Abstract
CT is the principal imaging modality used for the pre-operative 3D planning and assessment of total hip arthroplasty (THA).
The image quality offered by CT has a radiation penalty to the patient. Higher than necessary radiation exposure is of particular concern when imaging young patients and women of childbearing age, due to the greater risk of radiation-induced cancer in this group.
A harmonised low-dose CT protocol is needed, evidenced by the huge variability in the 17 protocols reviewed. The majority of the protocols were incomplete, leading to uncertainty among radiographers when performing the scans.
Only three protocols (20%) were optimised for both ‘field of view’ and image acquisition parameters. 10 protocols (60%) were optimised for ‘field of view’ only. These protocols included imaging of the relevant landmarks in the bony pelvis in addition to the knees – the reference for femoral anteversion.
CT parameters, including the scanner kilovoltage (kV), milliamperage–time product (mAs) and slice thickness, must be optimised with a ‘field of view’ that includes the relevant bony landmarks. The recommended kV and mAs values were very wide ranging from 100 to 150 and from 100 to 250, respectively.
The large variability that exists amongst the CT protocols illustrates the need for a more consistent low-dose CT protocol for the planning of THA. This must provide an optimal balance between image quality and radiation dose to the patient.
Current CT scanners do not allow for measurements of functional pelvic orientation and additional upright imaging modalities are needed to augment them.
Keywords: low dose, computed tomography, hip arthroplasty, primary hip replacement, protocol, primary hip reconstruction
Introduction
CT-based planning of total hip arthroplasty (THA) delivers 3D information regarding the patient’s anatomy which can be used to predict implant size and position (1). CT is also considered the gold standard for precise post-operative measurements of implant positioning, such as femoral and acetabular component version (2, 3). These are an indicator of the success of the surgery and clinical outcomes but are not routinely conducted due to radiation concerns (3).
Many studies have looked at the impact of manipulating CT acquisition parameters on the image quality and effective dose deposited to the patient (4, 5, 6). It has been observed that the average effective dose deposited to patients from a single pelvic CT scan can range from 4 to 20 mSv (7). This is equivalent to 1.5–7.4 times the average annual radiation exposure to the people living in the UK, as estimated by the Public Health England (8, 9).
Given that bony anatomy may easily be seen on CT scans, the current evidence suggests that an ideal trade-off between image resolution and dose to the patient must be established. To the authors’ knowledge, there has been no published appraisal of CT protocols offered by orthopaedic companies for the planning of THA. Despite acknowledging the need for a harmonised scanning protocol (7, 10), this issue has not been widely addressed.
This review sought to better understand the variability across different orthopaedic companies’ CT protocols currently used by UK surgeons, for the surgical planning of THA. First, we explored the factors affecting the effective dose deposited to patients, which include the field of view, scanner kilovoltage (kV), milliamperage–time product (mAs) and slice thickness. Second, we studied the variability in CT acquisition parameters for bony anatomy and estimated the radiation dose to the patient by means of dosimetry software.
Image quality vs radiation dose
CT constitutes the best imaging modality for the pre-operative planning of hip replacement. Accurate 3D models from bone segmentation are particularly important when using custom implants or patient-specific instrumentation (PSI) (11, 12). These are designed and produced from the 3D virtual plan to match the patient’s bony anatomy. Adequate image quality is thus paramount for bony landmarks of the pelvis to be clearly identified, since this will directly impact the fitting of the guides/implants and the long-term outcome of the surgery (13, 14).
CT scans used to plan size and position of prosthetic components differ from diagnostic radiographs or other imaging modalities that may be needed to image the soft tissues in the pelvic region. Pelvic radiographs cannot be used to estimate size and orientation of the implant components, as they are highly susceptible to errors in magnification and effects of patient positioning during imaging (15, 16). Although standard radiographs reduce the patient’s exposure to radiation, the 2D nature of the scans makes 3D planning of THA difficult (17).
A compromise between image quality and radiation dose must be considered with the use of CT as it offers superior image resolution but at the expense of higher radiation dose deposited to the patient (18). This problem is exacerbated by the poor choice of CT parameters by clinicians and the lack of a single low-dose CT protocol (19). Establishing this will help to address issues concerning variability in the radiation dose deposited to patients between (and within) different hospitals and institutions (20).
Image quality and Hounsfield units
The CT number, which is typically given in dimensionless Hounsfield units (HU), is a computed value that reflects the x-ray attenuation coefficient in an image voxel (21). HU values differ in different anatomical regions of the body and can be used to infer bone mineral density and quality (22, 23). The image contrast and greyscale visible on CT scans is produced by the varying levels of radiodensity within bone and soft tissue structures, which enables the visualisation of finer details. The radiodensity of water and cancellous bone is defined as 0 HU and +300–+400 HU, respectively. Metals are the most radiodense (>3000 HU), resulting in the bright appearance of hip implants on CT scans.
Visualising bone morphology
Although the lowering of the technical CT parameters (field of view, kV and mAs) assists the dose reduction process, image quality is compromised as a result. However, this is less problematic when studying bony structures under soft tissue. It has been shown that thicker subcutaneous layers of fat overlying the pubic tubercles and the anterior superior iliac spines does not necessarily correlate with less accurate anteversion measurements in the work of Ybinger et al. (24) The overlying soft-tissues surrounding the pelvic bone only pose challenges in extreme cases concerning obese patients (BMI > 30) (25) but does not reduce the accuracy of planning as patient size and obesity will affect the visualisation of soft tissues but generally not bone.
