Abstract
Analysis of a 2.4-kb cDNA of the cellulose-binding extracellular β-glucosidase (CBGL) from Phanerochaete chrysosporium suggested that CBGL is organized into two domains, an N-terminal cellulose-binding domain and a C-terminal catalytic domain. Genomic sequence analysis suggested that cbgl is encoded by 30 exons. Southern analysis of DNA from homokaryotic cultures indicated that CBGL is encoded by two alleles, cbgl-1 and cbgl-2, of a single gene.
Cellulose-degrading cultures of the white rot basidiomycete Phanerochaete chrysosporium apparently produce three different β-glucosidases—extracellular, intracellular, and cell wall bound—depending on the carbon source (9, 26). Deshpande et al. (9) reported that cellulose induces intracellular and cell wall-bound enzymes and purified five isozymes of extracellular β-glucosidases from cellulose-degrading cultures of P. chrysosporium. Molecular weights of these glucosidases ranged from 165,000 to 182,000. Smith and Gold (26) partially purified an extracellular β-glucosidase from P. chrysosporium OGC101 and charaterized it as a monomer with a molecular weight of 90,000. Recently, we purified and characterized a cellulose-binding extracellular β-glucosidase (CBGL) with a molecular mass of 114,000 from cellulose-supplemented cultures of P. chrysosporium OGC101. When CBGL was treated with papain, its molecular weight decreased to 95,000; it lost the ability to bind to cellulose, but its catalytic activity was unchanged. This suggested that CBGL is organized into two domains—a cellulose-binding domain (CBD) and a catalytic domain (20). The glucosidase isolated previously by Smith and Gold (26) from this strain might be the non-cellulose-binding form. The kinetic properties of the cellulose-binding and nonbinding forms were similar, indicating that the CBD was not involved in catalysis. Here cloning and characterization of a cDNA clone and a genomic clone of CBGL are reported. Sequence analysis confirmed our prediction that this β-glucosidase consists of a catalytic domain and a CBD.
Organism.
P. chrysosporium OGC101 (a derivative of BKM-F-1767) was obtained from Michael H. Gold (Oregon Graduate Institute) (1). Escherichia coli XL1-Blue MRF′ and SOLR were obtained from Stratagene (La Jolla, Calif.).
Nucleotides.
Oligonucleotides were prepared by the Oregon Regional Primate Research Center (Beaverton, Oreg.). The plasmid isolation kit was obtained from Qiagen, Inc. (Chatsworth, Calif.).
Isolation of a cDNA clone of cbgl.
The cDNA λZAP expression library, prepared as described previously (18), was screened with an anti-CBGL antibody and a secondary antibody labeled with alkaline phosphatase. The pBluescript SK(−) plasmid containing a putative β-glucosidase cDNA insert was rescued by in vivo excision with a helper phage. The plasmid was purified with a commercial plasmid isolation kit (Qiagen, Inc.). The cDNA was sequenced by the dideoxy method with the primer walking strategy (25, 27).
Isolation of a genomic clone of cbgl.
A λEMBL3 genomic library of P. chrysosporium OGC101 was screened at high stringency (4.8× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]–48% formamide at 50°C) with a 550-bp ApaI fragment from the 3′ end of the cbgl cDNA clone. Based on the restriction mapping of the genomic clones, four overlapping restriction fragments (3.6-kb SacI, 1.7-kb SacI, 4.5-kb SalI, and 1.2-kb SalI) covering the entire region of cbgl were subcloned into pBluescript SK (Stratagene) and sequenced by the primer walking method (27). Sequencing was performed with an automatic sequencer (model 377; Applied Biosystems) and AmpliTaq DNA polymerase, FS.
Isolation of a full-length cDNA clone of cbgl.
The cDNA library of P. chrysosporium was probed with a restriction fragment (66 to 324 bp) obtained by digesting cbgl-2 with MscI and NdeI. Hybridization was performed at high stringency (4.8× SSC, 48% formamide, 50°C). Positive clones were purified by further screening. The pBluescript II SK plasmid containing the putative cbgl cDNA insert was rescued by in vivo excision with a helper phage. The plasmid was purified with a plasmid midi kit (Qiagen, Inc.).
Isolation and analysis of homokaryons.
Single homokaryotic basidiospores were isolated as described previously (1, 12). DNAs from homokaryotic cultures were isolated by standard procedures and restriction digested with SalI, size fractionated in a 0.7% agarose gel, blotted onto a Magnagraph nylon transfer membrane (Micron Separations, Westboro, Mass.), and probed with a 32P-labeled 1.4-kb SacI fragment of cbgl (nucleotides 1446 to 2866).
