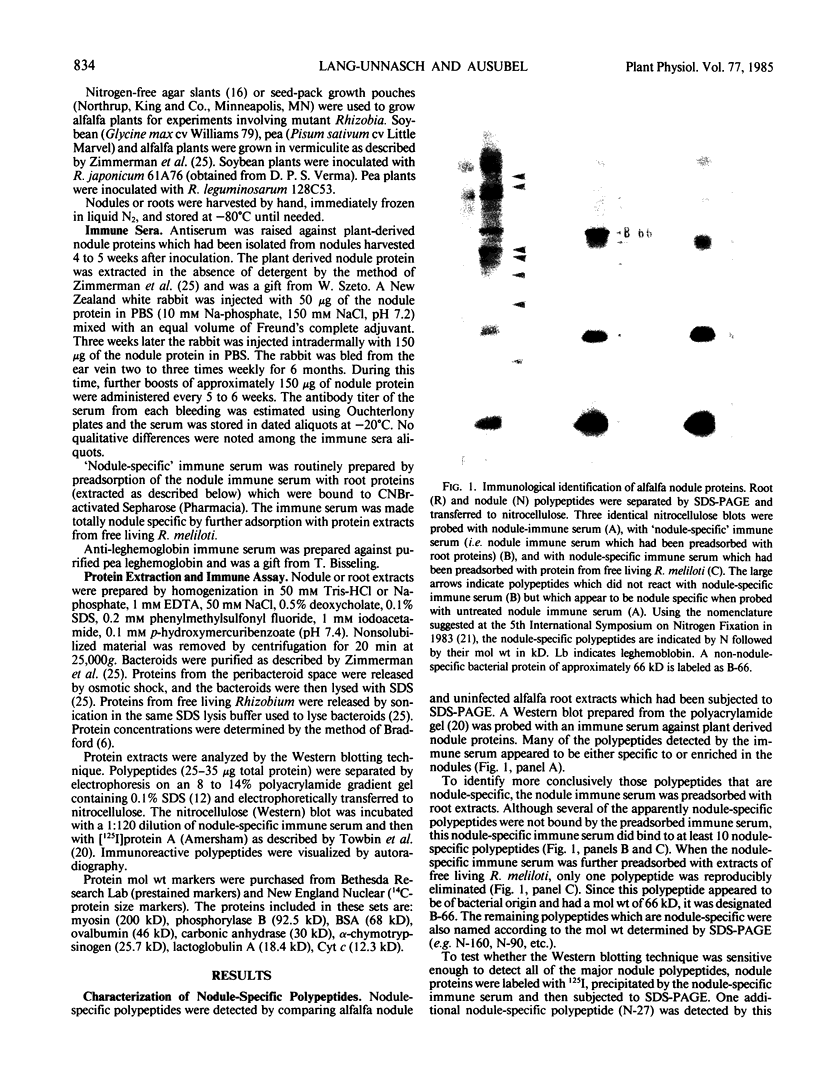
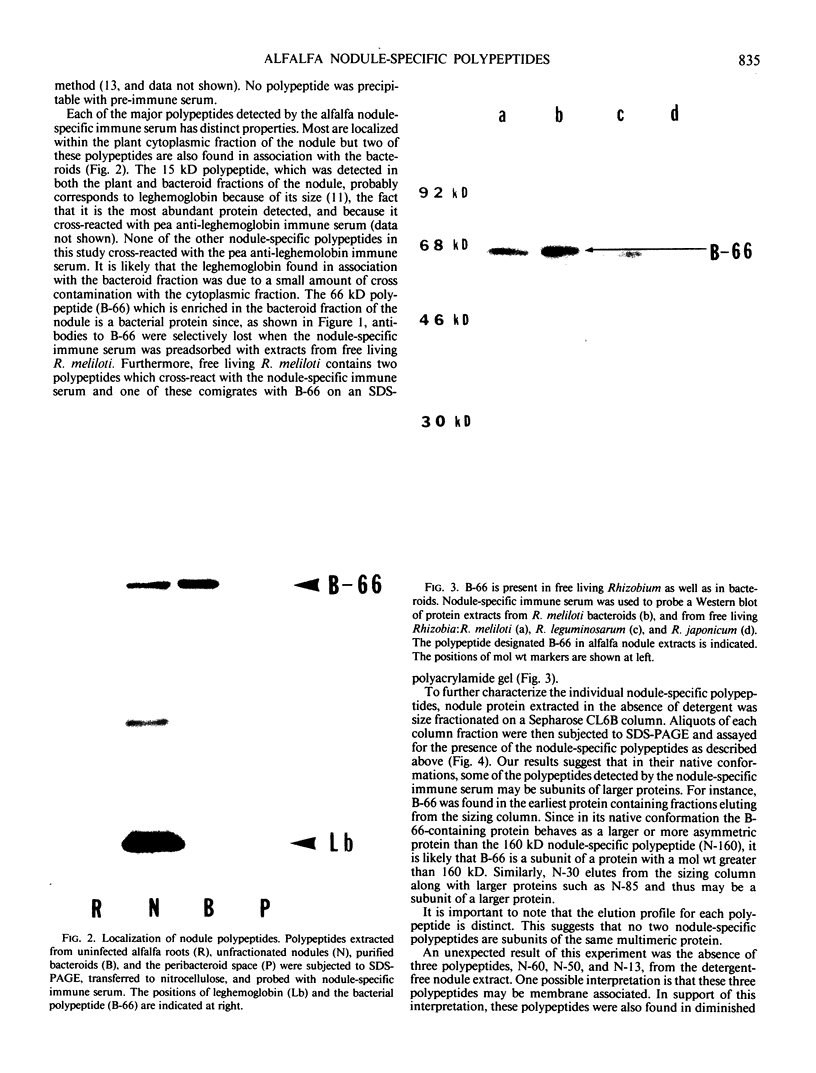
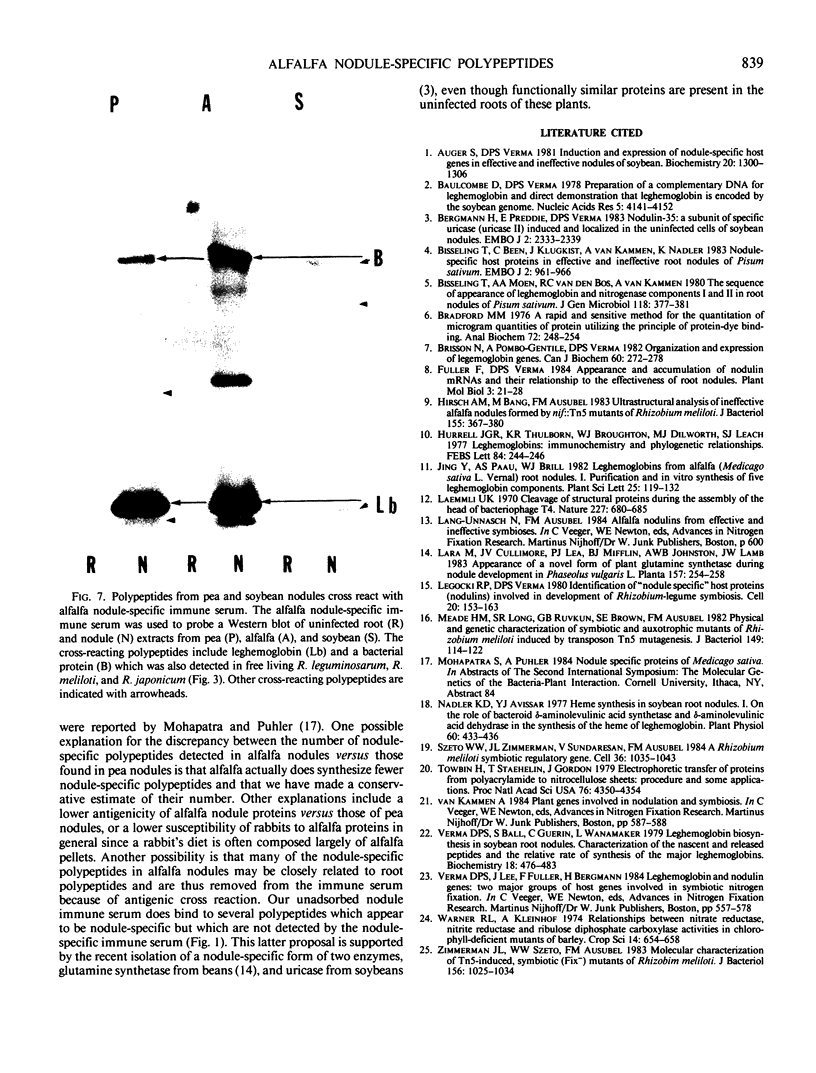
Abstract
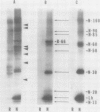
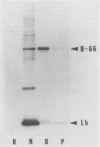
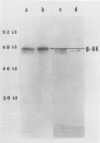
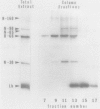
In addition to leghemoglobin, at least nine nodule-specific polypeptides from the alfalfa (Medicago sativa L.)-Rhizobium meliloti symbiosis were identified by immune assay. Some of these polypeptides may be subunits of larger proteins but none appeared to be subunits of the same multimeric protein. All nine of the nodule-specific polypeptides were localized to within the plant cytosol; they were not found in extracts of bacteroids or in the peribacteroid space. At least one of these nodule-specific polypeptides was found to be antigenically related to nodule-specific polypeptides in pea and/or soybean. Ineffective nodules elicited by R. meliloti strains containing mutations in four different genes required for nitrogenase synthesis contained reduced concentrations of leghemoglobin and of several of the nodule-specific polypeptides. Other nodule-specific polypeptides were unaltered or actually enriched in the ineffective nodules. Many of the differences between the ineffective and effective nodules were apparent in nodules harvested shortly after