Abstract
Background:
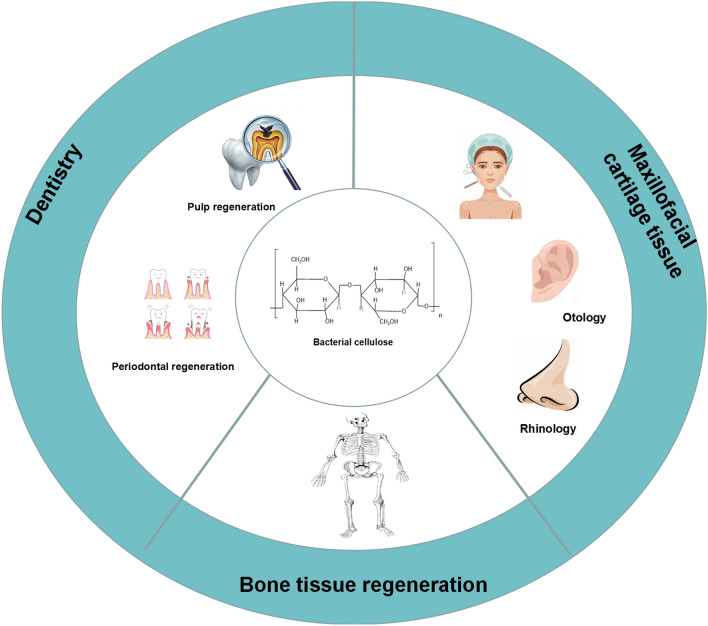
Cartilage, bone, and teeth, as the three primary hard tissues in the human body, have a significant application value in maintaining physical and mental health. Since the development of bacterial cellulose-based composite materials with excellent biomechanical strength and good biocompatibility, bacterial cellulose-based composites have been widely studied in hard tissue regenerative medicine. This paper provides an overview of the advantages of bacterial cellulose-based for hard tissue regeneration and reviews the recent progress in the preparation and research of bacterial cellulose-based composites in maxillofacial cartilage, dentistry, and bone.
Method:
A systematic review was performed by searching the PubMed and Web of Science databases using selected keywords and Medical Subject Headings search terms.
Results:
Ideal hard tissue regenerative medicine materials should be biocompatible, biodegradable, non-toxic, easy to use, and not burdensome to the human body; In addition, they should have good plasticity and processability and can be prepared into materials of different shapes; In addition, it should have good biological activity, promoting cell proliferation and regeneration. Bacterial cellulose materials have corresponding advantages and disadvantages due to their inherent properties. However, after being combined with other materials (natural/ synthetic materials) to form composite materials, they basically meet the requirements of hard tissue regenerative medicine materials. We believe that it is worth being widely promoted in clinical applications in the future.
Conclusion:
Bacterial cellulose-based composites hold great promise for clinical applications in hard tissue engineering. However, there are still several challenges that need to be addressed. Further research is needed to incorporate multiple disciplines and advance biological tissue engineering techniques. By enhancing the adhesion of materials to osteoblasts, providing cell stress stimulation through materials, and introducing controlled release systems into matrix materials, the practical application of bacterial cellulose-based composites in clinical settings will become more feasible in the near future.
Graphical abstract
Keywords: Bacterial cellulose, Hard tissue, Regenerative medicine, Dentistry, Bone
Introduction
Hard tissue defects or deficiencies in the human body, including cartilage, teeth, and bone, often arise due to various factors such as degenerative diseases, traumatic injuries, congenital defects, tumor resection, osteomyelitis debridement, aging, congenital diseases, and natural disasters [1]. These impairments have a significant impact on patients' quality of life, as well as their physical and mental well-being. Consequently, clinical interventions are necessary to address these issues, and promoting the regeneration of hard tissue has long been a formidable challenge in clinical surgery.
One specific challenge in the field of oral and maxillofacial surgery involves the biological regeneration and repair of hard tissue in this region. Oral and maxillofacial hard tissue includes cartilage and teeth, which are closely interconnected and mutually supportive. They play a crucial role in mastication, speech, and facial appearance, providing structural support for the maxillofacial region and contributing to the overall performance of the oral and maxillofacial system. However, conditions such as oral and maxillofacial tumors, trauma, and congenital deformities like jaw ameloblastoma, condylar dysplasia, and congenital jaw bone loss can result in varying degrees of cartilage and tooth loss in the maxillofacial region. Cartilage tissue poses unique challenges for regeneration due to its composition of a single cell type, low cell count, and limited proliferative activity of chondrocytes. Moreover, cartilage tissue lacks essential components such as blood vessels, nerves, and lymphatic vessels, making self-repair highly challenging. similarly, teeth, being the hardest tissues in the human body, consist of cementum, dentin, and enamel, but they also face difficulties in recovering from conditions like caries, trauma, or acid erosion. Despite advancements in correcting tooth and cartilage abnormalities, current techniques fall short of meeting clinical requirements. These approaches are often limited to laboratory animal models or have yielded unsatisfactory results and subsequent complications in clinical practice. Thus, there is a pressing need to improve the methods and concepts of maxillofacial hard tissue defect repair.
Compared to the unique characteristics of cartilage tissue and teeth, bone tissue defects are more prevalent. As one of the crucial hard tissues in the human body, bone tissue is primarily composed of type I collagen and hydroxyapatite (HAP) containing carbonate [2]. Each year, millions of patients worldwide suffer from bone defects resulting from severe trauma, fractures with infections, improper fracture treatments, bone tumors, and other complications. While bone tissue is active and possesses a certain degree of natural regenerative ability, the majority of bone injuries, such as fractures, abnormalities, and localized necrosis, can be managed through routine care, and minor bone damage areas often recover on their own. However, once a bone defect surpasses the critical size threshold (typically > 2 cm, depending on the anatomical location), it fails to heal spontaneously. Current bone reconstruction techniques often encounter issues such as complex procedures, lengthy surgical duration, prolonged bone healing time, delayed weight-bearing, low healing rates, poor tolerance, and numerous complications. Consequently, large bone deficiencies still necessitate treatment with bone transplants or artificial bone. In summary, the self-healing capacity of hard tissue is limited, and thus the regeneration of hard tissue requires the use of transplantation materials or substitutes. Bone transplantation remains the conventional method for bone regeneration [3]. The three primary approaches to bone transplantation include allogeneic bone transplantation, xenogeneic bone transplantation, and autologous bone transplantation. Allogeneic bone transplantation utilizes bones from other individuals, thereby introducing potential risks of disease transmission and immunological rejection, despite its improved effectiveness in generating new bone. Xenogeneic bone transplantation involves using animal bones, which exhibit relatively slow bone synthesis rates in the human body due to heterologous factors. In comparisonc to the previous two methods, autologous bone transplantation serves as the "gold standard" in traditional transplantation techniques [4]. It offers minimal and good bone bonding effects, but its supply is severely limited. This repair method typically involves two surgeries, which not only increases the risk of infection but may also result in deformities, pain, and even dysfunction [5].
Therefore, the search for an optimal artificial bone substitute has become a major focus in clinical research on regenerative medicine for hard tissue. The development of biodegradable and biocompatible biomaterials is crucial for enhancing the treatment of bone diseases. In response to pressing clinical needs, bone tissue engineering (BTE) has emerged as a promising field, offering a broad research platform for cartilage regeneration and the repair of bone tissue defects, instilling hope in patients. In recent years, BTE has evolved into a highly interdisciplinary field and has demonstrated success in addressing bone-related issues [6]. The approach involves creating artificial materials that replicate the biochemical and mechanical characteristics of natural bones and implanting them to restore their biological functions. In particular, the use of biocompatible materials with advanced functionality and design has been shown to enhance cell adhesion, proliferation, and differentiation [7, 8].
Recently, research in BTE primarily focuses on three main areas: seed cells, growth factors, and scaffold materials [9]. Among these, scaffold materials hold utmost importance as they provide a surface for growth factors to attach and facilitate cell proliferation [10]. The scaffold employed in BTE must fulfill certain requirements: (i) the materials used should be biocompatible and pose no harm to the host; (ii) natural degradation of thet material should obviate the need for further surgeries post-healing; (iii) the scaffold should possess interconnected pores to support cell attachment, vascularization, and the transport of nutrients and waste products [11]; and (iv) importantly, it must exhibit robust mechanical properties to withstand various stresses and maintain structural integrity. To address the limitations of scaffold materials, researchers are increasingly exploring modifications and doping techniques to improve their performance and utilize different scaffold options [12]. Currently, bone tissue engineering scaffolds are fabricated from diverse materials for bone replacement and healing, including metal materials, bioactive ceramics, and high molecular weight polymers. However, these materials possess inherent drawbacks, such as immune rejection, slow degradation, and suboptimal repair capabilities. Additionally, their physical, chemical, and structural properties significantly differ from those of human hard tissue, often leading to unsatisfactory repair outcomes.
The extracellular microbial substance called bacterial cellulose (BC) was initially discovered by British scientist Brown in 1886 [13]. BC is a naturally biodegradable nanostructured polymer produced by bacteria [14]. This nanomaterial consists of randomly arranged raw fibers with a width of < 100 nm, which aggregate into bundles of nanofibers with a width of 7–8 nm. In contrast to conventional materials, BC is composed of β-D-glucose units linked by β-1,4-glycosidic bonds, resulting in a specific stepwise assembly that gives rise to its remarkable properties. These properties include high purity, flexibility, and tensile strength (with Young's modulus of 15–18 GPa), a high surface area (due to the high aspect ratio of fibers with a diameter of 20–100 nm) [15], a high degree of polymerization, high crystallinity (84–89%), high water retention capacity (over 100 times its weight) [16], good biocompatibility, and controllable biodegradability and mechanical properties. Although BC shares the same chemical formula as plant cellulose((C6H10O5)n), it possesses a unique network structure with a width of approximately 0.01–0.10 µm, which is 2–3 orders of magnitude smaller in diameter than plant cellulose (generally 10 µm) [14]. Moreover, the fiber structure of BC closely resembles the collagen fibers found in bones. The combination of these excellent characteristics establishes BC as an appealing natural biopolymer material and an ideal candidate for bone regeneration scaffolds.
However, using BC alone presents many problems: (i) In maxillofacial cartilage tissue engineering, BC can be molded into precise shapes and possesses strong mechanical strength and chemical stability. While BC has great potential as a cartilage implant, its aperture is too small to promote chondrocyte proliferation and adhesion. (ii) In dentistry, BC itself exhibits excellent transparency, elasticity, durability, and easy formability, making it a good dental medical material. However, it lacks medical properties and antibacterial abilities and cannot accelerate the healing process or alleviate pain. (iii) In BTE, although BC demonstrates superior mechanical properties compared to other materials, it still falls slightly short of normal bone tissue and cannot support load-bearing bone implants. Additionally, it lacks biological activity, such as cellular recognition, intrinsic antibacterial activity, and antioxidant activity. This makes it challenging to adjust pore size and results in delayed degradation in vivo. Lastly, the BC scaffold itself lacks bone conduction effect. Therefore, the sole use of BC is often insufficient to meet all the requirements and expectations in the bone regeneration process [17]. These restrictions restrict the use of BC in hard tissue regenerative medicine. Hence, it becomes imperative to address the limitations of BC and explore strategies to enhance its potential and broaden its application in hard tissue regenerative medicine. In recent years, significant research efforts have been focused on modifying BC through in situ or non in-situ methods such as oxidation, esterification, etherification, and graft copolymerization. Another approach involves developing various BC-based composite scaffold materials to overcome its shortcomings and expand its utilization in BTE. These investigations have garnered significant attention and are considered a hot research topic [18–20].
This review primarily focuses on the application of BC-based composites (including natural polymers and synthetic polymers) in the field of hard tissue regenerative medicine, specifically in maxillofacial cartilage tissue, dentistry, and bone tissue. By summarizing the performance, advantages, and challenges associated with different BC-based composites, we aim to provide a comprehensive understanding of their capabilities. Ultimately, these composites can serve as a crucial strategy for the development of orthopedic and dental biomaterials.
Applications of BC-based composites in bone tissue regenerative medicine
Bone tissue, a rigid connective tissue, consists of cells, collagen fibers, and the bone matrix. The matrix primarily consists of solid inorganic salts, while the fibers act as binding agents. The deposition of calcium salts within the matrix contributes to the hardness of bone tissue, providing structural support to the body, facilitating bodily movement, maintaining mineralization balance, and safeguarding internal organs. Additionally, certain bones contribute to hematopoiesis and mineral balance regulation. Consequently, maintaining the integrity of bone tissue is vital for normal bodily functions. However, bone defects resulting from trauma, tumors, inflammation, and other factors are increasingly prevalent. Every year, millions of individuals worldwide require bone transplantation, with approximately 250,000 patients needing bone defect repair solely due to tumor resection.