Northern (RNA) blot analysis.
Total RNA was isolated from 11-day-old mycelia of P. chrysosporium cultured with 1% cotton linters, cellobiose, or glucose as the carbon source (2, 8). RNA was electrophoresed in 1.5% agarose gel containing 2.2 M formaldehyde, transferred to Magnagraph nylon membranes (Micron Separations), and probed with cDNA for CBGL at 42°C as described previously (6).
Southern blot analysis of cbgl.
DNA from P. chrysosporium was restriction digested and electrophoresed with a 0.7% agarose gel. The DNA was transferred to Magnagraph nylon membranes and hybridized to a 32P-labeled 1.4-kb SalI cDNA fragment of cbgl (5).
Seventy-two positive clones of cbgl were isolated by immunoscreening of the P. chrysosporium cDNA library. A full-length clone was isolated by screening the cDNA library with a MscI + NdeI fragment from the genomic clone cbgl-2. Genomic clones were isolated by screening a λEMBL3 genomic library of P. chrysosporium with a 500-bp ApaI fragment from the 3′ region of the cDNA sequence. This screening yielded ∼50 positive genomic clones. Restriction fragment analyses of five clones which hybridized strongly to the probe indicated that they were very similar, and one of them was subcloned and sequenced.
cbgl cDNA sequence.
Sequence analysis of the cDNA clone (2.4 kb) revealed an open reading frame consisting of 2,469 bp encoding 823 amino acids, including a 21-amino-acid N-terminal signal peptide sequence (Fig. 1). Prediction of the signal peptide cleavage site suggested that Gln22 was the N-terminal amino acid (22). The mature CBGL apparently consists of 802 amino acids and has an apparent molecular weight of 83,439. The CBGL molecular weight as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was 114,000. CBGL is a glycoprotein, and the difference in molecular weight could be attributable to the carbohydrate portion. The cDNA sequence revealed six potential N-glycosylation sites conforming to the general rule Asn-X-Thr/Ser, in which X is not a proline (4). In addition, numerous O-glycosylation sites are possible.
FIG. 1.
Nucleotide and deduced amino acid sequences of CBGL from P. chrysosporium. Genomic and amino acid sequences were derived from cbgl-2. The amino acid sequence deduced from the cDNA sequence of cbgl-1 was the same as that of cbgl-2 except at positions indicated in the line below the amino acid sequence in parentheses. The exon sequence of cbgl-1 is the same as that of cbgl-2 except at the positions indicated in the line above the gene sequence. Nucleotides and amino acids are numbered on the right. The potential signal peptide sequence is overlined. The potential CBD is boxed. Potential N-glycosylation sites are in boldface type.
CBD.
Analysis of the cbgl cDNA sequence suggested that the amino acid sequence from 22 to 57 has a high sequence similarity to the conserved cellulose-binding sequence of CBHII from P. chrysosporium and other fungal CBD sequences (11). This confirmed our prediction that CBGL possesses a CBD similar to that of cellulases (20). This domain is connected to the C-terminal catalytic domain via a 43-amino-acid linker region.
Catalytic domain.
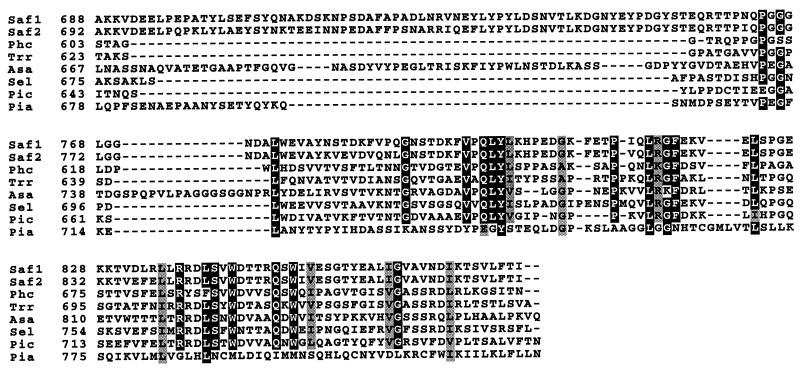
Approximately amino acids 101 to 823 at the C terminus form the catalytic domain. Sequence analysis suggests that CBGL should be categorized as a family 3 glycosylhydrolase along with the extracellular β-glucosidases from Trichoderma reesei, Aspergillus aculeatus, Saccharomycopsis fibuligera, and Pichia capsulata (3, 13, 14, 17, 21). Asp and Glu have been found in the active sites of numerous glycosidases, including β-glucosidase, cellulase, and amylase (7, 23, 28). Analysis of the catalytic domain sequences of eight family 3 fungal and yeast glycosidases indicated conservation of 13 acidic amino acid residues. Sequences surrounding seven conserved residues are preserved (Fig. 2). Potentially, any two of the conserved residues could be involved in catalysis.