A low-dose protocol proposed by Su et al. (26) for patients undergoing hip preservation surgery revealed that the use of the protocol with minimised acquisition parameters (100 kV and 100 mAs) produced scans with adequate image resolution for surgical planning and outcome assessment while inducing a 90% reduction in radiation exposure. Thus, when adopting a low-dose approach, it does not restrict the surgeon’s ability to identify crucial anatomical landmarks, further accentuating the need for a low-dose protocol for the preoperative planning of THA.
The defining factors of an optimal CT scan for THA planning
As the years have progressed, the need for THA in younger patients has become much more common, highlighting the need for longer-lasting implants. Since 75% of hip implants are expected to last between 15 and 20 years (27), revision hip surgery can be anticipated in patients under the age of 55 (28, 29), which is further confirmed by their higher revision rate. To lower the risk of implant failure, precise pre-operative planning of THA is thus crucial.
This review presents a comparison between different CT protocols based on the recommendations of the technical parameters required for conducting the pelvic scan. That is, how the CT scanner is used, rather than which scanner is used. These technical acquisition parameters include the scan field of view (length of the scan), kV, mAs, the presence of automated tube current modulation (ATCM) and the slice thickness. The significance of these parameters in allowing the visualisation of different tissues is presented in this section. The authors acknowledge that there are also dose variations as a result of patient and machine characteristics, and the institution type. However, in the work presented by Smith-Bindman et al. (10), accounting for such variations has proven to have comparatively minimal impact on reducing the dose variation.
Field of view
The scan volume in the CT acquisition of the whole pelvis should be as small as reasonably possible and include only the landmarks relevant for the surgical planning of THA (20). This includes the whole pelvis and the knees of the patient. Due to patient movement throughout the scan, the inclusion of the knees are essential to determine whether any femoral anteversion needs to be accounted for (30, 31). The different radio-sensitivities of the organs within the field of view stress the need for a limited scan length, to avoid the scan extending into regions containing highly radiosensitive organs. This is because pelvic scans are already in close proximity to radiosensitive organs, such as the ovaries in female subjects and testes in male subjects (32). Thus, scans that extend into the spine of the patient or run directly from the most superior part of the ilium to the mid knee joint are exposing sections of the patient that are not necessary for accurate surgical planning. Concomitantly, the patient receives an excessive amount of effective dosage.
Scanner kilovoltage
The selection of the peak potential (kVp) applied to the x-ray tube in a CT scanner is often dependent on patient size, as it correlates to the photon energy and hence the penetrating power of the x-ray beam. Common choices of tube voltages are 80 kV, 100 kV and 120–140 kV, for small-, average- and large-sized patients, respectively (33, 34). This is due to the varying amounts of the soft-tissue envelope surrounding the pelvis, so a higher kV compensates for the increased photon interaction in thicker layers.
Reduction of the tube potential is identified in the literature as an effective technique for optimising the dose to the patient, despite the increased image noise as a result. This is because the image contrast may be enhanced with the option of using ATCM or a slight increase in the mAs, which will result in a less significant increase in patient exposure (35).
Automated tube current modulation and mAs
According to studies looking at the effect of ATCM on patient dose, the ATCM technology works to lower the effective dose deposited to the patient. Most CT scanners today include an ATCM setting, however, the decision to use it during a CT scan assumes that the patient is positioned in the centre of the ring (6); if not, ATCM can have adverse effects and increase the dose to the patient. ATCM technology is commonly used for chest and pelvic CT examinations (36). In a comparative investigation between constant and modulated current in the work of Iball et al. (37), it has been demonstrated that the Siemens CARE Dose current modulation system is more effective in lowering patient dose without compromising image quality, when studying bony structures compared with less dense regions. Therefore, for the interest of this work, with ideal patient centring, it can be assumed that the application of an ATCM setting will not decrease the signal-to-noise ratio of the pelvic CT scans. When examining a patient’s pelvis or shoulder, this approach is especially useful due to the higher attenuation of x-rays in these bony cross sections (6).
As opposed to a fixed tube current, with this feature a pre-set peak tube current is modulated to maintain a good image quality throughout, as it accounts for the differing amounts of tissue attenuation as the x-ray tube and detectors rotate around the patient (33, 35, 38). The size and shape of the patient is factored when the mA of the x-ray tube is altered. This prevents excessive dose exposure to the patient in regions of low attenuation and permits a sufficient number of x-ray photons to reach the detector in regions of high attenuation (35). As the tube spins around the patient, the mA is automatically adjusted based on measurements from the online feedback system in angular increments (33, 38).
Slice thickness
CT slice thickness controls the beam width entering and, in turn, the number of x-rays detected by each detector in the CT system (39). A reduction in the slice thickness requires an increase in patient dose to maintain the same image resolution throughout the scan (40). This is because fewer photons can contribute to the image formation with thinner slice thickness, demanding an inverse change (increase) in the kV or mAs of the CT acquisition to reduce the noise in the image, which has adverse effects on patient dose.
Method
The protocol search
We conducted a systematic search of commercial CT protocols published between 2000 and 2022 by all orthopaedic companies that are currently offering CT planning for their hip replacements to UK surgeons. These include but are not limited to implant manufacturers. The terms used in the search were ‘CT protocol’, ‘CT hip plan’, ‘pelvic plan’ and ‘primary hip reconstruction’. First, the evidence of the protocols were searched for in any published literature. Then, any protocols not found through this method were looked for directly on the orthopaedic companies’ websites. Some companies needed to be contacted for their protocols as they were not publicly available online.
Result
The variability in CT acquisition parameters for bony anatomy
Seventeen different hip CT protocols published by orthopaedic companies were reviewed and are summarised in Table 1.
Table 1.
