Abstract
Background
Postoperative pain remains a significant problem following paediatric surgery. Premedication with a suitable agent may improve its management. Clonidine is an alpha‐2 adrenergic agonist which has sedative, anxiolytic and analgesic properties. It may therefore be a useful premedication for reducing postoperative pain in children.
Objectives
To evaluate the evidence for the effectiveness of clonidine, when given as a premedication, in reducing postoperative pain in children less than 18 years of age. We also sought evidence of any clinically significant side effects.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 12, 2012), Ovid MEDLINE (1966 to 21 December 2012) and Ovid EMBASE (1982 to 21 December 2012), as well as reference lists of other relevant articles and online trial registers.
Selection criteria
We included all randomized (or quasi‐randomized), controlled trials comparing clonidine premedication to placebo, a higher dose of clonidine, or another agent when used for surgical or other invasive procedures in children under the age of 18 years and where pain or a surrogate (principally the need for supplementary analgesia) was reported.
Data collection and analysis
Two authors independently performed the database search, decided on the inclusion eligibility of publications, ascertained study quality and extracted data. They then resolved any differences between their results by discussion. The data were entered into RevMan 5 for analyses and presentation. Sensitivity analyses were performed, as appropriate, to exclude studies with a high risk of bias.
Main results
We identified 11 trials investigating a total of 742 children in treatment arms relevant to our study question. Risks of bias in the studies were mainly low or unclear, but two studies had aspects of their methodology that had a high risk of bias. Overall, the quality of the evidence from pooled studies was low or had unclear risk of bias. Four trials compared clonidine with a placebo or no treatment, six trials compared clonidine with midazolam, and one trial compared clonidine with fentanyl. There was substantial methodological heterogeneity between trials; the dose and route of clonidine administration varied as did the patient populations, the types of surgery and the outcomes measured. It was therefore difficult to combine the outcomes of some trials for meta‐analysis.
When clonidine was compared to placebo, pooling studies of low or unclear risk of bias, the need for additional analgesia was reduced when clonidine premedication was given orally at 4 µg/kg (risk ratio (RR) 0.24, 95% confidence interval (CI) 0.11 to 0.51). Only one small trial (15 patients per arm) compared clonidine to midazolam for the same outcome; this also found a reduction in the need for additional postoperative analgesia (RR 0.25, 95% CI 0.09 to 0.71) when clonidine premedication was given orally at 2 or 4 µg/kg compared to oral midazolam at 0.5 mg/kg. A trial comparing oral clonidine at 4 µg/kg with intravenous fentanyl at 3 µg/kg found no statistically significant difference in the need for rescue analgesia (RR 0.89, 95% CI 0.56 to 1.42). When clonidine 4 µg/kg was compared to clonidine 2 µg/kg, there was a statistically significant difference in the number of patients requiring additional analgesia, in favour of the higher dose, as reported by a single, higher‐quality trial (RR 0.38, 95% CI 0.23 to 0.65).
The effect of clonidine on pain scores was hard to interpret due to differences in study methodology, the doses and route of drug administration, and the pain scale used. However, when given at a dose of 4 µg/kg, clonidine may have reduced analgesia requirements after surgery. There were no significant side effects of clonidine that were reported such as severe hypotension, bradycardia, or excessive sedation requiring intervention. However, several studies used atropine prophylactically with the aim of preventing such adverse effects.
Authors' conclusions
There were only 11 relevant trials studying 742 children having surgery where premedication with clonidine was compared to placebo or other drug treatment. Despite heterogeneity between trials, clonidine premedication in an adequate dosage (4 µg/kg) was likely to have a beneficial effect on postoperative pain in children. Side effects were minimal, but some of the studies used atropine prophylactically with the intention of preventing bradycardia and hypotension. Further research is required to determine under what conditions clonidine premedication is most effective in providing postoperative pain relief in children.
Plain language summary
Clonidine premedication for postoperative pain in children
Review question
We reviewed the evidence about the effect of giving clonidine before anaesthesia (that is, as a premedication) on postoperative pain in children.
Background
Pain after operations remains a major problem for children undergoing surgery. Premedication is the practice of giving a drug to reduce anxiety or provide sedation, or both, prior to anaesthesia. Premedications can also be used to provide pain relief after surgery. Clonidine is sometimes used as a premedication as it is believed to have some useful effects such as pain relief, sedation, and reducing anxiety. We investigated whether clonidine premedication provides pain relief after surgery in children.
Study characteristics
The evidence is current to December 2012. We identified a total of 11 controlled studies, including a total of 742 children, where clonidine was compared to another medication or to a dummy treatment (placebo).
Key results
We found evidence that when clonidine is given at an adequate dose (4 µg/kg) it is effective in reducing the need for pain relief after surgery for children (and probably reduces the children's pain) when compared to a placebo. The evidence is less clear when clonidine is compared to the sedative drug midazolam; this is likely to relate to differences in the design of the clinical trials. The side effects of clonidine did not seem to be a significant problem at the doses used, although in some of the studies the investigators took measures to prevent such side effects by the use of other medications.
Quality of the evidence
Overall, the evidence so far is of low or unclear quality. Further research is required to confirm under what conditions clonidine premedication is most effective in children.
Summary of findings
Summary of findings for the main comparison. Clonidine compared to placebo or no treatment for postoperative analgesia in children.
| Clonidine compared to placebo or no treatment for postoperative analgesia in children | ||||||
| Patient or population: patients with postoperative pain Settings: paediatric surgery Intervention: clonidine Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | Clonidine | |||||
| Number requiring additional analgesia at any time postoperatively ‐ low dose clonidine | 867 per 1000 | 867 per 1000 (676 to 1000) | RR 1 (0.78 to 1.28) | 45 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Number requiring additional analgesia at any time postoperatively ‐ high dose clonidine | 558 per 1000 | 134 per 1000 (61 to 285) | RR 0.24 (0.11 to 0.51) | 205 (3 studies) | ⊕⊕⊕⊝ moderate3,4 | |
| Number requiring additional analgesia at any time postoperatively ‐ high dose clonidine, studies with lower risk of bias | 700 per 1000 | 168 per 1000 (63 to 483) | RR 0.24 (0.09 to 0.69) | 160 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Postoperative pain score ‐ low dose clonidine | The mean postoperative pain score ‐ low dose clonidine in the intervention groups was 0.23 standard deviations higher (0.4 lower to 0.85 higher) | 45 (1 study) | ⊕⊕⊝⊝ low1,2 | SMD 0.23 (‐0.4 to 0.85) | ||
| Postoperative pain score ‐ high dose clonidine | The mean postoperative pain score ‐ high dose clonidine in the intervention groups was 1.11 standard deviations lower (1.46 to 0.75 lower) | 145 (2 studies) | ⊕⊕⊕⊝ moderate1 | SMD ‐1.11 (‐1.46 to ‐0.75) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Missing information on methods of randomization and allocation concealment. 2 Single, small study. 3 Missing information on methods of randomization and allocation concealment; no information at all for Georgiou 1999 study.
4 GRADE Quality of evidence is weighted towards 'Moderate' by the two studies judged to be at lower risk of bias.
Summary of findings 2. Clonidine compared to midazolam for postoperative analgesia in children.
| Clonidine compared to midazolam for postoperative analgesia in children | ||||||
| Patient or population: patients with postoperative pain Settings: paediatric surgery Intervention: clonidine Comparison: midazolam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Midazolam | Clonidine | |||||
| Number requiring additional analgesia at any time postoperatively ‐ low dose clonidine | 800 per 1000 | 200 per 1000 (72 to 568) | RR 0.25 (0.09 to 0.71) | 30 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Number requiring additional analgesia at any time postoperatively ‐ high dose clonidine | 800 per 1000 | 200 per 1000 (72 to 568) | RR 0.25 (0.09 to 0.71) | 30 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Haemodynamic or respiratory changes requiring intervention | 371 per 1000 | 204 per 1000 (115 to 360) | RR 0.55 (0.31 to 0.97) | 134 (1 study) | ⊕⊕⊕⊝ moderate3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Minimal or no information on methods of randomization, allocation concealment or blinding. 2 Very low patient and event numbers. 3 Missing information on concealment of randomizations.
Summary of findings 3. Clonidine compared to fentanyl for postoperative analgesia in children.
| Clonidine compared to fentanyl for postoperative analgesia in children | ||||||
| Patient or population: patients with postoperative pain Settings: paediatric surgery Intervention: clonidine Comparison: fentanyl | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fentanyl | Clonidine | |||||
| Number requiring additional analgesia at any time postoperatively | 706 per 1000 | 628 per 1000 (395 to 1000) | RR 0.89 (0.56 to 1.42) | 36 (1 study) | ⊕⊕⊕⊝ moderate1 | |
| Number requiring opioids postoperatively | 706 per 1000 | 628 per 1000 (395 to 1000) | RR 0.89 (0.56 to 1.42) | 36 (1 study) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Single, small study.
Summary of findings 4. High dose clonidine compared to low dose clonidine for postoperative analgesia in children.
| High dose clonidine compared to low dose clonidine for postoperative analgesia in children | ||||||
| Patient or population: patients with postoperative pain Settings: paediatric surgery Intervention: high dose clonidine Comparison: low dose clonidine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low dose clonidine | High dose clonidine | |||||
| Number requiring additional analgesia required postoperatively ‐ all studies | 644 per 1000 | 316 per 1000 (142 to 715) | RR 0.49 (0.22 to 1.11) | 90 (2 studies) | ⊕⊕⊝⊝ low1,2 | |
| Number requiring additional analgesia required postoperatively ‐ moderate quality studies | 867 per 1000 | 329 per 1000 (199 to 563) | RR 0.38 (0.23 to 0.65) | 60 (1 study) | ⊕⊕⊝⊝ low3,4 | |
| Postoperative pain score | The mean postoperative pain score in the intervention groups was 1.25 standard deviations lower (1.8 to 0.69 lower) | 60 (1 study) | ⊕⊕⊝⊝ low3,4 | SMD ‐1.25 (‐1.8 to ‐0.69) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Postoperative pain in children remains a considerable problem (Mattila 2005; Stewart 2012; Tomecka 2012). It causes distress to the patient and their parents, and may increase the time spent in the post‐anaesthetic care unit (PACU) and delay discharge from hospital. In addition, pain can be difficult to assess and to distinguish from agitation, delirium and anxiety. One strategy to reduce postoperative pain is to administer a suitable premedication.
Premedication for children prior to their surgery has a number of aims. Firstly, it is used to reduce anxiety and provide sedation. Secondly, it can enhance the anaesthetic and provide pain relief after surgery. Finally, premedications are given with the aim of preventing nausea and vomiting after surgery. Various agents have been used to achieve these different but associated aims. For anxiolysis and sedation, benzodiazepines such as midazolam or diazepam have been widely used but these agents have no pain relieving activity and can cause side effects such as paradoxical reactions (Golparvar 2004) and prolonged cognitive and behavioural problems (McGraw 1998). Paracetamol is another frequently used premedication which can improve postoperative pain relief. However, it has no effect on reducing anxiety or improving the cooperation of children during induction of anaesthesia.
Clonidine has useful effects in reducing anxiety, providing sedation and enhancing the pain relieving effects of other medications. It has, therefore, been proposed as a possible alternative premedication to traditional agents such as midazolam. Possible disadvantages of clonidine include prolonged postoperative sedation, a slowing of the heart rate (bradycardia) and lowering of blood pressure (hypotension) (Nishina 1999). The use of clonidine as a premedication in children has been previously reviewed (Bergendahl 2006) and reportedly it has beneficial effects on sedation (Frank 2000; Ramesh 1997); separation anxiety from parents (Mikawa 1993); mask acceptance (Mikawa 1993); postoperative analgesia (Bergendahl 2004; Mikawa 1996; Nishina 2000; Reimer 1998); postoperative shivering (Bergendahl 2004); nausea (Handa 2001; Motsch 1997); and agitation (Bergendahl 2004; Bock 2002; Kulka 2001). In addition, a reduction in the surgical stress response has been found (Nishina 1998).
Clonidine's analgesic activity has been questioned (Wallace 2006) with some studies finding a reduction in pain score or analgesic requirements and some failing to find any such differences. Part of the problem may lie in the wide variety of types of surgery, patient demographics, and pain assessment methods used. In addition, increasing sedation due to clonidine may be misinterpreted as improved analgesia (Bergendahl 2004). Although there have been reviews of clonidine's general properties as a premedication in paediatric surgery (Bergendahl 2006; Dahmani 2010), there has been no quantitative, systematic review to specifically address whether clonidine premedication in children can provide effective postoperative analgesia.
Description of the condition
Pain following nearly every type of surgical procedure is a common problem in the general population, and it continues to be a significant issue in paediatric surgery (Mattila 2005; Stewart 2012; Tomecka 2012). Uncontrolled pain can have significant adverse implications not only in the perioperative period but also in the long term. In turn, treatment of pain, particularly with opioids, can bring its own side effects (for reviews see Collins 2010 and Duedahl 2007). The management of pain in children requires specific techniques for assessment and therapy (Macintyre 2010). Therefore, it cannot be assumed that measures found to be effective in adults are optimal for children.
Description of the intervention
Clonidine may be given orally, per rectum or parenterally prior to surgery. It may be given as the sole premedication or in conjunction with other drugs.
How the intervention might work
Clonidine has central analgesic activity; pharmacologically, it is described as an alpha‐2 adrenergic agonist with a relative activity of 220:1 at alpha‐2 and alpha‐1 adrenergic receptors, respectively (Gentili 2007). Its analgesic effect is mediated via brainstem and spinal alpha‐2 adrenergic receptors, which are associated with descending pain inhibitory pathways originating in areas such as the locus ceruleus. These pathways control neurotransmitter release from primary afferent neurons in the dorsal horn of the spinal cord (D'Mello 2008; Gentili 2007). By utilizing a mechanism distinct from that involved in opioid analgesia, clonidine may spare opioid use and reduce associated side effects such as respiratory depression. Clonidine's anxiolytic activity may also help reduce distress in children postoperatively.
Why it is important to do this review
Postoperative pain is a significant problem in paediatric surgery. Inadequate analgesia increases patient distress in the short term and may have long‐term adverse effects. In addition, it may extend the time to discharge from both the PACU and the hospital, thereby increasing health costs to the community. Clonidine represents a promising treatment for improving postoperative analgesia. In addition, other outcome measures, such as agitation, may benefit from the use of clonidine and it therefore may represent a useful alternative to agents such as benzodiazepines.
Objectives
To evaluate the evidence for the effectiveness of clonidine, when given as a premedication, in reducing postoperative pain in children less than 18 years of age. We also sought evidence of any clinically significant side effects.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion
We included all randomized and quasi‐randomized, published and unpublished, controlled clinical studies. 'Quasi‐randomized' means trials in which allocations were made on the basis of some characteristic assumed not to be associated with the outcome, for example date of birth or hospital record number, but which meant the trials could not be said to have been truly randomized.
Exclusion
We excluded observational studies.
Types of participants
We included children less than 18 years of age presenting for anaesthesia for surgical or other invasive interventions.
Types of interventions
Inclusion
We included any study where clonidine had been given as a premedication for any type of anaesthetic, regardless of the type of surgery and regardless of the stated purpose of giving clonidine as long as pain or one of its surrogates had been reported as an outcome. We therefore included studies where the primary outcome measure was, for instance, anxiolysis but where postoperative pain or analgesic use was measured as a secondary outcome.
