Abstract
Objectives:
To evaluate the evolution of CT radiation dose in pediatric patients undergoing hybrid 2-[18F]fluoro-2-deoxy-D-glucose (2-[18F]FDG) PET/CT between 2007 and 2021.
Methods and materials:
Data from all pediatric patients aged 0–18 years who underwent hybrid 2-[18F]FDG PET/CT of the body between January 2007 and May 2021 were reviewed. Demographic and imaging parameters were collected. A board-certified radiologist reviewed all CT scans and measured image noise in the brain, liver, and adductor muscles.
Results:
294 scans from 167 children (72 females (43%); median age: 14 (IQR 10–15) years; BMI: median 17.5 (IQR 15–20.4) kg/m2) were included. CT dose index-volume (CTDIvol) and dose length product (DLP) both decreased significantly from 2007 to 2021 (both p < 0.001, Spearman’s rho coefficients −0.46 and −0.35, respectively). Specifically, from 2007 to 2009 to 2019–2021 CTDIvol and DLP decreased from 2.94 (2.14–2.99) mGy and 309 (230-371) mGy*cm, respectively, to 0.855 (0.568–1.11) mGy and 108 (65.6–207) mGy*cm, respectively. From 2007 to 2021, image noise in the brain and liver remained constant (p = 0.26 and p = 0.06), while it decreased in the adductor muscles (p = 0.007). Peak tube voltage selection (in kilovolt, kV) of CT scans shifted from high kV imaging (140 or 120kVp) to low kV imaging (100 or 80kVp) (p < 0.001) from 2007 to 2021.
Conclusion:
CT radiation dose in pediatric patients undergoing hybrid 2-[18F]FDG PET/CT has decreased in recent years equaling approximately one-third of the initial amount.
Advances in knowledge:
Over the past 15 years, CT radiation dose decreased considerably in pediatric patients undergoing hybrid imaging, while objective image quality may not have been compromised.
Introduction
Positron emission tomography (PET) has emerged as a landmark clinical imaging modality enabling the visualization and measurement of cellular metabolism and molecular interactions in the brain, heart, and in tumor tissue. Hybrid PET/CT (combination of PET and computed tomography) simultaneously provides functional and anatomical information. Thus, hybrid PET/CT imaging has revolutionized initial oncologic workup. It is considered of paramount importance for the diagnosis, staging, response assessment, and management of metabolically active malignancies, such as lymphoma, sarcoma, and melanoma. Beyond its role in oncologic imaging, hybrid PET/CT has also shown promise for the management of non-malignant diseases, such as chronic granulomatous disease or fever of unknown origin. 1–7
Despite its merits, the use of hybrid PET/CT imaging is associated with radiation exposure. Especially in children, radiation exposure is a concern, as they are particularly sensitive to ionizing radiation. In hybrid PET/CT imaging, radiation originates both from the administration of a radiopharmaceutical tracer, most often 2-[18F]fluoro-2-deoxy-D-glucose (2-[18F]FDG) , as well as from the CT scan. The latter merits particular focus, both because children may undergo several consecutive CT scans as part of their diagnostic workup and because even a single CT scan can be associated with a considerable amount of radiation dose. 8–10 Given these considerations, recent scientific endeavors, such as improved detector designs and tubes, low tube voltage imaging and iterative reconstruction algorithms, have aimed at reducing the radiation dose of CT scans without compromising image quality.
In our study, we sought to systematically evaluate the temporal evolution of the CT radiation dose in pediatric patients undergoing hybrid 2-[18F]FDG PET/CT between the years 2007 and 2021, hypothesizing that within these last 15 years, improvements in CT hardware and software have enabled considerable radiation dose savings without compromising the objective image quality.
Methods and Materials
Study subjects
The study design was approved by the local ethics committee (Kantonale Ethikkomission Zürich, Zurich, Switzerland). The study was performed in accordance with ICH-GCP-rules and the Declaration of Helsinki (2013). In patients scanned before January 2016, a waiver for written informed consent was granted. For patients scanned after this date, only those were included who provided written informed consent (or obtained from the responsible parental person) to the retrospective use of their data for research. In this retrospective study, we focused on the evaluation of all pediatric patients aged 0–18 years who underwent hybrid PET/CT of the body between January 2007 and May 2021 at the University Hospital Zurich, Zurich, Switzerland. A hospital-wide database search was conducted to identify all relevant patients and their clinical information, including age, gender, body height, body weight, body-mass index (BMI), international classification of diseases codes (ICD-10), and imaging parameters, such as volume computed tomography dose index (CTDIvol), dose-length product (DLP), and peak kilovoltage (kVp) of the CT scan and administered radiotracer activity in Mega-Becquerel (MBq).
