Abstract
Leaching bacteria such as Thiobacillus ferrooxidans attach to pyrite or sulfur by means of extracellular polymeric substances (EPS) (lipopolysaccharides). The primary attachment to pyrite at pH 2 is mediated by exopolymer-complexed iron(III) ions in an electrochemical interaction with the negatively charged pyrite surface. EPS from sulfur cells possess increased hydrophobic properties and do not attach to pyrite, indicating adaptability to the substrate or substratum.
The acidophilic, iron(II) ion-oxidizing bacteria Thiobacillus ferrooxidans and Leptospirillum ferrooxidans are the most important mesophiles for the extraction of metals from sulfidic ores by bioleaching (13, 27). Little is known about the interfacial processes leading to the degradation of metal sulfides (26), because of a complex interaction of electrochemical, biochemical, and surface-specific mechanisms (25, 33). Extracellular polymeric substances (EPS) mediate the contact between the bacterial cell and the sulfidic energy source, having a pivotal role in organic film formation and bacterium-substratum interactions (28). To understand their function, the chemical composition of EPS was analyzed.
(This article is based on the results of studies by T. Gehrke for a Ph.D. thesis at the Faculty of Biology, University of Hamburg, Hamburg, Germany.)
Microorganisms.
T. ferrooxidans R1 was used throughout this study (27). The bacteria were grown with iron(II) sulfate as the energy source (23) according to the method of Blake et al. (5).
Solid substrates.
Elemental sulfur (as finely divided powder; Merck) was sterilized in mineral salts medium at 112°C for 20 min. Flotation-grade pyrite grains (diameter, less than 100 μm) (30) were washed in acetone and in boiling 30% sulfuric acid and were rinsed to pH neutrality with deionized water. After drying, the grains were sterilized for 24 h at 120°C under nitrogen. Polished pyrite cubes (museum grade) for surface analysis were similarly treated.
Isolation and partial purification of EPS.
EPS were harvested by centrifugation at 12,000 × g (9). For EPS harvested from sessile cells, homogenization (3 min at 12,000 rpm [Ultra Turrax IKA model T 25 homogenizer]) of the substratum slurry (filter residue) in the presence of 10 mM Tris-HCl [pH 7]– 10−3 mM N-dodecyl-N,N-dimethyl-3-ammonio-1-propane-sulfonate (Zwittergent 3-12)–1 mM EGTA at 4°C (10) was additionally included. The supernatants with EPS were extensively dialyzed against pH 2 distilled water at 4°C in sterilized dialysis bags (cutoff, 3,500 Da). The remaining crude EPS were freeze-dried. The pellets consisting of bacteria without EPS are called EPS-deficient cells hereafter in this work. Contamination by membrane fragments was verified by 2-keto-3-deoxyoctonate analysis (21).
Chemical composition.
Aliquots of crude EPS were analyzed for phosphorous (according to DIN 38405-D11-2), nitrogen (34), iron species (3), and protein (8). Estimations of fatty acids and sugar monomers were achieved by gas-liquid chromatography (Carlo-Erba Fractovap 4160 with a flame ionization detector) using ultrapure standards (Sigma, PolyScience). Free fatty acids (FFA) were extracted with hexane from EPS samples, dried, and treated with 2 N dry methanolic hydrogen chloride for 1 h at 90°C under nitrogen, with dodecanoic acid used as the internal standard. After the addition of water the fatty acid methyl esters were extracted and dried. An aliquot was injected into a fused-silica capillary column (25 m) consisting of FFAP CB (Chrompack) at a split ratio of 1:25. A temperature program from 115°C to 240°C (4°C/min; initial holding period, 10 min) was used. Short-chain fatty acids were extracted with hexane after acidification of the EPS suspension (pH 2, HCl) and injected into the gas chromatograph without derivatization. Tightly bound fatty acids and the sugar composition of the crude EPS were analyzed according to the method of Hanson and Phillips (19). Neutral sugars, uronic acids, and hexosamines were analyzed as described by Chaplin (11, 12). Quantitative estimation of the uronic acids was spectrophotometrically performed (7).
Leaching experiments.
The media were inoculated with EPS-containing or -deficient cells (109/ml) supplemented with iron(III) sulfate. Degradation products were determined according to methods presented in references 3 and 14, and the reaction enthalpy was determined as heat output by microcalorimetry (31). To differentiate between chemical and biological activity, some samples were treated with chloroform and measured.
Attachment to solids.