FIG. 2.
Comparison of the catalytic domain sequence of CBGL with the β-glucosidases from T. reesei (Trr) (3), A. aculeatus (Asa) (15), S fibuligera (Saf1 and Saf2) (21), Pichia anomala (Pia) (17), P. capsulata (Pic) (14), and Septoria lycopersici (Sel) (24).
CBGL expression.
P. chrysosporium produced CBGL abundantly only when cellulose was provided as the sole carbon source (2, 20). CBGL production was not observed in cultures supplemented with either glucose or cellobiose (2). To further understand CBGL expression in P. chrysosporium, total RNA was isolated from 11-day-old cellulose, cellobiose, or glucose cultures and analyzed by Northern blotting (Fig. 3). A band corresponding to 2.4 kb was observed only with the RNA isolated from cellulose-grown cells. Also, the size of this RNA was very similar to the size of the cDNA insert. These preliminary findings suggest that either cellulose or one of its degradation products might be controlling the expression of CBGL at the transcriptional level.
FIG. 3.
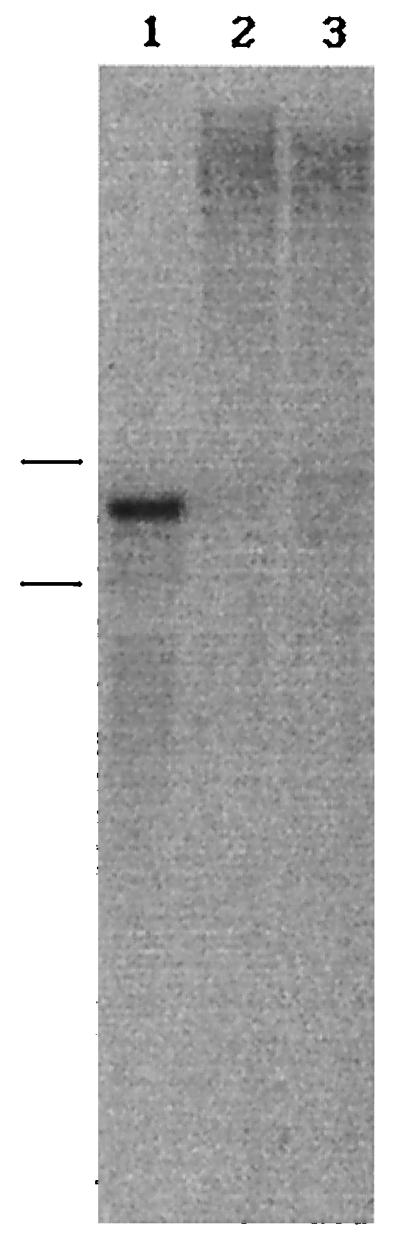
Northern blot analysis of P. chrysosporium RNA. Total RNA was isolated from 11-day-old mycelia obtained from 1% cellulose (lane 1), glucose (lane 2), and cellobiose (lane 3) cultures. The blot was probed with 32P-labeled CBGL cDNA. Bars to the left indicate the positions of 18S and 28S rRNA (from top to bottom).
Gene sequence of cbgl-2.