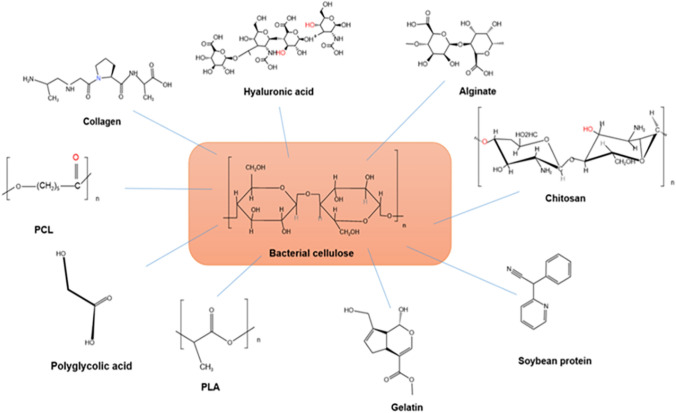
Unfortunately, many patients are unable to receive adequate treatment due to the lack of ideal replacement materials. Consequently, there is considerable clinical significance in the development of optimal artificial bone healing materials and related technologies. Autologous and allogeneic grafts used for bone defect repair often face limitations such as supply shortages, additional complications, and immune rejection reactions. Currently, BTE is deemed the most advanced approach to treating bone defects. It involves the cultivation and expansion of isolated autologous high concentration osteoblasts, bone marrow stromal stem cells, or chondrocytes in vitro. Subsequently, these cells are implanted onto a biocompatible scaffold or extracellular matrix, either naturally or artificially synthesized, that gradually degrades and is absorbed by the body. This process aims to repair bone tissue defects. Undoubtedly, the regeneration and repair of damaged bone tissue necessitate the coordinated use of three key components in tissue engineering: the scaffold, seed cells, and information factors. Among these components, the scaffold serves as the foundation and determinant of the success or failure of BTE. Regarding bone tissue engineering scaffolds, BC possesses a broad range of applications and is a focal point in international biomedical material research. This is owing to its distinctive three-dimensional fiber network structure resembling the extracellular matrix (ECM), biological compatibility, adaptability, high crystallinity, and excellent mechanical toughness. However, for scaffold materials in BTE, the generally accepted pore size ranges from 100 to 350 µm. Scaffolds with porosity exceeding 90% are more conducive to cellular and tissue ingrowth, promoting bone tissue regeneration. The natural nanofiber network structure of BC may be too dense for animal cells, thus, limiting its applicability in tissue engineering scaffolds. Furthermore, BC is less commonly employed as the sole scaffold in bone tissue synthesis due to its inadequate mechanical properties. So researchers often combine BC with various materials to create composite materials, thereby introducing new functionalities to BC and expanding its application in BTE, similar to other biomaterials. Synthetic polymers and natural polymers, both classified as degradable polymers, are commonly employed in conjunction with BC for bone tissue regeneration. However, each type of polymer presents its own set of advantages and disadvantages. For example, Poly(lactic acid) and polycaprolactone are examples of synthetic polymers that offer flexibility in terms of processing and modification. However, these polymers are often associated with drawbacks, including the risk of infection, immune response, and toxicity to the human body. On the other hand, natural polymers exhibit various desirable characteristics that mimic the ECM and are theoretically believed to be free from the defects associated with synthetic polymers [21]. Nevertheless, natural polymers still possess limitations, such as low mechanical strength, challenging processing, and fast degradation. Given these considerations, this chapter aims to investigate the advantages and disadvantages of incorporating BC with both synthetic and natural polymers in the context of BTE. This research endeavor is expected to make a valuable contribution to the field of BTE (Fig. 1).
Fig. 1.
Natural source polymer and synthetic polymer combined with BC
BC/synthetic polymer composites
In recent years, the combination of synthetic polymers with BC has led to significant advancements in the development of composite materials for bone regeneration [22]. Synthetic polymers, such as poly(lactic acid) (PLA) [23, 24], polyhydroxyalkanoate (PHB) [25], poly(vinyl alcohol) (PVA) [26, 27], polycaprolactone (PCL) [28], and polyacrylamide (PAM) [29], offer numerous advantages, including high chemical strength, excellent mechanical properties, and durability.
PLA, an aliphatic polyester, is a completely biodegradable and thermoplastic material. It can be metabolized into lactic acid within the human body without causing harm or triggering an inflammatory response. However, PLA's limited crystallinity and brittleness of its printed materials restrict its wide-ranging applications. Conversely, BC possesses good mechanical strength but lacks biodegradability within the body. To address these limitations, Wu et al. [23] developed a novel composite scaffold by combining BC with PLA. Compared to the pure BC membrane (25.61 MPa), the tensile strength of the PLA/BC composite scaffold increased to 66.49 MPa. Additionally, while the degradation rate of BC membrane in simulated bodily fluid was 14.38%, the degradation rate of the PLA/BC composite scaffold was 18.75%. This combination successfully resolved the issues of inadequate mechanical performance and non-biodegradability in vivo. Similarly, Zhang et al. employed vacuum freeze-drying and electrospinning techniques to fabricate a bilayer composite membrane consisting of polylactic-co-glycolic acid (PLGA), multi-walled carbon nanotubes (MWNTs), and bacterial cellulose (BC). This membrane served as a guided tissue regeneration (GTR) membrane [24]. After 12 weeks following GTR surgery, the experimental group exhibited a substantial amount of lamellar bone masses at the defect site, along with pleomorphic osteoblasts demonstrating active proliferation. These findings confirm the potential of PLA/BC composite materials for BTE.
Polyhydroxyalkanoate (PHB) is a commonly utilized bacterial-derived polymer that has garnered significant attention in bone regenerative medicine due to its exceptional mechanical properties, biodegradability, and favorable biocompatibility. Codreanu et al. investigated the in vitro biocompatibility of polyhydroxyalkanoate-modified bacterial cellulose (PHB/BC) scaffolds and their osteogenic potential in critical-sized mouse skull defects [25]. The study revealed that the PHB/BC scaffold promotes the proliferation of 3T3-L1 preadipocytes and exhibits a positive impact on osteoblast development in vivo, leading to the generation of new bone tissue 20 weeks post-implantation. Consequently, the newly developed PHB/BC scaffold holds promise as a suitable biomaterial for bone tissue engineering applications.
Poly(vinyl alcohol) (PVA) offers desirable characteristics such as flexibility, hydrophilicity, excellent chemical stability, high water content, and ease of molding, making it an ideal choice for bone regeneration scaffolds. Aki et al. employed 3D printing technology to fabricate a porous bone scaffold comprising poly(vinyl alcohol) (PVA), hexagonal boron nitride (hBN), and bacterial cellulose. This scaffold mimics the mechanical structure and physical properties of bone and was found to significantly enhance the activity of human osteoblasts [26]. Additionally, ChristyP et al. [27] explored a novel organic–inorganic hybrid CPBNC biological nanocomposite material by combining chitosan (C), poly(vinyl alcohol) (P), nano bioactive glass (B), and nano cellulose (NC) with the addition of 1%, 2%, and 3wt% nano cellulose as template materials for bone tissue applications. The resulting scaffold exhibited porosity comparable to cancellous bone (61–79%), making it an ideal candidate for cell inoculation. Furthermore, compared to the chitosan/PVA system, the scaffold demonstrated improved mechanical properties, thermal stability, osmotic characteristics, rigid network structure, cell adhesion, and mineralization enhancement.
Polycaprolactone (PCL) is a biodegradable polymer synthesized artificially, known for its good biocompatibility and processing properties. Although PCL exhibits slow degradation and produces fewer acidic decomposition products compared to other polyesters, it lacks bone induction capability, hydrophilicity, and sufficient biological activity, limiting its use in the field of bone regeneration. To overcome these limitations, the combination of PCL with bioactive particles to fabricate composite scaffolds has emerged as a promising approach. Cakmak et al. utilized polycaprolactone (PCL), gelatin (GEL), bacterial cellulose (BC), and hydroxyapatite (HA) as raw materials to develop a novel PCL/GEL/BC/HA composite scaffold using 3D printing technology [28]. The scaffold possessed an optimal pore size for BTE and demonstrated favorable cell survival and adhesion.
Polyacrylamide (PAM) is a widely used water-soluble polymer, obtained through homopolymerization or copolymerization of acrylamide with other monomers. Its structural units contain amide groups, allowing for the formation of hydrogen bonds, good water solubility, and high chemical activity. PAM can be easily modified by grafting or crosslinking, resulting in branched or network structures. Yuan et al. [29] employed bacterial cellulose nanofiber clusters (BCNC) as a novel reinforcing and physical crosslinking agent, replacing BCM, to polymerize acrylamide monomer in a polyacrylamide/BC nanofiber cluster suspension. This synthesis led to the formation of a polyacrylamide/bacterial cellulose (PAM/BC) hybrid hydrogel with high strength, toughness, and recyclability. The hybrid gel exhibited excellent biocompatibility, a breaking elongation of 2200%, and a breaking stress of 1.35 MPa. It could withstand nearly 99% strain without fracturing and immediately recover its original shape after the release of compressive forces. The potential of PAM/BC hydrogels as biomaterials for bone and cartilage repair shows promise.
In the creation of scaffolds for BTE, synthetic polymers offer several advantages compared to other materials. They possess predictable and consistent mechanical and physical properties, including tensile strength, compressive modulus, and degradation rate. By controlling factors such as degree of polymerization (molecular weight size), chemical composition, and crystallinity, the mechanical strength and degradation rate of the materials can be manipulated. Synthetic polymers can be shaped into various forms with desired pore structures, facilitating tissue growth. Consequently, the combination of BC with synthetic polymers has gained popularity in the construction of BTE scaffolds in recent years. However, it should be noted that these polymers undergo substantial erosion processes, leading to premature scaffold failure. Moreover, the sudden release of acidic breakdown products can trigger a significant inflammatory reaction. These considerations are crucial when composite scaffolds of this nature are frequently employed in clinical settings (Table 1).
Table 1.
Comparison of advantages and disadvantages of different synthetic polymers in BTE
| Composite | Advantage | Disadvantage | References |
|---|---|---|---|
| Poly(lactic acid) (PLA) | Materials with good mechanical pr-operties,good biocompatibility and biodegradability, low cost, toughness and elasticity | The heat resistance is general, and it starts to deform at a temperature above 60 °C, and it is not resistant to water or chemical corrosion | [23, 24] |
| Polyhydroxyalkanoate (PHB) | Good Biodegradability, biocompatibility,optical activity | Poor thermal stability, narrow processing window | [25] |
| Poly(vinyl alcohol) (PVA) | Good water solubility | If overheated, toxic vapor will be released; expensive | [26, 27] |
| Polycaprolactone (PCL) | Excellent mechanical strength and proper biodegradability, certain rigidity and strength | Insufficient strength of mechanical properties | [28] |
| Polyacrylamide (PAM) | It has good thermal stability, good water solubility and high chemical activity, small dosage and no odor and corrosivity when used | When the temperature is low, the dissolution time is long, and the dosage is too high, which will cause the water to be viscous | [29] |
BC/natural polymer composites
The primary limitation of synthetic polymers lies in their lack of cellular recognition signals and limited biological interactions with cells. Consequently, natural polymers have gained considerable attention in this regard due to their inherent biological and chemical resemblance to natural tissues, as well as their composition derived from living structures [30]. Natural polymers can form hydrogel structures that closely mimic the ECM, facilitating tissue formation [31]. Moreover, many natural polymers are integral components of the ECM and can enhance critical cellular processes such as cell division, adhesion, differentiation, and migration [32]. Natural biomaterials are derived from diverse sources, including animal proteins such as collagen and gelatin [33], seaweed-based alginate derived from the cytoplasm and cell wall [34], and chitosan obtained through chitin deacetylation. Currently, a wide range of natural polymers have been employed in the fabrication of BC/natural polymer composites. These include protein-based biopolymers such as collagen [35–37], gelatin [38, 39], soy protein [40], osteopontin [41], silk fibroin [42–46], as well as alginate [47–50], chitosan [51], among others. The BC/natural polymer composite material represents an innovative approach that harnesses the advantages of BC and natural polymers while compensating for their respective drawbacks. As a result, it has generated significant interest in the field of BTE.
Collagen and gelatin
The extracellular matrix (ECM) of bone tissue is a composite structure comprising inorganic compounds, primarily hydroxyapatite (HAp), and organic compounds, mainly collagen. In tissue engineering, there is a need to replicate the ECM, and collagen (Col) and gelatin derived from animal proteins have proven to be excellent biomimetic materials due to their composition.
