Abstract
INTRODUCTION
White matter hyperintensities (WMHs) increase with age and contribute to cognitive and motor function decline. Energy costs for mobility worsen with age, as the energetic cost of walking increases and energetic capacity declines. We examined the cross‐sectional associations of multiple measures of walking energetics with WMHs in mid‐ to late‐aged adults.
METHODS
A total of 601 cognitively unimpaired adults (mean age 66.9 ± 15.3 years, 54% women) underwent brain magnetic resonance imaging scans and completed standardized slow‐ and peak‐paced walking assessments with metabolic measurement (V̇O2). T1‐weighted scans and fluid‐attenuated inversion recovery images were used to quantify WMHs. Separate multivariable linear regression models examined associations adjusted for covariates.
RESULTS
Lower slow‐paced V̇O2 (B = 0.07; P = 0.030), higher peak‐paced V̇O2 (B = –0.10; P = 0.007), and lower cost‐to‐capacity ratio (B = .12; P < 0.0001) were all associated with lower WMH volumes.
DISCUSSION
The cost‐to‐capacity ratio, which describes the percentage of capacity required for ambulation, was the walking energetic measure most strongly associated with WMHs.
Keywords: cardiorespiratory fitness, cerebral small vessel disease, cerebral vascular health, gait, magnetic resonance imaging, metabolic cost of walking, motor control, neuroimaging, physical fitness, physical function, walking economy, walking efficiency
1. INTRODUCTION
Older adults experience age‐related declines in cognitive and motor function. 1 One common neuropathological change hypothesized to contribute to this decline is the accumulation of white matter hyperintensities (WMHs). 2 , 3 , 4 , 5 WMHs are generally observed in older age and are primarily considered markers of cerebral small vessel disease. 6 , 7 The appearance of WMHs, which is indicative of deteriorating white matter structure (e.g., myelin pallor, tissue rarefaction), 5 , 8 has been linked to Alzheimer's disease (AD)‐related pathology, brain atrophy, and cognitive decline. 9 , 10 , 11 , 12 Further, WMHs have been shown to be associated with the clinical onset and progression of AD and related dementias. 13 , 14 , 15
Greater volumes of WMHs consistently have been associated with poor motor function in older adults. 16 Specifically, older adults with higher WMH volume tend to display impaired balance, greater step length variability, and slower gait speed, 17 , 18 , 19 , 20 hallmarks of poor mobility and emerging indicators of adverse cognitive change. 21 , 22 , 23 Previous research has demonstrated that the energy costs for mobility, measured through oxygen consumption (V̇O2), worsen in older age and predict mobility decline. 24 , 25 , 26 More recent evidence suggests these measures of walking energetics are also associated with AD‐related pathology (amyloid beta [Aβ] deposition), white and gray matter brain volume, and cognitive performance in cognitively unimpaired mid‐ to late‐aged adults. 27 , 28 , 29 , 30 , 31 , 32 Collectively, these findings suggest that the energy costs for mobility may reflect underlying changes that precede adverse changes in cognitive and motor function. Moreover, given that cerebral vascular dysfunction likely contributes to age‐ and disease‐related pathology (e.g., brain atrophy, Aβ), it may underlie the previously observed walking energetics–brain associations. However, the connection between the energy costs for mobility and WMHs remains unexplored.
This study aims to expand upon this emerging area of research and examine the cross‐sectional associations of multiple measures of walking energetics with WMHs in cognitively unimpaired participants of the Baltimore Longitudinal Study of Aging (BLSA). We hypothesized that favorable walking energetics would be associated with lower WMH volume, independent of usual gait speed.
2. METHODS
2.1. Participants
The BLSA is a study of human aging initiated in 1958. All BLSA participants are community‐dwelling adults free of major chronic conditions and cognitive and functional impairment at the time of enrollment. Once enrolled, participants are followed for life and undergo comprehensive health, cognitive, neuroimaging, and functional assessments every 1 to 4 years depending on age (< 60: every 4 years, 60–79: every 2 years, ≥ 80: every year). Trained staff administer all assessments following standardized protocols. Additional study enrollment and design details have been previously described. 33 The sample for the present study consists of cognitively unimpaired participants free of Parkinson's disease or history of stroke who underwent physical examinations, health history assessments, functional testing, and magnetic resonance imaging (MRI) between 2008 and 2020. Data from the participant's most recent visit that contained all measures of interest were used for the present study. Clinical diagnoses are based on consensus diagnostic procedures implemented for many years at the BLSA, comparable to the National Institute on Aging–Alzheimer's Association criteria. 34 , 35 The clinical and cognitive data are first reviewed to determine a syndromic diagnosis (i.e., cognitively unimpaired, mild cognitive impairment [MCI], impaired not MCI, or dementia). Those judged to be cognitively impaired (e.g., MCI, dementia) are then further classified by presumed etiology (e.g., AD, frontotemporal dementia, dementia with Lewy bodies). More than one etiology can be endorsed (e.g., AD and vascular disease). Participants classified as cognitively impaired were excluded from the present study. The Internal Review Board of the Intramural Research Program of the National Institutes of Health approved the study protocol. Consent statement: all participants provided written informed consent.