Hybrid 2-[18F]FDG PET/CT Imaging
Hybrid PET/CT imaging was performed on state-of-the-art equipment with state-of-the art protocols at the time of image acquisition. In total, images were acquired on four different scanner platforms of one vendor (GE Healthcare, Waukesha, WI). The following platforms were used throughout the years: Discovery RX, Discovery STE/VCT, Discovery 690 VCT, and Discovery MI. A detailed overview of the technical parameters of all scanners is provided in the Supplementary Material. For all patients, a standard clinical 2-[18F]FDG dosage protocol was utilized. Specifically, until 2016, a body-weight-dependent amount of radiotracer activity was used while from 2017 onward a body-weight-dependent BMI-adapted protocol was used as recommended by relevant guidelines. 11,12 All patients were instructed to fast for at least 4 h prior to 2-[18F]FDG injection. The uptake time of 2-[18F]FDG was 60 min by default.
CT scans were acquired as whole-body exams or partial-body exams for hybrid PET/CT imaging, depending on the clinical indication. CT image reconstruction was performed with filtered back projection (FBP) in the earlier years, followed by iterative reconstruction (IR) in more recent years, in line with technological developments. Specifically, from 2008 onwards, three different IR algorithms were developed and implemented by the vendor (GE Healthcare): First, ASIR (Adaptive Statistical Iterative Reconstruction) which is a hybrid IR algorithm that works in the raw-data domain to model the noise and the object. Furthermore, a full model-based IR algorithm named Veo was introduced that implemented the modeling of the entire system with physics and optics modeling. Most recently, a further hybrid IR algorithm named ASIR-V was introduced which combines the characteristics of both previous IR algorithm to enable a more advanced noise and object modeling. Importantly, with ASIR-V, both noise and artifacts can be reduced and reconstruction times are shortened. 13,14 All CT scans were reconstructed with a standard soft tissue kernel, 3.75 mm slice thickness, 1.25 mm increment, and a 512 × 512 pixel matrix.
Image analysis
All CT scans were reviewed by a board-certified radiologist with 7 years of experience in a blinded and randomized manner. The radiologist performed an objective image quality assessment by placing regions of interest (ROI) into the thalamus of the brain, the liver, and the adductor muscles. The standard deviation of the attenuation (in HU) was recorded from each ROI and was considered as an objective image quality metric reflecting the image noise. 15
Statistical analysis
All statistical analyses were performed with the R statistical software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/). Data distribution was assessed visually. Jonckheere–Terpstra tests were computed to assess whether there was a specific trend in scores (i.e., radiation dose or objective image quality) between the years 2007 and 2021. To assess the association between year of image acquisition and radiation dose or objective image quality, Spearman’s rho was calculated. Two-sided p-values < 0.05 were considered significant. Data were summarized either as mean ± standard deviation or as median (interquartile range, IQR).
Results
Study subjects
Between January 2007 and May 2021, the data of 167 children, including 72 females (43%), and 95 males (57%), with a total of 294 scans were assessed. Indications for PET/CT imaging were neoplasms in 135 patients (80.8%), diseases of the blood and blood-forming organs in 17 patients (10.2%), infectious diseases in 11 patients (6.6%), and diseases of the musculoskeletal system and connective tissue in four patients (2.4%), respectively. The number of scans per patient ranged from 1 scan for 95 patients to 14 scans for one patient. At the time of image acquisition, patients were at median 14 (10 - 15) years old (mean age: 12 years, age range: 0–17 years), and exhibited a median BMI of 17.5 (15–20.4) kg/m2.
