Abstract
染色质许可和DNA复制因子1(Cdt1)是复制起始许可的主要调控因子,也是组成复制前复合物的核心成员。细胞通过依赖Cdt1的波动水平,且在每个周期中通过调节其总量以确保DNA仅复制一次。Cdt1功能缺陷会造成DNA过度复制,最终导致基因组不稳定。虽然酵母中cdt1和人类Meier-Gorlin综合征(MGS)患者中的CDT1已被广泛研究,但缺乏脊椎动物模型。我们发现在硬骨鱼类分支的几个鲤形目物种(包括斑马鱼)中,Cdt1蛋白在其N末端插入一段其他脊椎动物中没有的独特无序序列。通过分析在cdt1基因中携带移码缺失的遗传性斑马鱼突变体(命名为cdt1zju1 ),我们发现突变胚胎虽然几乎无任何早期胚胎表型异常,但成年突变斑马鱼却表现出侏儒症、生存能力降低的症状,以及性腺发育不全且不育。此外,我们同样发现除转录本cdt1-201外,斑马鱼还存在第二个cdt1转录本——cdt1-202,它是通过跳过外显子2产生,这在其他生物中暂无报道。有意思的是cdt1-202在cdt1-201纯合突变体中显著上调。上述研究结果表明,cdt1-202转录本可能可以补偿cdt1-201在早期发育过程中的功能损失,但不能补偿后期生长,这可支持斑马鱼作为研究人类MGS的遗传模型。
The cell cycle consists of four distinct phases: G0/G1, S (DNA synthesis), G2, and M (mitosis). The G1 to S transition is typified by an accumulation of 4',6-diamidino-2-phenylindole (DAPI) signal that indicates the rapid DNA synthesis initiated at special sites on DNA, generally called the Ori (origin of replication) (Alfa et al., 1989). The cell division cycle 10 (Cdc10)-dependent transcript 1 (Cdt1) is bestowed the term “replication origin licensing factor” for its role in warranting replication once (and only once) per round of the eukaryotic cell cycle, during the S phase (Pozo and Cook, 2017). In a normal cell cycle, Cdt1 is present only in the G1 and S entry phases, whereas Geminin, a protein that targets Cdt1 for S-phase-dependent proteolysis, is present in the S and G2 phases. Failure of this gatekeeping task as observed in cdt1 overexpression, for example in yeast, leads to inappropriate origin firing (also termed re-replication (Vaziri et al., 2003)) and eventually DNA damage checkpoint activation (Kanellou et al., 2020).
Cdt1 is a crucial cell-cycle determinant, and was first cloned in fission yeast via an immunoprecipitation-polymerase chain reaction (PCR) strategy. The goal was to search for a novel target sequence that interacts with Cdc10, a transcription factor belonging to a conserved DNA-binding complex (Hofmann and Beach, 1994). Due to its high conservation across eukaryotic kingdoms (Pozo and Cook, 2017), cdt1 homologues of Drosophila (Whittaker et al., 2000), Xenopus (Maiorano et al., 2000), and Schizosaccharomyces pombe (Nishitani et al., 2000) were successively cloned via homology or functional approaches. By employing its cycling nature during the cell cycle, the human homologue was eventually obtained through interaction with GEMININ (Wohlschlegel et al., 2000). While most functions were elucidated via experiments in lower organisms, especially in yeasts, the importance and significance of CDT1 were subsequently confirmed and extended to cell-cycle-related human diseases; this was made possible by the completion of the human genome project and the availability of large patient datasets (de Munnik et al., 2015). Specifically, mutations in genes of the pre-replication complex, including CDT1, have been detected in approximately 67%‒78% of patients with Meier-Gorlin syndrome (MGS), a rare autosomal recessive primordial dwarfism disorder which is characterized by impaired post-natal growth, short stature, and microcephaly (de Munnik et al., 2015). Using the model organism zebrafish, researchers found that overexpression of Cdc6 mutant forms mimicked the human CDC6 (T323R) mutation found in an MGS patient, raising the exciting possibility of using zebrafish mutants as animal models of MGS to identify tissue and organ defects in detail and develop medical treatment strategies for MGS patients (Yao et al., 2017). The recent discovery of gene compensation response (GCR) in the small teleost further supports the potential contributions of zebrafish genetics to human medicine (Ma et al., 2019).