RESEARCH IN CONTEXT
Systematic Review: The authors performed a traditional literature review related to energy costs for mobility and white matter hyperintensities (WMHs). There is evidence that poor motor function is associated with WMHs in older adults. However, whether energy costs for mobility, which have been shown to precede motor function decline, are linked to WMHs in older adults is currently unknown.
Interpretation: Within a cognitively unimpaired cohort of community‐dwelling older adults, lower energy costs for slow‐paced walking and higher peak‐paced walking energetic capacity are each separately associated with lower WMH volume. Notably, the cost‐to‐capacity ratio, which describes the percentage of capacity required for ambulation, was the energetic measure most strongly associated with WMH volume. These data add to a growing body of research investigating mobility with age‐ and brain disease‐related neuropathology and suggest the energy costs for mobility in older age may be a physiological indicator of compromised brain health.
Future Directions: Longitudinal studies that assess the temporality of the observed associations and biological mechanisms connecting walking energetics and WMHs are needed to better elucidate causality and directionality of the observed associations.
2.2. Walking energy expenditure measurements
All participants completed two separate walking assessments with indirect calorimetry, from which three walking energetic measures were derived. The energetic cost of slow‐paced walking was measured on a motor‐driven treadmill on which participants walked at 1.5 mph (0.67 m/s) at 0% grade for 5 minutes. This protocol was used for all participants, providing a standardized energy expenditure measure to slow‐paced walking. Oxygen consumption (V̇O2) levels were obtained during the slow‐paced treadmill walking test using a metabolic cart with indirect calorimetry (Medical Graphics Corp.) and two‐way non‐rebreathing mask. To calculate average slow‐paced walking energy expenditure (V̇O2 mL/kg/min), readings from the first 2 minutes of the walking test were discarded to allow the participant to adjust to the workload. The average V̇O2 (mL/kg/min) recorded during the final 3 minutes of the treadmill walk was used to derive a single measure of the energetic cost of slow‐paced walking.
The energetic cost of peak‐paced walking was assessed during the 400‐meter segment of the long‐distance corridor walk, a two‐part, self‐paced overground endurance walking test. 36 Participants were instructed to walk “as fast as possible, at a pace you can sustain for 400 meters” in a continuous loop around a 20‐meter course laid out in an uncarpeted corridor marked by traffic cones. Standardized encouragement was given with each lap along with the number of laps remaining. Values of V̇O2 were obtained during the peak‐paced overground walking test using a portable indirect calorimeter (Cosmed K4b2, Cosmed) and two‐way non‐rebreathing mask. To calculate average peak‐paced walking energy expenditure (V̇O2 mL/kg/min), readings from the first 1.5 minute of the 400‐meter walk were discarded to allow the participant to adjust to the workload. The average V̇O2 (mL/kg/min) recorded during the remainder of the 400‐meter walk was used to derive a single measure of the energetic cost of peak‐paced walking.
A ratio of the energetic cost of slow‐paced walking to peak‐paced walking (the “cost‐to‐capacity ratio”) was calculated to define the percentage of peak‐paced walking capacity needed for slow‐paced walking (slow‐paced V̇O2/peak‐paced V̇O2). The cost‐to‐capacity ratio is bounded at 0 and 1, with a higher ratio indicating poorer walking energy utilization (i.e., greater percentage of peak walking capacity needed for ambulation). Each metabolic system was calibrated using standard procedures prior to each test to ensure accuracy (i.e., volumetric, gas). All described walking energetic measures have been extensively used in the BLSA. 26
2.3. Neuroimaging protocol
The MRI scans were acquired on a 3T Philips Achieva scanner. Three‐dimensional T1‐weighted magnetization‐prepared rapid gradient echo scans, T2‐weighted dual‐echo scans, and fluid‐attenuated inversion recovery (FLAIR) scans were collected. T1‐weighted scans used the following parameters: echo time = 3.2 ms, repetition time = 6.8 ms, flip angle = 8°, image matrix = 256 × 256, 170 slices, pixel size 1 × 1 mm, slice thickness = 1.2 mm, sagittal acquisition. The structural images were processed using an automated multi‐atlas approach. After correction of intensity inhomogeneities 37 a multi‐atlas skull stripping algorithm was applied for the removal of extra‐cranial tissues. 38 WMHs were quantified using FLAIR and T1‐weighted images based on a deep learning–based method. 39 The MRI scans were conducted during the same visit as the walking assessments.
