Abstract
Cultures of soybean cells incorporate [5,6-3H]-l-fucose into various cellular components including lipids and proteins. The membrane glyco-proteins were digested with pronase to produce glycopeptides, and the glycopeptides were isolated on columns of Biogel P-4. The major fucoselabeled glycopeptide sized as a Hexose15-17-N-acetylglucosamine2 (GlcNAc2) on columns of Biogel P-4. Fucose incorporation was also examined in the presence of the processing inhibitor swainsonine, and the glycosylation inhibitor tunicamycin. In the presence of swainsonine, the incorporation of fucose was not reduced but the glycopeptides were smaller in size and migrated like Hexose12-13-GlcNAc2 structures. On the other hand, tunicamycin inhibited the incorporation of fucose into the glycopeptides by 70 to 80%, indicating that the l-fucose was present in N-linked oligosaccharides.
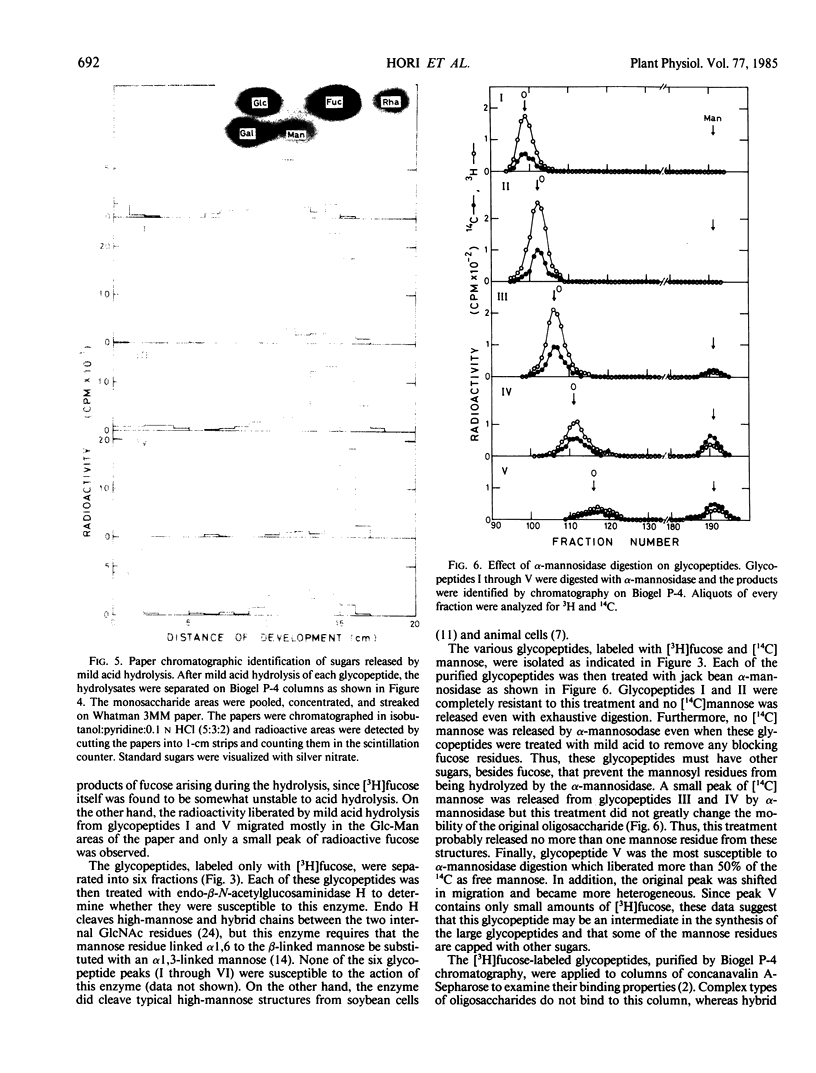
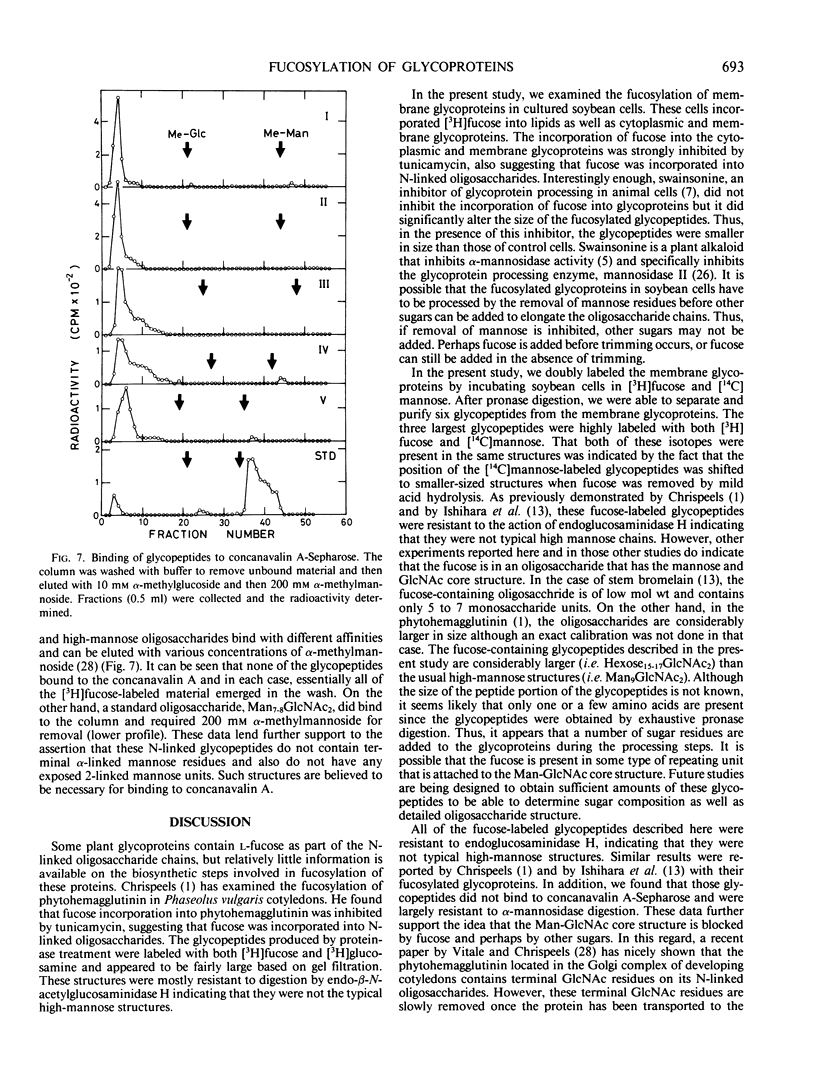
The membrane glycoproteins were doubly-labeled by incubating soybean cells in [3H]fucose and [14C]mannose. By repeated separation on Biogel P-4, six glycopeptides were purified that ranged in size from Hexose8GlcNAc2 to Hexose15-17-GlcNAc2. The three larger glycopeptides (I, II, III) were highly labeled with [3H]fucose and also contained [14C] mannose. Evidence that both isotopes were in the same glycopeptide was obtained by the finding that the mannose-labeled glycopeptides were shifted to smaller-sized structures when the [3H]fucose was removed by mild acid hydrolysis. Glycopeptide IV also contained [3H]fucose and [14C] mannose but only part of the [3H]fucose was released by mild hydrolysis. Glycopeptides V and VI contained only small amounts of tritium, but were labeled with [14C]mannose. None of the six glycopeptides was susceptible to the action of endo-β-N-acetylglucosaminidase H, and none of these glycopeptides was bound to columns of concanavalin A-sepharose. The smaller glycopeptides (IV, V, VI) were partially susceptible to α-mannosidase digestion, but this enzyme did not release any radioactive mannose from the larger-sized glycopeptides. These data indicate that the fucosylated glycopeptides are N-linked structures containing fucose, mannose, GlcNAc, and probably other sugars, and that the mannose units are blocked by other sugars. Thus, these results indicate that plant membrane glycoproteins contain a significant amount of modified oligosaccharide side chains.
