Abstract
We tested a number of inhibitory monovalent anions for their primary site of action on photosystem II(PSII) in chloroplasts. We find that the inhibitory effects of F−, HCO2−, NO2−, NO3−, and CH3CO2− are all reversed by addition of a high concentration of HCO3−. This class of anions competitively inhibits H14CO3− binding to PSII. All of those anions tested reduced H14CO3− binding more in the light than in the dark. We conclude that the primary inhibitory site of action of a number of monovalent anions is at the HCO3− binding site(s) on the PSII complex. The carbonic anhydrase inhibitor gold cyanide, and also azide, inhibit PSII but at a site other than the HCO3− binding site. We suggest that the unique ability of HCO3− to reverse the effects of inhibitory anions reflects its singular ability to act as a proton donor/acceptor at the anion binding site. A similar role has been proposed for non-substrate-bound HCO3− on carbonic anhydrase by Yeagle et al. (1975 Proc Natl Acad Sci USA 72: 454-458).
Full text
PDF
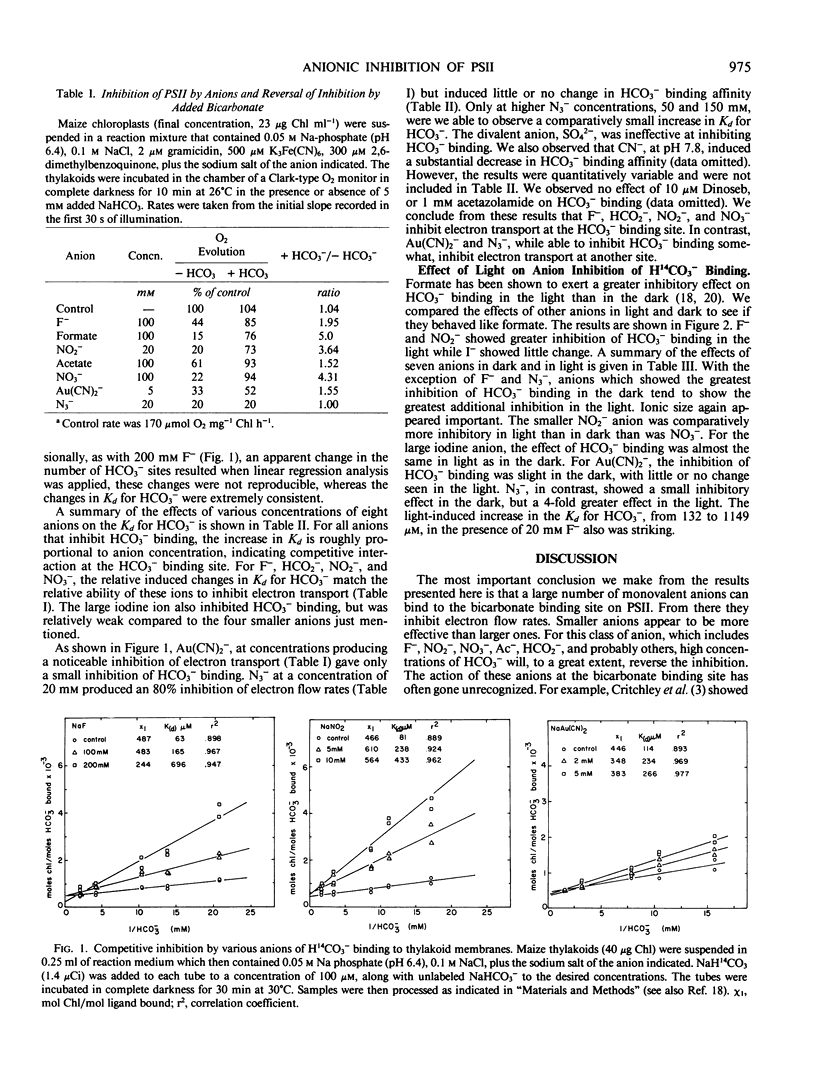
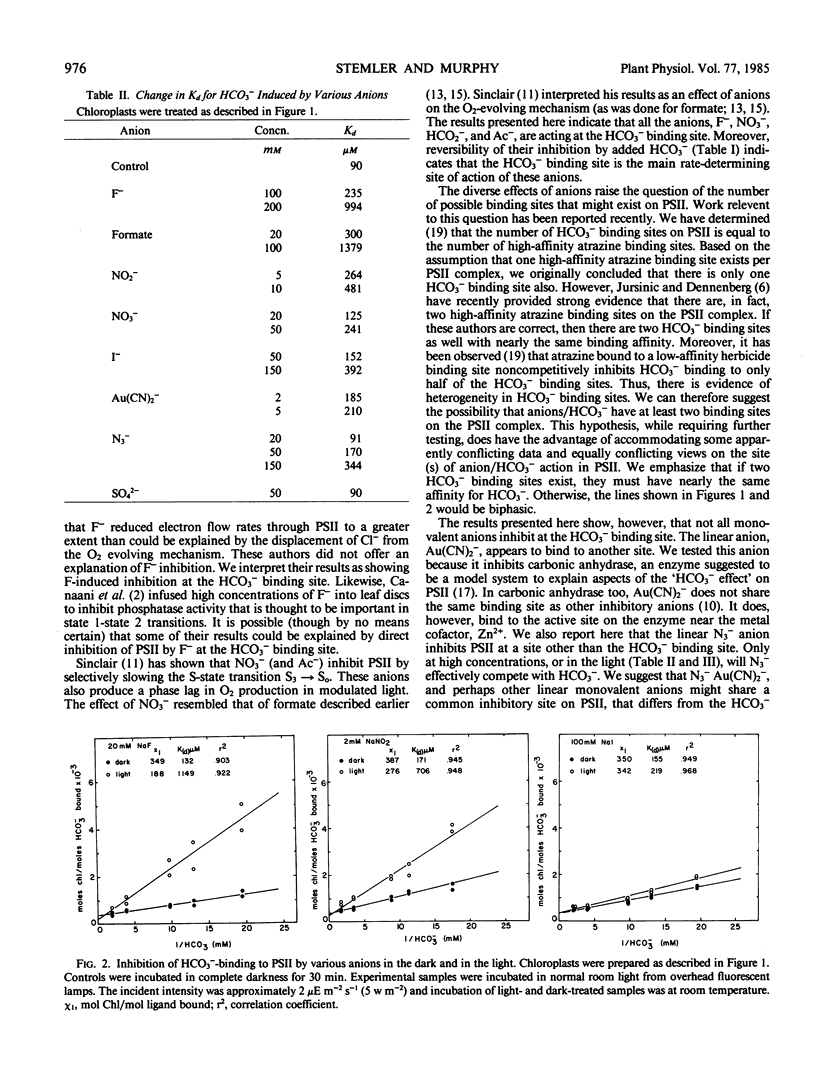
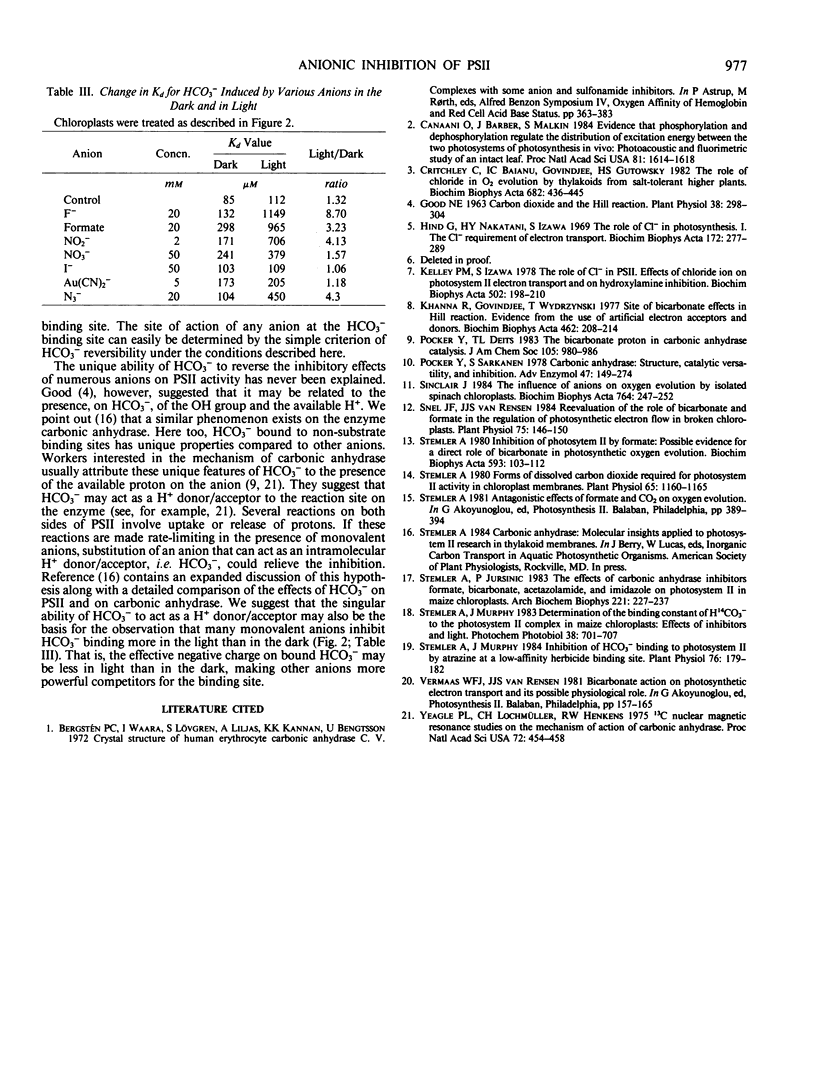