2.4. Covariate measures
All participants completed a variety of health‐related questionnaires and measurements at the study visit. Variables investigated as potential confounders included total white matter volume, age, sex, race, years of education, systolic blood pressure, height, body composition, comorbid conditions, and usual gait speed. Total white matter volume was measured through MRI. Age, sex, race, and years of education were determined by self‐report. Blood pressure and height were measured according to standard protocols. Body composition, specifically fat and lean mass (kg), were estimated using a dual‐energy x‐ray absorptiometry scan (Prodigy Scanner, GE). Comorbid conditions were defined as a history of two or more of the following: cardiovascular disease, lung disease, liver disease, kidney disease, peripheral neuropathy, hypertension, diabetes, cancer, and lower extremity arthritis pain. Usual gait speed was measured over a 6‐meter course in an uncarpeted corridor. Participants stood with their feet behind a taped starting line and were asked to walk at a “normal comfortable pace.” After a command of “Go,” timing was initiated with the first foot‐fall over the starting line and stopped after the first foot‐fall over the finish line. Two timed trials were conducted to derive usual gait speed in m/s; the faster of the two trials was used for analyses.
2.5. Maximal exercise testing
A subset of participants underwent a maximal graded exercise test on a motor‐driven treadmill using a modified Balke protocol. 40 All participants walked at a constant speed for the duration of the test, 3.0 mph for women and 3.5 mph for men. The exercise test began with a 2‐minute warm‐up at 0% grade that was increased by 3% every 2 minutes until the participant reached volitional exhaustion or indicated they could no longer continue. During the exercise test, metabolic data (e.g., V̇O2, V̇CO2) were measured using a metabolic cart with indirect calorimetry (Medical Graphics Corp.) and two‐way non‐rebreathing mask. Inclusion criteria for a valid exercise test included a respiratory exchange ratio (RER) value of 1.0 or greater. Aerobic capacity was defined as the highest oxygen consumption (V̇O2peak) value recorded during the final stage of a graded exercise test.
2.6. Statistical analyses
Participant characteristics were summarized with means, standard deviations, and frequencies. Prior to model fitting, WMH volume was log10‐transformed to normalize its distribution. All additional assumptions for linear regression were verified. Separate multivariable linear regression models were used to test the associations between walking energetics and WMH volume. A series of unadjusted and adjusted models that controlled for the effects of demographics, body composition, and comorbid conditions were performed. Model 1 adjusted for total white matter volume, age, sex, race, education, systolic blood pressure, height, lean body mass, fat body mass, and comorbid conditions; Model 2 included additional adjustment for usual gait speed. To improve the interpretability, we report standardized beta coefficients in all models. To verify the robustness of the results, we performed a series of sensitivity analyses by (1) removing participants with impaired mobility, defined as a usual gait speed ≤0.8 m/s, and (2) recalculating the cost‐to‐capacity ratio as: energetic cost of slow‐paced walking (V̇O2 mL/kg/min)/aerobic capacity (V̇O2peak mL/kg/min) in a subset of participants that underwent a maximal graded exercise treadmill test and achieved an RER of ≥ 1.0. These were conducted to confirm (1) results were not impacted by adults with mobility impairment, and (2) the robustness of the cost‐to‐capacity findings by using the gold standard measure of aerobic capacity. Analyses were conducted using Stata IC (15.1, Stata Corporation).
