Abstract
The objective of this investigation is to assess the efficacy of ropivacaine on intraoperative and postoperative endpoints like operative time, blood loss, pain, and bleeding among adult’s patients undergoing for tonsillectomy. PubMed, CENTRAL, Scopus, and Web of Science databases were screened from inception until November 2022. The included RCTs were evaluated for risk of bias via risk of bias tool (second version). All endpoints were summarized as mean difference (MD) or standardized mean difference (SMD) for continues outcomes, and risk ration (RR) for dichotomous outcomes, under random-effect model. Four RCTs met our PICOS criteria, comprising a total of 257 patients. Regarding postoperative pain, there was a significant difference that favor ropivacaine group compared with placebo group within hours (n = 4 RCTs, SMD = -0.92, 95% CI [-1.57, -0.26], p = 0.006), and within days (n = 4 RCTs, SMD = -050, 95% CI [-0.82, -0.18], p = 0.002). However, there were no significant difference between ropivacaine and placebo groups I terms of operative time (n = 3 RCTs, SMD = -0.17, 95% CI [-0.45, 0.11], p = 0.22), intraoperative blood loss (n = 2 RCTs, SMD = -0.37, 95% CI [-1.41, 0.67], p = 0.49), and postoperative bleeding (n = 4 RCTs, RR = 2.27, 95% CI [0.90, 5.73], p = 0.08). In conclusion, administration of ropivacaine was associated with less postoperative pain among adult’s patients who undergoing tonsillectomy. However, there were no benefit in term of reduction in operative time, intraoperative blood loss, and postoperative hemorrhage.
Keywords: Ropivacaine, Tonsillectomy, Pain, Bleeding, Adults
Introduction
Tonsillectomy is a common procedure used to treat various conditions in both adults and children [1]. The decision to perform this surgery is based on the severity and frequency of symptoms, as well as the patient’s health and medical history [1]. The surgery is generally safe but can have potential complications, including pain, bleeding, nausea, vomiting, and dehydration [2]. Postoperative pain is a common complication and can be managed with medications such as opioids, NSAIDs, and acetaminophen [3]. However, current treatments for post-tonsillectomy pain are insufficient.
Local anesthetics, such as lidocaine, bupivacaine, and ropivacaine, can be administered to the peritonsillar space to improve postoperative pain management [4]. Ropivacaine is a type of amide-type local anesthetic medication commonly used to manage pain and provide analgesia for surgical procedures. It works by blocking nerve impulses that carry pain signals to the brain, resulting in pain relief [5]. Ropivacaine has a toxicity profile intermediate between bupivacaine and lidocaine and can be administered in various ways, including injection, infusion, and topical application [5].
Several randomized placebo-controlled trials (RCTs) have scrutinized the clinical impact of ropivacaine for tonsillectomy among adult’s patients [6–9]. Therefore, the study aimed to perform a comprehensive systematic review and meta-analysis of RCTs to evaluate the efficacy of ropivacaine compared to placebo in alleviating postoperative pain and improving the outcomes during and after tonsillectomy procedure.
Materials and Methods
Protocol Registration
To conduct our systematic review, we followed the guidelines outlined in the Cochrane Handbook [10] and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [11]. Our study protocol was registered with PROSPERO, with the registration number [ID: CRD42022377190]. Since our study was a secondary analysis, we did not require any ethical approvals.
Literature Search Strategy
We conducted a systematic search of digital databases including PubMed, Scopus, Cochrane, and Web of Science. The search was performed from inception until November 2022. Our search strategy involved using the following keywords: (tonsillectom* OR adenotonsillectom* OR “tonsil surgery” OR “tonsil removal” OR “tonsillar surgery” OR “tonsillar removal”) AND (ropivacain* OR “ropivacaine hydrochloride” OR “ropivacaine monohydrochloride” OR naropeine OR naropin OR “LEA 103” OR “LEA-103” OR “AL 381” OR “1 Propyl 2’,6’ pipecoloxylidide” OR “(S)-Ropivacaine” OR “84057-95-4” OR “rocaine” OR “local anaesthesia” OR “local anesthesia” OR “local analgesia” OR “local anesthetic” OR “local anaesthetic”). To expand our search for relevant studies, we also reviewed the references of eligible trials and recent reviews. The process of selecting studies involved eliminating duplicate citations, screening titles and abstracts, and examining the full text of potential resources. Two authors independently performed the search and selection process. If any discrepancies arose, the principal investigator was consulted to make a final decision.
