Abstract
Background:
Injectable Platelet Rich Fibrin (I-PRF) and Advanced-Platelet Rich Fibrin (A-PRF) are autologous materials derived from patients’ blood and employed in periodontal regenerative surgery. Although I-PRF and A-PRF have different characteristics, their biological effects on gingival tissue fibroblasts remain unclear. This research aims to compare the in vitro capacity in inducing gene expression and proliferation of human gingival fibroblasts between A-PRF and I-PRF.
Methods:
Human donors undergoing dental implant surgery were sampled for normal human gingival fibroblasts (NHGFCs), followed by preparing A-PRF and I-PRF membranes. Enzyme-linked immunosorbent assay (ELISA) kit was used to assess the release of platelet-derived growth factor-AA (PDGF-AA), transforming growth factor-beta1 (TGF- β1), and insulin growth factor-1 (IGF-1) at different periods. Cell viability and proliferation of A-PRF and I-PRF were compared using CCK-8 assay. The impacts of platelet concentration on human gingival fibroblast cells (HGFCs) were evaluated by quantifying the level or amount of phosphorylated extracellular signal-regulated protein kinase (p-ERK), and Matrix metalloproteinases (MMPs), MMP-1 and MMP-3. The effects of PRF on aged human gingival fibroblast cells were examined retrospectively.
Results:
Overall, A-PRF demonstrated a higher release of TGF-B1 and PDGF-AA, while I-PRF reflected higher levels of IGF-1. A significantly higher level of cell proliferation was induced by higher cell proliferation by A-PRF and I-PRF. Additionally, in comparison to I-PRF, the expression of ERK phosphorylation and MMP-1 &MMP-3 in HGFCs was demonstrated by I-PRF and A-PRF. The increase in A-PRF was time-dependent (p < 0.05).
Conclusion:
Both I-PRF and A-PRF induced a stimulatory biological impact on the proliferation of human gingiva fibroblasts, with the latter demonstrating better capacity in facilitating the release of different growth factors. A-PRF also induced higher gene expression of p-ERK, MMP-1 &MMP-3, and the proliferation of fibroblasts.
Keywords: Gingival fibroblasts, Platelet concentrates, Regenerative surgery, Biomedical materials, Cellular proliferation
Introduction
Oral human fibroblasts from cell cultures are commonly utilized in tissue engineering studies [1] due to their gap-reducing property between the teeth and oral mucosa. Diverse methods, such as enzymatic and direct explant techniques, have been employed in cultivating human gingival fibroblast cells (HGFCs) [2–4]. Enzymes such as trypsin, dispase, or collagenase are commonly used in these enzymatic techniques by focusing on isolating human keratinocytes. Meanwhile, higher proliferation rates and fewer steps are observed upon applying the direct explant method, which has been used for more than four decades with some adjustments [5]. Presently, there is a growing interest in tissue engineering, regenerative medicine, and therapeutic cloning research. Specifically, various medical fields have benefitted from tissue engineering. Platelet-rich fibrin (PRF) was first introduced over a decade ago as an autogenous source of blood growth factors that can function as a tissue regeneration tool in advanced medicine [6, 7]. PRF appears as a fibrin network with efficient proliferation and cell migration, thereby leading to cicatrization. This unique structure may assist in transporting essential cells for tissue regeneration. Furthermore, tissue regeneration could be guided by using the PRF membrane could, which is also recognized as a resorbable membrane [8]. PRF has become a popular technique in regenerative dentistry, given its ability to exhibit natural coagulation when centrifuged and fractionated from the red thrombus [1]. The utilization of L-PRF according to a high-speed protocol of approximately 700 g for 12 min has also revolutionized PRF [1]. Thereafter, The aim of introducing injectable PRF (roughly 60 g for three minutes) and A-PRF (about 200 g for eight minutes) with reduced g-forces and centrifugation time was to boost the numbers of platelets and leucocytes. The aforementioned purpose was also achieved by employing centrifuges with swingout rotors [9].
Several growth factors are also released by Platelet-rich fibrin (PRF), including the platelet-derived growth factor (PDGF) and transforming growth factor (TGF-b). Recent studies reported that the PRF membrane possesses a substantial steady and slow, sustained release of important growth factors for a minimum of one week and extends to 28 days, indicating the capacity of the PRF membrane to stimulate its surrounding for a prolonged period during wound healing [6]. PRF has been used clinically in various fields, including oral surgery and implant dentistry. Likewise, the biological effects of PRF in managing periodontal infra-bony defects have also been reported [2, 3].
It was recently hypothesized that the population of white blood cells might be increased within the PRF matrix by reducing centrifugation G-force [10]. The hypothesis was based on the fact that high centrifugation forces shift the cells to the base of collection tubes while the top one-third layer accommodates the PRF. Hence, the total white blood cells within PRF matrix scaffolds increased significantly by decreasing centrifugation g-force, which is presently referred to as A-PRF or advanced-PRF [10]. Additionally, aligning with the aforementioned hypothesis, A-PRF elicited a higher release of numerous growth factors, such as PDGF, TGF-β1, VEGF, epidermal growth factor (EGF), and insulin-like growth factor (IGF) relative to L- PRF, and PRP [11].
There is a dearth of information comparing the effects between A-PRF and I-PRF on human gingival fibroblast cell vitality, proliferation, and differentiation. All the previous research either compared the effects between platelet-rich plasma and L-PRF on human gingival fibroblast. Hence, this study aims to achieve the following objectives: (1) determine the impact of A-PRF and I-PRF on vitality, proliferation, and differentiation of HGFCs (2) determine the levels of growth factors (i.e., IGF-1, TGF-b1, and PDGF-AA released from A-PRF and I-PRF over time, (3) assess the biological effects of I-PRF and A-PRF on aged HGFCs, and (4) Differentiate the concentration-dependent secretion and proliferation of neurotrophic factors.