3. RESULTS
A total of 601 cognitively unimpaired participants (mean age 66.9 ± 15.3 years, 54% women) completed walking energy expenditure and neuroimaging assessments. The average oxygen consumption (V̇O2) for the slow‐ and peak‐paced walking was 8.4 ± 1.5 and 16.5 ± 4.3 mL/kg/min, respectively. Additional participant characteristics are detailed in Table 1. In the unadjusted models, lower slow‐paced V̇O2, higher peak‐paced V̇O2, and lower cost‐to‐capacity were each significantly associated with less WMHs (all P < 0.0001; Table 2). All associations remained significant after adjusting for total white matter volume, age, sex, race, education, systolic blood pressure, height, body composition, and comorbid conditions (Model 1; Table 2). After additional adjustment for usual gait speed (Model 2), lower slow‐paced V̇O2 (B = 0.07; P = 0.030), higher peak‐paced V̇O2 (B = –0.10; P = 0.007), and lower cost‐to‐capacity ratio (B = 0.12; P < 0.0001), each remained significantly associated with lower WMH volume (Figure 1).
TABLE 1.
Characteristics of BLSA study participants.
| Baseline variables | Entire sample | Sensitivity analysis |
|---|---|---|
| N (%) or mean (SD) | N = 601 | N = 502 |
| Age (years) | 66.9 (15.3) | 65.9 (15.3) |
| Women | 326 (54%) | 268 (53%) |
| Non‐White | 204 (34%) | 173 (34%) |
| Education (years) | 17.6 (2.7) | 17.7 (2.6) |
| Fat mass (kg), % | 26.3 (10.2) | 26.1 (10.0) |
| Lean mass (kg), % | 47.0 (10.2) | 47.1 (10.3) |
| Height (cm) | 168.0 (9.4) | 168.2 (9.3) |
| Systolic blood pressure, mmHg | 129.9 (17.0) | 130.0 (17.2) |
| Comorbid conditions (≥2) | 287 (48%) | 228 (45%) |
| Usual gait speed (m/s) | 1.2 (0.2) | 1.2 (0.2) |
| Total white matter volume, cm3 | 469.6 (54.2) | 471.2 (53.8) |
| White matter hyperintensities, log10 | 3.0 (0.8) | 3.0 (0.9) |
| Walking energetics | ||
| Slow‐paced V̇O2, mL/kg/min | 8.4 (1.5) | 8.5 (1.5) |
| Peak‐paced V̇O2, mL/kg/min | 16.5 (4.3) | ─ |
| Cost‐to‐capacity, ratio | 0.54 (0.16) | ─ |
| V̇O2peak, mL/kg/min | ─ | 23.9 (6.9) |
| Cost‐to‐capacity a ratio | ─ | 0.38 (0.12) |
Note: Values indicate mean and standard deviation unless indicated otherwise.
Abbreviations: BLSA, Baltimore Longitudinal Study of Aging; cm, centimeters; kg, kilograms; m, meters; m/s, meters per second; mL, milliliters; mmHg, millimeters of mercury; V̇O2, oxygen consumption.
Ratio calculated with V̇O2peak.
TABLE 2.
Linear regression estimates of the association between walking energetics and WMHs (N = 601).
| Unadjusted | Model 1 | Model 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | B | (SE) | P‐value | B | B | (SE) | P‐value | B | B | (SE) | P‐value | |
| Slow‐paced V̇O2 → WMHs | 0.18 | 0.10 | (0.02) | <0.0001 | 0.07 | 0.04 | (0.02) | 0.022 | 0.07 | 0.04 | (0.02) | 0.030 |
| Peak‐paced V̇O2 → WMHs | –0.38 | –0.07 | (0.01) | <0.0001 | –0.12 | –0.02 | (0.01) | 0.001 | ‐0.10 | ‐0.02 | (0.01) | 0.007 |
| Cost‐to‐capacity → WMHs | 0.43 | 2.3 | (0.20) | <0.0001 | 0.13 | 0.73 | (0.18) | <0.0001 | 0.12 | 0.67 | (0.18) | <0.0001 |
Note: Model 1: adjusted for total white matter volume, age, sex, race, education, systolic blood pressure, height, lean body mass, fat body mass, and comorbid conditions; Model 2: Model 1 + Gait speed.
Abbreviations: B, standardized beta coefficient; B, unstandardized beta coefficient; SE, standard error; WMHs, white matter hyperintensities.
FIGURE 1.