Collagen, an abundant protein found in various animal tissues such as the skin [52], tendons, bones, muscles of mammals, and the skin, bones, and scales of aquatic animals, is a crucial component of the ECM itself. It possesses desirable characteristics, including non-toxicity, minimal immunogenicity, compatibility with human tissues, and the ability of the body to absorb degradation products, making it widely utilized in BTE. Lee et al. employed biomimetic technology to fabricate bacterial cellulose membranes (BCM) and compared them with absorbable collagen membranes for guiding bone regeneration [53]. The findings revealed that BC membranes exhibited superior mechanical properties, such as wet tensile strength, compared to collagen membranes. However, in vitro investigations demonstrated that cells cultured on BC membranes exhibited lower levels of morphological differentiation and cell proliferation rates compared to cells on collagen membranes. Consequently, combining and complementing the properties of both materials is anticipated to provide significant benefits in the field of BTE. Scholars have conducted research in this area. Zhang et al. employed Malaprade and Schiff base reactions to prepare three-dimensional porous microspheres comprising collagen (COL), bacterial cellulose (BC), and bone morphogenetic protein 2 (BMP-2) as a loaded growth factor [35]. The study showed that the 3D porous microspheres with multiple structures and components exhibited greater efficacy in promoting the adhesion, proliferation, and osteogenic differentiation of mouse MC3T3-E1 cells compared to BC alone. Noh et al. utilized freeze-drying techniques to fabricate BC/collagen composite scaffolds with varying volume ratios of BC and collagen [37]. The results demonstrated that scaffolds with a higher BC content exhibited improved physical stability, along with a water absorption rate of up to 400%, rendering them more resistant to shrinkage under humid conditions. In osteogenic induction experiments conducted on mesenchymal stem cells derived from umbilical cord blood (UCB-MSCs), the composite scaffold showed upregulation of osteogenic markers such as type I collagen, osteocalcin, and bone sialoprotein. Furthermore, the protein and calcium deposition were significantly increased on this composite scaffold. Saska et al. [36] synthesized and evaluated a nanocomposite for bone regeneration purposes, consisting of bacterial cellulose (BC), collagen (Col), apatite (Ap), and either osteogenic growth peptide (OGP) or its C-terminal pentapeptide [OGP (10–14)]. The findings indicated that the combination of collagen, apatite, and OGP peptide promoted cell proliferation more effectively than BC-Ap containing OGP. The composite also exhibited good bioactivity and osteoinductive properties. The aforementioned studies provide evidence that composite materials combining Col and BC have the potential to serve as scaffolds for BTE.
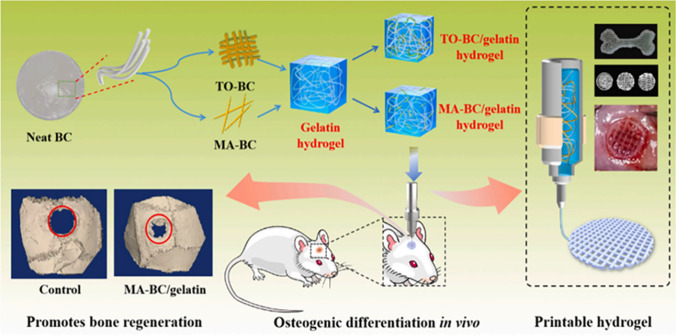
Gelatin is derived from collagen through the process of deformation induced by acid, alkali, or high temperature. It is a mixture of proteins and polypeptides. Gelatin exhibits lower antigenicity compared to collagen [54]. As a natural polymer, the molecular chains of gelatin contain repetitive arginine, glutamic acid, and aspartic acid (RGD) motifs, which facilitate the deposition of ECM and integrins. This property allows gelatin to modulate cell adhesion and physiological activities, including growth factor release, cell spreading, and blood vessel growth, thereby enhancing the overall biological behavior of biomaterials [55]. The advantage of gelatin lies in its ability to improve the bone-inductive capability that BC alone lacks. However, gelatin itself possesses poor mechanical properties and low heat resistance, rendering it unsuitable as a standalone material to meet the performance requirements of bone tissue engineering scaffolds. Thus, combining gelatin with BC, which exhibits excellent mechanical properties, presents a mutually beneficial solution. Research has demonstrated that the composite material formed by bonding BC and gelatin possesses a unique dual network structure, imparting it with remarkable mechanical strength. In comparison to pure BC and gelatin, the composite scaffold exhibits superior elastic modulus, compressive strength, and tensile strength [56]. Furthermore, compared to pure BC scaffold materials, the composite scaffold demonstrates enhanced biocompatibility, further highlighting its significant potential for BTE. Wang et al. [39] prepared an MA-BC-Gel composite scaffold by treating BC with TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl radical) and maleic acid (MA), following previous research protocols. This scaffold exhibited excellent performance in terms of increasing bone density in newly formed bone and trabecular thickness in rat skull bone regeneration, without causing any damage to visceral organs or serum in vivo.
Pure HAp bioceramics, when calcified at high temperatures, possess poor moldability, fragility, and lack bone induction activity, making it challenging to achieve a balance between their mechanical properties, biological functionality, and biodegradation. Gelatin, as a derivative of collagen, exhibits remarkable capabilities in promoting the self-assembly of synthetic bone nanocomposites. In their study, Yang et al. [38] employed a biomimetic approach to fabricate nanocomposites comprising oxidized bacterial cellulose (OBC), hydroxyapatite (HAp), and gelatin (Gel). OBC serves as the matrix, facilitating the fusion of gel and the coordination of HAp nanocrystals. This combination imparts the composite with enhanced mechanical strength and an increased capacity to withstand complexation with Ca2+. Due to the presence of numerous active groups (–COOH and –NH2) in crosslinked gelatin, the precipitated HAp is dispersed as microcrystals rather than HAp nanocrystals. The distributed and encapsulated HAp nanocrystals within the gelatin molecules effectively regulate the physiological activity of bone cells and control the release and degradation of HAp nanocrystals, thereby achieving sustained efficacy in bone defect healing (Fig. 2).
Fig. 2.
Preparation of bacterial cellulose-gelatin gel for in vivo bone regeneration [39]
Soybean protein, osteopontin, and silk fibroin
The self-assembly of proteins into functional nanoparticles has garnered significant interest as a means to fabricate novel biomaterials. Previous studies highlighted the intrinsic biological activity of gelatin and collagen proteins, demonstrating their potential as promising biomaterials in BTE without posing significant risks [57]. Protein-based biomaterials, due to their molecular structures resembling natural proteins found in tissues, hold promise for performing biological functions. Proteins that interact with cell receptors can serve as scaffolds for cellular growth, making surface modification of biomaterials with proteins a focus of research to enhance cell compatibility [58, 59]. While collagen and gelatin proteins offer numerous advantages, they also present practical challenges such as high cost, risk of cross-infection, difficulty in control, and unclear commercial sources [60]. Consequently, attention has shifted towards exploring other protein alternatives. In this section, we will primarily discuss other natural proteins utilized in BTE, including soy protein, osteopontin, and silk fibroin. These proteins have gained favor among experts in the field of BTE in recent years.
Soybean protein is a significant natural plant protein that has found various commercial applications as a food ingredient, additive, biodegradable film, and tissue engineering scaffold [61]. It possesses favorable characteristics such as abundant availability, toughness, excellent water solubility, high biocompatibility, good biodegradability, and non-immunogenicity [62]. Additionally, soybean protein contains bioactive compounds that resemble proteins found in the ECM. As a result, BC has been modified with soybean protein to develop bacterial cellulose/soy protein composites for bone tissue engineering applications. For example, Cai et al. [40] conducted a study where they modified BC electrospun nanofiber scaffolds with soybean protein. The results demonstrated that the surface modification with soybean protein did not significantly alter the crystalline structure of BC electrospun nanofibers, while improving their thermal stability, toughness, and tensile strength. Moreover, in vitro tests revealed that soybean protein-modified BC electrospun nanofiber scaffolds exhibited enhanced biodegradability and ALP activity in enzyme solutions compared to pure BC electrospun nanofiber scaffolds. Overall, the soybean protein-modified BC electrospun nanofiber composite scaffold exhibited increased biological activity and holds promise as a scaffold for bone tissue regeneration.
Osteopontin (OPN) is an extracellular glycosylated phosphoprotein that is present at the mineral/tissue interface of bones [63]. Its molecular weight ranges from 41 to 75 kDa due to differences in phosphorylation levels resulting from post-translational modifications [64]. Recently, recombinant human OPN (rhOPN) was successfully produced from Nicotiana benthamiana, a plant with high yield and low production costs. The structure of this plant-derived rhOPN (p-rhOPN) was found to be identical to that of commercially available rhOPN derived from mammalian cell lines. Coating a culture plate with p-rhOPN or chemically connecting it with a glass matrix grafted with poly(acrylic acid) (PAA) has been shown to promote the expression of osteoblast-related genes [65]. Based on these findings, Klnthoopthamrong et al. grafted a PAA brush onto a BCM, introducing multiple carboxyl groups that serve as active anchoring points for coupling with plant-derived recombinant human osteopontin p-rhOPN, resulting in p-rhOPN BCM [41]. Experimental results demonstrated that p-rhOPN-BCM exhibited enhanced hPDLSC adhesion and osteogenic differentiation compared to BCM alone. Initial research has indicated the potential of p-rhOPN-BCM as a GTR membrane for clinical applications, although further investigation into the mechanisms underlying bone regeneration and in vivo testing is necessary.
Silk fibroin (SF) is a natural protein polymer derived from silkworm cocoons that can be processed into various materials, including hydrogels, thin and thick films, 3D porous matrices, and adjustable diameter fibers. It exhibits excellent biocompatibility, minimal inflammatory reactions, water vapor permeability, and controllable biodegradability. Additionally, SF contains amino acids that act as cell receptors, facilitating critical connections between the ECM and mammalian cells. This promotes cell adhesion, proliferation, and possesses antimicrobial properties. However, regenerated silk fibroin has notable drawbacks such as brittleness, rapid degradation, and challenges in achieving consistent thickness. To overcome these limitations, researchers have explored the incorporation of BC to enhance the mechanical performance of SF. For instance, Choi et al. developed plate-like BC/SF composites and observed improved mechanical properties attributed to the addition of SF [43]. Lee et al. evaluated these composites in animal models and found that BC/SF materials enhanced new bone formation and significantly reduced the bone regeneration period, facilitating complete repair of zygomatic arch segmental abnormalities [46]. Chen et al. utilized a multi-stage freeze-drying method with a temperature gradient to obtain a three-dimensional SF/bacterial cellulose nano ribbon (BCNR) composite scaffold [45]. The results demonstrated that the incorporation of BCNRs into SF improved water absorption capacity and swelling rate of the composite scaffold. This biodegradable SF/BCNRs scaffold exhibited robust mechanical properties, enhanced cell adhesion, and promoted bone integration. Despite the mechanical suitability of BC/SF composites for bone regeneration, further investigation is required to ensure their biological safety. Barud et al. [44] conducted cytotoxicity experiments that confirmed the non-cytotoxic nature of BC/SF composites, providing additional evidence of their biosafety. In addition, the combination of BC with different types of silk fibroins can result in varying biological affinities and mechanical properties due to their distinct amino acid sequences. To explore this further, Jiang et al. [42] conducted an extensive investigation on Bombyx mori silk fibroin (BMSF) and Antheraea yamamai silk fibroin (AYSF). They synthesized BC-AYSF/HAp, BC-BMSF/HAp, and BC/HAp nanocomposites using a novel in situ hybridization method. The results revealed that BC-AYSF/HAp exhibited superior mechanical strength, interpenetration, and biocompatibility compared to BC/HAp and BC-BMSF/HAp. These characteristics are advantageous for nutrient and waste movement and promote cell adhesion. Thus, the BC-AYSF/HAp composite material holds great potential as an excellent platform for bone scaffolds or biomedical membranes in future applications, this research encourages further exploration of silk fibroin's utilization in bone regeneration, highlighting the importance of investigating different silk fibroin varieties.
Alginate
Alginate is a naturally derived anionic polysaccharide obtained from different bacteria and brown algae [66]. It has been reported that highly purified alginate does not elicit a significant foreign body reaction when transplanted into animals. Alginate exhibits excellent biocompatibility, low toxicity, and biodegradability. It is cost-effective, abundantly available, and can easily form a gel under mild conditions. When combined with calcium ions (Ca2+), alginate can form a calciumc alginate hydrogel with a porous structure, resembling a "box egg". This hydrogel inherits the advantageous properties of alginate and provides a protective environment for cells. It also facilitates the formation of interconnected 3D network pore structures, allowing for the transportation of cell nutrients and metabolic waste removal within the scaffold. Alginate has the potential for minimally invasive delivery into the human body to replenish damaged areas, making it advantageous for bone regeneration. However, pure alginate gel lacks the necessary cell adhesion sites for BTE and exhibits poor mechanical strength and slow biodegradability, limiting its practical applications [67]. To maximize its potential in bone regeneration, Yuan et al. [47] incorporated the antibacterial peptide β-defensin 2, which possesses antibacterial, osteogenic, and angiogenic properties, into a biomimetic nanofiber hybrid hydrogel composed of BCM and alginate/CaCl2. This composite membrane exhibited superior mechanical properties compared to pure BC and alginate alone, providing an appropriate environment for new bone formation. It also demonstrated prolonged antibacterial, angiogenic, and osteogenic effects, enabling the immobilization and sustained release of various growth factors or agents. This approach holds promise for achieving sustained release and enhancing the effectiveness of BTE and related biomedical applications. Zhu et al. [48] developed composite scaffolds using alginate and bacterial cellulose-chitosan (Alg/BC-CS). The addition of BC significantly reduced the pore sizes of Alg/BC-CS composite hydrogels, facilitating tissue ingrowth and ensuring mechanical integration. The excellent barrier performance, intermolecular hydrogen bonding, and dense fiber network structure of BC in combination with alginate allowed for precise control of the swelling behavior and prevented degradation of the Alg/BC-CS composite scaffold. Yan et al. [49] prepared composite scaffolds using alginate, bacterial cellulose nanocrystals, chitosan, and gelatin (Alg/BCNs CS-GT). These scaffolds exhibited superior compressive strength and biodegradability compared to pure BC. They also supported the attachment, proliferation, and differentiation of MC3T3-E1 osteoblasts. Similarly, Li et al. [50] developed composite scaffolds using alginate, bacterial cellulose nanocrystals, chitosan, and gelatin (Alg/BCNs CS-GT). The porous fiber network of BCNs faithfully mimicked the structure of the extracellular matrix, promoting cell adhesion and diffusion of MG63 and MC3T3-E1 cells on the Alg/BCNs CS-GT composite scaffold. These studies highlight the significant potential of alginate/BC composite scaffolds in the field of BTE.