Materials and methods
Gingival biopsy and human gingival fibroblast cultivation
Conducted between September 2019 and January 2021, this study was carried out at the stomatology school/hospital of Lanzhou University in China. Six healthy patients (three females and three males) with clinically normal gingivae who were scheduled for implant surgery were selected for the study. The patients had a mean age of 25 years. Gingival fragments were collected from them at the Institutional Department of a dental implant, and the analysis was performed in accordance with the ethical committee guidelines at the University (LZUKQ-2022–030).
The patients provided written and informed consent before the onset of the study. Boric acid (0.75%) was used to wipe the gingival tissue before it was explanted. This procedure is vital in protecting the tissue against infection. Explants were then harvested using a sharp scalpel under local anesthesia. The explants were minced in a 15 mL sterile centrifuge tube comprising 10% fetal bovine serum (Royacel SERUM, FBS, RY-F22-01, Shanghi, China), DMEM (Dulbecco's Modified Eagle Medium) from Hyclone DMEM, cytiva, USA was used in the experiment, and 10% antibiotics (10,000 U/mL penicillin G, 10000 µg/mL streptomycin, Solarbio, Shanghi, China), and transported to the laboratory for analysis [12].
A solution consisting of 20 mM sodium phosphate, 150 mM NaCl, and pH 7.2, commonly known as phosphate-buffered saline (PBS), was used for rinsing the collected samples twice. The PBS solution also contained 1% antibiotics (10,000 U/mL penicillin G, 10,000 µg/mL streptomycin, Solarbio). Sterile surgical scissors were then used to fractionate the samples into small pieces. Subsequently, the connective tissue pieces were placed into culture flasks alongside DMEM containing 20% fetal bovine serum (Royacel SERUM, FBS, RY-F22-01) and 1% antibiotics in an incubator at 37 °C under 5% CO2.
The cells were visualized around the tissue pieces on the 10th day. Upon attaining cell confluency, the fibroblasts were trypsinized (Trypsin – EDTA [0.25%] phenol red, Yeasen, China) and cultured in DMEM with 10% FBS. The cells from passages three through five were used in all the experiments [13]. The cells were assessed microscopically at × 40 magnification (OLYMPUS DP74, Tokyo, Japan). Finally, 0.25% trypsin/EDTA solution was used to subculture the cells upon attaining 80% confluency (Yeasen, China).
A-PRF and I-PRF preparation
A total of eight volunteer donors aged between 20 and 40 years old who provided informed consent were used in this experiment. Each donor provided three blood samples, thereby amounting to 24 samples. The blood samples were processed for I-PRF and A-PRF preparation.
For A-PRF preparation, a blood sample of approximately 9 mL was transferred into 10 mL EDTA tubes (Sanli, Hunan, China) and immediately centrifuged at 1300 rpm for 14 min, and RCF-max of 60 g at room temperature (Trausim AiPRF-08 Centrifuge, Jiangsu, China). Meanwhile, I-PRF was prepared by collecting 7 mL of a blood sample using 10 mL EDTA tubes (Kangjian, Taizhou, China) and immediately centrifuged at 700 rpm for three min at RCF-max of 60 g at room temperature (Trausim AiPRF-08 Centrifuge, Jiangsu, China). The centrifugation produced three distinct layers; the collected blood samples were divided into three distinct layers. The bottom layer consists of erythrocytes, the supernatant or upper layer contains the serum, and for the A-PRF samples, an additional layer was present interposed between the two adjacent layers, known as the A-PRF clot layer.".
The A-PRF membrane was then obtained by squeezing the samples between two pieces of sterile gauze. Meanwhile, the I-PRF was prepared by collecting the upper liquid layer of the centrifugation. Afterward, the samples were moved into culture dishes made of polystyrene 5 ml of culture media (DMEM from Gibco, Life Technologies, Carlsbad, CA, USA) was added to the container for subsequent analysis.
Determination of growth factor release in A-PRF and I- PRF by ELISA
This experiment was initiated by preparing six tubes comprising 4 mL of DMEM without PBS or serum. The concentration of TGF-B1, PDGF-AA, and IGF-1 was estimated by submerging the A-PRF in the first tube for 1 h. The A-PRF was suctioned and transferred into the next tube. These steps were performed again until the sixth tube. PDGF-AA, TGF-B1, and IGF-1 concentrations were estimated at 1, 8, 24, 48, and 72 h, and finally, on day 5 & day 7. An equivalent number of tubes were prepared for I-PRF, 4 mL of DMEM without serum or PBS was added to each container. A shaking incubator set at 60 rotations per minute and 37 °C was used to prepare the samples to facilitate the release of growth factors GF release into the DMEM. This procedure assisted in determining the concentration of growth factors released from I-PRF at different time intervals (1, 8, 24, 48, and 72 h and five &seven days).
To quantify the protein levels in A-PRF and I-PRF, ELISA kits (Meimian, Jiangsu, China, MM-0047R, MM-0190R, MM-0180R) were used in accordance with the manufacturer's instructions at predetermined time intervals. The absorbance of the samples was measured using a microplate reader (Infinite M Mano, 200Pro, Austria) at a wavelength of 450 nm. The findings were presented as the total weight of molecules (ng) per mL of supernatant volume. The concentration that produced the highest growth factor release was identified by measuring their levels at various time intervals.
All samples were analyzed in duplicates, and each platelet concentrate was evaluated in three separate experiments.
Evaluation of A-PRF treatment and I-PRF on the HGFCs proliferation by CCK-8
The initial step entailed the treatment with A-PRF extracts to limit the disparities among the A-PRF membranes. The A-PRF was submerged in fresh DMEM and incubated for five days at room temperature and 5% CO2 to facilitate the optimal release of the total growth factors for the aforementioned process. Then, conditioned mediums were collected, followed by supplementing the extracts with 10% FBS and 1% antibiotics. The conditioned mediums obtained from A-PRF were labeled as 100% A-PRF extract and stored at − 80 °C until use. The I-PRF samples were processed in a similar manner to A-PRF.