Hybrid 2-[18F]FDG PET/CT Imaging
Of all 294 scans, 159 (54.1%) were acquired on a Discovery RX (used between 2007 and 2016) , 85 (28.9%) on a Discovery STE/VCT (used between 2007 and 2016), 30 (10.2%) on a Discovery MI (used between 2017 and 2021), and 20 (6.8%) on the Discovery 690 VCT PET/CT system (used between 2011 and 2020), respectively. The median CTDIvol and DLP were 2.4 (1.3–2.9) mGy and 252.8 (124.7–346) mGy*cm across all scans and an a per-scanner basis 2.8 (1.3–2.9) mGy and 271 (131-346) mGy*cm for the Discovery RX, 2.6 (1.4–3.4) mGy and 277 (143-407) mGy*cm for the Discovery STE/VCT, 0.8 (0.5–1) mGy and 94.8 (56.3–145) mGy*cm for the Discovery MI and finally 2.6 (2.3–2.7) mGy and 299 (264-305) mGy*cm for the Discovery 690 VCT system. The median injected 2-[18F]FDG activity was 237.5 (166.8–307.8) MBq across all scans.
Temporal evolution of CT radiation dose
A detailed overview of the data is provided in Table 1 and Figure 1. CTDIvol and DLP both decreased significantly from 2007 to 2021 (both p < 0.001). Spearman’s rho coefficients between radiation dose metrics and year of scan were −0.46 for CTDIvol (p < 0.001) and −0.35 for DLP (p < 0.001). This trend of decreasing dose values across the years could generally also be shown per scanner within their respective periods of use (Spearman’s rho coefficients ranging from −0.59 to 0.043 with p-values of <0.001 to 0.89) (Supplementary Material).
Table 1.
Overview of patient and imaging metrics across all years. Data are shown as median (interquartile range)
| Year of scan | |||||
|---|---|---|---|---|---|
| 2007–2009 n = 91 |
2010–2012 n = 88 |
2013–2015 n = 65 |
2016–2018 n = 36 |
2019–2021 n = 14 |
|
| Body weight, kg | 45 (27–58) | 46.5 (30 - 58) | 41 (31–63) | 42 (24–65) | 49 (29–54) |
| Body height, cm | 165 (135–173) | 158 (14–175) | 157 (144–170) | 158 (122–172) | 158 (134–168) |
| BMI, kg/m2 | 16.3 (14.3–19.0) | 18.1 (15.4–20.8) | 17.9 (15.4–21.8) | 17.7 (15.8–21.5) | 17.4 (17.2–20) |
| CTDIvol (mGy) | 2.94 (2.14–2.99) | 2.42 (1.12–2.94) | 2.14 (1.29–2.8) | 1.24 (0.778–2.23) | 0.855 (0.568–1.11) |
| DLP (mGy*cm) | 309 (230–371) | 253 (104–346) | 231 (126–319) | 154 (89–264) | 108 (65.6–207) |
| CTDIvol/BMI (mGy*m2/kg) | 0.161 (0.137–0.195) | 0.119 (0.07–0.151) | 0.01 (0.081–0.136) | 0.065 (0.046–0.105) | 0.049 (0.033–0.057) |
| Image noise, HU | |||||
| Brain | 12 (9.7-14.4) | 13.4 (11.2-16.9) | 12.4 (9.1-14.8) | 14.5 (10.8-16) | 12.2 (10.5-15.7) |
| Liver | 17.1 (13-21.2) | 19 (15.1-23.8) | 17.1 (14.6-20.9) | 15.5 (13.9-17.1) | 13.5 (11.5-17.1) |
| Adductor muscles | 20.3 (12.9-26.8) | 22.6 (16.5-29.3) | 20 (15.5-23) | 15.6 (13.7-18.1) | 14.2 (11.9-17.1) |
BMI, body mass index; DLP, CTDIvol, HU; MBq, Mega-Becquerel; kg, kilogramm.
Values are given as absolute numbers and percentages in parenthesis or as median (25th to 75th percentile).
Figure 1.
Overview of CT radiation dose metrics including dose-length product (DLP) and computed tomography dose index (CTDIvol) of all scans (n = 294) across the years.
In order to account for body size and age, we calculated a normalized CTDIvol metric (named CTDInorm) by dividing CTDIvol by BMI and by grouping childrens’ ages as follows: 0–4 years (n = 13), 5–7 years (n = 23), 8–11 years (n = 52), 12–15 years (n = 137), and 16–18 years (n = 69). Irrespective of age, CTDInorm also decreased significantly from 2007 to 2021 (p < 0.001), with Spearman’s rho coefficients between CTDInorm and year of scan being −0.57 (p < 0.001). When stratified by age group, Spearman’s rho coefficients between CTDInorm and year of scan were −0.44 (p = 0.132),–0.6 (p = 0.002),–0.66 (p < 0.001),–0.52 (p < 0.001), and −0.7 (p < 0.001) for the age groups 0–4 years, 5–7 years, 8–11 years, 12–15 years, and 16–18 years, respectively, Figure 2.
