Abstract
BACKGROUND:
Memory impairments have profound implications for social communication and educational outcomes in children with autism spectrum disorder (ASD). However, the precise nature of memory dysfunction in children with ASD and the underlying neural circuit mechanisms remain poorly understood. The default mode network (DMN) is a brain network that is associated with memory and cognitive function, and DMN dysfunction is among the most replicable and robust brain signatures of ASD.
METHODS:
We employed a comprehensive battery of standardized episodic memory assessments and functional circuit analyses in 8–12-year-old 25 children with ASD and 29 matched typically developing controls.
RESULTS:
Memory performance was reduced in children with ASD, compared to controls. General and face memory emerged as distinct dimensions of memory difficulties in ASD. Importantly, findings of diminished episodic memory in children with ASD were replicated in two independent data sets. Analysis of intrinsic functional circuits associated with the DMN revealed that general and face memory deficits were associated with distinct, hyperconnected circuits: aberrant hippocampal connectivity predicted diminished general memory while aberrant posterior cingulate cortex connectivity predicted diminished face memory. Notably, aberrant hippocampal-posterior cingulate cortex circuitry was a common feature of diminished general and face memory in ASD.
CONCLUSIONS:
Results represent a comprehensive appraisal of episodic memory function in children with ASD and identify extensive and replicable patterns of memory reductions in children with ASD that are linked to dysfunction of distinct DMN-related circuits. Findings highlight a role for DMN dysfunction in ASD that extends beyond face memory to general memory function.
Keywords: Episodic memory, Autism, Default mode network, Hippocampus, Posterior cingulate cortex, Recollection and familiarity, Face memory
Introduction
Episodic memory function is critical for a wide range of tasks in children’s lives that extend from navigating their complex social world to classroom performance (1, 2). Aspects of episodic memory are reported to be diminished in children with autism spectrum disorder (ASD) (3), which may contribute to impairments in social function (4) and academic achievement (5). Little is known regarding the specific dimensions of memory function that are impaired in children with ASD and the brain mechanisms underlying these deficits.
Early research on episodic memory in ASD suggested that affected children may have memory deficits restricted to social content, particularly memory for faces, while general memory was largely intact (6, 7). However, subsequent studies have reported broader impairments in memory profiles in ASD beyond face stimuli (1, 8), supporting a general memory deficit model (9) in which face and general memory deficits may represent distinct factors influencing memory performance. Our detailed review of the behavioral literature points to inconsistent findings arising from a lack of comprehensive and standardized assessments of episodic memory, a lack of measures of general cognitive abilities, and a wide age range of study participants (Table S1). Additionally, no prior studies have examined the replicability of findings related to episodic memory dysfunction in ASD. It is unclear whether memory for faces and other stimuli constitute distinct dimensions of memory function in children with ASD, and, crucially, the underlying neural circuit bases of impairments in episodic memory remain poorly understood.
The default mode network (DMN) is a large-scale brain network that has been implicated in a wide range of cognitive deficits in ASD, including the ability to understand other people’s mental states (10–12). The DMN is comprised of distributed and interconnected nodes encompassing the posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), angular gyrus (AG), and hippocampus. Importantly, atypical functional connectivity of DMN is among the most replicable brain signatures of childhood ASD. This is evident not only in task-based functional magnetic brain imaging (fMRI) during theory of mind and mentalizing tasks, but also in studies examining intrinsic functional connectivity (13–15).
Beyond the established role of the DMN in social cognition, nodes of this network, most notably the hippocampus, are important for episodic memory function. An extensive literature has demonstrated a critical role for the hippocampus (16–21) and its neocortical circuits (22, 23) in encoding and recall of episodic memory. For example, previous research in neurotypical children has shown that intrinsic functional connectivity between the hippocampus and lateral prefrontal, temporal, and posterior parietal cortex is correlated with episodic memory performance (24, 25). In addition, the PCC, a hub node of the DMN, has also been implicated in both general (26) and social memory function (27). However, it is not known whether dysfunction of PCC and hippocampal circuits of the DMN are associated with impairments in different aspects of episodic memory, including face and general memory, in children with ASD.
Here we address crucial gaps in our knowledge of episodic memory impairments in children with ASD, and elucidate the underlying brain circuit mechanisms, with a focus on DMN circuits. We first sought to overcome limitations of previous behavioral studies by employing a comprehensive battery of standardized episodic memory assessments (Figure S1) in a well-characterized sample of 8–12-year-old children with ASD with normal intelligence quotient (IQ), and IQ-, age-, gender-matched typically developing (TD) children, and validating the main findings in independent cohorts of participants (Table 1 and Table S1). We used hierarchical clustering analysis to examine whether general and face memory constituted distinct dimensions of memory function in children with ASD. This analysis is critical to adjudicate between different theoretical models, including face-specific and general memory deficit models, associated with reduced memory performance in ASD. We then investigated links between distinct dimensions of memory function and brain connectivity in hippocampal and PCC nodes of the DMN. We hypothesized that children with ASD would show weaker performance in both face and general memory domains compared to their TD peers. Based on the centrality of DMN impairments to cognitive function and clinical symptomology in ASD (13), we hypothesized a primary role for PCC and hippocampal nodes of the DMN in distinct dimensions of memory functions.
Table 1.
Demographic, neuropsychological, and clinical measures
| Measure | ASD (n = 25) | TD (n = 29) | t / χ2 | df | Cohen’s d | P |
|---|---|---|---|---|---|---|
|
| ||||||
| Gender (Male/Female) | 21/4 | 24/5 | 0.01a | 1 | 0.01 | 0.903 |
| Age | 10.44 (1.29) | 10.41 (1.24) | 0.08 | 52 | 0.02 | 0.940 |
| WASI scale | ||||||
| Verbal IQ | 114.96 (16.00) | 120.41 (15.08) | −1.29 | 52 | −0.35 | 0.203 |
| Performance IQ | 113.76 (16.75) | 118.79 (16.56) | −1.11 | 52 | −0.30 | 0.273 |
| Full IQ | 116.00 (16.55) | 122.17 (15.99) | −1.39 | 52 | −0.38 | 0.170 |
| WRAML2 † | ||||||
| Immediate verbal recall | 10.23 (3.12) | 12.56 (2.62) | −2.89 | 49 | −0.81 | 0.006 |
| Immediate visual recall | 8.50 (2.61) | 10.20 (2.95) | −2.17 | 49 | −0.61 | 0.035 |
| Delayed verbal recall | 10.38 (2.84) | 12.39 (2.16) | −2.86 | 49 | −0.80 | 0.006 |
| Delayed verbal recognition | 10.50 (2.70) | 12.04 (1.51) | −2.55 | 49 | −0.71 | 0.014 |
| Delayed visual recognition | 10.48 (2.62) | 10.50 (2.48) | −0.03 | 49 | −0.01 | 0.977 |
| Total general memory | 10.02 (2.38) | 11.54 (1.79) | −2.59 | 49 | −0.73 | 0.012 |
| NEPSY-II †† | ||||||
| Immediate design recognition | 10.30 (3.14) | 12.07 (3.14) | −2.00 | 49 | −0.56 | 0.051 |
| Delayed design recognition | 9.96 (2.90) | 12.18 (3.02) | −2.66 | 49 | −0.75 | 0.010 |
| Total general memory | 10.13 (2.87) | 12.12 (2.89) | −2.46 | 49 | −0.69 | 0.017 |
| Immediate face recognition | 8.43 (2.94) | 11.71 (3.16) | −3.81 | 49 | −1.07 | <0.001 |
| Delayed face recognition | 9.43 (3.53) | 11.68 (2.68) | −2.58 | 49 | −0.73 | 0.013 |
| Total face memory | 8.93 (2.88) | 11.70 (2.52) | −3.65 | 49 | −1.03 | <0.001 |
| ADI-R ††† | ||||||
| Social | 18.83 (6.06) | |||||
| Verbal | 15.83 (4.90) | |||||
| Repetitive behavior | 5.21 (2.54) | |||||
| Development | 3.08 (1.59) | |||||
| Severity scores | 32.67 (8.58) | |||||
| ADOS †††† | ||||||
| Social/Affect | 8.00 (2.65) | |||||
| Restricted and repetitive behavior | 2.61 (1.44) | |||||
| Severity scores | 6.26 (1.79) | |||||
| Total | 10.61 (3.43) | |||||
The mean (standard deviation) of measure, t or χ2, effect size Cohen’s d and p value of two sample t-test/chi-square test are shown here. t statistics are obtained unless otherwise noted. ASD, Children with autism spectrum disorder; TD, typically developing children; IQ, Intelligence quotient; WASI, Wechsler Abbreviated Scale of Intelligence; WRAML2, Wide Range Assessment of Memory and Learning, Second Edition; NEPSY-II, Developmental Neuropsychological Assessment, Second Edition; ADI-R, Autism Diagnostic Interview-Revised (diagnostic scores); ADOS, Autism Diagnostic Observation Schedule-new algorithm.