Hip CT scan protocols used for the current review.
| Protocol | Orthopaedic company | CT plan | Clinical requirement |
|---|---|---|---|
| 1 | AQ Solutions | AQI Process | Custom |
| 2 | Conformis | Hip System | PSI |
| 3 | HipXpert | HipXpert System | Navigation |
| 4 | Implantcast | C-Fit 3D® | Custom |
| 5 | JRI | JRI-ICOS | Custom |
| 6 | Lima Corp. | PROMADE | Custom |
| 7 | Materialise | OrthoView | Custom |
| 8 | Medacta International | MyHip | PSI |
| 9 | OSSIS | Custom Implant | Custom |
| 10 | Smith & Nephew | Dyonics | PSI |
| 11 | Stryker | MAKO | Navigation |
| 12 | Symbios | Hip-Plan | Custom |
| 13 | Zimmer Biomet | Patient-Matched Implants | Custom |
| 14 | LEXI Co., Ltd | ZedHip | 3D planning |
| 15 | Adler Ortho | Adler Ortho | Custom |
| 16 | mediCAD, HecTec GmbH | Hip 3D | 3D planning |
| 17 | Signature Orthopaedics | Hip | Custom |
The protocols were not limited to those that are used to provide planning in 3D but also to produce custom-made implants, PSIs for the surgeon’s use intraoperatively and for CT image-based hip navigation systems. It is assumed that the image quality needed for all three cases is comparable, so the acquisition parameters recommended in all collected protocols should reveal low variability. Some of the companies were very specific and prescriptive in their published protocols, while others provided incomplete and ambiguous information, leaving the choice of acquisition parameters to the institution implementing their protocol.
Field of view
The field of view for capturing the regions required to plan THA is summarised in Table 2. The information is presented directly as it appears in the protocol documentation. Of the 17 protocols, five did not report quantitative information.
Table 2.
Recommended field of view as published in the orthopaedic companies’ CT protocol document.
| Orthopaedic company | Scan length |
|---|---|
| AQ Solutions | Proximal – ‘iliac crest to mid-femur’; knee – ‘Both condyles as rotational measurement of the femurs’; ankles – ‘Both ankles as rotational measurement of the tibiae in frontal plane’ |
| Conformis | ~38–45 cm (include full pelvis); ‘Top of iliac crest to mid knee joint’ |
| HipXpert | ‘Include the whole pelvis and the upper part of the femurs (approximately 20 cm below the tip of the greater trochanters)’; Femoral condyles: ‘50 mm of the left and right distal femurs’ |
| Implantcast | ‘Start: Above anterior superior ilia spine (ASIS); Stop: 200–375 mm below centre of trochanter major, depending on required stem length. Scan two femoral condyle slices’ |
| JRI | ‘>50 mm inferior of lesser trochanter up to superior origin of sacroiliac joints’ |
| Lima Corp. | 38–44 cm depending on patient size. ‘Start: Top of Iliac crest; Stop: Mid-femur or at least 3 cm below existing femoral implant. Perform two femoral condyle slices’ |
| Materialise | <40 cm ‘Include complete bony pelvis: from the most superior point of the ilium to the most inferior point of the ischium’ |
| Medacta | Whole pelvis and proximal femur (up to 60 cm). ‘Scan must start at least 2 cm above the iliac crests and continue to at least 10 cm below the lesser trochanter.’ Distal part of the femur in the affected side |
| OSSIS | ‘Captures all bone and soft tissue of the entire pelvis (from above iliac crests to below ischial tuberosity)’ |
| Smith & Nephew | 500 mm. Bilateral pelvis. ‘Start: Midpoint between the anterior inferior iliac spine (AIIS) and anterior superior iliac spine (ASIS). End: 30 mm below lesser trochanter’ |
| Stryker | Region 1 = pelvis + proximal femur. ‘The entire bilateral pelvis and at least 180mm below the lesser trochanter on the femur.’ Region 2 = bilateral knee-joint lines between femur and tibia and 10 cm proximal to joint line on femur |
| Symbios | 500 mm. ‘Pelvis series: Include the iliac crests as well as the distal femoral isthmus Knees series: Include the entire distal femoral epiphysis’ |
| Zimmer Biomet | ‘38–44 cm depending on patient size. Needs to include full pelvis. Start: Top of Iliac crest. Stop: Mid-femur or below existing femoral.’ Perform two femoral condyle slices (to show anteversion) |
| MediCAD | ‘CT scan in one pass through the entire pelvis and both knees.’ Pelvis: top of the iliac crest to 20 cm distally to the centre of the femoral head. |
| Signature Orthopaedics | ‘All bony regions of complete pelvis: from just above the most superior point of the ilium down to just below the most inferior point at the ischium’ |
| Adler | Not specified* |
| LEXI Co., Ltd. | ‘THA: entire pelvis and femur’ |
*Information left to the discretion of the imaging centre.
Adler does not specify any field of view in their protocol, whereas the field of view described by OSSIS, Signature Orthopaedics, LEXI and AQ Solutions is purely theoretical as no indications of the scan length are provided. This is in contrast to the remaining 12 protocols, which all include quantitative information on where to start and terminate the scan. However, the pelvic features used in the description of the scan areas vary across the studied protocols; while 11 of the protocols indicate the top of the iliac crest as the uppermost end of the scan, other manufacturers expect the clinicians to deduce this information from statements such as ‘include the whole pelvis’. This leaves the start and end point of the scans open to the radiographer’s interpretation which may cause them to request a CT scan that follows into the lower spine of the patient. This raises concerns regarding the unnecessary dose deposited to the patients as a result. On the contrary, the protocol issued by Smith & Nephew requests the scan to begin at the midpoint between the AIIS and the ASIS. Without the complete pelvis, an accurate 3D model of the patient’s anatomy may not be obtained, which in turn can lead to inaccurate measurements from which custom implants can be constructed.