The effects of various A-PRF and I-PRF concentrations on cellular proliferation were evaluated utilizing an established Cell counting kit-8 (CCK-8) test. The procedure was executed by seeding the 4th and 5th HGFC generations into 96-well plates at 5000 cells/well cell density and preserved and incubated for 24 h at 37 °C in a humidified atmosphere containing 5% CO2. The cultured cells were then grouped into four categories: 25%, 50%, 100%, and control. Meanwhile, a cell viability test was conducted on days 1, 2, and 3 using a CCK-8 kit (Yeasen, Biotech Co., Ltd, Shanghai, China). A microplate reader (Infinite 200Pro) was then employed to record the values obtained from measuring the absorbance at 450 nm. All samples were quantified in triplicates, with each platelet concentrate analyzed in three separate experiments.
Western blot for MMP-1, MMP-3, and p-ERK
HGFCs at confluency were treated with A-PRF and I-PRF following the same protocol described earlier, followed by trypsinization. The cells were counted and seeded at a concentration of 5 × 105 cells in a 60 mm culture dish to attain confluence. Afterward, the cells were lysed in radioimmunoprecipitation assay buffer (RIPA buffer, Solarbio, Life Sciences) containing protease inhibitors (PMSF, Solarbio, Life Sciences) and centrifuged at 12,000 rpm at 4 °C for 15 min. The protein content was measured utilizing a BCA colorimetric protein assay kit (Solarbio, Life Sciences). Equal amounts of total protein per sample of cell extracts were separated by 10% SDS-PAGE (SDS-PAGE gel kit Solarbio, Life Sciences), then transferred to nitrocellulose membranes (Solarbio, Life Sciences). The membranes were blocked with 5% diluted skim milk, washed with 1XTBST0.5Tween20 (Solarbio, Life Sciences), and then incubated with primary antibodies, namely anti-p-ERK (ImmunoWay Biotechnology Company, Plano, TX, USA) (1:1000), anti-MMP-1 (ImmunoWay Biotechnology Company) (1:1000), or anti-MMP-3 (ImmunoWay Biotechnology Company) (1:1000) in PBS containing 0.05% Tween20 for two hours. After washing with Tween20, the membranes were incubated for 60 min with a biotinylated secondary antibody (ImmunoWay Biotechnology Company) diluted 1:10,000 in the same buffer, followed by re-washing as described earlier. The reaction was developed using diaminobenzidine (Solarbio, Life Sciences) after a series of washing steps. The intensities of the bands were measured with a densitometer (AlphaImager 2000; Alpha Innotech, San Leandro, CA, USA). The densitometric values for each sample were expressed as mean and standard deviation.
Effectiveness of A-PRF and I-PRF in promoting the proliferation of older cells
Upon culturing the human gingival fibroblasts, The HGFCs were cultured for 14 to 16 generations and incubated at room temperature for 72 h (I-PRF) and five days (A-PRF). The cells were categorized into two groups; the DMEM medium was used to inoculate the HGFCs in the control group, while A-PRF Extract/I-PRF was utilized to inoculate the treatment group. Subsequently, the cell growth rate was visualized microscopically for four consecutive days. The same protocol of 14–16 generations of the HGFCs was treated with A-PRF and I-PRF extract, followed by performing the CCK-8 test (Yeasen, Biotech Co., Ltd) to assess the impacts of both PRFs on aged cell proliferation. This was achieved by seeding the HGFCs into 96-well plates at 5000 cells/well cell density and preserved in a CO2-regulated and humidified environment at 37 °C for 24 h. A microplate reader (Infinite 200Pro) was then utilized to record the absorbance at a 450 nm wavelength. Three separate experiments (i.e., in triplicates) were performed.
Results
Cultured fibroblast cells
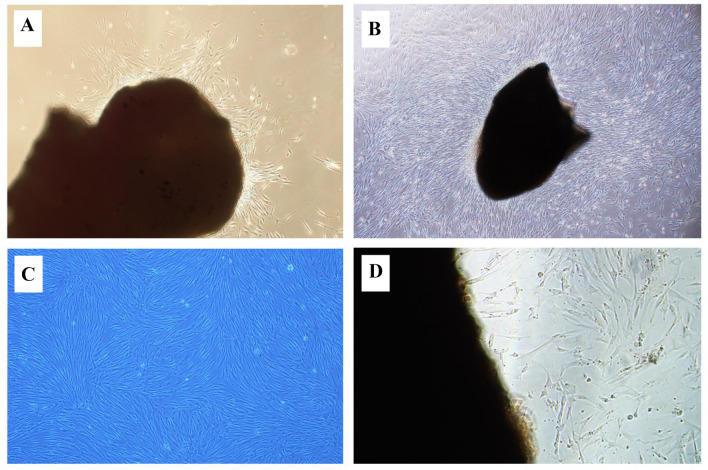
Patient biopsies were used to culture gingival tissues, which yielded HGFCs successfully. Adherent fibroblast cells were not visible until the eighth day of culturing. Using a light microscope, the primary cells exhibited a morphology displaying fibroblast-like spindle shapes (Fig. 1A).
Fig. 1.
Cultured HGFCs prepared from a gingival biopsy sample. A Spindle-shaped fibroblast cells start to appear after cell culture. B The cell line reached about 80% confluence without overlapping, which indicates the sub-culture. C Rapidly growing cells appeared radial or whorled after the first generation. D The explant technique offers a reusable gingival tissue after the first subculture and can be used for continuous cell culture for over two months. HGFCs—Human gingival fibroblast cells
A smaller-sized fibroblast cell with sharp edges is desirable for the explant technique [6]. The cells attained a complete single layer without overlapping cells and an 80% confluence after 10 days (Fig. 1B), which indicates the necessity for subsequent culturing. Thereafter, the cells developed rapidly and displayed whorled or radial following the 1st generation (Fig. 1C). Figure 1D depicts the abundant HGFCs with typical morphology; this was made possible by using the explant technique, which allowed for the reuse of gingival tissues after the first subculture and enabled cell culture that lasted for more than 60 days starting from the day of reculturing.
