Abstract
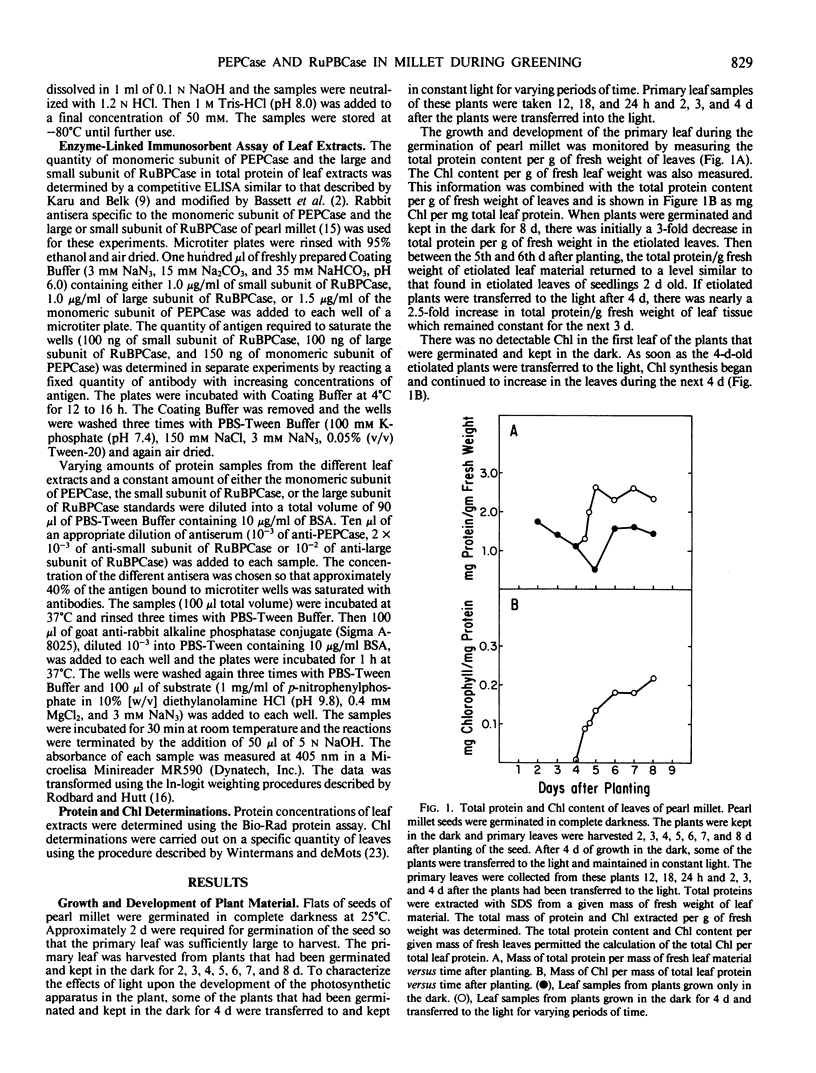
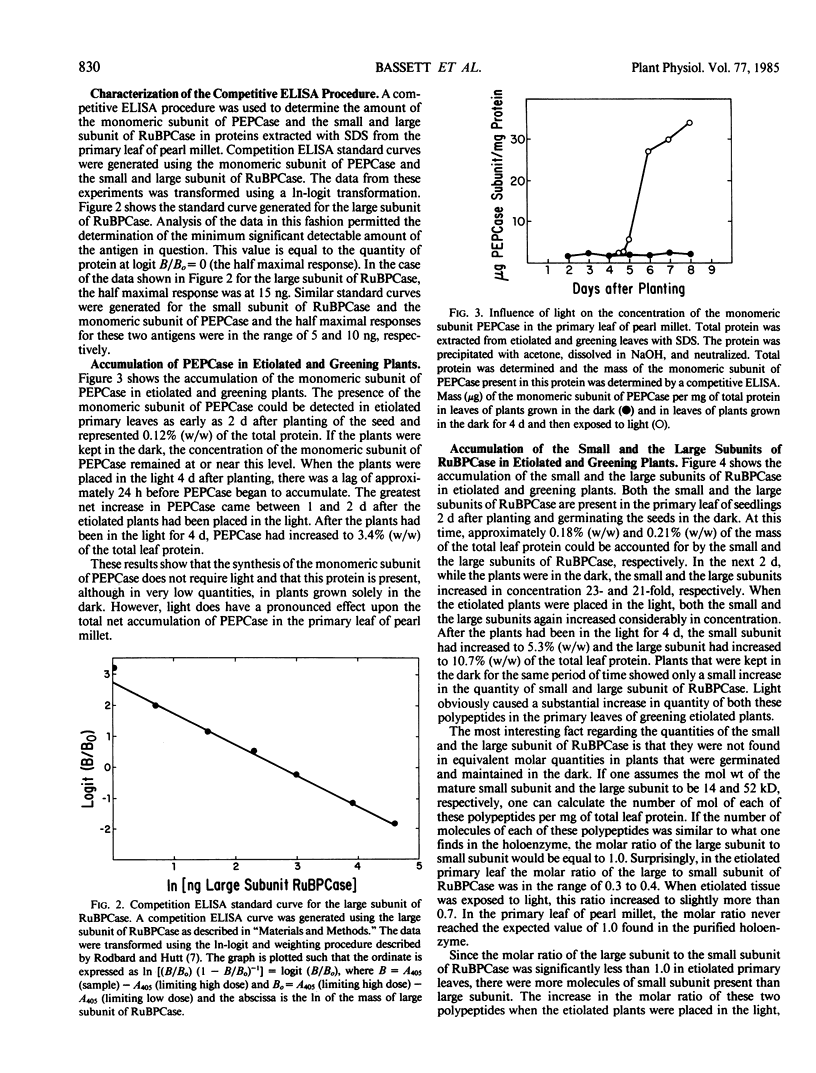
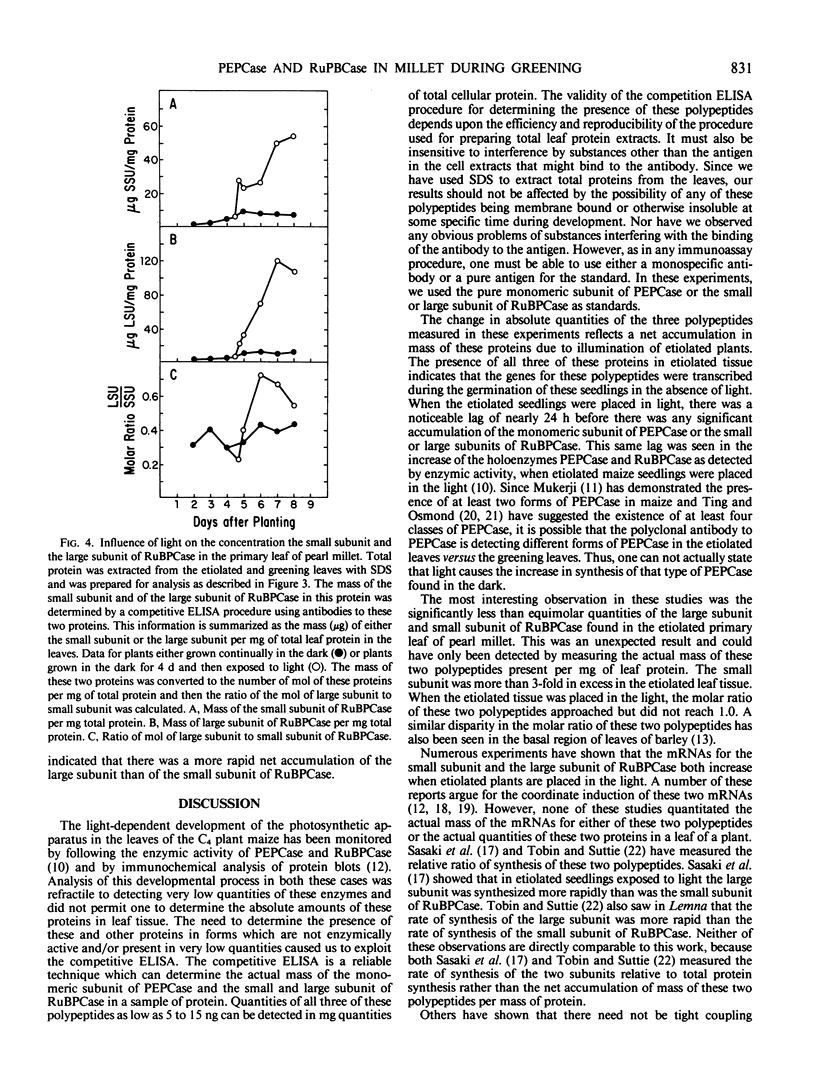
The light-dependent development of the photosynthetic apparatus in the first leaf of the C4 plant pearl millet (Pennisetum americanum) was monitored by immunologically determining the concentration of phospho-enolpyruvate carboxylase and ribulose 1,5-bisphosphate carboxylase. A competitive enzyme-linked immunosorbent assay procedure using antibodies to the monomeric subunit of phosphoenolpyruvate carboxylase and the large and small subunit of ribulose 1,5-bisphosphate carboxylase was used to quantitate the amounts of these polypeptides in the first leaf of etiolated seedlings and etiolated seedlings exposed to light for varying periods of time. Phosphoenolpyruvate carboxylase was present in etiolated tissue; however, light stimulated its synthesis nearly 23-fold. Maximum accumulation of phosphoenolpyruvate carboxylase occurred approximately 4 days after etiolated plants were placed in the light. Both the large subunit and the small subunit of ribulose 1,5-bisphosphate carboxylase were present in leaves of etiolated seedlings. Light also stimulated the synthesis of both of these polypeptides, but at different rates. In etiolated leaves there was approximately a 3-fold molar excess of the small subunit to large subunit. Exposure of the etiolated leaves to light resulted in the molar ratio of the large subunit to the small subunit increasing to approximately 0.72. These data indicate that the net synthesis of these two polypeptides is not coordinately regulated at all times.