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canaani O., Barber J., Malkin S. Evidence that phosphorylation and dephosphorylation regulate the distribution of excitation energy between the two photosystems of photosynthesis in vivo: Photoacoustic and fluorimetric study of an intact leaf. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1614–1618. doi: 10.1073/pnas.81.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E. Carbon Dioxide & the Hill Reaction. Plant Physiol. 1963 May;38(3):298–304. doi: 10.1104/pp.38.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind G., Nakatani H. Y., Izawa S. The role of Cl- in photosynthesis. I. The Cl- requirement of electron transport. Biochim Biophys Acta. 1969 Feb 25;172(2):277–289. doi: 10.1016/0005-2728(69)90070-x. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Izawa S. The role of chloride ion in photosystem II. I. Effects of chloride ion on photosystem II electron transport and on hydroxylamine inhibition. Biochim Biophys Acta. 1978 May 10;502(2):198–210. doi: 10.1016/0005-2728(78)90042-7. [DOI] [PubMed] [Google Scholar]
- Khanna R., Govindjee, Wydrzynski T. Site of bicarbonate effect in Hill reaction. Evidence from the use of artificial electron acceptors and donors. Biochim Biophys Acta. 1977 Oct 12;462(1):208–214. doi: 10.1016/0005-2728(77)90203-1. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Sarkanen S. Carbonic anhydrase: structure catalytic versatility, and inhibition. Adv Enzymol Relat Areas Mol Biol. 1978;47:149–274. doi: 10.1002/9780470122921.ch3. [DOI] [PubMed] [Google Scholar]
