Abstract
To determine the bacterial pathogens in chronic suppurative otitis media (CSOM) & the antibiotic susceptibility patterns of isolates among patients. A total of 400 patients clinically diagnosed with CSOM were interviewed &middle-ear effusion samples were collected using sterile swabs. All bacterial isolates were identified by conventional microbiological methods. Antibiotic susceptibility patterns of the isolates were determined by Kirby–Bauer disc diffusion. Pseudomonas aeruginosa (30.25%) were the most prevalent bacteria isolated, followed by S. aureus (MSSA) (18.5%) & MRSA (8.25%). The most effective antibiotic for treatment of bacterial CSOM was amikacin & ciprofloxacin. Statistical analysis showed no significant difference in bacterial infestations among CSOM patients & the antimicrobial susceptibility patterns of the bacterial isolates based on age (p > 0.05). The importance of a continuous & annual evaluation of bacteriological profile & antibiotic susceptibility patterns in CSOM patients is highlighted in our study.
Keywords: Ear infection, CSOM, Microbiology, Culture and sensitivity
Introduction
A WHO/CIBA Foundation workshop in 1996 defined “Chronic suppurative otitis media (CSOM) as stage of disease in which there is chronic infection of the middle ear cleft, i.e., Eustachian tube, middle ear and mastoid, and in which a non intact tympanic membrane (e.g., perforation or tympanostomy tube) and discharge (otorrhea) are present for at least 2 weeks or more.” It is also known as chronic active mucosal otitis media, chronic otomastoiditis, or chronic tympano-mastoiditis [1].
Chronic Suppurative Otitis Media (CSOM) is one of the most common diseases in clinical practice. It affects large number of people. Disease causes disability and mortality because of its ability to cause complications [2].
Frequent upper respiratory tract infections, overcrowded housing, poor hygiene, insertion of tympanostomy tubes, a history of recurrent AOM, older siblings, and attendants to child care center, young age, genetic predisposition, respiratory allergy, smoking in the household,lack of breastfeeding, familial predisposition, male sex, and method of feeding either bottle or breast are the risk factor for the development of CSOM [3].
CSOM is more common in developing countries. In our country burden of the disease is too high considering the huge population. Prevalence of CSOM in the world is around 65–330 million/year. Majority of world CSOM burden is attributed by Southeast Asia, Western pacific and African countries. India falls into countries with highest prevalence (prevalence > 4%) [2].
Most of the microbiological studies on CSOM have shown that the common bacteria found in CSOM are Escherichia coli, Pseudomonas aeruginosa, S. aureus (MSSA), Proteus spp. and Klebsiella spp., with methicillin-resistant S.aureus (MRSA) isolated in some cases. However, the type of bacteria associated with CSOM varies depending on the geo- graphical area and other factors [4, 5].
It is important to know the major bacterial aetiologies of CSOM and their antibiotic susceptibility patterns, both for selection of the most appropriate treatment regimen and prevention of the emergence of resistant strains. This study aimed on evaluation of the current bacterial pathogens and antibiogram in chronic suppurative otitis media patients visiting ENT opd, HIMS, Barabanki.
AIMS
To investigate the bacteriological flora of middle ear leading to CSOM in recent times and change in the antimicrobial sensitivity pattern.
Objectives
To determine the bacterial pathogens in adult CSOM patients.
To determine the antibiotic susceptibility patterns of isolates among patients.
To determine association between bacterial profile of CSOM in different age groups.
Material and Methods
Study Population
This cross-sectional analytical study comprised a total of 400 patients clinically diagnosed withCSOM in age group 18–60 years, who visited to the ENT opd of Hind Institute Of Medical Sciences, Barabanki. These patients had received no antibiotic treatment during the previous 3 days, and had not suffered any otitis externa, otomycosis, trauma, acute otitis media, congenital anomalies of ear, benign or malignant lesion of related area. CSOM was defined as otorrhoea through a perforated tympanic membrane, present for at least two to six weeks.
Ethical committee approval was obtained before starting the study. In addition, all 400 enrolled patients signed informed consent forms.