Figure 2.
Overview of normalized computed tomography dose index (CTDIvol) metric (named CTDInorm), derived by dividing CTDIvol by body-mass index (BMI) and by grouping childrens’ ages.
Temporal evolution of CT objective image quality
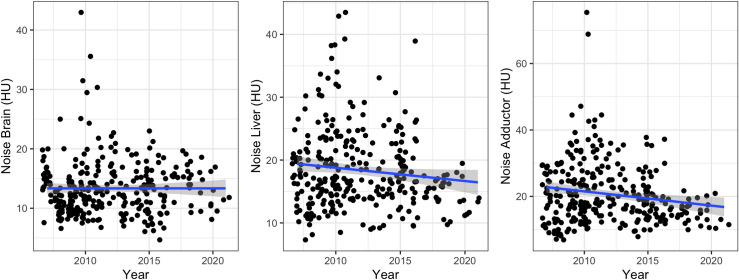
A detailed overview of the evolution of objective image quality is provided in Table 1 and Figure 3 . Image noise in the brain and liver remained constant from 2007 to 2021 (p = 0.26 and p = 0.06). Specifically, Spearman’s rho coefficients between image noise and the year of scan were 0.06 (p = 0.34) for the brain and −0.07 (p = 0.25) for the liver, respectively. For the adductor muscles, image noise decreased slightly from 2007 to 2021 (p = 0.007). Spearman’s rho coefficient between image noise and the year of scan was −0.12 (p = 0.04) for the adductor muscles. This trend of stable or even reduced image noise levels across the years could generally also be shown per scanner within their respective periods of use (Spearman’s rho coefficients ranging from −0.36 to 0.14 with p-values of 0.047 to 0.86) (Supplementary Material).
Figure 3.
Overview of objective image noise in the brain, liver and adductor muscles across the years.
Temporal evolution of CT tube voltage selection
An overview of the CT tube voltage across the scanners and years is provided in Figure 4. From 2007 to 2021, peak tube voltage selection shifted from high kV imaging (140 or 120 kVp) to low kV imaging (100 or 80 kVp) (p < 0.001). Specifically, in the years 2007–2009, 95% of scans were acquired with high kVp (140 or 120 kVp) and 5% of scans with low kVp (i.e., exclusively 100 kVp during this time). In the years 2019–2021, 64% of scans were acquired with high kVp (i.e., exclusively 120 kVp during this time) and 36% of scans with low kVp (100 or 80 kVp). On a per-scanner basis, a similar trend could be observed with more recent scanner models (Discovery MI) exhibiting only scans with tube voltages of 80, 100 or 120 kV while earlier models (Discovery RX and Discovery STE/VCT and to a lesser extent Discovery 690 VCT) exhibited scans with tube voltages ranging from 80 to 140 kV, whereby a large proportion of scans was acquired with 140 kV tube voltage on the two earliest scanner models (Discovery RX and Discovery STE/VCT).
Figure 4.
Overview of CT tube voltage selection across the scanners and years.
Discussion
In this study, we analyzed the temporal evolution of CT radiation dose in pediatric patients undergoing hybrid 2-[18F]FDG PET/CT between 2007 and 2021. To the best of our knowledge, this is the first study on this topic covering a large time span of 15 years.
Our results show that within the past few years, technological improvements have facilitated considerable radiation dose reduction while objective image quality may not have been compromised.
Notably, Ghoshhajra et al quantified the evolution of radiation dose in pediatric patients undergoing cardiac CT imaging at a tertiary medical center during an 8-year period (2004–2012). The authors found substantial dose reductions of up to 85.8% by employing state-of-the-art imaging hardware and software and the most recent imaging protocols. 16 Our results are in line with these previous observations given the fact that we also observed considerable dose reductions across the years, although in the context of CT imaging performed during hybrid PET/CT imaging.