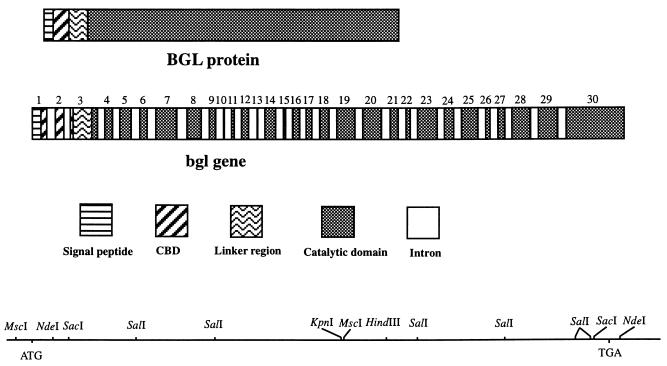
cbgl-2 consisted of 4,555 bp, including 182 bp in the 5′ flanking region and 339 bp in the 3′ flanking region (Fig. 1). The 5′ upstream region contained a potential TATAA box (TATAAGT) 64 bp upstream from the translation start codon. Comparison of the genomic and cDNA sequences of CBGL indicated the presence of 29 introns varying in size from 47 to 68 bp (Fig. 1). All of the intron splice junctions conformed to the GT–AG rule. Exon 1 codes for the signal peptide and a portion of the CBD (Fig. 4). Exon 2 codes only for the CBD. Exon 3 codes for the rest of the CBD, the linker peptide, and a small sequence of the catalytic domain. Exons 4 to 30 code for the catalytic domain. Interestingly, exons 10 and 13 code for one and two amino acids, respectively (Fig. 4). In contrast to cbgl, T. reesei bglu1 has only two introns (3).
FIG. 4.
Schematic representation of the protein and gene structures of CBGL and the restriction map of cbgl-2.
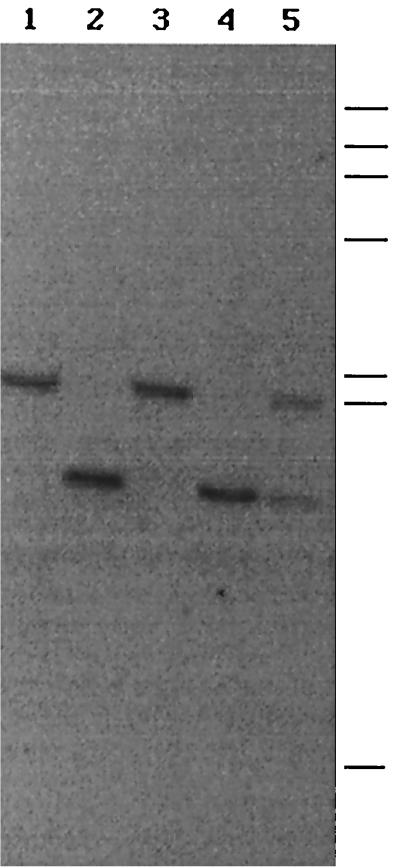
Exon sequences of cbgl-2 exhibited 98% similarity to the cbgl cDNA sequence. A total of 50 bp in the sequences (in the exon regions) did not match the cDNA sequence; however, the amino acid sequences differed only at four locations (Fig. 1). Restriction analysis of P. chrysosporium DNA indicated that, except for HindIII restriction, only one fragment from all the other restrictions hybridized to a 1.4-kb SalI fragment of cbgl-2 (Fig. 5). This result suggested that cbgl is probably encoded by two alleles (cbgl-1 and cbgl-2) of a single gene. The cDNA sequence was presumably derived from cbgl-1.
FIG. 5.
Southern analysis of genomic DNA from P. chrysosporium. Genomic DNA, isolated by standard procedures, was digested with restriction enzymes SalI (lane 1), HindIII (lane 2), BamHI (lane 3), NdeI (lane 4), EcoRI (lane 5), and SacI (lane 6). The blot was probed with a 32P-labeled 1.4-kb SalI fragment of cbgl-2. Bars indicate the positions of molecular size standards (from top to bottom: 23.1, 9.4, 6.6, 4.4, 2.3, 2.0, and 0.6 kb).
cbglu-1 and cbglu-2 allelism.