We included studies regardless of the route of administration of clonidine, as long as it had been used systemically. For example, we did not include studies where clonidine had been added to a regional block unless the study contained arms that had systemic clonidine versus a control as their only difference. We included studies where clonidine was compared to a placebo, those where it was compared to some other drug treatment, for example midazolam or a higher dose of clonidine, and those where it was compared to no intervention at all.
Exclusion
We excluded dose‐finding studies except where one arm of the trial equated to a placebo or comparison treatment, for example normal saline or midazolam, and all other aspects of the anaesthetic were the same between arms. We excluded studies where there were significant confounding factors. Specifically, we excluded studies where more than one treatment had been changed, for example clonidine and ketamine versus midazolam alone.
We excluded studies where clonidine had been given after induction of anaesthesia.
Types of outcome measures
Primary outcomes
The number of children requiring an additional analgesia intervention in the post‐anaesthesia care unit (PACU). This means any intervention in addition to the standard therapy administered to all patients, although for the purposes of combining data it may be useful to consider separately, where applicable, different types of analgesia e.g. simple analgesia versus opioids.
The number of children requiring an additional analgesia intervention at any time postoperatively, defined as for interventions in PACU, above.
The number of children with sedation requiring intervention. This can include simple measures e.g. supplemental oxygen or manual airway support, or more invasive techniques such as re‐intubation.
We reported primary outcomes 1 and 2 as a single outcome if there were insufficient trials for meta‐analysis of these outcomes separately.
Secondary outcomes
The number of children requiring opioid analgesia postoperatively.
The number of children pain‐free in PACU.
Postoperative pain as measured by the investigators. This can include a pain score, e.g. by visual analogue scale (VAS) or verbal numerical rating score (VNRS).
The number of children with emergence delirium or agitation postoperatively, or behavioural changes post‐discharge.
Time to first analgesic medication postoperatively, in minutes.
The number of children experiencing postoperative nausea and vomiting.
The number of children experiencing postoperative shivering.
The number of children with haemodynamic or respiratory changes requiring intervention. This can include simple measures (postural adjustment or supplemental oxygen) or medications e.g. atropine administration for bradycardia.
The number of children admitted to a high dependency unit (HDU) or intensive care unit (ICU).
The number of children with delayed discharge from PACU, according to criteria as defined by the authors.
Time to discharge from PACU.
The number of children with delayed discharge from hospital, according to criteria as defined by the authors.
Time to discharge from hospital.
We chose the primary outcomes because they were clearly defined, likely to apply in most studies, and clinically important. In view of clonidine's sedative activity, we judged the need for intervention for excessive sedation to be an easily decided and relevant negative outcome. We chose the secondary outcomes because while clinically relevant they were less likely to be based on universally consistent or agreed measures; some, like opioid dose or rate of opioid use, would be particularly sensitive to slight variations in the study protocol and therefore more difficult to subject to meta‐analysis.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 12, 2012), Ovid MEDLINE (1966 to 21 December 2012) and Ovid EMBASE (1982 to 21 December 2012). We did not apply language or publication restrictions.
We searched MEDLINE using the search strategy shown in Appendix 2. This includes appropriate MeSH headings and text words combined with the Cochrane highly sensitive search strategy as contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We adapted the search to other databases, as appropriate; the EMBASE strategy is shown in Appendix 3.
We searched for ongoing clinical trials and unpublished studies via Internet searches on the following sites: 1. http://www.controlled‐trials.com; 2. http://www.update‐software.com; 3. http://clinicalstudyresults.org; 4. http://centrewatch.com.
Searching other resources
We also searched for trials by: 1. manual searching, for example by searching relevant conference proceedings abstracts; 2. snowballing, that is by checking of the reference lists of relevant articles; 3. contacts; if relevant, we would contact trial authors by e‐mail to identify additional studies.
Data collection and analysis
Selection of studies
We identified titles and abstracts of studies in the initial search. We then retrieved potentially relevant studies in the full‐text version to be evaluated for inclusion by two authors (NK and PL) working independently. We resolved any disagreements by discussion between the two searching investigators, with arbitration by a third investigator (AMC) where necessary.
Data extraction and management
Two authors (NK and PL) independently extracted data from the relevant studies using a standardized data collection form (Appendix 4). We resolved any disagreements by discussion as for the selection process. If additional information was required we contacted the authors of the relevant study.
Assessment of risk of bias in included studies
We evaluated the selected studies using the Cochrane Collaboration's 'risk of bias' tool, which assesses risk of bias arising from any of six domains: random sequence generation; allocation concealment; blinding of participants, personnel and assessors; incomplete outcome data; selective outcome reporting; or other bias. We assessed the studies as at 'low risk', 'high risk' or 'unclear'. Where studies with varying risk of bias were identified, our strategy was to perform a sensitivity analysis to estimate the effect of including studies of high or unclear risk in the meta‐analysis.
We have included a 'Risk of bias' table as part of the table 'Characteristics of included studies' and a 'Risk of bias summary' figure, which details all of the judgements made for all included studies in the review.
Measures of treatment effect
We have presented the results from dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs); those from numerical data are presented as the standardized mean difference (SMD). We performed an initial overall meta‐analysis across all included studies for the primary outcome measures. It had been our intention to then perform, as applicable, meta‐analyses on subgroups of papers that had used similar outcome reporting strategies, for example all those reporting the number of children requiring supplementary analgesia in the first 24 hours. However, no such outcome subgroups were identified. Where it was not possible to combine data across studies, these data are presented in table format.
Unit of analysis issues
For studies reporting multiple or different time points, we have taken a representative time point considered to be comparable to the measured times used in the studies.
Dealing with missing data
Where it was evident that not all data had been presented in the text of a study, we contacted the relevant author(s) to obtain a complete data set. Where losses to follow‐up were > 15%, the study was considered to be at high risk of bias.
Assessment of heterogeneity
We applied the I2 statistic (Higgins 2002) to test for inconsistency among the results of studies, as an indication of the possible effects of heterogeneity. Where substantial inconsistency (I2 > 40%) was found, we investigated the reasons for this.
Assessment of reporting biases
We had intended, where feasible, to use a funnel plot or similar analytical methods to detect reporting bias. However, there were too few studies for this.
Data synthesis
We used the Cochrane Collaboration's software, Review Manager (RevMan 5.2), for quantitative analysis. The method of meta‐analysis depended on the nature of the outcomes. If no substantial inconsistency was identified between trial results (I2 < 40%), we pooled the results using a fixed‐effect model; where there were signs of a substantial effect of heterogeneity (I2 > 40%) we used either a random‐effects model or, if inconsistency was considerable (I2 > 75%), avoided pooling altogether. Where pooling was done, we calculated dichotomous data using relative risk (RR) and 95% CI. We performed, where appropriate, pooled outcome measures for continuous data only where the same scale was used. We calculated standardized mean difference (SMD) and 95% CI providing the pooled measure was at a similar time point. Where pooled analyses were not possible, we reported the trial results of the individual studies separately. We have presented other data as reported in the original trials.
Subgroup analysis and investigation of heterogeneity
We preplanned subgroup analyses to compare: different age groups; different types of surgery, emergency or elective surgery; other premedication adjuncts, especially other pre‐emptive analgesics; use of different opioids; use of other intraoperative analgesic adjuncts; and surgical versus less invasive techniques where there were sufficient numbers of studies.
The effects of differing opioid doses given in the operating theatre or in PACU were addressed by separately analysing those studies where the opioid dose was fixed between study arms and those where it was titrated to effect; in the latter case we would look to see whether the study in question found a difference in opioid dose between the clonidine and non‐clonidine arms. However, no such subgroups were identified for any of the outcomes in this review.
Where we found studies in which clonidine had been given at more than one dose, we considered the doses separately. These studies compared the different clonidine arms to a single control group; to avoid duplication of data (and therefore attribution of a falsely high number of patients to these studies) we divided such control groups equally between the clonidine arms.
The question may have arisen as to how to combine the results of studies recording the same outcome but using different criteria, for example blood pressure limits in determining the occurrence of hypotension. While such differences must be recorded, it would be reasonable to combine the results of different studies, as had been done previously, as long as the definitions are clear (Cyna 2006). However, this review returned no results requiring such interpretation.
Where inconsistency was found in the results from different studies (as might be indicated, for example, with an I2 > 40% in the meta‐analysis) we examined the characteristics of the studies concerned for methodological and clinical differences that might account for the disparate results. Subgroup analysis, where practicable and as specified in the protocol, provided an additional strategy for identifying the sources of difference between study conclusions.
Sensitivity analysis
Where there was uncertainty about the eligibility, validity or significance of study data, we undertook a sensitivity analysis to determine the significance of this to the outcome of the meta‐analysis. We did this by repeating the meta‐analysis excluding those studies found to be at higher risk of bias due to inadequate or unstated allocation, concealment, or blinding; high levels (> 15%) of exclusion; or lack of an intention‐to‐treat (ITT) analysis.
Summary of findings
We used the principles of the GRADE system (Guyatt 2008), where appropriate, to assess the quality of the body of evidence associated with the following specific outcomes.
Primary outcomes
The number of children requiring an additional analgesia intervention in the PACU postoperatively
The number of children requiring an additional analgesia intervention at any time postoperatively
The number of children with sedation requiring intervention
Secondary outcomes
The number of children requiring opioid analgesia postoperatively
Postoperative pain as measured by the investigators. This can include a pain score, e.g. by a visual analogue scale (VAS) or verbal numerical rating score (VNRS)
The number of children with emergence delirium or agitation postoperatively, or behavioural changes post‐discharge
The number of children with haemodynamic or respiratory changes requiring intervention
However, reporting the first and second primary outcomes separately proved impractical, as only one paper (Reimer 1998) distinguished between these two outcomes. In fact, in at least two of the studies (Mikawa 1996; Nishina 2000) the patients were returned directly to the ward from the operating theatre. We therefore examined as our sole positive primary outcome measure the incidence of supplementary analgesia at any time postoperatively, with no distinction between use in PACU and on the ward.
We could not include all of these outcomes in our summary of findings. In particular, no studies reported that any children needed intervention for excessive sedation as such; nor did any report on emergence delirium or agitation. Opioid requirement, as a dichotomous variable, was only reported by one study (comparing clonidine with fentanyl); and the only intervention relating to haemodynamic or respiratory changes was the application of supplementary oxygen, which was reported in one study only (comparing clonidine with midazolam).
We have constructed a 'Summary of findings' (SoF) table to present this assessment using the GRADE software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
See the tables describing Characteristics of included studies and Characteristics of excluded studies (below).
Results of the search
We searched the MEDLINE (1966 to 21 December 2012), EMBASE (1982 to 21 December 2012) and CENTRAL (The Cochrane Library 2012, Issue 12) databases as well as the reference lists of other relevant articles, and online trial registers.
Our initial search in MEDLINE returned 147 records; in EMBASE, 210 records; and in CENTRAL, 70 records. We inspected the titles and abstracts and eliminated those that we considered irrelevant as well as duplicates; this left a list of 36 unique entries, 34 of which were retrieved as the full publication (two were available only as conference abstracts). We subjected the 36 to further scrutiny, including reading of the manuscript and other background checking, and eliminated 25 (listed as excluded studies). One other abstract was found by cross‐referencing; this, however, was considered not relevant and was added to the excluded studies list. No further studies were found by checking of the references sections. Therefore, we included 11 studies investigating a total of 742 children in relevant treatment arms (Figure 1); and there were 27 Excluded studies.
1.
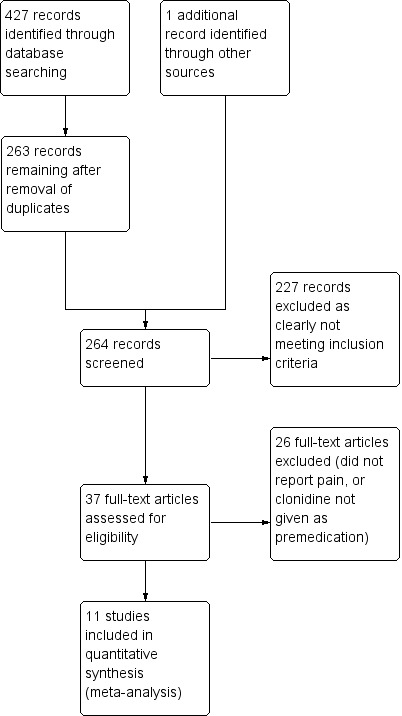
Study flow diagram.
Included studies
The included studies displayed some methodological heterogeneity. We grouped the included studies according to the comparisons made.
1. Clonidine versus placebo or no treatment
Four studies (Georgiou 1999; Hackmann 2003; Mikawa 1996; Nishina 2000) made this comparison. In the case of the last study, clonidine was given in conjunction with one of the non‐steroidal anti‐inflammatory drugs (NSAIDs), ibuprofen or flurbiprofen, but since there were arms of this study that were treated with only the relevant NSAID and a placebo instead of clonidine we considered the study was controlled in this respect.
2. Clonidine versus midazolam
Six studies (Bergendahl 2004; Cao 2009; Fazi 2001; Kuvaki 1998; Qteshat 2011; Schmidt 2007) belonged in this group. The study by Schmidt et al also contained an arm comparing the alpha‐2 agonist dexmedetomidine with the other two treatments, but this was not included in the analysis.
3. Clonidine versus fentanyl
Only one study (Reimer 1998) compared clonidine given as a premedication with intravenous fentanyl given post‐induction.
4. High dose versus low dose clonidine
This group was composed of two of the studies already listed: one of the clonidine versus placebo studies (Mikawa 1996) and one of the clonidine versus midazolam studies (Cao 2009). Both of these studies compared two different doses of clonidine, 2 µg/kg and 4 µg/kg. We included these as a separate comparison because they addressed a clinically important issue, and one that is a source of heterogeneity when the results are considered overall. Both of these studies reported the effects of clonidine on the need for additional analgesia postoperatively as well as the incidence of hypotension and bradycardia.
There were a number of sources of study heterogeneity. Firstly, there were differences between the studies in the doses of study drugs used. Details of these are given in the Characteristics of included studies table. Eight of the studies had treatment arms with clonidine 4 µg/kg orally (Cao 2009; Fazi 2001 (to a maximum of 300 μg), Georgiou 1999; Mikawa 1996; Nishina 2000; Qteshat 2011; Reimer 1998; Schmidt 2007); two of these also tested clonidine at 2 µg/kg orally (Cao 2009; Mikawa 1996). One study (Hackmann 2003) used oral clonidine at 5 µg/kg, with an additional dose having been given the night before surgery. Two studies used rectal administration of clonidine; one (Bergendahl 2004) used it at 5 µg/kg while the other (Kuvaki 1998) gave 2.5 µg/kg. In our analysis we found it useful to group together those studies using 4 to 5 µg/kg (by any route) as 'high dose clonidine' (Cao 2009; Fazi 2001 (to a maximum of 300 µg); Georgiou 1999; Mikawa 1996; Nishina 2000; Qteshat 2011; Reimer 1998; Schmidt 2007) and those using 2 to 2.5 µg/kg as 'low dose clonidine' (Cao 2009; Kuvaki 1998; Mikawa 1996). Note that these groups overlap where a study compared both high and low doses of clonidine. Midazolam was given orally at 0.5 mg/kg in four studies (Cao 2009; Fazi 2001 (to a maximum of 15 mg); Qteshat 2011; Schmidt 2007), and rectally at 0.3 mg/kg in one (Bergendahl 2004) and at 0.5 mg/kg in another (Kuvaki 1998). We did not subdivide the studies based on midazolam dose as firstly, there was less proportional variation in the dose; and secondly, it made comparisons of clonidine doses more difficult. There was also heterogeneity in the study populations. Four studies examined the use of clonidine in tonsillectomy or adenotonsillectomy (Bergendahl 2004; Fazi 2001; Qteshat 2011; Reimer 1998); two studies looked at patients having ophthalmological surgery (Mikawa 1996; Nishina 2000); one study examined patients having ventriculoperitoneal shunt insertion (Cao 2009); one involved maxillofacial surgery (Hackmann 2003); one looked at inguinal hernia repair (Kuvaki 1998); and two examined clonidine use in various minor surgical procedures. Local anaesthetic infiltration or regional blockade was used consistently in four of the studies (Georgiou 1999; Hackmann 2003; Kuvaki 1998; Reimer 1998) and for some of the patients in one study (Schmidt 2007). The agent used was bupivacaine with or without adrenaline in all except one study (Hackmann 2003) where lignocaine with adrenaline was used. Further details are given in the section Characteristics of included studies.