Pyrite-, sulfur-, or iron(II) sulfate-grown EPS-containing cells and the corresponding EPS extracts were used. Attachment was examined by column chromatography with a strongly acidic or a strongly basic ion exchanger or a hydrophobic adsorbent resin (Serdolit CSG, Serdolit ASG-II, or Serdolit PAD I [Serva]). Samples (0.5 ml) containing 108 cells or 10 mg of EPS were added. After rinsing, attached EPS were eluted with 0.5 M HCl (cation exchanger), 0.5 M NaCl (anion exchanger), or pure methanol (adsorbent). EPS-containing cells were treated and eluted in the same way; however, methanol was replaced by hexanol. Cell numbers were determined by counting or the most-probable-number method; the amount of EPS was determined colorimetrically with glucose as the standard (24). Attachment to natural substrata (pyrite and sulfur) was determined by reduction of the planktonic cell numbers or the decrease of suspended EPS.
Attachment sites on pyrite.
The maximal number of attached cells was estimated by a shake flask assay using a pyrite cube, iron(II) sulfate-grown bacteria, and iron(III) sulfate. The amount of pyrite coverage was calculated by assuming a bacterial attachment surface of 2 × 10−12 m2/cell, a total surface of 1.4 × 10−3 m2/pyrite cube, and a bacterial monolayer (as observed on scanning or transmission electron micrographs). Images of attached bacteria were achieved by atomic force microscopy (AFM) using a NanoScope III device (Digital Instruments) operated in the contact mode. The number of cathodic (and anodic) surface sites on pyrite was evaluated by the scanning vibrating electrode technique (20) comparing inoculated with sterile surfaces. The current density maps allow the user to localize defective areas by increased or decreased current densities (anodic and cathodic sites, respectively). The bacterial generation of a strongly oxidizing environment by iron(III) ion production was determined by a scanning vibrating Kelvin microprobe (32). A pyrite surface (working electrode) was covered with sterile washing solution or droplets containing bacteria (5 × 108 cells/ml). Either autoclaved or iron(II) sulfate-grown, EPS-containing or -deficient cells were used.
Statistical analysis.
Most experiments were carried out in triplicate; corrosion potential and current density measurements were performed in duplicate. Results are given as arithmetic mean values. Standard deviations (SD) generally amounted to ≤40% (cell numbers), to 4% (chemical and gas-chromatography analyses), or to 12% (electrochemical analyses).
EPS analysis.
The yield of EPS from cells of T. ferrooxidans was nutrient dependent (Table 1). Iron(II) sulfate-grown cells produced little EPS, whereas pyrite-grown cells produced 13 times as much. Neither extraction nor additions influenced the determination of the composition. The 2-keto-3-deoxyoctonate concentration, indicating contamination by cell wall fragments, remained below the limit of detection. The EPS consisted mainly of sugars and lipids (Table 2) besides small amounts of nitrogen, phosphorus, and FFA. The chemical compositions of the EPS from iron(II) sulfate- and pyrite-grown cells were alike, whereas a higher content of lipids, FFA, and phosphorus was detectable for sulfur-grown cells. Protein and hexosamines were not detectable (data not shown). EPS from iron(II) sulfate- and pyrite-grown cells contained neutral sugars, glucuronic acid, and iron exclusively as iron(III) ions. In contrast to the findings of Agate et al. (1), the iron species were not lipid associated. The molar ratio of 2 mol of glucuronic acid to 1 mol of iron(III) ions indicates the formation of glucuronic acid-iron ion complexes. In EPS from sulfur-grown cells only glucose remained. Glucuronic acid was reduced by about 85%. Iron species were not detectable. The lipids of all EPS preparations were qualitatively comparable. Stearic acid contributed about 55% to the total lipid fraction. Unsaturated fatty acids were not detectable. The EPS composition of planktonic cells was comparable with the one from sessile cells (data not shown).
TABLE 1.
Amount and chemical composition of EPS from 1010 cells of T. ferrooxidansa
| Substrate | Mean EPS ± SD (μg/ 1010 cells) | % (wt/wt) of total EPS
|
||||
|---|---|---|---|---|---|---|
| Sugars | Lipids | FFA | Nitrogen | Phosphorus | ||
| Iron(II) sulfate | 215 ± 30 | 52.2 | 36.9 | 5.5 | 0.5 | 0.7 |
| Pyrite | 2,760 ± 301 | 48.5 | 39.4 | 5.8 | 0.5 | 0.8 |
| Sulfur | 1,155 ± 94 | 40.9 | 53.8 | 8.0 | 0.6 | 2.8 |
T. ferrooxidans cells were grown with iron(II) sulfate, pyrite, or sulfur. Experiments were carried out in triplicate. Results are given as arithmetic mean values. Except where otherwise stated, SD amount to 4%.
