Abstract
Combined retrospective—prospective cohort study was done to know the risk of sensorineural hearing loss in patients of drug resistant Tuberculosis (TB) receiving Anti Tuberculous Treatment (ATT) at tertiary care centre in South Gujarat. Study was done by using retrospective and prospective data of the patients of drug resistant TB of NCHS who received injectable ATT and referred by department of Respiratory Medicine to ENT department for purpose of hearing evaluation pre and post treatment (Case cohort). Age and sex matched control cohort was also used which includes patients of non-drug resistant TB who were not receiving Injectable ATT. Incidence of SNHL in patients taking ATT for drug resistant tuberculosis in our study was 33.9%. The Relative Risk of SNHL was 14.3%. The Attributable Risk of SNHL (preventable SNHL) was 93%.
Keywords: Sensorineural hearing loss, Drug resistant Tuberculosis
Introduction
Tuberculosis is the disease that occurs in someone infected with Mycobacterium tuberculosis. It is characterized by signs or symptoms of TB disease, or both, and is distinct from TB infection, which occurs without signs or symptoms of TB.
Multidrug-resistant tuberculosis is a form of TB caused by bacteria that don’t respond to Isoniazid and Rifampicin, which are the two most effective first-line anti-TB drugs. MDR-TB can be treated by using second-line drugs. However, second-line treatment options are limited and require extensive chemotherapy with expensive and toxic medicines. A subcategory of extensively drug-resistant TB (XDR-TB) has additional resistance to Fluoroquinolone and to at least one of the 3 injectable anti-TB drugs: kanamycin, capreomycin, or amikacin.
MDR-TB treatment is poorly efficacious, prolonged, poorly tolerated and somewhat toxic. The mainstay of MDR-TB treatment contains one second-line injectable, an aminoglycoside (AG), for at least four months in combination with four oral drugs despite advances in injectable-sparing regimens. One of the main adverse effects from AGs is sensorineural hearing loss leading to permanent damage to auditory system.
The World Health Organization (WHO) estimates that, globally, there are 6,50,000 cases of multidrug-resistant (MDR) tuberculosis (TB) [1] India has the highest burden of tuberculosis infection globally, with nearly 35–40 crores Indian population having it, of which 26 lakhs people (18–36 lakh) are estimated to develop tuberculosis (TB) disease per year [2].
Detection of MDR-TB requires bacteriological confirmation of TB and testing for resistance to drug using rapid molecular tests, culture methods or sequencing technologies according to WHO. Treatment requires a course of the second-line drugs for at least nine months and up to twenty months, supported by counselling and monitoring for adverse events.
Only 57% of MDR-TB patients are currently successfully treated worldwide. In 2020, WHO recommended a new shorter (9–11 months) and fully-oral regimen for patients with MDR-TB. This research shows that patients find it easier to complete the shorter regimen, as compared to the longer regimens that last up to twenty months. Resistance to fluoroquinolones has to be excluded prior to the initiation of treatment with this regimen.
The monitoring of hearing loss is important for two reasons. First, if detected early it may be possible to change the regimen, to stop or reduce the dose of the drug which is responsible for it. Secondly, for preventing progression of hearing loss to the point where it would impact on communication skills of the patients. If significant hearing loss has developed and is detected, interventions/rehabilitations can be implemented to reverse/reduce the hearing loss or to assist in communication.
Despite the increasing literature on MDR-TB over the last 20 years, only few studies have investigated hearing loss in patients undergoing treatment. To the best of our knowledge, no cohort study (especially with Control-cohort) has been done to know the incidence of SNHL due to ototoxicity of injectable ATT (anti-tuberculous treatment) in South Gujarat region. So, we have planned this study to know the Incidence, Relative Risk (RR) and Attributable Risk (AR) of SNHL in patients of MDR-TB receiving injectable ototoxic drug in this part of world.
Aims and Objectives
To know the incidence of sensorineural hearing loss in patients receiving ATT for drug resistant TB.
To know the Relative Risk (RR) of sensorineural hearing loss in patients receiving ATT for drug resistant TB.
To know the Attributable Risk (AR) of sensorineural hearing loss in patients receiving ATT for drug resistant TB.
