Abstract
Feedback devices were developed to guide resuscitations as targets recommended by various guidelines are difficult to achieve. Yet, there is limited evidence to support their use for in-hospital cardiac arrests (IHCA), and they did not correlate with patient outcomes. Therefore, this study has investigated the compression quality and patient outcomes in IHCA with the use of a feedback device via a retrospective study of inpatient code blue activations in a Singapore hospital over one year. The primary outcome was compression quality and secondary outcomes were survival, downtime and neurological status. 64 of 110 (58.2%) cases were included. Most resuscitations (71.9%) met the recommended chest compression fraction (CCF, defined as the proportion of time spent on compressions during resuscitation) despite overall quality being suboptimal. Greater survival to discharge and better neurological status in resuscitated patients respectively correlated with higher median CCF (p = 0.040 and 0.026 respectively) and shorter downtime (p < 0.001 and 0.001 respectively); independently, a higher CCF correlated with a shorter downtime (p = 0.014). Overall, this study demonstrated that reducing interruptions is crucial for good outcomes in IHCA. However, compression quality remained suboptimal despite feedback device implementation, possibly requiring further simulation training and coaching. Future multicentre studies incorporating these measures should be explored.
Subject terms: Cardiology, Arrhythmias
Introduction
The provision of high-quality cardiopulmonary resuscitation (CPR) is a fundamental skill which has significant impact on patient’s survival and prognosis1–4. The 5 pillars of high-quality CPR as described by the American Heart Association (AHA) in 2015 are chest compression fraction (CCF) of above 80%, compression rate between 100 to 120 per minute, compression depth greater or equal to 5 cm but not exceeding 6 cm in an adult, no residual leaning and no excessive ventilation5. However, CPR providers often do not meet such standards6–8. Therefore, to help providers achieve such targets, the AHA stated that it was “reasonable” for CPR providers to utilise a feedback device during resuscitation3. This is especially important as deaths from cardiovascular disease, including sudden cardiac death, are the leading cause of mortality worldwide9,10.
Out-of-hospital cardiac arrests (OHCA) alone contributed to over 60% of cardiovascular deaths globally in 201010,11. Hence, many papers have reviewed the effectiveness of these feedback devices in the setting of OHCA. Other papers reviewed CPR quality when compressions were performed on mannikins in a controlled and simulated environment12–14. Based on these studies, some have recommended the use of automated feedback to improve CPR quality15,16.
However, studies on IHCA have been fairly limited despite these patients often having worse outcomes1,4,17. This could be contributed by the poorer baseline condition due to multiple comorbidities18. Altogether, few studies have been conducted on CPR feedback devices used in IHCA but they were unable to capture the full spectrum of compression parameters including CCF. It was also difficult to draw meaningful conclusions from these studies as results were often indeterminate19–21,23. These studies were also unable to establish a relationship between the provision of high-quality CPR with a feedback device and patient outcomes12,15,16,21. Hence, this study aims to examine CPR quality with the use of a feedback device during actual code blue activations and if the CPR quality correlates with outcomes in IHCA patients.
Methods
Study design
The waiver for this retrospective study was granted by the SingHealth Centralised Institutional Review Board (IRB) (Reference Number: 2019/2496). The methods employed in this study were in accordance to the relevant guidelines and regulations of the IRB. This manuscript was written in accordance to the STROBE checklist on cross-sectional studies.
All inpatient code blue activations from June 2020 to May 2021 in Sengkang General Hospital (SKH) was conducted using inpatient medical records. The inpatient resuscitation trolleys were equipped with the ZOLL® R Series® Plus defibrillator with ZOLL® Stat-padz® (ZOLL Medical Corporation, Chelmsford, MA, United States of America). The feedback device provided instantaneous audiovisual feedback via the International R Series® Software. Compression parameters from the feedback device were then uploaded via wifi for storage and then retrieved using the manufacturer’s software (RescueNet® CaseReview). Patients were subsequently reviewed for their survival and outcomes.
A prior study had been conducted by the ZOLL Medical Corporation to determine the accuracy of the feedback system24. In this study, 20 laypersons performed 45 to 60 s of continuous chest compressions on an instrumented manikin. Both the feedback device and the manikin recorded compression rate and depth independently. The data from the feedback device and manikin were analysed and compared against each other. Compared to the data from the manikin, the compression depth measured by the feedback device varied by a mean of 0.13 ± 0.64 cm (95% confidence interval) and the compression rate differed by an average of less than 2 compressions per minute.