Eligibility Criteria and Study Selection
We included the following criteria in our study: (i) adult patients who underwent tonsillectomy, (ii) the intervention was ropivacaine, (iii) comparison with a placebo solution, (iv) the study must report at least one of our specific endpoints, which were operation time, postoperative pain, and postoperative bleeding, and (v) the study design must be an RCT. We excluded studies that involved (i) procedures other than tonsillectomy, (ii) interventions other than ropivacaine such as lidocaine, bupivacaine, and dexamethasone, and (iii) study designs other than RCTs, such as case reports, observational studies, review articles, and letters.
Quality Assessment of the Included Studies
To evaluate the quality of each RCT and assess the risk of bias in the included trials, two authors utilized the second version of the Cochrane Risk of Bias assessment tool [12]. The tool examined domains such as randomization process, deviation from planned interventions, incomplete outcome data, outcome measurement, and selection of the reported result. The reviewers graded the risk of bias in each category and assessed the overall quality of the studies as “low,“ “some concerns,“ or “high.“ In case of disagreements, the group discussed until they reached a consensus. As per Egger et al. [13], determining publication bias using Egger’s test for funnel plot asymmetry is unreliable when fewer than ten studies are pooled. Therefore, we could not use this test to detect the presence of publication bias in our study.
Data Extraction and Outcomes
The eligible studies were analyzed by extracting data into a pre-designed Excel sheet, which included information on the characteristics of the studies, the risk of bias domains, and the outcomes. The summary sheet included details such as the study ID, country, recruitment duration, total sample size, intervention and control arms, and the method of tonsillectomy. The baseline characteristics of the trials consisted of information on the trial groups, timing of intervention or control administration, number of participants, age, sex, duration of follow-up, and the assessment tool used for pain outcomes. The outcomes sheet included the primary and secondary outcomes, such as postoperative pain in < 24 and > 24 h, operative time, intraoperative blood loss, and postoperative hemorrhage. The data was extracted independently by three investigators, and discrepancies were resolved through a consensus.
Meta-analysis
The Review Manager software 5.4, provided by the Cochrane collaboration, was used to analyze all study endpoints. For continuous variables, the standardized mean difference (SMD) and 95% confidence interval (CI) were reported using inverse variance and random-effect models. For dichotomous data, the risk ratio (RR) and 95% CI were reported using Mantel-Haenszel and random-effect models. Inconsistency among studies was assessed using two main tests: I-square test (I2) and the p-value of the Chi-square test of heterogeneity. A value of I²>50% and p < 0.1 were considered significant indicators of heterogeneity based on the Cochrane Handbook for Systematic Reviews of Interventions. Subgroup analyses were conducted based on the timing of postoperative pain (< 24 h and > 24 h), with further subgroups based on the specific time intervals of 1 h, 2 h, 4 h, 12 h, day 1, day 2, day 3, and day 7. Additionally, subgroup analyses were performed based on the timing of postoperative hemorrhage, either reactionary (< 24 h) or delayed (> 24 h).
Results
Summary of the Search Process and Included Trials
Literature search produced 1,209 citations, of which 403 citations were omitted due to duplication. Of the remaining 806 citations, 785 citations were omitted during title/abstract screening and the reaming 21 citations underwent full-text screening, of which 17 citations were excluded for various reasons. Finally, four RCTs with total of 257 patients were included in this systematic review and meta-analysis [6–9]. Figure 1. summarize the PRISMA flowchart for literature search and study selection. All the included RCTs were used conventional dissection as aa method of tonsillectomy. Two RCTs [6, 7] were executed in Turkey, one RCT [8] were executed in Nepal, and the last RCT [9] were executed in Finland. All the included RCTs were used a 10-point assessment tool wither visual analogue scale (VAS) or numeric rating scale (NRS). Summary of the included studies depicts in Table 1. and baseline characteristics of the included studies depicted in Table 2.
Fig. 1.
PRISMA flow flowchart for the literature search and study selections
Table 1.