Sample Collection
Middle-ear discharge was collected from the patients, by ENT specialist, under strict aseptic precautions using sterile swabs. The swab samples were immediately sent to the microbiology laboratory for bacterial studies.
Identification of Bacterial Isolates
The swab samples were cultured on blood agar, chocolate agar and Mac conkey agar for isolation of aerobic bacteria, and incubated aerobically at 37 °C for 24–48 h. The isolates grown were identified according to standard microbiological and biochemical methods.
Antibiotic Susceptibility of Bacterial Isolates
The antibiotic resistance profile of the isolates was determined by the Kirby–Bauer disc diffusion method and the results were interpreted according to Clinical and Laboratory Standards Institute guidelines.
Statistical Analysis
The data were analysed with chi-square test, using SPSS version 26, to evaluate the statistical significance of association between potential variables. P values of less than 0.05 were considered to be significant.
Results
A total of 400 patients with CSOM were investigated in this study. The mean age of patients was 32.94(range, 18–60 years), and the majority of the patients 177 (44.25%) were aged 18–28 years. Of the patients, 182 (49.5%) were male and 202 (50.5%)were female.
Bacterial Isolates
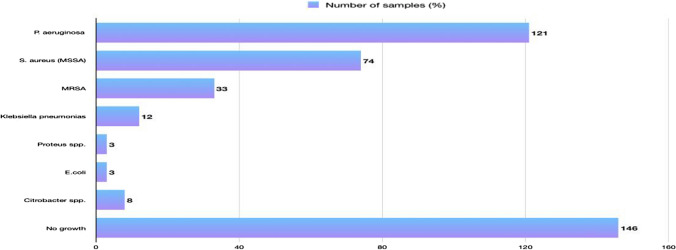
Of the 400 ear swabs processed, bacterial growth was found in samples 254 (63.5%), while 85 (21.25%) had no growth and 61 (15.25%) had fungal growth. The results revealed that Pseudomonas aeruginosa were the most prevalent bacteria isolated 121 (30.25%), followed by S. aureus (MSSA) 74 (18.5%) and MRSA 33(8.2%) (Fig. 1).
Fig. 1.
Frequency of bacterial species distribution among chronic suppurative otitis media patients
Statistical analysis indicated no significant difference between the type of bacteria causing CSOM and gender (p = 0.81). The highest frequency of bacteria isolated from patients belonged to those in the 18–28 Year age group 112 (44.25%). There was no significant difference between the type of bacteria isolated and patient age (p = 0.93). The frequency of bacteria isolated from the patients based on age and gender is shown in Table 1
Table 1.
Prevalence of bacterial isolates by age and gender among csom patients
| E.coli | Klebsiella spp. | Citrobacter spp. | Proteus spp. | Pseudomonas spp. | S. aureus (MSSA) | MRSA | Total | P value | |
|---|---|---|---|---|---|---|---|---|---|
| SEX | 0.81 | ||||||||
| Male | 1 | 4 | 3 | 1 | 61 | 31 | 13 | 114 | |
| Female | 2 | 8 | 5 | 2 | 60 | 36 | 20 | 133 | |
| AGE | 0.93 | ||||||||
| 18–28 yr | 2 | 7 | 2 | 1 | 52 | 36 | 12 | 112 | |
| 29–39 yr | 1 | 2 | 3 | 1 | 32 | 23 | 11 | 73 | |
| 40–50 yrs | – | 1 | 2 | – | 21 | 7 | 6 | 37 | |
| 51–60 yrs | – | 2 | 1 | 1 | 16 | 8 | 4 | 32 |
Antibiotic Susceptibility
In our study, Pseudomonas aeruginosa isolates demonstrated only 86.77% sensitivity towards amikacin and ciprofloxacin. S. aureus (MSSA)showed 100% sensitivity towards linezolid and vancomycin. MRSA showed 100% resistance towards penicillin, levofloxacin and erythromycin. Klebsiella, E.coli and Proteus exhibited relatively high resistance to ampicillin (Tables 2, 3).
Table 2.