Resuscitation model in Singapore
Code blue teams (CBT) in Singapore are activated for arrest and peri-arrest cases. Peri-arrest cases involve patients who are unstable and very likely go into cardiac arrest such as impending airway collapse and refractory shock. Upon cardiac arrest, the junior doctor of the ward team is notified and activated to attend to the patient. He or she attends swiftly to the collapsed patient and leads the basic resuscitation effort with the on-scene nursing staff and further activates the CBT at the soonest opportunity. The CBT then takes over the resuscitation effort from the ward team upon arrival. Most, if not all, patients who attain return of spontaneous circulation (ROSC) will be transferred to the Intensive Care Unit (ICU) for further management by the ICU team.
Patient population
Waiver for informed consent was granted by the SingHealth Centralised IRB (Reference Number: 2019/2496). All patients in SKH and Sengkang Community Hospital (SKCH) who had a cardiopulmonary collapse in areas covered by the inpatient CBT were included. These areas included the general ward, high dependency unit, endoscopy suite, dialysis centre, cardiovascular laboratory and SKCH inpatient wards.
Patients excluded were those who: (1) had achieved ROSC upon the CBT arrival; or (2) were on a Do Not Resuscitate (DNR) order. Peri-arrest activations which ultimately did not go into cardiac arrest were excluded from the analysis.
Measured outcomes
Primary outcome variables were CCF, proportion of compressions in target, mean compression rate and proportion of compressions in target rate, mean compression depth and proportion of compressions in target depth, and mean release velocity (RV). CCF was defined as proportion of time during resuscitation that chest compressions were performed. Compressions in target were defined as compressions that met both target rate and depth concurrently. RV was used as a surrogate marker for chest recoil. The compression targets were assessed using recommendations by AHA5.
Secondary outcome variables were downtime, survival and neurological outcomes. Downtime was defined as the interval from cardiopulmonary collapse to sustained ROSC, in which spontaneous circulation had been achieved without the need for further chest compressions for at least 20 min25. Survival of patients was recorded at hospital discharge.
A patient’s neurological function was assessed using the Cerebral Performance Categories (CPC). The CPC is a commonly used modality to prognosticate long-term neurological function after a cardiac arrest. The categories are: (1) good cerebral performance; (2) moderate cerebral disability; (3) severe cerebral disability; (4) coma, vegetative state; and (5) dead26.
Statistical analysis
Data was recorded using Microsoft® Excel® 365 software (Microsoft Corporation, Redmond, WA, United States of America). Missing data were reconciled where possible. Data which were unable to be reconciled were excluded from the analysis. Analysis was performed using IBM SPSS Statistics 28 software (IBM Corporation, Armonk, NY, United States of America). Shapiro–Wilk test was used to determine if continuous variables were normally distributed.
Due to the small sample size within subgroups, continuous variables were presented as median with interquartile range (IQR). Categorical variables were presented as numbers or percentages (%).
Mann–Whitney U and Kruskal–Wallis tests were performed to determine if there were significant differences between 2 or more than 2 outcome categories measured on a continuous scale. Measurements for compression parameters and downtime were collected. The data were analysed using the Kruskal–Wallis test to detect significant differences in medians of parameters and downtime for all survival outcome categories. Post-hoc tests were performed where necessary using Bonferroni correction, adjusting for multiple pairwise comparisons. Results were taken to be statistically significant if p < 0.05.
Results
Patient demographics
The data was collected from June 2020 to May 2021. Amongst the 110 code blue activations, 34 cases (30.9%) had no data captured and 5 cases (4.5%) had more than or equal to a 10% discrepancy in the number of data points captured for depth and rate. The exclusion criteria omitted 7 cases, of which, 2 cases (1.8%) had an active DNR order while 5 cases (4.5%) achieved ROSC upon CBT arrival. Altogether, 64 cases were ultimately included for analysis.
Overall, there were more males than females. The majority of patients were Chinese elderly (mean age: 71.4 ± 12.2 years). The most common organ system causing initial collapse was the cardiovascular system. Most patients had non-shockable rhythms (pulseless electrical activity or asystole) (74%) as their initial rhythms and 67% of patients did not receive any shock throughout resuscitation. The 10 discharged patients mostly returned home or were transferred to a stepdown care facility (Table 1).
Table 1.
Characteristics of patients included in this study. SD: Standard Deviation; IQR: Interquartile Range.