Growth factors release rate ELISA
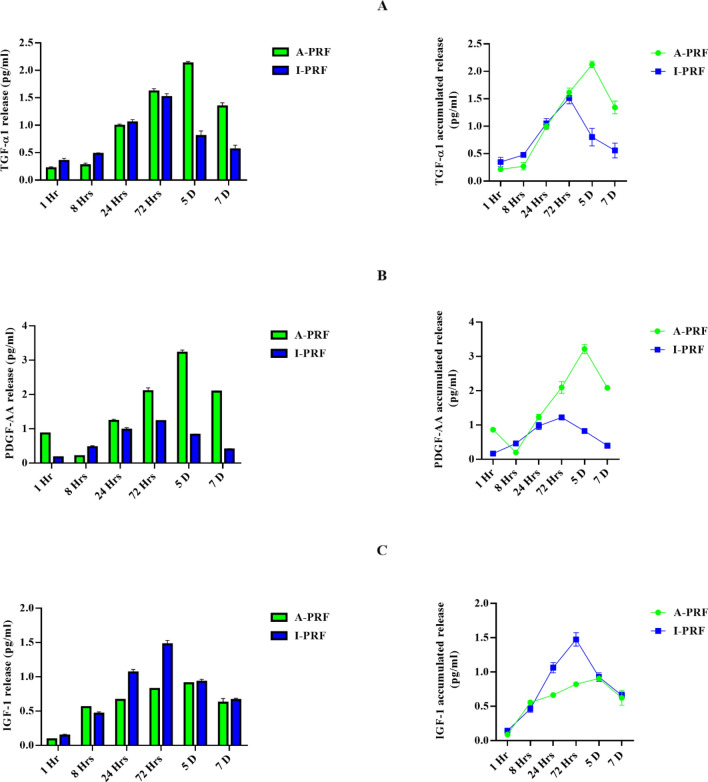
Despite reflecting a slow release of growth factors, A-PRF was better at enhancing the additional secretion of growth factors. Figure 2 depicts the quantification of PDGF-AA, TGF-b1, and IGF-1 concentrations at 1, 8, 24, and 72 h. and on 5&7 days, reflecting similar release patterns for PDGF-AA, IGF-1, and TGF-b1.
Fig. 2.
Immediate and cumulative release of growth factors from the A-PRF and i-PRF samples. A–C The released differential growth factors (TGF-β1, PDGF-AA, IGF-1) were quantified using Enzyme-linked Immunosorbent Assay method at various time points (1,8, 24, 72 h, 5 and 7 days). PDGF-AA—Platelet derived growth factor, TGF-β1—transforming growth factors-beta 1, IGF-1—Insulin growth factor-1
The release of growth factors (i.e., PDGF-AA, IGF-1, and TGF-b1) from A-PRF and I-PRF was evaluated by ELISA. Notably, the release of PDGF-AA and TGF-b1 was significantly higher for A-PRF in comparison to I-PRF (IGF-1 was significantly higher for I-PRF in comparison to A-PRF). Next, the cumulative release of growth factors was measured for up to seven days. Resultantly, TGF-b1 and PDGF-AA demonstrated a higher release of total growth factors from A-PRF relative to I-PRF. In contrast, the overall growth factor release of IGF-1 was significantly higher in I-PRF in comparison to A-PRF.
This experiment was conducted to assess the possibility of obtaining the best result from PRF extract preparation. I-PRF was then prepared within three days to obtain the best growth factors, while A-PRF was prepared after five days of incubation for the optimal result. These points are considered the appropriate time to prepare the conditioned medium for each type of PRF with the fibroblast cells.
Effect of A-PRF and I-PRF treatment on the proliferation of HGFCs
Excellent cell biocompatibility was displayed by all the A-PRF and I-PRF concentrations. This was evident by the presence of the highest population of living cells (Fig. 3). Hence, the A-PRF and I-PRF levels in each sample were completely biocompatible upon using the in vitro model of cell culture. The highest cellular proliferation levels were exhibited by the cells administered with 100% A-PRF and 100% I-PRF extracts. Overall, a higher cell population was induced by A-PRF induced at three days relative to the control and I-PRF (Fig. 3C).
Fig. 3.