Methodology
Combined retrospective—prospective cohort study was done at Dept of ENT, Govt Medical College & New Civil Hopital, Surat, Gujarat to know the risk of sensorineural hearing loss in patients of drug resistant TB receiving injectable Anti Tuberculous Treatment (ATT). Study was done between May 2020 to May 2021. A study population was selected from patients taking ATT for drug resistant tuberculosis which include injectable drug (Amikacin in all cases in this study) as Case-cohort and patients taking ATT for non- drug resistant tuberculosis which does not include injectable drugs as Control-cohort. Total 127 participants were enrolled in case-cohort and after age and sex match 127 participants were enrolled in control-cohort.
Hearing assessment of all participants was done before, during and after the treatment in both the groups.
Protocol of the study was approved by Institutional review board (scientific Review Committee and Ethical Committee). Date of approval is: 12 May, 2020.
Inclusion Criteria
Case-Cohort
Retrospective data: Patients of drug resistant TB receiving injectable ATT and whose pre-treatment Audiometry was done from December 2018 to 12th may 2020.
Prospective data: Patients of dug resistant-TB, receiving shorter MDR-TB regimen (includes injectable drugs) and who were referred for hearing evaluation by Pure Tone Audiometry (PTA) before and after starting ATT from 12th may 2020 till 30th Nov, 2020
If the patients receiving longer MDR-TB regimen (including Bedaquiline) develop intolerance to it, they are switched to injectable drugs. Such cases were also included in the study (till 30th Nov 2020).
Control-Cohort
1:1 control-cohort has been selected after matching the age and sex.
Newly diagnosed tuberculosis patients started with non-injectable ATT from the date of approval of this study till 30th Nov, 2020
Exclusion Criteria
Case-Cohort
Any patient whose pre-treatment PTA was not done.
Any patients of drug resistant TB between December 2018 to the date of approval of this study (retrospective data) in whom the required data were missing.
Patients whose pre-treatment PTA was found to have hearing loss.
Control-Cohort
The patients having any middle ear disease.
Candidates whose pre-treatment PTA was found to have hearing loss of any type.
Results
All the patients in this study were in the age group of 16 to 55 years (mean age = 30.1 years) with males constituting 54.3% (n = 69) and females constituting 45.7% (n = 58). Majority of the patients in case-cohort were from rural background 59% (n = 75) while 41% (n = 52) were from urban areas.
Total 127 patients belonging to 11 to 60 years were studied. Maximum number of patients i.e. 44 (34.6%) belonged to age group of 21–30 years. This is followed by 31 (24.4%) in 31–40 years, 25 (19.7%) patients in 11–20 years and 22 (17.4%) in 41–50 years. Least no of patients i.e. 5 (3.94%) belonged to 51–60 years. As we have taken age matched participants in Control cohort, the age wise distribution in Control cohort remains the same as Case cohort.
Out of 127 patients 69 (54.3%) were males and 58 (45.7%) were females. M: F ratio was 1.2:1. As we have taken sex matched participants in Control cohort, the sex wise distribution in Control cohort remains the same as Case cohort.
In Case cohort, it was found that most of the patients i.e. 44 (34.6%) were illiterate. 40 (31.5%) studied till schooling and 43 (33.9%) completed graduation. In Control cohort, 40 (31.5%) were illiterate, 36 (28.3%) patients studied till schooling and 51 (40.2%) patients studied till graduation.
In Case cohort, 52 (40.9%) belonged to urban areas and 75 (59.1%) belonged to rural areas. In Control cohort, 57 (44.9%) patients belonged to urban areas and 70 (55.1%) patients belonged to rural areas.
In Case cohort, 43 (33.9%) patients developed Hearing loss. Most of them i.e. 40 (31.5%) had bilateral hearing loss while only 3 (2.4%) had unilateral hearing loss. In control cohort, only 3 (2.4%) patients developed hearing loss. All the 3 patients had bilateral hearing loss (As shown in Table 1).
Table 1.
Distribution of Participants of Case & Control according to Hearing Loss
| Case cohort | Total | Control cohort | Total | |
|---|---|---|---|---|
| Both ear normal | 84 (66.1%) | 84 (66.1%) | 124 (97.6%) | 124 (97.6%) |
| Bilateral hearing loss | 40 (31.5%) | 43 (33.9%) | 3 (2.4%) | 3 (2.4%) |
| Only right ear hearing loss | 2 (1.6%) | 0 (00%) | ||
| Only left ear hearing loss | 1 (0.8%) | 0 (00%) |
In case cohort 43 patients (40 B/L and 3 U/L- i.e. 83 ears) developed SNHL. Amongst right side maximum 26 (30.2%) patients found to have high frequency SNHL. This was followed by moderate SNHL in 7 (8.1%) ears, mild SNHL in 5 (5.8%) ears and moderate-severe SNHL in 3 (3.5%) ears. Similarly, on left sider maximum 29 (33.7%) patients had high frequency SNHL which was followed by moderate SNHL in 6 (6.9%) ears, mild SNHL in 4 (4.7%) ears and moderate severe SNHL in 1 (1.2%) ear. 1 (1.2%) right ear and 2 (2.3%) left ears were not having any impairment and not a single ear had profound SNHL. (As shown in Table 2).