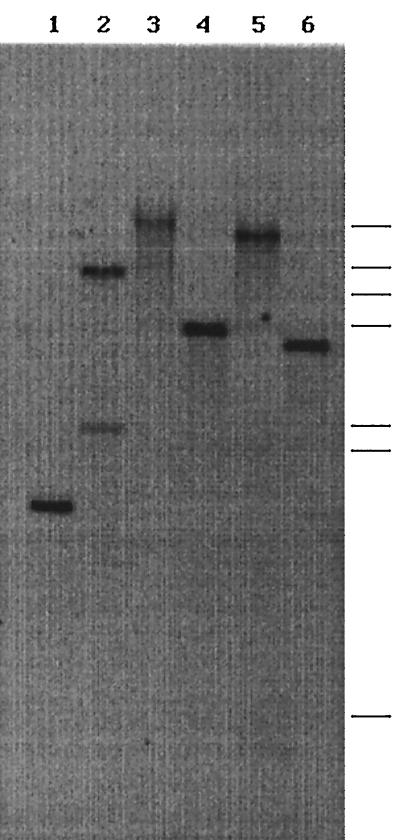
P. chrysosporium is a heterokaryon with two or more genetically distinct nuclei (1). The genomic library from which the cbgl clones were isolated was derived from such a heterokaryon. However, the basidiospores are homokaryons and contain two identical nuclei. If cbgl-1 and cbgl-2 are truly allelic, then they should segregate among homokaryons. Segregation of allelic variants of lignin peroxidase, glyoxal oxidase, and cellobiose dehydrogenase from P. chrysosporium is known (10, 16, 19). A comparison of the cDNA sequences of cbgl-1 and the exon sequences of cbgl-2 suggested that cbgl-2 has at least one extra SalI site at nucleotide 2866 (Fig. 4). This difference was utilized in identifying the two alleles. DNAs from homokaryotic and heterokaryotic cultures were restricted with SalI and probed with a 1.4-kb SalI fragment (bp 1446 to 2866). The probe was expected to hybridize to only a 2-kb fragment from cbgl-1, a 1.4-kb fragment from cbgl-2, and two fragments (1.4 and 2 kb) from the wild-type heterokaryon. Southern analysis suggested that only one fragment (1.4 or 2 kb) from homokaryon DNA and two fragments from wild-type DNA can hybridize to the probe (Fig. 6). These findings support the proposal that cbgl-1 and cbgl-2 are alleles.
FIG. 6.
Segregation of CBGL alleles into homokaryons. DNA from four separate single spore cultures (lanes 1 to 4) and one parenteral heterokaryon culture (lane 5) of P. chrysosporium OGC101 were restricted with SalI, size fractionated on an agarose gel, and probed with a 1.4-kb SalI of cbgl-2. Bars indicate the positions of molecular size standards (from top to bottom) of 23.1, 9.4, 6.6, 4.4, 2.3, 2.0, and 0.6 kb.
β-Glucosidase multiplicity.
Smith and Gold (26) partially purified an extracellular β-glucosidase (Mr, 90,000) from P. chrysosporium OGC101. CBGL is produced by the same strain in cultures optimized for low extracellular protease levels (2). Proteolytic hydrolysis of CBGL produces two non-cellulose-binding forms (Mrs, 96,000 and 98,000). Thus, the β-glucosidase isolated by Smith and Gold (26) was probably a degradation product of CBGL. At this time, there is no reason to believe that the low-molecular-weight glucosidase could arise from differential splicing of cbgl-1 or cbgl-2. Deshpande et al. (9) have reported five extracellular β-glucosidases from P. chrysosporium, with molecular weights ranging from 165,000 to 182,000. A comparison with the current findings is not worthwhile because of strain differences and the variation in culturing conditions.
Nucleotide sequence accession numbers. The P. chrysosporium OGC101 CBGL cDNA and gene sequence data reported here have been deposited in GenBank under accession no. AF036873 and AF036872.
Acknowledgments
This work was supported by grant DE-FG06-92ER20065 from the U.S. Department of Energy, Office of Basic Energy Sciences.
We thank Michael Gold of the Oregon Graduate Institute for providing the λEMBL3 genomic library of P. chrysosporium OGC101.
