Abstract
The role of Thiobacillus ferrooxidans in bacterial leaching of mineral sulfides is controversial. Much of the controversy is due to the fact that the solution conditions, especially the concentrations of ferric and ferrous ions, change during experiments. The role of the bacteria would be more easily discernible if the concentrations of ferric and ferrous ions were maintained at set values throughout the experimental period. In this paper we report results obtained by using the constant redox potential apparatus described previously (P. I. Harvey and F. K. Crundwell, Appl. Environ. Microbiol. 63:2586–2592, 1997). This apparatus is designed to control the redox potential in the leaching compartment of an electrolytic cell by reduction or oxidation of dissolved iron. By controlling the redox potential the apparatus maintains the concentrations of ferrous and ferric ions at their initial values. Experiments were conducted in the presence of T. ferrooxidans and under sterile conditions. Analysis of the conversion of zinc sulfide in the absence of the bacteria and analysis of the conversion of zinc sulfate in the presence of the bacteria produced the same results. This indicates that the only role of the bacteria under the conditions used is regeneration of ferric ions in solution. In this work we found no evidence that there is a direct mechanism for bacterial leaching.
Bacterial leaching is a commercially successful process used for pretreatment of refractory gold-bearing sulfides. The first commercial operation, at Fairview Gold Mine (South Africa), began in 1986 with a capacity of 10 tons per day (16). This plant now treats 35 tons of a pyrite-arsenopyrite concentrate per day. The largest bacterial leaching plant, at Ashanti in Ghana, presently treats 1,100 tons of gold-bearing pyritic concentrate per day. There are also operations at Sao Bento in Brazil and at Harbour Lights in Australia (16). More recently, a bacterial leaching project has been set up in Uganda to extract cobalt from a colbaltiferous pyrite ore (17).
In addition to the commercial value of bacterial leaching, bacterial interaction with sulfide minerals is a significant factor in the formation of acid mine drainage and acid rock drainage (8, 9, 30, 31). Acid mine drainage is a major environmental problem, and large amounts of effort are invested in the remediation of sites (20).
Knowledge of the mechanisms of bacterial oxidation of sulfide minerals could greatly improve the design and operation of bacterial leaching plants and may assist in the prevention of acid mine drainage.
Thiobacillus ferrooxidans, the microorganism that is primarily associated with these leaching processes, is able to oxidize ferrous ions and reduced sulfur compounds (9). The product of oxidation of ferrous ions, ferric ions, is a strong oxidant that is capable of oxidizing sulfide minerals (19). One of the two dominant views on the mechanism of bacterial leaching is that the overall leaching process occurs by bacterial oxidation of ferrous ions (13) and chemical leaching of the mineral (38, 39). This process is described by the following set of reactions for bacterial leaching of zinc sulfide:
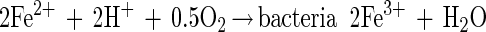 |
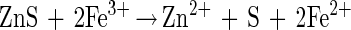 |
This mechanism is often referred to as the indirect mechanism (39), and it is clear that the iron couple plays a central and critical role in this mechanism.
The second proposed mechanism involves bacterial catalysis of the dissolution of sulfide minerals. It has been proposed that the bacteria are able to directly interact with the mineral and enhance the rate of dissolution of the mineral above the rate achieved during chemical leaching by ferric ions under the same conditions (21, 28, 39, 46). This mechanism is often referred to as the direct mechanism (39). In the case of zinc sulfide, the direct mechanism may be represented as follows (39):
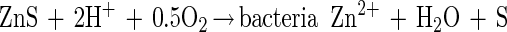 |
A distinction between the two mechanisms is often made on the basis of the role of iron in bacterial leaching. In the indirect mechanism, the presence of dissolved iron is critical, whereas the presence of dissolved iron in the direct mechanism is often considered superfluous (21, 37–39). However, there is the possibility that during the oxidation of sulfides, ferric ions act as the electron acceptors instead of oxygen (43). A large number of studies have been aimed at determining which of these two mechanisms dominates the bacterial leaching process (4, 5, 7, 10, 18, 21, 31, 34, 36, 48).
Boon et al. (5) recently studied bacterial leaching of pure zinc sulfide by using the experimental procedure of Verbaan and Huberts (48). These authors presented the results of only one leaching experiment performed over a period of 20 h, in which the level of leaching conversion was about 30%. The amount of zinc released into solution in the presence of bacteria is consistently higher than the amount of zinc released into solution in the absence of bacteria. This difference increases with reaction time. In spite of this difference in leaching results, Boon et al. concluded that the only mechanism of leaching is the indirect mechanism. In addition, they reported that the bacteria became inactive with respect to their ability to oxidize ferrous ions during the period of the experiment (5).