Summary of the included trials
| Study ID | Country | Study design | Recruitment duration | Total sample size, n | Trial arms | Tonsillectomy method | |
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| Arikan et al. 2006 | Turkey | RCT | Between January 2003 and September 2004 | n = 20 | Ropivacaine hydrochloride: 5 ml of (2%) with epinephrine (1:200,000) | Placebo: 0.9% normal saline with epinephrine (1:200,000) | Conventional dissection |
| Arikan et al. 2008 [7] | Turkey | RCT | Between January 2003 and December 2005 | n = 38 | Ropivacaine hydrochloride: 10 mL of (10 mg/mL) with epinephrine (1:200 000) | Placebo: 0.9% normal saline with epinephrine (1:200,000) | Conventional dissection |
| Bhandari et al. 2022 [8] | Nepal | RCT | Between June 2018 and May 2019 | n = 80 | Ropivacaine hydrochloride: 0.5% with epinephrine (1:100,000) | Placebo: 0.9% normal saline with epinephrine (1:200,000) | Conventional dissection |
| Tolska et al. 2017 [9] | Finland | RCT | NR | n = 119 | Ropivacaine hydrochloride: 20 mL (10 mg/mL) | Placebo: 0.9% normal saline (20 mL) | Conventional dissection |
Abbreviations: RCT = randomized controlled trial, NR = not reported.
Table 2.
Baseline characteristics of the included patients
| Study ID | Group | Time of administration | Participants | Age (range) |
Sex, n (M/F) |
Follow-up | Pain assessment tool |
|---|---|---|---|---|---|---|---|
| Arikan et al. 2006 | Ropivacaine | Pre-tonsillectomy | n = 20 | (18–37) | (12/8) | 10 days | 10-cm VAS |
| Placebo | Pre-tonsillectomy | n = 20 | (18–37) | (12/8) | |||
| Arikan et al. 2008 [7] | Ropivacaine | Pre-tonsillectomy | n = 19 | (24–42) | (11/8) | 10 days | 10-cm VAS |
| Placebo | Pre-tonsillectomy | n = 19 | (19–38) | (7/12) | |||
| Bhandari et al. 2022 [8] | Ropivacaine | Post-tonsillectomy | n = 40 | (18–50) | (18/22) | 24 h | 10-cm VAS |
| Placebo | Post-tonsillectomy | n = 40 | (18–50) | (21/19) | |||
| Tolska et al. 2017 [9] | Ropivacaine | Post-tonsillectomy | n = 62 | (18–47) | (27/35) | 14 days | 10-cm NRS |
| Placebo | Post-tonsillectomy | n = 57 | (18–59) | (21/36) |
Abbreviations: NR = not reported, VAS = visual analog scale, NRS = numeric rating scale.
Summary of the Quality Assessment of the Included Trials
Overall, two RCTs [6, 7] were evaluated as having “low” risk of bias, one RCT [8] as having “some concerns”, and one RCT [9] as having high risk of bias. Bhandari et al. [8] provide no information about the randomization and allocation concealment process. Also, Tolska et al. [9] were evaluate as having risk of bias in the deviations from intended treatment because there were unbalanced number of participants between the treatment and control groups. Risk of bias graph depicted in Fig. 2.
Fig. 2.
Risk of bias graph and summary
Summary of the Review Endpoints
Postoperative Pain
Regarding postoperative pain within hours, overall; there were a significant difference that favor ropivacaine group over placebo group (n = 4 RCTs, SMD= -0.92, 95% CI [-1.57, -0.26], p = 0.006). On 1-hour subgroup, there were a significant difference that factor ropivacaine group over placebo group (n = 2 RCTs, SMD= -1.26, 95% CI [-2.47, -0.05], p = 0.04). However, on 2-hours, 4-hours, and 12-hours subgroups there were no significant difference between ropivacaine and placebo groups (n = 2 RCTs, SMD= -1.87, 95% CI [-5.54, 1.80], p = 0.32), (n = 3 RCTs, SMD= -0.48, 95% CI [-1.56, 0.61], p = 0.39), and (n = 3 RCTs, SMD=-0.53, 95% CI [-1.51, 0.46], p = 0.30), respectively. The pooled analyses were heterogeneous (chi-square p < 0.1, I-square > 50%). (Fig. 3.)