| Overall (n = 64) | |
|---|---|
| Age (in years) | |
| Mean (SD) | 71.4 (12.2) |
| Median (IQR) | 72.5 (61.3–78.8) |
| Gender, n (%) | |
| Female | 25 (39.1) |
| Male | 39 (60.9) |
| Ethnicity, n (%) | |
| Chinese | 48 (75.0) |
| Malay | 11 (17.2) |
| Indian | 5 (7.8) |
| Organ system causing initial collapse, n (%) | |
| Cardiovascular | 25 (39.1) |
| Respiratory | 15 (23.4) |
| Metabolic/ renal | 5 (7.8) |
| Gastrointestinal | 1 (1.6) |
| Neurological | 1 (1.6) |
| Others | 17 (26.6) |
| Initial rhythms, n (%) | |
| Pulseless electrical activity | 24 (37.5) |
| Asystole | 23 (35.9) |
| Ventricular fibrillation | 7 (10.9) |
| Normal sinus rhythm | 3 (4.7) |
| Sinus tachycardia | 1 (1.6) |
| Ventricular tachycardia | 1 (1.6) |
| Unknown | 5 (7.8) |
| Discharge destination, n (%) | (n = 10) |
| Home | 4 (40.0) |
| Stepdown care facility | 4 (40.0) |
| Nursing home | 1 (10.0) |
| Other acute wards | 1 (10.0) |
The median response time from CBT activation to CBT arrival on the scene was 4.0 (3.0–5.0) minutes. The rates of ROSC and survival to hospital discharge were 64.1% and 13.7% respectively.
Compression quality was suboptimal
Compression parameters were retrieved from the manufacturer’s software. CPR performances were subsequently assessed using AHA recommendations.
71.9% of cases achieved CCF of above 80%. While 56.3% and 39.1% of compressions were within the target ranges of rate and depth respectively, only 13.7% (7.1–24.5%) of compressions met the composite of the two. No compression met the target RV.
Resuscitation outcome was not associated with any one compression parameter
With regards to initial survival outcome, we explored possible associations between compression parameters and outcome of resuscitation. Survival outcome from CPR was divided into those who: (1) did not attain ROSC; and (2) attained ROSC. No statistically significant difference was observed between CCF, depth, rate and RV for CPR survival outcome (p = 0.40, 0.93, 0.94 and 0.82 respectively) (Table 2).
Table 2.
Associations between the various chest compression parameters and CPR survival outcomes.
| CPR survival outcome | ||||
|---|---|---|---|---|
| Did not attain ROSC (n = 25) | Attained ROSC (n = 39) | Overall (n = 64) | p-value1 | |
| Chest compression fraction (in %) | ||||
| Median (IQR) | 84.7 (74.7–90.9) | 87.6 (77.2–93.1) | 86.6 (77.0–92.3) | 0.401 |
| Chest compression depth (in cm) | ||||
| Median (IQR) | 5.4 (5.0–5.8) | 5.5 (4.95–5.88) | 5.44 (5.02–5.84) | 0.934 |
| Chest compression rate (per minute) | ||||
| Median (IQR) | 121.9 (112.6–126.3) | 120.5 (113.5–126.6) | 120.8 (113.3–126.4) | 0.940 |
| Release velocity (in cm/s) | ||||
| Median (IQR) | 25.6 (23.3–27.5) | 25.6 (23.3–28.4) | 25.6 (23.8–28.0) | 0.820 |
CPR: Cardiopulmonary Resuscitation; ROSC: Return of Spontaneous Circulation; IQR: Interquartile Range.
1Mann-Whitney U test.
Patient prognosis after ROSC correlated with CCF and downtime
Next, we investigated if resuscitation parameters were associated with patient survival. The patients who attained ROSC were categorised into those who: (1) did not survive till hospital discharge; and (2) survived till hospital discharge.
The overall median CCF and downtime for patients who attained ROSC were 86.6% (77.0–92.3%) and 15.0 (7.5–20.3) minutes respectively. Amongst these patients who attained ROSC, the median CCF was higher in those who “survived till hospital discharge” (92.3%) than those who “did not survive till hospital discharge” (86.8%). Further, the median downtime was also shorter in the former, by a difference of 11.5 min. These differences in CCF and downtime, in terms of patients who survival till hospital discharge or not, were statistically different (p = 0.04 and < 0.001 respectively). However, no statistically significant difference was found for depth, rate and RV for these survival categories (p = 0.69, 0.96 and 0.79 respectively) (Table 3).
Table 3.
Associations between the various chest compression parameters and downtime, and survival till hospital discharge after ROSC.
| Ultimate survival outcome | ||||
|---|---|---|---|---|
| Did not survive till hospital discharge (n = 29) | Survived till hospital discharge (n = 10) | Overall (n = 39) | p-value1 | |
| Chest compression fraction (in %) | ||||
| Median (IQR) | 86.8 (76.3–90.7) | 92.3 (84.5–98.7) | 86.6 (77.0–92.3) | 0.040 |
| Chest compression depth (in cm) | ||||
| Median (IQR) | 5.5 (5.2–5.8) | 5.2 (4.9–6.1) | 5.4 (5.0–5.8) | 0.692 |
| Chest compression rate (per minute) | ||||
| Median (IQR) | 120.8 (113.4–127.3) | 119.9 (113.1–131.1) | 120.8 (113.3–126.4) | 0.962 |
| Release velocity (in cm/s) | ||||
| Median (IQR) | 26.3 (23.5–28.2) | 24.7 (22.1–29.5) | 25.6 (23.8–28.0) | 0.788 |
| Downtime (min) | (n = 28) | (n = 10) | (n = 38) | |
| Median (IQR) | 17.5 (10.5–22.0) | 6.0 (2.0–10.5) | 15.0 (7.5–20.3) | < 0.001 |
ROSC: Return of Spontaneous Circulation; IQR: Interquartile Range.