Radiation exposure by medical imaging, such as CT or hybrid PET/CT imaging, follows the ALARA principle 17,18 ; A patients’ exposure should be as low as reasonably achievable. This principle is of special importance for pediatric patients. It was estimated that children exhibit an increased radiation sensitivity for at least 25% of cancer types. Also, children have a longer life expectancy than adults, and hence late health effects caused by radiation exposure are more likely to manifest. Thus, recent epidemiological studies have confirmed that CT imaging during childhood may significantly be associated with the occurrence of central nervous system (CNS) tumors or leukemia. 19–22
While hybrid 2-[18F]FDG PET/CT imaging has emerged as a landmark imaging modality in pediatric patients with malignancies, the radiation exposure associated with this imaging modality is considerable: First, radiation originates both from the radiopharmaceutical tracer used for PET imaging as well as from CT imaging. Second, CT in PET/CT often represents a “full-body” scan, with images acquired from the brain to the mid-thighs. Thus, CT radiation exposure and its potential harmful effect is high, not only because the Z-axis coverage is particularly wide, but also because virtually all radiosensitive organs are irradiated. In this respect, a 5-year retrospective study on the cumulative radiation dose from PET/CT in children has estimated that the largest portion of radiation dose originates from the CT component of the examinations. 9 This finding further emphasizes the importance of optimizing CT protocols, hardware and software of hybrid PET/CT examinations in an effort to reduce the radiation dose.
Our study found considerable CT radiation dose reduction within the past 15 years irrespective of patient age or body size. Specifically, CTDIvol, DLP, and CTDIvol adjusted by BMI decreased significantly and considerably between 2007 and 2021. Importantly, this trend could generally also be shown across various age groups ranging from 0 to 4 years to 16–18 years with radiation dose decreasing in all age groups across the years. A visual analysis of Figure 2 also suggested that older children were generally subjected to higher dose values across the years, which can be expected from older children generally presenting with larger bodies. 23 Lastly, a further subgroup analysis partially presented in the supplementary material showed that older scanner models generally exhibited higher dose values and that radiation dose also decreased on a per-scanner level with dose values generally decreasing significantly within a scanners period of use, presumably due to factors such as software updates and protocol improvements. Nonetheless objective image quality as measured in the brain, liver, and adductor muscles remained constant or improved slightly across the past 15 years both globally and generally also on a per-scanner level.
This evolution accurately reflects the technological improvements that have transformed CT imaging during the past few years. In recent years, improved detector designs and tubes have been introduced. Furthermore, high quality, low(er) kV imaging has become available. This can also be seen in our study where a shift from higher to lower kV imaging has occurred over the years thus facilitating further dose reductions. Lastly, novel reconstruction algorithms, such as iterative reconstruction (IR), have been developed, that enable further dose reductions without compromising image quality. 24,25
In their systematic review and meta-analysis, Willemink et al reported dose reductions of 23 to 76% relative to FBP using various IR algorithms. 26 While the exact level of achievable dose reduction may largely depend on imaging hard- and software, initial dose and reconstruction settings and the anatomical area that is being imaged, it should be emphasized that dose cannot be lowered limitlessly. 14,27 Notably, various studies suggest that at least for abdominal imaging, reductions of beyond 30% of the previous standard-of-care may compromise low-contrast detectability and thus diagnostic efficacy even when using modern IR algorithms. 27 Furthermore, it has been shown that IR may alter the appearance and texture of images by impacting the noise frequency distribution. Specifically, the application of certain IR algorithms has been associated with “plastic-” or “oil-paint-“ like appearance due to a large amount of image smoothing. This again may impact diagnostic accuracy. 28,29 Nonetheless, IR algorithms have contributed significantly to radiation dose reductions in conventional CT imaging as well as in hybrid PET/CT imaging. In this regard, we hypothesize that a significant amount of the dose reduction demonstrated in the current study stems from the implementation of IR algorithms.
With novel advances in CT imaging, such as photon-counting detector CT 30,31 and artificial intelligence powered reconstruction algorithms, 32,33 the trend toward ever lower dosage will continue. The same will affect PET imaging, where novel hardware and software solutions will also allow for further reduction in the administrated radiotracer dose. 33 Lastly, it should be mentioned that the number of pediatric patients undergoing hybrid PET/CT at our institution decreased steadily from 2007 to 2021 despite the increasing use of hybrid PET/CT imaging in medicine. Specifically, in the years 2007–2009, 91 scans were performed, while in the years 2019–2021 only 14 scans were acquired.