Some companies split up the regions to be scanned into two sections: the bilateral pelvis and the bilateral knees. This approach ensures that instead of a single continuous scan running from the top of the iliac crest to the mid-femur, two shorter volume areas can be imaged to avoid irradiating the whole leg and restrict the scan to merely the areas necessary for preoperative planning. Examples of such protocols are Stryker MAKO and Medacta MyHip. On the other hand, the LEXI Co., Ltd. and Conformis Hip System CT protocols recommend a single scan running from the top of the pelvis to the knee joint. The variability in the field of view is larger when considering those which do not include the knees in the scans, such as OSSIS and Smith & Nephew.
The maximum and minimum scan lengths requested by the implant manufacturers were identified from the studied protocols and these field of views are presented in Fig. 1. As previously described, the smallest field of view was requested by Smith & Nephew, as they recommend a scan range of 50 cm, starting from below the ASIS to 3 cm below the lesser trochanter. Conversely, the largest field of view was associated with Conformis and LEXI Co., Ltd as there are no divisions in the scanned regions. However, Conformis also provides a quantitative value for the scan length of approximately 38-44 cm, which does not match with the start and end points which they specify, when considering an average-sized patient. Thus, greater care needs to be taken when writing these protocols as this can cause confusion among radiographers regarding which description to follow; the specified scan length or the start and end points of the scan? Interestingly, Smith & Nephew’s field of view which is visibly shorter than Conformis’s recommended field of view, is described to be 50 cm, which is numerically larger than the scan length of the latter.
Figure 1.
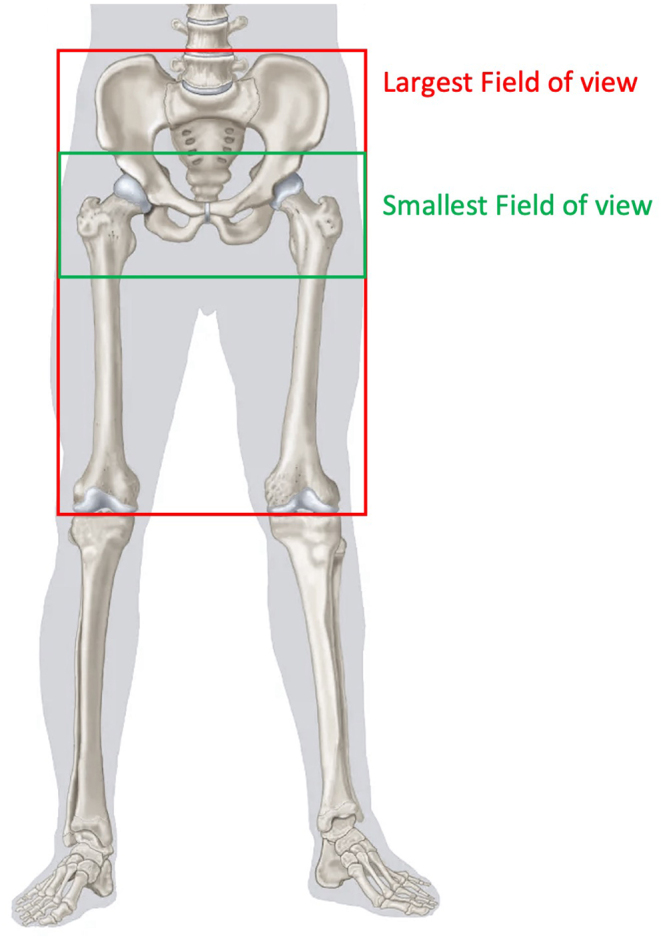
The maximum and minimum scan ranges identified from the 17 reviewed protocols.
kV, mAs and slice thickness
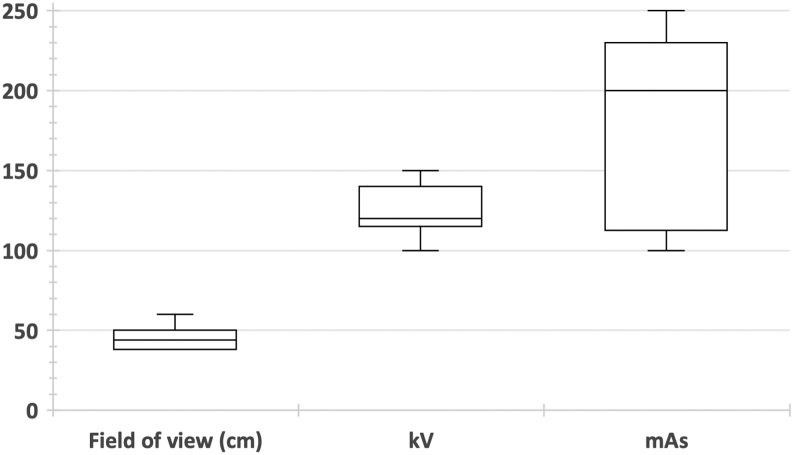
Seven of the reviewed protocols provide details on the scanner kV parameter which the radiologist should use when conducting the CT scan. Five of these recommend a tube potential equal to or greater than 120 kV, whereas Materialise and JRI both accept scans acquired at 100 kV.