1Mann-Whitney U test.
Significant values are in bold.
CPC correlated with CCF and downtime respectively
To investigate the extent in which neurological status is affected after resuscitation, we explored how the compression parameters correlated with CPC. We observed statistically significant differences in median CCF and downtime with CPC at discharge (p = 0.03 and 0.001 respectively). Median CCF (92.3%) was higher in patients with CPC = 1 than in patients with CPC = 5 (86.4%). Moreover, median downtime (6.0 min) was also shorter in patients with CPC = 1 than in patients with CPC = 5 (17.5 min). However, no statistically significant difference was observed in compression depth, rate and RV with CPC at discharge (p = 0.68, 0.97 and 0.77 respectively) (Table 4).
Table 4.
Associations between the various chest compression parameters and downtime, and CPC.
| Cerebral performance categories | ||||
|---|---|---|---|---|
| CPC = 1 (n = 10) | CPC = 5 (n = 54) | Overall (n = 64) | p-value1 | |
| Chest compression fraction (in %) | ||||
| Median (IQR) | 92.3 (84.5–98.7) | 86.4 (76.4–90.7) | 86.6 (77.0–92.3) | 0.026 |
| Chest compression depth (in cm) | ||||
| Median (IQR) | 5.2 (4.9–6.1) | 5.5 (5.1–5.8) | 5.4 (5.0–5.8) | 0.677 |
| Chest compression rate (per minute) | ||||
| Median (IQR) | 119.9 (113.1–131.1) | 121.4 (113.2–126.1) | 120.8 (113.3–126.4) | 0.971 |
| Release velocity (in cm/s) | ||||
| Median (IQR) | 24.4 (22.7–26.1) | 25.6 (23.5–28.4) | 25.6 (23.5–28.4) | 0.767 |
| Downtime (min) | (n = 10) | (n = 28) | (n = 38) | |
| Median (IQR) | 6.0 (2.0–10.5) | 17.5 (10.5–22.0) | 15.0 (7.5–20.3) | 0.001 |
CPC: Cerebral Performance Categories; IQR: Interquartile Range.
1Mann-Whitney U test.
Significant values are in bold.
CCF independently correlated with downtime
This study also examined whether compression parameters correlated with downtime. Downtimes were categorised into: (1) short (< 10 min); (2) moderate (10–20 min); and (3) long (> 20 min). We observed that a longer duration of downtime was associated with lower CCF (p = 0.014). Median CCF for: (1) short downtime was 92.3%; (2) moderate downtime was 87.0%; and (3) long downtime was 74.0%. Furthermore, post-hoc Bonferroni correction showed a statistically significantly higher CCF for those with short downtime than those with long downtime (p = 0.012) (Table 5).
Table 5.
Associations between the various chest compression parameters and downtime for patients who attained ROSC.
| Downtime | |||||
|---|---|---|---|---|---|
| Short (n = 14) | Moderate (n = 15) | Long (n = 9) | Overall (n = 38) | p-value1 | |
| Chest compression fraction (in %) | |||||
| Median (IQR) | 92.3 (84.7–98.4) | 87.0 (77.2–90.6) | 74.0 (58.1–88.4) | 86.6 (77.0–92.3) | 0.0142 |
| Chest compression depth (in cm) | |||||
| Median (IQR) | 5.4 (4.9–6.1) | 5.6 (5.2–6.1) | 5.5 (5.2–5.8) | 5.4 (5.0–5.8) | 0.877 |
| Chest compression rate (per minute) | |||||
| Median (IQR) | 122.1 (113.1–134.9) | 119.6 (113.5–125.2) | 119.4 (111.5–124.1) | 120.8 (113.3–126.4) | 0.510 |
| Release velocity (in cm/s) | |||||
| Median (IQR) | 25.8 (22.1–28.9) | 25.6 (23.3–29.2) | 26.3 (23.9–30.1) | 25.6 (23.8–28.0) | 0.904 |
ROSC: Return of Spontaneous Circulation; IQR: Interquartile Range.
1Kruskal-Wallis test.
2Bonferroni correction was performed for multiple tests between groups. There was statistically significant difference in CCF for those who had a short downtime than those who had a long downtime (p = 0.012).
Significant value is in bold.