Chitosan
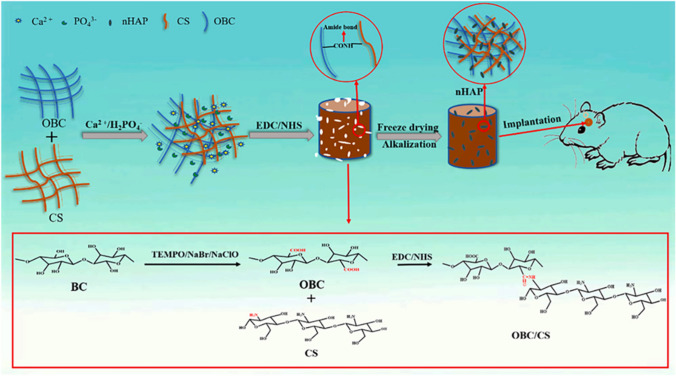
Chitosan is a linear polysaccharide derived from the deacetylation of chitin, consisting of glucosamine and N-acetylglucosamine units. It possesses a 1,4 glycosidic bond that promotes cell adhesion [68]. The presence of amino and hydroxyl groups on the chitosan molecular chain provides abundant active sites for covalent and ionic modifications [69]. Due to its non-toxicity, strong biocompatibility, biodegradability, and non-immunogenicity, chitosan holds great potential in the field of bone tissue engineering materials. When combined with BC, it further enhances its prospects. For instance, Cao et al. [51] incorporated nano hydroxyapatite (nHAP), obtained through in-situ crystallization of Ca2+/PO42− solution, uniformly into oxidized bacterial cellulose (OBC) and chitosan (CS) scaffolds to develop CS/OBC/nHAP bone healing composite scaffolds using freeze-drying and in-situ synthesis. The experimental results demonstrated that CS/OBC/nHAP scaffolds exhibited significantly higher mechanical properties and water retention performance compared to CS/nHAP scaffolds. Furthermore, they exhibited a more consistent degradation rate. These findings highlight the enhanced mechanical properties and water retention capabilities of CS/OBC/nHAP scaffolds, which contribute to their potential as effective materials for bone tissue engineering applications (Fig. 3; Table 2).
Fig. 3.
Preparation process of CS/OBC/nHAP scaffolds [51]
Table 2.
Comparison of advantages and disadvantages of polymers from different natural sources in BTE
| Composite | Advantage | Disadvantage | References |
|---|---|---|---|
| Collagen | No cytotoxicity, no irritation, good compatibility with human tissues, low immunogenicity | Low inflammatory symptoms, Low mechanical strength | [35–37] |
| Gelatin | It can regulate cell adhesion and physiological activity, as well as the release of growth factors, cell diffusion, and vascular growth, to improve the final biological behavior of biomaterials | Poor stability at high temperature, low mechanical strength | [38, 39] |
| Soybean protein | It has good mechanical toughness, ideal water solubility, good biodegradability, and non immunogenicity | Low compression strength and poor fatigue resistance | [40] |
| Osteopontin | High yield and low production costs | Poor and unstable mechanical strength | [41] |
| Silk fibroin | Good biocompatibility, rare inflammatory reactions, water vapor permeability, and controllable biodegradability | Easy to break and difficult to form uniform thickness | [42–46] |
| Alginate | It is non-toxic, has good biocompatibility, has no immunogenicity, is biodegradable, is cheap, has rich sources, and is easy to gel under mild conditions | It lacks tissue engineering cell adhesion sites, has poor and unstable mechanical strength, and has slow biodegradability, which greatly limits its practical application | [47–50] |
| Chitosan | It is non-toxic, biocompatible, biodegradable, non immunogenic, and can support cell adhesion | Unstable mechanical performance | [51] |
Applications of BC-based composites in maxillofacial hard tissue regenerative medicine
Cartilage tissue, a form of connective tissue in the human body [11], is primarily composed of collagen, a small number of cells, and 60–80% water. This slightly elastic and resilient tissue serves a crucial role in providing support and protection. In the adult body, maxillofacial cartilage tissue is primarily found in the auricle and nasal tip. Clinical practice frequently encounters injuries to facial cartilage tissue, such as those resulting from trauma, tumor resection, the aging population, and congenital conditions. However, due to the absence of a blood supply in mature cartilage tissue, it becomes challenging for undifferentiated cells to migrate to the injured area, impeding self-healing of the cartilage. Consequently, discovering an ideal approach to promote cartilage regeneration is an urgent challenge that plastic surgery must overcome.
Cartilage implants constitute a significant aspect of plastic surgery. Currently, the main traditional methods for treating cartilage abnormalities include autologous cartilage transplantation, autologous chondrocyte transplantation (ACI), cell culture therapy, and microfracture (bone marrow stimulation). While these technologies offer therapeutic benefits, they still possess limitations. These drawbacks encompass severe damage to the donor site, characteristics specific to the repair region, and subsurface healing of the surrounding cartilage and interface. For instance, the removal of cartilage from a patient's ribs to reconstruct facial structures may result in increased sensitivity in that area, as the newly created structure tends to be weaker than the original cartilage.
Given the pressing clinical need, cartilage tissue engineering has emerged as a promising approach, providing new possibilities for cartilage regeneration. An ideal material for cartilage tissue engineering must exhibit biocompatibility, sufficient porosity, and appropriate size to facilitate cell attachment, proliferation, and differentiation, thereby promoting cartilage tissue regeneration. It should also possess suitable flexibility and mechanical strength comparable to that of the surrounding healthy cartilage, preventing tissue damage caused by uneven tension between the repaired area and the neighboring normal cartilage. Furthermore, for scaffolds used in maxillofacial cartilage regeneration, it is crucial that their morphology aligns with the specific characteristics of the defect area.
BC has gained significant recognition in the field of maxillofacial cartilage tissue regeneration due to its three-dimensional network structure, remarkable mechanical properties, excellent chemical stability, high water absorption capacity, high crystallinity, and high purity [70]. Moreover, its structure is controllable, and it can be shaped into various forms, such as membranes and granules. These characteristics have garnered widespread attention in the field of regenerative engineering for maxillofacial cartilage tissue. However, as a nanoscale scaffold, BC itself has inherent limitations in terms of its three-dimensional nanostructures and small pore size, ranging from 100 to 300 nm, which are inadequate for cell adhesion and proliferation [71]. Furthermore, BC lacks biological functionality, further hindering its use in cartilage tissue engineering [72]. Therefore, BC needs to be modified or combined with other suitable materials [73]. Consequently, researchers are actively exploring composite materials that can be utilized in conjunction with BC to address its limitations and meet the requirements of cartilage tissue regeneration scaffolds, thereby expanding its applications in cartilage tissue engineering. This section focuses on the utilization of BC-based composite materials in ear and nasal cartilage regeneration.
Currently, in the field of otology, biodegradable materials such as polylactic acid, polyglycolic acid, and collagen [74] have been explored for ear cartilage engineering [75]. However, the poor shape stability and immune responses caused by degradation by-products have limited successful research outcomes thus far. Additionally, the use of non-viscoelastic and rigid biomaterials like metal or high-density polyethylene (HDPE) may pose problems, particularly considering the high extrusion rates observed in clinical outcomes when using standard HDPE allografts [76]. In comparison, BC exhibits good chemical stability and can provide ear cartilage with the necessary long-lasting mechanical properties and chemical stability due to its inherent characteristics [77]. Therefore, it is considered a potential and effective scaffold material for auricle cartilage regeneration [77]. Nevertheless, BC itself is electrically neutral, while cartilage contains charged groups [78] due to the presence of glycosaminoglycans (GAGs) in its collagen matrix. These charged groups in cartilage may influence its relaxation behavior. Hence, the use of BC alone cannot adequately simulate the mechanical properties of natural cartilage, limiting its application as an ear implant. Based on this understanding, Nimeskern et al. [79] suggest that charges can be introduced into bacterial nanocellulose (BNC) based composite structures by incorporating GAG produced by cells or chemically modifying BNC, thereby better mimicking the mechanical properties of natural ear cartilage, particularly enhancing its relaxation kinetics. The BC-based composite material developed using this approach exhibits mechanical characteristics highly similar to those of human ear cartilage. Furthermore, BC itself possesses a dense nanocellulose network, fulfilling the mechanical requirements for artificial ear grafts. However, the porosity of BC is limited, which hinders cell penetration into the material. To address this issue, Ávila et al. [80] developed a double-layer BNC composite scaffold by combining it with alginate. This scaffold offers a balance between mechanical stability and high porosity. The incorporation of alginate not only enables cell suspension, reducing cell loss after implantation, but also provides a three-dimensional environment that inhibits cell dedifferentiation.
Nasal cartilage repair remains challenging in nasal surgeries. Currently, rhinoplasty procedures commonly employ autologous rib cartilage grafts or other homologous or heterologous grafts for nasal stent reconstruction. Although autologous cartilage is considered the optimal transplant material, several significant limitations should be considered, including donor area scarring, meticulous preoperative cartilage carving requirements, and limited availability of well-differentiated autologous chondrocytes. Martinez Neto et al. [81] conducted research confirming the potential use of BC as an inert material for nasal septum repair. BC exhibits corrosion resistance, insolubility, and excellent mechanical properties, enabling the penetration of liquids and gases. Rabbit studies have indicated tissue tolerance when replacing septal cartilage with BC, thereby suggesting its suitability as a foundation for re-myelination of perforation boundaries. However, BC itself does not promote cartilage cell adhesion and proliferation. In light of this, Sang et al. introduced gelatin, known for its ability to enhance cell migration and differentiation, into BC, resulting in the creation of a Gelatin methacryloyl (GelMA)/hydroxyronic acid methacryloyl (HAMA)/BC composite hydrogel [82]. Gelatin modified with methacrylic anhydride exhibits excellent thermal sensitivity and photocrosslinking ability, thereby compensating for the limitations of using BC alone. Compared to autologous rib cartilage transplantation for nasal cartilage restoration, this BC-based composite material not only avoids donor site injury but also minimizes technical complexity and eliminates the need for time-consuming carving.
Currently, research on scaffold materials for maxillofacial cartilage tissue engineering encounters certain challenges, including striking a balance between material porosity and mechanical strength, as well as effectively integrating materials with cartilage tissue post-implantation. In this section, we aim to offer insights and inspiration to researchers by suggesting that modifying BC or combining it with other materials of interest can enhance its mechanical strength and biocompatibility while preserving its inherent advantages. This approach renders BC more suitable for the regeneration and repair of maxillofacial cartilage tissue. Currently, the majority of BC research in cartilage tissue regeneration focuses on articular cartilage, with limited investigation in the regeneration of maxillofacial cartilage. Future BC studies should therefore emphasize maxillofacial cartilage regeneration, enabling BC to have a more significant impact in the field of maxillofacial plastic surgery.
Applications of BC-based composites in dentistry
Human teeth consist of both hard tissue components (enamel, dentin, and cementum) and soft tissue components (pulp, periodontal ligament, and alveolar bone), all of which are comprised of connective tissue originating from the basement membrane [83].
Currently, limited research has been conducted on the direct use of BC in regenerating tooth hard tissue, specifically enamel and dentin. Instead, the application of BC in the oral field predominantly focuses on pulp regeneration and periodontal regeneration. This section aims to extensively discuss the advantages and challenges associated with BC application in the oral field, specifically in the context of pulp regeneration and periodontal regeneration. We hope that this section will provide valuable insights for future researchers interested in advancing direct tooth hard tissue regeneration (enamel, dentin) utilizing BC.
Pulp regeneration
Current research status of dental pulp regeneration
The dental pulp, the only vascularized connective tissue within the tooth structure, is shielded from external forces by highly mineralized enamel, cementum, and dentin [84]. Root canal therapy is a conventional and effective treatment approach for irreversible pulpitis and periapical diseases resulting from severe trauma, pulp infection, or necrosis. Although current root canal filling materials exhibit good biocompatibility and effectively seal the apical foramina [85], the treated tooth hard tissue is compromised, and the pulp loses its vitality, making it prone to complications such as tooth fracture. Moreover, microleakage of the crown can lead to secondary infections, while the loss of strain capacity makes the tooth susceptible to external stimuli. The presence of a vital pulp acts as a biological barrier, maintaining internal pressure, preventing bacterial re-invasion, reducing the risk of tooth fracture, and extending tooth lifespan. Consequently, replacing traditional root canal therapy with a biologically functional pulp represents a future trend in the treatment of pulp and periapical diseases.