- Snel J. F., van Rensen J. J. Reevaluation of the role of bicarbonate and formate in the regulation of photosynthetic electron flow in broken chloroplasts. Plant Physiol. 1984 May;75(1):146–150. doi: 10.1104/pp.75.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemler A. Forms of Dissolved Carbon Dioxide Required for Photosystem II Activity in Chloroplast Membranes. Plant Physiol. 1980 Jun;65(6):1160–1165. doi: 10.1104/pp.65.6.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemler A. Inhibition of photosystem II by formate. Possible evidence for a direct role of bicarbonate in photosynthetic oxygen evolution. Biochim Biophys Acta. 1980 Nov 5;593(1):103–112. doi: 10.1016/0005-2728(80)90011-0. [DOI] [PubMed] [Google Scholar]
- Stemler A., Jursinic P. The effects of carbonic anhydrase inhibitors formate, bicarbonate, acetazolamide, and imidazole on photosystem II in maize chloroplasts. Arch Biochem Biophys. 1983 Feb 15;221(1):227–237. doi: 10.1016/0003-9861(83)90139-x. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L., Lochmüller C. H., Henkens R. W. 13-C nuclear magnetic resonance studies on the mechanism of action of carbonic anhydrase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):454–458. doi: 10.1073/pnas.72.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]