Chi-squared statistic.
Missing data from 3 participants (ASD: n = 1; TD n = 2).
Missing data from 3 participants (ASD: n = 2; TD n = 1).
Missing data from 1 participant (ASD: n = 1).
Missing data from 2 participants (ASD: n = 2).
Materials and Methods
Participants
All study protocols were approved by the Stanford University Institutional Review Board, and informed written consent was obtained from the legal guardian of each child. Fifty-four children (25 children with ASD and 29 matched TD children) aged 8 to 12 years completed the study (Table 1). The diagnosis of ASD was confirmed by an experienced clinical psychologist using the standard criteria based on Autism Diagnostic Interview-Revised (ADI-R) (29) and/or the Autism Diagnostic Observation Schedule (ADOS) (30). Detail inclusion criteria and demographic information can be found in Supplementary Materials. The number of children included was based on the availability of high-quality data for each analysis (Table S2).
Memory assessments
To characterize children’s episodic memory profile across multiple dimensions – content domain (general/face), retrieval type (recall/recognition), type of material (verbal/visual), and delay interval (short/long) (Figure S1) – subtests of Wide Range Assessment of Memory and Learning, Second Edition (WRAML2) (31) and A Developmental NEuroPSYchological Assessment, Second Edition (NEPSY-II) (32) were administered by trained assessors (Supplementary Methods).
WRAML2.
Ten subtests were administered. We generated five memory subscores based on relevant subtests (Figure S1): i) immediate verbal; ii) delayed verbal recall; iii) immediate visual recall; iv) delayed verbal recognition; v) delayed visual recognition. A composite total memory score of WRAML2 was generated by averaging five memory subscores to represent general memory performance.
NEPSY-II.
Four subtests were administered. Four memory subscores were defined by the scaled scores of these four subtests. A composite general memory score was generated by averaging the scaled scores of two design subtests. A composite face memory score was generated by averaging the scaled scores of two face subtests.
Behavioral analysis
Group differences and interaction between group and memory dimensions in general memory scores (WRAML2).
We used a linear mixed model to examine overall memory function in the ASD group as well as the effects of retrieval type, type of material, delay interval and their interactions with group. Here, we modeled the unbalanced design of five memory subscores in which only verbal recall had both immediate and delayed versions, as follows:
where is the observed memory subscore for subject and is the residual of subject .
Group differences and interaction between group and memory dimensions in general and face memory scores (NEPSY-II).
A 2×2×2 mixed-design ANOVA was performed with group as a between-subject factor and content domain and delay interval as within-subject factors.
Replication analysis with NDA cohort data.
Using an open-source dataset, the National Institute of Mental Health Data Archive (NDA; https://nda.nih.gov/), we identified two cohorts: i) WRAML replication cohort, and ii) NEPSY replication cohort. Details about the procedures used to generate the replication cohorts are shown in Figure S2.
Relation between memory measures.
Pearson’s correlation coefficients were computed for pairs of WRAML2 and NEPSY-II memory subscores in each group.
Hierarchical clustering analysis of memory measures.
Hierarchical clustering analysis with Euclidean distance and complete-linkage criterion (33–35) was used to investigate the relation between memory measures. The optimal number of clusters was determined on the basis of the majority vote of 19 indices of internal validity measures on the number of clusters from one to eight (NbClust 3.0.1 package in R 4.1.0)(36).
General and face memory reduction scores in ASD.
For each child with ASD, two composite memory reduction scores were generated. A general memory reduction score was obtained by averaging the scaled reduction scores of all general memory assessments from WRAML2 and NEPSY-II. A face memory reduction score was obtained by averaging the scaled reduction scores of NEPSY-II for two faces memory assessments. The scaled reduction score was calculated as follows:
Greater negative values indicated greater memory reduction relative to the TD group.
Statistical analysis
Planned two-sample -tests were used to test group differences. Cohen’s or was calculated to estimate effect sizes.
Brain imaging analysis
Functional connectivity analysis.
Details of fMRI data acquisition and preprocessing are described in Supplementary Methods. For each child, voxel-wise whole-brain functional connectivity analysis was performed for using hippocampus and PCC regions of interest (ROIs; Figure S3). The PCC ROI (MNI: 4 −38 32) was defined using coordinates from the largest meta-analysis of fMRI studies on social cognition deficits in ASD, which encompassed 50 studies and included 675 individuals with ASD and 695 TD individuals (Patriquin et al., 2016). Voxels in non-grey matter areas in the PCC ROI were excluded using the Harvard-Oxford atlas as the reference. There is currently no similar meta-analysis of fMRI studies on episodic memory deficits in ASD. To define hippocampal ROIs, we first conducted a meta-analysis using Neurosynth (Yarkoni et al., 2011), with the search term ‘episodic memory’, which identified a large cluster in the medial temporal lobe including the hippocampus. ROIs in the left (MNI: −24 14 −20) and right (MNI: 24 −14 −20) hemisphere were then selected based on overlap of the meta-analysis-derived cluster with hippocampus coordinates from our previous study of medial temporal lobe connectivity patterns along the long axis of the hippocampus (Qin et al., 2016). Please see Supplementary Methods for definition of control ROIs. Two sample t-tests examined group differences in connectivity for each ROI. Results were corrected for multiple comparisons using a height threshold of and family-wise error rate correction at (cluster extent of 128 voxels) based on Monte Carlo simulations using a custom MATLAB script.
Multivariate brain-behavior association analysis.
We employed an epsilon-insensitive support vector regression (SVR) analysis with a non-linear sigmoid kernel (41) to investigate brain-behavior association. This supervised machine-learning approach is widely used in the field and was selected for its robust performance (41–45). First, for each ROI, we identified all target brain regions which showed significant differences in connectivity between the ASD and TD groups. Connectivity values for each ROI served as the feature vector in the SVR analysis. The SVR model was trained using a leave-one-out cross-validation strategy. The predicted score for each child was generated by a model that was trained on data from all other children in the group, with the connectivity features serving as predictors and the observed memory performance serving as the predicted variable. The correlation between predicted and actual scores was then computed. Significance levels were computed using permutation testing. Bonferroni correction was used to correct for multiple comparison ( for two regions of interest and two memory scores in each group). We performed additional control analysis using matrix reasoning score of the WASI to examine the specificity of our findings with respect to memory measures.
Results
General memory reductions in children with ASD examined using WRAMI2
A linear mixed model showed a significant main effect of group . There was no significant main effect of retrieval type, type of material, or delay interval, or their interactions with group (, ; Table S3). A planned two-sample -test on total general memory score from the WRAML.2 revealed that children with ASD had significantly rechuced scores relative to TD children (, , Cohens’ ) (Figure 1A, left). Planned -tests also revealed significantly lower scores in children with ASD compared to TD children on immediate and delayed verbal recall, immediate visual recall, and delayed verbal recognition subscores (, , Cohens’ ; Figure 1A, right and Table 1), but not on delayed visual recognition (, ; Cohen’s . Together, these results provided converging evidence for weak memory function across recognition and recall, verbal and visual materials, and short and long delay intervals in children with ASD compared to TD peers, highlighting general memory reductions in these individuals.