With the extensive utilization of tissue engineering technology, regenerated tissue plays a pivotal role in restoring the morphology and function of damaged tissues and organs in the human body. Achieving pulp regeneration through tissue engineering involves the implantation of stem cells, appropriate signaling factors, and biological scaffold complexes into the prepared pulp cavity. The pulp's stem cells proliferate and differentiate into various cell types with the assistance of the scaffold and signaling molecules. Throughout this process, the scaffold components assimilate, contributing to the development of new pulp tissue. Replacing infected and necrotic dental pulp with a reconstructed dental pulp system represents a prospective trend in the future treatment of dental pulp diseases. This innovative technology for pulp regeneration shows promising results in closing the apical hole, thickening the root canal wall, and lengthening the root in young permanent teeth affected by pulp inflammation or necrosis. To enhance the success rate of dental pulp regeneration, the selection of an appropriate scaffold is crucial as it provides a suitable three-dimensional space and regulates stem cell differentiation, proliferation, and metabolism.
Currently, there are two main categories of scaffold materials employed in dental pulp regeneration research: synthetic polymers and natural materials. Synthetic biomaterials such as poly(lactic acid) (PLA) and poly(glycolic acid) (PGA) offer favorable mechanical strength and processing properties. They undergo degradation via simple hydrolysis, posing a low risk of immune reactions. Furthermore, they allow for the fabrication of scaffolds with diverse forms, mechanical characteristics, and controlled degradation times, making them suitable for clinical applications. However, these synthetic materials lack the biological cues required for cell proliferation and tissue formation, as well as the bioactive chemicals necessary for cell recognition and interaction within the natural extracellular matrix [86]. They also exhibit poor hydrophilicity and cellular activity, resulting in slow degradation in vivo. On the other hand, natural substances such as collagen, fibrin, silk, and alginate possess specific binding sites and the ability to engage cell receptors. These materials offer good biological activity, hydrophilicity, and compatibility with cell interfaces. However, their impurities, limited antigenicity, and inferior mechanical properties have hindered their further development in the field of regenerative medicine for dental pulp tissue [87].
Applications of BC-based composites in pulp regeneration
BC is a recently developed scaffold material with exceptional mechanical properties, versatility in form and porosity, achieved through the manipulation of bacterial fermentation [88]. Moreover, BC membranes can mimic the structural components of collagen, exhibiting nanoscale fibrils similar to those observed in collagen under a microscope [89]. This characteristic provides a favorable environment for cells, making BC an ideal material for dental pulp regeneration scaffolds. However, BC itself lacks bone-inducing properties, and its use alone does not stimulate the formation of mineralized tissue, limiting its application in dental pulp regeneration. Therefore, it is necessary to select a material that can compensate for this deficiency. Hydroxyapatite has traditionally been a focus due to its low biodegradation rate, excellent bone conduction, and biological activity, making it widely used in bone defect repair [90, 91]. However, recent studies have revealed that hydroxyapatite poses a hidden carcinogenic risk, prompting the search for alternative materials [92].
Researchers have explored a novel material called Nanolith, derived from fish inner ears, which consists of a gel matrix and calcium carbonate. It possesses minerals crucial for bone mineralization, similar to hydroxyapatite, but with reduced toxicity. Previous reports have shown that otoliths, the source of Nanolith, are an ideal material for promoting dental pulp regeneration, demonstrating in vivo biocompatibility with dental pulp tissue. They are comparable to calcium hydroxide and can be directly applied to the pulp to maintain vitality, promote the synthesis of mineralized tissue barriers, and initiate pulp reactions that facilitate healing. This biomaterial exhibits promising potential for future use in oral treatments [93, 94]. Building on this, Olyveira et al. [95] developed a composite scaffold composed of natural nanotopolith and biological cellulose. This scaffold demonstrates moderate in vivo degradation, biocompatibility, low cost, and inherent cell interactions, making it highly suitable for dental pulp tissue regeneration. However, further investigation is needed to address the subpar adhesion and cell viability of this scaffold, making it an area of focus for future research. Gelatin nanofibers play a vital role in maintaining the biological and structural integrity of various tissues and organs, such as bones, skin, tendons, blood vessels, and cartilage. However, the sol–gel transition temperature of gelatin is around 30 °C, which necessitates chemical crosslinking to prevent dissolution at body temperature. Acasigua et al. utilized a fermentation approach to combine gelatin and hyaluronic acid with BC to produce scaffolds with drug delivery capabilities, a porous structure, and improved cell adhesion. By establishing a connection between fermentation, surface morphology, and cell adhesion, they achieved scaffolds with diverse surface morphologies while maintaining consistent cell adhesion and attachment [96]. The findings suggest that BC/gelatin scaffolds exhibit excellent long-term cell adhesion and are highly effective for dental pulp tissue regeneration.
Pulp regeneration holds promise as a future approach for managing periapical disorders. However, there are still challenges to overcome in the clinical translation of tissue engineering in dental pulp, such as the selection of appropriate scaffolds, blood supply, growth factors, signaling mechanisms, cell migration, and differentiation, and the precise activities involved in the formation and regeneration of new tissues. Although BC has shown promising results in dental pulp regeneration, the widespread use of these scaffolds in clinical practice requires further improvement in scaffold-cell surface adhesion, including better control of scaffold production, fermentation, and filler aggregation. There is still considerable progress to be made in exploring the application of BC in this field.
Periodontal regeneration
Current research status of dental, periodontal regeneration
Periodontal tissue is a complex supporting structure of the teeth, consisting of the gingiva, periodontal ligament (PDL) [97], cementum, and alveolar bone. It plays a crucial role in tooth stability, load transmission, and nutrient supply [98]. Periodontal disease, identified as the most prevalent chronic inflammatory disease in the oral cavity, gradually deteriorates the tissues supporting the teeth, leading to tooth mobility and eventual loss. The prevalence of periodontal disease in adults is alarmingly high, reaching up to 80% [99]. Although mechanical plaque removal and drug control can reduce inflammation, the damage caused by bone loss, particularly alveolar bone loss, is irreversible [100].
The restoration of periodontal tissue defects is the primary goal of periodontal therapy. Conventional periodontal treatments and flap surgeries are effective in controlling periodontitis, but they face challenges in regenerating alveolar bone, cementum, and functional periodontal ligament fibers. Common regeneration techniques include GTR, GBR, and periodontal tissue engineering. In periodontal surgery, the GTR technique involves the placement of a barrier membrane between the gingival flap and the exposed root surface. By preventing the migration of gingival epithelial cells along the root surface, it hinders excessive contact between gingival connective tissue and the root, allowing periodontal membrane cells to occupy the root surface and create a regenerative space. This facilitates the guided formation of new cementum and the attachment of collagen fibers, ultimately leading to the formation of new attachment between the tooth and surrounding tissues. The concept of GBR is derived from GTR and involves the placement of a barrier membrane between soft tissue and bone tissue to establish a biological barrier. The membrane impedes the infiltration of connective tissue and epithelial cells, allowing precursor osteoblasts with slower migration but the greater regenerative potential to populate the bone defect area first. This promotes the regeneration of bone tissue by facilitating the entry of osteoblasts and subsequent bone formation. The ideal membrane or scaffold for GTR and GBR should mimic the composition of the extracellular matrix, attract stem cells from neighboring tissues, and promote their proliferation and differentiation [101]. Additionally, these membranes or scaffolds should exhibit excellent biocompatibility and a suitable degradation rate that aligns with the regeneration rate of the damaged tissue. They should also possess sufficient mechanical strength for surgical procedures [102] and possess osteoinductive properties to induce stem cell differentiation into osteogenic tissue, facilitating alveolar bone regeneration.
Currently, there are two main types of barrier membranes used in GTR/GBR technology: non-absorbable and absorbable membranes. Non-absorbable membranes, such as expanded polytetrafluoroethylene (e-PTFE) and titanium mesh, are rigid and require additional surgery for their removal. This not only carries the risk of damaging regenerated tissue and introducing new infections but also places psychological pressure on patients [103]. Absorbable membranes can be categorized into natural bioactive materials and synthetic polyester polymers. While these membranes degrade on their own without the need for secondary surgery, the rate of absorption during the degradation process is uncontrollable, leading to unstable membrane volume and inadequate maintenance of the desired spatial capacity. Furthermore, the generation of degradation by-products and poor mechanical properties are also challenges that need to be addressed [104]. Therefore, the search for absorbable membranes with improved mechanical properties is currently a focus of research in this field.
Applications of BC-based composites in periodontal regeneration
In recent years, there has been extensive research on the use of BC as a material for GTR/GBR membranes. BC is an excellent choice for barrier membranes due to its unique structural and biological properties. It consists of a three-dimensional nanofiber network composed of linear polysaccharide polymers connected by β-(1,4) glycosidic bonds. The surface morphology of BC closely resembles that of the ECM, facilitating interactions between cells and the material. Compared to other natural materials, BC exhibits superior mechanical characteristics, higher crystallinity, and enhanced water absorption. Moreover, BC can be shaped during the biosynthesis process, allowing it to conform better to the size and contour of the defect area when used as a GTR/GBR membrane [105]. However, BC has a high degree of crystallinity and lacks the β-(1,4) glycosidic bond enzyme present in the human body, making it non-biodegradable in vivo. While this non-degradability can be advantageous for long-term scaffolding, it limits its application as an absorbable membrane. Furthermore, although BC demonstrates excellent mechanical properties compared to other materials, it still falls slightly short of matching the properties of natural bone. These two significant limitations restrict the use of BC in GTR applications.
The biodegradability of BC can be significantly improved through appropriate oxidative modification. Luz et al. conducted a study using oxidation to promote BC degradation and introduced Sr ions to mimic the mineral composition of bone. The results showed that the OxBC/SrAp composite material exhibited stable degradation in vivo and could be removed without the need for a second surgery after tissue repair, making it an ideal absorbable GBR membrane [106]. Similarly, Gorgieva et al. treated BC-GEL using continuous periodate oxidation and freeze–thaw/carbodiimide crosslinking procedures. The composite membrane demonstrated favorable characteristics such as high swelling, maintainability, proper degradation, pH stability, and support for fibroblast proliferation and attachment on gel sites, making it a promising GTR membrane [107]. Various techniques have been proposed to enhance the biodegradability of BC in vivo by accelerating cellulose hydrolysis, including acid hydrolysis, alkali hydrolysis, oxidative delignification, organic solvent pretreatment, and ionic liquid pretreatment [108]. However, these methods have limitations. Residual chemicals from these treatments may have potential cytotoxicity, and controlling the degradation process accurately is challenging. Based on this, some scholars have attempted to control the interaction between BC and cells and the biodegradability of BC by introducing electron beam irradiation (EI). High-energy electron beam technology can effectively disrupt polymer chains and is now widely used for crosslinking or degrading polymers, as well as killing microorganisms [109]. The results of Lee et al. [110] indicate that BC films (EI-BCM) irradiated with electron beams have higher porosity and similar wet tensile strength compared to the most popular absorbable collagen films (CM) in the current dental field. The excellent mechanical strength, good cell adhesion and proliferation, and the capacity of the membrane to regenerate bone in bone defects surrounding the implant suggest that the irradiated EI-BCM may replace the current absorbable barrier membrane. An et al. [111] irradiated the BC membrane with 100 kGy or 300 kGy, respectively, to determine the optimal electron beam dose. The outcomes demonstrated that the application of a high-energy electron beam to BCM decreased wet tensile strength but enhanced in vitro cell responsiveness and in vivo bone repair of skull lesions. In clinical applications, EI-BCM irradiated with 100 kGy is more effective than BCM irradiated with 300 kGy as an absorbable membrane for GBR.
Multiwalled carbon nanotubes (MWNTs) are well-known for their strong biocompatibility with blood, bone, cartilage, and soft tissue, and their mechanical properties can significantly enhance the strength and toughness of composite materials [112]. In an effort to improve the mechanical properties of BC, Zhang et al. [113] developed a bilayer-polylactic-co-glycolic acid (PLGA)/MWNTs/BC composite membrane through vacuum freeze-drying and electrospinning techniques. The experimental results demonstrated that after 12 weeks of GTR surgery using this composite membrane, a significant amount of lamellar bone masses were observed at the defect site, and osteoblasts near the bone edge exhibited active proliferation, indicating a positive effect on promoting periodontal tissue regeneration.