Excluded studies
Although it was possible to exclude most of the studies found in the original searches on the basis of the title or abstract alone, it was necessary to examine 37 more closely before making the decision to include or exclude them (Figure 1). Of the 27 studies that were excluded, most were excluded because they did not report pain as an outcome, although five (Akin 2010; Cao 2011; Freeman 2002; Lankinen 2006; Sfyra 2005) were excluded because the clonidine was given post‐induction rather than as a premedication. For further details, see Characteristics of excluded studies.
Risk of bias in included studies
All studies were reported as randomized, controlled and blinded except one (Schmidt 2007), which was described as open‐label. One was published as a conference abstract (Georgiou 1999) and details of methodology relating to randomization, concealment of allocation, blinding, and use of a placebo were not given. We were unsuccessful in our attempts to contact the authors of this study. The 'Risk of bias' tables summarize the relevant qualities of the various studies (Figure 2; Figure 3).
2.
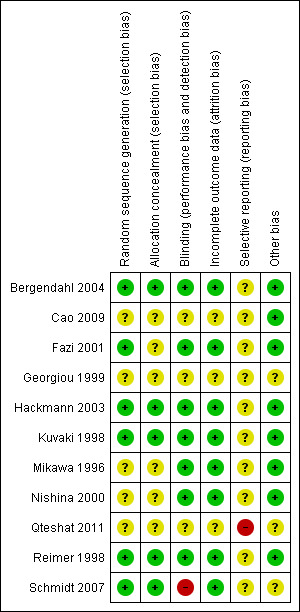
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
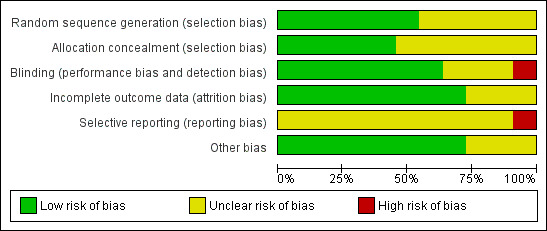
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We considered allocation by randomization to be adequate in six studies (Bergendahl 2004; Fazi 2001; Hackmann 2003; Kuvaki 1998; Reimer 1998; Schmidt 2007); five of these explicitly gave evidence of allocation concealment (or following contacting the authors) that we considered adequate (Bergendahl 2004; Hackmann 2003; Kuvaki 1998; Reimer 1998; Schmidt 2007).
Blinding
Seven of the studies reported adequate blinding for participants, anaesthesia personnel and assessors (Bergendahl 2004; Fazi 2001; Hackmann 2003; Kuvaki 1998; Mikawa 1996; Nishina 2000; Reimer 1998); in one additional study (Cao 2009) there was evidence that the assessors were blinded. One study was open‐label (Schmidt 2007). The reports by Georgiou 1999 and Qteshat 2011 contained no information related to blinding.
Incomplete outcome data
Eight of the studies explicitly accounted for loss of patient data after randomization (Bergendahl 2004; Fazi 2001; Hackmann 2003; Kuvaki 1998; Mikawa 1996; Nishina 2000; Reimer 1998; Schmidt 2007); the short‐term nature of the studies in this review meant that loss of patient data was due to exclusion for methodological reasons, for example breach of protocol, rather than losses to follow‐up. In these eight studies, exclusion rates were generally satisfactory; all were less than 15% with the exception of Hackmann 2003, where the exclusion rate was 15.2%; however, at least six out of seven of these appeared to have been excluded before randomization. This study stated that six patients were excluded because of patient refusal, language barrier, or weight greater than 80 kg, while an additional patient, who had originally agreed to participate, refused to take the study drug. Cao 2009 stated that any child who refused or spat out the study drug was excluded from the analysis. However, it was unclear whether any additional patients had been excluded before arriving at the 45 patients whose data were analysed. Information was also unavailable for this criterion for the Georgiou 1999 paper.
Selective reporting
Generally, all studies reported the outcomes stated in the respective materials and methods section, with the exception of Georgiou 1999, for which no information was available, and Qteshat 2011, which reported time to first postoperative analgesia without explicitly naming this outcome in the materials and methods section. In the Schmidt 2007 study, mention was made in the materials and methods section that non‐opioid analgesics were given for slight to moderate pain, and opioids for intense pain, but the actual use was not reported. However, analgesic use had not been stated as an outcome measure; rather, the numbers of patients with no pain or mild, moderate or severe pain by a verbal pain scale, or with no to mild pain or moderate to severe pain measured by a visual analogue scale were reported. We therefore marked this trial as 'Unclear' with respect to reporting bias.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Comparison 1: clonidine versus placebo or no treatment
Four studies (Georgiou 1999; Hackmann 2003; Mikawa 1996; Nishina 2000) made this comparison.
Primary outcome 1: analgesia intervention in the post‐anaesthesia care unit (PACU)
Combined into Outcome 2 (see 'Summary of findings' section of 'Methods', above).
Primary outcome 2: additional analgesia at any time postoperatively
Three studies addressed this outcome (Georgiou 1999; Mikawa 1996; Nishina 2000); all gave clonidine or placebo orally (see Analysis 1.1).
1.1. Analysis.
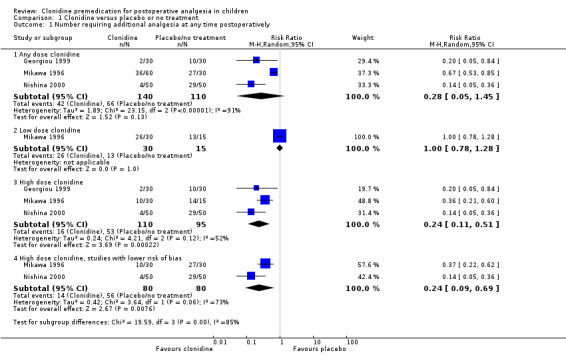
Comparison 1 Clonidine versus placebo or no treatment, Outcome 1 Number requiring additional analgesia at any time postoperatively.
Overall, no statistically significant differences were seen between the clonidine and placebo groups (RR 0.28, 95% CI 0.05 to 1.45). However, this result showed substantial heterogeneity (I2 = 91%). This heterogeneity was attenuated when we analysed the results by dosage subgroups, although it still remained high in the larger dose (4µg/kg) subgroup. The test for subgroup differences was positive, suggesting a difference in effect between the low and high dose groups (see below).
One of the studies (Mikawa 1996) tested clonidine at 2 μg/kg and 4 μg/kg orally, while the others tested it at 4 μg/kg only. There was no statistically significant difference in postoperative analgesia requirement when clonidine was given at a 2 μg/kg (low dose), but when it was given at 4 μg/kg (high dose) a difference emerged (RR 0.24, 95% CI 0.12 to 0.51; I2 = 52%) when the data were pooled across the three studies. Given that the incidence of additional analgesia use in the pooled control groups was 60.0%, this represents an absolute risk reduction of 45.6% or a number needed to treat for benefit (NNTB) of 2.2.
Given that there was very little information available on the design of the Georgiou 1999 study, we considered this to be at higher risk of bias than the other two studies, so we analysed the high dose clonidine data for the pooled Mikawa 1996 and Nishina 2000 studies alone. This restricted analysis made no difference to the risk ratio (RR), although the CI widened slightly and the I2 value increased (RR 0.24, 95% CI 0.09 to 0.69; I2 = 73%).
Primary outcome 3: excessive sedation
No studies reported on this outcome.
Secondary outcome 1: number requiring opioids postoperatively
This outcome was not addressed by any of these studies.
Secondary outcome 2: number pain‐free in PACU
Two studies (Mikawa 1996; Nishina 2000) using clonidine given orally at 2 or 4 μg/kg (Mikawa 1996) or 4 μg/kg only (Nishina 2000) recorded the number of pain‐free children (see Analysis 1.2). Note that the children were sent directly to the ward from the operating theatre and the numbers of children pain‐free in the first 12 hours were reported. A beneficial effect of clonidine was only quantifiable at the higher dose (no patient in the clonidine 2 µg/kg or control groups was pain‐free). Clonidine at 4 μg/kg (versus no clonidine) led to a pooled RR of 11.50 (95% CI 1.57 to 84.38).
1.2. Analysis.
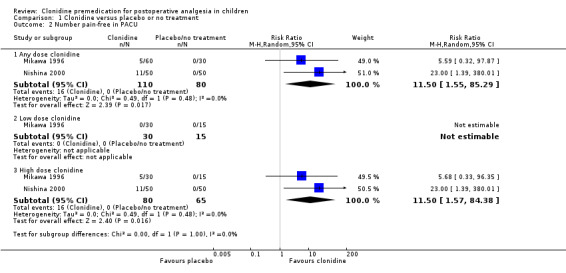
Comparison 1 Clonidine versus placebo or no treatment, Outcome 2 Number pain‐free in PACU.
Secondary outcome 3: postoperative pain score
Two studies (Mikawa 1996; Nishina 2000) addressed this outcome, using the Objective Pain Scale (OPS). In both cases, the pain scores reported were the maximum score for each child over the 12 hours following surgery, expressed as the median and range.
In the case of the Mikawa 1996 study, the peak pain scores (median and (range)) were: for placebo, 6.5 (3 to 10); for clonidine 2 µg/kg, 7 (1 to 10); and for clonidine 4 µg/kg, 4 (0 to 10) indicating a statistically significant difference (P < 0.05) for the higher dose clonidine versus placebo or 2 µg/kg, but not for 2 µg/kg versus placebo.
In the Nishina 2000 study, comparisons were made between clonidine (4 µg/kg orally) alone, diclofenac (2 mg/kg rectally), flurbiprofen (1 mg/kg intravenously), clonidine plus diclofenac, and clonidine plus flurbiprofen. The maximum OPS scores (median and (range)) were, respectively: 5 (1 to 8); 5 (1 to 8); 3 (0 to 8); 2 (0 to 6); and 2 (0 to 7). This amounted to a reduction in the pain scores for the clonidine groups compared with the diclofenac or flurbiprofen groups alone.
We used median and range to estimate the mean and standard deviation, using the method of Hozo et al (Hozo 2005), for the purposes of meta‐analysis. When the high dose arm of the Mikawa 1996 study and the clonidine arms of the Nishina 2000 study were pooled and compared to the pooled placebo data, there was a significant difference in the pain scores, in favour of clonidine: the standardized mean difference (SMD) was ‐1.11 (95% CI ‐1.46 to ‐0.75). The results appear graphically in Analysis 1.3.
1.3. Analysis.
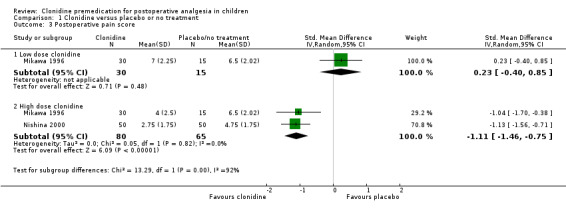
Comparison 1 Clonidine versus placebo or no treatment, Outcome 3 Postoperative pain score.
Secondary outcome 4: emergence delirium or agitation
This outcome was not addressed by any of these studies.
Secondary outcome 5: time to first postoperative analgesia dose
This outcome was not addressed by any of these studies.
Secondary outcome 6: nausea and vomiting
One study (Nishina 2000) addressed this outcome for clonidine versus placebo (on a background of analgesia with one of two NSAIDs). Pooling the two NSAID groups for clonidine (at 4 μg/kg orally) and the controls, there was a reduction in postoperative nausea and vomiting for the clonidine group (12%) versus the controls (46%). This equated to a RR of 0.26 (95% CI 0.1 to 0.59), or an absolute risk reduction of 34% (NNT = 2.9).
Secondary outcome 7: postoperative shivering
This outcome was not addressed by any of these studies.
Secondary outcome 8: haemodynamic or respiratory changes requiring intervention
One study (Mikawa 1996) addressed the issue of hypotension and bradycardia, using clonidine orally at 2 or 4 μg/kg. The authors defined hypotension as an intraoperative or postoperative change in systolic blood pressure of 20% or greater from the preoperative ward value, and bradycardia as a corresponding change in heart rate of greater than 20%. They also defined severe hypotension as a systolic blood pressure less than 70 mmHg, and severe hypotension as a heart rate less than 60 beats/min. None of the children developed these severe signs.
None of the children in the control group developed hypotension or tachycardia. With clonidine at 2 μg/kg, none of the children developed hypotension and only one child developed bradycardia. At 4 μg/kg, three children exhibited hypotension and six had bradycardia. Again, however, none of these occurrences appeared to have required intervention; the authors concluded: "...these differences in vital signs between the three groups were of little clinical significance". It is important to recognise, though, that all of the children in the study received atropine as prophylaxis against bradycardia.
Secondary outcome 9: admission to a high dependency unit (HDU) or intensive care unit (ICU)
This outcome was not reported by any of the studies.
Secondary outcome 10: delayed discharge from PACU
This was not reported by any of the studies.
Secondary outcome 11: time to discharge from PACU
Only the study by Hackmann 2003 addressed this outcome. There was a reduction in the length of stay in PACU for the clonidine group (mean 191 min, SD 43 min) compared to the placebo group (mean 228 min, SD 59 min) (P = 0.02).
Secondary outcome 12: delayed discharge from hospital
This comparison was not addressed by any of these studies.
Secondary outcome 13: time to discharge from hospital
This comparison was not addressed by any of these studies.
Comparison 2: clonidine versus midazolam
Six studies (Bergendahl 2004; Cao 2009; Fazi 2001; Kuvaki 1998; Qteshat 2011; Schmidt 2007) compared clonidine with midazolam.
Primary outcome 1: analgesia intervention in the post‐anaesthesia care unit (PACU)
Combined into Outcome 2 (see 'Summary of findings' section of 'Methods', above).
Primary outcome 2: additional analgesia at any time postoperatively
Only one study addressed this outcome for this comparison (Cao 2009). This small (15 patients per study arm) study compared midazolam 0.5 mg/kg given orally with clonidine via the same route at either 2 or 4 µg/kg. Rescue analgesia (rectal paracetamol) was given to children who complained of pain or dysphoria, or who exhibited 'frequent crying' postoperatively. The overall incidence of rescue analgesia was 20% (3/15) in each of the clonidine arms and 80% (12/15) in the midazolam group (RR 0.25, 95% CI 0.09 to 0.71), for both the 2 µg/kg and 4 µg/kg doses.
Primary outcome 3: excessive sedation
None of the studies specifically addressed this question, although no child was reported to exhibit sedation requiring an increased level of care, prolonged stay in PACU or hospital, or pharmacological intervention.