TABLE 2.
Chemical constituents of various fractions of EPS from cells of T. ferrooxidansa
| EPS fraction | Constituentb | % (wt/vol) of total EPS from cells grown on:
|
||
|---|---|---|---|---|
| Iron(II) sulfate | Pyrite | Sulfur | ||
| Sugar | Rhamnose | 13.9 | 10.8 | NDc |
| Fucose | 20.5 | 17.1 | ND | |
| Xylose | 0.9 | 0.8 | ND | |
| Mannose | 0.4 | 0.7 | ND | |
| Glucose | 11.4 | 15.2 | 40.4 | |
| Glucuronic acid | 4.4 | 3.3 | 0.6 | |
| Iron(III) ions | 0.7 | 0.5 | ND | |
| Lipid | C12:0 | 1.9 | 2.0 | 2.7 |
| C14:0 | 0.4 | 0.4 | 0.6 | |
| C16:0 | 8.8 | 9.4 | 12.9 | |
| C17:0 | 0.9 | 1.0 | 1.3 | |
| C18:0 | 20.2 | 21.6 | 29.5 | |
| C19:0 | 3.9 | 4.2 | 5.7 | |
| C20:0 | 0.8 | 0.8 | 1.1 | |
| FFA | C16:0 | 1.7 | 1.8 | 2.4 |
| C18:0 | 3.8 | 4.0 | 5.6 | |
T. ferrooxidans cells were grown with iron(II) sulfate, pyrite, or sulfur. Experiments were carried out in triplicate. Results are given as arithmetic mean values. SD amount to 4%.
C’s with subscripts show equivalent chain lengths of fatty acids.
ND, not detected (<0.08%).
Leaching experiments.
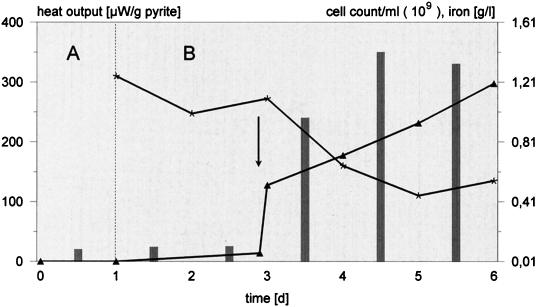
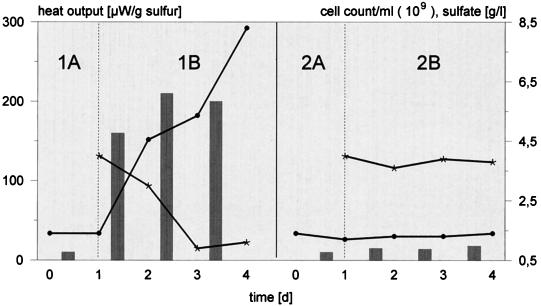
Leaching experiments were conducted with EPS-deficient cells and pyrite. These cells replenish their capsular material within a few hours (18). Furthermore, a sufficient amount of iron(III) ions in the medium (≥0.2 g/liter), to be complexed by the uronic acids in the exopolymers, was needed. Figure 1 indicates that pyrite oxidation remained negligible without iron(III) ion supplementation. Afterwards, iron concentration and heat output significantly increased, whereas the cell count of planktonic bacteria fell by about 50%. The attack on sulfur was independent of iron(III) ions. EPS-deficient cells with or without supplemented iron(III) ions exhibited comparable dissolution rates of about 2.5 g/liter of sulfate/ day (data not shown). Iron(II) sulfate-grown cells neither attached to nor metabolized sulfur unless their iron-containing EPS were removed (Fig. 2).
FIG. 1.
Importance of iron(III) ions for the onset of pyrite dissolution (bioleaching) by cells of T. ferrooxidans. Pyrite dissolution was measured before (A) and after (B) inoculation with EPS-deficient cells as an increase of total iron concentration in the medium (triangles) and heat output on the substratum (bars). Asterisks show the cell concentration of planktonic bacteria. The arrow indicates the addition of 0.5 g of iron(III) ions per liter. d, days.
FIG. 2.
