Abstract
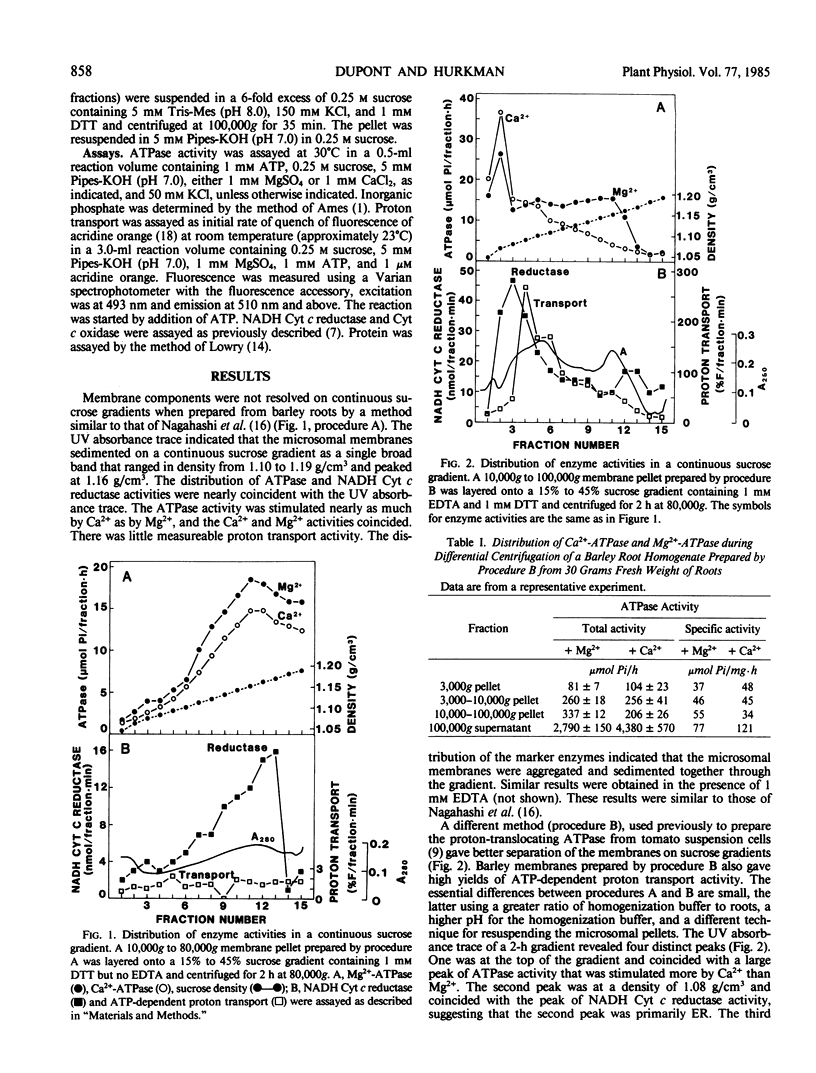
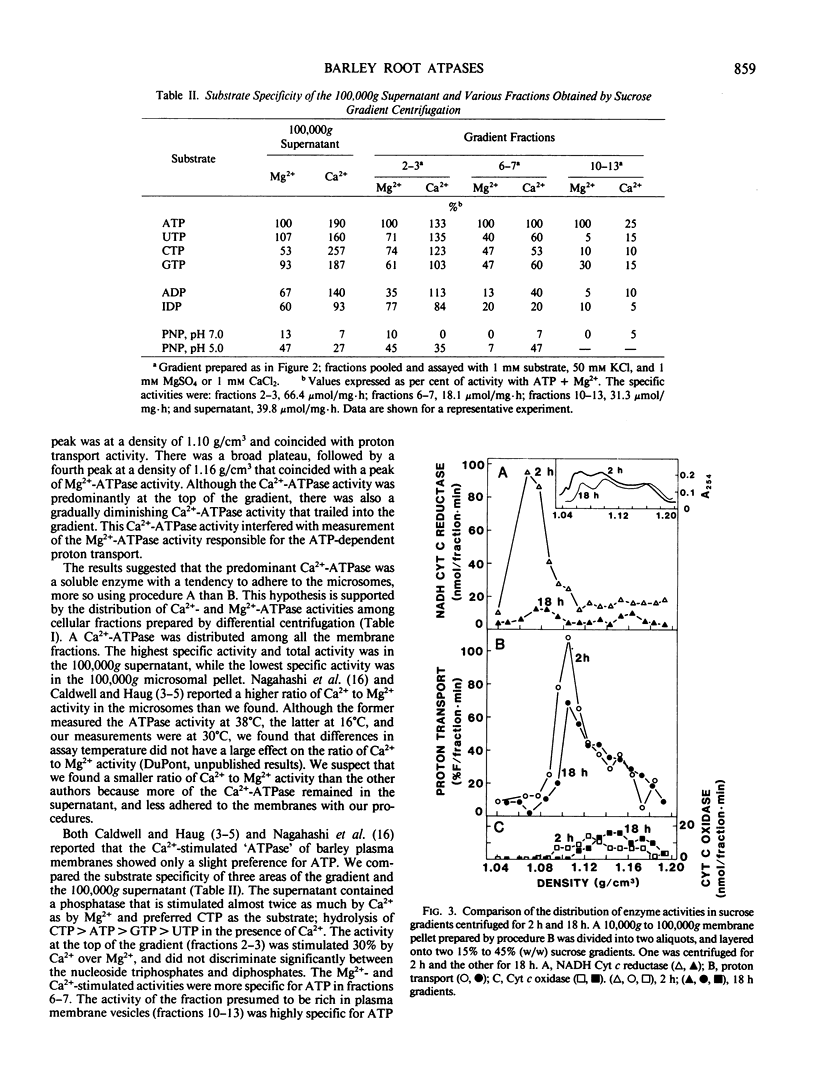
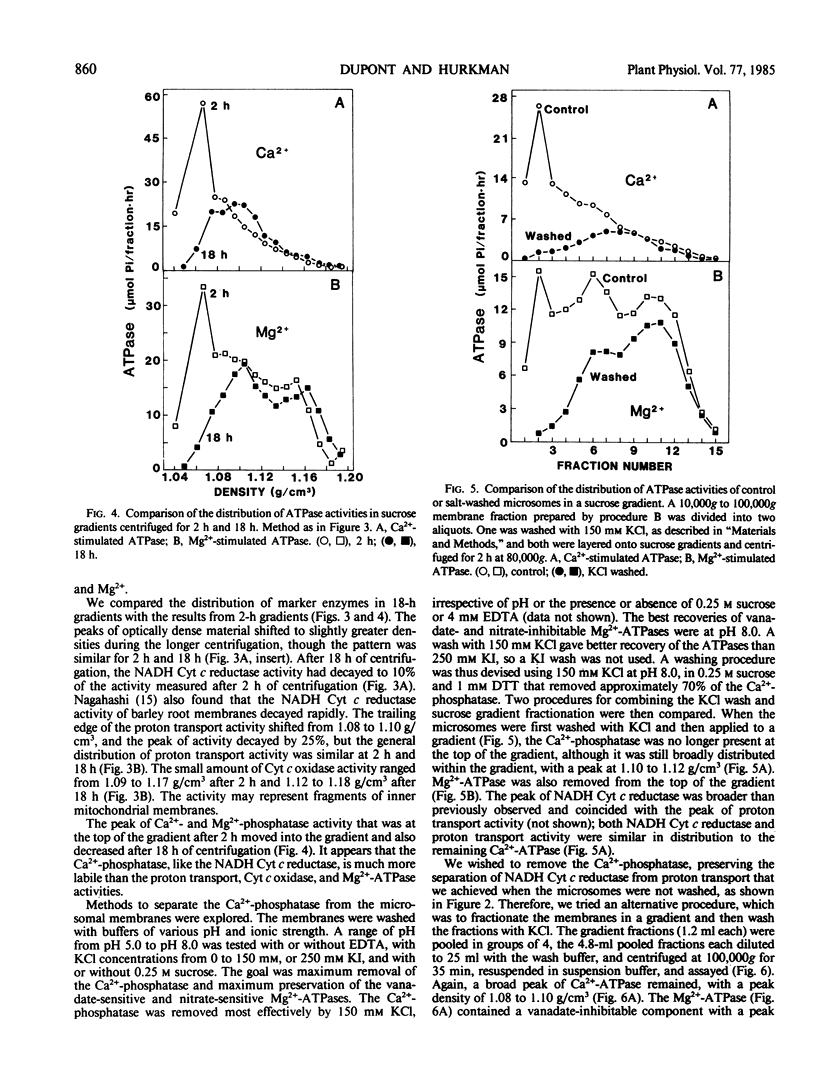
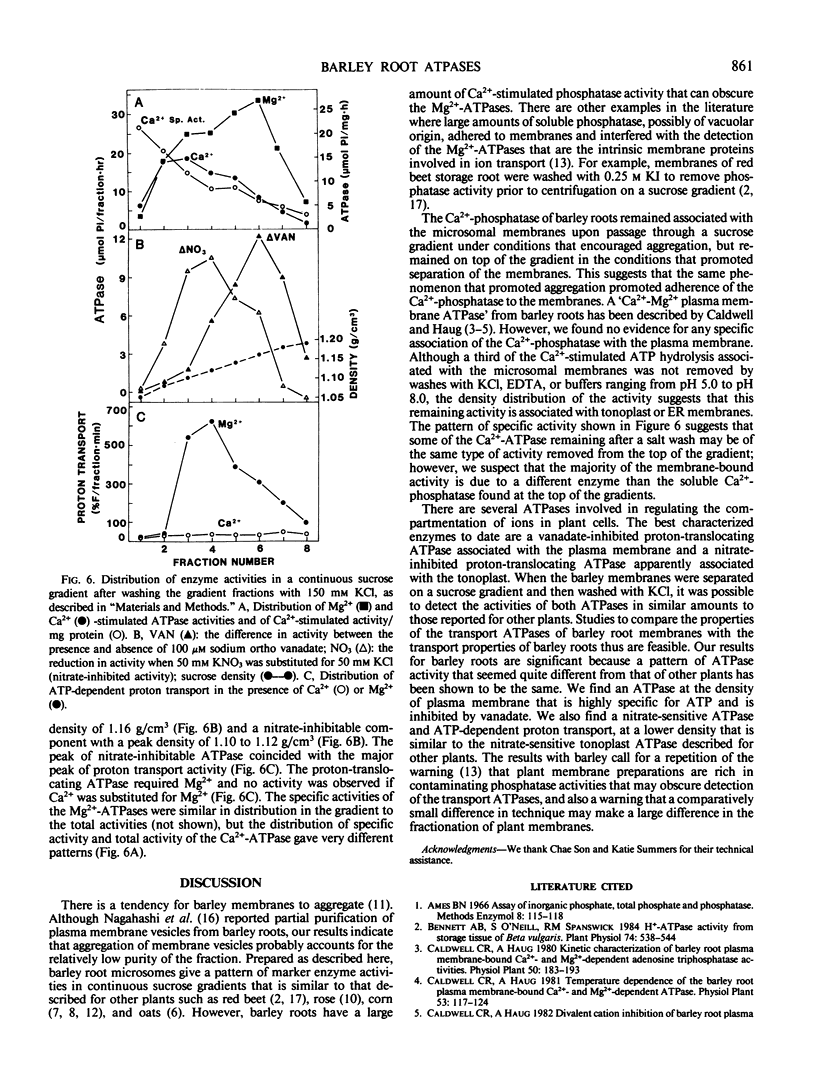
Two methods for preparing membrane fractions from barley (Hordeum vulgare cv California Mariout 72) roots were compared in order to resolve reported differences between the characteristics of the plasma membrane ATPase of barley and that of other species. When microsomal membranes were prepared by a published procedure and applied to a continuous sucrose gradient, the membranes sedimented as a single broad band with a peak density of 1.16 grams per cubic centimeter (g/cm3). Activities of NADH cytochrome (Cyt) c reductase, Ca2+-ATPase, and Mg2+-ATPase were coincident and there was little ATP-dependent proton transport anywhere on the gradient. When the homogenization