It is important to point out, however, some of the results from the study of Fazi 2001 in which tonsillectomy patients received clonidine (4 µg/kg orally to a maximum of 300 µg) or midazolam (0.5 mg/kg orally to a maximum of 15 mg). The authors' overall conclusion was to recommend midazolam over clonidine on the basis of better preoperative sedation and pain scores measured on the Children's Hospital of Eastern Ontario Pain Scale (CHEOPS) (see below). They reported a shorter time to extubation in the clonidine group (7.2 ± 4.9 min) versus midazolam (8.7 ± 3.9 min) (P < 0.05) and a reduced incidence of the requirement for supplementary oxygen in the clonidine group (20% versus 37%, P < 0.05). However, there was no evidence of a difference in the discharge times, either from PACU or from hospital (see below).
Secondary outcome 1: number requiring opioids postoperatively
This outcome was not addressed by any of the studies comparing clonidine with midazolam.
Secondary outcome 2: number pain‐free in PACU
This was addressed by two studies (Kuvaki 1998; Schmidt 2007). The former group used a rectal dose of clonidine at 2.5 µg/kg with midazolam via the same route at 0.5 mg/kg, 30 min before surgery. The latter (an open‐label study) used oral dosing with clonidine at 4 µg/kg and midazolam at 0.5 mg/kg, also 30 min before surgery. Neither group found a statistically significant difference in the number of pain‐free patients between the study arms (overall RR 1.21, 95% CI 0.61 to 2.38) (see Analysis 2.1).
2.1. Analysis.
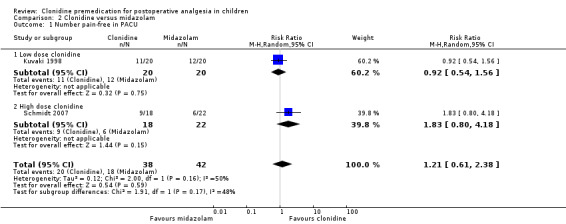
Comparison 2 Clonidine versus midazolam, Outcome 1 Number pain‐free in PACU.
Secondary outcome 3: postoperative pain score
Pain scores for the comparison of clonidine with midazolam were reported in four studies (Bergendahl 2004; Fazi 2001; Kuvaki 1998; Schmidt 2007). It was not possible to aggregate the results of these studies due to differences in drug doses and routes, the pain scores used, and the format of the reported data.
A results summary for each study is detailed below.
| Study | Clonidine dose and route | Midazolam dose and route | Pain score used | Data format | Favours |
| Bergendahl 2004 | 5 µg/kg rectally | 0.3 mg/kg rectally | OPS (see text) | 1. OPS components (versus time) (mean/SEM) 2. OPS score (versus time) (mean/SEM) 2. Sum of OPS scores in PACU |
Clonidine |
| Fazi 2001 | 4 µg/kg orally (to maximum of 300 µg) | 0.5 mg/kg orally (to maximum of 15 mg) | CHEOPS (see text) | Maximum score (median and range) | Midazolam |
| Kuvaki 1998 | 2.5 µg/kg rectally | 0.5 mg rectally | Parental rating scale | Numbers of patients achieving score | Neither |
| Schmidt 2007 | 4 µg/kg orally | 0.5 mg orally | 1. Verbal pain scale 2. Visual analogue scale |
1. Numbers of patients achieving score (VPS or VAS) 2. Mean (SD) (VAS) |
Clonidine |
In addition to their differing experimental methodologies, these studies used varying methods to report their data. For example, while Schmidt 2007 reported mean and standard deviation for 'any pain' reported by the child on the VAS or verbal pain scale (VPS) Bergendahl 2004 reported time points for components and totals of the OPS but used a scatter plot to present the sums of OPS scores across time points (from which the median and range could be derived); Fazi 2001 presented median and interquartile range for the maximum CHEOPS pain score taken at 15 minute intervals for 60 minutes. While it is possible to estimate the mean and standard deviation from the median and range for the Bergendahl 2004 study, these aggregate time point data are not directly comparable to the other studies, and in any case the same parameters cannot reliably be derived from the median and interquartile range for Fazi 2001 where the data are likely to be skewed (Higgins 2011).
In the study by Bergendahl 2004, the only statistically significant difference in the individual components of the OPS (Hannallah 1987; Hannallah 1992) was at 60 min, where significantly (P = 0.02) more patients in the clonidine group had a score of zero on the verbal evaluation, body language component than did patients in the midazolam group. This study showed a plot of OPS scores at 0, 30, 60, 90 and 120 minutes, in which the total score for clonidine was less than that for midazolam at every time point. The median of the sum of OPS scores favoured clonidine (8 versus 11.5, P = 0.012).
The study by Fazi 2001 used the CHEOPS (Beyer 1990; Tyler 1993) and found a median score for clonidine of 10 with an interquartile range (IQR) of 8 to 12; the median score for midazolam was 8, with an IQR of 4 to 12. This difference in favour of midazolam was significant at a level of P < 0.05.
In the study by Kuvaki 1998, pain was rated according to a parental scale and the number of patients with a particular score from 0 to 3 was tallied at 60, 120, 180 and 240 min. The results were presented as a simple column graph; the authors stated that there was no difference in the results between the groups.
Finally, in the Schmidt 2007 study, two different pain scales were used: a VPS and a VAS. On the VPS, proportionally more patients (6/22, 27.3%) had a pain score of zero in the clonidine group than in the midazolam group (9/18, 50%); the authors reported this to be significant at P = 0.05 when a Mann‐Whitney U test was used. On the VAS, a higher proportion of patients in the clonidine group (11/18, 61.1%) had a pain category of 'None‐mild' compared to the midazolam group (4/22, 18.2%), which was significant by the same test at P = 0.0021. Importantly, this study actually compared three treatment arms: the third tested dexmedetomidine given transmucosally at 1 µg/kg. The authors stated in their materials and methods section that they used the Mann‐Whitney test as a post hoc test following analysis by the Kruskal‐Wallis test; the potential problem being addressed here is the increased chance of making a type I error when more than two sets of data are compared. Corroborating the analysis of the VAS scores, the scores expressed as mean (SD) were 3.5 (4.4) for clonidine and 8.3 (7.1) for midazolam; analysis by ANOVA plus Tukey's test returned statistical significance at P = 0.046.
The varying conclusions of these studies are likely to have arisen as a result of methodological differences, as described above.
Secondary outcome 4: emergence delirium or agitation.
The only outcome related to this was examined by Bergendahl 2004, who reported a mean of the sum of confusion scores taken at five time points, rather than numbers of children experiencing confusion as specified in our protocol. Clonidine appeared to have a beneficial effect on the level of confusion in children less than 5 years old.
Secondary outcome 5: time to first postoperative analgesia dose
Two studies (Fazi 2001; Qteshat 2011) reported on this outcome: in the former study, midazolam at 0.5 mg/kg orally led to an increased time to first analgesia dosing (mean 28 min, SD 45 min) when compared to clonidine at 4 µg/kg by the same route (mean 13 min, SD 19 min) (P = 0.01). In the latter report there was no difference between the clonidine and midazolam groups (12 ± 16 min versus 12 ± 4 min, respectively). Because these studies were so widely divergent in their findings (I2 = 80%) we presented them here separately without meta‐analysis.
Secondary outcome 6: nausea and vomiting
Three studies (Bergendahl 2004; Cao 2009; Fazi 2001) addressed this outcome: none found a significant difference with clonidine, as opposed to midazolam, on this outcome. The pooled effect size for clonidine at 4 µg/kg was RR 0.67 (95% CI 0.32 to 1.40) (Analysis 2.2).
2.2. Analysis.
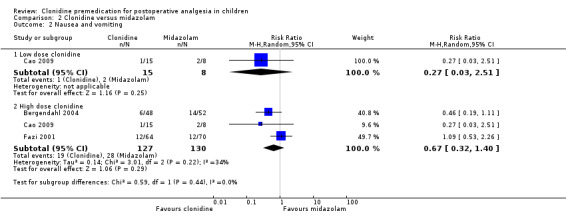
Comparison 2 Clonidine versus midazolam, Outcome 2 Nausea and vomiting.
Secondary outcome 7: postoperative shivering
Two studies (Bergendahl 2004; Cao 2009) addressed this outcome (see Analysis 2.3). The pooled results from the two studies for 4 µg/kg clonidine showed a beneficial effect of clonidine, with a RR of 0.09 (95% CI 0.01 to 0.67).
2.3. Analysis.
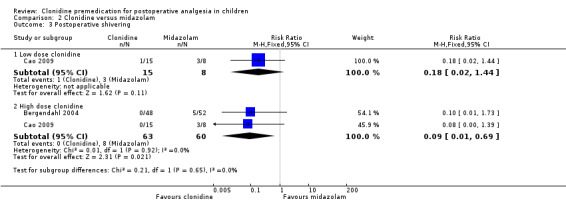
Comparison 2 Clonidine versus midazolam, Outcome 3 Postoperative shivering.
Secondary outcome 8: haemodynamic or respiratory changes requiring intervention
Only the Cao 2009 study presented any quantitative results for haemodynamic outcomes: there was no significant difference in rates of hypotension or bradycardia between the clonidine and midazolam groups. The Bergendahl 2004 and Kuvaki 1998 studies both employed atropine prophylaxis against bradycardia. No children were reported to have been in need of intervention.
Fazi 2001 showed a reduction (RR 0.55, 95% CI 0.31 to 0.97) in the need for supplemental oxygen in the clonidine arm.
Secondary outcome 9: admission to a high dependency unit (HDU) or intensive care unit (ICU)
None of these studies reported any admissions to HDU or ICU.
Secondary outcome 10: delayed discharge from PACU
None of the studies reported on this outcome.
Secondary outcome 11: time to discharge from PACU
Fazi 2001 and Schmidt 2007 found a statistically significant difference in favour of clonidine of ‐9.85 minutes (95% CI ‐19.61 to ‐0.09) in the time to discharge (see Analysis 2.4).
2.4. Analysis.
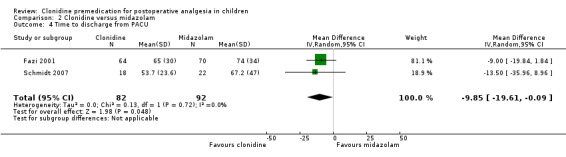
Comparison 2 Clonidine versus midazolam, Outcome 4 Time to discharge from PACU.
Secondary outcome 12: delayed discharge from hospital
None of the studies reported on this outcome.
Secondary outcome 13: time to discharge from hospital
The only study that examined this, Fazi 2001, found no significant difference between the groups: the median time for clonidine was 195 min (IQR 154 to 236 min) while for midazolam the median time was 201 min (IQR 152 to 240 min).
Comparison 3: clonidine versus fentanyl
This comparison was only addressed by the one study (Reimer 1998). The clonidine dose used was 4 µg/kg orally and that of fentanyl was 3 µg/kg intravenously, given post‐induction.
Primary outcome 1: analgesia intervention in the post‐anaesthesia care unit (PACU)
Combined into Outcome 2 (see 'Summary of findings' section of 'Methods', above).
Primary outcome 2: additional analgesia at any time postoperatively
There was no statistically significant difference seen between the clonidine and fentanyl groups in the requirement for additional analgesia (RR 0.89, 95% CI 0.56 to 1.42; n = 36).
Primary outcome 3: excessive sedation
Excessive sedation was recorded in two ways: (1) during the hospital (day) stay, using the criterion of a sedation score greater than two; and (2) by 24 hour postoperative follow‐up by a phone call to determine whether there was 'excessive sedation' according to the child's parents. A definition for excessive sedation in the latter context was not stated. The authors noted in the text of their results section that three (out of 19) in the clonidine group were sedated in hospital compared with none in the fentanyl group (n = 17). None of these children were required to stay in hospital for longer than the six hours that was mandated post‐tonsillectomy. Three of the clonidine group children and two of the fentanyl group were considered by their parents to be excessively sedated in the first 24 hours postoperatively.
Secondary outcome 1: number requiring opioids postoperatively
There was no significant difference in the number of children requiring opioids postoperatively, as judged either by the use of morphine in PACU or of codeine in the day care unit (DCU). Twelve out of 19 patients from the clonidine group and 12 out of 17 in the fentanyl group received morphine in PACU (P = 0.65); the incidence of codeine use was 6/19 (33%) for clonidine and 4/17 (24%) for fentanyl (P = 0.71).
Secondary outcome 2: number pain‐free in PACU
This outcome was not measured.
Secondary outcome 3: postoperative pain score
The authors of this study assessed pain by a VAS, every 10 min for the first 30 min following surgery and then every 15 min until a total of 2 hours had elapsed. To clarify the data presented graphically in the paper, we contacted the author of the study who kindly supplied tabulated data showing the median and range of the pain scores at each time point. At no point was there any significant difference between the treatment arms.
Secondary outcome 4: emergence delirium or agitation
This outcome was not examined by this study.
Secondary outcome 5: time to first postoperative analgesia dose
This outcome was not examined.
Secondary outcome 6: nausea and vomiting
There was no difference seen in the incidence of nausea and vomiting between the clonidine (3 out of 19) and fentanyl (5 out of 17) groups.
Secondary outcome 7: postoperative shivering
This outcome was not measured.
Secondary outcome 8: haemodynamic or respiratory changes requiring intervention
Eleven out of 19 in the clonidine group and 6 out of 17 in the fentanyl group had changes in mean arterial pressure (MAP) or heart rate (HR) of greater than 20%. This did not amount to a statistically significant difference (P = 0.20). None of the children required intervention.
Secondary outcome 9: admission to a high dependency unit (HDU) or intensive care unit (ICU)
This outcome was not reported.
Secondary outcome 10: delayed discharge from PACU
This outcome was not reported.
Secondary outcome 11: time to discharge from PACU
This outcome was not examined.
Secondary outcome 12: delayed discharge from hospital
None of the children were required to stay in hospital longer than the compulsory six hours following adenotonsillectomy.
Secondary outcome 13: time to discharge from hospital
This outcome was not reported.
Comparison 4: high dose versus low dose clonidine
Two studies made this comparison: Cao 2009 and Mikawa 1996. Both included arms using oral clonidine at 2 µg/kg or 4 µg/kg.
Primary outcome 1: analgesia intervention in the post‐anaesthesia care unit (PACU)
Combined into Outcome 2 (see 'Summary of findings' section of 'Methods', above).
Primary outcome 2: additional analgesia at any time postoperatively
The pooled effect did not show a statistically significant difference between the two doses (RR 0.49, 95% CI 0.22 to 1.11) (see Analysis 3.1).
3.1. Analysis.
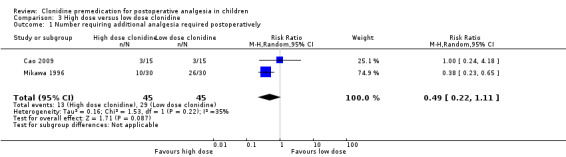
Comparison 3 High dose versus low dose clonidine, Outcome 1 Number requiring additional analgesia required postoperatively.
Primary outcome 3: excessive sedation
Neither trial reported this outcome.
Secondary outcome 1: number requiring opioids postoperatively
Neither trial reported this outcome.
Secondary outcome 2: number pain‐free in PACU
In Mikawa 1996 five out of 30 children were pain‐free postoperatively with 4 µg/kg clonidine compared with none out 30 children receiving 2 µg/kg (RR 11.00, 95% CI 0.64 to 190.53).
Secondary outcome 3: postoperative pain score
Mikawa 1996 reported this outcome and found a benefit with the higher dose, with a median pain score (range) for the high dose of 4 (0 to 10); for the low dose of 7 (1 to 10); and for placebo 6.5 (3 to 10). There were significantly lower OPS scores in children given clonidine 4 µg/kg than with the placebo (control) group (P < 0.05); and a significantly lower score in the clonidine 4 µg/kg group than in the 2 µg/kg group (P < 0.05).