Figure 1. General and face memory deficits in children with autism spectrum disorder (ASD) relative to typically developing (TD) children.
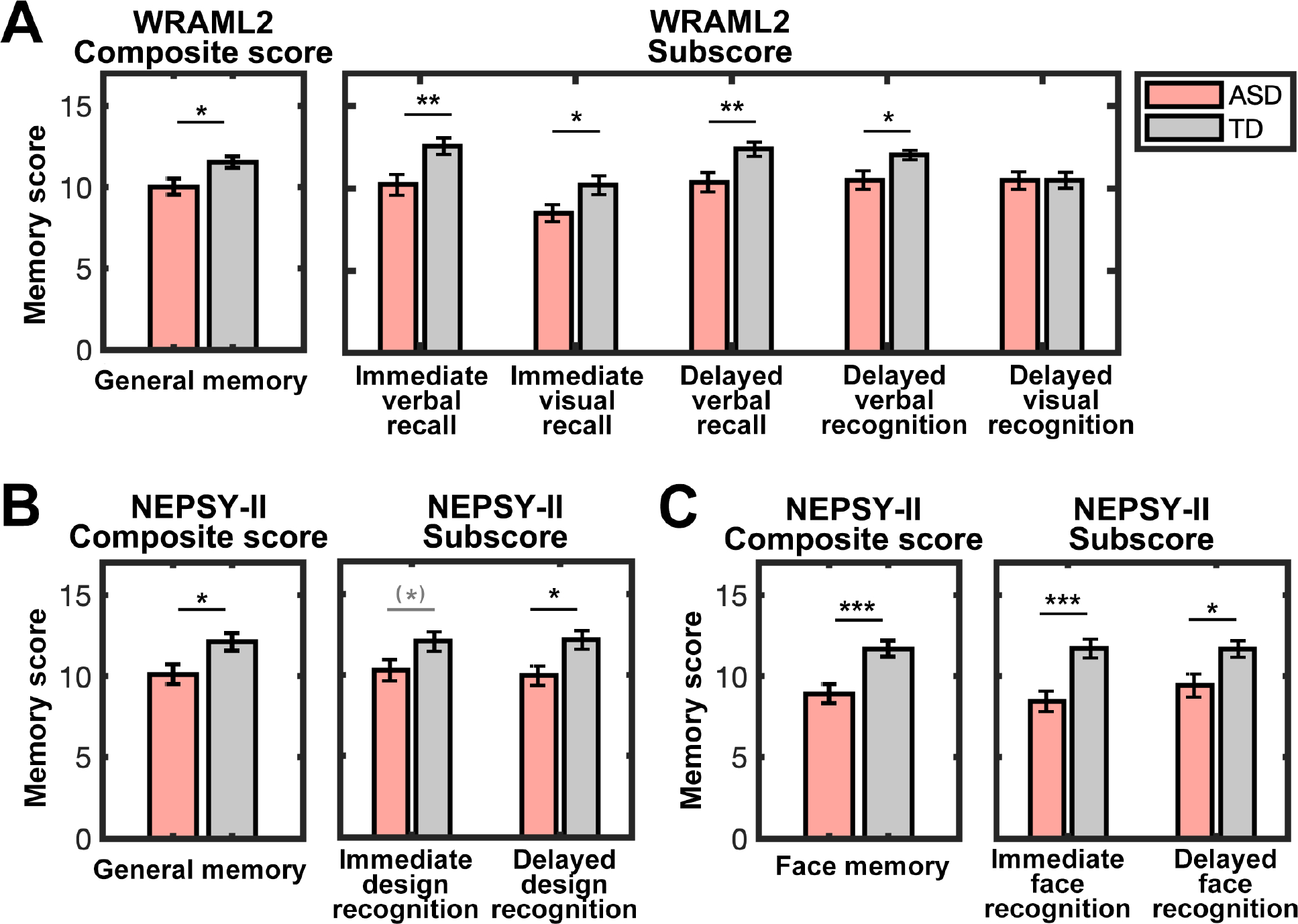
(A) General memory performance across multiple dimensions (see also Figure S1), as measured by WRAML2, was significantly lower in children with ASD compared to well-matched TD children. Immediate verbal and visual recall, delayed verbal recall, and delayed verbal recognition abilities were weaker in children with ASD, relative to TD children. (B) General memory, assessed by NEPSY-II, was also weaker, with significantly lower performance on delayed design recognition, in children with ASD compared to TD children. (C) Face memory performance after both immediate and delayed intervals, measured by NEPSY-II, was significantly lower in children with ASD. Error bars represent the standard error of the mean. WRAML2, Wide Range Assessment of Memory and Learning, Second Edition; NEPSY-II, Developmental Neuropsychological Assessment, Second Edition; *** p < 0.001; ** p < 0.01; * p < 0.05; (*) p < 0.10.
General and face memory reductions in children with ASD examined using NEPSY-II
A mixed-design ANOVA with group, content domain, and delay interval revealed a significant effect of group , with reduced performance in children with ASD compared to TD children. No significant main effect of content domain or delay interval or their interactions with group was observed (; Table S4). In planned two-sample -tests on total general and face memory scores from the NEPSY-II, we observed similar pattens of rechuced performance in the ASD group compared to the TD group ( Cohen’s ; Figure 1B&C, left). Additional analyses confirmed reduced performance across short and long delay interval for general and face memory in the ASD group compared to the TD group. Significantly lower scores were observed in children with ASD for all subscores , except for immediate design recognition (Figure 1B&C, right and Table 1). Results from the NEPSY-I extend the findings from the WRAML 2 and provide additional evidence for both general and face memory reductions in children with ASD.
Replication of general and face memory reductions from independent samples
We queried the NDA dataset for WRAML 2 and NEPSY-I measures, which assess general and face memory, respectively (Figure S2). The WRAMI2 replication sample comprised and participants. We observed significant lower scores in general memory in childten with ASD compared to TD children (, ; Figure 2A). The NEPSY-II replication sample comprised , which were compared to the TD sample from the Stanford cohort. We observed significantly reduced performance in face memory in children with ASD compared to TD children (, ; Figure 2B). These findings demonstrate replicable patterns of reduced scores in general and face memory abilities in children with ASD across independent datasets.
Figure 2. Replication of general and face memory deficits in children with autism spectrum disorder (ASD) from NDA.