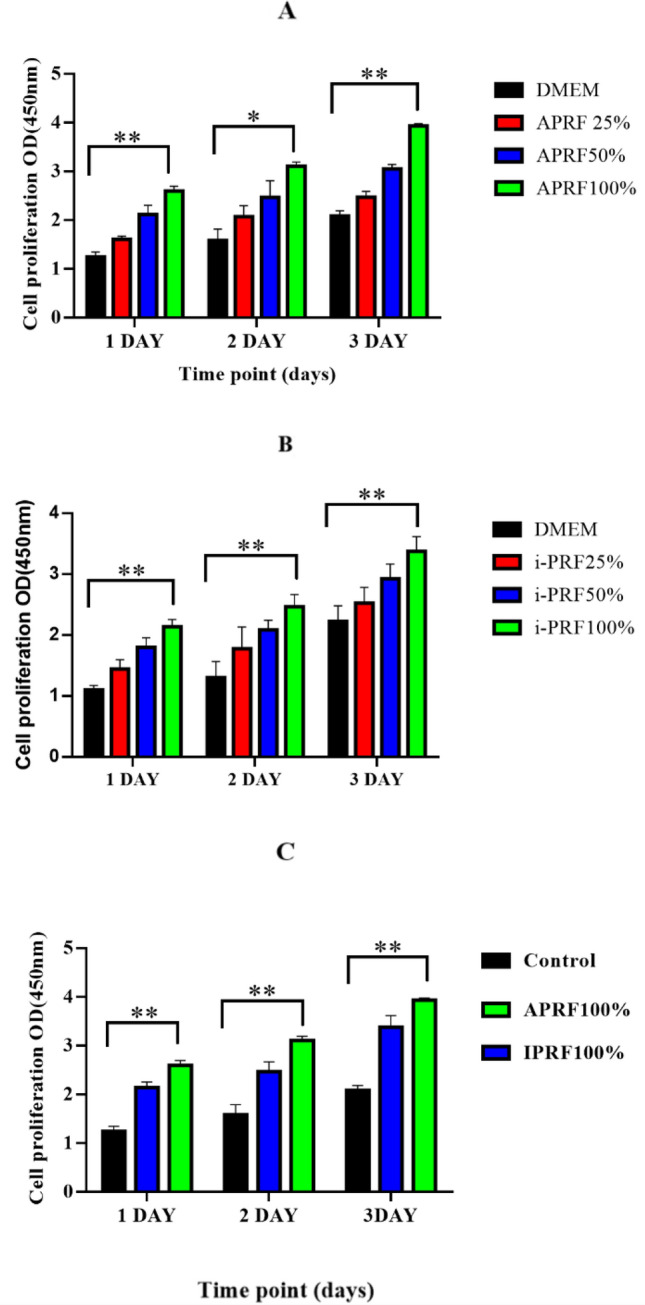
Effect of different concentrations of PCs on HGFCs proliferation. A, B Effect of different concentrations of (A-PRF and i-PRF) on HGFCs proliferation from day 1 to day 3. C Effect of full percentage concentrations of A-PRF and i-PRF on HGFCs proliferation from day 1 to day 3. Values are presented as mean ± SD (n = 6). Significant difference was indicated by *p < 0.01 and **p < 0.001
Western blot for p-ERK, MMP-1, and MMP-3
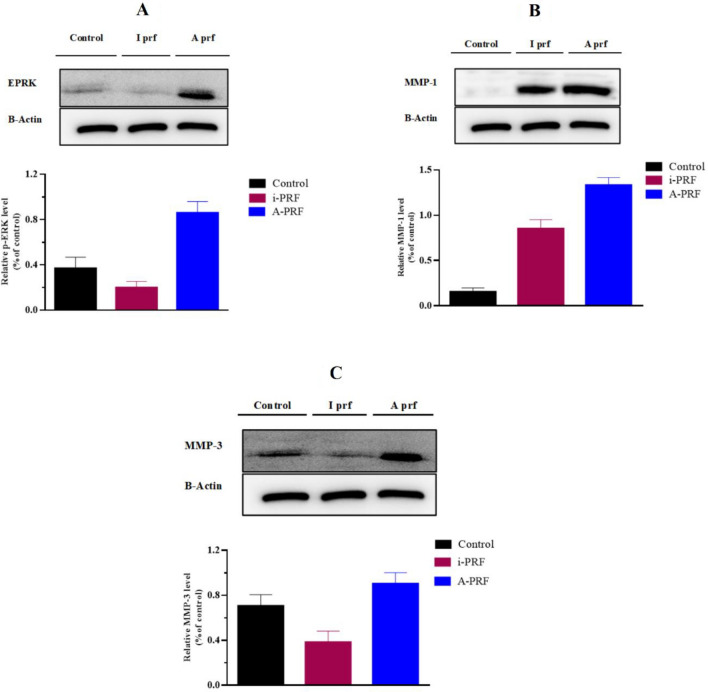
The p-ERK, MMP-1, and MMP-3 genes were upregulated several-fold by A-PRF and I-PRF. To determine whether the production of MMPs and p-ERK increased at the protein level, this study employed culture media from NHGFs treated with or without A-PRF and I-PRF, as well as Western blot analysis of cell extracts. As a result, only A-PRF induced a significant and time-dependent increase in the phosphorylation of p-ERK, MMP-1, and MMP-3 in HGFCs (p < 0.05). In comparison with injectable I-PRF, the collection of p-ERK and MMPs in the cell culture medium was significantly increased by the gene expression of A-PRF. Nevertheless, the increased protein levels in the cell layer and the control samples were not significantly different. The quantities of p-ERK, MMP-1, and MMP-3 activity increased immediately and significantly (p < 0.05) following the exposure to PRFs treatment. Furthermore, the -ERK protein expression in NHGFs was upregulated by PRF (Fig. 4) depicts The levels of phosphorylated ERK, MMP-1, and MMP-3 proteins treated with PRF, which were quantified as described by AlphaImager (2000).
Fig. 4.
Alpha Imager 2000 for performing B-actin to monitor the protein loading (of p-ERK, MMP-1 &MMP-3). A A-PRF was found to increase ERK phosphorylation in HGFCs when compared to I-PRF B A-PRF was found to increase MMP-1 in HGFCs when compared to I-PRF (C) A-PRF was found to increase MMP-3 in HGFCs when compared to I-PRF
Effect of A-PRF Extract and I-PRF on aged cells
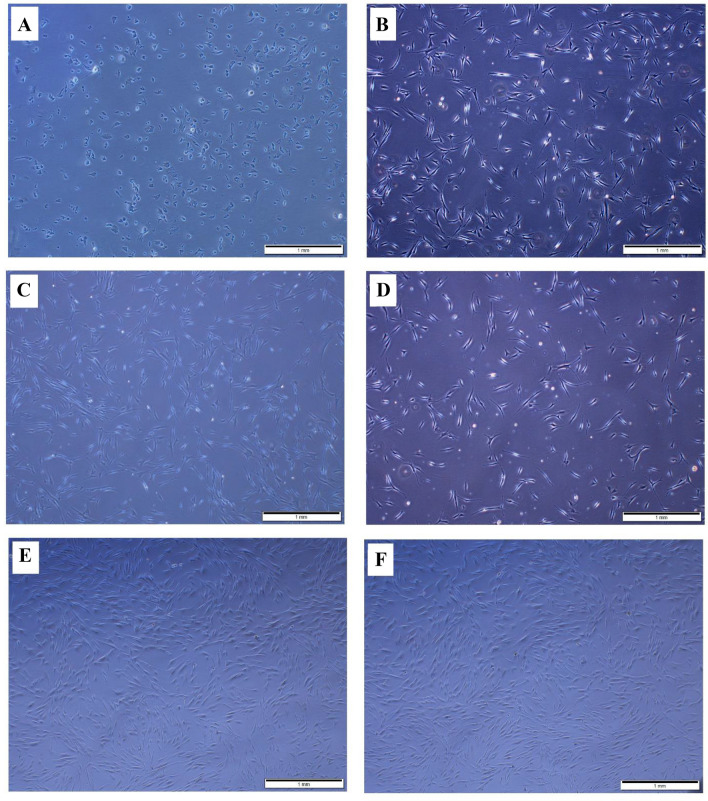
Microscopically, The CCK-8 test showed that the aged cells were able to maintain their attachment for two days, although with poor cell morphology (Fig. 5A). In contrast, after two days, significant cell proliferation was observed in HGFCs treated with A-PRF extract and I-PRF conditioned medium, and these cells exhibited usual elongated and spindle-shaped appearance (Fig. 5B, C). Slow proliferation continued in the aging HGFCs after four days (Fig. 5D) in comparison to the intense proliferation in those treated with I-PRF conditioned medium and A-PRF extract (Fig. 5E, F). The CCK-8 test also revealed superior results from the A-PRF extract than I-PRF, whereas both enhanced the cellular proliferation compared to the control group (Fig. 6).