Boon et al. (2) measured the specific oxygen uptake rates of T. ferrooxidans in the presence of ferrous ions and pyrite. The bacteria were exposed to various amounts of pyrite for periods of time ranging from 3 to 20 h. Boon et al. concluded that the oxygen uptake rates observed supported the conclusion that the mechanism of leaching was the indirect mechanism. However, there are significant differences between the two sets of data under the range of conditions under which a comparison is possible. In addition, the experimental method used assumed that oxygen is the only electron acceptor in the bacterial electron transport chain.
The concentrations of the ferric and ferrous ions in almost all of the previously described experiments varied over a wide range during the course of the experiments (4, 5, 7, 48). Since one of the possible mechanisms is strongly dependent on the concentrations of ferrous and ferric ions, changes in the concentrations of these species during the experiments made it difficult to determine which of the two mechanisms was dominant. This led to contradictory conclusions concerning the role of the bacteria in dissolution of sulfide minerals (4, 5, 31, 36).
On the other hand, if the concentrations of ferrous and ferric ions are controlled at constant values, bacterial and chemical leaching experiments can be conducted under conditions under which the concentrations in solution are the same for the duration of the experiment. This means that the contribution of each of the proposed mechanisms of bacterial leaching can be directly determined from a comparison of the extents of dissolution in bacterial and chemical leaching experiments conducted under the same conditions (23). Therefore, it is possible in this way to determine unambiguously the relative importance of the two mechanisms of bacterial leaching.
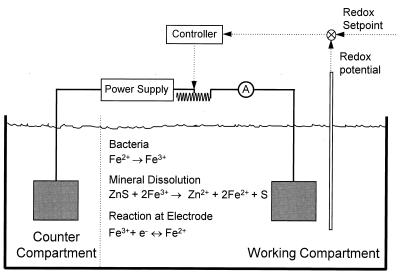
We described in a previous paper a unique apparatus that was designed to control the concentrations of ferric and ferrous ions in solution during batch growth and bacterial leaching experiments (23). This apparatus is a two-compartment electrolysis cell in which the two compartments are separated by an ion-exchange membrane. The leaching reactions, either chemical or bacterial, take place in one of the compartments. The redox potential, which is a measure of the concentrations of ferrous and ferric ions in solution, is maintained at a constant value in the working compartment by manipulating the electrolytic current. This is achieved by the measurement and control loop shown in Fig. 1. The current through the cell is the difference between rate of production of ferrous ions by the chemical leaching reaction and the rate of consumption of ferrous ions by the bacteria. In principle, this reactor is a fed-batch reactor in which the concentrations are replenished electrolytically. Experiments performed with this reactor have the advantage that no chemical reagents are added to the solution, so that the conditions of the chemical and bacterial leaching experiments are the same for the duration of the experiments.
FIG. 1.
Schematic diagram of the experimental apparatus. The working compartment is the compartment in which leaching experiments are conducted. The flow of current is regulated by adjusting the variable resistor so that the redox potential remains at the setpoint value.
The objective of this study was to investigate the mechanism of dissolution of sulfide ores in the presence of T. ferrooxidans under conditions under which the concentrations of ferric and ferrous ions were controlled for the duration of the experiment. In this paper we report the results of a study aimed at determining the mechanism of bacterial leaching of zinc sulfide (sphalerite).
MATERIALS AND METHODS
Apparatus.
The electrolysis cell was made of Plexigas and was divided into two sections by an anion-exchange membrane (Sybron Chemicals Inc., Birmingham, N.J.). The electrolysis cell was fitted with a perspex lid to minimize evaporation of the solution. The working volume of the cell was 2 liters. The contents of the working compartment were stirred by a three-bladed impeller driven by an overhead motor, and the compartment was sparged with air. Electrodes to measure the redox potential and the concentration of oxygen were suspended in the solution in the working compartment.
Redox potential measurements were made with a platinum electrode and an Ag-AgCl reference electrode by using a high-impedance galvanically isolated differential amplifier and an analog-digital control card (type PC30; Eagle Technology, Cape Town, South Africa) recorded by computer. A computer program determined the values of the output signals from the PC30 card to the relay switch and the variable resistor that varied the direction and magnitude of the current. The current was measured by determining the potential difference across a precision resistor.
The redox potential was controlled within 0.1% of the setpoint for the period of the experiment. All of the solution samples were analyzed for ferrous ions in order to confirm that the control of the redox potential maintained the concentration of ferrous ions at a constant value. Typical results showed that the concentration of ferrous ions differed from the initial value by less than 0.8%.