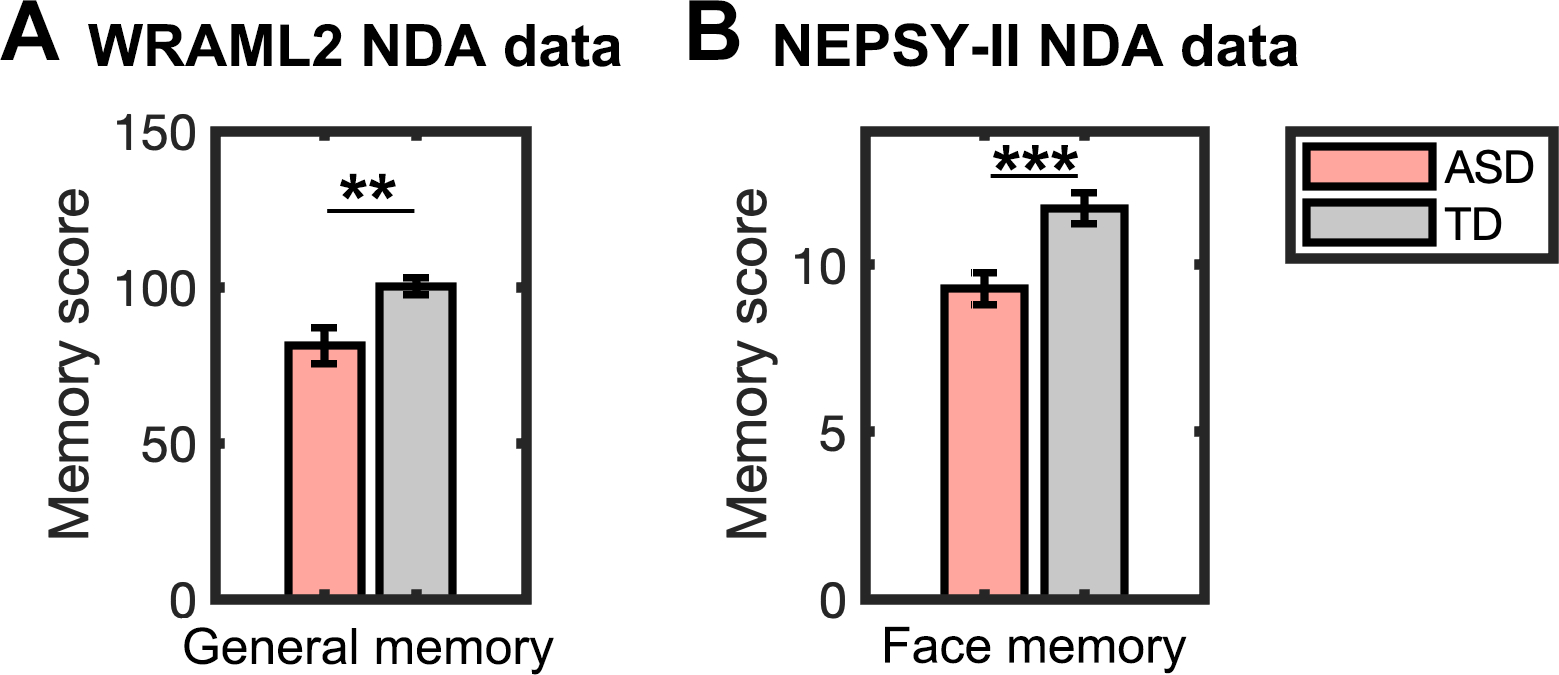
(A) General memory, assessed by WRAML2, was weaker in children with ASD compared to typically developing (TD) children in the WRAML2 NDA replication sample . (B) Face memory, measured by NEPSY-II, was also weaker in children with ASD in the NEPSY-II NDA replication sample () compared to TD sample from the Stanford cohort (no TD sample was available from NDA for NEPSY-II measure). Error bars represent standard error of mean. WRAML2, Wide Range Assessment of Memory and Learning, Second Edition; NEPSY-II, Developmental Neuropsychological Assessment, Second Edition; NDA, National Institute of Mental Health Data Archive (https://nda.nih.gov/); *** p < 0.001; ** p < 0.01.
Hierarchical relations between general and face memory measures in children with ASD
We first examined inter-relations between general and face memory measures in children with ASD by computing a correlation matrix of all memory measures. Two distinct blocks of interrelation between memory measures emerged in the ASD group: all general memory measures were highly correlated with each other (, ; Figure 3A, top; Table S5), and the two face memory measures were highly correlated with each other . More importantly, the correlations between the two domains (i.e., general vs. face) were generally low in this group (, ; the orange frame in Figure 3A, top).
Figure 3. Stronger dissociation between general and face memory in children with autism spectrum disorder (ASD) compared to typically developing (TD) children.
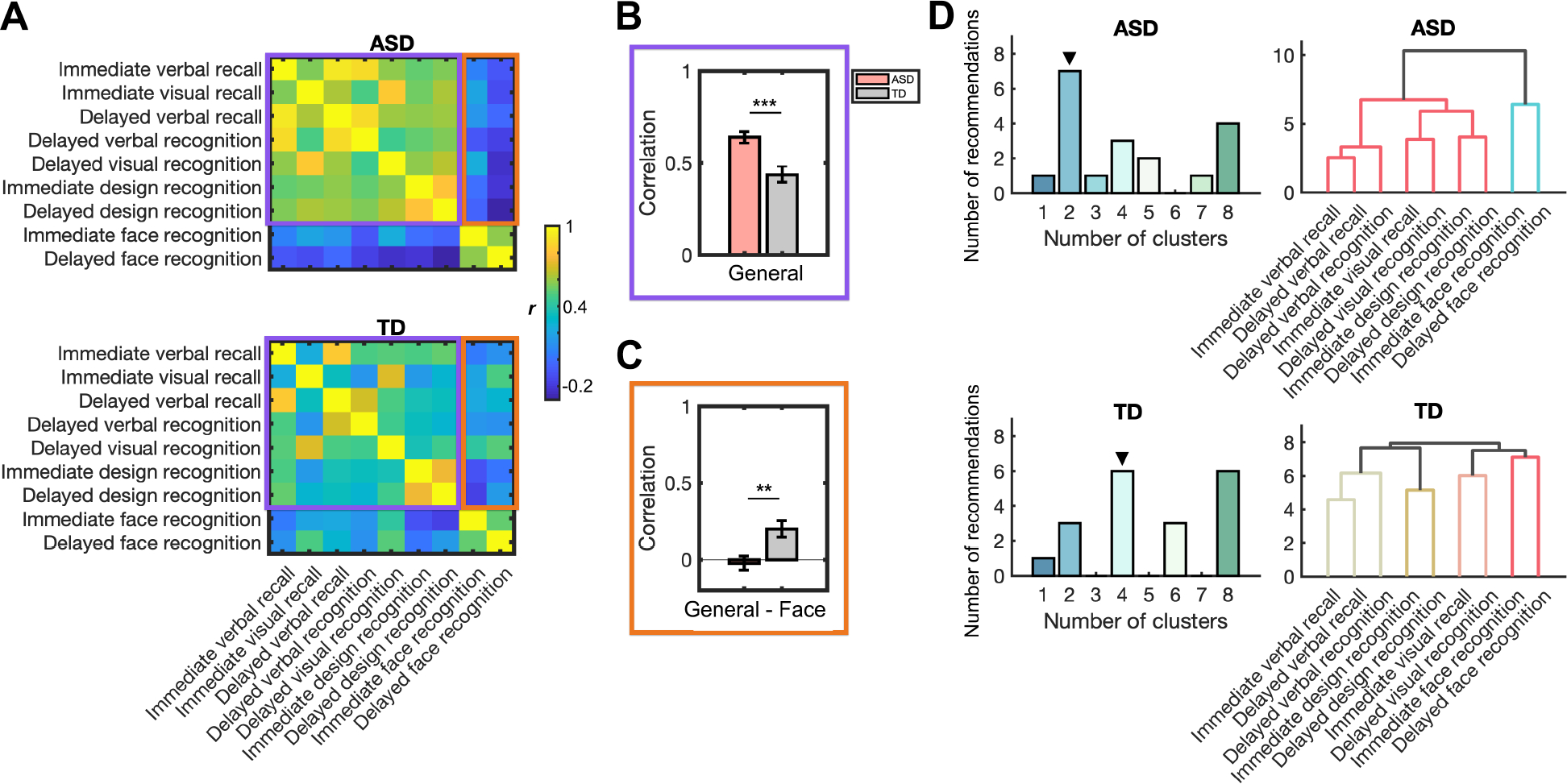
(A) Correlations between memory subscores assessed by WRAML2 and NEPSY-II. Purple frames show correlations between general memory measures. Orange frames show correlations between general and face memory measures. (B) Compared to TD children, children with ASD showed higher correlations between general memory measures (purple frames in Figure 3A). (C) Children with ASD, relative to TD children, showed lower correlations between general and face memory measures (orange frames in Figure 3A). (D) Results of hierarchical clustering analysis of memory subscores measured from WRAML2 and NEPSY-II in each group. ▼ indicates the optimal cluster solution supported by consensus analysis (see details in Methods). Dendrogram shows clear hierarchical clustering into general and face domains in children with ASD. In TD children, no single cluster had all general memory measures (see also Supplementary Results). WRAML2, Wide Range Assessment of Memory and Learning, Second Edition; NEPSY-II, Developmental Neuropsychological Assessment, Second Edition; *** p < 0.001; ** p < 0.01.
