Abstract
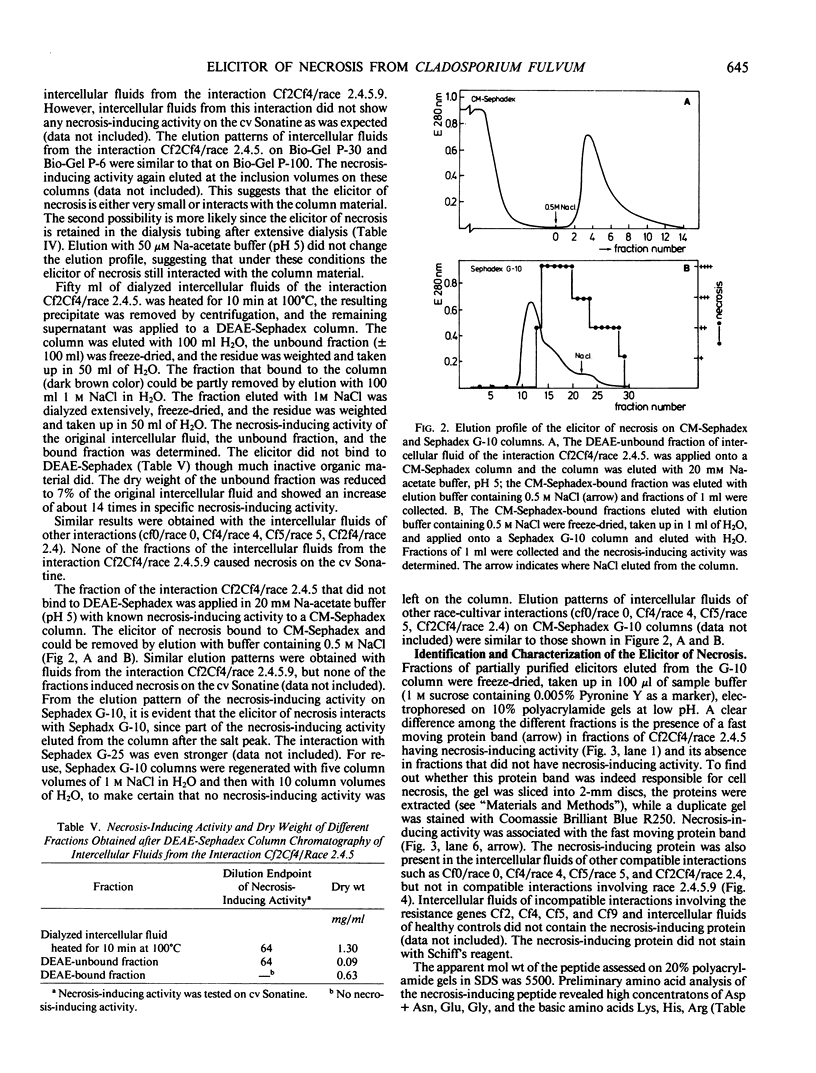
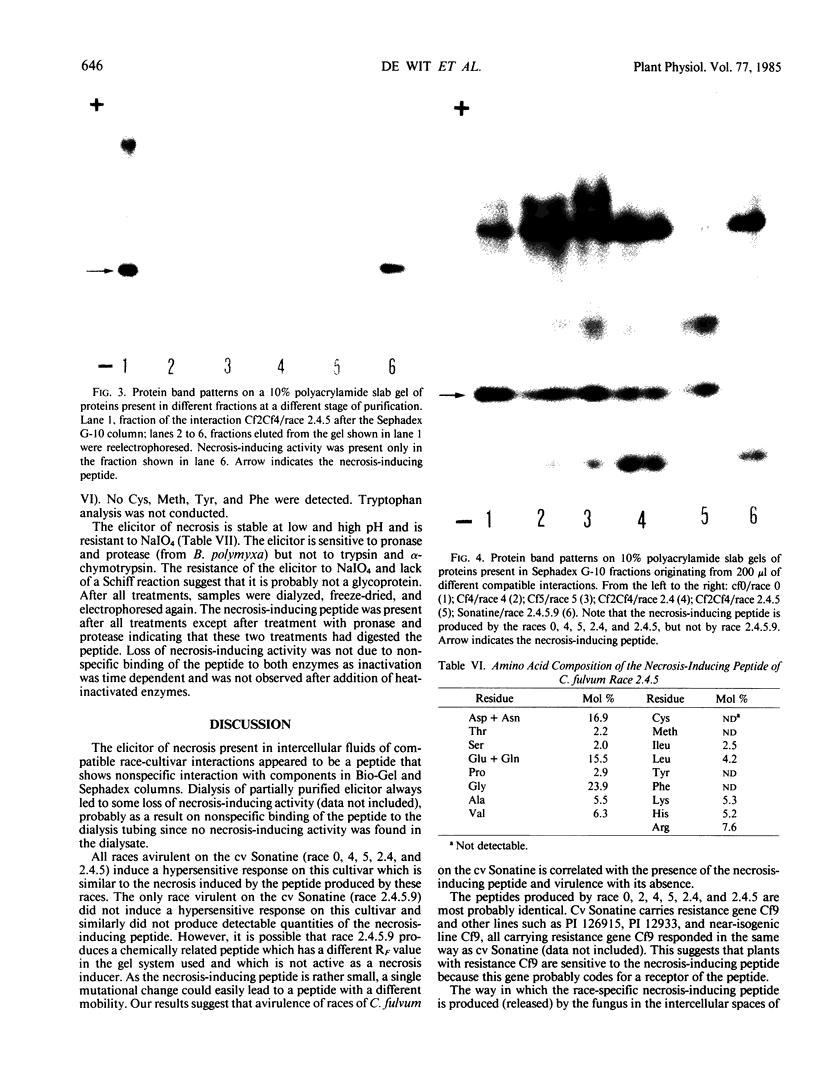
Intercellular fluids of compatible race-cultivar interactions of Cladosporium fulvum and tomato contain specific elicitors of necrosis. These elicitors which are of fungal origin induce chlorosis and necrosis in resistant but not in susceptible plants. With the tomato cultivar Sonatine (carrying resistance gene Cf9, resistant to the fungal races 0, 4, 5, 2, 2.4, and 2.4.5 but susceptible to race 2.4.5.9) as the test plant for assaying necrosis-inducing activity, we isolated and partially characterized an elicitor of necrosis on this cultivar. The elicitor bound to CM-Sephadex but not to DEAE-Sephadex; it was stable to heat (10 minutes at 100°C), HCl (0.01 normal), NaOH (0.01 normal), and NaIO4 (0.02 molar), sensitive to pronase and protease (from Bacillus polymyxa) but not to other proteases such as α-chymotrypsin and trypsin. After electrophoresis of partially purified elicitor preparations under low pH conditions, the necrosis-inducing activity was association with a peptide with an apparent molecular weight of 5500. Races 0, 4, 5, 2.4, and 2.4.5 but not race 2.4.5.9 produced this elicitor in high yields. The elicitor is probably a product of avirulence gene A9 which is present in all races except in race 2.4.5.9 and induces necrosis in cultivars carrying resistance gene Cf9.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Yoshikawa M., Wang M. C. Phytoalexin Elicitor Activity of Carbohydrates from Phytophthora megasperma f.sp. glycinea and Other Sources. Plant Physiol. 1983 Mar;71(3):466–471. doi: 10.1104/pp.71.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Yoshikawa M. beta-1,3-Endoglucanase from Soybean Releases Elicitor-Active Carbohydrates from Fungus Cell Walls. Plant Physiol. 1983 Mar;71(3):460–465. doi: 10.1104/pp.71.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Steck G., Leuthard P., Bürk R. R. Detection of basic proteins and low molecular weight peptides in polyacrylamide gels by formaldehyde fixation. Anal Biochem. 1980 Sep 1;107(1):21–24. doi: 10.1016/0003-2697(80)90486-8. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Matama M., Masago H. Release of a Soluble Phytoalexin Elicitor from Mycelial Walls of Phytophthora megasperma var. sojae by Soybean Tissues. Plant Physiol. 1981 May;67(5):1032–1035. doi: 10.1104/pp.67.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]