The pH of the solution in the working compartment was 1.6. The pH was measured throughout each experiment and remained within 0.05 pH unit of the initial value. The concentration of dissolved oxygen was measured (Hanna Instruments) and was maintained manually at 5.9 mg/liter. The electrolysis cell was placed in a water bath, and the temperature was maintained at 35 ± 0.1°C.
Bacterial culture.
A pure strain of T. ferrooxidans (strain FC1) was used. This organism, which was supplied by D. Rawlings of the University of Cape Town, Cape Town, South Africa, has been thoroughly characterized (35). The bacteria were cultured on a medium which contained (per liter) 1.5 g of (NH4)2SO4, 0.5 g of K2HPO4, 0.5 g of MgSO4 · 7H2O, and 45 g of FeSO4 · 7H2O (40). The pH of the medium was adjusted to 1.6 by adding H2SO4. The bacteria were maintained in the exponential growth phase by subculturing one-third of the culture volume on a daily basis.
Preparation and characterization of the ore.
The sphalerite concentrate, which was from the Gamsberg deposit, was supplied by Gold Fields Research Laboratories. This sample was milled and wet screened to a size fraction of −53+45 μm. The ore contained 53.3% Zn, 7.84% Fe, 32.5% S, 1.19% Mn, and 0.24% Pb. The metallic element contents were determined by analyzing the solution by atomic adsorption spectrophotometry with a Varian Spectra AA30 spectrophotometer after acid digestion. The sulfur content was determined with a LECO model SC32 DB64 Sulphur Determinator. X-ray diffraction at a scan rate of 0.05°/s indicated that the sample contained 98% sphalerite, which supported the conclusion that the iron was present in the solid solution as (Zn,Fe)S rather than as a separate mineral (12). The ore sample was washed with a 0.5 M solution of sodium sulfide to remove flotation agents and to sulfidize the mineral surface (11, 24, 41, 44).
Reagents.
Analytical reagents were used throughout this work.
Analytical techniques.
The number of bacterial cells in a solution was determined by counting with a hemacytometer (depth, 0.1 mm; area, 0.0025 mm2). The cells were stained by using crystal violet in a citric acid solution as the stain. The standard deviation for the cell number determinations was 1.2% of the mean (10 replicates).
The concentration of ferrous ions in solution was determined by titration with potassium dichromate by using sodium diphenylamine sulfonate as the indicator (49). The standard deviation for the determinations of ferrous ion concentrations was 1.1% of the mean (10 replicates). The total concentration of iron in solution was determined by using the titration for ferrous ions once the iron had been reduced to the ferrous state with stannous chloride. The concentration of ferric ions was calculated by determining the difference. (Note that measurements of the redox potential were used for control purposes only; they were not used for chemical analysis.)
The concentration of zinc in solution was determined by atomic adsorption spectrophotometry with the Varian Spectra AA30 spectrophotometer. The standard deviation for the determinations of the concentration of zinc in solution was 0.3% of the mean (10 replicates).
Procedure.
All experiments were conducted in the same medium but with different ferric ion concentrations. The concentration of ferrous ions was 1 g/liter for all experiments. The solids loading concentration was 5 g of sphalerite per liter. The bacterial leaching experiment preparations were inoculated with inocula equivalent to 10% (by volume) of the reactor size. Samples were withdrawn from the working compartment at the same time intervals for every leaching experiment performed.
Part of each sample was used to determine the bacterial cell population in suspension. The remaining part of each sample was immediately filtered by using a Millipore filter (Sterifil aseptic system with sterile, individually sealed, 0.45-μm-pore-size filter membrane). The filtrate was used to determine the concentrations of ferrous and ferric ions and the concentration of zinc ions in solution. The solid residues on the filter membrane were immersed in a 95% solution of ethanol to fix the bacteria (in bacterial leaching experiments) on the surfaces of the mineral particles. These preparations were critical point dried and coated for investigation with a scanning electron microscope (SEM). Samples were withdrawn at regular intervals from the counter compartment. These samples were also analyzed to determine the concentration of zinc ions in solution. Analyses revealed that no zinc was transferred from the working compartment to the counter compartment through the anion-exchange membrane.
The solution samples obtained from the sterile (chemical leaching) experiments were checked for contamination by T. ferrooxidans. No bacteria were detected by a microscopic investigation. Between successive experiments, the electrolysis cell was soaked in hydrochloric acid, rinsed with water, cleaned with an ammonia-based cleaning solution (pH 10), and finally rinsed with distilled water.
RESULTS
Experiments to examine bacterial and chemical leaching of sphalerite were conducted under exactly the same solution conditions in the controlled-redox-potential apparatus. Greater conversion of the mineral in a bacterial leaching experiment than in the corresponding chemical leaching experiment would have indicated that the bacteria contributed to the dissolution of the mineral beyond their ability to oxidize ferrous ions.
