Abstract
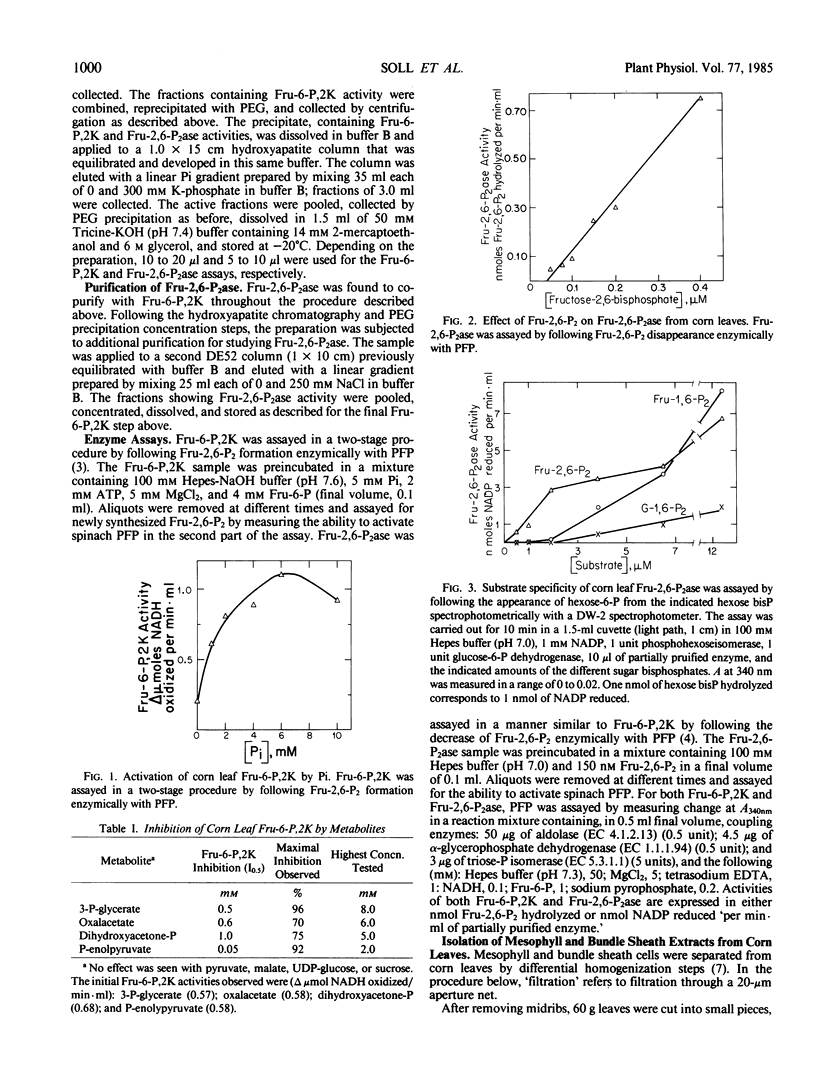
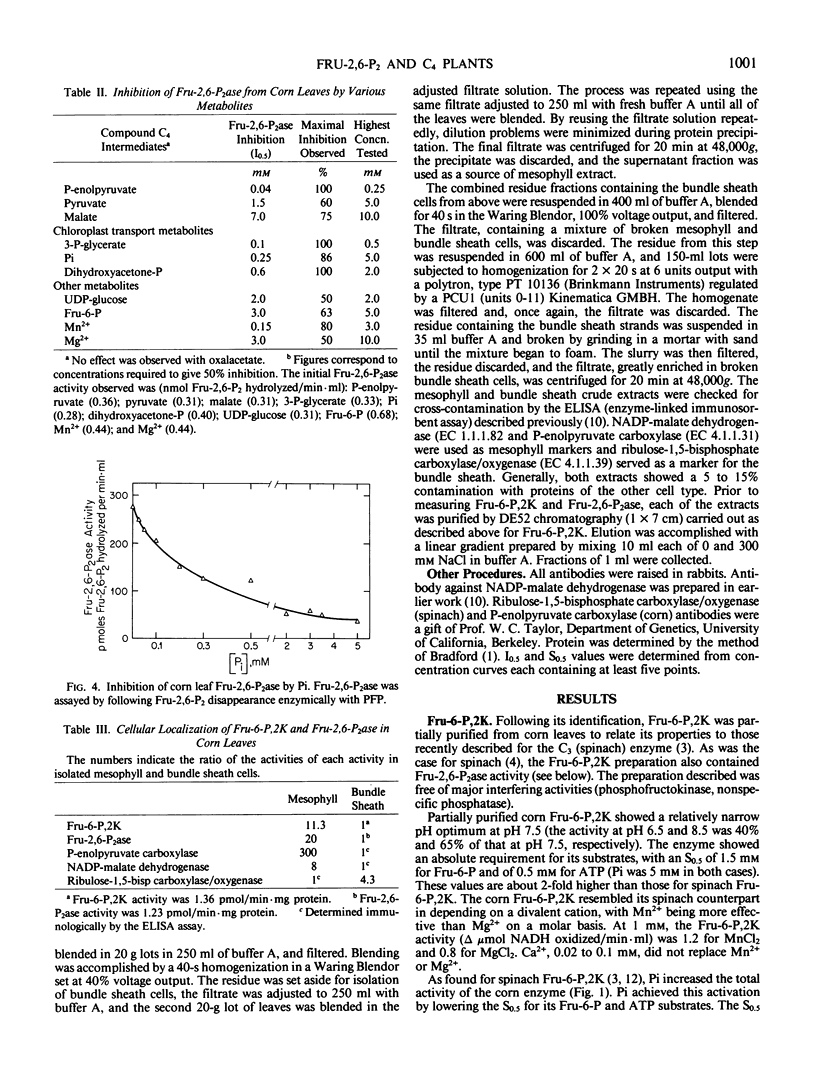
Activities catalyzing the synthesis of fructose-2,6-bisphosphate (fructose-6-phosphate,2-kinase or Fru-6-P,2K) and its breakdown (fructose-2,6-bisphosphatase or Fru-2,6-P2ase) were identified in leaves of corn (Zea mays), a C4 plant. Fru-6-P,2K and Fru-2,6-P2ase were both localized mainly, if not entirely, in the leaf mesophyll cells. A partially purified preparation containing the two activities revealed that the kinase and phosphatase were regulated by metabolite effectors in a manner generally similar to their counterparts in C3 species. Thus, corn Fru-6-P,2K was activated by inorganic phosphate (Pi) and fructose-6-phosphate, and was inhibited by 3-phosphoglycerate and dihydroxyacetone phosphate. Fru-2,6-P2ase was inhibited by its products, fructose-6-phosphate and Pi. However, unlike its spinach equivalent, corn Fru-2,6-P2ase was also inhibited by 3-phosphoglycerate and, less effectively, by dihydroxyacetone phosphate. The C4 Fru-6-P,2K and Fru-2,6-P2ase were also quite sensitive to inhibition by phosphoenolpyruvate, and each enzyme was also selectively inhibited by certain other metabolites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maghrabi M. R., Claus T. H., Pilkis J., Fox E., Pilkis S. J. Regulation of rat liver fructose 2,6-bisphosphatase. J Biol Chem. 1982 Jul 10;257(13):7603–7607. [PubMed] [Google Scholar]