Table 2.
Distribution of participants according to degree of hearing loss in case cohort (n = 83)
| Right ear | left ear | |
|---|---|---|
| No impairment | 1 (1.2%) | 2 (2.3%) |
| High frequency SNHL | 26 (30.2%) | 29 (33.7%) |
| Mild SNHL | 5 (5.8%) | 4 (4.7%) |
| Moderate SNHL | 7 (8.1%) | 6 (6.9%) |
| Moderate severe SNHL | 3 (3.5%) | 1 (1.2%) |
| Severe SNHL | 1 (1.2%) | 1 (1.2%) |
| Profound SNHL | 0 (0.0%) | 0 (0.0%) |
In Control cohort, all 3 patients (6 ears) developed bilateral SNHL, amongst which 2 (33.3%) right ears & 2 (33.3%) left ears developed mild SNHL and 1(16.7%) right and 1 (16.7%) left ears developed moderate SNHL.
Amongst the 43 patients who developed SNHL, maximum 13 (30.2%) patients belonged to 31—40 years age group. This was followed by11 (25.6%) patients in 21–30 years, 9 (20.9%) patients in 41–50 years, 7(16.3%) patients in 11–20 years and 3(7.0%) patients in 51–60 years age groups. In control-cohort, all 3 patients affected were in 41–50 years age group. In rest of the age groups, patients were found to have normal hearing.
In case cohort, out of 43 patients who developed SNHL 23 (53.5%) patients were males and 20 (46.5%) patients were females. In control cohort, 3 patients who developed SNHL 2 (66.67%) were males and 1(33.3%) was female. (As shown in Figs. 1 and 2).
Fig. 1.
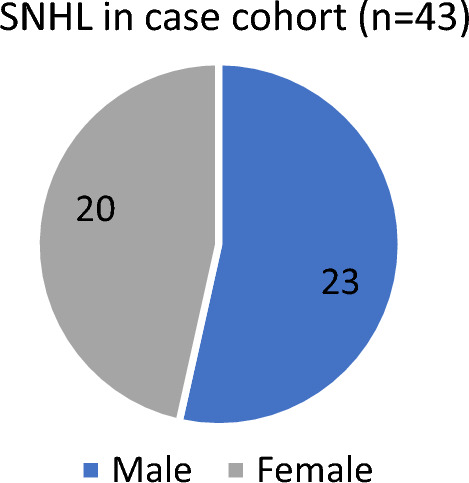
Sex distribution of participants in case (n = 43)
Fig. 2.
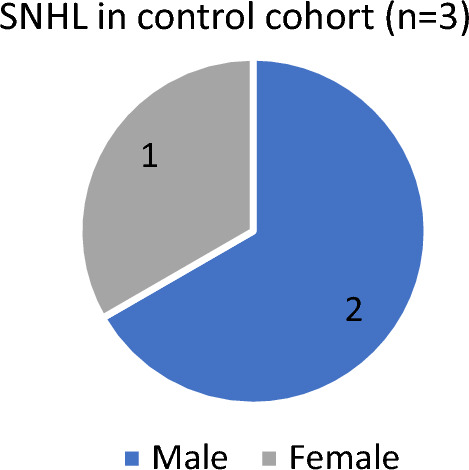
Sex distribution of participants in control (n = 3)
In case cohort, 43 patients who developed SNHL 13 (30.2%) were from urban areas and 30 (69.7%) were from rural areas. In control cohort, 3 pts who developed SNHL 01 (33.3%) were from urban areas and 02 (66.7%) were from rural areas.
Maximum number of patients i.e. 15 (34.8%) patients developed SNHL after 6 months. This was followed by 13 (30.2%) patients after 5 months, 9 (20.9%) patients after 4 months, 3 (7.1%) patients after 3 months, 2 (4.7%) patients after 2 months and 1 (2.3%) patient after 1 month. (As shown in Fig. 3).