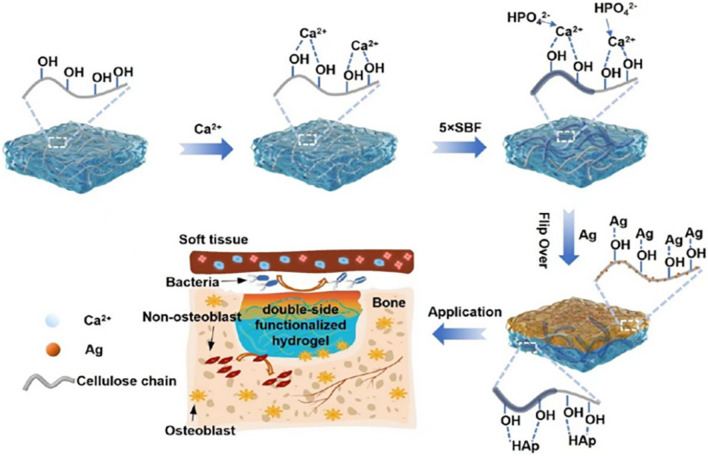
In addition to addressing the inherent limitations of BC, it is also important to explore its inherent advantages through composite materials. Collagen is a primary component of the ECM and collagen-based materials possess angiogenic properties that can stimulate mesenchymal stem cells to differentiate into osteoblasts and enhance the alveolar ridge [114]. To enhance BC's biological performance and make it more similar to the biological activity and surface morphology of the ECM, Saska et al. synthesized a BC COL-OGP (10–14) composite membrane, which incorporates osteogenic growth peptide [115]. The results indicated that the BC COL-OGP (10–14) composite membrane generated a higher proportion of bone tissue in the repair area compared to other membranes at 2 and 4 weeks. These biopolymer-based membranes show promise for bone regeneration applications. Moreover, loading bone morphogenetic protein-2 (BMP-2) onto the BC membrane can facilitate alveolar bone regeneration. Koike et al. conducted a study in a severe frontal bone defect model using male Japanese white rabbits to evaluate the bone regeneration ability of BC with BMP-2. The findings demonstrated that BC, combined with BMP-2, served as a barrier membrane, maintained the space, and acted as a sustained-release carrier for BMP-2, leading to excellent bone regeneration ability. This approach holds potential for enhancing maxillary sinus bone prior to clinical implantation [116]. To address the lack of antibacterial properties in BC itself and enable it to inhibit potential infections during periodontal tissue regeneration, silver (Ag) has garnered significant interest due to its non-drug resistance, broad-spectrum antibacterial action, and low toxicity to the human body [56]. Leveraging the antibacterial ability of Ag, Yang et al. developed a bionic silver/bacterial cellulose/hydroxyapatite (Ag/BC@HAp) composite membrane with a double-sided functional hydrogel network [117]. This composite membrane exhibits strong antibacterial activity, preventing infection, and also restricts the migration of endothelial cells and fibroblasts to the defect site. Furthermore, it demonstrates biocompatibility with MC3T3-E1 osteoblasts, achieving optimal GBR effects (Fig. 4).
Fig. 4.
Double-sided functionalized Ag/BC@HAp hydrogel preparation with GBR performance and antibacterial properties [117]
The advancement of materials science has driven the development of periodontal and bone tissue regeneration technologies, progressing from non-absorbable membranes to absorbable membranes and eventually to new multifunctional absorbable biological barrier membranes that combine the advantages of both. Although numerous cells and animal models have provided a foundation for clinical applications of multifunctional biological barrier membranes, their specific biological mechanisms, and clinical applications require further exploration. Considering clinical needs, it is essential to develop time-programmable barrier membrane materials, membranes with specific antibacterial and osteogenic functions during different stages of healing, multilayer barrier membranes with distinct functions in each layer, and biological barrier membranes with adjustable degradation rates. These advancements will contribute to the support of periodontal and bone tissue regeneration. Furthermore, periodontal tissue engineering technology plays an indispensable role in periodontal regenerative medicine. It involves three crucial factors: scaffold materials, seed cells, and growth factors. Among these factors, scaffold materials play a central role as they not only provide a suitable biological microenvironment for seed cells but also guide and regulate tissue regeneration.
In addition to utilizing GTR/GBR technology for periodontal regeneration, tissue engineering technology and biomaterials have emerged as research focal points in the field. Bioscaffold materials play a crucial role in providing the necessary space for regeneration, guiding cell proliferation and migration, and have become well-established components of tissue engineering. Researchers have explored the use of BC in the development of GTR scaffolds. For example, Pomegranate peel extract (PG extract), known for its wound-healing properties, is a bioactive phytochemical derived from pomegranate fruit peels. Bokhari et al. developed a freeze-dried composite GTR scaffold using BC, chitosan, hydroxyapatite, and PG extract. The scaffold demonstrated a microbicidal effect, high biocompatibility, and wound healing ability [118].
BCM, a membrane-like substance with a structure resembling collagen, is commonly used as an absorbable barrier membrane to promote tissue regeneration [119]. By incorporating active substances, the osteogenic performance of BC membrane can be enhanced for its application as a GTR/GBR membrane. Alternatively, by stacking multiple layers of fibers and modifying the manufacturing process, a three-dimensional structure can be created, serving as a tissue engineering scaffold. These approaches provide sustainable and biocompatible materials for periodontal regeneration applications. This section primarily focuses on the advantages and challenges associated with the use of BC in periodontal tissue regeneration, serving as a valuable addition to the field of periodontal regeneration (Fig. 5).
Table 3.
Applications of BC-based composites in dentistry
| Dental field | Application | Composites | Advantage | References |
|---|---|---|---|---|
| Pulp regeneration | Dental pulp regeneration scaffold | Nanotolith/BC | Moderate degradation rate, biocompatibility, low cost, and inherent cellular interactions in the body | [95] |
| BC/Gelatin | Good cell adhesion | [96] | ||
| Periodontal regeneration | GBR/GTR membrane | OxBC/SrAp | Stimulate osteoblasts in vivo and in vitro, with a stable degradation rate in vivo | [106] |
| OBC/Gel | Suitable degradability and pH stability | [107] | ||
| EI-BCM | Excellent mechanical strength, good cell adhesion and proliferation, and bone regeneration ability | [110] | ||
| EI-BCM | Increased in vitro cellular response and in vivo bone regeneration for skull defects | [111] | ||
| PLGA/MWNTs/BC | Good mechanical properties promote periodontal tissue regeneration | [113] | ||
| BC–COL OGP (10–14) | Inducing bone tissue formation | [115] | ||
| Ag/BC@HAp | Suppress potential infections and promote new bone formation | [117] | ||
| BC + BMP-2 | BC maintains graft space and continuously releases BMP-2, promoting optimal bone formation | [116] | ||
| GTR scaffold | CH–BC–HA–PG | This scaffold has bactericidal effect, high biocompatibility, and wound healing ability | [118] |
Fig. 5.
Rat calvarial defects were created by an in vivo surgical method. A, B In the middle of the cranium, create a defect model with a diameter of 8 mm using a trephine bur. C Using HA/β-TCP bone graft material to treat the defect site and then covered with 100 or 300 kGy irradiated BCM (D) [111] (Table 3)
Conclusion and future aspects
With the continuous progress in the fields of biomedicine and materials science, BC-based composite scaffold materials have made significant advancements in the application research of regenerative medicine and hard tissue engineering. These materials exhibit excellent properties such as biocompatibility, degradability, ease of processing and molding, and promote cell adhesion. As a result, they have begun to be implemented in clinical practice.
However, there are still challenges that need to be addressed for the widespread use of these composite scaffolds in clinical settings. Firstly, there are limitations in large-scale production, variations in finished products across different batches, and difficulties in controlling material factors such as mechanical strength and degradation rate. Secondly, the underlying mechanisms through which these scaffolds promote angiogenesis and osteogenic differentiation require further exploration and clarification. Although in vivo studies on mice and rabbits have been conducted, more research is needed to establish their suitability for human use. Additionally, bone defects caused by trauma or tumors often involve accompanying muscle and cartilage defects, making it necessary to consider multiphase repair strategies. Therefore, it is important to explore how these scaffolds can facilitate integrated repair of multiple tissue defects, such as bone-muscle or bone-cartilage integrated repair, to meet the clinical needs effectively.
In conclusion, BC-based composites hold great promise for clinical applications in hard tissue engineering. They have the potential to improve rehabilitation outcomes and enhance the quality of life for patients with various bone disorders and abnormalities. However, there are still several challenges that need to be addressed in BC-based composite materials. Further research is required, incorporating multiple disciplines and advancing biological tissue engineering technology. By enhancing the adhesion of materials to osteoblasts, providing cell stress stimulation through materials, and introducing controlled release systems into matrix materials, the practical application of BC-based composites in clinical settings will become more feasible in the near future.
Acknowledgements
This work is supported by Science and Technology Innovation Leader and Key Talent Team Project of Shanxi Province (202204051002034), Key Research and Development Plan of Shanxi Province (202102130501002), Scientific Research Project for Returned Overseas Professionals of Shanxi Province (2022-120), Key national science and technology cooperation project of Shanxi Provincial Department of Science and Technology (202204041101004) and the Shanxi Province Basic Research Program (free exploration) Project (20210302124085); Programs of Higher Education Institutions in Shanxi (2020L0212).
Author contributions
YL designed the structure and content,assembled the literature and wrote this review. HL, SG, JQ, RZ, XL, HC, LS, MZ, XW and BL provided suggestions and revised this review.
Declarations
Conflict of interest
There are no conflicts to declare.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yingyu Liu, Haiyan Liu and Susu Guo have contributed equally to this work.
Contributor Information
Xiuping Wu, Email: 77wxp@163.com.
Bing Li, Email: libing-1975@163.com.
References
- 1.Hu D, Ren Q, Li ZC, Zhang LL. Chitosan-based biomimetically mineralized composite materials in human hard tissue repair. Molecules. 2020;25:4758. doi: 10.3390/molecules25204785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarkar SK, Lee BT. Hard tissue regeneration using bone substitutes: an update on innovations in materials. Korean J Intern Med. 2015;30:279. doi: 10.3904/kjim.2015.30.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordana F, Le Visage C, Weiss P. Substituts osseux. Med Sci (Paris) 2017;33:60–65. doi: 10.1051/medsci/20173301010. [DOI] [PubMed] [Google Scholar]
- 4.Zhang HL, Zhou YL, Yu N, Ma HR, Wang KR, Liu JS, et al. Construction of vascularized tissue-engineered bone with polylysine-modified coral hydroxyapatite and a double cell-sheet complex to repair a large radius bone defect in rabbits. Acta Biomater. 2019;91:82–98. doi: 10.1016/j.actbio.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Deng JP, Song Q, Liu SY, Pei WH, Wang P, Zheng LM, et al. Advanced applications of cellulose-based composites in fighting bone diseases. Compos B Eng. 2022;245:110221. doi: 10.1016/j.compositesb.2022.110221. [DOI] [Google Scholar]
- 6.Stumpf TR, Yang XY, Zhang JC, Cao XD. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;82:372–383. doi: 10.1016/j.msec.2016.11.121. [DOI] [PubMed] [Google Scholar]
- 7.Saltzman WM. Drug delivery. Oxford University Press; 2001. Controlled drug delivery systems; pp. 235–280. [Google Scholar]
- 8.Biomimetic Materials. In: Tissue Engineering. CRC Press, 2007. p. 165–72.
- 9.Peng ZL, Zhao TS, Zhou YQ, Li SH, Li JJ, Leblanc RM. Bone tissue engineering via carbon-based nanomaterials. Adv Healthcare Mater. 2020;9:1901495. doi: 10.1002/adhm.201901495. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharjee P, Kundu B, Naskar D, Kim HW, Maiti TK, Bhattacharya D, et al. Silk scaffolds in bone tissue engineering: an overview. Acta Biomater. 2017;63:1–17. doi: 10.1016/j.actbio.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Khan S, Ul-Islam M, Ullah MW, Zhu YL, Narayanan KB, Han SS, et al. Fabrication strategies and biomedical applications of three-dimensional bacterial cellulose-based scaffolds: a review. Int J Biol Macromol. 2022;209:9–30. doi: 10.1016/j.ijbiomac.2022.03.191. [DOI] [PubMed] [Google Scholar]
- 12.Cohen IR, Lajtha A, Lambris JD, Paoletti R, Rezaei N. Biomimetic medical materials: from nanotechnology to 3D bioprinting. Noh I, editors. Adv Exp Med Biol. 2018;1064:1–399.