The correlation matrix in the TD group showed stronger association between general and face memory measures compared to the ASD group. Specifically, in the TD group, correlations between general memory measures were lower (, ; the purple frame in Figure 3A, bottom; Table S6) while correlations between general and face memory measures were higher (, ; the orange frame in Figure 3A, bottom). Direct comparisons of correlation matrices of ASD and TD groups confirmed that correlations between general memory measures are stronger in the ASD group compared to the TD group (, , Cohens’ ; Figure 3B), but the correlation between general and face memory measures was lower in the ASD group compared to the TD group (, , Cohens’ ; Figure 3C). These findings suggest that distinct structures of general and face memory might underlie broadly diminished memory performance in children with ASD.
Next, we conducted a hierarchical clustering analysis which revealed a two-cluster solution, one for general memory and the other for face memory in children with ASD (Figure 3D, top and Table S7). The dendrogram produced by the hierarchical clustering suggested that one cluster included all measures of general memory while another cluster included face memory measures. In cluster, clustering analysis in TD children revealed different patterns (Figure 3D, bottom, Figure S4 and Table S8), which suggest four- or eight-cluster solutions. Unlike what was observed in the ASD group, no single cluster had all general memory measures in TD children (Supplementary Results).
Together, convergent results suggested general and face memory as two different underlying constructs contributing to broad memory impairments in children with ASD.
Functional connectivity of the hippocampus predicts general memory in children with ASD
Compared to TD children, children with ASD showed greater functional connectivity between the left hippocampus and posterior fusiform gyrus, thalamus and cerebellum. Children with ASD also showed greater connectivity between the right hippocampus and fusiform gyrus, anterior cingulate cortex, medial prefrontal cortex, supramarginal gyrus, cerebellum and PCC (Figure 4A and Table S9). No regions showed decreased connectivity with the bilateral hippocampus in ASD compared to the TD group. Results from SVR showed that functional connectivity between the hippocampus and hyperconnected brain regions predicted general memory performance in children with ASD (correlation between predicted and observed values: , ; Figure 4B). In contrast, these hippocampal connectivity features did not predict general memory performance in TD children .
Figure 4. Aberrant hippocampal connectivity predicts general memory deficits in children with autism spectrum disorder (ASD).
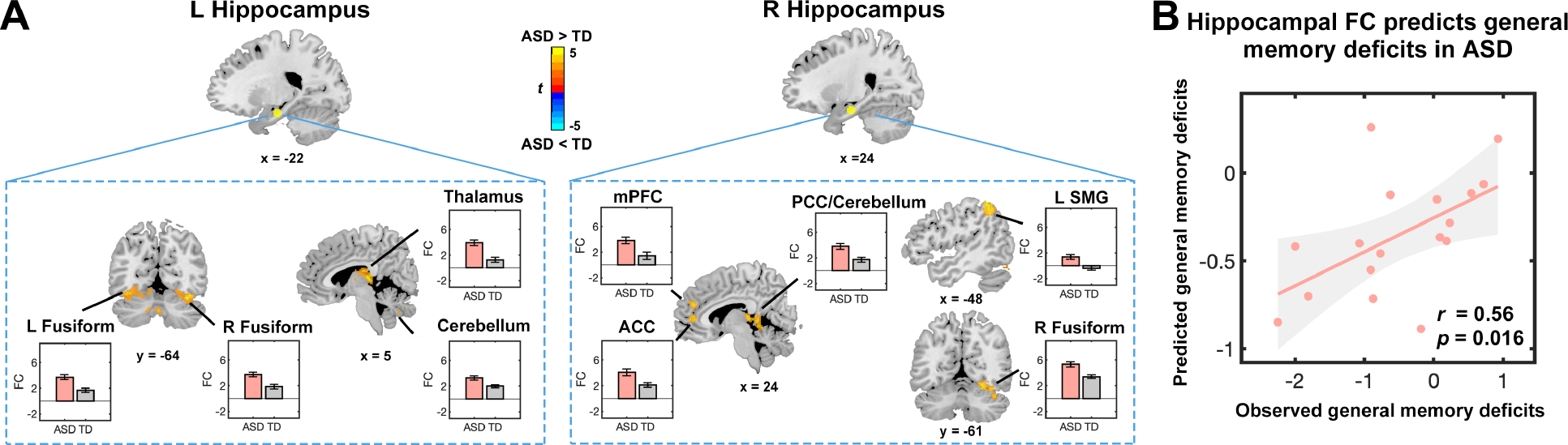
(A) Brain areas showing aberrant connectivity of the hippocampus in children with ASD (height threshold at , with family-wise error rate correction at for cluster extent). Significant hyperconnectivity in children with ASD compared to typically developing (TD) children was observed between the hippocampus and anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), supramarginal gyrus (SMG), medial prefrontal cortex (mPFC), fusiform gyrus, thalamus, and cerebellum. No brain areas showed reduced connectivity with the hippocampus in children with ASD, compared to TD children. Error bars represent standard error of mean. (B) Support vector regression analysis reveals that aberrant connectivity of hippocampus predicts deficits in general memory in children with ASD. Each dot represents data from one child. r is the correlation between observed and predicted general memory deficits scores. p was obtained using permutation testing, and results were Bonferroni corrected for multiple comparisons across regions of interests and memory measures. FC, functional connectivity.
To examine whether this prediction is specific for general memory, we performed additional analysis which revealed that hippocampal connectivity features did not predict face memory in children with autism or in TD children (; Table S10). To further examine specificity of our findings, we additionally examined children’s performance on matrix reasoning from the Wechsler Abbreviated Scale of Intelligence (WASI), which utilized similar visual stimuli (eg., shapes, designs, etc.) as those used in memory tasks in the current study, but without requirements for memory recall. We found that hippocampal connectivity features did not predict variance in matrix reasoning performance in children with ASD .
Finally, we examined functional connectivity of other brain regions implicated in episodic memory, including the prefrontal cortex and posterior parietal cortex (Table S11). No significant associations between connectivity features and general memory scores were observed for these brain regions in children with ASD (; Supplementary Results). Together, these findings identify a specific link between hyperconnected hippocampal circuits and general memory dysfunction in children with ASD.
Functional connectivity of the PCC predicts face memory in children with ASD
Compared to TD children, children with ASD showed greater functional connectivity between the PCC and the amygdala, hippocampus, caudate, thalamus, fusiform, and middle occipital gyrus (Figure 5A and Table S12). Results from SVR analysis showed that these hyperconnected links predicted face memory performance in children with ASD (; Figure 5B). These PCC connectivity features were not predictive of general memory performance in children with ASD ( ) nor were they predictive of general or face memory in TD children (; Table S10). We additionally examined WASI matrix reasoning task performance and did not find significant relation with PCC connectivity features in children with ASD (. Functional connectivity of other brain regions inplicated in social cognition, including the fusiform face area, anrygdala, and temporoparietal jumction (Table S11) did not significantly predict face memory performance scores in children with ASD (; Supplementary Results). Together, these findings identify a specific link between hyperconnected PCC circuits and face memory dysfunction in children with ASD.
Figure 5. Aberrant posterior cingulate cortex connectivity predicts face memory deficits in children with autism spectrum disorder (ASD).