REFERENCES
- 1.Alic M A, Letzring C, Gold M H. Mating system and basidiospore formation in the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1987;53:1464–1469. doi: 10.1128/aem.53.7.1464-1469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao W, Lymar E, Renganathan V. Production of cellobiose dehydrogenase and β-glucosidase by the cellulose-degrading cultures of Phanerochaete chrysosporium in shake flasks. Appl Microbiol Biotechnol. 1994;42:642–646. [Google Scholar]
- 3.Barnett C C, Berka R M, Fowler T. Cloning and amplification of the gene encoding an extracellular β-glucosidase from Trichoderma reesei: evidence for improved rates of saccharification of cellulosic substrates. Bio/Technology. 1991;9:562–567. doi: 10.1038/nbt0691-562. [DOI] [PubMed] [Google Scholar]
- 4.Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem J. 1983;209:331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown T. Analysis of DNA sequences by blotting and hybridization. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1994. pp. 2.9.1–2.9.15. [Google Scholar]
- 6.Brown T. Analysis of RNA by northern and slot blot hybridization. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1994. pp. 4.9.1–4.9.16. [Google Scholar]
- 7.Buisson G, Duee E, Haser R, Payan F. Three dimensional structure of porcine pancreatic α-amylase at 2.9 Å resolution. Role of calcium in structure and activity. EMBO J. 1987;6:3909–3916. doi: 10.1002/j.1460-2075.1987.tb02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande V, Eriksson K-E, Pettersson B. Production and purification of 1,4-β-glucosidase enzymes from Sporotrichum pulverulentum. Eur J Biochem. 1978;90:191–198. doi: 10.1111/j.1432-1033.1978.tb12590.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaskell J, Dieperink E, Cullen D. Genomic organization of lignin peroxidase genes of Phanerochaete chrysosporium. Nucleic Acids Res. 1991;19:599–603. doi: 10.1093/nar/19.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilkes N R, Henrissat B, Kilburn D G, Miller R C, Warren R A J. Domains in microbial β-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991;55:303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold M H, Alic M. Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol Rev. 1993;57:605–622. doi: 10.1128/mr.57.3.605-622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrissat B, Bairoch A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1993;293:781–788. doi: 10.1042/bj2930781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janbon G, Magnet R, Arnaud A, Galzy P. Cloning and sequencing of β-glucosidase gene from Candida molischiana 35M5N. Gene. 1995;165:109–113. doi: 10.1016/0378-1119(95)00428-9. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi T, Enoki T, Tsurumaki S, Sumitani J, Ueda M, Arai M. Cloning and sequencing of the cDNA encoding β-glucosidase from Aspergillus aculeatus. Gene. 1996;173:287–288. doi: 10.1016/0378-1119(96)00179-5. [DOI] [PubMed] [Google Scholar]
- 16.Kersten P J, Witek C, Vanden Wymelenberg A, Cullen D. Phanerochaete chrysosporium glyoxal oxidase is encoded by two allelic variants: structure, genomic organization, and heterologous expression of glx1 and glx2. J Bacteriol. 1995;177:6106–6110. doi: 10.1128/jb.177.21.6106-6110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohchi C, Toh-e A. Nucleotide sequence of Candida pelliculosa β-glucosidase gene. Nucleic Acids Res. 1985;13:6273–6282. doi: 10.1093/nar/13.17.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Nagalla S R, Renganathan V. Cloning of a cDNA encoding cellobiose dehydrogenase, a hemoflavoenzyme from Phanerochaete chrysosporium. Appl Environ Microbiol. 1996;62:1329–1335. doi: 10.1128/aem.62.4.1329-1335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Nagalla S R, Renganathan V. Cellobiose dehydrogenase from Phanerochaete chrysosporium is encoded by two allelic variants. Appl Environ Microbiol. 1997;63:796–799. doi: 10.1128/aem.63.2.796-799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lymar E S, Li B, Renganathan V. Purification and characterization of a cellulose-binding β-glucosidase from cellulose-degrading cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1995;61:2976–2980. doi: 10.1128/aem.61.8.2976-2980.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machida M, Ohtsuki I, Fukui S, Yamashita I. Nucleotide sequences of Saccharomycopsis fibuligera genes for extracellular β-glucosidases as expressed in Saccharomyces cerevisiae. Appl Environ Microbiol. 1988;54:3147–3155. doi: 10.1128/aem.54.12.3147-3155.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen H, Engelbrecht J, Brunak S, Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Rouvinen J, Bergfors T, Teeri T, Knowles J K C, Jones T A. Three dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science. 1990;249:380–386. doi: 10.1126/science.2377893. [DOI] [PubMed] [Google Scholar]
- 24.Sandrock R W, Dellapenna D, Van Etten H D. Purification and characterization of β-2-tomatinase, an enzyme involved in the degradation of α-tomatine, and isolation of the gene encoding β-2-tomatinase from Septoria lycopersici. Mol Plant-Microbe Interact. 1995;8:960–970. doi: 10.1094/mpmi-8-0960. [DOI] [PubMed] [Google Scholar]
- 25.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith M H, Gold M H. Phanerochaete chrysosporium β-glucosidase: induction, cellular localization, and physical characterization. Appl Environ Microbiol. 1979;37:938–942. doi: 10.1128/aem.37.5.938-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strauss E C, Kobori J A, Siu G, Hood L E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986;154:353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Trimbur D, Graham R, Warren R A J, Withers S G. Identification of the acid/base catalyst in Agrobacterium fecalis β-glucosidase by kinetic analysis of mutants. Biochemistry. 1995;34:14554–14562. doi: 10.1021/bi00044a034. [DOI] [PubMed] [Google Scholar]