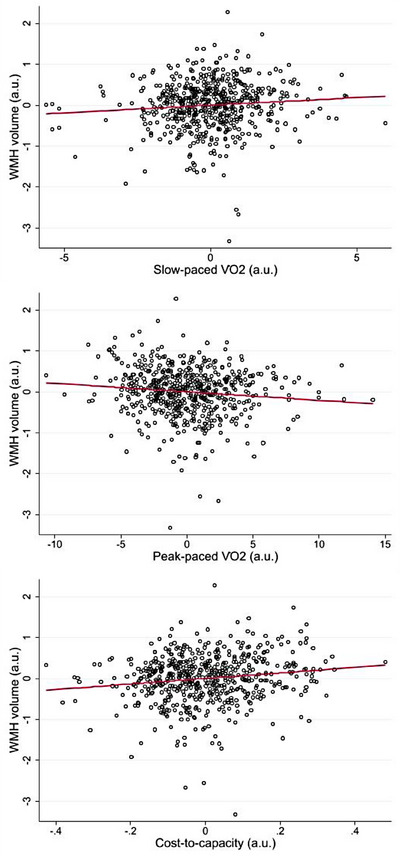
Associations among walking energetics and WMH volume (N = 601). Data are partial regression plots accounting for covariates in Model 2. Regression slopes are indicated with the solid line. a.u., arbirtary units; WMH, white matter hyperintensity
In sensitivity analyses, all observed unadjusted and adjusted associations among walking energetics and WMH volume persisted after the removal of 32 participants with impaired mobility (Table S1 in supporting information). Further, in a subset of participants that underwent a maximal graded exercise test (Table 1; N = 502), sensitivity analyses revealed using aerobic capacity (V̇O2peak mL/kg/min) in the cost‐to‐capacity ratio did not modify the findings with WMH volume in the unadjusted model (B = 0.49; P < 0.0001), adjusted Model 1 (B = .12; P = 0.003), or Model 2 (B = .11; P = 0.008).
4. DISCUSSION
We examined the cross‐sectional associations among three separate measures of walking energetics with WMHs in a sample of cognitively unimpaired community‐dwelling adults free of neurological disease. Our findings demonstrate that lower energy costs for slow‐paced walking and higher peak‐paced walking energetic capacity are each separately associated with lower WMH volume. These findings persisted after controlling for demographics, comorbid conditions, and usual gait speed. Notably, the cost‐to‐capacity ratio, which describes the percentage of capacity required for ambulation, was the energetic measure most strongly associated with WMH volume in all statistical models. Collectively, these data add to a growing body of research investigating mobility with age‐ and brain disease–related neuropathology and suggest the energetic cost for walking in older age may be a physiological indicator of compromised brain health.
The energetic costs of mobility have been studied for more than a century (see review in Poole and Jones 41 ). Much of the research to date has revolved around an individual's capacity for exercise (i.e., aerobic capacity), which is objectively measured as the highest rate of oxygen consumption during a maximal exercise test (V̇O2peak). With respect to ambulation, adults have a strong tendency to walk in ways that reduce metabolic energy costs. The established U‐shaped relationship between V̇O2 and walking speed demonstrates that the self‐selected speed for customary‐paced walking tends to minimize the energetic cost per distance traveled. 42 , 43 Work from BLSA investigators and others have detailed energetic changes throughout the adult lifespan. Aerobic capacity declines with aging, and the rate of this decline accelerates at older ages. 44 , 45 The energetic cost of walking remains relatively constant throughout young‐middle age and begins to rise once adults reach older adulthood (> 65 years old). 25 , 46 The decline in aerobic capacity, accompanied with an increased cost of walking, results in a diminished functional reserve (i.e., cost‐to‐capacity ratio), with the energy required for ambulation accounting for a larger proportion of capacity. 26 , 47 This cost‐to‐capacity ratio has been proposed as a phenotypic marker of aging, due to its strong association with chronological age and physical functioning. 33 Although the energetic costs of mobility have been long studied, investigations into whether walking energetics associate with neuroimaging measures of age‐ and disease‐related pathology are just beginning to emerge.
In the present study we found that adults with lower slow‐paced walking V̇O2 (i.e., more efficient energy use) had less WMHs. This finding complements our recent research that found a lower energetic cost of walking was associated with less Aβ deposition, a defining pathophysiologic feature of AD, and an attenuated rate of brain atrophy within regions that are vulnerable to age‐ and AD‐related neurodegeneration. 27 , 28 This study also observed that peak‐paced walking V̇O2, an estimate of aerobic capacity, was negatively associated with WMHs. This is in agreement with previous studies showing older adults with higher aerobic capacity tend to have lower WMH burden 48 , 49 (but see also Burzynska et al. 50 ).