Dependence of sulfur dissolution (bioleaching) on EPS composition for cells of T. ferrooxidans. Dissolution was measured before (sterile) (A) and after (B) inoculation with EPS-deficient (1) or -containing (2) cells of iron(II) sulfate-grown bacteria by sulfate concentration in the medium (circles), heat output on the substratum (bars) and cell concentration of planktonic bacteria (asterisks). d, days.
Mechanism of attachment.
In Table 3 the data indicate that attachment was dependent on the growth substrate. Whereas sulfur-grown cells attached only to hydrophobic surfaces (sulfur, adsorbent resin), iron(II) sulfate-grown cells adhered exclusively to negatively charged substrata (pyrite [6, 15], cation-exchange resin). Pyrite-grown cells accepted both substrata. The same results were obtained with cell-free EPS (data not shown). Obviously, charge effects are involved in attachment, a theory which is corroborated by earlier studies on the molecular structure of pyrite (22). Those cations or molecules acting as Lewis acids (accepting the unshared electron pair of pyritic sulfur), like EPS-complexed iron species, will be preferentially attracted. Attachment to hydrophobic substrata such as sulfur is dominated by van der Waal’s attraction forces. The pyrite-grown cells possess an intermediate chemical EPS composition, allowing them consequently to attach to both substrata.
TABLE 3.
Attachment of differently grown cells of T. ferrooxidans to various substrataa
| Substratum | Cells grown with:
|
||
|---|---|---|---|
| Iron(II) sulfate | Pyrite | Sulfur | |
| Pyriteb | ++ | ++ | − |
| Sulfurb | − | + | ++ |
| Cation exchangerb | ++ | ++ | − |
| Anion exchangerc | − | − | − |
| Hydrophobic adsorberb | − | + | ++ |
The attachment of T. ferrooxidans cells (108 cells/assay) to natural (pyrite and sulfur) and artificial substrata (ion-exchange resins and hydrophobic adsorbent resin) was assayed. ++, strong attachment (80 to 95% of inoculated cells attached to mineral); +, weak attachment (25 to 35%); −, no attachment (0%).
Tested at pH 2.
Tested at pH 7.
Pyrite colonization.
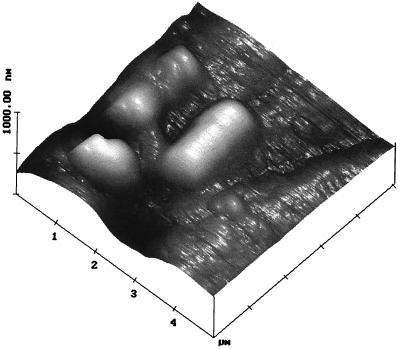
AFM images demonstrated that T. ferrooxidans specifically attached to dislocation sites on pyrite, such as cracks and grain boundaries (4) (Fig. 3). A statistical evaluation indicated that 76% of all cells adhered to (visible) surface imperfections. Furthermore, calculations based on the decrease of the planktonic cell count during initial cell-mineral interaction yielded a maximal surface utilization of 40%. This finding agrees with the previous one, since the amount of dislocations and cracks is limited. Andrews (2) suggested that selective attachment to pyrite is associated with the occurrence of distinct dislocation sites, where sulfur atoms are accumulated, constituting cathodically active regions. On the pyrite surface cathodic and anodic sites exist, but their diameters are (as opposed to steel [diameter, 10 to 50 μm]) less than 10 μm. Hence, scanning vibrating electrode technique experiments with a lateral minimal resolution of 10 μm remained without result (data not shown). Obviously, flat-spread corrosion phenomena occur on pyrite.
FIG. 3.
AFM image of a cell of T. ferrooxidans specifically attached to a dislocation area (surface fault).
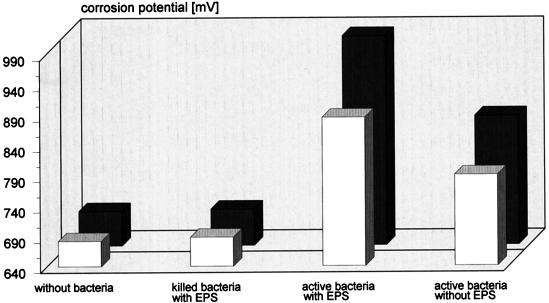
Surface potential measurements by the Kelvin electrode demonstrated that the process of pyrite degradation is electrochemical in nature (Fig. 4). With EPS-containing bacteria the potential strongly increased, whereas EPS-deficient cells caused only a slight increase. Dead cells, with or without EPS or sterile nutrient solution, only negligibly influenced the surface potential.