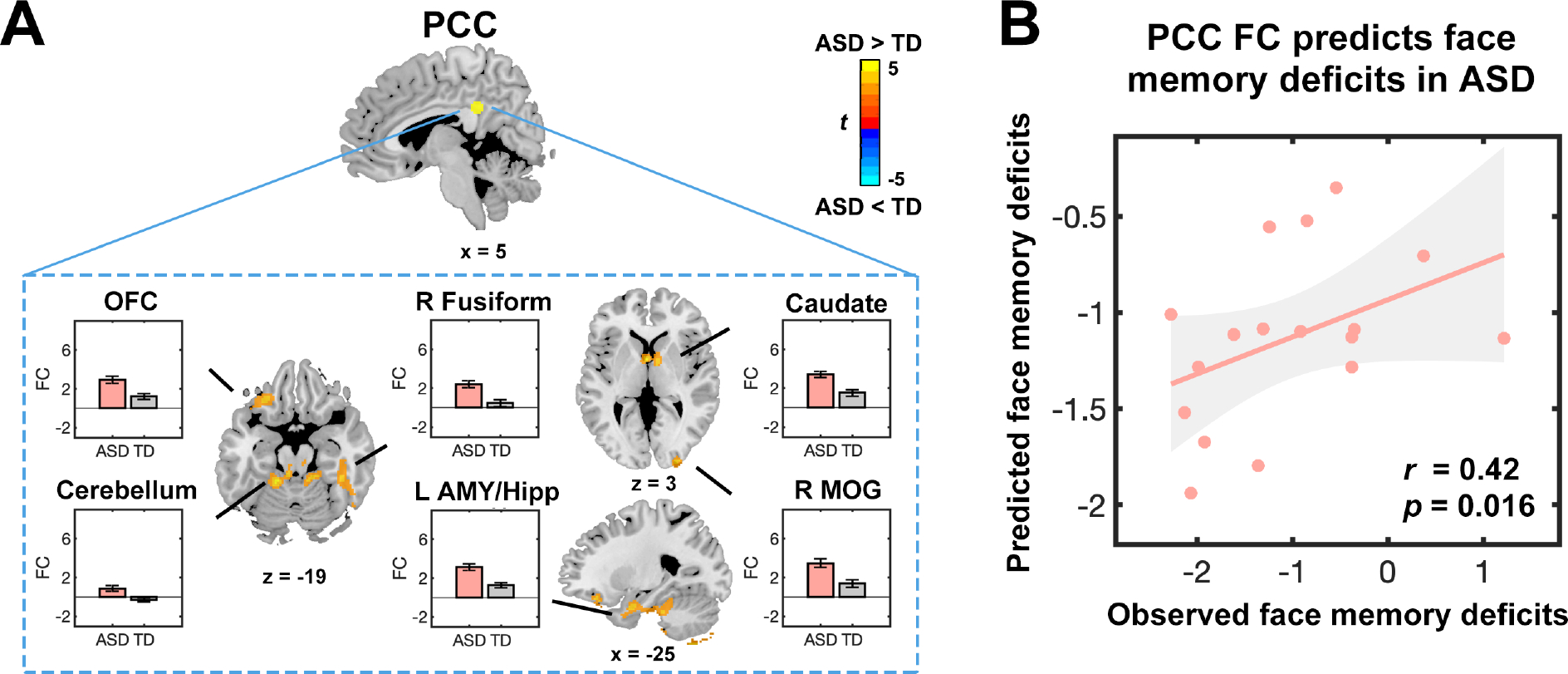
(A) Brain areas showing aberrant connectivity with the posterior cingulate cortex (PCC) in children with ASD (height threshold at , with family-wise error rate correction at for cluster extent). Significant hyperconnectivity in children with ASD compared to typically developing (TD) children was observed between the PCC and orbitofrontal cortex (OFC), fusiform gyrus, amygdala (AMY), hippocampus (Hipp), caudate nucleus, and middle occipital gyrus (MOG). No brain areas showed reduced connectivity with the PCC in children with ASD, compared to TD children. Error bars represent standard error of mean. (B) Support vector regression analysis reveals that aberrant PCC connectivity predicts face memory deficits in children with ASD. Each dot presents the data from one child. r represents the correlation between observed and predicted face memory deficits scores. p was obtained using permutation testing and results were Bonferroni corrected for multiple comparisons across regions of interests and memory measures. FC, functional connectivity.
Discussion
Memory abilities and their links to functional brain circuitry are a crucial but understudied area of childhood ASD. Our survey of the extant literature revealed lack of comprehensive assessments of general and face memory in a within-subjects design, inconsistent behavioral findings, limited characterization of the underlying neural circuitry, and lack of replication (Table S1). We used a comprehensive battery of standardized memory assessments and functional circuit analyses in a well-characterized sample of children with ASD and matched TD children. We show that children with ASD have broad, diminished performance in memory function. Critically, general and face memory reductions were identified as distinct dimensions of memory function in children with ASD, but not in TD children. Functional circuit analysis identified the nodes of the DMN associated with different dimensions of memory impairments in ASD: while hippocampal brain circuits predicted general memory performance, PCC circuitry predicted face memory performance. Our study represents the first comprehensive appraisal of both general and face memory function in independent cohorts of children with ASD, and identify replicable patterns of memory reductions in children with ASD that are linked to dysfunction of distinct DMN circuits. Findings highlight a crucial role of the DMN in ASD that extends beyond social cognition.
Memory play a key role in cognitive, social, and academic development (46,47). We examined both general and face memory in children with ASD compared to TD children, and we sought to determine whether they form distinct components of aberrant memory in affected children. A plausible hypothesis is that general and face memory tasks rely on shared cognitive mechanisms, given the similarity of task procedures and requirements. For example, both general and face memory tasks require that participants encode, and then subsequently recall or recognize, specified target stimuli, with the only difference being the type of stimulus encoded. Across two independent cohorts, we found replicable evidence for reduced episodic memory for both faces and general stimuli in children with ASD. Our findings help resolve inconsistent findings on episodic memory in childhood ASD reported in prior literature (Table S1).
Findings converge on prior findings of face memory deficits in adolescents and adults with ASD (7, 48). Research has consistently identified a relationship between face memory performance and autistic symptom severity in adolescents (49, 50). Moreover, face memory deficits have been recognized as a core aspect of symptom profiles based on meta-analysis (51) and a potential endophenotype in ASD based on findings from a recent study in adults (52). Furthermore, our results showed that face memory was independent of IQ in children with ASD (Supplementary Results). Similarly, face memory deficits consistently identified among child and adult studies have been independent from IQ (51). These findings point to a pattern of developmentally stable deficits in episodic memory for faces in ASD, consistent with reports suggesting lack of improvement in face memory over development in ASD (53).
Beyond face memory, children with ASD also showed significant deficits on visual recognition subtests of NEPSY-II, but not in the delayed visual recognition performance on WRAML2. This discrepancy is likely due to the different task designs in the two assessments. Although both use simple geometric designs as stimuli, WRAML2 recognition subtests display one stimulus at a time and requires children to make a binary response of “Yes” or “NO” during the response phase, while NEPSY-II recognition subtests present a series of stimuli and requires children to choose one. The latter may impose relatively high cognitive demands for children with ASD, as it requires them to suppress interference from other options. Children with ASD showed both diminished recall and recognition in our study, in contrast to adults who report relatively unaffected recognition (54). Further studies are needed to systematically examine the impact of task requirements on memory performance, which is critical for gaining a clearer understanding of the core memory deficits in affected children and their developmental progression (55).
Crucially, hierarchical cluster analysis revealed that shared cognitive mechanisms underlie general and face memory abilities in TD children, who showed significant correlations between these two aspects of memory function. In contrast, children with ASD did not show a significant relationship between general and face memory abilities, which suggests that these memory components are driven by distinct cognitive mechanisms in ASD. One possible explanation for these findings is that an element of ASD symptomatology affects face memory performance that is independent from general memory abilities. For example, reduced eye contact and time spent socially interacting may be factors that contribute to specific face memory deficits in ASD (48, 49). Another plausible explanation is that restricted and circumscribed interests and excessive attention to details associated with ASD may impair pattern separation for face stimuli which tend to be more similar than other classes of visual stimuli (56, 57). Together, our findings support a broad memory deficit model of ASD in which face and general memory reductions represent distinct factors influencing memory performance (9).
Next, we examined the role of the PCC and hippocampus nodes of the DMN, a large-scale brain network implicated in cognitive and social dysfunction in ASD. Aberrant function of the DMN has been consistently linked to impaired social function in ASD (13, 58). However, the hippocampus and PCC nodes of the DMN, have also been implicated in memory function in neurotypical individuals (16–21, 26, 27), and it is unknown whether the integrity of these functional circuits is related to distinct dimensions of memory function in children with ASD. Our results revealed that hyperconnectivity of the hippocampus predicted general memory reductions in children with ASD. In contrast, hyperconnectivity of the PCC predicted face memory reductions in children with ASD. Notably, hippocampal-PCC circuitry was a common feature of general and face memory reductions in ASD.