- Jacquot J. P., Gadal P., Nishizawa A. N., Yee B. C., Crawford N. A., Buchanan B. B. Enzyme regulation in C4 photosynthesis: mechanism of activation of NADP-malate dehydrogenase by reduced thioredoxin. Arch Biochem Biophys. 1984 Jan;228(1):170–178. doi: 10.1016/0003-9861(84)90058-4. [DOI] [PubMed] [Google Scholar]
- Stitt M., Cseke C., Buchanan B. B. Regulation of fructose 2,6-bisphosphate concentration in spinach leaves. Eur J Biochem. 1984 Aug 15;143(1):89–93. doi: 10.1111/j.1432-1033.1984.tb08345.x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Gerhardt R., Kürzel B., Heldt H. W. A role for fructose 2,6-bisphosphate in the regulation of sucrose synthesis in spinach leaves. Plant Physiol. 1983 Aug;72(4):1139–1141. doi: 10.1104/pp.72.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Wirtz W., Heldt H. W. Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta. 1980 Nov 5;593(1):85–102. doi: 10.1016/0005-2728(80)90010-9. [DOI] [PubMed] [Google Scholar]
- Usuda H., Edwards G. E. Localization of glycerate kinase and some enzymes for sucrose synthesis in c(3) and c(4) plants. Plant Physiol. 1980 May;65(5):1017–1022. doi: 10.1104/pp.65.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaftingen E., Davies D. R., Hers H. G. Fructose-2,6-bisphosphatase from rat liver. Eur J Biochem. 1982 May;124(1):143–149. doi: 10.1111/j.1432-1033.1982.tb05917.x. [DOI] [PubMed] [Google Scholar]