Discussion
This study examined CPR quality with a feedback device and how CPR performance correlated with outcomes of IHCA patients. Herein, under the guidance of the CPR feedback device, we identified that increased CCF and shorter downtime were associated with improved CPR survivability and neurological prognosis of patients. This study provided nascent insights and thorough evaluation on the execution of CPR. It also explored the potential avenues in resuscitation medicine in which CPR training could be further modified to potentially improve patient outcomes.
Compressions required further guidance
Abella et al. reported in 2005 that CPR providers averaged a mean compression rate of 105 per minute while Wiks et al. reported a mean compression rate of 121 per minute which improved to 109 compressions per minute with feedback6–8. Similarly, the former reported an average depth of 4.3 cm while the latter observed an average depth of 3.4 cm, which improved to 3.8 cm with feedback6–8. Compression depth in target also improved from 28 to 53% with feedback, as demonstrated by studies in 2005 and 2006 respectively7,8. Overall, it was observed that despite the use of a similar feedback device in 2006, compressions remained inadequate even for the guidelines at that time6–8.
Kovacs et al. found that an RV equal to or exceeding 40 cm/s was associated with improved survival and neurological outcomes27. Based on the abovementioned study and AHA 2015 guidelines, the CPR quality in this study was suboptimal. There was inadequate chest recoil and the median compression rate was also above target5. Overall, there was a low percentage of compressions in target. These occurred despite real-time feedback. Achieving full chest recoil is difficult28. High levels of self-reported stress were also reported to be associated with decreased CPR performance29. Even with the implementation of the feedback device, quality was still inadequate and calls for further intervention to improve CPR quality.
Thus, one solution is for CBT to introduce the role of a CPR coach. The CPR coach deduces compression quality based on the feedback generated from the CPR feedback device and first-hand observation to guide the provider in making adjustments to achieve the CPR targets. The CPR coach may be the CBT leader or assigned to another CBT member. Over the years, some had attempted to review the effectiveness of CPR coaching in resuscitation. While these studies were mostly limited to OHCA and paediatric IHCA, they demonstrated that CPR coaching improved adherence to resuscitation guidelines30–33. Most pertinently, a 2022 South Korean study which looked into a novel OHCA resuscitation protocol (which included CPR coaching) showed substantial improvements in the rates of prehospital ROSC, survival till hospital discharge and favourable neurological status34. It is crucial that future reviews of CPR data explore whether the introduction of a CPR coach, especially in adult resuscitation with prolonged downtime, leads to improved compression parameters and if such improvements correlate with better survival and neurological outcomes.
Moreover, with the variability of CPR quality between providers, more training to standardise quality is likely necessary. Simulation training was reported to be most effective in training to improve CPR quality16,35. Therefore, training sessions should also include the CPR coach in simulations to enable the CBT to incorporate this role while mimicking stressful real-life conditions.
Minimising interruptions during CPR is essential for good outcomes in IHCA patients
This study demonstrated that a higher CCF was associated with shorter downtime. Current evidence corroborates with our findings where a higher CCF reduces mortality and morbidity in patients who suffered from a cardiac arrest36–38. Additionally, a shorter downtime was also associated with better survival to hospital discharge and neurological status, supporting our findings39,40.
It is noteworthy that the current AHA recommendation to minimise interruptions during CPR are based on papers which studied OHCA patients5,41. Past studies also did not find reduced interruptions to be associated with improved survival and neurological status in IHCA patients38. Hence, it is reasonable to postulate that achieving uninterrupted compressions is important for good outcomes in IHCA patients, and this further strengthens AHA recommendation to minimise interruptions during CPR for OHCA and now IHCA patients (Fig. 1).
Figure 1.
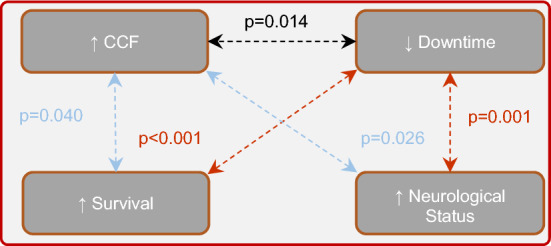
Associations between CCF and downtime, and patient survival and neurological status | Increased survival and better neurological status of patients were individually associated with a higher CCF (p = 0.040 and 0.026 respectively) and a shorter downtime (p < 0.001 and 0.001 respectively). A shorter downtime was independently associated with a higher CCF (p = 0.014).
With a median CCF of above 80%, this study has demonstrated that such a target is achievable within the hospital setting when performing resuscitations on IHCA patients. This supports current expert consensus41. This is an important finding since high CCF is associated with better patient outcomes.
However, it is currently unclear whether CCF decreases over time with prolonged CPR due to fatigue, or whether having a high CCF directly reduces downtime. Therefore, future studies can further explore this association.