There is greater discrepancy when considering the recommended mAs in these protocols. This is because Symbios and Materialise do not present an approximative value in their protocols, but only mention the use of ATCM if available. This may not be sufficient for the radiologist to conduct the scan as a pre-set mAs value is required, even when ATCM is used. Smith & Nephew are identified as the only orthopaedic company who offer three different mAs values according to patient size, which is a helpful inclusion in the protocol for radiologists to appropriately choose exposure settings depending on the patient undergoing the scan. This may also prevent the need for repeat CT scans, since using the wrong exposure settings may hinder the surgeons and manufacturers from planning the surgery accurately. Additionally, if the image is not good enough, repeat scans are likely to be requested, which exposes the patient to double the radiation. However, for the bony anatomy which is of interest, the soft tissue envelope should not pose major challenges. Among the four protocols which provide data on the mAs, the recommended values ranged from 100-250 mAs (with a rotation time of 1 s considered for Stryker’s protocol). Information on the slice thickness was provided by 16 protocols and ranged considerably from 0.5-3.0 mm. Signature Orthopaedics are the only company that do not provide any recommended value for the slice thickness in their protocol.
The technical scanner parameters that were found in the protocol documents are outlined in Table 3 and are presented graphically in Fig. 2.
Table 3.
Technical CT scanner parameters defined by different orthopaedic companies in their hip CT protocols.
| Company | Field of view | kV range | mAs | Slice thickness at the pelvis | Effective dose (mSv) |
|---|---|---|---|---|---|
| Conformis | Iliac crest to mid knee joint | 120 | ~100–200 | 0.5–1.5 mm | 2.55 (male); 3.99 (female) |
| Smith & Nephew | Midpoint between AIIS and ASIS to 3 cm below lesser trochanter | 120 | Small patients: 100; medium patients: 150; large patients: 200 | 2.0–2.5 mm | 3.41–6.83 (male); 4.07–8.14 (female) |
| Medacta | Whole pelvis to proximal femur and knees | >120 | >120 mA with 2 s exposure time | 0.5–1.0 mm | 8.11 (male); 9.76 (female) |
| Stryker | Whole pelvis to proximal femur and knees | 120–140 | 200–250 mA | 0.5–1.0 mm | 6.76–12.94 (male); 8.13–15.80 (female) |
| Materialise | Whole pelvis; the most superior point of ilium to the most inferior point of ischium | 100–140 (or auto selection) | Automated modulation | 1 - 1.50 mm (preferred); <3 mm (acceptable) | Cannot be computed** |
| Symbios | Pelvis to mid-shaft femur, knees and ankles | 120 | Adapted to patient morphology | 1.25–2 mm (pelvis) | Cannot be computed** |
| Zimmer Biomet | Iliac crest to mid-femur and knees | Not specified* | Not specified* | 2 mm by 2 mm; 2.5 mm by 2.5 mm or 3 mm by 3 mm | Cannot be computed** |
| Lima Corp. | Iliac crest to mid-femur and knees | Not specified* | Not specified* | 1–2 mm | Cannot be computed** |
| JRI | Superior origin of sacroiliac joints to 5 cm below lesser trochanter | 100–150 | Not specified* | <1 mm | Cannot be computed** |
| HipXpert | Whole pelvis to proximal femur and knees | Not specified* | Not specified* | 2–2.5 mm | Cannot be computed** |
| Implantcast | Above Iliac crest to below ischial tuberosity | Not specified* | Not specified* | 1 mm | Cannot be computed** |
| OSSIS | Above Iliac crest to below ischial tuberosity | Not specified* | Not specified* | <1.25 mm | Cannot be computed** |
| LEXI ZedHip | Whole pelvis and femur | Not specified* | Not specified* | <2 mm | Cannot be computed** |
| AQ Solutions | Iliac crest to mid-femur, knees and ankles | Not specified* | Not specified* | 2 mm | Cannot be computed** |
| MediCAD | Top of iliac crest to 20 cm distally to the centre of femoral head and knees | Not specified* | Not specified* | 2.0 mm; 1.25–2.0 mm (acceptable) | Cannot be computed** |
| Adler | Not specified* | Not specified* | Not specified* | 2 mm | Cannot be computed** |
| Signature Orthopaedics | Most superior point of ilium to just below most inferior point of ischium | Not specified* | Not specified* | Not specified* | Cannot be computed** |
*Information left to the discretion of the imaging centre; **Not enough information provided for effective dose calculations to be made.
Figure 2.
Box and whisker plot showing the variability in field of view, kV and mAs across the studied protocols.
Effective dose variability
Merely four out of the 17 protocols provide a complete set of technical parameters in their protocols i.e. the field of view, kV and mAs setting at which the CT scanner should be operated in. Thus, the variability across these protocols was studied in terms of the typical effective dose that would be deposited to an average-sized patient. We used a dedicated dosimetry software, VirtualDoseTM to estimate CT dose to a ‘virtual patient’ based on the CT scanner, technical factors and the characteristics of the patient. For the purpose of this review, the dose calculations were based on the Philips Ingenuity CT scanner series, to match our institution’s scanner and doses were measured for both, male and female patients. Out of the four protocols, Smith & Nephew and Stryker provide a range for these parameters, enabling a maximum and minimum effective dose to be estimated from them, whereas Conformis and Medacta simply state a kV of >120 kV, so no maximum value could be inferred.
The estimated effective doses are presented in Table 4. Figure 3 presents this data in a box and whisker plot. For comparison purposes, the effective dose associated with a single pelvic x-ray (41, 42), 3 pelvic x-rays and annual background radiation to someone living in the UK (43) are also plotted. A large variability across the four protocols can be seen; the minimum effective dose to a female patient following the Stryker MAKO protocol (8.13 mSv) is equivalent to the maximum effective dose to a female patient following the Smith & Nephew Dyonics plan (8.14 mSv). Similarly, the maximum effective dose corresponding to the Smith & Nephew protocol, for male and female patients (6.83 and 8.14 mSv, respectively), is smaller than the minimum patient exposure imposed by the Medacta MyHip protocol. Thus, the inconsistency previously seen across the protocols has a direct impact on patient dose, emphasising the need for harmonisation among the different orthopaedic companies and the protocols that they distribute to hospitals and institutions. Figure 3 highlights that even the minimum effective dose estimated from the CT protocols is greater than the recommended annual dose received by UK citizens from natural background radiation.