Fig. 3.
Meta-analysis of the mean postoperative pain within hours
Regarding postoperative pain within days, overall; there were a significant difference that favor ropivacaine group over placebo group (n = 4 RCTs, SMD=-050, 95% CI [-0.82, -0.18], p = 0.002). On subgroup analyses day-1, day-2, day-3, and day-7 there were no significant difference between ropivacaine and placebo groups (n = 4 RCTs, SMD=-0.32, 95% CI [-0.95, 0.26], p = 0.26), (n = 3 RCTs, SMD= -0.66, 95% CI [-1.64, 0.32], p = 0.19), (n = 3 RCTs, SMD= -0.58, 95% CI [-1.45, 0.28], p = 0.19), and (n = 3 RCTs, SMD= -0.56, 95% CI [-1.28, 0.17], p = 0.13), respectively. The pooled analyses were heterogeneous (chi-square p < 0.1, I-square > 50%). (Fig. 4.)
Fig. 4.
Meta-analysis of the mean postoperative pain within days
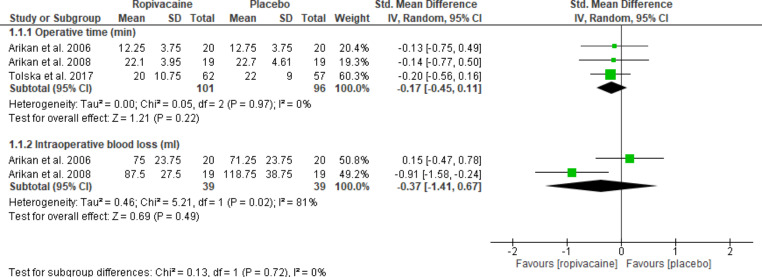
Operative Time and Intraoperative Blood Loss
Regarding operative time, there were no significant difference between ropivacaine and placebo groups (n = 3 RCTs, SMD= -0.17, 95% CI [-0.45, 0.11], p = 0.22). Similarly, regarding intraoperative blood loss, there were no significant difference between ropivacaine and placebo groups (n = 2 RCTs, SMD= -0.37, 95% CI [-1.41, 0.67], p = 0.49). The pooled analyses were heterogeneous (chi-square p < 0.1, I-square > 50%). (Fig. 5.)
Fig. 5.
Meta-analysis of the mean operative time (min) and intraoperative blood loss (ml)
Postoperative Hemorrhage
Overall, there were no significant difference between both ropivacaine and placebo groups (n = 4 RCTs, RR = 2.27, 95% CI [0.90, 5.73], p = 0.08). On subgroup analyses, there were no significant difference between both ropivacaine and placebo groups in reactionary and delayed hemorrhage subgroups (n = 4 RCTs, RR = 0.97, 95% CI [0.06, 15.30], p = 0.99) and (n = 3 RCTs, RR = 2.53, 95% CI [0.95, 6.76], p = 0.06). The pooled analyses were homogeneous (chi-square p > 0.1, I-square < 50%). (Fig. 6.)
Fig. 6.
Meta-analysis of the rate of postoperative hemorrhage (%)
Discussion
This study has employed a meta-analysis approach to compare the effectiveness of ropivacaine and placebo group, using normal saline, in reducing postoperative pain, intraoperative blood loss, operation time, and postoperative bleeding in tonsillectomy procedures in adults. The findings revealed a significant difference between the ropivacaine group and control group in reducing overall postoperative pain within hours and days following the surgery. However, subgroup analysis based on pain reduction within hours and days showed no statistically significant difference between two groups. The study also found no statistically significant difference between the groups regarding the mean operation time, intraoperative and postoperative bleeding.
Ropivacaine is a local anesthetic agent that act by blocking the initial painful stimuli and therefore limit signal transmission from the surgical field limiting pain sensation that is explaining its overall efficacy in reducing postoperative pain [5]. Statistically non-significant results of the subgroups analysis between the ropivacaine and control group regarding pain reduction within hours and days could be explained by small sample size variation of each subgroup which was not able to explore this relationship effectively but by summation of all this results by the meta-analysis showed the significance of ropivacaine in reducing overall postoperative pain within hours and days. This should emphasize the significance of the overall all effect size beside the subgroup analysis interpretation of meta-analysis results. This variation between the total effect size and subgroup effect size could be explained by differences in patient characteristics, administration time and the fact that statistically non-significance truly does not mean clinical non-significance therefore clinical relevance of the effect size is more important than statistical significance.