Other confounders
The use of analysis of means difference does not exclude other confounders from contributing to the differences. Therefore, spurious correlations may be generated. For example, the quality of post-resuscitation care may be different among various patients, which is an important factor that affects the prognosis of patients42. It is thus included as one of the steps in the chain of survival for all cardiac arrest patients43,44. Moreover, non-modifiable variables such as the patient’s gender, age and prior comorbidities affect outcomes, but cannot be reliably excluded for comparison and discussion45.
Limitations
Firstly, the sample size was small (n = 64). As such, this study is potentially underpowered to conclude the effects of compression parameters. A future multicentre study can increase the sample size. Secondly, there was significant data loss (n = 34; 31%). Data loss over wifi is being rectified for future evaluations. Thirdly, this study was unable to verify self-reported variables like patient downtime and CPC. Such variables were entered as a free-text note. Unlike retrieving compression parameters via the manufacturer’s software, a retrospective accuracy check for free-text notes was not possible. Lastly, this study was unable to capture pauses during CPR (for example, pulse check, perishock and intubation) and time from collapse to CPR. These affect survival and patient outcomes3,46–48. The data collection methodology will be revised to include these in future studies. This study was ultimately a retrospective one. It cannot control for all confounders or support causal relationships49.
Areas for further research
Only a few studies utilising a feedback device have been conducted on humans6,8,19–21,50. Few were conducted on IHCA6,19–21. This is perhaps the first study to review the effectiveness of a CPR feedback device which provided depth, rate and RV feedback during IHCA. At the time of writing, there are ongoing studies using real-time feedback on these parameters conducted in Japan and England51,52.
Conclusions
CPR quality was suboptimal despite using a CPR feedback device to guide resuscitation. CPR providers and the CBT included in this study require further training with on-site CPR coaching. Despite its limitations, this study revealed that achieving higher CCF is achievable and essential for good outcomes in IHCA patients who achieve ROSC, further strengthening AHA recommendation to minimise interruptions during CPR. Nonetheless, future multicentre studies with a greater sample size exploring the clinical effects of CPR feedback device implementation will be helpful.
However, it should be emphasised that providing high-quality CPR is merely one factor which impacts the outcomes of IHCA patients. Patient outcomes are also dependent on a robust system involving training, protocols and post-resuscitation care16,35,43,44. Therefore, optimisation of post-resuscitation care and review of current systems are also essential in improving the prognosis of cardiac arrest patients43,44,53.
Acknowledgements
We thank our colleagues who attended the code blue activations and the Clinical Governance team at SKH (Smita Pathare, Ong Li Rong and Nur Athirah Azhari) for helping to compile and organise the data.
Author contributions
V.K.H. conceptualised this study, formulated the methodology, performed the investigations and provided supervision for the study. W.Z.L., D.C. and H.C.T. curated and performed a formal analysis of the data. W.Z.L. wrote the initial draft and V.K.H., D.C. and H.C.T. reviewed and edited the drafts. All authors have read and approved the final manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tortolani AJ, Risucci DA, Rosati RJ, Dixon R. In-hospital cardiopulmonary resuscitation: patient, arrest and resuscitation factors associated with survival. Resuscitation. 1990;20:115–128. doi: 10.1016/0300-9572(90)90047-i. [DOI] [PubMed] [Google Scholar]
- 2.Valenzuela TD, et al. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation. 2005;112:1259–1265. doi: 10.1161/circulationaha.105.537282. [DOI] [PubMed] [Google Scholar]
- 3.Neumar RW, et al. Part 1: executive summary: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S315–S367. doi: 10.1161/cir.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 4.Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med. 2007;33:237–245. doi: 10.1007/s00134-006-0326-z. [DOI] [PubMed] [Google Scholar]