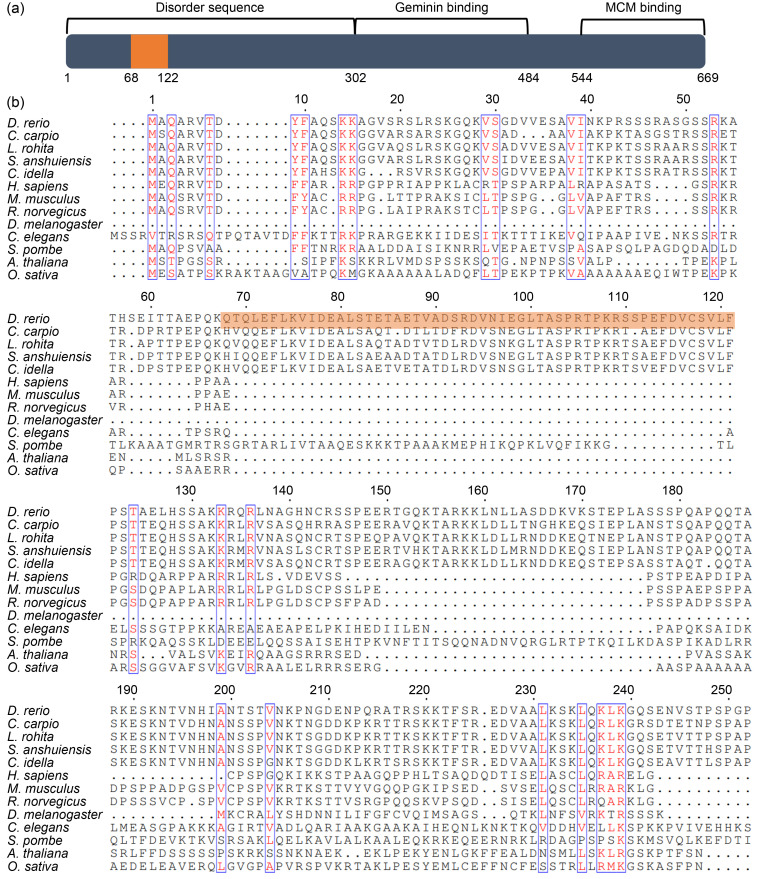
In this study, we first analyzed the full-length zebrafish Cdt1 protein sequence decoded from a cdt1 transcript (XP_695164.3) deposited in the National Center for Biotechnology Information (NCBI) database. Protein domain prediction using the Uniprot program revealed that the C-terminus contains well characterized functional domains, including the minichromosome maintenance protein (MCM)-binding domain (544‒669 aa) and Geminin-binding domain (303‒487 aa). These are responsible for preventing untimely MCM loading onto chromatin and origin licensing, respectively, during the cell cycle (Pozo and Cook, 2017); and they support the conserved role of Cdt1 in DNA replication (Fig. 1a). Interestingly, no known domain was returned for the N-terminal sequence (1‒301 aa). Instead, we primarily found disordered residues (Fig. 1a). Upon multiple sequence alignment, we found that while the full length (1‒678 aa) and C-terminal (302‒678 aa) of zebrafish Cdt1 shared high homologies, for example, with their mouse CDT1 counterparts, with approximately 45.6% and 59.6%, respectively, alignment of the N-terminal (with 1‒301 aa of zebrafish Cdt1 as the reference) yielded an identity value of only 19.4% with low significant E-value (Table 1), suggesting a unique N-terminus.
Fig. 1. A unique N-terminal found in zebrafish cell division cycle 10 (Cdc10)-dependent transcript 1 (Cdt1), specific to bony fishes. (a) Functional-domain prediction of zebrafish Cdt1 protein. The grey box represents Cdt1 protein; numbers depict amino acids. The 53 unique amino acids in the N-terminal domain are boxed in orange. (b) Multiple alignment of Cdt1 protein sequences obtained from thirteen organisms including: zebrafish (Danio rerio), four species in the Cypriniformes order (Cyprinus carpio, Labeo rohita, Sinocyclocheilus anshuiensis, and Ctenopharyngodon idella), human (Homo sapiens), mouse (Mus musculus), rat (Rattus norvegicus), fruitfly (Drosophila melanogaster), worm (Caenorhabditis elegans), fission yeast (Schizosaccharomyces pombe), thale cress (Arabidopsis thaliana), and rice (Oryza sativa), at N-termini. Red text: similarity within a group; Blue box: similarity across groups; Dots: gaps.
Table 1.
Homologies of cell division cycle 10 (Cdc10)-dependent transcript 1 (Cdt1) sequences from 52 species at full length, N-terminal, and C-terminal.