Fig. 5.
The effect of A-PRF and i-PRF extract on the proliferation of aged gingival fibroblasts. A Aging HGFCs did not proliferate well after two days of adherence. B, C After A-PRF and i-PRF induction, aged HGFCs began to proliferate two days later. D The control group proliferated slowly in the first four days. E, F Aged HGFCs proliferate rapidly on Day 4 after A-PRF and i-PRF induction
Fig. 6.

The effect of A-PRF and i-PRF extract on the proliferation of aged gingival fibroblasts using CCK-8 test. Based on the one-way analysis (ANOVA) the result showed that the use of A-PRF and i-PRF extract were significantly different compared to control. *p < 0.01. CCK-8: cell counting kit-8
Discussion
The field of dentistry has witnessed a wide application of tissue engineering technology and implantology. The current study evaluated a rapid method and simple modification of HGFC primary tissue culture. Specifically, the in vitro culture of HGFCs obtained from the biopsy sample depicted enhanced efficiency. The advantages of the fibroblast isolation method employed in this experiment are as follows: (1) HGFCs can be fastly produced given that a sufficient population of fibroblasts is obtainable in less than 11 days, (2) the fibroblasts could be uninterruptedly synthesized and re-used, (3) migrating cells benefit significantly from the companion tissue. The absence of proteolytic enzymes facilitates consistent attachment of the cell to the extracellular matrix (ECM), thereby causing a longer time for isolation, and (4) Isolation of cells through explant culture of HGFCs: no digestion step is required in placing the tissue pieces into the culture dish, and the wound condition facilitates the migration of the cells out of the tissue [12].
Choukroun's technique is employed to generate PRF through natural means without using thrombin. PRF is also believed to contain a natural fibrin scaffold that shields growth factors from proteolysis.[14]. Hence, their activities can be maintained for a relatively longer duration by growth factors, thereby stimulating efficient tissue regeneration [15].
Several clinicians in the implant dentistry field now consider the utilization of widely accepted regenerative methods in dental practice [16]. Presently, numerous biomaterials are routinely used, such as barrier membranes, bioactive growth factors, and bone grafting materials, to support new tissue regeneration. Notably, one of the prominent challenges for clinicians is the dimensional alterations of alveolar bone after tooth loss. As a result, numerous regenerative procedures have been employed [17–20].
To evaluate the effect of PRFs on the expression of wound healing-related genes in NHGFs, the NHGFs were treated with PRFs obtained from donors, while untreated NHGFs were used as a control. These wound healing-related genes include crucial fibroblast genes such as growth factors, extracellular matrix (ECM) proteins, matrix-degrading enzymes, cytokines, and those involved in regulating the fibroblast phenotype [21].
The present findings corroborate the hypothesis as A-PRF and I-PRF induced a sustainable release of growth factors. The micrographs of A-PRF and I-PRF revealed an innate fibrin framework that suggested the presence of a network of numerous erythrocytes, leukocytes, and platelets. These networks contain various cells and platelet components that are enriched with exceptional biological properties, which could contribute to the release of growth factors.
The growth factor levels released from I-PRF and A-PRF were evaluated in the current experiment. Specifically, TGF-b1, PDGF-AA, and IGF-1 were the main growth factors on account of their angiogenesis-promoting and anti-inflammatory effects [22]. The characteristic of being handled as a solid biomaterial is reported as one of the advantages of A-PRF relative to platelet concentrates [23]. Meanwhile, fibrin is the most critical adjunct molecule in A-PRF, which elicits better treatment compared to traditional PRP. Hence, A-PRF is considered a suitable delivery vehicle and biomaterial. On the other hand, I-PRF is an injectable liquid.
Both A-PRF and I- PRF were also significant in enhancing the proliferation of HGFCs, which was evident by the increased fibroblast migration. This finding might be linked to the mediating role of the released growth factors, thereby promoting the production of diverse cell types. Prior research reported that TGF-b1 enhanced the proliferation of cancer cells [24], while PDGF stimulated the multiplication of epithelial cells of the retinal pigment [25]. The production of various peripheral cells was also promoted by IGF-I. [26] The quick proliferation of HGFCs results in the production of crucial bioactive compounds necessary for promoting epithelial growth, which aids in the healing and repair of surgical areas. As displayed in the CCK-8 assay, the present study highlighted the crucial role of I-PRF and A-PRF in promoting HGFC proliferation [27]. Furthermore, the aforementioned function was propagated in a dose-dependent manner, with A-PRF eliciting a significantly higher level of HGFC proliferation compared to I-PRF. Both PRFs at a concentration of 100% induced the highest beneficial effect on fibroblast cells. Thus, A-PRF and I- PRF demonstrated a synergistic effect on cell proliferation.