the nodules became visible. These differences were greatly amplified in older nodules. When the four ineffective nodule types were compared to one another, there were clear quantitative differences in the concentrations of several of the nodule-specific polypeptides. These differences suggest that lack of a functional nitrogenase does not have a single direct effect on nodule development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger S., Verma D. P. Induction and expression of nodule-specific host genes in effective and ineffective root nodules of soybean. Biochemistry. 1981 Mar 3;20(5):1300–1306. doi: 10.1021/bi00508a040. [DOI] [PubMed] [Google Scholar]
- Baulcombe D., Verma D. P. Preparation of a complementary DNA for leghaemoglobin and direct demonstration that leghaemoglobin is encoded by the soybean genome. Nucleic Acids Res. 1978 Nov;5(11):4141–4155. doi: 10.1093/nar/5.11.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseling T., Been C., Klugkist J., Kammen A., Nadler K. Nodule-specific host proteins in effective and ineffective root nodules of Pisum sativum. EMBO J. 1983;2(6):961–966. doi: 10.1002/j.1460-2075.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brisson N., Pombo-Gentile A., Verma D. P. Organization and expression of leghaemoglobin genes. Can J Biochem. 1982 Mar;60(3):272–278. doi: 10.1139/o82-032. [DOI] [PubMed] [Google Scholar]