An important finding of the present study was that the cost‐to‐capacity ratio, which combines both slow‐paced and peak‐paced V̇O2, was most strongly associated with WMH volume. This expands upon previous research and suggests examining ambulation costs as a function of capacity results in a stronger predictor than either measure individually. Recent research from the BLSA found that participants with a higher cost‐to‐capacity ratio displayed greater brain atrophy and lower cognitive performance across several domains including executive function, memory, attention, language, and visuospatial ability. 29 , 30 Meta‐analytic data suggest WMHs are most consistently associated with declines in global cognition and executive function. 4 , 5 Future studies will examine whether the cost‐to‐capacity ratio modifies the association between WMHs and cognition.
Because WMHs appear in mid‐to‐late adulthood, and this accumulation of white matter brain damage is associated with future cognitive impairment, 4 , 5 , 6 our findings raise the question of whether preserving energy utilization may attenuate WMHs and protect against future cognitive decline. One potential pathway through which favorable energetics may attenuate the accumulation of WMHs is through cerebral vascular function. Indeed, WMHs are presumed to be, in part, the result of chronic hypoperfusion (i.e., reduced cerebral blood flow) 5 , 6 , 7 and adults with higher aerobic capacity appear to have greater cerebral blood flow in brain regions vulnerable to hypoperfusion. 51 Therefore, preserving and/or improving energy utilization through exercise training may positively impact cerebral vascular function. 52 , 53 Alternatively, WMHs may lead to walking energetic changes. Pathological findings in regions of WMHs include loss of myelin and axons, 5 which may disrupt corticospinal pathways necessary for neuromuscular signaling/recruitment, resulting in higher energy costs for ambulation. Another possible explanation is that the observed associations among walking energetics and WMHs may be a consequence of an associated biological process, such as mitochondrial function. It is hypothesized that neuronal mitochondrial deficits contribute to the accumulation of AD‐related pathology, 54 and mitochondrial deficits in skeletal muscle have been shown to be associated with decreased aerobic capacity in older adults. 55 Future longitudinal studies that assess changes in walking energetics and WMHs are needed to better elucidate causality and directionality of the observed associations. Additional research into the biological mechanisms connecting walking energetics with age‐ and disease‐related neuropathology is also warranted.
Although the cross‐sectional design of the present study precludes temporal inferences, several attempts to verify the robustness of the findings were made. All observed associations persisted after accounting for key covariates, including systolic blood pressure, which is known to have a strong influence on WMH progression. 56 We also demonstrated associations with each of the three walking energetic measures were independent of usual gait speed, suggesting the physiological underpinnings of walking explains unique variance not captured through gross motor movements. Additionally, sensitivity analyses confirmed the findings were not driven by participants with mobility impairment. To further scrutinize our cost‐to‐capacity findings, we replaced the peak‐paced walking V̇O2 measure (estimate of aerobic capacity), with the gold‐standard measure of aerobic capacity (V̇O2peak) in a subset of our sample that underwent maximal graded exercise testing. The results remained essentially unchanged; a reduced functional reserve measured through the modified cost‐to‐capacity ratio was the energetic measure most strongly associated with higher WMH volume.
While this study has notable strengths, the BLSA is a well‐characterized sample of highly educated healthy mid‐ to late‐aged adults, making it uncertain whether our results generalize to more socioeconomic diverse populations. To be included in the present study adults had to be healthy enough to undergo a 400 meter peak‐paced walk test, which may have underestimated the magnitudes of effects. Although this study focused on WMHs, there are many neuroimaging markers of cerebral small vessel disease that were not evaluated such as cerebral microbleeds, lacunar infarcts, and perivascular spaces. 57 Research investigating additional markers/measures of cerebral small vessel disease (e.g., Fazekas scale) is needed to determine the specificity of the association between walking energetics and cerebral vascular health. These limitations should be considered in the context of the study novelties, which include investigation of several objectively measured walking energetic measures and examination of WMHs.
In summary, the present study provides evidence that in cognitively unimpaired mid‐ to late‐aged community‐dwelling adults, favorable walking energetics are associated with lower WMH volume. Notably, the cost‐to‐capacity ratio was the walking energetic measure most strongly associated with WMH volume, demonstrating the utility of examining ambulation costs as a function of capacity. Efforts to maintain aerobic capacity and walking efficiency may preserve white matter structure throughout mid‐to‐late life.
CONFLICTS OF INTEREST STATEMENT
All authors report no conflicts of interest. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors have nothing to report. This research was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health and by grant U01AG057545. Data used in the analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging Intramural Research Program (blsa.nih.gov). Ryan J. Dougherty was supported by grant K01AG080122.