Secondary outcome 4: emergence delirium or agitation
This outcome was not examined by either study.
Secondary outcome 5: time to first postoperative analgesia dose
Neither study reported this outcome.
Secondary outcome 6: nausea and vomiting
Cao 2009 recorded that one patient in each group of 15 experienced nausea and vomiting.
Secondary outcome 7: postoperative shivering
Similarly, Cao 2009 recorded no difference in the occurrence of shivering between the high and low dose groups.
Secondary outcome 8: haemodynamic or respiratory changes requiring intervention
Both studies reported no difference between the high and low dose groups for hypotension and bradycardia.
Secondary outcome 9: admission to a high dependency unit (HDU) or intensive care unit (ICU)
This outcome was not reported.
Secondary outcome 10: delayed discharge from PACU
Neither study reported this outcome.
Secondary outcome 11: time to discharge from PACU
Neither study reported this outcome.
Secondary outcome 12: delayed discharge from hospital
Neither study reported this outcome.
Secondary outcome 13: time to discharge from hospital
Neither study reported this outcome.
Discussion
This review represents the most comprehensive study to date of the effects of clonidine premedication on postoperative pain in children.
Summary of main results
This review presents the findings of four main comparisons, clonidine versus placebo or no treatment; clonidine versus midazolam; clonidine versus fentanyl; and low dose (2 µg/kg) versus high dose (4 µg/kg) clonidine.
Comparison 1: clonidine versus placebo or no treatment
Clonidine appears to have a postoperative analgesic effect when given as a premedication in adequate dose (4 µg/kg dose). This was also reflected in lower pain scores, which were lower for clonidine versus placebo only at the higher dose.
Comparison 2: clonidine versus midazolam
Our primary outcome measure of postoperative additional analgesic use was only reported in one trial (Cao 2009), which reported results in favour of clonidine at 2 or 4 µg/kg. The other primary outcome measure, excessive sedation, was not reported by any study. In Fazi 2001 there was an increased need for oxygen supplementation in PACU in the midazolam group, consistent with a shorter time to discharge from PACU in the clonidine group. The increased need for supplemental oxygen and the longer times to discharge from PACU that were found for the midazolam group in the Fazi 2001 study are also consistent with a greater sedating tendency for midazolam at the doses used.
Comparison 3: clonidine versus fentanyl
In the single study comparing clonidine and fentanyl (Reimer 1998) no statistically significant differences were found.
Comparison 4: high dose versus low dose clonidine
Taken together, Cao 2009 and Mikawa 1996 show no significant difference in the need for additional analgesia between clonidine 2 µg/kg and 4 µg/kg, although available data were limited. However, in the Cao 2009 study the numbers of participants are few and the effect indicated by the larger, better characterized Mikawa 1996 study may have been obscured possibly explaining the inconsistent finding with Comparison 1.
Overall, there is evidence to suggest that clonidine premedication may reduce the use of postoperative analgesia in children. Additionally, clonidine premedication may reduce nausea and vomiting and the time in the recovery or PACU. Clonidine premedication may also provide similar analgesia to intraoperative fentanyl for children undergoing tonsillectomy. With respect to midazolam, comparisons were difficult to interpret due to differing methodologies in the studies. In addition, differentiating analgesia from sedation was problematic as different studies use pain scales that place differing emphasis on a sedation component.
At the doses used in the studies examined here, clonidine does not appear to be associated with appreciable side effects although several studies used atropine prophylaxis against hypotension and bradycardia.
Overall completeness and applicability of evidence
There have been few published randomized controlled trials that address whether clonidine premedication in children is an effective way of providing postoperative analgesia. Overall, the evidence so far is of low to moderate quality (see below for a discussion of risk of bias). Although some trials appear to have been well conducted, the total numbers of patients are low. Also, the methodologies of the trials are heterogeneous. Nevertheless, clonidine when given at an adequate dose (4 to 5 µg/kg) appears to be an effective means of supplementing postoperative pain relief in children. The limited number of studies to date prevented us from performing subgroup analysis to define which children, having which procedure, might benefit the most. However, we have found studies where children having adenotonsillectomy, a reasonably painful procedure, did benefit from clonidine premedication. Although there appears to be a low risk of cardiovascular side effects at the dose of clonidine studied, this conclusion is made in the context of some studies using prophylactic atropine to mitigate such adverse effects.
Quality of the evidence
The overall risk of bias of the individual studies was in the range from 'low' to 'unclear'. According to the criteria used in this review for assessment of quality, the studies by Bergendahl 2004; Hackmann 2003; Kuvaki 1998; and Reimer 1998 can be described as at low risk of bias and of generally high quality. That by Schmidt 2007 appears to have been thoroughly conducted but is an open‐label study. Those by Fazi 2001; Mikawa 1996; and Nishina 2000 may also have been well conducted but there is missing information relating to blinding and allocation concealment. There is no information on randomization, blinding, allocation, or attrition for the studies by Cao 2009; Georgiou 1999; and Qteshat 2011, so the risk of bias of these studies is unclear. Although the design and execution of at least some of the trials seems to have been of a high standard, the low overall number of patients studied remains another factor contributing to the 'low' or 'unclear' rating of the overall evidence given in the GRADE analysis.
Potential biases in the review process
The potential biases are few. We acknowledge the difficulty in contacting some of the authors for supplementary information, the absence of which may have led to a downgrading of the evidence for the studies concerned.
Agreements and disagreements with other studies or reviews
We know of two other systematic reviews that numerically evaluate clonidine as a premedication (Bergendahl 2006; Dahmani 2010). Both of these compare clonidine principally with benzodiazepines but make reference to other comparisons in their texts. The first of these takes into account the studies of Bergendahl 2004; Mikawa 1996; Nishina 2000; and Reimer 1998 with findings in favour of the analgesic properties of clonidine. The latter is also in favour of analgesia with clonidine, specifically comparing to midazolam, but takes into account the studies of Bergendahl 2004; Schmidt 2007; and Tazeroualti 2007; although Fazi 2001 is included in the paper its conclusions regarding pain are not mentioned. We would disagree with the inclusion of Tazeroualti 2007 on the subject of analgesia as this study was designed to examine emergence agitation in circumcision patients and a modified form of the Objective Pain Scale (OPS) was used for this purpose. Any patient without a functioning regional block was excluded, specifically because pain would confound the results for agitation, which is a potential source of considerable bias.
In considering analgesic effects as judged from the other pain related outcomes, the Fazi 2001 study generally favoured midazolam 0.5 mg/kg over clonidine 4 µg/kg orally, based on the CHEOPS pain score and time to first analgesia. In contrast, Bergendahl 2004 favoured clonidine 5 µg/kg rectally (versus midazolam 0.3 mg/kg) based on the OPS pain scale. The differences in the design of these studies no doubt contribute greatly to the disparate conclusions. As pointed out, in Bergendahl 2004 these different findings could at least partly relate to the doses used; the Bergendahl 2004 study used rectal dosing at 0.3 mg/kg for midazolam and 5 µg/kg for clonidine, while the Fazi 2001 study used oral dosing at 0.5 mg/kg and 4 µg/kg respectively. The bioavailability of midazolam is 27% to 36% when given orally, and 16% to 18% rectally (Bergendahl 2004). On the other hand, the oral bioavailability of clonidine in children has recently been reported as 55.4%, which is lower than the previously determined value in adults of 75% to 100% (Larsson 2011). Its rectal bioavailability has been measured to be 95% (Lonnqvist 1994). The Fazi 2001 study would, therefore, have used a much higher dose of midazolam in comparison to clonidine than was used in the Bergendahl 2004 study. In addition, the authors of the latter study make the point that the CHEOPS is very sensitive to the effects of sedation. Since midazolam is strongly sedating, this effect may have contributed to the increased apparent efficacy of midazolam in the Fazi 2001 study. In fact, two of the papers in this review (Bergendahl 2004; Mikawa 1996) showed a correlation between clonidine's analgesic effect and its sedative effect. Both of these studies used the OPS to measure pain. The use of a different scale might also help account for the opposite conclusions of the Fazi 2001 and Schmidt 2007 studies, which both used the same doses and routes of administration. The absence of a detectable difference between clonidine and midazolam in the Kuvaki 1998 study, which we judged to be at low risk of bias, may relate to the pain score used but could also be partly due to the lower dose of clonidine compared to the other studies (2.5 µg/kg rectally) with a similar midazolam dose (0.5 mg/kg rectally) .
Authors' conclusions
Implications for practice.
Clonidine premedication at a dose of 4 to 5 µg/kg, as part of a multimodal analgesia strategy, is likely to be an effective way of providing postoperative pain relief in children. The available evidence indicates that, compared to placebo, it reduces the need for supplemental analgesia. Its use can also lead to lower postoperative pain scores and to a greater proportion of patients being pain‐free postoperatively.
Implications for research.
Further research relating to the analgesic efficacy of clonidine premedication in children is required with attention to minimising bias, such as the rigorous conduct and reporting of randomization, allocation concealment, blinding and analyses. One strategy might be to conduct a large study that includes a range of patient demographics, surgeries and adjuvant measures (for example regional block), with numbers sufficient to allow subgroup analyses and conclusions to be drawn about the circumstances under which clonidine might be most beneficial. Smaller studies would also be useful if clearly focused on one clinical situation, for example children from two to 12 years old having tonsillectomies. The outcomes sought for any study ought to be relevant, in common use and clearly specified. Opioid dose requirements as an outcome are difficult to interpret due to variations in the opioid used, route of delivery, and age differences in their pharmacodynamics. Dichotomous outcome measures such as a requirement for supplemental analgesia are well understood and comparable across studies. A standardised measure for assessing pain relief in children, for example the Objective Pain Scale or face scale, is required so that meta‐analyses for this outcome can be properly assessed in future.
More research is needed to assess whether clonidine premedication, versus midazolam or fentanyl, provides more effective pain relief in children postoperatively. In particular, studies should be designed in such a way that it is clear that analgesia, or pain, is being measured and differentiated from sedation or emergence agitation. This would require the careful choice and interpretation of assessment tools for analgesia or pain.
Acknowledgements
The authors would like to thank Drs T Hackmann, B Kuvaki, E Reimer, and A Schmidt for their kind assistance in providing additional details of their studies.
We would also like to thank Mark Neuman (content editor), Cathal Walsh (statistical editor), Donald R Mattison, William McIlvaine, Scott Strassels (peer reviewers) and Tracey Lloyd (Cochrane Consumer Network) for their help and editorial advice during the preparation of this protocol. We also appreciate the constructive feedback, of Dominic A Cave, Jeffrey K Lu, William McIlvaine, Vaughan Thomas and Marialena Trivella on the draft version of this systematic review.
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Anesthesia explode all trees #2 MeSH descriptor Perioperative Care explode all trees #3 an?esthe* or perioperative:ti,ab #4 (#1 OR #2 OR #3) #5 MeSH descriptor Child explode all trees #6 MeSH descriptor Pediatrics explode all trees #7 child* or p?ediatric* #8 (#5 OR #6 OR #7) #9 (#4 AND #8) #10 MeSH descriptor Pain, Postoperative explode all trees #11 MeSH descriptor Postoperative Complications explode all trees #12 MeSH descriptor Postoperative Nausea and Vomiting explode all trees #13 (postoperative near (pain or nausea or vomiting or analgesia)) or (delirium or agitation or pruritus or itch* or sedation or hypotensi* or bradycardi*) #14 (#10 OR #11 OR #12 OR #13) #15 MeSH descriptor Clonidine explode all trees #16 clonidin* #17 (#15 OR #16) #18 (#9 AND #14 AND #17)
Appendix 2. Ovid MEDLINE search strategy
1. (exp Anesthesia/ or exp Perioperative care/ or an?esthe*.mp. or perioperative.ti,ab.) and (exp child/ or child*.af. or exp Pediatrics/ or p?ediatric*.af.) 2. exp Pain, Postoperative/ or exp Postoperative Complications/ or exp "Postoperative Nausea and Vomiting"/ or (postoperative adj5 (pain or nausea or vomiting or analgesia)).mp. or (delirium or agitation or pruritus or itch* or sedation or hypotensi* or bradycardi*).mp. 3. exp Clonidine/ or clonidin*.af. 4. 1 and 2 and 3
Appendix 3. Ovid EMBASE search strategy
1. (exp anesthesia/ or exp perioperative period/ or an?esthe*.mp. or perioperative.ti,ab.) and (exp child/ or child*.af. or exp pediatrics/ or p?ediatric*.af.) 2. exp postoperative pain/ or exp postoperative complication/ or exp postoperative vomiting/ or postoperative nausea/ or (postoperative adj5 (pain or nausea or vomiting or analgesia)).mp. or (delirium or agitation or pruritus or itch* or sedation or hypotensi* or bradycardi*).mp. 3. exp clonidine/ or clonidin*.af. 4. 1 and 2 and 3 5. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 6. 4 and 5
Appendix 4. Study Selection, Quality Assessment & Data Extraction Form
Clonidine premedication for postoperative analgesia in children
Study Selection, Quality Assessment & Data Extraction Form
version3
| Reviewer |
| First author | Journal/Conference Proceedings etc | Year |
|
|
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear |
Yes / No / Unclear |
Yes / No / Unclear |
Yes / No* / Unclear |
* Iissue relates to selective reporting when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should then be excluded.
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| |
| Other comments |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year |
| The paper listed above | |||
| Further papers | |||
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers) | |
| Surgical/procedure type | |
| Trial characteristics | |
| Further details | |
| Single centre / multicentre | |
| Country / Countries | |
| How was participant eligibility defined? |
|
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose / frequency of administration | |
| Route drugs given | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in Meta‐View | |
| Trial design (e.g. parallel / cross‐over*) | |
| Other | |
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Low risk (Random) |
| High risk (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Low risk | |
| High risk | |
| Unclear | |
| Blinding | |
| Person responsible for participants care | Yes / No / Unclear |
| Participant | Yes / No / Unclear |
| Outcome assessor | Yes / No / Unclear |
| Other (please specify) | Yes / No / Unclear |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes ? No ? not clear ?
Discuss if appropriate
Selective outcome reporting
Is there any suspicion of selective reporting of previously stated outcomes?
Other sources of bias
Are there any other possible sources of bias?