| Classification | Name | Identity of full length (%) | Identity of N-terminal (%) | Identity of C-terminal (%) |
|---|---|---|---|---|
| Mammalias | Homo sapiens | 46.5 | 20.4 | 58.8 |
| Macaca mulatta | 45.9 | 16.9 | 59.8 | |
| Mus musculus | 45.6 | 19.4 | 59.6 | |
| Sus scrofa | 46.4 | 22.1 | 60.0 | |
| Canis lupus familiaris | 43.7 | 17.9 | 60.5 | |
| Myotis lucifugus | 48.0 | 23.5 | 58.0 | |
| Microcebus murinus | 38.5 | 14.2 | 54.8 | |
| Oryctolagus cuniculus | 41.6 | 13.7 | 54.0 | |
| Ochotona princeps | 46.1 | 17.9 | 60.0 | |
| Felis catus | 46.7 | 20.6 | 60.1 | |
| Erinaceus europaeus | 44.0 | 15.6 | 62.5 | |
| Sarcophilus harrisii | 43.9 | 16.6 | 67.0 | |
| Ornithorhynchus anatinus | 47.7 | 20.5 | 60.0 | |
| Equus caballus | 47.0 | 21.4 | 59.6 | |
| Dasypus novemcinctus | 45.2 | 17.0 | 56.7 | |
| Echinops telfairi | 44.3 | 19.0 | 60.2 | |
| Loxodonta africana | 45.3 | 21.6 | 59.0 | |
| Rattus norvegicus | 45.0 | 19.6 | 61.8 | |
| Aves | Anas platyrhynchos | 47.5 | 19.4 | 62.6 |
| Gallus gallus | 45.1 | 18.3 | 61.6 | |
| Meleagris gallopavo | 49.6 | 17.2 | 59.6 | |
| Taeniopygia guttata | 42.3 | 19.7 | 68.2 | |
| Reptilia | Chrysemys picta bellii | 45.3 | 20.5 | 61.6 |
| Thamnophis elegans | 44.5 | 18.9 | 60.6 | |
| Crotalus tigris | 41.9 | 17.4 | 63.3 | |
| Anolis carolinensis | 47.1 | 19.7 | 64.0 | |
| Gavialis gangeticus | 53.3 | 24.5 | 67.8 | |
| Amphibias | Xenopus tropicalis | 48.5 | 20.1 | 68.4 |
| Xenopus laevis | 48.2 | 20.5 | 66.5 | |
| Xenopus laevis | 48.1 | 19.8 | 73.3 | |
| Osteichthyes | Lepisosteus oculatus | 54.3 | 29.5 | 65.4 |
| Oncorhynchus mykiss | 54.4 | 26.5 | 77.3 | |
| Oncorhynchus mykiss | 54.6 | 21.3 | 89.6 | |
| Cyprinus carpio | 79.4 | 72.6 | 89.6 | |
| Cyprinus carpio | 82.1 | 78.8 | 64.9 | |
| Labeo rohita | 91.9 | 71.6 | 66.5 | |
| Sinocyclocheilus anshuiensis | 90.9 | 70.3 | 65.3 | |
| Ctenopharyngodon idella | 90.9 | 70.3 | 65.5 | |
| Chondrichthyes | Rhincodon typus | 43.4 | 18.3 | 62.6 |
| Callorhinchus milii | 42.2 | 17.5 | 70.7 | |
| Cyclostomata | Petromyzon marinus | 32.6 | 15.6 | 42.8 |
| Ascidiacea | Ciona intestinalis | 30.1 | 13.6 | 45.2 |
| Leptocardii | Branchiostoma belcheri | 40.2 | 20.3 | 45.2 |
| Branchiostoma floridae | 30.2 | 16.9 | 44.1 | |
| Echinodermata | Asterias rubens | 30.2 | 13.1 | 45.5 |
| Patiria miniata | 31.3 | 14.6 | 46.5 | |
| Acanthaster planci | 31.4 | 14.2 | 42.6 | |
| Strongylocentrotus purpuratus | 29.4 | 15.8 | 44.1 | |
| Anneissia japonica | 31.0 | 15.2 | 47.3 | |
| Enteropneusta | Saccoglossus kowalevskii | 32.4 | 15.6 | 47.3 |