Full text
PDF





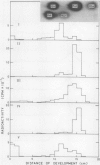
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cummings R. D., Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J Biol Chem. 1982 Oct 10;257(19):11235–11240. [PubMed] [Google Scholar]
- Dankert M., Wright A., Kelley W. S., Robbins P. W. Isolation, purification, and properties of the lipid-linked intermediates of O-antigen biosynthesis. Arch Biochem Biophys. 1966 Sep 26;116(1):425–435. doi: 10.1016/0003-9861(66)90049-x. [DOI] [PubMed] [Google Scholar]
- Dorland L., van Halbeek H., Vleigenthart J. F., Lis H., Sharon N. Primary structure of the carbohydrate chain of soybean agglutinin. A reinvestigation by high resolution 1H NMR spectroscopy. J Biol Chem. 1981 Aug 10;256(15):7708–7711. [PubMed] [Google Scholar]
- Dorling P. R., Huxtable C. R., Colegate S. M. Inhibition of lysosomal alpha-mannosidase by swainsonine, an indolizidine alkaloid isolated from Swainsona canescens. Biochem J. 1980 Nov 1;191(2):649–651. doi: 10.1042/bj1910649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D., Dorling P. R., Vosbeck K., Horisberger M. Swainsonine prevents the processing of the oligosaccharide chains of influenza virus hemagglutinin. J Biol Chem. 1982 Feb 25;257(4):1573–1576. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of membrane and secretory glycoproteins. Arch Biochem Biophys. 1981 Oct 1;211(1):1–19. doi: 10.1016/0003-9861(81)90423-9. [DOI] [PubMed] [Google Scholar]
- Hori H., Elbein A. D. Characterization of the oligosaccharides from lipid-linked oligosaccharides of mung bean seedlings. Plant Physiol. 1982 Jul;70(1):12–20. doi: 10.1104/pp.70.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Elbein A. D. Processing of N-linked oligosaccharides in soybean cultured cells. Arch Biochem Biophys. 1983 Feb 1;220(2):415–425. doi: 10.1016/0003-9861(83)90431-9. [DOI] [PubMed] [Google Scholar]
- Hori H., Elbein A. D. Tunicamycin inhibits protein glycosylation in suspension cultured soybean cells. Plant Physiol. 1981 May;67(5):882–886. doi: 10.1104/pp.67.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Takahashi N., Oguri S., Tejima S. Complete structure of the carbohydrate moiety of stem bromelain. An application of the almond glycopeptidase for structural studies of glycopeptides. J Biol Chem. 1979 Nov 10;254(21):10715–10719. [PubMed] [Google Scholar]
- Kobata A. endo-beta-N-Acetylglucosaminidases CI and CII from Clostridium perfringens. Methods Enzymol. 1978;50:567–574. doi: 10.1016/0076-6879(78)50064-5. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Longmore G. D., Schachter H. Product-identification and substrate-specificity studies of the GDP-L-fucose:2-acetamido-2-deoxy-beta-D-glucoside (FUC goes to Asn-linked GlcNAc) 6-alpha-L-fucosyltransferase in a Golgi-rich fraction from porcine liver. Carbohydr Res. 1982 Mar 1;100:365–392. doi: 10.1016/s0008-6215(00)81049-6. [DOI] [PubMed] [Google Scholar]
- Misaki A., Goldstein I. J. Glycosyl moiety of the lima bean lectin. J Biol Chem. 1977 Oct 25;252(20):6995–6999. [PubMed] [Google Scholar]
- Robbins P. W., Krag S. S., Liu T. Effects of UDP-glucose addition on the synthesis of mannosyl lipid-linked oligosaccharides by cell-free fibroblast preparations. J Biol Chem. 1977 Mar 10;252(5):1780–1785. [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Ugalde R. A., Staneloni R. J., Leloir L. F. Action of glycosidases on the saccharide moiety of the glucose--containing dolichyl diphosphate oligosaccharide. FEBS Lett. 1978 Jul 15;91(2):209–212. doi: 10.1016/0014-5793(78)81174-0. [DOI] [PubMed] [Google Scholar]
- Vitale A., Chrispeels M. J. Transient N-acetylglucosamine in the biosynthesis of phytohemagglutinin: attachment in the Golgi apparatus and removal in protein bodies. J Cell Biol. 1984 Jul;99(1 Pt 1):133–140. doi: 10.1083/jcb.99.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. EFFECT OF CARBON SOURCES ON FORMATION OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Oct;86:681–686. doi: 10.1128/jb.86.4.681-686.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]