- 5.Kleinman ME, et al. Part 5: adult basic life support and cardiopulmonary resuscitation quality: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S414–S435. doi: 10.1161/cir.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 6.Abella BS. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 7.Wik L. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 8.Kramer-Johansen J, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: A prospective interventional study. Resuscitation. 2006;71:283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Mehra R. Global public health problem of sudden cardiac death. J. Electrocardiol. 2007;40:S118–S122. doi: 10.1016/j.jelectrocard.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat. Rev. Cardiol. 2010;7:216–225. doi: 10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berdowski J, Berg RA, Tijssen JGP, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Kirkbright S, et al. Audiovisual feedback device use by health care professionals during CPR: A systematic review and meta-analysis of randomised and non-randomised trials. Resuscitation. 2014;85:460–471. doi: 10.1016/j.resuscitation.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Yeung J, et al. The use of CPR feedback/prompt devices during training and CPR performance: A systematic review. Resuscitation. 2009;80:743–751. doi: 10.1016/j.resuscitation.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Gugelmin-Almeida D, Tobase L, Polastri TF, Peres HHC, Timerman S. Do automated real-time feedback devices improve CPR quality? A systematic review of literature. Resuscitation Plus. 2021;6:100108. doi: 10.1016/j.resplu.2021.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nassar BS, Kerber R. Improving CPR performance. Chest. 2017;152:1061–1069. doi: 10.1016/j.chest.2017.04.178. [DOI] [PubMed] [Google Scholar]
- 16.Seethala Raghu R, E. E. C., Abella Benjamin S. Approaches to improving cardiac arrest resuscitation performance. Curr. Op. Crit. Care16, 196–202, 10.1097/MCC.0b013e328338c121 (2010). [DOI] [PubMed]
- 17.Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: A review. JAMA. 2019;321:1200. doi: 10.1001/jama.2019.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirlekar G, et al. Survival and neurological outcome in the elderly after in-hospital cardiac arrest. Resuscitation. 2017;118:101–106. doi: 10.1016/j.resuscitation.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Vahedian-Azimi, A. et al. Effect of the Cardio First Angel™ device on CPR indices: a randomized controlled clinical trial. Critical Care20, 10.1186/s13054-016-1296-3 (2016). [DOI] [PMC free article] [PubMed]
- 20.Goharani, R. et al. Real-time compression feedback for patients with in-hospital cardiac arrest: a multi-center randomized controlled clinical trial. J. Int. Care7, 10.1186/s40560-019-0357-5 (2019). [DOI] [PMC free article] [PubMed]
- 21.Amir Vahedian-Azimi, F. R., Andrew C Miller. A comparison of cardiopulmonary resuscitation with standard manual compressions versus compressions with real-time audiovisual feedback: A randomized controlled pilot study. Int. J. Crit. Illness Injury Sci.10, 32–37, 10.4103/IJCIIS.IJCIIS_84_19 (2020). [DOI] [PMC free article] [PubMed]
- 22.Yuanshan Liu ZH, Li H, Zheng G, Ling Q, Tang W, Zhengfei Yang CPR, feedback, prompt device improves the quality of hands-only CPR performed in manikin by laypersons following the, AHA guidelines. Am. J. Emerg. Med. 2015;36(1980–1985):2018. doi: 10.1016/j.ajem.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Abella BS, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73:54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Malanga, B. & Geheb, F. J. Emerging technologies A Primer to Real CPR Help ® Technology. (2007).
- 25.Jacobs I, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports. Circulation. 2004;110:3385–3397. doi: 10.1161/01.cir.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 26.Temple A, Porter R. Predicting neurological outcome and survival after cardiac arrest. Cont. Educ. Anaesthesia Crit. Care Pain. 2012;12:283–287. doi: 10.1093/bjaceaccp/mks029. [DOI] [Google Scholar]
- 27.Kovacs A, et al. Chest compression release velocity: Association with survival and favorable neurologic outcome after out-of-hospital cardiac arrest. Resuscitation. 2015;92:107–114. doi: 10.1016/j.resuscitation.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Niles DE, et al. Prevalence and hemodynamic effects of leaning during CPR. Resuscitation. 2011;82:S23–S26. doi: 10.1016/s0300-9572(11)70147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent A, et al. Does stress influence the performance of cardiopulmonary resuscitation? A narrative review of the literature. J. Crit. Care. 2021;63:223–230. doi: 10.1016/j.jcrc.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Allison E. Infinger, S. V., Jonathan R. Studnek. Introduction of performance coaching during cardiopulmonary resuscitation improves compression depth and time to defibrillation in out-of-hospital cardiac arrest. Resuscitation85, 1752–1758, 10.1016/j.resuscitation.2014.09.016 (2014). [DOI] [PubMed]
- 31.Adam Cheng, J. P. D., David Kessler, Nancy M. Tofil, Jennifer Davidson, Yiqun Lin, Jenny Chatfield, Linda L. Brown, Elizabeth A. Hunt. Optimizing CPR performance with CPR coaching for pediatric cardiac arrest: A randomized simulation-based clinical trial. Resuscitation132, 33–40, 10.1016/j.resuscitation.2018.08.021 (2018). [DOI] [PubMed]
- 32.Michael Buyck, Y. S., Jocelyn Gravel, Elizabeth A. Hunt, Adam Cheng, Arielle Levy. CPR coaching during cardiac arrest improves adherence to PALS guidelines: a prospective, simulation-based trial. Resuscitation Plus100058, 10.1016/j.resplu.2020.100058 (2021). [DOI] [PMC free article] [PubMed]