Our study provides new insights into DMN dysfunction and its links to memory abilities in children with ASD. Importantly, we found that memory impairments in ASD are associated with hyperconnected, rather than hypo-connected, hippocampal and PCC circuits. This result is consistent with a growing literature highlighting the prevalence of hyperconnected brain circuitry in children with ASD, including the DMN (58–60). Previous studies have shown that functional hyperconnectivity in children with ASD is associated with increased symptom severity and elevated regional brain fluctuations (60). This hyperconnectivity is hypothesized to arise from an imbalance of neural excitation and inhibition and may explain a key component of neurophysiology in ASD (61–64). Our findings add to this evidence by suggesting that memory impairments in ASD may result from hyperconnectivity of DMN circuits, which could be similarly impacted by a similar excitation-inhibition imbalance. Moreover, hyperconnectivity in these circuits may hinder appropriate task-related modulation and cause overlap between distinct memories, thereby affecting memory abilities.
Our study sheds light on the impact of hyperconnected hippocampus circuits in episodic memory function in children with ASD. Previous research on neurotypical individuals has demonstrated that the hippocampus, in conjunction with prefrontal and parietal brain systems, is integral to the encoding and retrieval of episodic memory (22, 23). Our findings converge on previous studies which have provided evidence for a relation between the functional connectivity of the hippocampus and episodic memory performance across different age groups spanning childhood, adolescence, and adulthood (65–67). However, the magnitude of memory impairments and the relationship between aberrant hippocampus circuitry and memory in ASD have not been consistent across studies, likely due to differences in the developmental stage, wide age ranges of study participants, as well as differences between task- and resting-state fMRI connectivity. Results from the current study suggest that aberrations in the intrinsic circuitry of the hippocampus underlie general memory reductions during a developmental stage closer to the onset and diagnosis of ASD.
Results also provide new information regarding a role for the PCC in memory function in children with ASD. The PCC is a primary node or hub of the DMN and has a key role in autobiographical memory and social cognitive functions (21, 68), including social memory (27). Our results demonstrate that aberrant PCC circuitry is a significant predictor of face memory reductions in ASD and highlight PCC functions that extend beyond social memory to general episodic memory. These results suggest that dysfunctional PCC circuits are not only associated with deficits in social communication in ASD, but also extend to a broad range of episodic memory deficits.
Individuals with ASD achieve lower levels of post-secondary education and independent living compared to other clinical populations (69). Our findings of distinct general and face memory dysfunction in children with ASD may have practical implications for these individuals. While many ASD interventions are focused on improving social and language function (70), a more comprehensive intervention that takes general episodic memory deficits into account may further improve cognitive function in these children. Moreover, if parents, caregivers, and teachers are aware of multiple dimensions of memory deficits in children with ASD, it may help them better understand the challenges these children face in their daily lives and may inform their interactions to support their learning and development.
Several limitations of this study warrant consideration and suggest avenues for future work. First, larger sample sizes are needed to validate the observed memory impairments and to further characterize heterogeneity of memory function in children with ASD. Second, our study focused on memory reductions and related neural circuits in children with ASD who do not have an intellectual disability, and it is unclear whether these mechanisms also apply to children with more severe forms of ASD. Additional research that includes a broader spectrum of participants is required to address this question. Third, while our findings demonstrate a relation between hippocampal/PCC functional circuits and memory reductions in ASD, a causal relationship remains unclear. Further investigations with appropriate task-based fMRI studies and longitudinal designs are needed to explore the interaction between brain circuits, neural representations, and memory abilities, and their developmental trajectory. Lastly, future studies should address the suitability of standardized psychological instruments for atypical populations (55), including children with autism, as different developmental trajectories may impact the measurement of comparable psychological abilities.
In conclusion, our study reveals that children with ASD have an array of memory reductions that affect both general and face memory, and that distinct, but overlapping, DMN circuits predict performance in these two areas of memory function. Our findings identify novel neurobiological targets for memory intervention in children with ASD and point to a potentially outsized role for the DMN in neurocognitive dysfunction in affected children. More broadly, our findings provide a renewed focus on areas of impairments in ASD, and further elucidate various challenges that individuals with ASD may experience as they navigate their social, educational, and professional environments.
Supplementary Material
Acknowledgements
We thank participating families, and Drs. Jennifer Phillips and Kaustubh Supekar for assistance with the study.
Funding
This research was supported by the United States National Institutes of Health to V.M. (HD059205, MH084164, HD094623, MH121069), and by the Stanford Maternal & Child Health Research Institute Postdoctoral Support Awards to H.C. and J.L.
Footnotes
Competing interests
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Southwick JS, Bigler ED, Froehlich A, DuBray MB, Alexander AL, Lange N, et al. (2011): Memory functioning in children and adolescents with autism. Neuropsychology. 25:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutchins TL, Prelock PA (2018): Using Story-Based Interventions to Improve Episodic Memory in Autism Spectrum Disorder. Semin Speech Lang. 39:125–143. [DOI] [PubMed] [Google Scholar]
- 3.Cooper RA, Simons JS (2019): Exploring the neurocognitive basis of episodic recollection in autism. Psychon Bull Rev. 26:163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buitelaar JK, van der Wees M, Swaab-Barneveld H, van der Gaag RJ (1999): Verbal memory and Performance IQ predict theory of mind and emotion recognition ability in children with autistic spectrum disorders and in psychiatric control children. J Child Psychol Psychiatry. 40:869–881. [PubMed] [Google Scholar]
- 5.Chen L, Abrams DA, Rosenberg-Lee M, Iuculano T, Wakeman HN, Prathap S, et al. (2019): Quantitative analysis of heterogeneity in academic achievement of children with autism. Clin Psychol Sci. 7:362–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauck M, Fein D, Maltby N, Waterhouse L, Feinstein C (1998): Memory for faces in children with autism. Child Neuropsychology. 4:187–198. [Google Scholar]
- 7.Weigelt S, Koldewyn K, Kanwisher N (2012): Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci Biobehav Rev. 36:1060–1084. [DOI] [PubMed] [Google Scholar]
- 8.Williams DL, Goldstein G, Minshew NJ (2006): The profile of memory function in children with autism. Neuropsychology. 20:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewing L, Pellicano E, Rhodes G (2013): Reevaluating the selectivity of face-processing difficulties in children and adolescents with autism. J Exp Child Psychol. 115:342–355. [DOI] [PubMed] [Google Scholar]
- 10.Nair A, Jolliffe M, Lograsso YSS, Bearden CE (2020): A Review of Default Mode Network Connectivity and Its Association With Social Cognition in Adolescents With Autism Spectrum Disorder and Early-Onset Psychosis. Front Psychiatry. 11:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schurz M, Radua J, Tholen MG, Maliske L, Margulies DS, Mars RB, et al. (2021): Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol Bull. 147:293–327. [DOI] [PubMed] [Google Scholar]
- 12.Xie X, Mulej Bratec S, Schmid G, Meng C, Doll A, Wohlschlager A, et al. (2016): How do you make me feel better? Social cognitive emotion regulation and the default mode network. Neuroimage. 134:270–280. [DOI] [PubMed] [Google Scholar]
- 13.Padmanabhan A, Lynch CJ, Schaer M, Menon V (2017): The Default Mode Network in Autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uddin LQ, Supekar K, Menon V (2013): Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. (2013): Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 70:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davachi L, Mitchell JP, Wagner AD (2003): Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 100:2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichenbaum H, Yonelinas AP, Ranganath C (2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci. 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckner RL, DiNicola LM (2019): The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 20:593–608. [DOI] [PubMed] [Google Scholar]