Rates of antibiotic resistance in gram negative
| Pseudomonas spp. (%) | Klebsiella spp. (%) | Proteus spp. (%) | |
|---|---|---|---|
| AK | 7.78 | ||
| AT | 3.30 | ||
| CAZ | 50.41 | 8.33 | |
| CFS | 28.92 | ||
| CIP | 4.95 | ||
| CPM | 23.96 | ||
| DOR | 11.57 | ||
| GEN | 19.00 | ||
| IPM | 11.57 | ||
| LE | 19.00 | ||
| MRP | 19.00 | ||
| PB | 100 | ||
| PIT | 4.95 | ||
| TOB | 7.43 | ||
| PN | 34.71 | ||
| AMP | 54.54 | 100 | 100 |
| A/S | 2.47 | ||
| TE | 4.95 | 100 |
Table 3.
Rates of antibiotic resistance in staphylococci species
| S. aureus (MSSA) (%) | MRSA (%) | |
|---|---|---|
| AK | 50 | 30.31 |
| CD | 10.81 | 69.69 |
| CFS | 50 | 30.31 |
| TE | 33.78 | 69.69 |
| PN | 89.18 | 100 |
| E | 22.97 | 100 |
| CIP | 9.45 | 69.69 |
| LE | 40.54 | 100 |
| GEN | 13.51 | 9.09 |
| AMC | 5.40 |
Discussion
Chronic Suppurative Otitis Media is one of the most common hearing problems, with a 0.4% prevalence [2]. Chronic suppurative otitis media can cause many complications, including mas- toiditis and meningitis, if not treated properly. Therefore, identification of the causative organisms is essential for proper management of CSOM.
In our study, bacterial growth was seen in 254 (63.5 per cent) of CSOM cases, while 85 (21.25%) had no growth and 61 (15.25%) had fungal growth. The results of the current study are in line with the results of other research [6–11].Our study showed that Pseudomonas aeruginosa were the most common bacteria isolated in CSOM, followed by S. aureus (MSSA) and MRSA, which is in line with reports of some other studies in different parts of the world [12–15].However other researchers have reported that S. aureus (MSSA)are the most common isolated pathogenic bacteria in CSOM patients [16, 17]. It is noteworthy that very few studies have been conducted in this field in this part of U.P. The differences in the spectra of bacteria causing CSOM imply that sole reliance on empirical antimicrobial therapy is not appropriate for effective treatment of affected patients.
The gender and age analysis of this study showed a variation in the ratio of suspected CSOM patients and their bacterial infestations. The highest numbers of suspected CSOM patients were aged 18–28 years and the highest bacterial infestations were also found in this age group.
In this study, younger groups 44.25% (18–28 yrs.) were more affected as compared to the older aged group 12.25% (51–60 yrs.). This is similar to the study [18–21] conducted where majority of the patients were from younger aged group as compared to the older patients.
In our study, there was a dominancy of female compared to male CSOM patients, which is consistent with some other studies [8].The comparison of age and sex profile of patient in present study thus highlight the dominance of young adults and females as the characteristic distinguishing feature of present study.
Antibiotic susceptibility patterns serve as a useful guideline for choosing the appropriate antibiotic for CSOM treatment. The high incidence of MRSA in our study was not surprising to us, because the patients had undergone several courses of antibiotic therapy.
The rising prevalence of antibiotic resistance (especially in developing countries) can be attributed to the overuse and incorrect use of antibiotics. The commonest bacteria isolated in this study was Pseudomonas aeruginosa 121(30.2%) which showed 86.7% sensitive to amikacin, 86.7% sensitive to ciprofloxacin and 76.85% sensitive to gentamicin. A previous study [22] reported maximum sensitivity for colistin, piperacillin-tazobactam and ceftazidime, while according to old studies [17] it is observed that the most effective antibiotics for Pseudomonas aeruginosa was piperacillin and piperacillin tazobactam.
Next most common isolate was S. aureus (MSSA)74(18.5%) which showed 100% sensitive to linezolid, vancomycin and cefoxitin, 89.18% sensitive to clindamycin, 50% to amikacin, and cefoperazone sulbactam. These results were comparable with the other studies [16]also (Table 4).
Table 4.
