Abstract
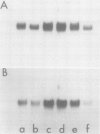
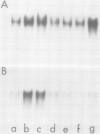
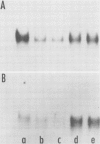
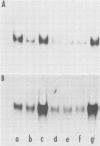
We have examined the effects of cytokinin, fusicoccin, and ethylene on auxin-induced changes in gene expression during auxin-promoted cell elongation in soybean (Glycine max L. Merr. cv Wayne) using cloned cDNAs to two auxin-responsive mRNAs (Walker, Key 1982 Proc Natl Acad Sci USA 79: 7185-7989). RNA blot analyses demonstrate that under conditions of cytokinin inhibition of auxin-promoted cell elongation the levels of these two auxin-responsive mRNAs is unaltered. Fusicoccin-promoted elongation is not associated with an enhanced expression of these two mRNAs, suggesting that the increased levels of these mRNAs observed during auxin-promoted cell elongation are not simply due to enhanced rates of cell elongation. We have also determined that ethylene plays no apparent role in the regulation of expression of these mRNAs. However, the auxins indole-3-acetic acid, 2,4-dichlorophenoxyacetic acid, and α-naphthalene acetic acid all enhance an accumulation of these mRNAs. We conclude that the regulation of these mRNAs is directly dependent on auxin. That auxin-promoted cell elongation is dependent upon the increased accumulation of these mRNAs remains to be determined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Christoffersen R. E., Laties G. G. Ethylene regulation of gene expression in carrots. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4060–4063. doi: 10.1073/pnas.79.13.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Barnett N. M., Lin C. Y. RNA and protein biosynthesis and the regulation of cell elongation by auxin. Ann N Y Acad Sci. 1967 Aug 9;144(1):49–62. doi: 10.1111/j.1749-6632.1967.tb34000.x. [DOI] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Hammett J. R., Key J. L. Sequence complexity of polyadenylated ribonucleic acid from soybean suspension culture cells. Biochemistry. 1979 Jun 26;18(13):2725–2731. doi: 10.1021/bi00580a006. [DOI] [PubMed] [Google Scholar]
- Theologis A., Ray P. M. Early auxin-regulated polyadenylylated mRNA sequences in pea stem tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):418–421. doi: 10.1073/pnas.79.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Key J. L. Isolation of cloned cDNAs to auxin-responsive poly(A)RNAs of elongating soybean hypocotyl. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7185–7189. doi: 10.1073/pnas.79.23.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the population of translatable messenger RNA in elongating sections of soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):332–337. doi: 10.1104/pp.69.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]