We believe that this may partially be attributed to the introduction of a clinical PET/MR system at our institution, where hybrid PET imaging can be performed without the radiation exposure from a CT scan. 34–36 When considering the harmful effects of ionizing radiation in children, the shift from PET/CT to PET/MR in pediatric imaging may continue in the coming years.
Our study has several limitations: First, this was a retrospective single-center study. Specifically, longitudinal data from other institutions would be of great interest to confirm our observations. Second, all imaging data originated from scanners from a single vendor. This may affect the generalizability of our results. In this regard, it should be noted that due to lack of availability, we could not include data from all scanner models of the manufacturer. Third, due to a lack of reference standard we did not evaluate whether diagnostic accuracy was affected by the reduction in radiation dose over the years. Fourth, although common in the current field of research, we acknowledge that our method of objectifying image quality was limited. We acknowledge that there may be better and more sophisticated methods of measuring objective image quality. Fifth, due to the long timespan of 15 years we were unable to gather in-depth details on the imaging protocols of all scanners which prohibited us from performing further subgroup analyses. Specifically, this prohibited us from providing insight into the impact of certain protocol changes or acquisition parameters on radiation dose or image quality.
In conclusion, we evaluated the temporal evolution of CT radiation dose in pediatric patients undergoing hybrid 2-[18F]FDG PET/CT imaging between 2007 and 2021. Our findings suggest that technological advancements have resulted in substantial reductions in radiation dose over the past 15 years, while also indicating that objective image quality may not have been compromised.
Footnotes
Acknowledgements: Moritz Schwyzer, Stephan Skawran, Thomas Sartoretti and Michael Messerli are suported by a research grant from the Palatin Foundation, Switzerland. Antonio G. Gennari, Martin Huellner and Michael Messerli are supported by a grant from the CRPP “AI Oncological Imaging Network” of the University of Zurich. The authors would like to thank Freya Klein, Josephine Trinckauf, Corina Weyermann, Marlena Hofbauer, Victoria Schober, Edlira Loga, Melanie Thüringer, Nina Bächle, Sabrina Epp, Ana-Mari Gaspar, Michèle Hug, Juliana Koller and Eirini Leivaditaki for their excellent support. Further, Michael Messerli would like to thank Prof. G. K. von Schulthess for his invaluable support of this work.
Funding: Open access funding provided by Universitat Zurich.
The authors Stephan Waelti and Michael Messerli contributed equally to the work.
Contributor Information
Stephan Skawran, Email: Stephan.Skawran@usz.ch.
Thomas Sartoretti, Email: Thomas.Sartoretti@usz.ch.
Antonio G. Gennari, Email: gennari_antonio@libero.it.
Moritz Schwyzer, Email: moritz.schwyzer@usz.ch.
Elisabeth Sartoretti, Email: elisabeth.sartoretti@uzh.ch.
Valerie Treyer, Email: Valerie.Treyer@usz.ch.
Alexander Maurer, Email: Alexander.Maurer@usz.ch.
Martin W. Huellner, Email: Martin.Huellner@usz.ch.
Stephan Waelti, Email: Stephan.Waelti@kssg.ch.
Michael Messerli, Email: michael.messerli@usz.ch.