| Ascomycotina | Schizosaccharomyces pombe | 14.2 | 16.9 | 14.5 |
| Nematoda | Caenorhabditis elegans | 14.7 | 14.0 | 17.8 |
| Arthropoda | Drosophila melanogaster | 24.6 | 12.8 | 35.6 |
| Angiospermae | Arabidopsis thaliana | 13.6 | 17.9 | 12.2 |
| Arabidopsis thaliana | 12.6 | 24.3 | 12.1 |
To be continued
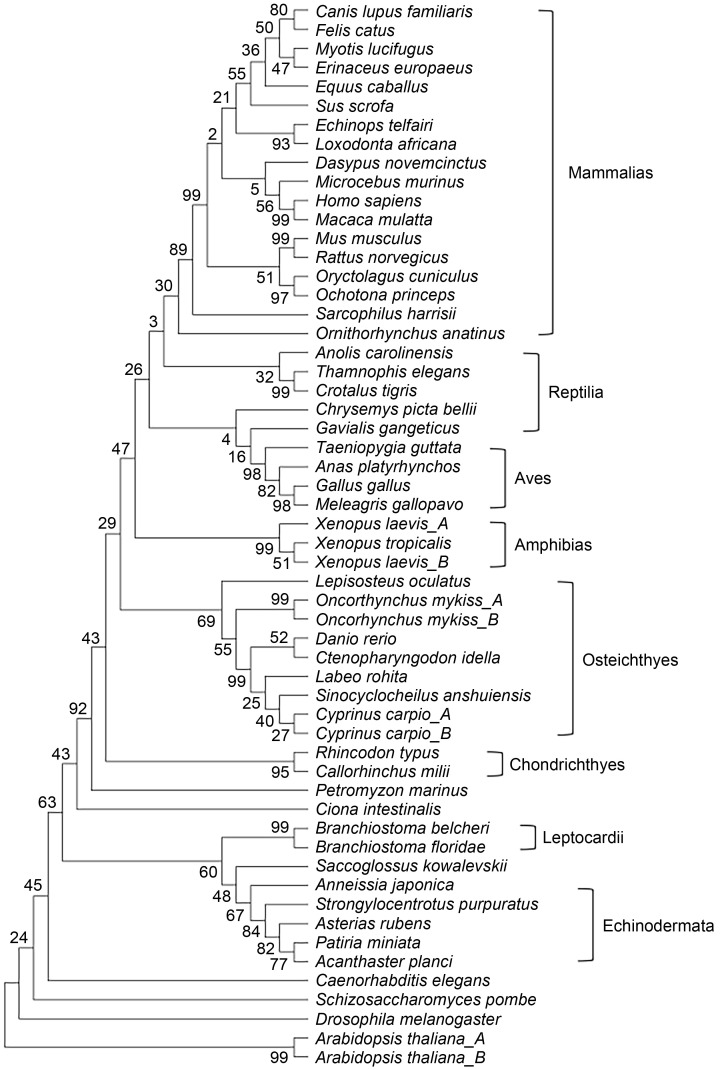
To further explore the distinctiveness of this sequence, we constructed a phylogenetic tree involving 56 sequences from 52 species, encompassing dicot, protozoan, invertebrate, and vertebrate phyla. We then used MEGA7 to explore the evolutionary distance between Cdt1/CDT1 in these species, including seven species from the bony fish clade (Fig. 2). While the full-length protein was fully consistent with the classic classification based on anatomic characteristics, phylogenetic analysis of the Cdt1/CDT1 N-termini from these 52 species failed to yield a proper evolutionary relationship, mainly due to the divergent sequences from different phyla (Table 1). Strikingly, aligning the N-termini of Cdt1/CDT1 revealed a 53-aa insertion (69‒121 aa in zebrafish Cdt1) which is only found in the four species in the Cypriniformes order. It is not found in other species such as human, mouse, rat, fly, worm, rice, Arabidopsis, or yeast (Fig. 1b), suggesting a unique origin of this sequence retained by this clade during evolution.
Fig. 2. Phylogenetic tree-derived cladogram of cell division cycle 10 (Cdc10)-dependent transcript 1 (Cdt1) at full length, constructed using 56 Cdt1 protein sequences obtained from 52 organisms (listed in Materials and methods) using MEGA7. Length of line represents evolutionary distance. Number represents bootstrap value, an indication of distance reliability (Bootstrap value of >70 means reliable distance predicted).
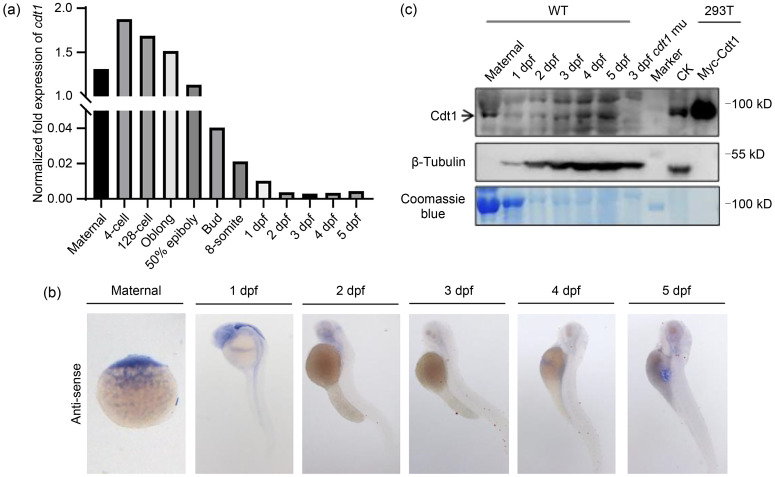
While quantitative real-time PCR (qPCR) (Fig. 3a), whole-mount in situ hybridization (WISH) (Fig. 3b), and western blot (Fig. 3c) showed that cdt1/Cdt1 at both the transcript and protein levels were maternal in nature and zygotic expression was maintained at low and ubiquitous levels, RNA sequencing (RNA-seq) data revealed that the cdt1 was particularly enriched in adult testis and ovary tissues (Fig. 4d, lower panel), suggesting important roles in zebrafish reproductive organs. In contrast, the cdt1 transcript was present at relatively low levels in other organs/tissues (transcripts per million (TPM)<2.5) (Fig. 4d, lower panel), indicating limited cell proliferation in these organs/tissues in adult fish.