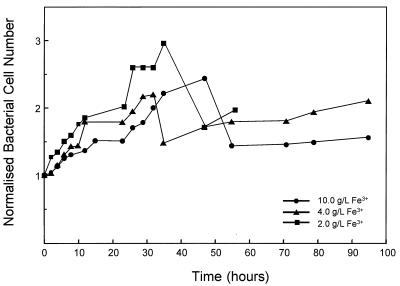
Figure 2 is a plot of the normalized number of bacteria in suspension versus time for three of the bacterial leaching experiments and shows the effect of the concentration of ferric ions in solution. The normalized bacterial cell number is the bacterial cell count divided by the initial bacterial cell count. The number of bacteria in suspension is an underestimate of the total number of bacteria present as the bacteria attach to the surfaces of mineral particles. Figure 2 shows that the number of bacteria in solution increased with time for the first 35 h of the experiment. The normalized bacterial cell number was greater at lower concentrations of ferric ions in solution, showing that the bacteria were inhibited by the ferric ions in solution. This effect was expected (15, 23, 26, 32). After the initial period of growth, the cell population in solution decreased substantially.
FIG. 2.
Effect of the concentration of the ferric ions in solution on the normalized bacterial cell number in suspension. The initial cell numbers were 4.2 × 108 cells/ml (•), 3.4 × 108 cells/ml (▴), and 3.4 × 108 cells/ml (■).
The bacteria on the surfaces of the mineral particles were examined by SEM. SEM analysis of samples taken after 2 h showed that the bacteria were present on the surfaces as single cells, indicating that they attached rapidly to the mineral surfaces. The concentration of bacteria on the surfaces increased for the first 35 h of the experiment. These bacteria were all present on the surfaces as single cells; there was no indication of microcolonies or biofilms on the surfaces. The mineral surfaces were also covered with sulfur, a product of the dissolution reaction. After about 47 h, the appearance of the bacteria on the mineral particles changed; this corresponded to the point in Fig. 2 at which there was a dramatic change in the bacterial population in solution. Samples of the mineral particles were covered with a large amount of exopolymer, suggesting that large numbers of bacteria were present on the surfaces. In addition, the sulfur that was present previously was no longer present, suggesting that the bacteria oxidized the sulfur to sulfate (34, 42, 45). The SEM analysis indicated that the bacteria were attached to the surfaces for the duration of the experiment; however, after about 47 h large numbers of bacteria were present on the mineral particles. This seems to account for the decrease in the number of bacteria in suspension.
Five bacterial and five chemical leaching experiments were performed with various concentrations of ferric ions. The purpose of varying the concentration of ferric ions was to change the rate of bacterial growth in solution and the rate of dissolution of zinc sulfide. At low concentrations of ferric ions, the rate of chemical dissolution of zinc sulfide is low (11, 14, 47) and the rate of bacterial growth in solution is not inhibited by ferric ions. Since the rate of chemical leaching is low, any contribution to the dissolution by direct bacterial action should be easily discernible. On the other hand, the rate of bacterial growth in solution is inhibited at high concentrations of ferric ions (15, 26, 32). Under these adverse conditions for bacterial growth in solution, the bacteria should prefer a different substrate, such as that proposed for direct action of the bacteria on the mineral sulfide. Therefore, by performing experiments with a range of concentrations of ferric ions, we should be able to detect the contribution of direct bacterial action.
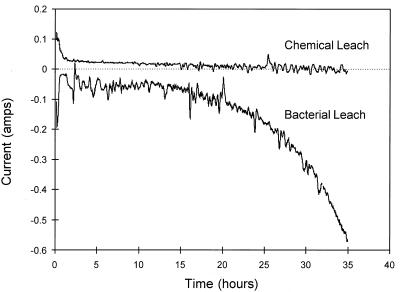
The solution conditions were controlled throughout each experiment. The current through the electrolytic cell changed due to the reactions that occurred in the working compartment. Figure 3 shows typical values for the current that passed through the electrolytic cell. Positive currents represent the oxidation of ferrous ions, while negative currents represent the reduction of ferric ions. Thus, the current is positive when chemical leaching is dominant, while the current is negative when bacterial oxidation of ferrous ions is dominant. In a chemical leaching experiment, only one reaction is possible, and the current is a measure of the rate of the chemical leaching reaction. In the bacterial leaching experiment, both chemical leaching and bacterial oxidation of ferrous ions occurred. In this case, the current was the difference between the rates of these two reactions. Therefore, the rate of bacterial oxidation of ferrous ions was the difference between the current for the bacterial leaching experiment and the current for the chemical leaching experiment. Inspection of results similar to those shown in Fig. 3 for each experiment and the SEM micrographs of the mineral particles indicated that the bacteria were active in oxidation of both ferrous ions and elemental sulfur.
