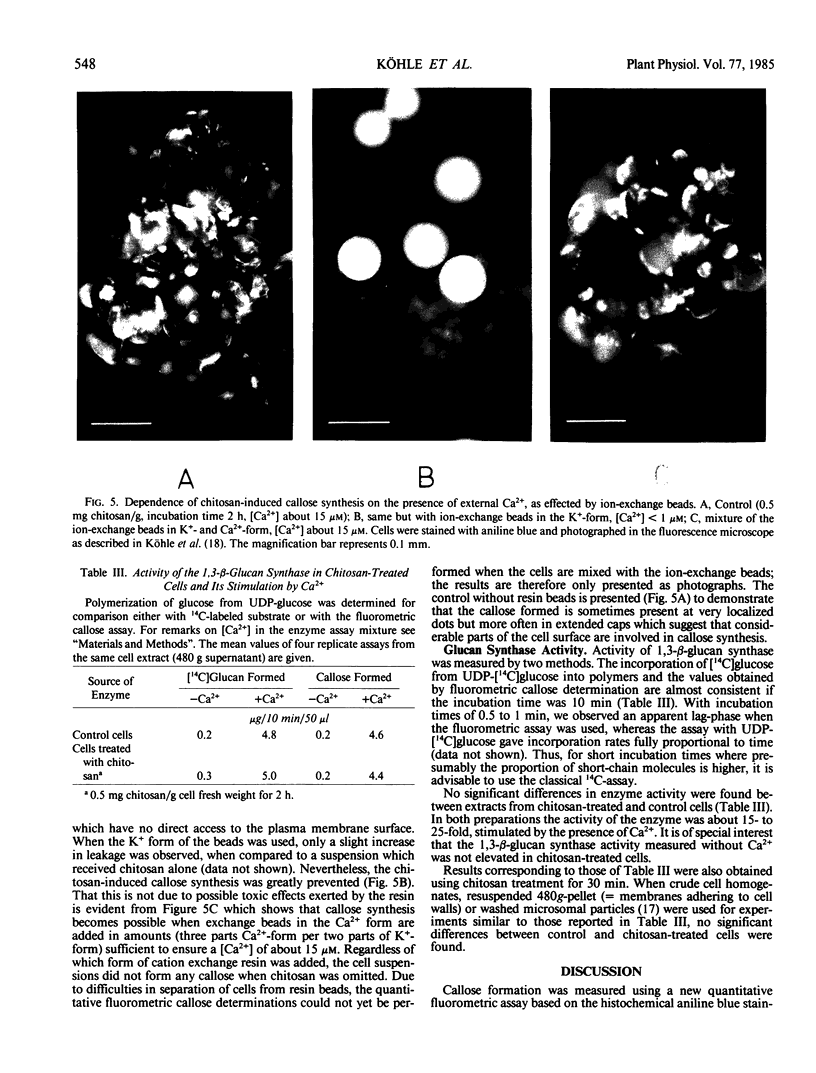


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Hoggart R. D., Clarke A. E. The possible role of lectins in mediating plant cell-cell interactions. Prog Clin Biol Res. 1983;138:143–161. [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brett C. T. Synthesis of beta-(1-->3)-Glucan from Extracellular Uridine Diphosphate Glucose as a Wound Response in Suspension-cultured Soybean Cells. Plant Physiol. 1978 Sep;62(3):377–382. doi: 10.1104/pp.62.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad A. E. Cationic polypeptide-induced fusion of acidic liposomes. Biochim Biophys Acta. 1983 Mar 9;728(3):377–382. doi: 10.1016/0005-2736(83)90509-6. [DOI] [PubMed] [Google Scholar]
- Jaffe M. J., Leopold A. C. Callose deposition during gravitropism of Zea mays and Pisum sativum and its inhibition by 2-deoxy-D-glucose. Planta. 1984 May;161(1):20–26. doi: 10.1007/BF00951455. [DOI] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Tighe D. M., Heath M. C. Callose induction in cowpea by uridine diphosphate glucose and calcium phosphate-boric Acid treatments. Plant Physiol. 1982 Feb;69(2):366–370. doi: 10.1104/pp.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Hadwiger L., Ryan C. A. Chitosans and pectic polysaccharides both induce the accumulation of the antifungal phytoalexin pisatin in pea pods and antinutrient proteinase inhibitors in tomato leaves. Biochem Biophys Res Commun. 1983 Jan 14;110(1):194–199. doi: 10.1016/0006-291x(83)91279-2. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Fulcher R. G. Dye interactions. A basis for specific detection and histochemistry of polysaccharides. J Histochem Cytochem. 1983 Jun;31(6):823–826. doi: 10.1177/31.6.6841974. [DOI] [PubMed] [Google Scholar]
- Young D. H., Köhle H., Kauss H. Effect of Chitosan on Membrane Permeability of Suspension-Cultured Glycine max and Phaseolus vulgaris Cells. Plant Physiol. 1982 Nov;70(5):1449–1454. doi: 10.1104/pp.70.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]