In addition, this study emphasized the effect of A-PRF and I-PRF on the multiplication of HGFCs that have aged. The findings demonstrated that both A-PRF and I-PRF were able to recover and reinstate the biological features of elderly HGFCs and stimulate cell growth accordingly. This occurrence can be explained by the abundant release of growth factors. There is a possibility that these growth factors can revitalize the metabolic function of senescent cells. Given this, it is essential to further examine the impact of A-PRF and I-PRF on aged cells and their particular mechanism; as highlighted in this study, Fibroblast multiplication is essential for tissue recovery, and both A-PRF and I-PRF have been linked to this process, suggesting that they may have the ability to promote tissue healing.
A vital mitogen-activated protein kinase cascade, the extracellular signal-regulated protein kinase (ERK) signaling pathway, is indispensable for governing cell proliferation and differentiation. The influence of A & I-PRF on HGFCs was measured by assessing the expression of the p-ERK protein, where cell proliferation is a known function of ERK [8]. This study found that A-PRF&I-PRF can upregulate the expression of p-ERK in HGFCs because it enhances the phosphorylation of ERK in HGFCs. Consequently, A-PRF and I-PRF may use the p-ERK signal transduction pathway to enhance cell proliferation. In implant dentistry, PRF is frequently employed as a medium for cell growth factors as it is derived from a concentrated platelet autogenous preparation. This study found that A-PRF increased p-ERK expression higher than I-PRF. The inflammation can be modulated by MMPs by stimulating or inhibiting inflammatory mediators, which contribute to matrix remodeling. An upregulation of MMPs has been documented during the early stages of scarless wound healing in both fetal and adult gingiva. L-PRF was observed to markedly elevate the expression of MMP-1 and MMP-3 in early wounds and NHGFs, which exert broad influence on the regulation of chemokine activity, including the synthesis of receptor-blocking agents for CCL2, -5, -7, -8, and -13, which may reduce inflammation; furthermore, MMPs degrade matrix barriers, leaving space for cell migration, which might provide a possible mechanism for gingival fibroblast cell migration mediated by L-PRF. Additionally, by secreting growth factors that are immobilized in the matrix, including TGF-β, FGF-2, and VEGF, as well as by activating CXCL8/IL-8, FGF-2, and VEGF are powerful angiogenic agents that control neovascularization; MMPs modulate this angiogenesis. We investigated how A-PRF and I-PRF influenced the genetic expression of MMP-linked genes in NHGFs since MMPs are essential for regulating inflammation as well as ECM degradation in wound healing. MMP-1 and MMP-3 were both substantially elevated. [21] In conclusion, A-PRF therapy of NHGFs regulated multiple genes and behaviors related to wound healing. In the current study, MMP-1 and MMP-3 showed several-fold upregulation and were among the genes that exhibited this pattern by A-PRF than I-PRF. Moreover, to investigate if these MMPs also increase at the level of the protein, Western blotting was performed on cell extracts and culture media collected from NHGFs that were treated with PRF or left untreated. The results indicated that A-PRF led to a greater increase in the accumulation of MMP-1 and MMP-3 in the cell culture media than I-PRF.
The findings of this study showed that PRF treatment resulted in an elevation of ERK phosphorylation in HGFCs. This finding is consistent with previous research indicating the involvement of ERK in promoting cell proliferation, and the finding coincides with that of Chang et al. [28] MMPs are among the genes that are upregulated several-fold by PRF. Western blotting of cell extracts was used in this study. The protein level expression of these MMPs was examined by collecting culture media from NHGFs that were treated with or without PRF therapy [21]. Overall, only A-PRF increased p-ERK phosphorylation, MMP-1, and MMP-3 in HGFCs in a way that was dependent on time.
Conclusively, this study showed that both A-PRF and I-PRF possess several growth factors with fibroblast-inducing behavior and tissue-regenerating properties. A-PRF and I-PRF exhibited different levels of growth factor release and cellular effects, which might be linked to the different addition of anti-coagulants and centrifugation protocols. Nevertheless, A-PRF was superior in eliciting growth factors release compared to I-PRF. Future studies should consider investigating the utilization of liquid formulation of blood concentrates to elucidate their benefits in regenerative dentistry for possible clinical advantage. The present results will boost the utilization of I-PRF, A-PRF, and HGFCs in the tissue engineering field.
Acknowledgements
This research was funded by the 1) Lanzhou Talent Innovation and Venture Project (2017-RC-31), 2) Competitive Projects for Science and Technology Innovation and Development of Gansu Province (2018ZX-10), and 3) Gansu Key Technologies R&D Program for International S&T Cooperation and Exchange (18YF1WA116).
Author contributions
Conceptualization: LY, SHA, and MM; methodology: SHA, and MM; software: RA-A, OAA-A; validation: OAA-A; formal analysis: LY; investigation: MM; resources, and data curation: SHA and LY; writing—original draft preparation: SHA; writing—review and editing: MM, OAA-A, and WL; supervision: LY and WL; project administration: OAA-A, RA-A; funding acquisition: LY.
Data availability statement
All relevant study data are available in the tables and figures of the manuscript. If you need more details, please contact the corresponding author.
Declarations
Conflict of interest
The authors have no competing interests to disclose.
Ethical statement
The study was approved by the Ethics Committee of the School/Hospital of Stomatology, Lanzhou University, China (LZUKQ-2022-030).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reid CB, Cloos J, Snow GB, Braakhuis BJ. A simple and reliable technique for culturing of human oral keratinocytes and fibroblasts. Acta Otolaryngol. 1997;117:628–33. [DOI] [PubMed]
- 2.Wanichpakorn S, Kedjarune-Laggat U. Primary cell culture from human oral tissue: gingival keratinocytes, gingival fibroblasts and periodontal ligament fibroblasts. Songklanakarin J Sci Technol. 2010;32:327–31.