FIG. 3.
Typical current required to maintain the redox potential at its setpoint value. The solution conditions were as follows: 10% (vol/vol) T. ferrooxidans inoculum; Fe2+ concentration, 8.0 g/liter; Fe3+ concentration, 1.0 g/liter; solids density, 5 g/liter; temperature, 35°C; pH, ca. 1.6; O2 concentration, ca. 5.9 mg/liter.
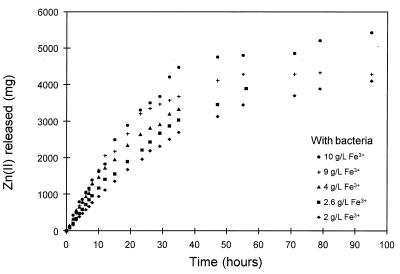
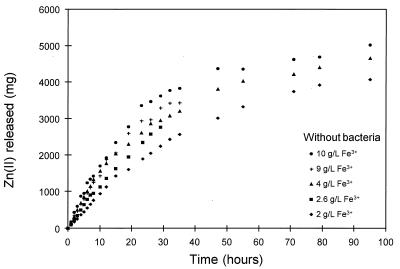
The amounts of zinc released from the mineral with time in the bacterial and chemical leaching experiments are shown in Fig. 4 and 5, respectively, for five different concentrations of ferric ions. A value of 5,220 mg of Zn released from the mineral represented complete conversion of the mineral. The results of the chemical leaching experiments were similar to the results of the bacterial leaching experiments. The chemical leaching results show the expected trend due to the kinetics of the leaching reaction, in which the rate of dissolution increases with increasing concentrations of ferric ions. The results are consistent with previously published data on the chemical dissolution of sphalerite (11, 12, 47).
FIG. 4.
Effect of the concentration of ferric ions in solution on the bacterial leaching of sphalerite. The solution conditions were as follows: 10% (vol/vol) T. ferrooxidans inoculum; Fe2+ concentration, 1.0 g/liter; solids density, 5 g/liter; temperature, 35°C; pH ca. 1.6; O2 concentration, ca. 5.9 mg/liter.
FIG. 5.
Effect of the concentration of ferric ions in solution on the chemical leaching of sphalerite. The solution conditions were as follows: Fe2+ concentration, 1.0 g/liter; solids density, 5 g/liter; temperature, 35°C; pH, ca. 1.6; O2 concentration, ca. 5.9 mg/liter.
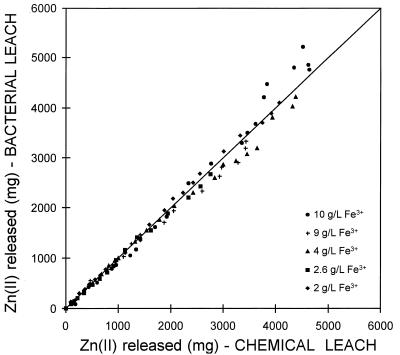
Since the solution conditions were the same in the chemical and bacterial leaching experiments, the effect that the presence of the bacteria had on the mineral could be determined by plotting the conversion obtained in the bacterial experiments against the conversion obtained in the corresponding chemical experiment at the same time. These results are shown in Fig. 6.
FIG. 6.
Comparison of the conversion of sphalerite obtained in a bacterial leaching experiment and the conversion of sphalerite obtained in a chemical leaching experiment. The same conditions were used for both experiments.
Figure 6 shows that the conversion for bacterial leaching of sphalerite was the same as the conversion observed during chemical leaching of the sphalerite. This result is significant; it is unambiguous evidence that there is no direct mechanism for dissolution of sphalerite and that the role of bacteria is the oxidation of ferrous ions in solution.
DISCUSSION
The results for bacterial leaching of zinc sulfide obtained in this study are different from the results described previously (6, 7, 22, 25, 28, 46). Data presented previously showed that bacterial leaching results in a much higher level of extraction of metal than a sterile control (chemical leaching) under the same initial solution conditions. On the basis of these results, most of the researchers concluded that the bacteria act as a catalyst for the leaching reaction by the direct mechanism. This interpretation is typical of much of the work done previously on bacterial leaching of sulfide minerals (28, 29, 45). However, in this previous work, the ferric ions in the sterile control experiment were rapidly consumed, and the reaction stopped because of a lack of reagent, while in the presence of bacteria ferric ions were regenerated by the bacteria in sufficient quantities to ensure that the reaction was not halted. The previous experiments could not distinguish between the direct and indirect mechanisms of bacterial leaching because of the different conditions used.