- 33.Kessler DO, et al. Influence of cardiopulmonary resuscitation coaching on interruptions in chest compressions during simulated pediatric cardiac arrest*. Pediatric Crit. Care Med. 2021;22:345–353. doi: 10.1097/pcc.0000000000002623. [DOI] [PubMed] [Google Scholar]
- 34.Gi Woon Kim, H. J. M., Hoon Lim, Yu Jin Kim, Choung Ah. Lee, Yong Jin Park, Kyoung Mi Lee, Jae Hyug Woo, Jin Seong Cho, Won Jung Jeong, Hyuk Joong Choi, Chang Sun Kim, Han Joo Choi, Il Kug Choi, Nam Hun Heo, Jung Soo Park, Young Hwan Lee, Seung Min Park, Dong Kil Jeong. Effects of Smart Advanced Life Support protocol implementation including CPR coaching during out-of-hospital cardiac arrest. Am. J. Emerg. Med.56, 211–217, 10.1016/j.ajem.2022.03.050 (2022). [DOI] [PubMed]
- 35.Cheng, A. et al. Part 6: Resuscitation Education Science: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation142, 10.1161/cir.0000000000000903 (2020). [DOI] [PubMed]
- 36.Christenson J, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009;120:1241–1247. doi: 10.1161/circulationaha.109.852202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaillancourt C, et al. The impact of increased chest compression fraction on return of spontaneous circulation for out-of-hospital cardiac arrest patients not in ventricular fibrillation. Resuscitation. 2011;82:1501–1507. doi: 10.1016/j.resuscitation.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milena Talikowska, H. T., Judith Finn. Cardiopulmonary resuscitation quality and patient survival outcome in cardiac arrest: A systematic review and meta-analysis. Resuscitation96, 66–77, 10.1016/j.resuscitation.2015.07.036 (2015). [DOI] [PubMed]
- 39.Cheema MA, et al. Duration of in-hospital cardiopulmonary resuscitation and its effect on survival. Indian Heart J. 2019;71:314–319. doi: 10.1016/j.ihj.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welbourn, C. & Efstathiou, N. How does the length of cardiopulmonary resuscitation affect brain damage in patients surviving cardiac arrest? A systematic review. Scandinavian J. Trauma, Resuscit. Emerg. Med.26, 10.1186/s13049-018-0476-3 (2018). [DOI] [PMC free article] [PubMed]
- 41.Meaney PA, et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital. Circulation. 2013;128:417–435. doi: 10.1161/cir.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 42.Girotra S, Chan PS, Bradley SM. Post-resuscitation care following out-of-hospital and in-hospital cardiac arrest. Heart. 2015;101:1943–1949. doi: 10.1136/heartjnl-2015-307450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merchant, R. M. et al. Part 1: Executive summary: 2020 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation142, 10.1161/cir.0000000000000918 (2020). [DOI] [PubMed]
- 44.Panchal, A. R. et al. Part 3: Adult basic and advanced life support: 2020 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation142, 10.1161/cir.0000000000000916 (2020). [DOI] [PubMed]
- 45.Fernando, S. M. et al. Pre-arrest and intra-arrest prognostic factors associated with survival after in-hospital cardiac arrest: systematic review and meta-analysis. BMJ, l6373, 10.1136/bmj.l6373 (2019). [DOI] [PMC free article] [PubMed]
- 46.Cheskes S, et al. Perishock pause. Circulation. 2011;124:58–66. doi: 10.1161/circulationaha.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deakin CD, R. W. K. Chest compression pauses during defibrillation attempts. Curr. Op. Crit. Care. 2016;22:206–211. doi: 10.1097/MCC.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 48.Hara, M., Hayashi, K., Hikoso, S., Sakata, Y. & Kitamura, T. Different Impacts of Time From Collapse to First Cardiopulmonary Resuscitation on Outcomes After Witnessed Out-of-Hospital Cardiac Arrest in Adults. Circulation: Cardiovas. Qual. Outcomes8, 277–284, 10.1161/circoutcomes.115.001864 (2015). [DOI] [PubMed]
- 49.Keerthi Talari MG. Retrospective studies - utility and caveats. J. R. Coll. Phys. Edinburgh. 2020;50:398–402. doi: 10.4997/JRCPE.2020.409. [DOI] [PubMed] [Google Scholar]
- 50.Hostler D, et al. Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, cluster-randomised trial. BMJ. 2011;342:d512–d512. doi: 10.1136/bmj.d512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirakawa, A. et al. Real-time feedback, debriefing, and retraining system of cardiopulmonary resuscitation for out-of-hospital cardiac arrests: a study protocol for a cluster parallel-group randomized controlled trial. Trials19, 10.1186/s13063-018-2852-8 (2018). [DOI] [PMC free article] [PubMed]
- 52.Perkins GD, et al. The effect of real-time CPR feedback and post event debriefing on patient and processes focused outcomes: A cohort study: trial protocol. Scandinavian J. Trauma, Resuscit. Emerg. Med. 2011;19:58. doi: 10.1186/1757-7241-19-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berg, K. M. et al. Part 7: Systems of Care: 2020 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation142, 10.1161/cir.0000000000000899 (2020). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