Fig. 3. Expression of cdt1 in zebrafish. (a) Quantitative real-time polymerase chain reaction (qPCR) analysis of expression levels of the cell division cycle 10 (Cdc10)-dependent transcript 1 (cdt1)messenger RNA (mRNA) in maternal, 4-cell stage to 5 dpf zebrafish embryos. (b) Representative whole-mount in situ hybridization (WISH) images showing expression patterns of cdt1 mRNA in maternal and 1 to 5 dpf zebrafish embryos. (c) Western blot analysis of expression levels of Cdt1 protein in maternal and 1 to 5 dpf zebrafish embryos. Top panel: Cdt1 antibody; middle panel: β-tubulin antibody; bottom panel: Coomassie blue staining. Negative control: cdt1 mutant (mu); positive control: Myc-Cdt1 overexpression in 293T cells; CK: empty vector; dpf: days post fertilization; WT: wild type.
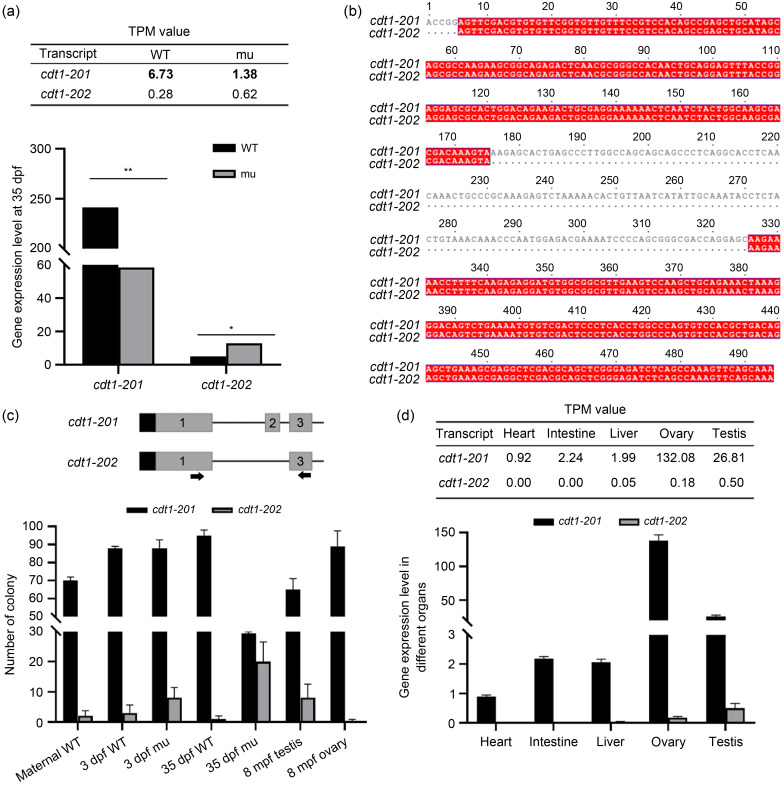
Fig. 4. Transcript cell division cycle 10 (Cdc10)-dependent transcript 1 (cdt1) -202 is barely detectable in wild type (WT) but increases in cdt1-201 mutant. (a) Expression levels of cdt1-201 and cdt1-202 transcripts in WT vs. mutant (mu) fries, obtained by RNA sequencing (RNA-seq). The transcripts per million (TPM) values derived from RNA-seq data are shown (upper panel). (b) Alignment of cdt1-201 and cdt1-202 reverse transcription-polymerase chain reaction (RT-PCR) products amplified by primers on exon 1 and exon 3. (c) Expression levels of cdt1-201 and cdt1-202 transcripts in WT vs. mu embryos or fries, WT testis, and WT ovary, obtained by RNA reverse transcription followed by single-colony PCR. Positions of primers are indicated. (d) Expression levels of cdt1-201 and cdt1-202 transcripts in WT organs, obtained by RNA-seq. The TPM values derived from RNA-seq data are shown in tables (upper panel). Data are expressed as mean±standard deviation (SD), n=3 to 10 based on specific tissue size (e.g., three fishes vs. ten hearts). * P<0.05, ** P<0.01, two-tailed t-test. dpf: days post fertilization; mpf: months post fertilization.