One of the experimental strategies that has been adopted to determine the mechanism of bacterial leaching is to limit the amount of iron added to the system (33). Pistorio et al. (33) concluded that bacterial leaching of zinc sulfide occurred by the direct mechanism since the mineral dissolved in the absence of dissolved iron. However, the absence of dissolved iron does not actually mean that the direct mechanism is operative. It is well-known that metal sulfides react with acids to form hydrogen sulfide in the absence of an oxidant (14). Therefore, it is possible that in the absence of ferric ions, the mineral will be attacked by the acid and produce hydrogen sulfide, which the bacteria oxidize. This mechanism for bacterial leaching in the absence of ferric ions has not been discussed previously to our knowledge. However, it is a possible explanation for the results of Pistorio et al. (33).
Other experimental approaches have been used to distinguish between the direct and indirect mechanisms (1, 21, 31, 34, 36, 37). The conclusions that have been drawn from the research to date are (i) that either the direct mechanism is dominant (21, 27, 37, 46) or the indirect mechanism is dominant (1, 4, 36) and (ii) that some sulfide minerals are leached by the direct mechanism, whereas others are leached by the indirect mechanism (3, 10, 34). These contradictory conclusions have been drawn because of the different types of experiments that have been performed. A characteristic of all the experiments described previously is that the solution conditions are different. The differences are either that the solution conditions for a bacterial leaching experiment and a chemical leaching experiment are different (27, 46), that solution conditions change during the course of the experiment (4, 5), or that bacterial leaching experiments are performed under different solution conditions (31).
We used an experimental procedure that allowed us to distinguish between the two mechanisms. This was achieved by controlling the redox potential, which ensured that bacterial leaching and sterile control experiments were performed under conditions under which the concentrations of ferric and ferrous ions were constant throughout the experiment. A comparison of the conversion data obtained in the presence and absence of bacteria allowed us to determine the contributions of the direct and indirect mechanisms of leaching. We clearly demonstrated that the indirect mechanism plays a central role in the leaching of zinc sulfide, and we found no evidence that there is a direct mechanism over a wide range of conditions.
ACKNOWLEDGMENTS
We thank Billiton Process Research and the Foundation for Research Development for funding this project.
We also thank D. Rawlings (University of Cape Town) and E. Lawson (University of the Witwatersrand, Johannesburg, South Africa) for supplying the bacterial culture and valuable assistance.
REFERENCES
- 1.Boon M. Theoretical and experimental methods in the modelling of bio-oxidation kinetics of sulphide minerals. Ph.D. thesis. Delft, The Netherlands: Technical University of Delft; 1996. [Google Scholar]
- 2.Boon M, Hansford G S, Heijnen J J. The role of bacterial ferrous iron oxidation in the bio-oxidation of pyrite. In: Jerez C A, Vargas T, Toledo H, Wiertz J V, editors. Biohydrometallurgical processing. II. Santiago, Chile: University of Chile Press; 1995. pp. 153–164. [Google Scholar]
- 3.Boon M, Heijnen J J. Mechanisms and rate limiting steps in bioleaching of sphalerite, chalcopyrite and pyrite with Thiobacillus ferrooxidans. In: Torma A E, Wey J E, Lakshmanan V L, editors. Biohydrometallurgical technologies. Warrendale, Pa: The Minerals, Metals and Materials Society; 1993. pp. 217–235. [Google Scholar]
- 4.Boon M, Heijnen J J. Chemical oxidation kinetics of pyrite in bioleaching processes. Hydrometallurgy. 1998;48:27–41. [Google Scholar]
- 5.Boon M, Snijder M, Hansford G S, Heijnen J J. The oxidation kinetics of zinc sulphide with Thiobacillus ferrooxidans. Hydrometallurgy. 1998;48:171–186. [Google Scholar]
- 6.Chaudhury G R, Sukla L B, Das R P. Kinetics of bio-chemical leaching of sphalerite concentrate. Metall Trans B. 1985;16B:667–670. [Google Scholar]
- 7.Choi W K, Torma A E, Ohline R W, Ghali E. Electrochemical aspects of zinc sulphide leaching by Thiobacillus ferrooxidans. Hydrometallurgy. 1993;33:137–152. [Google Scholar]