- Hirsch A. M., Bang M., Ausubel F. M. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):367–380. doi: 10.1128/jb.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurrell J. G., Thulborn K. R., Broughton W. J., Dilworth M. J., Leach S. J. Leghemoglobins: immunochemistry and phylogenetic relationships. FEBS Lett. 1977 Dec 15;84(2):244–246. doi: 10.1016/0014-5793(77)80698-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K. D., Avissar Y. J. Heme Synthesis in Soybean Root Nodules: I. On the Role of Bacteroid delta-Aminolevulinic Acid Synthase and delta-Aminolevulinic Acid Dehydrase in the Synthesis of the Heme of Leghemoglobin. Plant Physiol. 1977 Sep;60(3):433–436. doi: 10.1104/pp.60.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Ball S., Guérin C., Wanamaker L. Leghemoglobin biosynthesis in soybean root nodules. Characterization of the nascent and released peptides and the relative rate of synthesis of the major leghemoglobins. Biochemistry. 1979 Feb 6;18(3):476–483. doi: 10.1021/bi00570a016. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Szeto W. W., Ausubel F. M. Molecular characterization of Tn5-induced symbiotic (Fix-) mutants of Rhizobium meliloti. J Bacteriol. 1983 Dec;156(3):1025–1034. doi: 10.1128/jb.156.3.1025-1034.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]