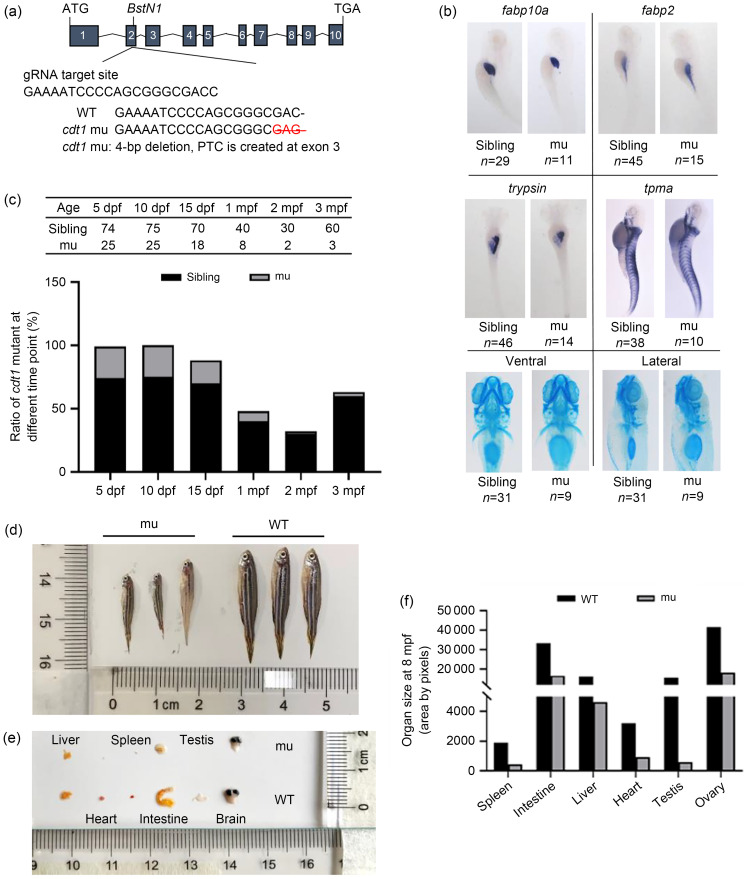
To explore the function of cdt1 in zebrafish, we designed a guide RNA (gRNA)-targeting zebrafish cdt1 exon 2. We then generated a mutant allele (cdt1zju1 / zju1 ) of 4-bp deletion in exon 2, resulting in an early stop codon in exon 3 (Fig. 5a). This gave rise to a truncated and presumably unstable protein, leading to a null mutant (Fig. 3c). The protein was undetected in 3-day post fertilization (dpf) mutant embryos. Next, we sought to investigate the developmental status of the three basic germ layers during the early embryonic stage of the cdt1zju1 / zju1 mutant, by systematically scoring them with specific markers via WISH. No noticeable differences were detected in ectoderm-derived cranioskeletons (by Alcine blue staining), mesoderm-derived muscles (by α-tropomyosin, (tpma)), or endoderm-derived liver (by fabp10a), intestine (by fabp2), or exocrine pancreas (by trypsin) (Fig. 5b). We proceeded to raise the embryos laid by both heterozygous parents and traced cdt1zju1 / zju1 mutant lethality, if any. We found that while full survival was exhibited by 25% of the population up to 15 dpf, the cdt1zju1 / zju1 mutant ratio was drastically reduced at 1 month post fertilization (mpf), with only a few surviving homozygous fish by 2 and 3 mpf (sexual maturity of zebrafish occurs around 3 mpf) (Fig. 5c). Notably, we found that the surviving cdt1zju1 / zju1 mutants experienced a growth disorder, in which their body size was less than 50% of that of the wild type (WT) (Fig. 5d). This observation reminded us of human MGS patients, who characteristically carry mutations in multiple components of the pre-replication complex that bring about extreme growth failure (Bicknell et al., 2011). Next, we sacrificed the cdt1zju1 / zju1 mutant fish and dissected some major organs, including the liver, heart, spleen, intestine, testes, and brain, for size/weight comparison (Figs. 5e and 5f). Although these major organs exhibited no obvious phenotypes during the embryonic stages, as shown by normal germ-layer development, their growth, especially that of the testis, was severely arrested in cdt1zju1 / zju1 mutant adult fish (Figs. 5e and 5f).
Fig. 5. Zebrafish cell division cycle 10 (Cdc10)-dependent transcript 1 (cdt1)-201 mutant is viable but suffers from growth retardation and infertile. (a) Schematic diagram depicting generation of the cdt1-201 mutant (mu) with guide RNA (gRNA) targeting of exon 2 of cdt1-201. (b) Expression of molecular markers from the three germ layers in 5 dpf wild type (WT) siblings (left panel) and mutant (mu) (right panel). Fraction represents number of observed phenotypes/total embryos scored. (c) Table shows the actual numbers of viable siblings and mutants (mu) scored from 5 dpf to 3 mpf. Bar chart shows the survival scores (%) of mutant from 5 dpf to 3 mpf. (d, e) Comparison of 8 mpf WT and mutant (males) in overall body size and size of various major organs including liver, heart, spleen, intestine, testis, and brain. Scale is in cm. Sample number in each group is three. (f) The mean area of each organ is tabulated into a bar graph. Standard deviation is not calculated due to too small sample size (n=3, for each organ). dpf: days post fertilization; mpf: months post fertilization.
While impaired replication might be expected to affect growth of all tissues equally, it is noteworthy that in MGS patients, specific tissues are disproportionately reduced in size, particularly the ears and patellae (Nazarenko et al., 2022). The severity observed in the cdt1zju1 / zju1 mutant testis coincides with a high cdt1 expression level in the reproductive organs revealed in our RNA-seq data (Fig. 4d).