Dougherty RJ, Wanigatunga AA, An Y, et al. Walking energetics and white matter hyperintensities in mid‐to‐late adulthood. Alzheimer's Dement. 2023;15:e12501. 10.1002/dad2.12501
REFERENCES
- 1. Ferrucci L, Cooper R, Shardell M, et al. Age‐Related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci. 2016;71(9):1184‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moon SY, de Souto Barreto P, Rolland Y, et al. Prospective associations between white matter hyperintensities and lower extremity function. Neurology. 2018;90(15):e1291‐e1297. [DOI] [PubMed] [Google Scholar]
- 3. Maltais M, de Souto Barreto P, Moon SY, Rolland Y, Vellas B. Prospective association of white matter hyperintensity volume and frailty in older adults. Exp Gerontol. 2019;118:51‐54. [DOI] [PubMed] [Google Scholar]
- 4. Roseborough AD, Saad L, Goodman M, Cipriano LE, Hachinski VC, Whitehead SN. White matter hyperintensities and longitudinal cognitive decline in cognitively normal populations and across diagnostic categories: a meta‐analysis, systematic review, and recommendations for future study harmonization. Alzheimers Dement. 2023;19(1):194‐207. [DOI] [PubMed] [Google Scholar]
- 5. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157‐165. [DOI] [PubMed] [Google Scholar]
- 7. Brickman AM, Zahra A, Muraskin J, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172(2):117‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Humphreys CA, Smith, C, Wardlaw JM. Correlations in post‐mortem imaging‐histopathology studies of sporadic human cerebral small vessel disease: a systematic review. Neuropathol Appl Neurobiol. 2021;47(7):910‐930. [DOI] [PubMed] [Google Scholar]
- 9. Raji C, Lopez OL, Kuller LH, et al. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. 2012;33(4):834.e7‐834.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65(1):94‐100. [DOI] [PubMed] [Google Scholar]
- 11. Soldan A, Pettigrew C, Zhu Y, et al. Cognitive reserve and midlife vascular risk: cognitive and clinical outcomes. Ann Clin Transl Neurol. 2020;7(8):1307‐1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graff‐Radford J, Arenaza‐Urquijo EM, Knopman DS, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain. 2019;142(8):2483‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brickman AM, Provenzano FA, Muraskin J, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain, 2019. 142(8):2483‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boyle P, Yu L, Fleischman DA, et al. White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann Clin Transl Neurol. 2016;3(10):791‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee S, Viqar F, Zimmerman ME, et al. White matter hyperintensities are a core feature of Alzheimer's disease: evidence from the dominantly inherited Alzheimer network. Ann Neurol. 2016;79(6):929‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng JJ, Delbaere K, Close JC, Sachdev PS, Lord SR. Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke. 2011;42(7):2086‐2090. [DOI] [PubMed] [Google Scholar]
- 17. Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74(1):94‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosano C, Brach J, Studenski S, Longstreth WT Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high‐functioning older adults. Neuroepidemiology. 2007;29(3‐4):193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosano C, Sigurdsson S, Siggeirsdottir K, et al. Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging. 2010;31(7):1197‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolandzadeh N, Liu‐Ambrose T, Aizenstein H, et al. Pathways linking regional hyperintensities in the brain and slower gait. Neuroimage. 2014;99:7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tian Q, Resnick SM, Studenski SA, Ferrucci L. Lap time variability from a 400‐m walk is associated with future mild cognitive impairment and Alzheimer's disease. J Am Med Dir Assoc. 2019;20(12):1535‐1539.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian Q, An Y, Resnick SM, Studenski S. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: results from the Baltimore Longitudinal Study of Aging. Age Ageing. 2017;46(3):445‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quan M, Xun P, Chen C, et al. Walking pace and the risk of cognitive decline and dementia in elderly populations: a meta‐analysis of prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2017;72(2):266‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schrack JA, Simonsick EM, Chaves PH, Ferrucci L. The role of energetic cost in the age‐related slowing of gait speed. J Am Geriatr Soc. 2012;60(10):1811‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schrack JA, Zipunnikov V, Simonsick EM, Studenski S, Ferrucci L. Rising energetic cost of walking predicts gait speed decline with aging. J Gerontol A Biol Sci Med Sci. 2016;71(7):947‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(Suppl 2):S329‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dougherty RJ, Ramachandran J, Liu F, et al. Association of walking energetics with amyloid beta status: findings from the Baltimore Longitudinal Study of Aging. Alzheimer's Dement. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dougherty RJ, Liu F, An Y, et al. Energetic cost of walking and brain atrophy in mid‐to‐late life. J Gerontol A Biol Sci Med Sci. 2022; 77(10):2068‐2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiao Y, Wanigatunga AA, An Y,et al. Longitudinal associations between energy utilization and brain volumes in cognitively normal middle aged and older adults. Sci Rep. 2022;12(1):6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuo PL, An Y, Gross AL, et al. Association between walking energy utilisation and longitudinal cognitive performance in older adults. Age Ageing. 2022(12):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dougherty RJ, Schultz SA, Boots EA, et al. Relationships between cardiorespiratory fitness, hippocampal volume, and episodic memory in a population at risk for Alzheimer's disease. Brain Behav. 2017;7(3):e00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dougherty RJ, Jonaitis EM, Gaitán JM, et al. Cardiorespiratory fitness mitigates brain atrophy and cognitive decline in adults at risk for Alzheimer's disease. Alzheimers Dement. 2021;13(1):e12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuo PL, Schrack JA, Shardell MD, et al. A roadmap to build a phenotypic metric of ageing: insights from the Baltimore Longitudinal Study of Aging. J Intern Med. 2020;287(4):373‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well‐functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54(1):127‐132. [DOI] [PubMed] [Google Scholar]
- 37. Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi‐atlas skull‐stripping. Acad Radiol. 2013;20(12):1566‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Habes M, Pomponio R, Shou H, et al. The brain chart of aging: machine‐learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans. Alzheimers Dement. 2021;17(1):89‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. BALKE B, WARE RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10(6):675‐688. [PubMed] [Google Scholar]
- 41. Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol. 2012;2(2):933‐996. [DOI] [PubMed] [Google Scholar]
- 42. McNeill Alexander R. Energetics and optimization of human walking and running: the 2000 Raymond Pearl memorial lecture. Am J Hum Biol. 2002;14(5):641‐648. [DOI] [PubMed] [Google Scholar]
- 43. Saibene F, Minetti AE. Biomechanical and physiological aspects of legged locomotion in humans. Eur J Appl Physiol. 2003;88(4‐5):297‐316. [DOI] [PubMed] [Google Scholar]
- 44. Dougherty R, Lose SR, Gaitán JM, et al. Five‐year changes in objectively measured cardiorespiratory fitness, physical activity, and sedentary time in mid‐to‐late adulthood. Appl Physiol Nutr Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674‐682. [DOI] [PubMed] [Google Scholar]
- 46. Malatesta D, Simar D, Dauvilliers Y, et al. Energy cost of walking and gait instability in healthy 65‐ and 80‐yr‐olds. J Appl Physiol (1985). 2003;95(6):2248‐2256. [DOI] [PubMed] [Google Scholar]
- 47. Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture. 1999;9(3):207‐231. [DOI] [PubMed] [Google Scholar]
- 48. Vesperman CJ, Pozorski V, Dougherty RJ, et al. Cardiorespiratory fitness attenuates age‐associated aggregation of white matter hyperintensities in an at‐risk cohort. Alzheimers Res Ther. 2018;10(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tseng BY, Gundapuneedi T, Khan MA, et al. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burzynska AZ, Chaddock‐Heyman L, Voss MW, et al. Physical activity and cardiorespiratory fitness are beneficial for white matter in low‐fit older adults. PLoS One. 2014;9(9):e107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dougherty RJ, Boots EA, Lindheimer JB, et al. Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for Alzheimer's disease. Brain Imaging Behav. 2020;14(4):1154‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gaitán JM, Boots EA, Dougherty RJ, et al. Brain Glucose metabolism, cognition, and cardiorespiratory fitness following exercise training in adults at risk for Alzheimer's disease. Brain Plast. 2019;5(1):83‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barnes JN, Pearson AG, Corkery AT, Eisenmann NA, Miller KB. Exercise, arterial stiffness, and cerebral vascular function: potential impact on brain health. J Int Neuropsychol Soc. 2021;27(8):761‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weidling I, Swerdlow RH. Mitochondrial dysfunction and stress responses in Alzheimer's disease. Biology. 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Conley KE, Esselman PC, Jubrias SA, et al. Ageing, muscle properties and maximal O(2) uptake rate in humans. J Physiol. 2000;526(Pt 1)211‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gottesman RF, Coresh J, Catellier DJ. Blood pressure and white‐matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41(1):3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duering M, Biessels GJ, Brodtmann A, et al. Neuroimaging standards for research into small vessel disease‐advances since 2013. Lancet Neurol. 2023;22(7):602‐618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


