Abstract
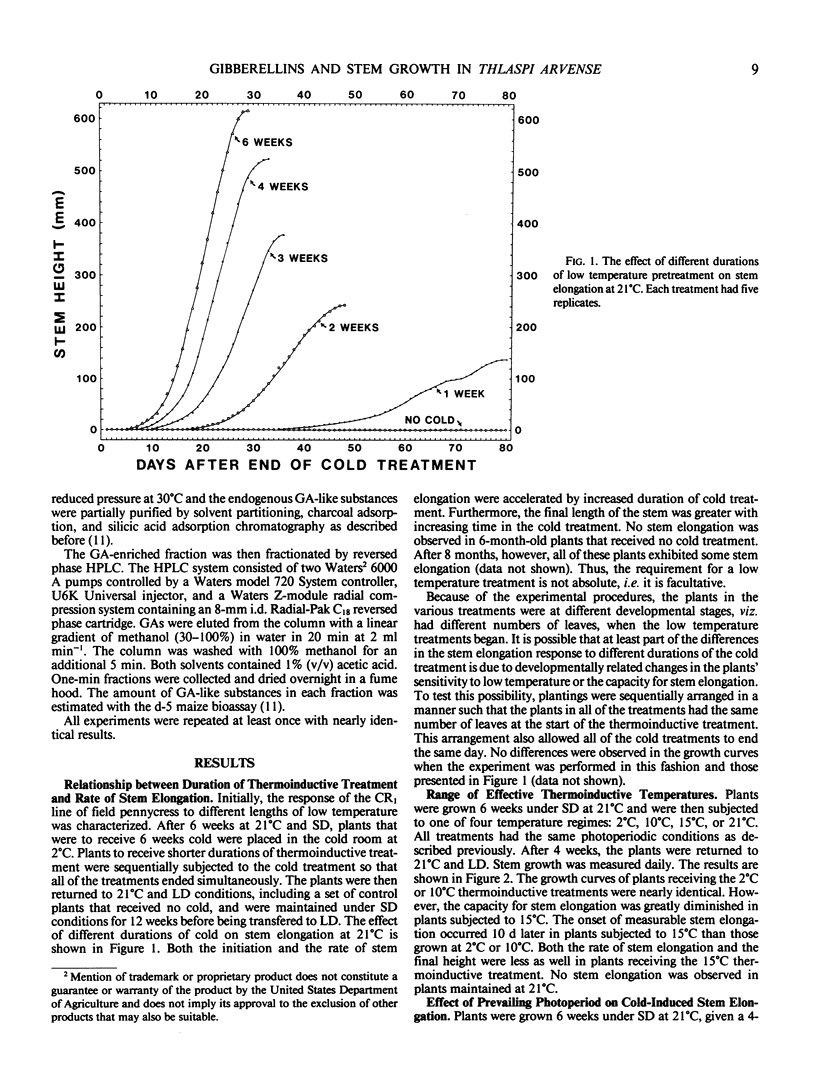
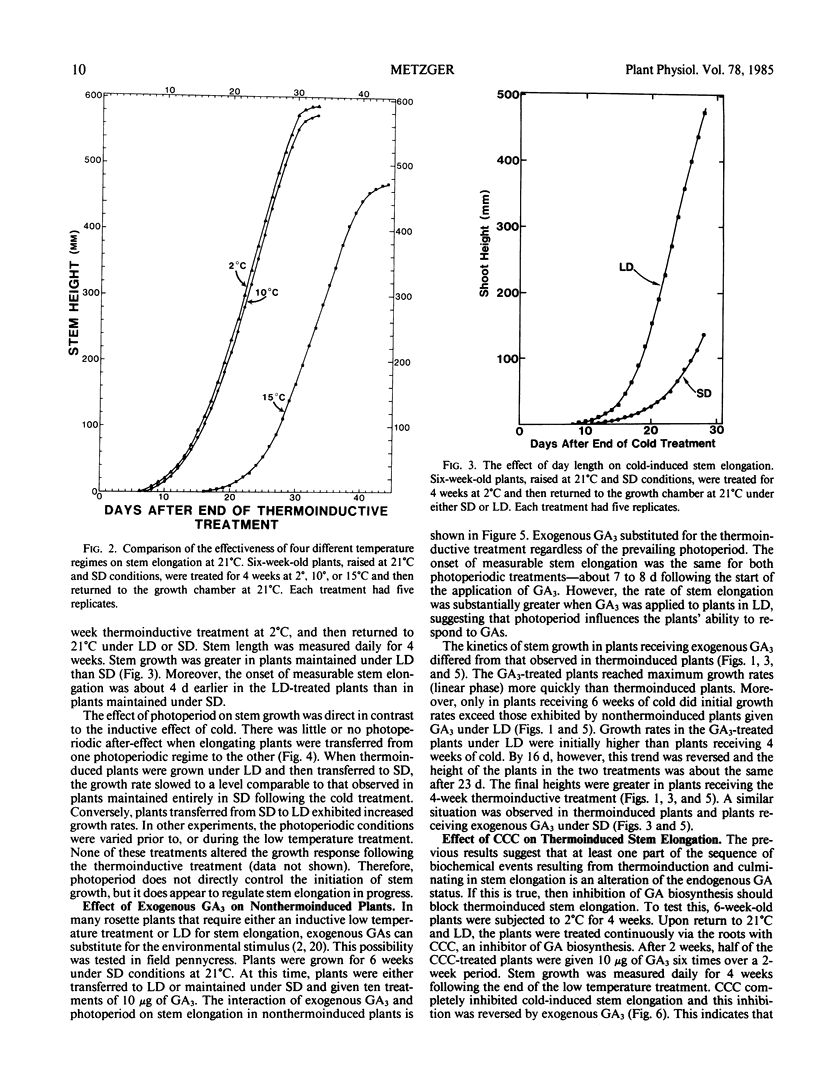
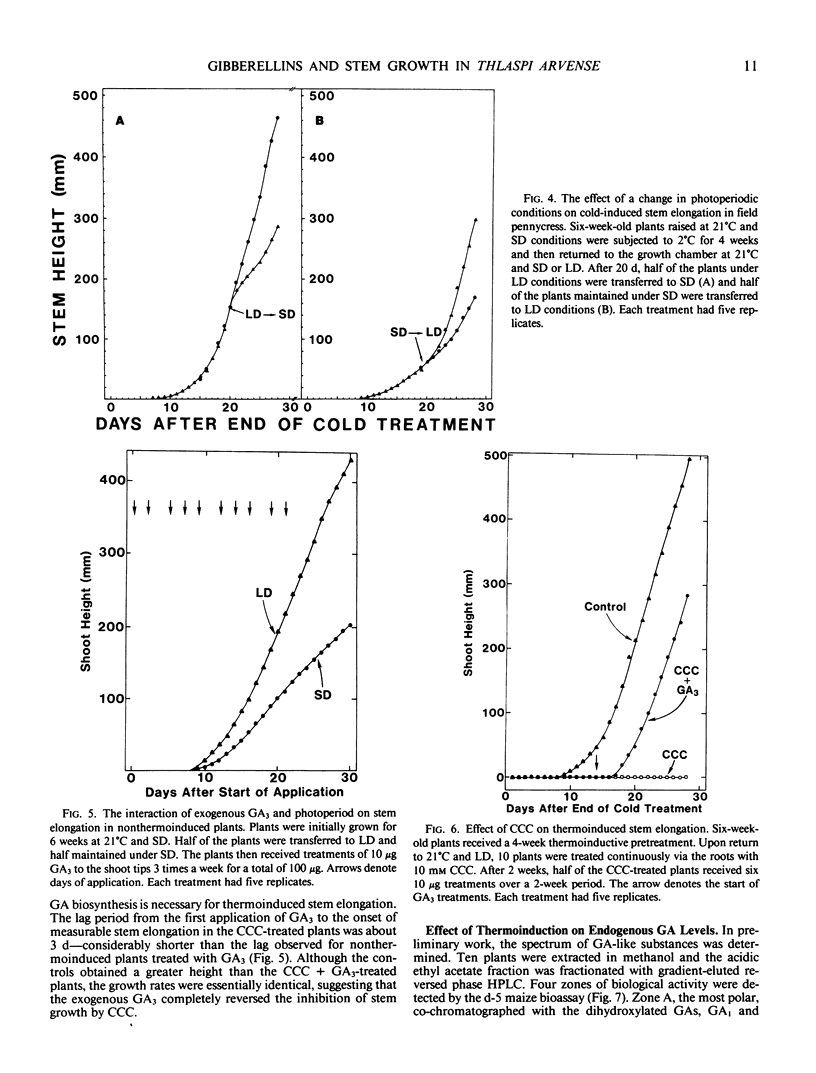
Field pennycress (Thlaspi arvense L.) is a winter annual that requires a cold treatment for the induction of stem elongation. An inbred line was selected in which no stem elongation was observed in plants grown for 6 months at 21°C regardless of the prevailing photoperiod. Increased exposure time of plants grown initially at 21°C to cold (2°C) induced a greater rate of stem elongation when the plants were returned to 21°C. Moreover, longer cold treatments resulted in a greater maximum stem height and reduced the lag period for the onset of measurable internode elongation. The optimal temperature range for thermoinduced stem growth was broad: rates of stem growth in plants maintained for 4 weeks at either 2° or 10°C were virtually identical. However, a 4-week thermoinductive treatment at 15°C resulted in a greater lag period for the initiation of stem elongation and a decreased growth rate. The rate of cold-induced stem elongation was greater in plants subjected to long days than for plants subjected to short days following the cold treatment. Thus, photoperiod does not control the induction of stem elongation, but does regulate stem elongation in progress. Exogenous gibberellin A3 (GA3) was able to substitute for the cold requirement, but elicited a greater response in plants maintained under long days than short days. This indicates that photoperiod influences the plant's sensitivity to GAs. The GA biosynthesis inhibitor, 2-chloroethyltrimethyl ammonium chloride, inhibited low temperature-induced stem elongation, and this inhibition was completely reversed by exogenous GA3. These results suggest that cold-induced stem elongation in field pennycress is mediated by some change in the endogenous GA status.