To determine the gene-expression profiles altered by cdt1 mutation, we extracted total RNA from both WT and cdt1zju1 / zju1 mutant fish at 35 dpf and performed an RNA-seq analysis. Analysis of RNA-seq data revealed that cdt1 transcript levels were greatly reduced in cdt1zju1 / zju1 mutant fish (Fig. 4a), suggesting that cdt1zju1 mutant messenger RNA (mRNA) is subject to degradation by the nonsense-mRNA-mediated RNA decay pathway (Wittkopp et al., 2009). Surprisingly, detailed sequence analysis revealed that some cdt1 sequence counts joined exon 1 and exon 3 directly, skipping exon 2 (Fig. 4b). Through a database search, we discovered that two zebrafish cdt1 transcripts were deposited in the Ensembl database: the first one corresponds to the authentic cdt1 transcript (designated as cdt1-201) which encodes a full length of 678-aa peptide, whereas the second one lacks exon 2 (here in cdt1-202) and encodes a 458-aa peptide (Fig. 4b). To confirm the presence of the cdt1-202 transcript, we designed a pair of genotyping primers that differentiated the two transcripts according to size. cdt1-201 gave 521 bp and cdt1-202 yielded a fragment of 371 bp (Fig. 4c, upper panel). These primers were used to amplify corresponding DNA fragments from the complementary DNA (cDNA) generated using total RNA extracted from the WT unfertilized eggs, embryos at 3 dpf, fish at 35 dpf, and adult ovary and testis at 8 mpf. We cloned the PCR products to a T-vector and prepared plasmid DNA from single colonies for sequencing analysis. The sequencing results showed that while cdt1-201 was the predominant form, transcript cdt1-202 was present at all stages examined (Fig. 4c, lower panel).
Next, we performed a detailed analysis of the RNA-seq data obtained from heart, intestine, liver, ovary, and testis tissues to specifically identify both cdt1-201 and cdt1-202 transcripts. We found that cdt1-202 was present at a very low level (TPM<0.5) in the liver, ovary, and testis but was undetectable in the heart or intestine (Fig. 4d, upper panel). These results suggest that cdt1-202 is a genuine transcript. In addition, it appeared that cdt1-202 exhibited differential expression in different organs (Fig. 4d, upper panel), which might offer one explanation for the different sensitivity of various organs affected in the cdt1zju1 / zju1 mutant. In relation to this, it is interesting to note that heterogeneous symptoms are observed in patients suffering from MGS. It is also noteworthy that cdt1-202 seems to be enriched in WT testis tissue, in comparison to WT ovary tissue (Fig. 4d, upper panel), a possible biological significance worth pursuing in the future.
Considering that the exon 2 mutation in cdt1zju1 / zju1 exists only in the coding frame of cdt1-201 and not that of cdt1-202 (Fig. 5a), we wondered if there were any alterations to cdt1-202 transcript levels. We first compared the transcript levels of cdt1-202 in the RNA-seq data obtained from WT and cdt1zju1 / zju1 fish at 35 dpf, and found an approximately 2.2-fold increase in the cdt1zju1 / zju1 mutant (Fig. 4a). We next compared the levels of cdt1-202 between WT and cdt1zju1 / zju1 at 3 and 35 dpf through single-colony DNA sequencing (Fig. 4c). We found that the frequency of cdt1-202 colonies, compared to that in the WT control, increased obviously in cdt1zju1 / zju1 at 3 dpf (4 folds) and dramatically at 35 dpf (20 folds) (Fig. 4c), likely due to the steep reduction of cdt1-201 transcript levels in cdt1zju1 / zju1 that altered the template ratio of cdt1-201 versus cdt1-202 (Fig. 4a). Thus, we would expect to detect Cdt1-202 on western blot if 35-dpf mutant protein samples were used instead of 3-dpf samples (Fig. 3c). This observation highlights a possible link between mutant survival through functional compensation by Cdt1-202, a hypothesis worth probing in the future. However, dosage of the upregulated cdt1-202 might not be sufficient to compensate for normal growth processes. Considering the infertility phenotype suffered by the cdt1zju1 / zju1 mutant, we are currently unable to exclude the possibility that Cdt1-202 acts as a dominant-negative regulator of testis development.
In summary, using zebrafish as a model system, our work puts forward three preliminary but interesting discoveries on cdt1, a well-studied player in the pre-replication complex. First, it is worth pursuing whether the Cypriniformes-specific N-terminal has a function or act as an “evolutionary tag.” Second, it is of great interest whether cdt1-202 transcript exists in other organisms and what its biological functions might be. Third, because the zebrafish cdt1 mutant exhibits phenotypes that mimic MGS patients, this genetic mutant may serve as a model for drug screening to treat or alleviate MGS.
Materials and methods
Detailed methods are provided in the electronic supplementary materials of this paper.