Table 4.
Maximum and minimum effective dose measurements computed using VirtualDoseTM for four protocols.
| Hip CT protocol | Minimum effective dose (mSv) | Maximum effective dose (mSv) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Stryker MAKO | 6.76 | 8.13 | 12.94 | 15.80 |
| Smith & Nephew Dyonics | 3.41 | 4.07 | 6.83 | 8.14 |
| Conformis Hip System | 2.55 | 3.99 | Cannot be computed* | Cannot be computed* |
| Medacta MyHip | 8.11 | 9.76 | Cannot be computed* | Cannot be computed* |
*Not enough information provided for effective dose calculations to be made.
Figure 3.
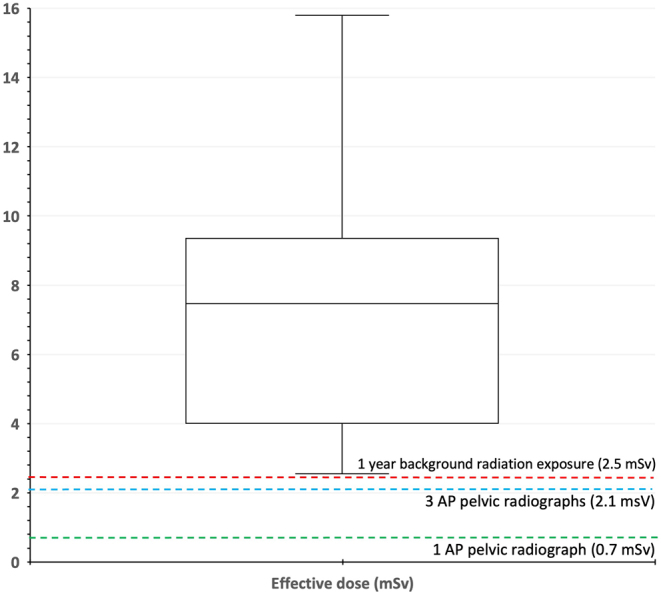
Box and whisker plot showing the variability in the effective dose estimated from four CT protocols.
Although the lifetime attributable risks of cancer associated with the range of the calculated doses are estimated to be small, the number needed to harm (NNH) is relatively low compared with that of a standard pelvic radiograph, raising a concern, as the risk increases for younger ages (44, 45). Given that bony anatomy may easily be seen on CT scans, the current evidence suggests that an ideal trade-off between image resolution and dose to the patient must be established.
Consideration of pelvic tilt
In order to plan adequate implant position and pelvic orientation, tilt in all three planes is increasingly needed in the CT protocols used for planning (46). However, while the analysis of spinal sagittal balance is useful, current CT scanners do not allow functional measurement of pelvic tilt as the patient is supine in the scanner. Additional upright imaging is needed with the consequent additional radiation burden to the patient.
Conclusion
This is the first study to present the large variability that exists among the CT protocols published and distributed by orthopaedic companies offering CT planning to UK surgeons. There is a lack of harmonisation between the studied protocols used for planning THA. The majority of the protocols presented incomplete information, which can cause confusion among radiographers when performing the requested scans. While ensuring that the patient is not overexposed to ionising radiation, obtaining CT scans with an appropriate field of view should decrease manufacturers’ requests for repeat scans. Since poorly chosen acquisition parameters have adverse radiation impacts, optimal imaging will offer an ideal balance between image quality and radiation dose to the patient. This, in turn, will help lower cancer risks in young adult hips, particularly in women who are of childbearing age (aged between 12 and 55 years old). Establishing a single harmonised low-dose protocol will help reduce the radiation dose to a limit that is tolerable while enabling bony anatomy to be clearly identified for accurate preoperative THA planning, PSI and custom implants.
ICMJE Conflict of Interest Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding Statement
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
This research was supported by the Arthroplasty for Arthritis Charity, the Maurice Hatter Foundation, the RNOH Charity, the Rosetrees Trust and the Stoneygate Trust and by researchers at the National Institute for Health Research, University College London Hospitals Biomedical Research Centre.