Fig. 3.
Distribution according to time interval of development of hearing impairment Case cohort (N = 43)
Time interval of development of SNHL was calculated from the day of onset of injectable to the day of diagnosing SNHL by Pure Tone Audiometry.
Calculation of Incidence, Relative Risk (RR) and Attributable Risk (AR) of SNHL in pts taking Injectable ATT is shown in Table 3
In present study incidence of SNHL due to injectable ATT (Amikacin) is found to be 33.9%.
The relative risk in our study is 14.3 which means that patients of drug resistant tuberculosis taking injectable ATT have 14.3 times greater risk of developing hearing loss as compared to those without injectable ATT.
In our study AR for development of SNHL in patients taking injectable ATT is 93% which means that 93% of SNHL amongst the pts on injectable ATT, was attributed to injectable ototoxic drugs (i.e 93% of SNHL is preventable).
Table 3.
Incidence, relative risk (RR) and attributable risk (AR) of SNHL in pts taking Injectable ATT
| Injectable ATT given (Case cohort) | Injectable ATT not given (Control cohort) | |
|---|---|---|
| SNHL present | 43 | 3 |
| SNHL absent | 84 | 124 |
Discussion
In our study, we had male preponderance (M:F = 1.2:1). Duggal et al. [3], Achakzai et al. [4] and Sogebi et al. [5] also detected male preponderance (M:F ratio 1.6, 1.9:1 and 2:1 respectively). The higher M:F ratio in our study and many others is because the higher prevalence of tuberculosis in male which is corroborated by many studies on epidemiology of tuberculosis.
Among the total patients in case-cohort, maximum patients were from age group 21–30 years (34.6%), followed by 31–40 years (24.4%), 11–20 years (19.7%), 41–50 years (17.4%) and 51–60 years (3.9%). In our study we selected patients whose pre-treatment audiogram was normal. As presbycusis is common in elderly patients, and we eliminated the patients having any abnormality in the pre-treatment audiogram, the number of patients in 51 to 60 years’ age group was smaller. Hence, in our study we had more participants from the younger age groups.
In case-cohort, it was found that most of the patients i.e. 34.64% were illiterate while only 31.50% studied till schooling. In control-cohort also 31.50% were illiterate and 28.35% patients studied till schooling. This indicate that tuberculosis is still a disease of the poor socio-economic class and illiterate people.
In our study, in both the groups, more participants belonged to rural group. In Case cohort 40.9% participants belonged to urban group and 59.1% participants belonged to rural group. In Control cohort, 44.9% and 55.1% participants were from urban and rural groups respectively. In a study conducted by Kumar et al.[6], also noted that most of the patients (65.3%) were from rural area while 34.7% belonged to urban area. Verma et al. [7] also reported higher prevalence in rural as compared to urban area. i.e. 61.7% vs 38.3%.
In our study in case-cohort group, 31.5% patients developed bilateral hearing loss. Only 1.6% and 0.8% patients developed right sided and left sided unilateral hearing loss respectively. Sharma et al. [8] also reported bilateral hearing loss in majority cases i.e. 72.2%. Similarly, in studies conducted by Sogebi et al. [5] and Adoga et al.[9] maximum number of patients developed bilateral hearing loss.
In case cohort 43 patients (40 bilateral and 3 unilateral- i.e. 83 ears) developed SNHL. Amongst right side maximum 30.2% patients found to have high frequency SNHL. On left side also max. 33.7% patients had high frequency SNHL. Verma et al. [7] in their study including 60 participants reported overall incidence of High frequency loss (HFL) was 28.3%.
Maximum number of patients i.e. 15 (34.8%) patients developed SNHL after 6 months. This was followed by 13 (30.2%) pts after 5 months, 9 (20.9%) pts after 4 months, 3 (7.1%) pts after 3 months, 2 (4.7%) pts after 2 months and 1 (2.3%) pts after 1 month. This indicate that as the duration of injectable drug increase the chances of SNHL also increase.
In present study incidence of SNHL due to injectable ATT (Amikacin) is found to be 33.9%. Meta-analysis done by Wrohan et al.[10] has detected that amongst injectable ATT incidence of SNHL is highest in Amikacin (33.4%, 95% CI, 18.2–48.6) and lowest among those treated with Capreomycin (2.0%, 95% CI, 0–5.5). Unfortunately in our study all participants included were given Amikacin only so we could not find incidence of SNHL for Capreomycin.