Risk of Bias Summary Table
Data extraction
|
Outcomes relevant to your review | |
| Reported in paper (circle) | |
| The number of children requiring an additional analgesia intervention (over routine) in the post anaesthesia care unit (PACU). This means any intervention over standard therapy administered to all patients, although for the purposes of combining data it may be useful to consider separately, where applicable, different types of analgesia e.g. simple analgesia versus opioids. | Yes / No |
| The number of children requiring an additional analgesia intervention (over routine) at any time postoperatively, defined as for interventions in PACU, above. | Yes / No |
| The number of children with sedation requiring intervention..This can include simple measures e.g. supplemental oxygen or manual airway support, or more invasive techniques such as re‐intubation. | Yes / No |
| The number of children requiring opioid analgesia postoperatively | Yes / No |
| The number of children pain‐free in PACU. | |
| Postoperative pain as measured by the investigators. This can include a pain score, e.g. by visual analogue scale (VAS) or verbal numerical rating score (VNRS) | Yes / No |
| The number of children with emergence delirium, agitation, or behavioural changes post‐discharge | |
| Time to first analgesic medication postoperatively in minutes | Yes / No |
| The number of children experiencing postoperative nausea and vomiting | Yes / No |
| The number of children experiencing postoperative shivering | |
| The number of children with haemodynamic or respiratory changes requiring intervention. This can include simple measures (postural adjustment or supplemental oxygen) or medications e.g. atropine administration for bradycardia. | Yes / No |
| The number of children admitted to a high dependency unit (HDU) or intensive care unit (ICU). | |
| The number of children with delayed discharge from PACU | Yes / No |
| Time to discharge from PACU | Yes / No |
| The number of children with delayed discharge from hospital | Yes / No |
| Time to discharge from hospital | Yes / No |
| For Continuous data | |||||||
| Code of paper |
Outcomes |
Unit of measurement |
Clonidine group | No clonidine group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| Postoperative pain as measured by the investigators. This can include a pain score, e.g. by visual analogue scale (VAS) or verbal numerical rating score (VNRS) | |||||||
| Time to first analgesic medication postoperatively in minutes | |||||||
| Time to discharge from PACU | |||||||
| Time to discharge from hospital | |||||||
| For Dichotomous data | |||
| Code of paper | Outcomes | clonidine group (n) n = number of participants, not number of events |
No clonidine group (n) n = number of participants, not number of events |
| The number of children requiring additional analgesia intervention in the post anaesthesia care unit (PACU) postoperatively. | |||
| The number of children requiring additional analgesia intervention at any time postoperatively. | |||
| The number of children with sedation requiring intervention. | |||
| The number of children requiring opioid analgesia postoperatively | |||
| The number of children pain‐free in PACU | |||
| The number of children with delayed discharge from PACU | |||
| The number of children experiencing postoperative nausea and vomiting | |||
| The number of children experiencing postoperative shivering | |||
| The number of children with haemodynamic or respiratory changes requiring intervention | |||
| The number of children with emergence delirium, agitation, or behavioural changes post‐discharge | |||
| The number of children admitted to HDU or ICU | |||
| The number of children with delayed discharge from hospital | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
|
|
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
Data and analyses
Comparison 1. Clonidine versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number requiring additional analgesia at any time postoperatively | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Any dose clonidine | 3 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.05, 1.45] |
| 1.2 Low dose clonidine | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.78, 1.28] |
| 1.3 High dose clonidine | 3 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.11, 0.51] |
| 1.4 High dose clonidine, studies with lower risk of bias | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.09, 0.69] |
| 2 Number pain‐free in PACU | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Any dose clonidine | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 11.50 [1.55, 85.29] |
| 2.2 Low dose clonidine | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 High dose clonidine | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 11.50 [1.57, 84.38] |
| 3 Postoperative pain score | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Low dose clonidine | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.40, 0.85] |
| 3.2 High dose clonidine | 2 | 145 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.46, ‐0.75] |
Comparison 2. Clonidine versus midazolam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number pain‐free in PACU | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.61, 2.38] |
| 1.1 Low dose clonidine | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.56] |
| 1.2 High dose clonidine | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.80, 4.18] |
| 2 Nausea and vomiting | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low dose clonidine | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.03, 2.51] |
| 2.2 High dose clonidine | 3 | 257 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.32, 1.40] |
| 3 Postoperative shivering | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Low dose clonidine | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.44] |
| 3.2 High dose clonidine | 2 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.69] |
| 4 Time to discharge from PACU | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐9.85 [‐19.61, ‐0.09] |
Comparison 3. High dose versus low dose clonidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number requiring additional analgesia required postoperatively | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.22, 1.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bergendahl 2004.
| Methods | Randomized, double‐blinded, controlled clinical trial | |
| Participants | ASA I patients, age 1‐11 years, for adeno‐tonsillectomy One hundred and four (104) children enrolled; 100 participated (48 in clonidine group, 52 in midazolam group) Four patients excluded (refusal to accept rectal premedication, unexpected severe haemorrhage (>15% of calculated blood volume, requiring blood transfusion), surgery cancelled due to severe post‐intubation bronchospasm, refusal to accept mask induction) | |
| Interventions | Group C: clonidine 5 ug/kg/atropine 40 ug/kg rectally, 30‐60 min pre‐induction Group M: midazolam 300 ug/kg/atropine 40 ug/kg rectally, 30‐60 min pre‐induction | |
| Outcomes | Pain by Objective Pain Scale (Hannallah 1992) at 0, 30, 60, 90, 120 min; then during first 24 hours by parents (pain and paracetamol requirements); sedation at same time points
Shivering and vomiting reported in text as present or absent Confusion score at same time points, but summed and presented as one number |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Text states: "Both patients and investigators were blinded with respect to the randomized treatment and the study code was opened once the entire study was concluded." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Person responsible for participants care, participant and outcome assessor all blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 4 patients were excluded, each for a different reason |
| Selective reporting (reporting bias) | Unclear risk | None evident |
| Other bias | Low risk | No other obvious source of bias; control and experimental patient characteristics appear similar |
Cao 2009.
| Methods | Randomized, double‐blinded, controlled trial | |
| Participants | ASA I‐II patients, aged 2‐8 years, undergoing ventriculoperitoneal shunt insertion Forty‐five (45) children: 15 in each of two clonidine groups; 15 in midazolam group Excluded any children who refused or spat out the medication Other exclusion criteria: abnormal liver function, renal and mental disease | |
| Interventions | Group C2: clonidine 2 μg/kg, orally Group C4: clonidine 4 μg/kg, orally Group M: midazolam 0.5 mg/kg, orally All medications given in 5 ml of syrup, 60 min pre‐induction | |
| Outcomes | Main outcomes of study were: preoperative sedation, mask acceptance, separation from parents; also included postoperative analgesia, haemodynamic status and adverse effects (hypotension, bradycardia, respiratory depression, nausea/vomiting, shivering) Pain is reported as whether or not rescue analgesia was required postoperatively | |
| Notes | Rescue analgesia was given in the cases of child complaints of pain, frequent crying, or dysphoria after operation; analgesia was via a rectal loading dose of paracetamol of 30‐40 mg/kg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial is described as "Randomized" but no description of this given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear as to whether the person responsible for the patient's care, or the patient, were blinded. Outcome assessor appears to have been blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Children who spat out drug were excluded but no details on frequency of this |
| Selective reporting (reporting bias) | Unclear risk | None evident |
| Other bias | Low risk | No other obvious source of bias; control and experimental patient characteristics appear similar |
Fazi 2001.
| Methods | Randomized, double‐blinded, controlled clinical trial | |
| Participants | ASA I‐II children, 4‐12 years old, scheduled for tonsillectomy with or without adenoidectomy. One hundred and thirty‐four (134) children enrolled (64 in clonidine group; 70 in midazolam group) Trial excluded patients with: hypertension, CNS disorders, obesity (weight > 95th percentile for age), GI disorders affecting drug absorption, those with previous reactions to clonidine or benzodiazepines | |
| Interventions | Clonidine 4 ug/kg to 300 ug max, 60‐90 min pre‐induction Midazolam 0.5 mg/kg to 15 mg max, 30 min pre‐induction | |
| Outcomes | Pain assessed using Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) (Beyer 1990; Tyler 1993) on admission to the PACU and then each 15 minutes for the first hour (maximum score recorded) Morphine dose in PACU (given if CHEOPS score ≥ 8) Emesis Supplemental oxygen given in PACU Actual discharge time (PACU and hospital) Excitement score in PACU Discharge readiness time (PACU and hospital) Unanticipated hospital admission Return to baseline preoperative activity Number of parents not completely satisfied | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as "Double blind"; design of experiment, with drugs formulated to identical volumes by pharmacist, strongly implies that the anaesthetist and patient were also blinded. Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients appear to have been excluded |
| Selective reporting (reporting bias) | Unclear risk | None evident |
| Other bias | Low risk | None apparent; group demographics appear similar |
Georgiou 1999.
| Methods | Controlled trial | |
| Participants | Sixty (60) children, aged 6‐12 years, undergoing minor surgical procedures. No information in abstract as to how these were divided | |
| Interventions | Experimental group: clonidine 4 μg/kg orally, plus atropine Control group: atropine only | |
| Outcomes | Pain by Visual Analogue Scale at 1, 3 and 6 hours postoperatively Need for analgesia |
|
| Notes | Abstract does not report pain scores; only reports numbers of children needing analgesia at 1, 3 and 6 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information available |
| Selective reporting (reporting bias) | Unclear risk | No information available |
| Other bias | Unclear risk | No information available |
Hackmann 2003.
| Methods | Randomized, double‐blind, controlled trial | |
| Participants | Healthy adolescent patients scheduled for orthognathic surgery. Forty‐six (46) consecutive patients considered eligible; excluded 7 as below, leaving 19 for the clonidine group and 20 for the placebo group Exclusion criteria: significant heart disease that contraindicated the use of controlled hypotension, medically important liver or kidney dysfunction, allergy to clonidine, allergy or contraindication to the use of labetalol or β‐blocking drugs, weight heavier than 80 kg, and inability to comply with the protocol, i.e., a language barrier Excluded 6 patients (patient refusal, language barrier, or weight heavier than 80 kg). One patient who had initially consented to participate refused to take the study drug, and his data were not included in the analysis | |
| Interventions | Experimental group: clonidine 5 μg/kg orally at bedtime the night before surgery and 90 min prior to induction (rounded to nearest 50 μg) Control group: identical‐looking placebo at the same times | |
| Outcomes | Morphine or codeine given in PACU PACU length of stay Haemodynamic values before surgery and at induction Preoperative sedation Temperature Time to arrival in PACU Time to eye opening Time to movement to command Extubation | |
| Notes | Primary outcome of this study was controlled hypotension perioperatively, but also reported on analgesic requirements postoperatively; note that fentanyl was given intraoperatively to control BP (different amounts to each group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by hospital pharmacy using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Communication with Dr. Hackmann: "Only the pharmacists knew to which study group the patients were assigned." "The randomization code did not have to be broken for any of the study participants." "Only after all patients had completed the study were the assignments revealed to the authors so that statistical analysis could be performed." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Explicitly states that "the patients, investigators, surgeons, and nurses involved in the patients’ care were blinded to the nature of the assignment ". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be satisfactory; 7 out of 46 patients excluded for various reasons (distribution between groups not stated) |
| Selective reporting (reporting bias) | Unclear risk | None evident |
| Other bias | Low risk | None considered to be significant. Height in control group slightly less than that in clonidine group (159 ± 6.9 versus 166 ± 10.4, P=0.02) but no difference in weight or other demographical and surgical characteristics |
Kuvaki 1998.
| Methods | Randomized controlled trial | |
| Participants | ASA I‐II children from 2‐10 years old, undergoing inguinal hernia repair. Forty children divided into two groups of 20 No exclusion criteria given | |
| Interventions | Experimental group: clonidine 2.5 μg/kg Control group: midazolam 0.5 mg/kg Premedications given rectally 30 min before the procedure | |
| Outcomes | Pain as judged by a parental pain rating scale from 0‐3
Level of sedation Mask acceptance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes drawn at random from a box |
| Allocation concealment (selection bias) | Low risk | Contents of the envelope read by an independent doctor, who administered the treatment as read and had no other involvement in the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators, patients and parents all blinded (information from original publication and from correspondence with Dr. Kuvaki) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one patient excluded |
| Selective reporting (reporting bias) | Unclear risk | None evident |
| Other bias | Low risk | None evident; group demographics appear similar |
Mikawa 1996.
| Methods | Randomized, placebo‐controlled trial; anaesthetists, patients and observers blinded to treatment | |
| Participants | ASA I children from 5‐12 years old, undergoing minor surgery (ophthalmic, otological or urological). Ninety (90) children divided into 3 groups of 30: one group for placebo, the others for two different doses of clonidine No exclusion criteria given | |
| Interventions | Clonidine 2 μg/kg or Clonidine 4 μg/kg or Control: placebo All premedications given in apple juice, 105 min before estimated time of induction of anaesthesia. Atropine 0.03 mg/kg in apple juice given to all children 60 min before estimated time of induction | |
| Outcomes | Pain by Objective Pain Scale; presented as overall highest OPS score
Number of children requiring rescue analgesia (diclofenac suppository) in the first 12 hours after surgery
Number of children pain‐free in the first 12 hours after surgery
Sedation score Perioperative vital signs also recorded at various points throughout course of anaesthesia, surgery and recovery Time to extubation Time to eye opening Time to obeying commands Postoperative recovery score (Aldrete) on return to ward |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized" but method not given |
| Allocation concealment (selection bias) | Unclear risk | Not given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Anaesthetist and observers blinded to group assignment; premedication or placebo given to patient in apple juice so patient appears also to have been blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients appear to have been included |
| Selective reporting (reporting bias) | Unclear risk | None evident |
| Other bias | Low risk | None evident; patient demographics and types of surgery are evenly distributed among between groups |
Nishina 2000.
| Methods | Randomized, placebo‐controlled trial; anaesthetists, patients and observers blinded to treatment. Rectal or intravenous medications for different study arms were prepared and administered by an anaesthetist who had no other involvement in either in anaesthesia or data collection | |
| Participants | ASA I children from 2‐12 years old, undergoing ophthalmological surgery. One hundred and twenty five children were allocated to 5 groups of 25 No exclusion criteria given | |
| Interventions | Oral placebo followed by rectal diclofenac 2 mg/kg
Oral placebo followed by intravenous flurbiprofen 1 mg/kg
Oral clonidine 4 μg/kg alone
Oral clonidine 4 μg/kg followed by rectal diclofenac 2 mg/kg
Oral clonidine 4 μg/kg followed by intravenous flurbiprofen 1 mg/kg Clonidine given 105 min before estimated time of induction; diclofenac and flurbiprofen given immediately post‐induction |
|
| Outcomes | Pain by modified Objective Pain Scale (OPS) (blood pressure component removed to avoid confounding by clonidine's antihypertensive effect; presented as overall highest OPS score Number of children requiring rescue analgesia (diclofenac suppository) in the first 12 hours after surgery Number of children pain‐free in the first 12 hours after surgery Postoperative nausea and vomiting Time to extubation Time to eye opening Time to obeying commands Postoperative recovery score (Aldrete) on return to ward | |
| Notes | For the purposes of comparison, we have pooled the diclofenac/placebo and flurbiprofen/placebo groups together, and compared these to the pooled diclofenac/clonidine and flurbiprofen/clonidine groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States only "were randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Report states that "the individual anaesthetist ... was blinded to the group assignment ". Pain was rated by an "independent observer" and other postoperative variables were recorded by nurses and anaesthetists blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions given: all patients appear to have been included |
| Selective reporting (reporting bias) | Unclear risk | None evident |
| Other bias | Low risk | No evident other bias; demographic characteristics appear evenly distributed across groups |
Qteshat 2011.
| Methods | Mainly unclear: states only "double blind randomized study" without giving further details of design | |
| Participants | 54 children, age 6‐14, presenting for tonsillectomy; no data relating to age or gender, but states that there were no statistically significant differences between study arms. Divided into equal groups; no exclusion criteria given | |
| Interventions | Clonidine 4 μg/kg versus midazolam 0.5 mg/kg | |
| Outcomes | Time to first analgesia postoperatively, in minutes | |
| Notes | Also states that there was no difference in morphine use between groups and no clinically significant episodes of hypotension or bradycardia, but that the mean intraoperative blood pressure was lower in the clonidine group; however, no data were presented for any of these outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States only "double‐blind randomized study" without giving methods for achieving this |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | See "Random sequence generation". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data on excluded/lost data |
| Selective reporting (reporting bias) | High risk | Time to first analgesia is not explicitly stated as an outcome in the Methods section; however, the Methods section does not refer to any intended outcomes relevant to this meta‐analysis |
| Other bias | Unclear risk | States no statistically significant difference in age and gender between groups, but no details of this or other baseline characteristics given |
Reimer 1998.