The study showed that there is no difference between the ropivacaine group and control group in reducing the mean operation time, intraoperative and postoperative blood loss. This could be explained by the mechanism of action of ropivacaine which may not directly related to the duration of surgical process and controlling blood loss despite of its effect on pain reduction. Also, the statistical power of the included studies could explain this non-significance as the power of included studies may be low too be able to detect the significance of the ropivacaine in reduction of operation time and blood loss.
There have been various studies conducted to determine the effectiveness of using peritonsillar infiltration of ropivacaine prior to tonsillectomy for pain control. However, these studies have produced varying results. For instance, a study conducted by Apostolopoulos et al. [14], discovered that pre-incisional infiltration of ropivacaine was more effective than lidocaine for providing postoperative pain relief in adults undergoing local tonsillectomy. However, the study did not investigate long-lasting pain. On the other hand, Giannoni et al. [15], found that pre-incisional injection of ropivacaine reduced postoperative pain and opioid use in children. Furthermore, the addition of clonidine further improved pain and recovery. This finding is consistent with our own results.
However, some studies have found no advantage of ropivacaine infiltration over saline beyond the postoperative period [15–17]. In Unal et al. [16] and Akoglu et al. [17] found that pre-incisional infiltration of ropivacaine did not significantly reduce postoperative pain compared with isotonic saline. We believe this inadequacy was due to the low concentration of ropivacaine solution used in these studies compared to other studies as [7]. The duration of postoperative pain assessment in some studies may also be a limitation, and it is possible that these results were influenced by altered pain perception or expression in children.
Future researches should investigate other factors that could control postoperative pain such as technique of surgery and its effect on pain. A recently published meta-analysis comparing Coblation and Laser tonsillectomy investigating the effect of different tonsillectomy maneuver on surgical outcomes revealed that the main focus of the surgery was on reducing intraoperative blood loss and operation times rather than reducing postoperative pain and hemorrhage [18]. Therefore, targeting a new approach such as Platelet-rich plasma (PRP) has been recommended for reducing postoperative pain. A recently published meta-analysis showed that the use of PRP during the surgery significantly reduces postoperative pain and hemorrhage. However, these findings should be interpreted with caution due to potential limitations. Therefore, investigating these new approach is highly recommended to optimize the efficacy of tonsillectomy outcome by reducing post-operative pain and hemorrhage [19].
As far as we know, this is the first met-analysis comparing the effects of ropivacaine with placebo, normal saline, regarding its effect on postoperative pain. However, our study has certain drawbacks, such as a smaller sample size and limited power, so we advise researchers to carry out more trials to comprehensively evaluate the evidence and this relationship effectively.
Conclusion
This systematic review and meta-analysis compared the clinical outcomes of ropivacaine administration in adult’s patients undergoing tonsillectomy. Generally, ropivacaine was associated with reduction in postoperative pain compared with control group. However, there was no statistical or clinical benefit regarding administration of ropivacaine in term of reduction in operative time, intraoperative blood loss, and postoperative hemorrhage. Additional RCTs are needed to consolidate the power and quality of the presented evidence.
Acknowledgements
None.
Author Contributions
Ebraheem Albazee contributed to study conception, study design, data collection, data analysis, write up of original draft of manuscript, and review of manuscript for editorial and intellectual contents. Rehab Adel Diab, Mostafa A Soliman, Ahmed Abdelaziz, Adel Mouffokes, Sara Desouki, Rahma Ibrahim contributed to literature review, data collection, and review of manuscript for editorial and intellectual contents.
Funding
None.
Data Availability
All data are available within the manuscript and can be obtained from the corresponding author upon a reasonable request.
Declarations
Conflict of Interest
None.
Ethical Approval
Not applicable.
