Abstract
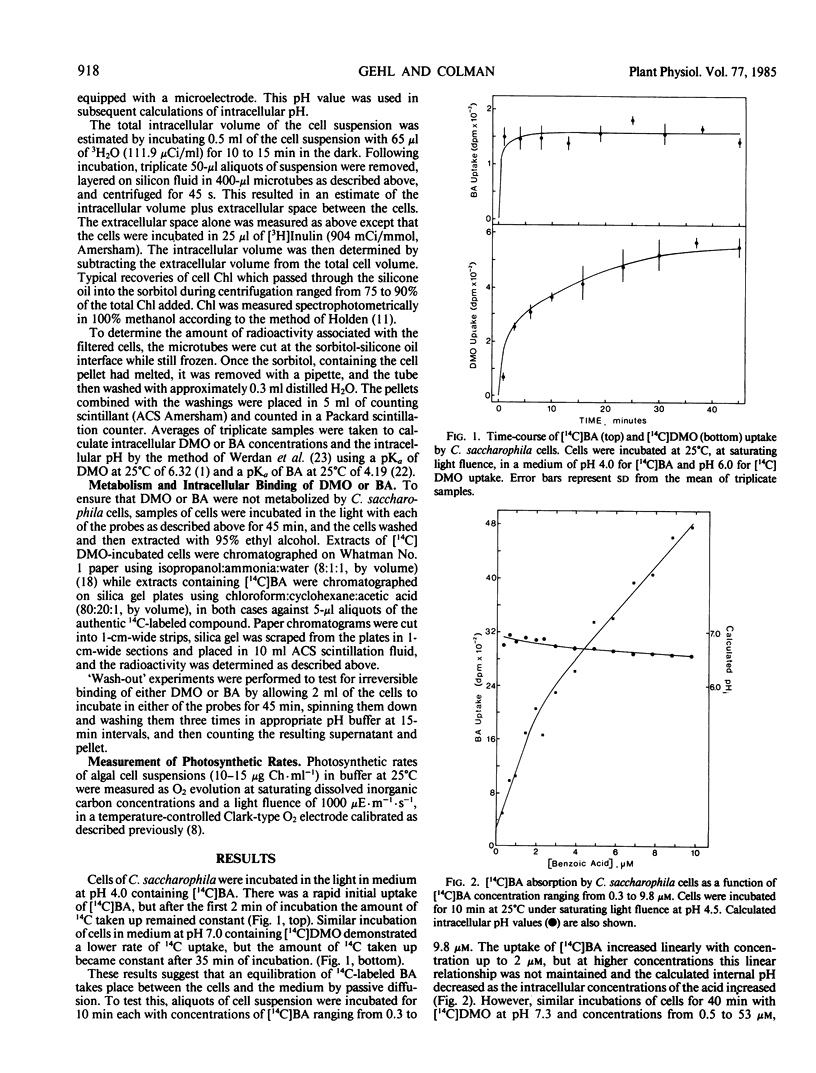
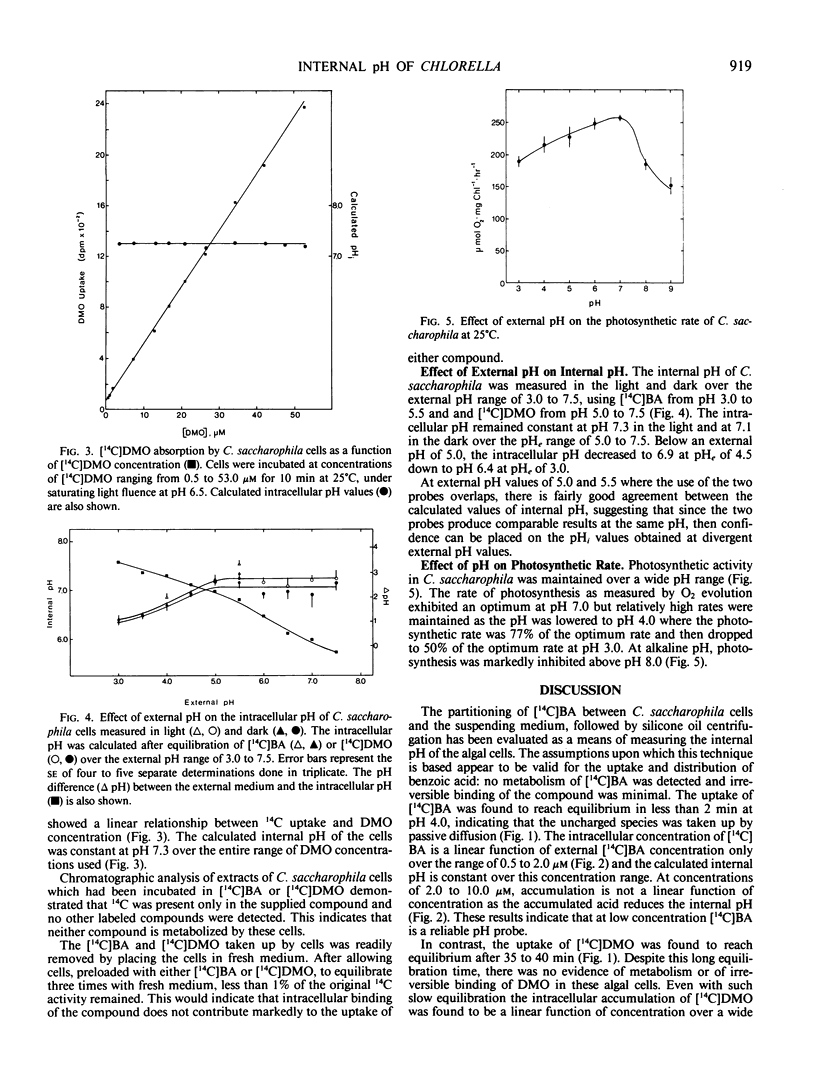
The overall internal pH of the acid-tolerant green alga, Chlorella saccharophila, was determined in the light and in the dark by the distribution of 5,5-dimethyl-2-[14C]oxazolidine-2,4-dione ([14C]DMO) or [14C]benzoic acid ([14C]BA) between the cells and the surrounding medium. [14C]DMO was used at external pH of 5.0 to 7.5 while [14C]BA was used in the range pH 3.0 to pH 5.5. Neither compound was metabolized by the algal cells and intracellular binding was minimal. The internal pH of the algae obtained with the two compounds at external pH values of 5.0 and 5.5 were in good agreement. The internal pH of C. saccharophila remained relatively constant at pH 7.3 over the external pH range of pH 5.0 to 7.5. Below pH 5.0, however, there was a gradual decrease in the internal pH to 6.4 at an external pH of 3.0. The maintenance of a constant internal pH requires energy and the downward drift of internal pH with a drop in external pH may be a mechanism to conserve energy and allow growth at acid pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- Boron W. F., Roos A. Comparison of microelectrode, DMO, and methylamine methods for measuring intracellular pH. Am J Physiol. 1976 Sep;231(3):799–809. doi: 10.1152/ajplegacy.1976.231.3.799. [DOI] [PubMed] [Google Scholar]
- Cobley J. G., Cox J. C. Energy conservation in acidophilic bacteria. Microbiol Rev. 1983 Dec;47(4):579–595. doi: 10.1128/mr.47.4.579-595.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Colman B. Inorganic Carbon Accumulation and Photosynthesis in a Blue-green Alga as a Function of External pH. Plant Physiol. 1981 May;67(5):917–921. doi: 10.1104/pp.67.