- 8.Colmer A R, Hinkle M E. The role of microorganisms in acid mine drainage: a preliminary report. Science. 1947;106:253–256. doi: 10.1126/science.106.2751.253. [DOI] [PubMed] [Google Scholar]
- 9.Colmer A R, Temple K L, Hinkle M E. An iron-oxidizing bacterium from the acid drainage of some bituminous coal mines. J Bacteriol. 1950;59:317–328. doi: 10.1128/jb.59.3.317-328.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrans I J, Harris B, Ralph B J. Bacterial leaching: an introduction to its application and theory and a study on its mechanism of operation. J S Afr Inst Mining Metall. 1972;72:221–230. [Google Scholar]
- 11.Crundwell F K. Kinetics and mechanism of the oxidative dissolution of a zinc sulphide concentrate in ferric sulphate solution. Hydrometallurgy. 1987;19:227–242. [Google Scholar]
- 12.Crundwell F K. Effect of iron impurity in zinc sulphide concentrates on the rate of dissolution. Am Inst Chem Eng J. 1988;34:1128–1134. [Google Scholar]
- 13.Crundwell F K. The kinetics of the chemiosmotic proton circuit of the iron-oxidising bacterium Thiobacillus ferrooxidans. Bioelectrochem Bioenerg. 1997;43:115–122. [Google Scholar]
- 14.Crundwell F K, Verbaan B. Kinetics and mechanisms of the non-oxidative dissolution of sphalerite (zinc sulphide) Hydrometallurgy. 1987;17:369–384. [Google Scholar]
- 15.Curutchet G, Pogliani C, Donati E, Tedesco P. Effect of iron(III) and its hydrolysis products (jarosites) on Thiobacillus ferrooxidans growth and on bacterial leaching. Biotechnol Lett. 1992;14:329–334. [Google Scholar]
- 16.Dew D W, Lawson E N, Broadhurst J L. The BIOX® process for biooxidation of gold-bearing ore or concentrates. In: Rawlings D E, editor. Biomining: theory, microbes and industrial processes. Berlin, Germany: Springer-Verlag; 1998. pp. 45–80. [Google Scholar]
- 17.D’Hughes P, Cezac P, Cabral T, Battaglia F, Truong-Meyer X M, Morin D. Bioleaching of a cobaltiferous pyrite: a continuous laboratory-scale study at high solids concentration. Miner Eng. 1997;10:507–527. [Google Scholar]
- 18.Duncan D W, Landesman J, Walden C C. Role of Thiobacillus ferrooxidans in the oxidation of sulfide minerals. Can J Microbiol. 1967;13:397–403. doi: 10.1139/m67-052. [DOI] [PubMed] [Google Scholar]
- 19.Dutrizac J E, MacDonald R J C. Ferric ion as a leaching medium. Miner Sci Eng. 1974;6:59–100. [Google Scholar]
- 20.Evangelou V P. Pyrite oxidation and its control: solution chemistry, surface chemistry, acid mine drainage (AMD), molecular oxidation mechanisms. Boca Raton, Fla: CRC Press; 1995. p. 1. [Google Scholar]
- 21.Free M L, Oolman T, Nagpal S, Dahlstroom D A. Bioleaching of sulphide ores—distinguishing between indirect and direct mechanisms. In: Smith R W, Misra M, editors. Mineral bioprocessing. Warrendale, Pa: The Minerals, Metals and Materials Society; 1991. pp. 485–495. [Google Scholar]
- 22.Garcia O, Bigham J M, Tuovinen O H. Sphalerite oxidation by Thiobacillus ferrooxidans and Thiobacillus thiooxidans. Can J Microbiol. 1995;41:578–584. [Google Scholar]
- 23.Harvey P I, Crundwell F K. Growth of Thiobacillus ferrooxidans: a novel experimental design for batch growth and bacterial leaching studies. Appl Environ Microbiol. 1997;63:2586–2592. doi: 10.1128/aem.63.7.2586-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin Z-M, Warren G W, Henein H. Reaction kinetics of the ferric chloride leaching of sphalerite—an experimental study. Metall Trans B. 1984;15B:5–12. [Google Scholar]
- 25.Konishi Y, Kubo H, Asai S. Bioleaching of zinc sulfide concentrate by Thiobacillus ferrooxidans. Biotechnol Bioeng. 1992;39:66–74. doi: 10.1002/bit.260390111. [DOI] [PubMed] [Google Scholar]
- 26.Lizama H M, Suzuki I. Synergistic competitive inhibition of ferrous iron oxidation by Thiobacillus ferrooxidans by increasing concentrations of ferric iron and cells. Appl Environ Microbiol. 1989;55:2588–2591. doi: 10.1128/aem.55.10.2588-2591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lizama H M, Suzuki I. Rate equations and kinetic parameters of the reactions involved in pyrite oxidation by Thiobacillus ferrooxidans. Appl Environ Microbiol. 1989;55:2918–2923. doi: 10.1128/aem.55.11.2918-2923.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lizama H M, Suzuki I. Interaction of chalcopyrite and sphalerite with pyrite during leaching by Thiobacillus ferrooxidans and Thiobacillus thiooxidans. Can J Microbiol. 1991;37:304–311. [Google Scholar]