REFERENCES
- 1. Kluge R, Kurch L, Georgi T, Metzger M. Current role of FDG-PET in pediatric Hodgkin’s lymphoma. Semin Nucl Med 2017; 47: 242–57. doi: 10.1053/j.semnuclmed.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 2. Harrison DJ, Parisi MT, Shulkin BL. The role of 18 F-FDG-PET/CT in pediatric sarcoma. Seminars in Nuclear Medicine 2017; 47: 229–41. doi: 10.1053/j.semnuclmed.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 3. Daldrup-Link HE, Theruvath AJ, Baratto L, Hawk KE. One-stop local and whole-body staging of children with cancer. Pediatr Radiol 2022; 52: 391–400. doi: 10.1007/s00247-021-05076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agrawal A, Shah S, Gnanasegaran G, Rajkotia S, Purandare N, Puranik A, et al. PET/CT normal variants and pitfalls in pediatric disorders. Semin Nucl Med 2021; 51: 572–83. doi: 10.1053/j.semnuclmed.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 5. Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med 2006; 354: 496–507. doi: 10.1056/NEJMra050276 [DOI] [PubMed] [Google Scholar]
- 6. Biermann M, Schwarzlmüller T, Fasmer KE, Reitan BC, Johnsen B, Rosendahl K. Is there a role for PET-CT and SPECT-CT in pediatric oncology. Acta Radiol 2013; 54: 1037–45. doi: 10.1258/ar.2012.120616 [DOI] [PubMed] [Google Scholar]
- 7. Biassoni L, Easty M. Paediatric nuclear medicine imaging. Br Med Bull 2017; 123: 127–48. doi: 10.1093/bmb/ldx025 [DOI] [PubMed] [Google Scholar]
- 8. Magill D, Alavi A. Radiation safety concerns related to PET/computed tomography imaging for assessing pediatric diseases and disorders. PET Clin 2020; 15: 293–98. doi: 10.1016/j.cpet.2020.03.012 [DOI] [PubMed] [Google Scholar]
- 9. Chawla SC, Federman N, Zhang D, Nagata K, Nuthakki S, McNitt-Gray M, et al. Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review. Pediatr Radiol 2010; 40: 681–86. doi: 10.1007/s00247-009-1434-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dorfman AL, Fazel R, Einstein AJ, Applegate KE, Krumholz HM, Wang Y, et al. Use of medical imaging procedures with ionizing radiation in children. Arch Pediatr Adolesc Med 2011; 165: 458–64. doi: 10.1001/archpediatrics.2010.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lassmann M, Treves ST. Pediatric Radiopharmaceutical administration: harmonization of the 2007 EANM Paediatric dosage card (version 1.5.2008) and the 2010 North American consensus guideline. Eur J Nucl Med Mol Imaging 2014; 41(): 1636. doi: 10.1007/s00259-014-2817-4 [DOI] [PubMed] [Google Scholar]
- 12. Treves ST, Gelfand MJ, Fahey FH, Parisi MT. 2016 update of the North American consensus guidelines for pediatric administered Radiopharmaceutical activities. J Nucl Med 2016; 57: 15N–18N. [PubMed] [Google Scholar]
- 13. De Marco P, Origgi D. New adaptive statistical Iterative reconstruction Asir-V: assessment of noise performance in comparison to Asir. J Appl Clin Med Phys 2018; 19: 275–86. doi: 10.1002/acm2.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willemink MJ, Noël PB. The evolution of image reconstruction for CT-from filtered back projection to artificial intelligence. Eur Radiol 2019; 29: 2185–95. doi: 10.1007/s00330-018-5810-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higashigaito K, Euler A, Eberhard M, Flohr TG, Schmidt B, Alkadhi H. Contrast-enhanced abdominal CT with clinical photon-counting detector CT: assessment of image quality and comparison with energy-integrating detector CT. Acad Radiol 2022; 29: 689–97. doi: 10.1016/j.acra.2021.06.018 [DOI] [PubMed] [Google Scholar]
- 16. Ghoshhajra BB, Lee AM, Engel L-C, Celeng C, Kalra MK, Brady TJ, et al. Radiation dose reduction in pediatric cardiac computed tomography: experience from a tertiary medical center. Pediatr Cardiol 2014; 35: 171–79. doi: 10.1007/s00246-013-0758-5 [DOI] [PubMed] [Google Scholar]
- 17. Eddy FK, Ngano SO, Jervé FA, Serge A. Radiation dose evaluation of pediatric patients in CT brain examination: multi-center study. Sci Rep 2021; 11: 4663. doi: 10.1038/s41598-021-84078-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brody AS, Frush DP, Huda W, Brent RL. Radiation risk to children from computed tomography. Pediatrics 2007; 120: 677–82. doi: 10.1542/peds.2007-1910 [DOI] [PubMed] [Google Scholar]