FIG. 4.
Importance of EPS for the onset of pyrite degradation (bioleaching) by cells of T. ferrooxidans. Pyrite degradation was measured 4 h (open bars) and 18 h (shaded bars) after inoculation with EPS-containing or -deficient cells of living or dead iron(II) sulfate-grown bacteria as an increase of the surface potential by using a Kelvin electrode.
Summarizing, the importance of the EPS for the first steps in metal sulfide dissolution became obvious. The lipopolysaccharide-containing EPS appear to be a prerequisite for an attachment to solid substrates such as pyrite and sulfur. The substrate or substratum influenced the chemical composition of the exopolymers. The mode of adhesion is different for either sulfur- or iron-grown cells and will trigger the expression of different EPS genes (16). A crucial factor is the availability of iron ions.
Sulfur-grown cells exhibit purely hydrophobic surface properties and do not attach to charged particles such as pyrite. On the contrary, the primary attachment to pyrite is mediated by positively charged exopolymer-complexed iron(III) ions, allowing an electrochemical interaction with the negatively charged surface. The iron(III) ions occur stochiometrically with the complexing glucuronic acid. Similar observations are described by Geesey and Lang (17).
The bacteria regenerate the iron(III) ions and use the energy for growth. Consequently, the main bacterial contribution to this corrosion system is to keep the iron ions in the oxidized state. Because the primary attack of the EPS-bound iron(III) ions occurs outside the cells in the EPS, it may be hypothesized that this layer constitutes a special, enlarged reaction space, allowing the cells to considerably extend action. Consequently, only the indirect leaching mechanism, i.e., the catalytic effect of iron(III) ions, can sufficiently explain the accumulated data. These results are completely in agreement with those of Sand et al. (26) and Schippers et al. (29).
Acknowledgments
We appreciate the excellent help of A. Nazarov, F. Zou, and Z. Keresztes with potential measurements and atomic force microscopy.
REFERENCES
- 1.Agate A D, Korczynski M S, Lundgren D G. Extracellular complex from the culture filtrate of Thiobacillus ferrooxidans. Can J Microbiol. 1969;15:259–264. doi: 10.1139/m69-048. [DOI] [PubMed] [Google Scholar]
- 2.Andrews G F. The selective adsorption of thiobacilli to dislocation sites on pyrite surfaces. Biotechnol Bioeng. 1988;31:378–381. doi: 10.1002/bit.260310414. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung. Weinheim, Germany: Verlag Chemie; 1984. Bestimmung von Eisen, E1; pp. 1–10. [Google Scholar]
- 4.Bagdigian R M, Myerson A S. The adsorption of Thiobacillus ferrooxidans on coal surfaces. Biotechnol Bioeng. 1986;28:467–479. doi: 10.1002/bit.260280402. [DOI] [PubMed] [Google Scholar]
- 5.Blake R C, II, Howard G T, McGinness S. Enhanced yields of iron-oxidizing bacteria by in situ electrochemical reduction of soluble iron in the growth medium. Appl Environ Microbiol. 1994;60:2704–2710. doi: 10.1128/aem.60.8.2704-2710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake R C, Shute E A, Howard G T. Solubilization of minerals by bacteria: electrophoretic mobility of Thiobacillus ferrooxidans in the presence of iron, pyrite, and sulfur. Appl Environ Microbiol. 1994;60:3349–3357. doi: 10.1128/aem.60.9.3349-3357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein, utilizing the principle of protein-dye binding. Ann Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Brown M J, Lester J N. Comparison of bacterial extracellular polymer extraction methods. Appl Environ Microbiol. 1980;40:179–185. doi: 10.1128/aem.40.2.179-185.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camper A K, LeChevallier M W, Broadaway S C, McFeters G A. Evaluation of procedures to desorb bacteria from granular activated carbon. J Microbiol Methods. 1985;3:187–198. [Google Scholar]
- 11.Chaplin M F. Monosaccharides. In: Chaplin M F, Kennedy J F, editors. Carbohydrate analysis: a practical approach. Oxford, England: IRL Press; 1986. pp. 1–36. [Google Scholar]