Full text
PDF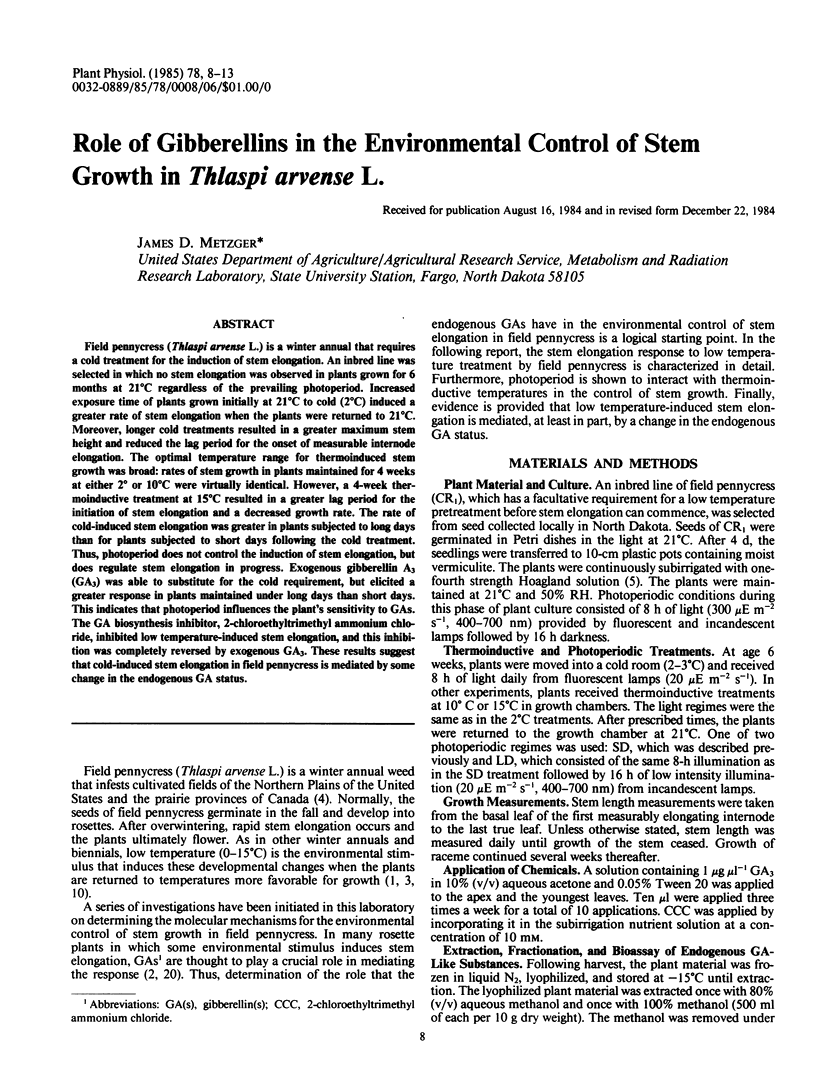

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland C. F., Zeevaart J. A. Gibberellins in Relation to Flowering and Stem Elongation in the Long Day Plant Silene armeria. Plant Physiol. 1970 Sep;46(3):392–400. doi: 10.1104/pp.46.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller L. K., Kelly W. C., Powell L. E. Temperature Interactions with Growth Regulators and Endogenous Gibberellin-like Activity during Seedstalk Elongation in Carrots. Plant Physiol. 1979 Jun;63(6):1055–1061. doi: 10.1104/pp.63.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D., Zeevaart J. A. Effect of Photoperiod on the Levels of Endogenous Gibberellins in Spinach as Measured by Combined Gas Chromatography-selected Ion Current Monitoring. Plant Physiol. 1980 Nov;66(5):844–846. doi: 10.1104/pp.66.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D., Zeevaart J. A. Identification of six endogenous gibberellins in spinach shoots. Plant Physiol. 1980 Apr;65(4):623–626. doi: 10.1104/pp.65.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. P., Paleg L. G. Low Temperature-Induced GA(3) Sensitivity of Wheat : I. Characterization of the Low Temperature Effect on Isolated Aleurone OF KITE. Plant Physiol. 1984 Sep;76(1):139–142. doi: 10.1104/pp.76.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. P., Paleg L. G. Low temperature induction of hormonal sensitivity in genotypically gibberellic Acid-insensitive aleurone tissue. Plant Physiol. 1984 Feb;74(2):437–438. doi: 10.1104/pp.74.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suge H., Rappaport L. Role of gibberellins in stem elongation and flowering in radish. Plant Physiol. 1968 Aug;43(8):1208–1214. doi: 10.1104/pp.43.8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Effects of photoperiod on growth rate and endogenous gibberellins in the long-day rosette plant spinach. Plant Physiol. 1971 Jun;47(6):821–827. doi: 10.1104/pp.47.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]