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Windfuhr JP, Toepfner N, Steffen G, et al. Clinical practice guideline: tonsillitis II. Surgical management. Eur Arch Oto-Rhino-Laryngology. 2016;273:989–1009. doi: 10.1007/s00405-016-3904-x. [DOI] [PubMed] [Google Scholar]
- 2.Naja MZ, El-Rajab M, Sidani H, et al. Modified infiltration technique in tonsillectomy: expanded case report of 25 children. Int J Pediatr Otorhinolaryngol. 2005;69:35–41. doi: 10.1016/j.ijporl.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Aldamluji N, Burgess A, Pogatzki-Zahn E, et al. PROSPECT guideline for tonsillectomy: systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2021;76:947–961. doi: 10.1111/anae.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Özkiriş M, Kapusuz Z, Saydam L. Comparison of ropivacaine, bupivacaine and lidocaine in the management of post-tonsillectomy pain. Int J Pediatr Otorhinolaryngol. 2012;76:1831–1834. doi: 10.1016/j.ijporl.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Patel N. Ropivacaine. Essence Analg Analg. 2010;65:276–279. doi: 10.1017/CBO9780511841378.066. [DOI] [Google Scholar]
- 6.Relief PP (2006) Preincisional Infiltration of Tonsils with. 35:167–173 [PubMed]
- 7.Arikan OK, Sahin S, Kazkayasi M, et al. High-dose ropivacaine versus bupivacaine for posttonsillectomy pain relief in adults. J Otolaryngol - Head Neck Surg. 2008;37:836–843. doi: 10.2310/7070.2008.OA0189. [DOI] [PubMed] [Google Scholar]
- 8.Bhandari C, Pokharel A, Mayya NJ, Thapa AS. Peritonsillar Infiltration of Ropivacaine and Pain Control in Immediate Postoperative Period following Tonsillectomy surgery in adult patients. J Coll Med Sci. 2022;18:215–220. doi: 10.3126/jcmsn.v18i3.47527. [DOI] [Google Scholar]
- 9.Tolska HK, Takala A, Blomgren K, et al. Topical ropivacaine in prevention of post-tonsillectomy pain in adults. Anesth Analg. 2017;124:1459–1466. doi: 10.1213/ANE.0000000000002015. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thomas J, Chandler J et al (2019) Cochrane handbook for systematic reviews of interventions [DOI] [PMC free article] [PubMed]
- 11.Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88. 10.1016/j.ijsu.2021.105906 [DOI] [PubMed]
- 12.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apostolopoulos K, Labropoulou E, Samaan R, Bogris K. Ropivacaine compared to lidocaine for tonsillectomy under local anaesthesia. Eur Arch Oto-Rhino-Laryngology. 2003;260:355–357. doi: 10.1007/s00405-002-0567-6. [DOI] [PubMed] [Google Scholar]
- 15.Giannoni C, White S, Enneking FK, Morey T. Ropivacaine with or without clonidine improves pediatric tonsillectomy pain. Arch Otolaryngol - Head Neck Surg. 2001;127:1265–1270. doi: 10.1001/archotol.127.10.1265. [DOI] [PubMed] [Google Scholar]
- 16.Unal Y, Pampal K, Korkmaz S, et al. Comparison of bupivacaine and ropivacaine on postoperative pain after tonsillectomy in paediatric patients. Int J Pediatr Otorhinolaryngol. 2007;71:83–87. doi: 10.1016/j.ijporl.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Akoglu E, Akkurt BCO, Inanoglu K, et al. Ropivacaine compared to bupivacaine for post-tonsillectomy pain relief in children: a randomized controlled study. Int J Pediatr Otorhinolaryngol. 2006;70:1169–1173. doi: 10.1016/j.ijporl.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Albazee E, Al-Sebeih KH, Alkhaldi F, et al. Coblation tonsillectomy versus laser tonsillectomy: a systematic review and meta-analysis of randomized controlled trials. Eur Arch Oto-Rhino-Laryngology. 2022;279:5511–5520. doi: 10.1007/s00405-022-07534-0. [DOI] [PubMed] [Google Scholar]
- 19.Albazee E, Diab S, Awad AK, et al. The analgesic and anti-haemorrhagic efficacy of platelet-rich plasma in tonsillectomy: a systematic review and meta-analysis of randomised controlled trials. Clin Otolaryngol. 2023;48:1–9. doi: 10.1111/coa.13977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available within the manuscript and can be obtained from the corresponding author upon a reasonable request.