Data comparison with previous studies
| Studies | Pseudomonas spp. | S. aureus (MSSA) | MRSA | Klebsiella spp. |
|---|---|---|---|---|
| Present study | 121 (30.25%) | 74 (18.5%) | 33 (8.25%) | 12 (3%) |
| Prakash R | 38 (19.8%) | 93 (48.6%) | 18 (9.4%) | |
| Samanth | 31% | 35% | 17% | |
| Kumar D [23] | 38 (26%) | 54 (38%) | 8 (6%) | |
| Rangaiah ST [24] | 38 (28.1%) | 43 (31.8%) | 6 (4.19%) | 3 (2.2%) |
| Dharmendra Kumar [25] | 21 (29.16%) | 22 (30.55%) | 3 (4.6%) |
Conclusion
Our findings highlight the importance of Pseudomonas followed by Staphylococcus. aureus & MRSA as the most common causative bacterial agents of CSOM. The high incidence rate of MRSA among CSOM patients is a cause of concern. Pseudomonas was 86.77% sensitive to Amikacin and Ciprofloxacin, whereas S. aureus (MSSA)was 100% sensitive to Linezolid, Cefoxitin and Vancomycin. MRSA was sensitive to Vancomycin and Linezolid 100%, whereas 100% resistance was seen towards Penicillin, Cefoxitin, Levofloxacin and Erythromycin.
Funding
No funding was received for the study.
Declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical Approval
Ethical committee approval was received for this study from the Institutional Review Board of Ethical committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gupta P, Varshney S, Kumar SK, Mohanty A, Jha MK. Chronic suppurative otitis media: a microbiological review of 20 years Uttarakhand. Indian J Otol. 2020;26:59–67. [Google Scholar]
- 2.Chandrashekharayya SH, Kavitha MM, Handi P, Khavasi SSP, Doddmani, Riyas M. To study the level of awareness about complications of chronic suppurative otitis media (CSOM) in CSOM patients. J Clin Diagn Res. 2014;8(2):59–61. doi: 10.7860/JCDR/2014/8009.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molla.et al R. Pathogenic bacteria profile & antimicrobial susceptibility patterns of chronic suppurative otitis media at university of gondar referral hospital, northwest ethiopia2017. BMC Res Not. 2019;12:414. doi: 10.1186/s13104-019-4452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loy A, Tan A, Lu P. Microbiology of chronic suppurative otitis media in Singapore. Singapore Med J. 2002;43:296–299. [PubMed] [Google Scholar]
- 5.Kumar R, Srivastava P, Sharma M, Rishi S, Nirwan S, Hemwaniand K. Isolation and antimicrobial sensitivity profile of bacterial agents in chronic suppurative otitis media patients at NIMS hospital. Jaipur IJPBS. 2013;3:265–269. [Google Scholar]
- 6.Jain V, Jain S, Shah RK. Bacteriological profile of CSOM and antibiotic susceptibility pattern of aerobic isolates in a tertiary care hospital of central India. Int J Curr Microbiol App Sci. 2019;8(01):2240–2246. doi: 10.20546/ijcmas.2019.801.234. [DOI] [Google Scholar]
- 7.Saitabau Z, Abraham.et al Prevalence and etiological agents for chronic suppurative otitis media in a tertiary hospital in Tanzania. BMC Res Not. 2019;12:429. doi: 10.1186/s13104-019-4483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basnet R, Sharma S, Rana JC, Shah PK. Bacteriological study of otitis media and its antibiotic susceptibility pattern. J Nepal Health Res Counc. 2017;15(36):124–129. doi: 10.3126/jnhrc.v15i2.18186. [DOI] [PubMed] [Google Scholar]
- 9.Khomtchouk KM.et al. (2021) Treatment with a neutrophil elastase inhibitor and ofloxacin reduces P. aeruginosa burden in a mouse model of chronic suppurative otitis media. NPJ Biofilms Microbiomes. 7(1). [DOI] [PMC free article] [PubMed]