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barraclough R., Ellis R. J. The biosynthesis of ribulose bisphosphate carboxylase. Uncoupling of the synthesis of the large and small subunits in isolated soybean leaf cells. Eur J Biochem. 1979 Feb 15;94(1):165–177. doi: 10.1111/j.1432-1033.1979.tb12883.x. [DOI] [PubMed] [Google Scholar]
- Boffey S. A., Leech R. M. Chloroplast DNA levels and the control of chloroplast division in light-grown wheat leaves. Plant Physiol. 1982 Jun;69(6):1387–1391. doi: 10.1104/pp.69.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu A. E., Belk E. D. Induction of E. coli recA protein via recBC and alternate pathways: quantitation by enzyme-linked immunosorbent assay (ELISA). Mol Gen Genet. 1982;185(2):275–282. doi: 10.1007/BF00330798. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Asami S., Akazawa T. Development of Enzymes Involved in Photosynthetic Carbon Assimilation in Greening Seedlings of Maize (Zea mays). Plant Physiol. 1980 Feb;65(2):198–203. doi: 10.1104/pp.65.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S. K. Corn leaf phosphoenolpyruvate carboxylases. Purification and properties of two isoenzymes. Arch Biochem Biophys. 1977 Jul;182(1):343–351. doi: 10.1016/0003-9861(77)90315-0. [DOI] [PubMed] [Google Scholar]