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Colman B. Photosynthesis and inorganic carbon transport in isolated asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):649–654. doi: 10.1104/pp.70.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner G., Horner F. pH Changes in the Cytoplasm of the Blue-Green Alga Anacystis nidulans Caused by Light-dependent Proton Flux into the Thylakoid Space. Plant Physiol. 1976 Dec;58(6):717–718. doi: 10.1104/pp.58.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallas T., Castenholz R. W. Internal pH and ATP-ADP pools in the cyanobacterium Synechococcus sp. during exposure to growth-inhibiting low pH. J Bacteriol. 1982 Jan;149(1):229–236. doi: 10.1128/jb.149.1.229-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallas T., Castenholz R. W. Rapid transient growth at low pH in the cyanobacterium Synechococcus sp. J Bacteriol. 1982 Jan;149(1):237–246. doi: 10.1128/jb.149.1.237-246.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A. E., Burris J. E. Effects of Environmental pH on the Internal pH of Chlorella pyrenoidosa, Scenedesmus quadricauda, and Euglena mutabilis. Plant Physiol. 1981 Aug;68(2):439–442. doi: 10.1104/pp.68.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguay J. J. The 5,5-dimethyloxazolidine-2[14C],4-dione distribution technique and the measurement of intracellular pH in Acer pseudoplatanus cells. Biochim Biophys Acta. 1977 Mar 29;497(1):329–333. doi: 10.1016/0304-4165(77)90167-2. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M., Miller A. G. Measurement of the Cytoplasmic pH in Nitella translucens: Comparison of Values Obtained by Microelectrode and Weak Acid Methods. Plant Physiol. 1977 Apr;59(4):664–666. doi: 10.1104/pp.59.4.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]