- 19.Shapira-Lichter I, Oren N, Jacob Y, Gruberger M, Hendler T (2013): Portraying the unique contribution of the default mode network to internally driven mnemonic processes. Proc Natl Acad Sci U S A. 110:4950–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sestieri C, Corbetta M, Romani GL, Shulman GL (2011): Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 31:4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philippi CL, Tranel D, Duff M, Rudrauf D (2015): Damage to the default mode network disrupts autobiographical memory retrieval. Soc Cogn Affect Neurosci. 10:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitlock JR, Sutherland RJ, Witter MP, Moser MB, Moser EI (2008): Navigating from hippocampus to parietal cortex. Proc Natl Acad Sci U S A. 105:14755–14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavenex P, Banta Lavenex P (2013): Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav Brain Res. 254:8–21. [DOI] [PubMed] [Google Scholar]
- 24.Riggins T, Geng F, Blankenship SL, Redcay E (2016): Hippocampal functional connectivity and episodic memory in early childhood. Dev Cogn Neurosci. 19:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghetti S, Bunge SA (2012): Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci. 2:381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natu VS, Lin JJ, Burks A, Arora A, Rugg MD, Lega B (2019): Stimulation of the Posterior Cingulate Cortex Impairs Episodic Memory Encoding. J Neurosci. 39:7173–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer ML, Spunt RP, Berkman ET, Taylor SE, Lieberman MD (2012): Evidence for social working memory from a parametric functional MRI study. Proc Natl Acad Sci U S A. 109:1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler D (1999): Abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 29.Lord C, Rutter M, Le Couteur A (1994): Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24:659–685. [DOI] [PubMed] [Google Scholar]
- 30.Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, et al. (2009): The Autism Diagnostic Observation Schedule-toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J Autism Dev Disord. 39:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheslow D, Adams W (2003): Wide range assessment of memory and learning (WRAML). NCS Pearson; Bloomington, MN. [Google Scholar]
- 32.Brooks BL, Sherman EM, Strauss E (2009): NEPSY-II: a developmental neuropsychological assessment. Child Neuropsychology. 16:80–101. [Google Scholar]
- 33.Murtagh F, Contreras P (2012): Algorithms for hierarchical clustering: an overview. Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discovery. 2:86–97. [Google Scholar]
- 34.Szekely GJ, Rizzo ML (2005): Hierarchical clustering via joint between-within distances: Extending Ward’s minimum variance method. Journal of classification. 22:151–184. [Google Scholar]
- 35.Ward JH Jr (1963): Hierarchical grouping to optimize an objective function. Journal of the American statistical association. 58:236–244. [Google Scholar]
- 36.Charrad M, Ghazzali N, Boiteau V, Niknafs A (2014): NbClust: an R package for determining the relevant number of clusters in a data set. Journal of statistical software. 61:1–36. [Google Scholar]
- 37.Glover GH, Lai S (1998): Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 39:361–368. [DOI] [PubMed] [Google Scholar]
- 38.Qin S, Duan X, Supekar K, Chen H, Chen T, Menon V (2016): Large-scale intrinsic functional network organization along the long axis of the human medial temporal lobe. Brain Struct Funct. 221:3237–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL (2008): Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 100:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patriquin MA, DeRamus T, Libero LE, Laird A, Kana RK (2016): Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum Brain Mapp. 37:3957–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. (2010): Prediction of individual brain maturity using fMRI. Science. 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Liao X, Xia M, He Y (2018): Chronnectome fingerprinting: Identifying individuals and predicting higher cognitive functions using dynamic brain connectivity patterns. Hum Brain Mapp. 39:902–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erus G, Battapady H, Satterthwaite TD, Hakonarson H, Gur RE, Davatzikos C, et al. (2015): Imaging patterns of brain development and their relationship to cognition. Cereb Cortex. 25:1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Q, Xue G, Chen C, Chen C, Lu ZL, Dong Q (2013): Decoding the neuroanatomical basis of reading ability: a multivoxel morphometric study. J Neurosci. 33:12835–12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui Z, Su M, Li L, Shu H, Gong G (2018): Individualized Prediction of Reading Comprehension Ability Using Gray Matter Volume. Cereb Cortex. 28:1656–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pluck G (2018): Lexical reading ability predicts academic achievement at university level. Cognition, Brain, Behavior. 22. [Google Scholar]
- 47.Pluck G, Bravo Mancero P, Maldonado Gavilanez CE, Urquizo Alcivar AM, Ortiz Encalada PA, Tello Carrasco E, et al. (2019): Modulation of striatum based non-declarative and medial temporal lobe based declarative memory predicts academic achievement at university level. Trends Neurosci Educ. 14:1–10. [DOI] [PubMed] [Google Scholar]
- 48.Tehrani-Doost M, Salmanian M, Ghanbari-Motlagh M, Shahrivar Z (2012): Delayed face recognition in children and adolescents with autism spectrum disorders. Iran J Psychiatry. 7:52–56. [PMC free article] [PubMed] [Google Scholar]
- 49.Eussen ML, Louwerse A, Herba CM, Van Gool AR, Verheij F, Verhulst FC, et al. (2015): Childhood Facial Recognition Predicts Adolescent Symptom Severity in Autism Spectrum Disorder. Autism Res. 8:261–271. [DOI] [PubMed] [Google Scholar]
- 50.Scherf KS, Elbich D, Minshew N, Behrmann M (2015): Individual differences in symptom severity and behavior predict neural activation during face processing in adolescents with autism. Neuroimage Clin. 7:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffin JW, Bauer R, Scherf KS (2021): A quantitative meta-analysis of face recognition deficits in autism: 40 years of research. Psychol Bull. 147:268–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minio-Paluello I, Porciello G, Pascual-Leone A, Baron-Cohen S (2020): Face individual identity recognition: a potential endophenotype in autism. Mol Autism. 11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Hearn K, Schroer E, Minshew N, Luna B (2010): Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia. 48:3955–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desaunay P, Briant AR, Bowler DM, Ring M, Gerardin P, Baleyte JM, et al. (2020): Memory in autism spectrum disorder: A meta-analysis of experimental studies. Psychol Bull. 146:377–410. [DOI] [PubMed] [Google Scholar]
- 55.Boucher J, Bowler D (2008): Memory In Autism-Theory and Evidence. [Google Scholar]
- 56.Gauthier I, Behrmann M, Tarr MJ (1999): Can face recognition really be dissociated from object recognition? J Cogn Neurosci. 11:349–370. [DOI] [PubMed] [Google Scholar]
- 57.Busigny T, Graf M, Mayer E, Rossion B (2010): Acquired prosopagnosia as a face-specific disorder: ruling out the general visual similarity account. Neuropsychologia. 48:2051–2067. [DOI] [PubMed] [Google Scholar]
- 58.Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V (2013): Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry. 74:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nomi JS, Uddin LQ (2015): Developmental changes in large-scale network connectivity in autism. Neuroimage Clin. 7:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, et al. (2013): Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep. 5:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubenstein JL (2010): Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr Opin Neurol. 23:118–123. [DOI] [PubMed] [Google Scholar]
- 62.Rubenstein JL, Merzenich MM (2003): Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vattikuti S, Chow CC (2010): A computational model for cerebral cortical dysfunction in autism spectrum disorders. Biol Psychiatry. 67:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. (2011): Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 477:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hashimoto T, Yokota S, Matsuzaki Y, Kawashima R (2021): Intrinsic hippocampal functional connectivity underlying rigid memory in children and adolescents with autism spectrum disorder: A case-control study. Autism.13623613211004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hogeveen J, Krug MK, Geddert RM, Ragland JD, Solomon M (2020): Compensatory Hippocampal Recruitment Supports Preserved Episodic Memory in Autism Spectrum Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 5:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooper RA, Richter FR, Bays PM, Plaisted-Grant KC, Baron-Cohen S, Simons JS (2017): Reduced Hippocampal Functional Connectivity During Episodic Memory Retrieval in Autism. Cereb Cortex. 27:888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saxe R, Powell LJ (2006): It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 17:692–699. [DOI] [PubMed] [Google Scholar]
- 69.Newman L, Wagner M, Knokey A-M, Marder C, Nagle K, Shaver D, et al. (2011): The Post-High School Outcomes of Young Adults with Disabilities up to 8 Years after High School: A Report from the National Longitudinal Transition Study-2 (NLTS2). NCSER 2011–3005. National Center for Special Education Research. [Google Scholar]
- 70.Kodak T, Bergmann S (2020): Autism Spectrum Disorder: Characteristics, Associated Behaviors, and Early Intervention. Pediatr Clin North Am. 67:525–535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