- 29.Lundgren D G, Silver M. Ore leaching by bacteria. Annu Rev Microbiol. 1980;34:263–283. doi: 10.1146/annurev.mi.34.100180.001403. [DOI] [PubMed] [Google Scholar]
- 30.Muyzer G, De Bryn A C, Schmedding D J M, Bos P, Westbroek P, Kuenen G J. A combined immunofluorescence-DNA-fluorescence staining technique for enumeration of Thiobacillus ferrooxidans in a population of acidophilic bacteria. Appl Environ Microbiol. 1987;53:660–664. doi: 10.1128/aem.53.4.660-664.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyavor K, Egiebor N O, Fedorak P M. Bacteria oxidation of sulphides during acid mine drainage formation: a mechanistic study. In: Warren G W, editor. EPD Congress 1996. Warrendale, Pa: Minerals, Metals and Materials Society; 1996. pp. 269–287. [Google Scholar]
- 32.Nyavor K, Egiebor N O, Fedorak P M. The effect of ferric ion on the rate of ferrous oxidation by Thiobacillus ferrooxidans. Appl Microbiol Biotechnol. 1996;45:688–691. [Google Scholar]
- 33.Pistorio M, Curutchet G, Donati E, Tedesco P. Direct zinc sulphide bioleaching by Thiobacillus ferrooxidans and Thiobacillus thiooxidans. Biotechnol Lett. 1994;16:419–424. [Google Scholar]
- 34.Porro S, Ramirez S, Reche C, Curutchet G, Alonso-Romanowski S, Donati E. Bacterial attachment—its role in bioleaching processes. Process Biochem. 1997;32:573–578. [Google Scholar]
- 35.Rawlings D E. Restriction enzyme analysis of 16S rRNA genes for the rapid identification of Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Leptospirillum ferrooxidans strains in leaching environments. In: Jerez C A, Vargas T, Toledo H, Wiertz J V, editors. Biohydrometallurgical processing. II. Santiago, Chile: University of Chile Press; 1995. pp. 9–17. [Google Scholar]
- 36.Sand W, Gerke T, Hallmann R, Schippers A. Sulfur chemistry, biofilm, and the (in)direct attack mechanism—a critical evaluation of bioleaching. Appl Microbiol Biotechnol. 1995;43:961–966. [Google Scholar]
- 37.Shrihari J, Modak M, Kumar R, Gandhi K S. Dissolution of particles of pyrite mineral by direct attachment of Thiobacillus ferrooxidans. Hydrometallurgy. 1995;38:175–187. [Google Scholar]
- 38.Silverman M P. Mechanism of bacterial pyrite oxidation. J Bacteriol. 1967;94:1046–1051. doi: 10.1128/jb.94.4.1046-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverman M P, Ehrlich H L. Microbial formation and degradation of minerals. Adv Appl Microbiol. 1964;6:181–183. [Google Scholar]
- 40.Silverman M P, Lundgren D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1954;77:642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somasundaran P, Moudgil B M. Reagents in mineral technology. New York, N.Y: Marcel Dekker Inc.; 1988. [Google Scholar]
- 42.Sugio T, Hirose T, Oto A, Inagaki K, Tano T. The regulation of sulfur use by ferrous ion in Thiobacillus ferrooxidans. Agric Biol Chem. 1990;54:2017–2022. [Google Scholar]
- 43.Sugio T, Katagiri T, Moriyama M, Zhen Y L, Inagaki K, Tano T. Existence of a new type of sulfite oxidase which utilizes ferric ion as an electron acceptor in Thiobacillus ferrooxidans. Appl Environ Microbiol. 1988;54:153–157. doi: 10.1128/aem.54.1.153-157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suni J, Henein H, Warren G W, Reddy D. Modelling the leaching kinetics of a sphalerite concentrate size distribution in ferric chloride solution. Hydrometallurgy. 1989;22:22–38. [Google Scholar]
- 45.Torma A E. The role of Thiobacillus ferrooxidans in hydrometallurgical processes. Adv Biochem Eng. 1977;6:1–37. [Google Scholar]
- 46.Torma A E, Walden C C, Branion R M R. Microbiological leaching of a zinc sulfide concentrate. Biotechnol Bioeng. 1970;12:501–517. doi: 10.1002/bit.260120403. [DOI] [PubMed] [Google Scholar]
- 47.Verbaan B, Crundwell F K. An electrochemical model for the leaching of a sphalerite concentrate. Hydrometallurgy. 1986;16:345–359. [Google Scholar]
- 48.Verbaan B, Huberts R. An electrochemical study of the bacterial leaching of synthetic Ni3S2. Int J Miner Process. 1988;24:185–202. [Google Scholar]
- 49.Vogel A I. A textbook of quantitative inorganic analysis. London, United Kingdom: Longman; 1962. p. 309 and 319. [Google Scholar]