| Methods | Randomized, controlled, double‐blinded study | |
| Participants | ASA I‐II children from 7‐12 years old, undergoing adenotonsillectomy. 41 enrolled but only 36 in final analysis. Removals due to breach of protocol (3); inability to cooperate with VAS due to ADD (1) and bronchospasm on extubation requiring adrenaline nebuliser (1) Exclusions: inability to understand English, contraindications to any of the medications in the study, obesity (weight > 90th percentile by nomogram), inability to use a visual analogue scale, use of any preoperative sedative, hypnotic or analgesic medications | |
| Interventions | Clonidine group: clonidine 4 μg/kg orally to maximum 200 μg, 60‐90 min preoperatively; then intravenous placebo immediately post‐induction Fentanyl group: placebo orally, 60‐90 min preoperatively; then intravenous fentanyl 3 μg/kg immediately post‐induction | |
| Outcomes | Patients receiving morphine (0.05 mg/kg) in PACU
Total morphine given
Number of morphine doses given
Number receiving codeine and/or paracetamol (acetaminophen) in day care unit
Vomiting within 24 hours
Use of analgesia post‐discharge
Excessive sedation Also measured preoperative anxiety and sedation scores, satisfaction scores, and vital signs |
|
| Notes | Local anaesthetic infiltration of tonsillar bed by surgeon prior to incision | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, done by pharmacy |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. Randomization code kept by pharmacy; also held in sealed envelopes by one investigator in case the information was needed after hours |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All parties, including anaesthetist, patient, and observer blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | None evident |
| Other bias | Low risk | None apparent; patient demographics and surgery type distributed evenly between groups |
Schmidt 2007.
| Methods | Randomized open‐label trial | |
| Participants | ASA I‐II children, 7‐12 years old, undergoing general or combined general/regional anaesthesia for various surgeries. Sixty (60) children enrolled (clonidine 18; dexmedetomidine 20; midazolam 22) Exclusions: chronic pain, cerebral palsy, autism, difficulty understanding verbal commands, preoperative use of analgesics or anticonvulsants, pre‐anaesthesia medications prior to evaluation | |
| Interventions | Experimental group (1): clonidine 4 μg/kg orally, 90 min before surgery Experimental group (2): dexmedetomidine 1 μg/kg transmucosally, 45 min before surgery Comparison group: midazolam 0.5 mg/kg orally, 30 min before surgery | |
| Outcomes | Pain by verbal pain scale (reported as categorical data: numbers with none, mild, moderate or severe pain)
Pain by visual analogue scale (categorically as none‐mild and moderate‐severe, and as continuous data for average score)
Sedation
Anxiety
Time to discharge from PACU Recovery time Mean arterial pressure and heart rate |
|
| Notes | Children received regional blocks pre‐incision according to routine: individual numbers not specified but overall rates of blockade for each treatment group given as a percentage The data from the dexmedetomidine group have not been included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Described as concealed by communication with principal author |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants' data analysed |
| Selective reporting (reporting bias) | Unclear risk | Analgesic use was reportedly recorded, but this does not appear in Results section |
| Other bias | Unclear risk | None evident; patient demographics, type of surgery, and use of regional anaesthesia evenly distributed between groups |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akin 2010 | Compares caudal bupivacaine, caudal bupivacaine plus caudal clonidine, and caudal bupivacaine plus intravenous clonidine in children 2‐8 years old having orchidopexy or inguinal hernia surgery. However, clonidine is given post‐induction, not as a premedication (premedication was with midazolam in all children) |
| Almenrader 2007a | Compares oral and nasal clonidine only; no clonidine‐free control. Does not measure pain or related variables |
| Almenrader 2007b | Measures several outcomes including agitation and parental satisfaction, comparing clonidine to midazolam, but does not measure pain or analgesic use |
| Cao 2011 | Compares intrathecal bupivacaine, intrathecal bupivacaine plus intrathecal clonidine, and intrathecal bupivacaine plus intravenous clonidine in children 6‐8 years old having orthopaedic surgery. However, intravenous clonidine is given post‐induction, at the time of subarachnoid block placement |
| Constant 2004 | Compares midazolam and clonidine as premedication, measures only agitation, BIS and EEG parameters, not pain or its associated outcomes |
| De Kort 2007 | Measures emergence agitation, not pain. Also, clonidine is given intravenously, post‐induction |
| Freeman 2002 | Measures pain and analgesic use after tonsillectomy/adenoidectomy, but clonidine is given intramuscularly following induction, not as a premedication |
| Fujii 2000 | Compares clonidine to diazepam in children having surgery for inguinal hernia or phimosis, but measures only haemodynamic response to extubation, not pain or related outcomes |
| Ghai 2010 | Measures effect of intravenous clonidine on emergence agitation after cataract surgery. Although it uses the Pain Discomfort Score this is modified (items 3‐5 only) to assess agitation, and patients without an effective regional block are excluded from the analysis |
| Gulhas 2001 | Compares clonidine with placebo as premedication but measures only postoperative nausea and vomiting |
| Gulhas 2003 | Measures postoperative nausea and vomiting with clonidine versus placebo, but not pain |
| Handa 2001 | Measures postoperative nausea and vomiting with clonidine versus diazepam, but not pain |
| Jatti 1998 | Compares clonidine with diazepam premedication but measures only psychomotor function, which does not include pain or its surrogates |
| Kihara 2000 | Compares clonidine premedication with placebo, but measures MAC‐awake, not pain or its surrogates |
| Lankinen 2006 | Clonidine, tropisetron or placebo given intravenously post‐induction, not as a premedication. Modified pain scale used to assess specifically agitation (although time to first dose of oxycodone is reported ‐ no difference between groups) |
| Mikawa 1993 | Clonidine versus diazepam; reports preoperative anxiolysis, mask acceptance, separation from parents, haemodynamic responses to tracheal intubation; no report of pain or analgesic use |
| Mikawa 1995 | Clonidine versus placebo or diazepam; assesses nausea and vomiting, not pain |
| Nishina 1994 | Compares clonidine premedication versus placebo for its effect on induction dose of thiamylal, but does not measure pain or associated events |
| Nishina 1995 | Clonidine premedication versus placebo but only measures effect on cardiovascular response to atropine; does not measure pain |
| Ramesh 1997 | Clonidine versus diazepam; assesses sedation, haemodynamic response to tracheal intubation and recovery score, not pain |
| Sfyra 2005 | Clonidine versus control given post‐induction, not as a premedication |
| Shiga 2000 | Clonidine versus placebo, but looks at effect on response to intravenous adrenaline or isoproterenol; no measurement of pain or its associated events |
| Sumiya 2003 | Kinetic study looking at plasma clonidine concentrations after oral dosing |
| Tazeroualti 2007 | Modified objective pain scale used; this excludes the arterial pressure and verbal/bodily expression components of the original and is used to assess postoperative agitation, not pain |
| Tesoro 2005 | Assessed clonidine's effects on postoperative agitation following sevoflurane anaesthesia, using a modified Pain Discomfort Score. Measures were taken specifically to exclude pain as a contributor to agitation |
| Trevor 2012 | Compares clonidine and midazolam premedication, but looks only at anxiety and sedation, not pain or related variables |
| Yun 2003 | Examines the effect of clonidine given intravenously 10 minutes pre‐induction, but only on sedation, separation anxiety and haemodynamic variables, not pain or its surrogates |
Differences between protocol and review
The principal difference between the protocol (Lambert 2012) and review that emerged on writing of the review was the inability to separate supplementary analgesia use in PACU from that at any time postoperatively. Hence, as described above, only the latter outcome was analysed and reported as our positive principal outcome measure.
Additionally, the studies were few in number, relatively small overall, and had such a degree of heterogeneity that we did not consider that extensive subgroup analysis for example by type of surgery, presence or absence of regional anaesthesia, age of child etc. would be a meaningful exercise. The only reasonable subgroup analysis pertained to the different doses of clonidine used, where we considered doses of 2 to 2.5 µg/kg to be 'low dose', and doses of 4 to 5 µg/kg to be 'high dose'.
While we had intended to initially pool studies across different doses of clonidine for the same outcome measure before subdividing them, there was a difference between those categorized as 'low dose' compared to the high dose' studies. With this in mind, it was apparent that pooling served only to obscure the effects (or lack thereof) at each dose, and that it made more sense to present the different doses separately from the beginning. In fact, given the difference between the 'low dose' and 'high dose' studies, we considered it reasonable to examine an additional comparison to those initially planned (clonidine versus placebo, another drug, or no treatment), namely 'high dose' versus 'low dose' clonidine.
It was not meaningful to pool the results of methodologically and statistically disparate studies in forest plots as intended, or in the 'Summary of findings' tables. Therefore, we have had to present these results descriptively in the text of our review.
Contributions of authors
Paul Lambert (PL), Nicholas Knight (NK), Philippa Middleton (PM), Allan M Cyna (AC)
Conceiving the review: AMC, NK, PL
Co‐ordinating the review: AMC
Undertaking manual searches: PM, NK, PL
Screening search results: NK, PL
Organizing retrieval of papers: NK, PL
Screening retrieved papers against inclusion criteria: NK, PL, PM
Appraising quality of papers: AMC, NK, PL, PM
Abstracting data from papers: NK, PL
Writing to authors of papers for additional information: AMC, PL
Providing additional data about papers: NK, PL
Obtaining and screening data on unpublished studies: NK, PL, PM
Data management for the review: NK, PL, PM
Entering data into Review Manager (RevMan 5.2): NK, PL, PM
RevMan statistical data: PM
Other statistical analysis not using RevMan: PM
Double entry of data: (data entered by person one: NK; data entered by person two: PL)
Interpretation of data: AMC, NK, PL, PM
Statistical inferences: PL, NK, PM, AMC
Writing the review: NK, PL, AMC
Securing funding for the review:
Performing previous work that was the foundation of the present study:
Guarantor for the review (one author): AMC
Person responsible for reading and checking review before submission: AMC, PL
Sources of support
Internal sources
-
Anaesthesia Research at the Women's and Children's Hospital, Australia.
In house support
External sources
No sources of support supplied
Declarations of interest
Paul Lambert: none known
Nicholas Knight: none known
Philippa Middleton: none known
Allan M Cyna: received travel, accommodation and meeting expenses unrelated to this research. (He was an invited keynote speaker for the Association of Anaesthetists (AAGBI) in London, invited to speak for the Malaysian Society of Anaesthetists in Malaysia (2011/2012) and a keynote at the New Zealand Society of Hypnosis, November 2013.)
New
References
References to studies included in this review
Bergendahl 2004 {published data only}
- Bergendahl HT, Lonnqvist PA, Eksborg S, Ruthstrom E, Nordenberg L, Zetterqvist H, et al. Clonidine vs. midazolam as premedication in children undergoing adeno‐tonsillectomy: a prospective, randomized, controlled clinical trial. Acta Anaesthesiologica Scandinavica 2004;48(10):1292‐300. [PUBMED: 15504191] [DOI] [PubMed] [Google Scholar]
Cao 2009 {published data only}
- Cao J, Shi X, Miao X, Xu J. Effects of premedication of midazolam or clonidine on perioperative anxiety and pain in children. Bioscience Trends 2009;3(3):115‐8. [PUBMED: 20103833] [PubMed] [Google Scholar]
Fazi 2001 {published data only}
- Fazi L, Jantzen EC, Rose JB, Kurth CD, Watcha MF. A comparison of oral clonidine and oral midazolam as preanesthetic medications in the pediatric tonsillectomy patient. Anesthesia and Analgesia 2001;92(1):56‐61. [PUBMED: 11133600] [DOI] [PubMed] [Google Scholar]
Georgiou 1999 {published data only}
- Georgiou M, Petropoulou P, Kaprini E, Pistofidou K, Farmakis H. Clonidine as premedication in combination with surgical wound infiltration ‐ an effective method for postoperative analgesia in children [abstract]. British Journal of Anaesthesia 1999; Vol. 82 Suppl 1:148. [CN‐00305983]
Hackmann 2003 {published data only}
- Hackmann T, Friesen M, Allen S, Precious DS. Clonidine facilitates controlled hypotension in adolescent children. Anesthesia and Analgesia 2003;96(4):976‐81, table of contents. [PUBMED: 12651645] [DOI] [PubMed] [Google Scholar]
Kuvaki 1998 {published data only}
- Kuvaki B, Kucukguclu S, Senturk Z, Turkoz A. [Rectal premedication in children: clonidine compared with midazolam]. Turk Anesteziyoloji Ve Reanimasyon 1998; Vol. 26, issue 1:30‐3. [CN‐00455903]
Mikawa 1996 {published data only}
- Mikawa K, Nishina K, Maekawa N, Obara H. Oral clonidine premedication reduces postoperative pain in children. Anesthesia and Analgesia 1996;82(2):225‐30. [PUBMED: 8561317] [DOI] [PubMed] [Google Scholar]
Nishina 2000 {published data only}
- Nishina K, Mikawa K, Shiga M, Takao Y, Maekawa N, Obara H. Diclofenac and flurbiprofen with or without clonidine for postoperative analgesia in children undergoing elective ophthalmological surgery. Paediatric Anaesthesia 2000;10(6):645‐51. [PUBMED: 11119198] [DOI] [PubMed] [Google Scholar]
Qteshat 2011 {published data only}
- Qteshat BS. Use of oral clonidine and oral midazolam as preanesthetic medications in the pediatric patient undergoing tonsillectomy. Rawal Medical Journal. Pakistan: Pakistan Medical Association (Garden Road, Karachi ‐ 3, Pakistan), 2011; Vol. 36, issue 2:114‐5.
Reimer 1998 {published data only}
- Reimer EJ, Dunn GS, Montgomery CJ, Sanderson PM, Scheepers LD, Merrick PM. The effectiveness of clonidine as an analgesic in paediatric adenotonsillectomy. Canadian Journal of Anaesthesia = Journal Canadien d'Anesthesie 1998;45(12):1162‐7. [PUBMED: 10051933] [DOI] [PubMed] [Google Scholar]
Schmidt 2007 {published data only}
- Schmidt AP, Valinetti EA, Bandeira D, Bertacchi MF, Simoes CM, Auler JO Jr. Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatric Anaesthesia 2007;17(7):667‐74. [PUBMED: 17564649] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akin 2010 {published data only}
- Akin A, Ocalan S, Esmaoglu A, Boyaci A. The effects of caudal or intravenous clonidine on postoperative analgesia produced by caudal levobupivacaine in children. Paediatric Anaesthesia 2010;20(4):350‐5. [PUBMED: 20158620] [DOI] [PubMed] [Google Scholar]
Almenrader 2007a {published data only}
- Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P. Steal‐induction after clonidine premedication: a comparison of the oral and nasal route. Paediatric Anaesthesia 2007;17(3):230‐4. [PUBMED: 17263737] [DOI] [PubMed] [Google Scholar]
Almenrader 2007b {published data only}
- Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P. Premedication in children: a comparison of oral midazolam and oral clonidine. Paediatric Anaesthesia 2007;17(12):1143‐9. [PUBMED: 17986032] [DOI] [PubMed] [Google Scholar]
Cao 2011 {published data only}
- Cao JP, Miao XY, Liu J, Shi XY. An evaluation of intrathecal bupivacaine combined with intrathecal or intravenous clonidine in children undergoing orthopedic surgery: a randomized double‐blinded study. Paediatric Anaesthesia 2011;21(4):399‐405. [PUBMED: 21371167] [DOI] [PubMed] [Google Scholar]
Constant 2004 {published data only}
- Constant I, Leport Y, Richard P, Moutard ML, Murat I. Agitation and changes of Bispectral Index and electroencephalographic‐derived variables during sevoflurane induction in children: clonidine premedication reduces agitation compared with midazolam. British Journal of Anaesthesia 2004;92(4):504‐11. [PUBMED: 14977793] [DOI] [PubMed] [Google Scholar]
De Kort 2007 {published data only}
- Kort R, Vandevelde A, Lauwers M, Camu F. Comparison of sufentanil and clonidine in the prevention of agitation in children after sevoflurane anesthesia. Acta Anaesthesiologica Belgica. Belgium: ARSMB‐KVBMG, 2007; Vol. 58, issue 2:148.