- Nelson T., Harpster M. H., Mayfield S. P., Taylor W. C. Light-regulated gene expression during maize leaf development. J Cell Biol. 1984 Feb;98(2):558–564. doi: 10.1083/jcb.98.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivison H. T., Stocking C. R. Ribulose bisphosphate carboxylase synthesis in barley leaves: a developmental approach to the question of coordinated subunit synthesis. Plant Physiol. 1983 Dec;73(4):906–911. doi: 10.1104/pp.73.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Sakihama T., Kamikubo T., Shinozaki K. Phytochrome-mediated regulation of two mRNAs, encoded by nuclei and chloroplasts of ribulose-1,5-bisphosphate carboxylase/oxygenase. Eur J Biochem. 1983 Jul 1;133(3):617–620. doi: 10.1111/j.1432-1033.1983.tb07507.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Sasaki Y., Sakihama T., Kamikubo T. Coordinate light-induction of two mRNAs, encoded in nuclei and chloroplasts, of ribulose 1,5-bisphosphate carboxylase/oxygenase. FEBS Lett. 1982 Jul 19;144(1):73–76. doi: 10.1016/0014-5793(82)80571-1. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Ellis R. J. Light-stimulated accumulation of transcripts of nuclear and chloroplast genes for ribulosebisphosphate carboxylase. J Mol Appl Genet. 1981;1(2):127–137. [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Multiple forms of plant phosphoenolpyruvate carboxylase associated with different metabolic pathways. Plant Physiol. 1973 Mar;51(3):448–453. doi: 10.1104/pp.51.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M., Suttie J. L. Light Effects on the Synthesis of Ribulose-1,5-Bisphosphate Carboxylase in Lemna gibba L. G-3. Plant Physiol. 1980 Apr;65(4):641–647. doi: 10.1104/pp.65.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