- 19. Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012; 380: 499–505. doi: 10.1016/S0140-6736(12)60815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013; 346: f2360. doi: 10.1136/bmj.f2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nikkilä A, Raitanen J, Lohi O, Auvinen A. Radiation exposure from computerized tomography and risk of childhood leukemia: Finnish register-based case-control study of childhood leukemia (FRECCLE). Haematologica 2018; 103: 1873–80. doi: 10.3324/haematol.2018.187716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foucault A, Ancelet S, Dreuil S, Caër-Lorho S, Ducou Le Pointe H, Brisse H, et al. Childhood cancer risks estimates following CT scans: an update of the French CT cohort study. Eur Radiol 2022; 32: 5491–98. doi: 10.1007/s00330-022-08602-z [DOI] [PubMed] [Google Scholar]
- 23. Thomas KE, Wang B. Age-specific effective doses for pediatric MSCT examinations at a large children’s hospital using DLP conversion coefficients: a simple estimation method. Pediatr Radiol 2008; 38: 645–56. doi: 10.1007/s00247-008-0794-0 [DOI] [PubMed] [Google Scholar]
- 24. Lell MM, Wildberger JE, Alkadhi H, Damilakis J, Kachelriess M. Evolution in computed tomography: the battle for speed and dose. Invest Radiol 2015; 50: 629–44. doi: 10.1097/RLI.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 25. Runge VM, Marquez H, Andreisek G, Valavanis A, Alkadhi H. Recent technological advances in computed tomography and the clinical impact therein. Invest Radiol 2015; 50: 119–27. doi: 10.1097/RLI.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 26. Willemink MJ, Leiner T, de Jong PA, de Heer LM, Nievelstein RAJ, Schilham AMR, et al. Iterative reconstruction techniques for computed tomography part 2: initial results in dose reduction and image quality. Eur Radiol 2013; 23: 1632–42. doi: 10.1007/s00330-012-2764-z [DOI] [PubMed] [Google Scholar]
- 27. Mileto A, Guimaraes LS, McCollough CH, Fletcher JG, Yu L. State of the art in abdominal CT: the limits of Iterative reconstruction Algorithms. Radiology 2019; 293: 491–503. doi: 10.1148/radiol.2019191422 [DOI] [PubMed] [Google Scholar]
- 28. Ehman EC, Yu L, Manduca A, Hara AK, Shiung MM, Jondal D, et al. Methods for clinical evaluation of noise reduction techniques in Abdominopelvic CT. Radiographics 2014; 34: 849–62. doi: 10.1148/rg.344135128 [DOI] [PubMed] [Google Scholar]
- 29. Schaller F, Sedlmair M, Raupach R, Uder M, Lell M. Noise reduction in abdominal computed tomography applying Iterative reconstruction (ADMIRE). Acad Radiol 2016; 23: 1230–38. doi: 10.1016/j.acra.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 30. Rajendran K, Petersilka M, Henning A, Shanblatt ER, Schmidt B, Flohr TG, et al. First clinical photon-counting detector CT system: technical evaluation. Radiology 2022; 303: 130–38. doi: 10.1148/radiol.212579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sartoretti T, Landsmann A, Nakhostin D, Eberhard M, Roeren C, Mergen V, et al. Quantum Iterative reconstruction for abdominal photon-counting detector CT improves image quality. Radiology 2022; 303: 339–48. doi: 10.1148/radiol.211931 [DOI] [PubMed] [Google Scholar]
- 32. Brady SL, Trout AT, Somasundaram E, Anton CG, Li Y, Dillman JR. Improving image quality and reducing radiation dose for pediatric CT by using deep learning reconstruction. Radiology 2021; 298: 180–88. doi: 10.1148/radiol.2020202317 [DOI] [PubMed] [Google Scholar]
- 33. Theruvath AJ, Siedek F, Yerneni K, Muehe AM, Spunt SL, Pribnow A, et al. Validation of deep learning-based augmentation for reduced 18F-FDG dose for PET/MRI in children and young adults with lymphoma. Radiol Artif Intell 2021; 3: e200232. doi: 10.1148/ryai.2021200232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schäfer JF, Gatidis S, Schmidt H, Gückel B, Bezrukov I, Pfannenberg CA, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology 2014; 273: 220–31. doi: 10.1148/radiol.14131732 [DOI] [PubMed] [Google Scholar]
- 35. Frush D. The cumulative radiation dose paradigm in pediatric imaging. BJR 2021; 94: 20210478. doi: 10.1259/bjr.20210478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sepehrizadeh T, Jong I, DeVeer M, Malhotra A. PET/MRI in Paediatric disease. Eur J Radiol 2021; 144: 109987. doi: 10.1016/j.ejrad.2021.109987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.