Freeman 2002 {published data only}
- Freeman KO, Connelly NR, Schwartz D, Jacobs BR, Schreibstein JM, Gibson C. Analgesia for paediatric tonsillectomy and adenoidectomy with intramuscular clonidine. Paediatric Anaesthesia 2002;12(7):617‐20. [PUBMED: 12358658] [DOI] [PubMed] [Google Scholar]
Fujii 2000 {published data only}
- Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Pretreatment with oral clonidine attenuates cardiovascular responses to tracheal extubation in children. Paediatric Anaesthesia 2000;10(1):65‐7. [PUBMED: 10632912] [DOI] [PubMed] [Google Scholar]
Ghai 2010 {published data only}
- Ghai B, Ram J, Chauhan S, Wig J. Effects of clonidine on recovery after sevoflurane anaesthesia in children undergoing cataract surgery. Anaesthesia and Intensive Care 2010;38(3):530‐7. [PUBMED: 20514964] [DOI] [PubMed] [Google Scholar]
Gulhas 2001 {published data only}
- Gulhas N, Turkoz A, Bayramlar H, Durmus M, Gedik E, Daglioglu MC, et al. The effect of oral clonidine on postoperative nausea and vomiting in children undergoing strabismus surgery. Turk Anesteziyoloji Ve Reanimasyon 2001; Vol. 29, issue 5:202‐5. [CN‐00442465]
Gulhas 2003 {published data only}
- Gulhas N, Turkoz A, Durmus M, Togal T, Gedik E, Ersoy MO. Oral clonidine premedication does not reduce postoperative vomiting in children undergoing strabismus surgery. Acta Anaesthesiologica Scandinavica 2003;47(1):90‐3. [PUBMED: 12492804] [DOI] [PubMed] [Google Scholar]
Handa 2001 {published data only}
- Handa F, Fujii Y. The efficacy of oral clonidine premedication in the prevention of postoperative vomiting in children following strabismus surgery. Paediatric Anaesthesia 2001;11(1):71‐4. [PUBMED: 11123735] [DOI] [PubMed] [Google Scholar]
Jatti 1998 {published data only}
- Jatti K, Batra YK, Bhardwaj N, Malhotra S. Comparison of psychomotor functions and sedation following premedication with oral diazepam and clonidine in children. International Journal of Clinical Pharmacology and Therapeutics 1998;36(6):336‐9. [PUBMED: 9660042] [PubMed] [Google Scholar]
Kihara 2000 {published data only}
- Kihara S, Inomata S, Yaguchi Y, Toyooka H, Baba Y, Kohda Y. The awakening concentration of sevoflurane in children. Anesthesia and Analgesia 2000;91(2):305‐8. [PUBMED: 10910838] [DOI] [PubMed] [Google Scholar]
Lankinen 2006 {published data only}
- Lankinen U, Avela R, Tarkkila P. The prevention of emergence agitation with tropisetron or clonidine after sevoflurane anesthesia in small children undergoing adenoidectomy. Anesthesia and Analgesia 2006;102(5):1383‐6. [PUBMED: 16632814] [DOI] [PubMed] [Google Scholar]
Mikawa 1993 {published data only}
- Mikawa K, Maekawa N, Nishina K, Takao Y, Yaku H, Obara H. Efficacy of oral clonidine premedication in children. Anesthesiology 1993;79(5):926‐31. [PUBMED: 8239010] [DOI] [PubMed] [Google Scholar]
Mikawa 1995 {published data only}
- Mikawa K, Nishina K, Maekawa N, Asano M, Obara H. Oral clonidine premedication reduces vomiting in children after strabismus surgery. Canadian Journal of Anaesthesia = Journal Canadien d'Anesthesie 1995;42(11):977‐81. [PUBMED: 8590507] [DOI] [PubMed] [Google Scholar]
Nishina 1994 {published data only}
- Nishina K, Mikawa K, Maekawa N, Takao Y, Obara H. Clonidine decreases the dose of thiamylal required to induce anesthesia in children. Anesthesia and Analgesia 1994;79(4):766‐8. [PUBMED: 7943789] [DOI] [PubMed] [Google Scholar]
Nishina 1995 {published data only}
- Nishina K, Mikawa K, Maekawa N, Obara H. Oral clonidine premedication blunts the heart rate response to intravenous atropine in awake children. Anesthesiology 1995;82(5):1126‐30. [PUBMED: 7741287] [DOI] [PubMed] [Google Scholar]
Ramesh 1997 {published data only}
- Ramesh VJ, Bhardwaj N, Batra YK. Comparative study of oral clonidine and diazepam as premedicants in children. International Journal of Clinical Pharmacology and Therapeutics 1997;35(5):218‐21. [PUBMED: 9174878] [PubMed] [Google Scholar]
Sfyra 2005 {published data only}
- Sfyra E, Soumpassis I, Soilemezi E, Marinou S, Georgiou M, Kanakoudis, et al. Preincisional i.v. clonidine prevents agitation during recovery from sevoflurane anesthesia in children. European Journal of Anaesthesiology 2005;22 Suppl 34:141. [Google Scholar]
Shiga 2000 {published data only}
- Shiga M, Nishina K, Mikawa K, Uesugi T, Maekawa N, Obara H. Oral clonidine premedication does not change efficacy of simulated epidural test dose in sevoflurane‐anesthetized children. Anesthesiology 2000;93(4):954‐8. [PUBMED: 11020745] [DOI] [PubMed] [Google Scholar]
Sumiya 2003 {published data only}
- Sumiya K, Homma M, Watanabe M, Baba Y, Inomata S, Kihara S, et al. Sedation and plasma concentration of clonidine hydrochloride for pre‐anesthetic medication in pediatric surgery. Biological & Pharmaceutical Bulletin 2003;26(4):421‐3. [PUBMED: 12673018] [DOI] [PubMed] [Google Scholar]
Tazeroualti 2007 {published data only}
- Tazeroualti N, Groote F, Hert S, Ville A, Dierick A, Linden P. Oral clonidine vs midazolam in the prevention of sevoflurane‐induced agitation in children. a prospective, randomized, controlled trial. British Journal of Anaesthesia 2007;98(5):667‐71. [PUBMED: 17416907] [DOI] [PubMed] [Google Scholar]
Tesoro 2005 {published data only}
- Tesoro S, Mezzetti D, Marchesini L, Peduto VA. Clonidine treatment for agitation in children after sevoflurane anesthesia. Anesthesia and Analgesia 2005;101(6):1619‐22. [PUBMED: 16301230] [DOI] [PubMed] [Google Scholar]
Trevor 2012 {published data only}
- Trevor S, Upadya M, Sinha C, Kaur M. A comparison of midazolam and clonidine as an oral premedication in pediatric patients. Saudi Journal of Anaesthesia 2012;6(1):8‐11. [PUBMED: 22412769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2003 {published data only}
- Yun MJ. [The effect of intravenous clonidine premedication on pediatric adenotonsillectomy]. Korean Journal of Anesthesiology 2003; Vol. 45, issue 6:715‐9. [CN‐00508427]
Additional references
Bergendahl 2006
- Bergendahl H, Lonnqvist PA, Eksborg S. Clonidine in paediatric anaesthesia: review of the literature and comparison with benzodiazepines for premedication. Acta Anaesthesiologica Scandinavica 2006;50(2):135‐43. [PUBMED: 16430532] [DOI] [PubMed] [Google Scholar]
Beyer 1990
- Beyer JE, McGrath PJ, Berde CB. Discordance between self‐report and behavioral pain measures in children aged 3‐7 years after surgery. Journal of Pain and Symptom Management 1990;5(6):350‐6. [PUBMED: 2269802] [DOI] [PubMed] [Google Scholar]
Bock 2002
- Bock M, Kunz P, Schreckenberger R, Graf BM, Martin E, Motsch J. Comparison of caudal and intravenous clonidine in the prevention of agitation after sevoflurane in children. British Journal of Anaesthesia 2002;88(6):790‐6. [PUBMED: 12173195] [DOI] [PubMed] [Google Scholar]
Collins 2010
- Collins CE, Everett LL. Challenges in pediatric ambulatory anesthesia: kids are different. Anesthesiology Clinics 2010;28(2):315‐28. [PUBMED: 20488397] [DOI] [PubMed] [Google Scholar]
Cyna 2006
- Cyna AM, Andrew M, Emmett RS, Middleton P, Simmons SW. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD002251.pub2] [DOI] [PubMed] [Google Scholar]
D'Mello 2008
- D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. British Journal of Anaesthesia 2008;101(1):8‐16. [PUBMED: 18417503] [DOI] [PubMed] [Google Scholar]
Dahmani 2010
- Dahmani S, Brasher C, Stany I, Golmard J, Skhiri A, Bruneau B, et al. Premedication with clonidine is superior to benzodiazepines. A meta analysis of published studies. Acta Anaesthesiologica Scandinavica 2010;54(4):397‐402. [PUBMED: 20085541] [DOI] [PubMed] [Google Scholar]
Duedahl 2007
- Duedahl TH, Hansen EH. A qualitative systematic review of morphine treatment in children with postoperative pain. Paediatric Anaesthesia 2007;17(8):756‐74. [PUBMED: 17596221] [DOI] [PubMed] [Google Scholar]
Frank 2000
- Frank T, Thieme V, Radow L. Premedication in maxillofacial surgery under total intravenous anesthesia. Effects of clonidine compared to midazolam on the perioperative course [Pramedikation im Rahmen einer TIVA bei kieferchirurgischen Operationen‐‐Vergleich der perioperativen Verlaufe nach Clonidin versus Midazolam.]. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie: AINS 2000;35(7):428‐34. [PUBMED: 10949680] [DOI] [PubMed] [Google Scholar]
Gentili 2007
- Gentili F, Pigini M, Piergentili A, Giannella M. Agonists and antagonists targeting the different alpha2‐adrenoceptor subtypes. Current Topics in Medicinal Chemistry 2007;7(2):163‐86. [PUBMED: 17266604] [DOI] [PubMed] [Google Scholar]
Golparvar 2004
- Golparvar M, Saghaei M, Sajedi P, Razavi SS. Paradoxical reaction following intravenous midazolam premedication in pediatric patients ‐ a randomized placebo controlled trial of ketamine for rapid tranquilization. Paediatric Anaesthesia 2004;14(11):924‐30. [PUBMED: 15500492] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 208;336:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannallah 1987
- Hannallah RS, Broadman LM, Belman AB, Abramowitz MD, Epstein BS. Comparison of caudal and ilioinguinal/iliohypogastric nerve blocks for control of post‐orchiopexy pain in pediatric ambulatory surgery. Anesthesiology 1987;66(6):832‐4. [PUBMED: 2884900] [DOI] [PubMed] [Google Scholar]
Hannallah 1992
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5(13):http://www.biomedcentral.com/1471‐2288/5/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulka 2001
- Kulka PJ, Bressem M, Tryba M. Clonidine prevents sevoflurane‐induced agitation in children. Anesthesia and Analgesia 2001;93(2):335‐8, 2nd contents page. [PUBMED: 11473855] [PubMed] [Google Scholar]
Larsson 2011
- Larsson P, Nordlinder A, Bergendahl HT, Lonnqvist PA, Eksborg S, Almenrader N, et al. Oral bioavailability of clonidine in children. Paediatric Anaesthesia 2011;21(3):335‐40. [PUBMED: 20735802] [DOI] [PubMed] [Google Scholar]
Lonnqvist 1994
- Lonnqvist PA, Bergendahl HT, Eksborg S. Pharmacokinetics of clonidine after rectal administration in children. Anesthesiology 1994;81(5):1097‐101. [PUBMED: 7978467] [DOI] [PubMed] [Google Scholar]
Macintyre 2010
- Macintyre PE, Schug SA, Scott DA, Visser EJ, Walker SM, APM:SE Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine (2010). Acute Pain Management: Scientific Evidence. 3rd Edition. Melbourne: ANZCA & FPM, 2010. [Google Scholar]
Mattila 2005
- Mattila K, Toivonen J, Janhunen L, Rosenberg PH, Hynynen M. Postdischarge symptoms after ambulatory surgery: first‐week incidence, intensity, and risk factors. Anesthesia and Analgesia 2005;101(6):1643‐50. [PUBMED: 16301235] [DOI] [PubMed] [Google Scholar]
McGraw 1998
- McGraw T, Kendrick A. Oral midazolam premedication and postoperative behaviour in children. Paediatric Anaesthesia 1998;8(2):117‐21. [PUBMED: 9549736] [DOI] [PubMed] [Google Scholar]
Motsch 1997
- Motsch J, Bottiger BW, Bach A, Bohrer H, Skoberne T, Martin E. Caudal clonidine and bupivacaine for combined epidural and general anaesthesia in children. Acta Anaesthesiologica Scandinavica 1997;41(7):877‐83. [PUBMED: 9265931] [DOI] [PubMed] [Google Scholar]
Nishina 1998
- Nishina K, Mikawa K, Maekawa N, Shiga M, Obara H. Effects of oral clonidine premedication on plasma glucose and lipid homeostasis associated with exogenous glucose infusion in children. Anesthesiology 1998;88(4):922‐7. [PUBMED: 9579500] [DOI] [PubMed] [Google Scholar]
Nishina 1999
- Nishina K, Mikawa K, Shiga M, Obara H. Clonidine in paediatric anaesthesia. Paediatric Anaesthesia 1999;9(3):187‐202. [PUBMED: 10320597] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Stewart 2012
- Stewart DW, Ragg PG, Sheppard S, Chalkiadis GA. The severity and duration of postoperative pain and analgesia requirements in children after tonsillectomy, orchidopexy, or inguinal hernia repair. Paediatric Anaesthesia 2012;22(2):136‐43. [PUBMED: 22023485] [DOI] [PubMed] [Google Scholar]
Tomecka 2012
- Tomecka MJ, Bortsov AV, Miller NR, Solano N, Narron J, McNaull PP, et al. Substantial postoperative pain is common among children undergoing laparoscopic appendectomy. Paediatric Anaesthesia 2012;22(2):130‐5. [PUBMED: 21958060] [DOI] [PubMed] [Google Scholar]
Tyler 1993
- Tyler DC, Tu A, Douthit J, Chapman CR. Toward validation of pain measurement tools for children: a pilot study. Pain 1993;52(3):301‐9. [PUBMED: 8460048] [DOI] [PubMed] [Google Scholar]
Wallace 2006
- Wallace AW. Clonidine and modification of perioperative outcome. Current Opinion in Anaesthesiology 2006;19(4):411‐7. [PUBMED: 16829723] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lambert 2012
- Lambert P, Knight N, Middleton P, Cyna AM. Clonidine premedication for postoperative analgesia in children. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009633] [DOI] [PMC free article] [PubMed] [Google Scholar]