- 13.Andrade FK, Morais JPS, Muniz CR, Vieira JHONR, Gama FMP, Rosa MF. Stable microfluidized bacterial cellulose suspension. Cellulose. 2019;26:5851–5864. doi: 10.1007/s10570-019-02512-y. [DOI] [Google Scholar]
- 14.Pu Y, Jiang H, Zhang Y, Cao J, Jiang W. Advances in propolis and propolis functionalized coatings and films for fruits and vegetables preservation. Food Chem. 2023;414:135662. doi: 10.1016/j.foodchem.2023.135662. [DOI] [PubMed] [Google Scholar]
- 15.Gorgieva S, Trček J. Bacterial cellulose: production, modification and perspectives in biomedical applications. Nanomaterials. 2019;9:1352. doi: 10.3390/nano9101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteiro AS, De Oliveira M, Santagneli S, Carcel C, Gutmann T, Buntkowsky G, et al. Modification of bacterial cellulose membrane with 1,4-bis(triethoxysilyl)benzene: a thorough physical-chemical characterization study. J Phys Chem C. 2021;125:4498–4508. doi: 10.1021/acs.jpcc.0c09837. [DOI] [Google Scholar]
- 17.Wang J, Zhu Y, Du J. Bacterial cellulose: a natural nanomaterial for biomedical applications. J Mech Med Biol. 2011;11:285–306. doi: 10.1142/S0219519411004058. [DOI] [Google Scholar]
- 18.Ullah H, Santos HA, Khan T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose. 2016;23:2291–2314. doi: 10.1007/s10570-016-0986-y. [DOI] [Google Scholar]
- 19.Pandit A, Kumar R. A review on production, characterization and application of bacterial cellulose and its biocomposites. J Polym Environ. 2021;29:2738–2755. doi: 10.1007/s10924-021-02079-5. [DOI] [Google Scholar]
- 20.Deng J, Song Q, Liu S, Pei W, Wang P, Zheng L, et al. Advanced applications of cellulose-based composites in fighting bone diseases. Compos Pt B-Eng. 2022;245:110221. doi: 10.1016/j.compositesb.2022.110221. [DOI] [Google Scholar]
- 21.Bujoli B, Scimeca J-C, Verron E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics. 2019;11:556. doi: 10.3390/pharmaceutics11110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung K, Corrigan N, Wong EHH, Boyer C. Bioactive synthetic polymers. Adv Mater. 2022;34:2105063. doi: 10.1002/adma.202105063. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Wang Y, Wang F, Huang Y, He J. Preparation of 3D printed polylactic acid/bacterial cellulose composite scaffold for tissue engineering applications. Polymers. 2022;14:4756. doi: 10.3390/polym14214756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Wang J, Wang K, Xu L. A bilayered PLGA/multiwall carbon nanotubes/bacterial cellulose composite membrane for tissue regeneration of maxillary canine periodontal bone defects. Mater Lett. 2018;212:118. doi: 10.1016/j.matlet.2017.10.058. [DOI] [Google Scholar]
- 25.Codreanu A, Balta C, Herman H, Cotoraci C, Mihali CV, Zurbau N, et al. Bacterial cellulose-modified polyhydroxyalkanoates scaffolds promotes bone formation in critical size calvarial defects in mice. Materials. 2020;13:1433. doi: 10.3390/ma13061433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aki D, Ulag S, Unal S, Sengor M, Ekren N, Lin CC, et al. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater Des. 2020;196:109094. doi: 10.1016/j.matdes.2020.109094. [DOI] [Google Scholar]
- 27.Christy PN, Basha SK, Kumari VS. Multifunctional organic and inorganic hybrid bionanocomposite of chitosan/poly(vinyl alcohol)/nanobioactive glass/nanocellulose for bone tissue engineering. J Mech Behav Biomed Mater. 2022;135:105427. doi: 10.1016/j.jmbbm.2022.105427. [DOI] [PubMed] [Google Scholar]
- 28.Cakmak AM, Unal S, Sahin A, Oktar FN, Sengor M, Ekren N, et al. 3D Printed polycaprolactone/gelatin/bacterial cellulose/hydroxyapatite composite scaffold for bone tissue engineering. Polymers. 2020;12:1962. doi: 10.3390/polym12091962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan N, Xu L, Zhang L, Ye H, Zhao J, et al. Superior hybrid hydrogels of polyacrylamide enhanced by bacterial cellulose nanofiber clusters. Mater Sci Eng C Mater Biol Appl. 2016;67:221–230. doi: 10.1016/j.msec.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 30.Jabbari E. Challenges for natural hydrogels in tissue engineering. Gels. 2019;5:30. doi: 10.3390/gels5020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishnan B, Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev. 2011;111:4453–4474. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 32.Grande DA, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997;34:211–220. doi: 10.1002/(SICI)1097-4636(199702)34:2<211::AID-JBM10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.León-López A, Morales-Peñaloza A, Martínez-Juárez VM, Vargas-Torres A, Zeugolis DI, Aguirre-Álvarez G. Hydrolyzed collagen—sources and applications. Molecules. 2019;24:4031. doi: 10.3390/molecules24224031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reakasame S, Boccaccini AR. Oxidized alginate-based hydrogels for tissue engineering applications: a review. Biomacromolecules. 2018;19:3–21. doi: 10.1021/acs.biomac.7b01331. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Wang XC, Li XY, Zhang LL, Jiang F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr Polym. 2020;236:116043. doi: 10.1016/j.carbpol.2020.116043. [DOI] [PubMed] [Google Scholar]
- 36.Saska S, Teixeira LN, Raucci LMSdC, Scarel-Caminaga RM, Santos RAD, Santagneli SH, et al. Nanocellulose-collagen-apatite composite associated with osteogenic growth peptide bone regeneration. Int J Biol Macromol. 2017;103:467–476. doi: 10.1016/j.ijbiomac.2017.05.086. [DOI] [PubMed] [Google Scholar]
- 37.Noh YK, Costa ADSD, Park YS, Du P, Kim IH, Park K. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr Polym. 2019;219:210–218. doi: 10.1016/j.carbpol.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Zhen WJ, Chen H, Shan Z. Biomimetic design of oxidized bacterial cellulose-gelatin-hydroxyapatite nanocomposites. J Bionic Eng. 2016;13:631–640. doi: 10.1016/S1672-6529(16)60334-7. [DOI] [Google Scholar]
- 39.Wang X, Tang S, Chai S, Wang P, Qin J, Pei W, et al. Preparing printable bacterial cellulose based gelatin gel to promote in vivo bone regeneration. Carbohydr Polym. 2021;270:118342. doi: 10.1016/j.carbpol.2021.118342. [DOI] [PubMed] [Google Scholar]
- 40.Zhijiang C, Ping X, Shiqi H, Cong Z. Soy protein nanoparticles modified bacterial cellulose electrospun nanofiber membrane scaffold by ultrasound-induced self-assembly technique: characterization and cytocompatibility. Cellulose. 2019;26:6133–6150. doi: 10.1007/s10570-019-02513-x. [DOI] [Google Scholar]
- 41.Klinthoopthamrong N, Chaikiawkeaw D, Phoolcharoen W, Rattanapisit K, Kaewpungsup P, Pavasant P, et al. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int J Biol Macromol. 2020;149:51–59. doi: 10.1016/j.ijbiomac.2020.01.158. [DOI] [PubMed] [Google Scholar]
- 42.Jiang P, Ran J, Yan P, Zheng L, Shen X, Tong H. Rational design of a high-strength bone scaffold platform based on in situ hybridization of bacterial cellulose/nano-hydroxyapatite framework and silk fibroin reinforcing phase. J Biomater Sci Polym Ed. 2018;29:107–124. doi: 10.1080/09205063.2017.1403149. [DOI] [PubMed] [Google Scholar]
- 43.Choi Y, Cho SY, Heo S, Jin HJ. Enhanced mechanical properties of silk fibroin-based composite plates for fractured bone healing. Fibers Polym. 2013;14:266–270. doi: 10.1007/s12221-013-0266-5. [DOI] [Google Scholar]
- 44.Barud HGO, Barud HS, Cavicchioli M, Amaral TS, de Oliveira Junior OB, Santos DM, et al. Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaffold for tissue regeneration. Carbohydr Polym. 2015;128:41–51. doi: 10.1016/j.carbpol.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Zhuang A, Shao H, Hu X, Zhang Y. Robust silk fibroin/bacterial cellulose nanoribbon composite scaffolds with radial lamellae and intercalation structure for bone regeneration. J Mat Chem B. 2017;5:3640–3650. doi: 10.1039/C7TB00485K. [DOI] [PubMed] [Google Scholar]
- 46.Lee JM, Kim JH, Lee OJ, Park CH. The fixation effect of a silk fibroin-bacterial cellulose composite plate in segmental defects of the zygomatic arch. JAMA Otolaryngol Head Neck Surg. 2013;139:629–635. doi: 10.1001/jamaoto.2013.3044. [DOI] [PubMed] [Google Scholar]
- 47.Yuan Q, Li L, Peng Y, Zhuang A, Wei W, Zhang D, et al. Biomimetic nanofibrous hybrid hydrogel membranes with sustained growth factor release for guided bone regeneration. Biomater Sci. 2021;9:1256. doi: 10.1039/D0BM01821J. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Q, Chen X, Liu Z, Li Z, Li D, Lin Q. Development of alginate-chitosan composite scaffold incorporation of bacterial cellulose for bone tissue engineering. Int J Polym Mater Polym Biomater. 2021;72:296–307. doi: 10.1080/00914037.2021.2007384. [DOI] [Google Scholar]
- 49.Yan H, Chen X, Feng M, Shi Z, Zhang D, Lin Q. Layer-by-layer assembly of 3D alginate-chitosan-gelatin composite scaffold incorporating bacterial cellulose nanocrystals for bone tissue engineering. Mater Lett. 2017;209:492–496. doi: 10.1016/j.matlet.2017.08.093. [DOI] [Google Scholar]
- 50.Li Z, Chen X, Bao C, Liu C, Liu C, Li D, et al. Fabrication and evaluation of alginate/bacterial cellulose nanocrystals-chitosan-gelatin composite scaffolds. Molecules. 2021;26:5003. doi: 10.3390/molecules26165003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao S, Li Q, Zhang S, Liu K, Yang Y, Chen J. Oxidized bacterial cellulose reinforced nanocomposite scaffolds for bone repair. Colloid Surf B Biointerfaces. 2022;211:112316. doi: 10.1016/j.colsurfb.2021.112316. [DOI] [PubMed] [Google Scholar]
- 52.Hong H, Fan H, Chalamaiah M, Wu J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): current progress, challenges, and future perspectives. Food Chem. 2019;301:125222. doi: 10.1016/j.foodchem.2019.125222. [DOI] [PubMed] [Google Scholar]
- 53.Lee SH, Lim YM, Jeong S, An SJ, Kang SS, Jeong CM, et al. The effect of bacterial cellulose membrane compared with collagen membrane on guided bone regeneration. J Adv Prosthodont. 2015;7:484–495. doi: 10.4047/jap.2015.7.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez-Rodríguez R, Espinosa-Andrews H, Velasquillo-Martínez C, García-Carvajal ZY. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: a review. Int J Polym Mater Polym Biomater. 2020;69:1–20. doi: 10.1080/00914037.2019.1581780. [DOI] [Google Scholar]
- 55.Neffe AT, Loebus A, Zaupa A, Stoetzel C, Müller FA, Lendlein A. Gelatin functionalization with tyrosine derived moieties to increase the interaction with hydroxyapatite fillers. Acta Biomater. 2011;7:1693–1701. doi: 10.1016/j.actbio.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 56.Shi P, Sun H, Zhang H, Li S, Liang S, Lv Y, et al. Highly effective antibacterial properties of self-formed Ag nanoparticles/Zr-Ag granular films. Appl Surf Sci. 2023;622:156929. doi: 10.1016/j.apsusc.2023.156929. [DOI] [Google Scholar]
- 57.Zhu Y, Yang Q, Yang M, Zhan X, Lan F, He J, et al. Protein corona of magnetic hydroxyapatite scaffold improves cell proliferation via activation of mitogen-activated protein kinase signaling pathway. ACS Nano. 2017;11:3690–3704. doi: 10.1021/acsnano.6b08193. [DOI] [PubMed] [Google Scholar]
- 58.Chen WS, Guo LY, Masroujeh AM, Augustine AM, Tsai CK, Chin TY, et al. A single-step surface modification of electrospun silica nanofibers using a silica binding protein fused with an RGD motif for enhanced PC12 cell growth and differentiation. Materials (Basel) 2018;11:927. doi: 10.3390/ma11060927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Mao J, Chen Y, Song D, Gao Z, Zhang X, et al. Design of antibacterial biointerfaces by surface modification of poly (ε-caprolactone) with fusion protein containing hydrophobin and PA-1. Colloids Surf B. 2017;151:255–263. doi: 10.1016/j.colsurfb.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 60.Kong XD, Cui FZ, Wang XM, Zhang M, Zhang W. Silk fibroin regulated mineralization of hydroxyapatite nanocrystals. J Cryst Growth. 2004;270:197–202. doi: 10.1016/j.jcrysgro.2004.06.007. [DOI] [Google Scholar]
- 61.Li MC, Wu Q, Song K, Lee S, Jin C, Ren S, et al. Soy protein isolate as fluid loss additive in bentonite–water-based drilling fluids. ACS Appl Mater Interfaces. 2015;7:24799–24809. doi: 10.1021/acsami.5b07883. [DOI] [PubMed] [Google Scholar]
- 62.De Angelis E, Pilolli R, Bavaro SL, Monaci L. Insight into the gastro-duodenal digestion resistance of soybean proteins and potential implications for residual immunogenicity. Food Funct. 2017;8:1599–1610. doi: 10.1039/C6FO01788F. [DOI] [PubMed] [Google Scholar]
- 63.Martin SM, Ganapathy R, Kim TK, Leach-Scampavia D, Giachelli CM, Ratner BD. Characterization and analysis of osteopontin-immobilized poly(2-hydroxyethyl methacrylate) surfaces. J Biomed Mater Res A. 2003;67:334–343. doi: 10.1002/jbm.a.10060. [DOI] [PubMed] [Google Scholar]