Supplementary information
Acknowledgments
We thank Jinrong PENG (Zhejiang University, Hangzhou, China) for critical suggestions to this work. We thank Mrs. Xiangfeng SHEN (Zhejiang University) for her expertise in zebrafish husbandry and Mrs. Jingwei LU (Zhejiang University) for laboratory management. This work was supported by the National Natural Science Foundation of China (Nos. 31830113 and 31771596).
Author contributions
Yinan HE, Yong WANG, Yanqing ZHU, and Li Jan LO performed the conceptualization and methodology; Yinan HE performed the investigation; Yong WANG, Yinan HE, and Li Jan LO contributed to formal analysis; Li Jan LO contributed to the resources; Yinan HE was responsible for the data curation; Yinan HE and Li Jan LO wrote the original draft; Yong WANG and Li Jan LO contributed to visualization and funding acquisition. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Yinan HE, Yong WANG, Yanqing ZHU, and Li Jan LO declare that they have no conflict of interest.
All animal experiments were approved by the Animal Care Committee of Zhejiang University, and all methods were performed in accordance with the Guidelines for the Care and Use of Animals for Research and Teaching at Zhejiang University (ETHICS CODE Permit No. ZJU20220031).
Data availability statement
The sequencing data were deposited in the NCBI Sequence Read Archive (submission ID: SUB13301727; BioProject ID: PRJNA970210).
References
- Alfa CE, Booher R, Beach D, et al. , 1989. Fission yeast cyclin: subcellular localisation and cell cycle regulation. J Cell Sci, 1989(S12): 9-19. 10.1242/jcs.1989.supplement_12.2 [DOI] [PubMed] [Google Scholar]
- Bicknell LS, Walker S, Klingseisen A, et al. , 2011. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat Genet, 43(4): 350-355. 10.1038/ng.776 [DOI] [PubMed] [Google Scholar]
- de Munnik SA, Hoefsloot EH, Roukema J, et al. , 2015. Meier-Gorlin syndrome. Orphanet J Rare Dis, 10: 114. 10.1186/s13023-015-0322-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JF, Beach D, 1994. cdt1 is an essential target of the Cdc10/Sct1 transcription factor: requirement for DNA replication and inhibition of mitosis. EMBO J, 13(2): 425-434. 10.1002/j.1460-2075.1994.tb06277.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellou A, Giakoumakis NN, Panagopoulos A, et al. , 2020. The licensing factor Cdt1 links cell cycle progression to the DNA damage response. Anticancer Res, 40(5): 2449-2456. 10.21873/anticanres.14214 [DOI] [PubMed] [Google Scholar]
- Ma ZP, Zhu PP, Shi H, et al. , 2019. PTC-bearing mRNA elicits a genetic compensation response via Upf3a and COMPASS components. Nature, 568(7751): 259-263. 10.1038/s41586-019-1057-y [DOI] [PubMed] [Google Scholar]
- Maiorano D, Moreau J, Méchali M, 2000. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis . Nature, 404(6778): 622-625. 10.1038/35007104 [DOI] [PubMed] [Google Scholar]
- Nazarenko MS, Viakhireva IV, Skoblov MY, et al. , 2022. Meier-Gorlin syndrome: clinical misdiagnosis, genetic testing and functional analysis of ORC6 mutations and the development of a prenatal test. Int J Mol Sci, 23(16): 9234. 10.3390/ijms23169234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H, Lygerou Z, Nishimoto T, et al. , 2000. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature, 404(6778): 625-628. 10.1038/35007110 [DOI] [PubMed] [Google Scholar]
- Pozo PN, Cook JG, 2017. Regulation and function of Cdt1; a key factor in cell proliferation and genome stability. Genes (Basel), 8(1): 2. 10.3390/genes8010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri C, Saxena S, Jeon Y, et al. , 2003. A p53-dependent checkpoint pathway prevents rereplication. Mol Cell, 11(4): 997-1008. 10.1016/s1097-2765(03)00099-6 [DOI] [PubMed] [Google Scholar]
- Whittaker AJ, Royzman I, Orr-Weaver TL, 2000. Drosophila Double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev, 14(14): 1765-1776. 10.1101/gad.14.14.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopp N, Huntzinger E, Weiler C, et al. , 2009. Nonsense-mediated mRNA decay effectors are essential for zebrafish embryonic development and survival. Mol Cell Biol, 29(13): 3517-3528. 10.1128/MCB.00177-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dwyer BT, Dhar SK, et al. , 2000. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science, 290(5500): 2309-2312. 10.1126/science.290.5500.2309 [DOI] [PubMed] [Google Scholar]
- Yao LK, Chen J, Wu XT, et al. , 2017. Zebrafish cdc6 hypomorphic mutation causes Meier-Gorlin syndrome-like phenotype. Hum Mol Genet, 26(21): 4168-4180. 10.1093/hmg/ddx305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data were deposited in the NCBI Sequence Read Archive (submission ID: SUB13301727; BioProject ID: PRJNA970210).