References
- 1.Chen X Wang Y Ma R Peng H Zhu S Li S Li S Dong X Qiu G & Qian W. Validation of CT-based three-dimensional preoperative planning in comparison with acetate templating for primary total hip arthroplasty. Orthopaedic Surgery 2022141152–1160. ( 10.1111/os.13298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujishiro T Hayashi S Kanzaki N Hashimoto S Kurosaka M Kanno T & Masuda T. Computed tomographic measurement of acetabular and femoral component version in total hip arthroplasty. International Orthopaedics 201438941–946. ( 10.1007/s00264-013-2264-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson CG Brilliant ZR Jang SJ Sokrab R Mayman DJ Vigdorchik JM Sculco PK & Jerabek SA. Validating the use of 3D biplanar radiography versus CT when measuring femoral anteversion after total hip arthroplasty: a comparative study. Bone and Joint Journal 2022104–B1196–1201. ( 10.1302/0301-620X.104B11.BJJ-2022-0194.R2) [DOI] [PubMed] [Google Scholar]
- 4.McCollough CH Primak AN Braun N Kofler J Yu L & Christner J. Strategies for reducing radiation dose in CT. Radiologic Clinics of North America 20094727–40. ( 10.1016/j.rcl.2008.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadakis AE Perisinakis K & Damilakis J. Angular on-line tube current modulation in multidetector CT examinations of children and adults: the influence of different scanning parameters on dose reduction. Medical Physics 2007342864–2874. ( 10.1118/1.2747048) [DOI] [PubMed] [Google Scholar]
- 6.Gervaise A Teixeira P Villani N Lecocq S Louis M & Blum A. CT dose optimisation and reduction in osteoarticular disease. Diagnostic and Interventional Imaging 201394371–388. ( 10.1016/j.diii.2012.05.017) [DOI] [PubMed] [Google Scholar]
- 7.Lattanzi R Baruffaldi F Zannoni C & Viceconti M. Specialised CT scan protocols for 3-D pre-operative planning of total hip replacement. Medical Engineering and Physics 200426237–245. ( 10.1016/j.medengphy.2003.11.008) [DOI] [PubMed] [Google Scholar]
- 8.Firmin L & Steward MJ. Explaining radiation risks to patients. British Journal of Hospital Medicine 201374C162–C165. ( 10.12968/hmed.2013.74.sup11.c162) [DOI] [PubMed] [Google Scholar]
- 9.UK Health Security Agency. Ionising radiation: dose comparisons 2011. Available at: https://www.gov.uk/government/publications/ionising-radiation-dose-comparisons/ionising-radiation-dose-comparisons (date last accessed 24 November 2022). [Google Scholar]
- 10.Smith-Bindman R, Wang Y, Chu P, Chung R, Einstein AJ, Balcombe J, Cocker M, Das M, Delman BN, Flynn M, et al. International variation in radiation dose for computed tomography examinations: prospective Cohort Study. BMJ 2019364k4931. ( 10.1136/bmj.k4931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong KC. 3D-printed patient-specific applications in orthopedics. Orthopedic Research and Reviews 2016857–66. ( 10.2147/ORR.S99614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia RZ Zhai ZJ Chang YY & Li HW. Clinical applications of 3-dimensional printing technology in hip joint. Orthopaedic Surgery 201911533–544. ( 10.1111/os.12468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa T Takao M Sakai T & Sugano N. Factors related to disagreement in implant size between preoperative CT-based planning and the actual implants used intraoperatively for total hip arthroplasty. International Journal of Computer Assisted Radiology and Surgery 201813551–562. ( 10.1007/s11548-017-1693-3) [DOI] [PubMed] [Google Scholar]
- 14.Constantinescu DS Costello JP Dalling AD Wagner JD Al-Hardan W & Carvajal JA. The efficacy of patient specific instrumentation (PSI) in total hip arthroplasty (THA): a systematic review and meta-analysis. Journal of Orthopaedics 202234404–413. ( 10.1016/j.jor.2022.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komeno M Hasegawa M Sudo A & Uchida A. Computed tomographic evaluation of component position on dislocation after total hip arthroplasty. Orthopedics 2006291104–1108. ( 10.3928/01477447-20061201-05) [DOI] [PubMed] [Google Scholar]
- 16.Colombi A Schena D & Castelli CC. Total hip arthroplasty planning. EFORT Open Reviews 20194626–632. ( 10.1302/2058-5241.4.180075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huppertz A Radmer S Wagner M Roessler T Hamm B & Sparmann M. Computed tomography for preoperative planning in total hip arthroplasty: what radiologists need to know. Skeletal Radiology 2014431041–1051. ( 10.1007/s00256-014-1853-2) [DOI] [PubMed] [Google Scholar]
- 18.D’Amore T Klein G & Lonner J. The use of computerized tomography scans in elective knee and hip arthroplasty—what do they tell us and at what risk? Arthroplasty Today 202215132–138. ( 10.1016/j.artd.2022.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackenbroch C Feilhuber M Halt D Riesner HJ Beer M & Wunderlich A. Low-dose CT in pelvic imaging: comparing dose and image quality in relation to clinical value in a phantom study. American Journal of Roentgenology 2021216453–463. ( 10.2214/AJR.20.22907) [DOI] [PubMed] [Google Scholar]
- 20.Henckel J, Richards R, Lozhkin K, Harris S, Baena FM, Barrett AR, et al. Very low-dose computed tomography for planning and outcome measurement in knee replacement. Journal of Bone and Joint Surgery. British Volume 200688-B1513–1518. ( 10.1302/0301-620X.88B11.17986) [DOI] [PubMed] [Google Scholar]
- 21.He T Qian X Zhai R & Yang Z. Computed tomography number measurement consistency under different beam hardening conditions: Comparison Between Dual-Energy Spectral Computed Tomography and Conventional Computed Tomography Imaging in Phantom Experiment. Journal of Computer Assisted Tomography 201539981–985. ( 10.1097/RCT.0000000000000287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gausden EB Nwachukwu BU Schreiber JJ Lorich DG & Lane JM. Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: a qualitative systematic review. Journal of Bone and Joint Surgery. American Volume 2017991580–1590. ( 10.2106/JBJS.16.00749) [DOI] [PubMed] [Google Scholar]