- 12.Chaplin M F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982;123:336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- 13.Collmer A R, Temple K T, Hinkle M E. An iron-oxidizing bacterium from the acid mine drainage of some bituminous coal mines. J Bacteriol. 1950;59:317–328. doi: 10.1128/jb.59.3.317-328.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunk R, Mostyn R A, Hoarl H C. The determination of sulfate by indirect atomic absorption spectroscopy. At Absorp Newsl. 1969;8:79–81. [Google Scholar]
- 15.Evangelou V P. Pyrite oxidation and its control. Boca Raton, Fla: CRC Press; 1995. [Google Scholar]
- 16.Fletcher M, editor. Bacterial adhesion: molecular and ecological diversity. New York, N.Y: Wiley-Liss; 1996. pp. 1–24. [Google Scholar]
- 17.Geesey G G, Lang L. Interactions between metal ions and capsular polymers. In: Beveridge T J, Doyle R J, editors. Metal ions and bacteria. New York, N.Y: John Wiley & Sons; 1989. pp. 325–357. [Google Scholar]
- 18.Gehrke T, Hallmann R, Sand W. Importance of exopolymers from Thiobacillus ferrooxidans and Leptospirillum ferrooxidans for bioleaching. In: Vargas T, Jerez C A, Wiertz J V, Toledo H, editors. Biohydrometallurgical processing. Santiago: University of Chile; 1995. pp. 1–11. [Google Scholar]
- 19.Hanson R S, Phillips J A. Chemical composition. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. pp. 328–364. [Google Scholar]
- 20.Isaacs H S, Ishikawa Y. Applications of the vibration probe to localized current measurements. 1985. pp. 1–9. . Paper 55. In Corrosion 1985. NACE International, Houston, Texas. [Google Scholar]
- 21.Karkhanis Y D, Zeltner J Y, Jackson J J, Carlo D J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharides of gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 22.Luther G W., III . The frontier-molecular-orbital theory approach in geotechnical processes. In: Stumm W, editor. Aquatic chemical kinetics. New York, N.Y: John Wiley & Sons; 1990. pp. 173–181. [Google Scholar]
- 23.Mackintosh M E. Nitrogen fixation by Thiobacillus ferrooxidans. J Gen Microbiol. 1978;105:215–218. [Google Scholar]
- 24.Merchant D J, Kahn R H, Murphy W H. Handbook of cell and organ culture. 2nd ed. Minneapolis, Minn: Burgess; 1969. [Google Scholar]
- 25.Murr L E, Torma A E, Brierley J A. Metallurgical applications of bacterial leaching and related microbiological phenomena. New York, N.Y: Academic Press; 1978. [Google Scholar]
- 26.Sand W, Gehrke T, Hallmann R, Schippers A. Sulfur chemistry, biofilm, and the (in)direct attack mechanism—a critical evaluation of bacterial leaching. Appl Microbiol Biotechnol. 1995;43:961–966. [Google Scholar]
- 27.Sand W, Rhode K, Sobotke B, Zenneck C. Evaluation of Leptospirillum ferrooxidans for leaching. Appl Environ Microbiol. 1992;58:85–92. doi: 10.1128/aem.58.1.85-92.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage D C, Fletcher M. Bacterial adhesion. New York, N.Y: Plenum Press; 1985. pp. 349–351. [Google Scholar]
- 29.Schippers A, Jozsa P-G, Sand W. Sulfur chemistry in bacterial leaching of pyrite. Appl Environ Microbiol. 1996;62:3424–3431. doi: 10.1128/aem.62.9.3424-3431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schröter A W, Sand W. Estimations on the degradability of ores and bacterial leaching activity using short-time microcalorimetric tests. FEMS Microbiol Rev. 1993;11:79–86. [Google Scholar]
- 31.Schröter A W, Sand W. Investigations on leaching bacteria by microcalorimetry. In: Sally J, McCready R G L, Wichlacz P L, editors. Biohydrometallurgy. Ottawa, Canada: Canmet; 1989. pp. 427–438. [Google Scholar]
- 32.Stratmann M, Streckel H. On the atmospheric corrosion of metals which are covered with thin electrolyte layers. I. Verification of the experimental technique. Corrosion Sci. 1990;30:681–696. [Google Scholar]
- 33.Tributsch H, Bennett J C. Semiconductor-electrochemical aspects of bacterial leaching. I. Oxidation of metals. J Chem Technol Biotechnol. 1981;31:565–577. [Google Scholar]
- 34.Velghe N, Claeys A. Rapid spectrophotometric determination of nitrate in mineral water with resorcinol. Analyst. 1985;110:313–314. [Google Scholar]