procedure was modified by increasing the pH of the buffer and the ratio of buffer to roots, the microsomal membranes separated as several components on a continuous sucrose gradient. A Ca2+-phosphatase was at the top of the gradient, NADH Cyt c reductase at 1.08 g/cm3, a peak of ATP-dependent proton transport at 1.09 to 1.12 g/cm3, a peak of nitrate-inhibited ATPase at 1.09 to 1.12 g/cm3, and of vanadate-inhibited ATPase at 1.16 g/cm3. The Ca2+-phosphatase had no preference for ATP over other nucleoside di- and tri-phosphates and was separated from the vanadate-inhibited ATPase on a sucrose gradient; approximately 70% of the Ca2+-phosphatase was removed from the microsomes by washing with 150 millimolar KCl. The vanadate-sensitive ATPase required Mg2+, was highly specific for ATP, and was not affected by the KCl wash. These results show that barley roots have a plasma membrane ATPase similar to that of other plant species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Zabala M. de G. Preparation of Membrane Vesicles Enriched in ATP-Dependent Proton Transport from Suspension Cultures of Tomato Cells. Plant Physiol. 1985 Jan;77(1):69–73. doi: 10.1104/pp.77.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi G., Leonard R. T., Thomson W. W. Purification of plasma membranes from roots of barley: specificity of the phosphotungstic Acid-chromic Acid stain. Plant Physiol. 1978 Jun;61(6):993–999. doi: 10.1104/pp.61.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Kinetic properties of the KCl transport at the secreting apical membrane of the oxyntic cell. J Membr Biol. 1983;71(3):195–207. doi: 10.1007/BF01875461. [DOI] [PubMed] [Google Scholar]