- 23.Yaprak G Gemici C Seseogullari OO Karabag IS& Cini N. CT derived Hounsfield Unit: an easy way to determine osteoporosis and radiation related fracture risk in irradiated patients. Frontiers in Oncology 2020101–7. ( 10.3389/fonc.2020.00742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ybinger T Kumpan W Hoffart HE Muschalik B Bullmann W & Zweymüller K. Accuracy of navigation-assisted acetabular component positioning studied by computed tomography measurements: Methods and results. Journal of Arthroplasty 200722812–817. ( 10.1016/j.arth.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 25.Hohmann E Bryant A & Tetsworth K. Accuracy of acetabular Cup positioning using imageless navigation. Journal of Orthopaedic Surgery and Research 2011640. ( 10.1186/1749-799X-6-40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su AW Hillen TJ Eutsler EP Bedi A Ross JR Larson CM Clohisy JC & Nepple JJ. Low-dose computed tomography reduces radiation exposure by 90% compared with traditional computed tomography among patients undergoing hip-preservation surgery. Arthroscopy 2019351385–1392. ( 10.1016/j.arthro.2018.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeed MK. A comparison of the CT-dosimetry software packages based on stylized and boundary representation phantoms. Radiography 202026e214–e222. ( 10.1016/j.radi.2020.02.011) [DOI] [PubMed] [Google Scholar]
- 28.Rahm S Hoch A Tondelli T Fuchs J & Zingg PO. Revision rate of THA in patients younger than 40 years depends on primary diagnosis – a retrospective analysis with a minimum follow-up of 10 years. European Journal of Orthopaedic Surgery and Traumatology: Orthopedie Traumatologie 2021311335–1344. ( 10.1007/s00590-021-02881-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deere K Whitehouse MR Kunutsor SK Sayers A Mason J & Blom AW. How long do revised and multiply revised hip replacements last? A retrospective observational study of the National Joint Registry. Lancet Rheumatology 20224e468–e479. ( 10.1016/S2665-9913(2200097-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouyoumdjian P Mansour J Assi C Caton J Lustig S & Coulomb R. Current concepts in robotic total hip arthroplasty. SICOT-J 2020645. ( 10.1051/sicotj/2020041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi XT Li CF Cheng CM Feng CY Li SX & Liu JG. Preoperative planning for total hip arthroplasty for neglected developmental dysplasia of the hip. Orthopaedic Surgery 201911348–355. ( 10.1111/os.12472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huppertz A, Lembcke A, Sariali E, Durmus T, Schwenke C, Hamm B, Sparmann M, Baur AGJ. Low dose computed tomography for 3D planning of total hip arthroplasty. Journal of Computer Assisted Tomography 201539649–656. ( 10.1097/rct.0000000000000271) [DOI] [PubMed] [Google Scholar]
- 33.Martin CJ & Sookpeng S. Setting up computed tomography automatic tube current modulation systems. Journal of Radiological Protection 201636R74–R95. ( 10.1088/0952-4746/36/3/R74) [DOI] [PubMed] [Google Scholar]
- 34.Bebbington NA Jørgensen T Dupont E & Micheelsen MA. Validation of care KV automated tube voltage selection for PET-CT: pet quantification and CT radiation dose reduction in phantoms. EJNMMI Physics 2021829. ( 10.1186/s40658-021-00373-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lira D Padole A Kalra MK & Singh S. Tube potential and CT radiation dose optimization. American Journal of Roentgenology 2015204W4–10. ( 10.2214/AJR.14.13281) [DOI] [PubMed] [Google Scholar]
- 36.Li X Segars WP & Samei E. The impact on CT dose of the variability in tube current modulation technology: a theoretical investigation. Physics in Medicine and Biology 2014594525–4548. ( 10.1088/0031-9155/59/16/4525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iball GR Brettle DS & Moore AC. Assessment of tube current modulation in pelvic CT. British Journal of Radiology 20067962–70. ( 10.1259/bjr/50019934) [DOI] [PubMed] [Google Scholar]
- 38.Kalender WA Wolf H & Suess C. Dose reduction in CT by anatomically adapted tube current modulation. II. Phantom measurements. Medical Physics 1999262248–2253. ( 10.1118/1.598738) [DOI] [PubMed] [Google Scholar]
- 39.Goldman LW. Principles of CT: radiation Dose and image quality. Journal of Nuclear Medicine Technology 200735213–226. ( 10.2967/jnmt.106.037846) [DOI] [PubMed] [Google Scholar]
- 40.Maldjian PD & Goldman AR. Reducing radiation dose in body CT: a Primer on dose metrics and key CT technical parameters. American Journal of Roentgenology 2013200741–747. ( 10.2214/AJR.12.9768) [DOI] [PubMed] [Google Scholar]
- 41.Mraity HA. Optimisation of Radiation Dose and Image Quality for AP Pelvis Radiographic Examination [Thesis]. University of Salford; 2015. Available at: https://usir.salford.ac.uk/id/eprint/36914/1/Hussein%20Abid%20Ali%20Bakir%20Mraity%20PhD%20Thesis.pdf (date last accessed 20 November 2022). [Google Scholar]
- 42.Public Health England. Patient dose information: guidance 2008. Available at: https://www.gov.uk/government/publications/medical-radiation-patient-doses/patient-dose-information-guidance (Date last accessed 20 November 2022). [Google Scholar]
- 43.Harrison RM. The role of the medical physicist in radiation protection in Hospitals. Physics Education 198924222–226. ( 10.1088/0031-9120/24/4/311) [DOI] [Google Scholar]
- 44.Wylie JD Jenkins PA Beckmann JT Peters CL Aoki SK & Maak TG. Computed tomography scans in patients with young adult hip pain carry a lifetime risk of malignancy. Arthroscopy 201834155–163.e3. ( 10.1016/j.arthro.2017.08.235) [DOI] [PubMed] [Google Scholar]
- 45.Hall EJ & Brenner DJ. Cancer risks from Diagnostic Radiology. British Journal of Radiology 200881362–378. ( 10.1259/bjr/01948454) [DOI] [PubMed] [Google Scholar]
- 46.Hamada H Uemura K Takashima K Ando W Takao M & Sugano N. What changes in pelvic sagittal tilt occur 20 years after THA? Clinical Orthopaedics and Related Research 2023481690–699. ( 10.1097/CORR.0000000000002382) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a