The relative risk is 14.3 which means that patients of drug resistant tuberculosis taking injectable ATT have 14.3 times greater risk of developing SNHL as compared to those without injectable ATT.
Attributable risk of SNHL in patients taking injectable ATT is 93% which means that 93% of SNHL amongst the pts on injectable ATT, is attributed to injectable ototoxic drugs which further mean that if we remove injectable drugs from the ATT then 93% of the SNHL in patients taking ATT will be prevented.
Conclusion
In our study, incidence of SNHL due to injectable ATT is 33.9%.
The relative risk is 14.3 which means that patients of drug resistant tuberculosis taking injectable ATT have 14.3 times greater risk of developing SNHL as compared to those without injectable ATT.
Attributable risk of SNHL in patients taking injectable ATT is 93% which means that 93% of SNHL amongst the pts on injectable ATT, is attributed to ototoxic drug viz. amikacin which further mean that if we remove injectable drugs from the ATT then 93% of the SNHL in patients taking ATT will be prevented.
Limitation
Sensory-neural hearing loss due to ototoxicity start earliest in high frequency. In most audiology set up including ours the pure tone audiometer used includes frequency range of 250 Hz to 8000 Hz. So, if the SNHL is at frequencies more than 8000 Hz, it cannot be measured. So those patients in whom SNHL develops at frequency above 8000 Hz may be missed.
Acknowledgements
The full-time regular Audiologist Mr. Ishwarbhai Chaudhari in our department for conducting audiometry and counselling the participants.
Funding
The authors have not received any kind of support from any organization.
Declarations
Conflict of interest
The authors have no financial or non-financial intrest to disclose.
Ethical approval
This is combined Retrospective and Prospective cohort design. It was started only after Ethical approval was taken from institutional ethical committee of Govt Medical college, Surat and date of approval was 12th may 2020. Retrospective Data includes patients of drug resistant TB from December 2018 to 12th may 2020. Prospective Data includes patients of drug resistant TB from 12th may 2020 till 30th Nov, 2020. This research is done complying all ethical standard of our institue, nation and with 1964 Helsinki declaration.
Informed Consent
All prospectively added participants were included in the study only after obtaining informed written consent for the participation in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO [PubMed]
- 2.India. Ministry of Health & Family Welfare. Guidelines of Programmatic Management of Tuberculosis preventive treatment in India. New Delhi: Central TB Division; July 2021, 90. https://tbcindia.gov.in
- 3.Duggal P, Sarkar M. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC Ear Nose Throat Disord. 2007;7:5. doi: 10.1186/1472-6815-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achakzai A, Achakzai MA, Achakzai H, Baqi A, Achakzai M, Professors A. Frequency of sensorineural hearing loss in patients with drug resistant pulmonary tuberculosis. PJMHS. 2020;14(2):478–479. [Google Scholar]
- 5.Sogebi OA, Fadeyi MO, Adefuye BO, Soyinka FO. Hearing thresholds in patients with drug-resistant tuberculosis: Baseline audiogram configurations and associations. J Bras Pneumol. 2017;43(3):195–201. doi: 10.1590/s1806-37562016000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh DSP, Varunkumar TD. Audiologic monitoring of multi-drug resistant tuberculosis patient on aminoglycoside treatment with long term follow-up. IOSR-JDMS. 2017;16(10):10–15. doi: 10.1186/1472-6815-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma J, Syed MT. Evaluating hearing loss in patients undergoing second line anti tubercular treatment. IJOHNS. 2019;71(Suppl 2):S1202–S1206. doi: 10.1007/s12070-018-1266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma V, Bhagat S, Verma B, Singh R, Singh S. Audiological evaluation of patients taking kanamycin for multidrug resistant tuberculosis. Iranan J Otorhinolaryngol. 2016;28(3):203–208. [PMC free article] [PubMed] [Google Scholar]
- 9.Adoga AS, Chun-Gyang SD, Okoh AF, Ukoli CO, Benjamin B. Treatment of multi-drug resistant tuberculosis and the associated hearing loss. J Res Basic Sci. 2019;1(4):315–321. [Google Scholar]
- 10.Wrohan I, et al. Ototoxicity among multi-drug resistant TB patients: a systemic review and meta-analysis. Int J Tuberc Lung Dis. 2021;25(1):23–30. doi: 10.5588/ijtld.20.0217. [DOI] [PubMed] [Google Scholar]