- 64.Hao C, Cui Y, Owen S, Li W, Cheng S, Jiang WG. Human osteopontin: potential clinical applications in cancer (review) Int J Mol Med. 2017;39:1327–1337. doi: 10.3892/ijmm.2017.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rattanapisit K, Abdulheem S, Chaikeawkaew D, Kubera A, Mason HS, Ma JK-C, et al. Recombinant human osteopontin expressed in Nicotiana benthamiana stimulates osteogenesis related genes in human periodontal ligament cells. Sci Rep. 2017;7:17358. doi: 10.1038/s41598-017-17666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phatchayawat PP, Khamkeaw A, Yodmuang S, Phisalaphong M. 3D bacterial cellulose-chitosan-alginate-gelatin hydrogel scaffold for cartilage tissue engineering. Biochem Eng J. 2022;184:108476. doi: 10.1016/j.bej.2022.108476. [DOI] [Google Scholar]
- 67.Lee J, Lee KY. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009;26:1739–1744. doi: 10.1007/s11095-009-9884-4. [DOI] [PubMed] [Google Scholar]
- 68.Nishimura K, Nishimura S, Nishi N, Tokura S, Azuma I. Immunological activity of chitin derivatives. Vaccine. 1984;2:93–9. [DOI] [PubMed]
- 69.Bagher Z, Ehterami A, Safdel MH, Khastar H, Semiari H, Asefnejad A, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J Drug Deliv Sci Technol. 2020;55:101379. doi: 10.1016/j.jddst.2019.101379. [DOI] [Google Scholar]
- 70.Yuan H, Chen L, Hong FF. A biodegradable antibacterial nanocomposite based on oxidized bacterial nanocellulose for rapid hemostasis and wound healing. ACS Appl Mater Interfaces. 2020;12:3382–3392. doi: 10.1021/acsami.9b17732. [DOI] [PubMed] [Google Scholar]
- 71.Wang J, Yang C, Wan Y, Luo H, Fang H, Kerong D, et al. Laser patterning of bacterial cellulose hydrogel and its modification with gelatin and hydroxyapatite for bone tissue engineering. Soft Mater. 2013;11:173–180. doi: 10.1080/1539445X.2011.611204. [DOI] [Google Scholar]
- 72.Janmohammadi M, Nazemi Z, Salehi AOM, Seyfoori A, John JV, Nourbakhsh MS, et al. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioactive Mater. 2023;20:137–163. doi: 10.1016/j.bioactmat.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldmann EM, Sundberg J, Bobbili B, Schwarz S, Gatenholm P, Rotter N. 4Description of a novel approach to engineer cartilage with porous bacterial nanocellulose for reconstruction of a human auricle. J Biomater Appl. 2013;28:626–640. doi: 10.1177/0885328212472547. [DOI] [PubMed] [Google Scholar]
- 74.Bartlett W, Skinner JA, Gooding CR, Carrington R, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87B:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 75.Bichara DA, O'Sullivan NA, Pomerantseva I, Zhao X, Sundback CA, Vacanti JP, et al. The tissue-engineered auricle: past, present, and future. Tissue Eng Part B Rev. 2012;18:51–61. doi: 10.1089/ten.teb.2011.0326. [DOI] [PubMed] [Google Scholar]
- 76.Cenzi R, Farina A, Zuccarino L, Carinci F. Clinical outcome of 285 medpor grafts used for craniofacial reconstruction. J Craniofac Surg. 2005;16:526–530. doi: 10.1097/01.scs.0000168761.46700.dc. [DOI] [PubMed] [Google Scholar]
- 77.Martínez Ávila H, Schwarz S, Feldmann E-M, Mantas A, Bomhard A, Gatenholm P, et al. 4Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl Microbiol Biotechnol. 2014;98:7423–7435. doi: 10.1007/s00253-014-5819-z. [DOI] [PubMed] [Google Scholar]
- 78.Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Annu Rev Biomed Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 79.Nimeskern L, Martínez Ávila H, Sundberg J, Gatenholm P, Müller R, Stok KS. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J Mech Behav Biomed Mater. 2013;22:12–21. doi: 10.1016/j.jmbbm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 80.Martínez Ávila H, Feldmann EM, Pleumeekers MM, Nimeskern L, Kuo W, de Jong WC, et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials. 2015;44:122–133. doi: 10.1016/j.biomaterials.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 81.Martinez Neto EE, Dolci JEL. Fechamento de perfuração septal nasal em coelhos com celulose bacteriana. Braz J Otorhinolaryngol. 2010;76:442–449. doi: 10.1590/S1808-86942010000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sang S, Ma Z, Cao Y, Shen Z, Duan J, Zhang Y, et al. BC enhanced photocurable hydrogel based on 3D bioprinting for nasal cartilage repair. Int J Polym Mater Polym Biomater. 2023;72:702–713. doi: 10.1080/00914037.2022.2052727. [DOI] [Google Scholar]
- 83.Reinholt FP, Hultenby K, Oldberg A, Heinegård D. Osteopontin: a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang B, Xiao M, Cheng X, Bai Y, Chen H, Yu C, et al. Enamel matrix derivative enhances the odontoblastic differentiation of dental pulp stem cells via activating MAPK signaling pathways. Stem Cells Int. 2022;2022:2236250. doi: 10.1155/2022/2236250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang GTJ. Dental pulp and dentin tissue engineering and regeneration advancement and challenge. Front Biosci. 2011;E3:788–800. doi: 10.2741/e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gong T, Heng BC, Lo ECM, Zhang C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells Int. 2016;2016:9204574. doi: 10.1155/2016/9204574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cañas-Gutiérrez A, Osorio M, Molina-Ramírez C, Arboleda-Toro D, Castro-Herazo C. Bacterial cellulose: a biomaterial with high potential in dental and oral applications. Cellulose. 2020;27:9737–9754. doi: 10.1007/s10570-020-03456-4. [DOI] [Google Scholar]
- 88.De Oliveira Barud HG, Da Silva RR, Da Silva BH, Tercjak A, Gutierrez J, Lustri WR, et al. A multipurpose natural and renewable polymer in medical applications: bacterial cellulose. Carbohydr Polym. 2016;153:406–420. doi: 10.1016/j.carbpol.2016.07.059. [DOI] [PubMed] [Google Scholar]
- 89.Bäckdahl H, Esguerra M, Delbro D, Risberg B, Gatenholm P. Engineering microporosity in bacterial cellulose scaffolds. J Tissue Eng Regen Med. 2008;2:320–330. doi: 10.1002/term.97. [DOI] [PubMed] [Google Scholar]
- 90.Sopyan I, Mel M, Ramesh S, Khalid KA. Porous hydroxyapatite for artificial bone applications. Sci Technol Adv Mater. 2007;8:116–123. doi: 10.1016/j.stam.2006.11.017. [DOI] [Google Scholar]
- 91.Kattimani VS, Kondaka S, Lingamaneni KP. Hydroxyapatite: past, present, and future in bone regeneration. Bone Tissue Regener Insights. 2016;7:BTRI.S36138. doi: 10.4137/BTRI.S36138. [DOI] [Google Scholar]
- 92.Remya NS, Syama S, Sabareeswaran A, Mohanan PV. Investigation of chronic toxicity of hydroxyapatite nanoparticles administered orally for one year in wistar rats. Mater Sci Mater Biol Appl. 2017;76:518–527. doi: 10.1016/j.msec.2017.03.076. [DOI] [PubMed] [Google Scholar]
- 93.Costa LMM, de Olyveira GM, Basmaji P, Valido D. Novel otoliths/bacterial cellulose nanocomposites as a potential natural product for direct dental pulp capping. J Biomater Tissue Eng. 2012;2:48–53. doi: 10.1166/jbt.2012.1031. [DOI] [Google Scholar]
- 94.de Olyveira GM, Valido DP, Costa LMM, Gois PBP, Filho LX, Basmaji P. First otoliths/collagen/bacterial cellulose nanocomposites as a potential scaffold for bone tissue regeneration. J Biomater Nanobiotechnol. 2011;2:239–243. doi: 10.4236/jbnb.2011.23030. [DOI] [Google Scholar]
- 95.Olyveira GM, Acasigua GAX, Costa LMM, Scher CR, Filho LX, Pranke PHL, et al. Human dental pulp stem cell behavior using natural nanotolith/bacterial cellulose scaffolds for regenerative medicine. J Biomed Nanotechnol. 2013;9:1370–1377. doi: 10.1166/jbn.2013.1620. [DOI] [PubMed] [Google Scholar]
- 96.Acasigua GAX, de Olyveira GM, Costa LMM, Braghirolli DI, Fossati ACM, Guastaldi AC, et al. Novel chemically modified bacterial cellulose nanocomposite as potential biomaterial for stem cell therapy applications. Curr Stem Cell Res Ther. 2014;9:117–123. doi: 10.2174/1574888X08666131124135654. [DOI] [PubMed] [Google Scholar]
- 97.Raju R, Oshima M, Inoue M, Morita T, Yan H, Waskitho A, et al. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci Rep. 2020;10:1656. doi: 10.1038/s41598-020-58222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park CH. Biomaterial-based approaches for regeneration of periodontal ligament and cementum using 3D platforms. Int J Mol Sci. 2019;20:4364. doi: 10.3390/ijms20184364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis: a comprehensive review. J Clin Periodontol. 2017;44:S94–S105. doi: 10.1111/jcpe.12677. [DOI] [PubMed] [Google Scholar]
- 100.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of severe Periodontitis in 1990–2010: a systematic review and Meta- regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin YM, Yang HS, Chun HJ. Directional cell migration guide for improved tissue regeneration. In: Chun HJ, Reis RL, Motta A, Khang G, editors. Bioinspired biomaterials. Singapore: Springer; 2020. pp. 131–140. [DOI] [PubMed] [Google Scholar]
- 102.Abdelaziz D, Hefnawy A, Al-Wakeel E, El-Fallal A, El-Sherbiny IM. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J Adv Res. 2021;28:51–62. doi: 10.1016/j.jare.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. doi: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang Q, Huang X, Gu L. Periodontal bifunctional biomaterials: progress and perspectives. Materials. 2021;14:7588. doi: 10.3390/ma14247588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klemm D, Schumann D, Udhardt U, Marsch S. Bacterial synthesized cellulose — artificial blood vessels for microsurgery. Prog Polym Sci. 2001;26:1561–603. doi: 10.1016/S0079-6700(01)00021-1. [DOI] [Google Scholar]
- 106.Luz EPCG, Das Chagas BS, De Almeida NT, Andrade FK, Muniz CR, et al. Resorbable bacterial cellulose membranes with strontium release for guided bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;116:111175. doi: 10.1016/j.msec.2020.111175. [DOI] [PubMed] [Google Scholar]
- 107.Gorgieva S, Hribernik S. Microstructured and degradable bacterial cellulose-gelatin composite membranes: mineralization aspects and biomedical relevance. Nanomaterials. 2019;9:303. doi: 10.3390/nano9020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Czaja W, Kyryliouk D, DePaula CA, Buechter DD. Oxidation of γ-irradiated microbial cellulose results in bioresorbable, highly conformable biomaterial. J Appl Polym Sci. 2014;131:39995. doi: 10.1002/app.39995. [DOI] [Google Scholar]
- 109.Kim SM, Fan H, Cho YJ, et al. Electron beam effect on biomaterials I: focusing on bone graft materials. Biomater Res. 2015;19:10. doi: 10.1186/s40824-015-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee SH, An SJ, Lim YM, Huh JB. The efficacy of electron beam irradiated bacterial cellulose membranes as compared with collagen membranes on guided bone regeneration in peri-implant bone defects. Materials. 2017;10:1018. doi: 10.3390/ma10091018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.An SJ, Lee SH, Huh JB, Jeong SI, Park JS, Gwon HJ, et al. Preparation and characterization of resorbable bacterial cellulose membranes treated by electron beam irradiation for guided bone regeneration. Int J Mol Sci. 2017;18:2236. doi: 10.3390/ijms18112236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang H, Chen Z. Fabrication and characterization of electrospun PLGA/MWNTs/hydroxyapatite biocomposite scaffolds for bone tissue engineering. J Bioact Compat Polym. 2010;25:241–259. doi: 10.1177/0883911509359486. [DOI] [Google Scholar]
- 113.Zhang H, Wang J, Wang K, Xu L. A bilayered PLGA/multiwall carbon nanotubes/bacterial cellulose composite membrane for tissue regeneration of maxillary canine periodontal bone defects. Mater Lett. 2018;212:118–21. doi: 10.1016/j.matlet.2017.10.058. [DOI] [Google Scholar]
- 114.Schneider RK, Puellen A, Kramann R, Raupach K, Bornemann J, Knuechel R, et al. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2010;31:467–480. doi: 10.1016/j.biomaterials.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 115.Saska S, Pigossi SC, Oliveira GJPL, Teixeira LN, Capela MV, Gonçalves A, et al. Biopolymer-based membranes associated with osteogenic growth peptide for guided bone regeneration. Biomed Mater. 2018;13:035009. doi: 10.1088/1748-605X/aaaa2d. [DOI] [PubMed] [Google Scholar]
- 116.Koike T, Sha JJ, Bai YP, Matsuda YH, Hideshima K, Yamada T, et al. Efficacy of bacterial cellulose as a carrier of BMP-2 for bone regeneration in a rabbit frontal sinus model. Materials. 2019;12:2489. doi: 10.3390/ma12152489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang X, Huang J, Chen C, Zhou L, Ren H, Sun D. Biomimetic design of double-sided functionalized silver nanoparticle/bacterial cellulose/hydroxyapatite hydrogel mesh for temporary cranioplasty. ACS Appl Mater Interfaces. 2023;15:10506–10519. doi: 10.1021/acsami.2c22771. [DOI] [PubMed] [Google Scholar]
- 118.Bokhari N, Fatima T, Nosheen S, Iqbal F, Moeen F, Sharif F. Bioactive bacterial cellulose–chitosan composite scaffolds for prospective periodontal tissue regeneration. J Mater Res. 2023;38:1952–1962. doi: 10.1557/s43578-023-00930-0. [DOI] [Google Scholar]
- 119.Dutta SD, Patel DK, Lim KT. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J Biol Eng. 2019;13:55. doi: 10.1186/s13036-019-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]