- 10.Hassett DJ, Sutton MD, Schurr MJ, Herr AB, Caldwell CC, Matu JO. Pseudomonas aeruginosa hypoxic or anaerobic biofilm infections within cystic fibrosis airways. Trends Microbiol. 2009;17(3):130–138. doi: 10.1016/j.tim.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Sierra A.et al. (2011) Non-typeable Haemophilus influenzae and Streptococcus pneumoniae as primary causes of acute otitis media in colombian children: a prospective study. BMC Infect Dis. 11. [DOI] [PMC free article] [PubMed]
- 12.Vimal S, Rathod.et al Study of bacteriological profile and its antibiotic susceptibility in patients of chronic suppurative otitis media in Nanded Maharashtra. Int J Health Sci Res. 2016;6(3):68–72. [Google Scholar]
- 13.Gupta P, Varshney S, Kumar SK, Mohanty A, Jha MK. Chronic suppurative otitis media: a microbiological review of 20 years. Indian J Otol. 2020;26(2):59–67. [Google Scholar]
- 14.Rovers MM, Straatman H, Zielhuis GA, Ingels K and van der Wilt GJ. Seasonal variation in the prevalence of persistent otitis media with effusion in one-year-old infants. [DOI] [PubMed]
- 15.Chirwa M, Mulwafu W, Aswani JM, Masinde PW, Mkakosya R, Soko D. Microbiology of chronic suppurative otitis media at queen Elizabeth central hospital, Blantyre, Malawi: a cross-sectional descriptive study. Malawi Med J. 2015;27(4):120–124. doi: 10.4314/mmj.v27i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash R, Juyal D, Negi V, Pal S, Adekhandi S, Sharma M, et al. Microbiology of chronic suppurative otitis media in a tertiary care setup of Uttarakhand state India. N Am J Med Sci. 2013;5:282–287. doi: 10.4103/1947-2714.110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samanth T, Jha S, Sinha Vand Dadhich S. Bacteriology and drug susceptibility in chronic suppurative otitis media in ear, nose, and throat outpatient and inpatient department of tertiary care Hospital Bhavnagar. Indian J Otol. 2017;23(4):252–255. doi: 10.4103/indianjotol.INDIANJOTOL_132_16. [DOI] [Google Scholar]
- 18.Suneha S, Kumar M, Bhavana K. Clinical and sociodemographic profiles of patients with chronic otitis media seeking health care at a tertiary care center of bihar: a prescription-based analysis. Otorhinolaryngol Clin Int J. 2022;1:10003–11419. [Google Scholar]
- 19.Khatoon A.et al. (2015) Chronic suppurative otitis media: a clinico-microbiological menace. Int J Res Med Sci, 3 (8)
- 20.Shrestha B, Amatya R, Shrestha I, Ghosh I. Microbiological profile of chronic suppurative otitis media. Nepalese J Head Neck Surg. 2011;2(2):6–8. doi: 10.3126/njenthns.v2i2.6793. [DOI] [Google Scholar]
- 21.Agrawal R, Khatri P, Parihar R, Shah H. Microbial assessment of chronic suppurative otitis media in a tertiary care center of Rajasthan. Int J Health Sci Res. 2017;7(2):120. [Google Scholar]
- 22.Kaur P, Sood AS, Sharma S and Awal G. Microbiological profile and antimicrobial susceptibility pattern of chronic suppurative otitis media in a tertiary care centre. Orig Res Art Pathol Update: Tropical J Pathol Microbiol.
- 23.Kumar D, Priyadarshini AMK, Prakash P. Bacteriological profile of chronic suppurative otitis media in patients at a tertiary level hospital. Eastern J Med Sci. 2016;1(1):5–7. doi: 10.32677/EJMS.2016.v01.i01.002. [DOI] [Google Scholar]
- 24.Rangaiah ST, Dudda R, Prasad MH, Balaji NK, Sumangala B, Gudikote MM. Bacteriological profile of chronic suppurative otitis media in a tertiary care hospital. Int J Otorhinolaryngol Head Neck Surg. 2017;3:601–605. doi: 10.18203/issn.2454-5929.ijohns20173031. [DOI] [Google Scholar]
- 25.Kumar D. A study of the microbiological profile of CSOM in a tertiary care Centre Of North India. IOSR J Dent Med Sci (IOSR-JDMS) 2019;18(5):20–24. [Google Scholar]