- 3.Kedjarune U, Pongprerachok S, Arpornmaeklong P, Ungkusonmongkhon K. Culturing primary human gingival epithelial cells: comparison of two isolation techniques. J Craniomaxillofac Surg. 2001;29:224–31. [DOI] [PubMed]
- 4.Klingbeil MFG, Herson MR, Cristo EB, dos Santos Pinto D, Yoshito D, Mathor MB. Comparison of two cellular harvesting methods for primary human oral culture of keratinocytes. Cell Tissue Bank. 2009;10:197–204. [DOI] [PubMed]
- 5.Lauer G, Otten JE, von Specht BU, Schilli W. Cultured gingival epithelium: a possible suitable material for pre-prosthetic surgery. J Craniomaxillofac Surg. 1991;19:21–6. [DOI] [PubMed]
- 6.Pinto N, Quirynen M. RE: Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J Periodontol. 2019;90:119–21. [DOI] [PubMed]
- 7.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet‐rich fibrin with the low‐speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–21. [DOI] [PubMed]
- 8.Chang YC, Zhao JH. Effects of platelet‐rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dental J. 2011;56:365–71. [DOI] [PubMed]
- 9.Strauss FJ, Nasirzade J, Kargarpoor Z, Stähli A, Gruber R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: a systematic review of in vitro studies. Clin Oral Investiga. 2020;24:569–584. doi: 10.1007/s00784-019-03156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rut-kowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–89. [DOI] [PubMed]
- 11.Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. Comparative release of growth factors from PRP PRF, and advanced-PRF. Clin Oral Investiga. 2016;20:2353–2360. doi: 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 12.Mudalal M, Wang Z, Mustafa S, Liu Y, Wang Y, Yu J, et al. Effect of Leukocyte-Platelet Rich Fibrin (L-PRF) on Tissue Regeneration and Proliferation of Human Gingival Fibroblast Cells Cultured Using a Modified Method. Tissue Eng Regen Med. 2021;18:895–904. doi: 10.1007/s13770-021-00360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Yang Y, Zhang Y, Miron RJ. Fluid platelet-rich fibrin stimulates greater dermal skin fibroblast cell migration, proliferation, and collagen synthesis when compared to platelet-rich plasma. J Cosmet Dermatol. 2019;18:2004–2010. doi: 10.1111/jocd.12955. [DOI] [PubMed] [Google Scholar]
- 14.Lundquist R, Dziegiel MH, Ågren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regen. 2008;16:356–363. doi: 10.1111/j.1524-475X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang YC, Zhao JH. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dent J. 2011;56:365–371. doi: 10.1111/j.1834-7819.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 16.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Invest. 2017;21:2619–27. [DOI] [PubMed]
- 17.Horowitz R, Holtzclaw D, Rosen PS. A review on alveolar ridge preservation following tooth extraction. J Eviden Based Dental Pract. 2012;12:149–160. doi: 10.1016/S1532-3382(12)70029-5. [DOI] [PubMed] [Google Scholar]
- 18.Lee CT, Chiu TS, Chuang SK, Tarnow D, Stoupel J. Alterations of the bone dimension following immediate implant placement into extraction socket: systematic review and meta-analysis. J Clin Periodontol. 2014;41:914–926. doi: 10.1111/jcpe.12276. [DOI] [PubMed] [Google Scholar]
- 19.Morjaria KR, Wilson R, Palmer RM. Bone healing after tooth extraction with or without an intervention: a systematic review of randomized controlled trials. Clin Implant Dent Relat Res. 2014;16:1–20. doi: 10.1111/j.1708-8208.2012.00450.x. [DOI] [PubMed] [Google Scholar]
- 20.Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implant Res. 2012;23:1–21. doi: 10.1111/j.1600-0501.2011.02375.x. [DOI] [PubMed] [Google Scholar]
- 21.Bi J, Intriago MFB, Koivisto L, Jiang G, Häkkinen L, Larjava H. Leucocyte-and platelet-rich fibrin regulates expression of genes related to early wound healing in human gingival fibroblasts. J Clin Periodontol. 2020;47:851–862. doi: 10.1111/jcpe.13293. [DOI] [PubMed] [Google Scholar]
- 22.Mudalal M, Sun X, Li X, Zhou Y. The evaluation of leukocyte-platelet rich fibrin as an anti-inflammatory autologous biological additive: a novel in vitro study. Saudi Med J. 2019;40:657. doi: 10.15537/smj.2019.7.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boora P, Rathee M, Bhoria M. Effect of platelet rich fibrin (PRF) on peri-implant soft tissue and crestal bone in one-stage implant placement: a randomized controlled trial. J Clin Diagnostic Res. 2015;9:ZC18. doi: 10.7860/JCDR/2015/12636.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Mudalal M, Sun Y, Liu Y, Wang J, Wang Y, et al. The effects of leukocyte-platelet rich fibrin (L-PRF) on suppression of the expressions of the pro-inflammatory cytokines, and proliferation of Schwann cell, and neurotrophic factors. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-59319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan C-M, Chang H-H, Wang V-C, Huang C-L, Hung C-F. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathways. PLoS One. 2013;8:e56819. doi: 10.1371/journal.pone.0056819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maucksch C, McGregor A, Yang M, Gordon R, Connor B. IGF-I redirects doublecortin-positive cell migration in the normal adult rat brain. Neuroscience. 2013;241:106–115. doi: 10.1016/j.neuroscience.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Mudalal M, Wang Z, Mustafa S, Liu Y, Wang Y, Yu J, et al. Effect of leukocyte-platelet rich fibrin (L-PRF) on tissue regeneration and proliferation of human gingival fibroblast cells cultured using a modified method. Tissue Eng Regen Med. 2021;18:895–904. doi: 10.1007/s13770-021-00360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang IC, Tsai CH, Chang YC. Platelet-rich fibrin modulates the expression of extracellular signal-regulated protein kinase and osteoprotegerin in human osteoblasts. J Biomed Mater Res, Part A. 2010;95:327–332. doi: 10.1002/jbm.a.32839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant study data are available in the tables and figures of the manuscript. If you need more details, please contact the corresponding author.